Principles for the dynamic maintenance of cortical polarity (original) (raw)
. Author manuscript; available in PMC: 2008 Apr 20.
Summary
Diverse cell types require the ability to dynamically maintain polarized membrane protein distributions through balancing transport and diffusion. However, design principles underlying dynamically maintained cortical polarity are not well understood. Here we constructed a mathematical model for characterizing the morphology of dynamically polarized protein distributions. We developed analytical approaches for measuring all model parameters from single-cell experiments. We applied our methods to a well-characterized system for studying polarized membrane proteins: budding yeast cells expressing activated Cdc42. We found that balanced diffusion and colocalized transport to and from the plasma membrane were sufficient for accurately describing polarization morphologies. Surprisingly, the model predicts that polarized regions are defined with a precision that is nearly optimal for measured transport rates, and that polarity can be dynamically stabilized through positive feedback with directed transport. Our approach provides a step towards understanding how biological systems shape spatially precise, unambiguous cortical polarity domains using dynamic processes.
Introduction
Many cellular processes such as cell polarization, cellular morphogenesis and cell division depend critically on the stable maintenance of polarized, i.e. spatially asymmetric, distributions of molecules that regulate or execute polarized cell functions. Polarized distributions of molecules can in principle be maintained through two mutually non-exclusive mechanisms: statically, through immobilization via scaffolds, or dynamically, through constant recycling. A state in which cortical polarity is dynamically maintained has the advantage of enabling flexible and rapid cellular responses to an often wide range of input signals.
The dynamic maintenance of cortical polarity plays a central role in the development of many organisms. Membrane associated polarity markers including PH-domain containing proteins in Dictyostelium and neutrophils (Affolter and Weijer, 2005), PAR-2 and PAR-6 in C. elegans zygotes (Cheeks et al., 2004), Pon and Numb in Drosophila sensory organ precursor cells (Mayer et al., 2005), and Cdc42 and Bem1 in budding yeast (Wedlich-Soldner et al., 2004), are maintained in dynamically polarized states. Transport processes, such as cytoskeleton-mediated exocytosis and endocytosis, are critical for regulating the membrane concentrations of polarity proteins in a variety of systems. For example, the maintenance of polarity has been shown to require microtubule-based transport in fission yeast (Snaith and Sawin, 2003), and actin-based transport in Drosophila neuroblasts (Petritsch et al., 2003) and budding yeast (Pruyne and Bretscher, 2000; Wedlich-Soldner et al., 2003). Endocytosis is required for the maintenance of polarization in budding yeast (Pruyne and Bretscher, 2000; Valdez-Taubas and Pelham, 2003) and Hela cells (Bretscher and Thomson, 1983). Disruption of the actin cytoskeleton, affecting both secretion and endocytosis, leads to the loss of polarity of Numb in Drosophila (Knoblich et al., 1997), PAR-2 and PAR-3 in C. elegans zygotes (Severson and Bowerman, 2003), and Rho GTPases in yeast (Pruyne et al., 2004; Wedlich-Soldner et al., 2004). Finally, lateral diffusion within the membrane strongly influences the distribution of polarized proteins (Greenberg and Axelrod, 1993; Valdez-Taubas and Pelham, 2003).
Based on the results mentioned above, we hypothesize that dynamic polarization of membrane proteins can be achieved by balancing three redistribution mechanisms. First, proteins are redistributed from the cytoplasm to the plasma membrane via vesicle transport along actin or microtubule tracks and subsequent vesicle fusion to the plasma membrane. We will refer to this mechanism as directed transport. Second, the deposited proteins are spread within the plasma membrane via two-dimensional diffusion. Third, the proteins are redistributed from the plasma membrane back to the cytoplasm via endocytic uptake and subsequent intracellular membrane recycling. For simplicity we will refer to this mechanism as endocytosis.
The necessity of these three mechanisms for the formation of dynamically polarized steady-states is well established. However, it remains unclear whether these mechanisms are sufficient to maintain steady-state distributions of polarized membrane proteins. If they are, what is the relationship between the respective rates of redistribution and polarization morphology? Are there “special” or “optimal” rates selected by cellular systems? And, what is the role of positive feedback in maintaining the polarized state for polarity regulators (such as Cdc42) that interact with mechanisms of establishing directed transport? Here we develop mathematical models to quantitatively explore the impact of changes in transport rates and feedback strengths on polarization morphology.
To experimentally validate predictions of our model in a biological system, accurate estimates of parameters are needed to experimentally validate predictions. In most studies, model parameters are separately estimated from experiments that rely on intrusive biochemical or genetic approaches to disentangle parameters from each other (Chen et al., 2000; Lee et al., 2003). Here, we estimated all parameters but diffusion on each individual cell using a single, non-intrusive, photobleaching experiment designed to tie closely with the model. We tested our approach in a prototypical system for polarized membrane proteins: polarized yeast cells expressing a constitutively activated form of Cdc42. This Rho-type GTPase is anchored in the membrane through a C-terminal geranylgeranyl group (Johnson, 1999; Wedlich-Soldner et al., 2003; Ziman et al., 1993) and its constitutively activated form Cdc42Q61L has been shown to localize exclusively to membranes (Wedlich-Soldner et al., 2003). We previously showed that the activated Cdc42, when overexpressed, is capable of inducing spontaneous polarization in otherwise non-polarized G1 yeast cells. The ability of Cdc42Q61L in regulating the polarized state, its broad polarization morphology, and its lack of membrane dissociation make it a desirable marker for understanding dynamic polarization of membrane-bound proteins that define cortical polarity.
Results
Model for the dynamic redistribution of polarized membrane proteins
We developed a mathematical model combining the effects of three redistribution mechanisms needed to maintain a distribution of cortically polarized proteins at steady-state (Figure 1). First, our model incorporated directed transport of the protein from a homogeneous cytoplasmic pool to a small region of the plasma membrane. Second, we incorporated lateral diffusion of the protein on the plasma membrane using a uniform diffusion constant. Finally, we incorporated endocytosis at and away from the region of directed transport, with rates proportional to the amount of the protein at each membrane position.
Figure 1. Mathematical model for the dynamic redistribution of polarized membrane proteins.
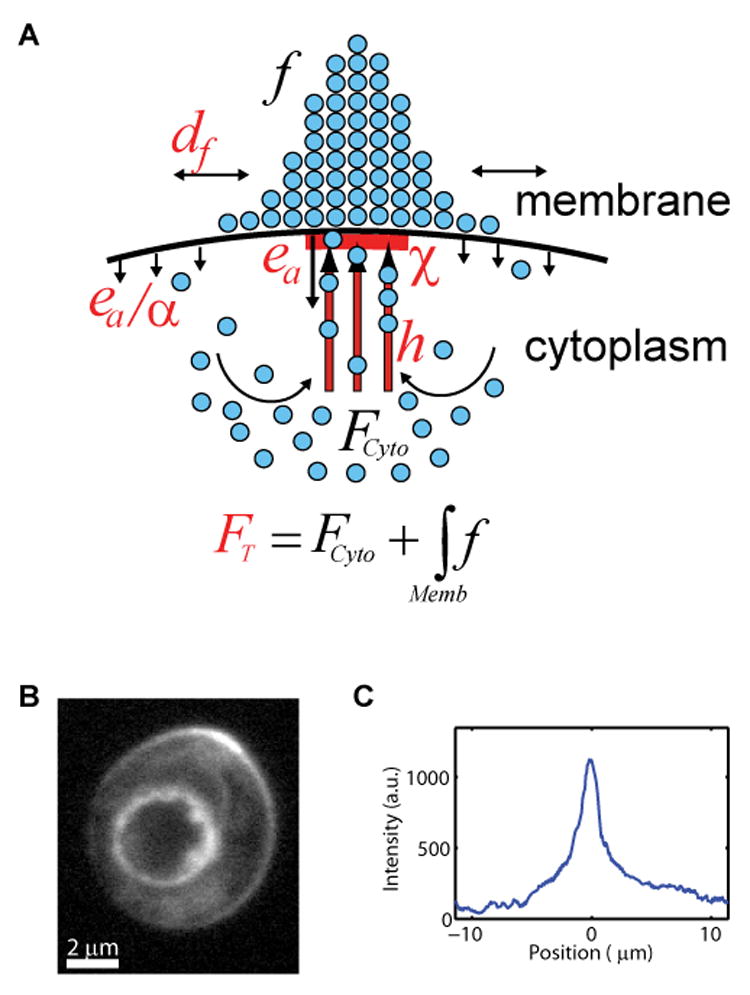
(A) Schematic model of dynamically polarized membrane proteins (blue circles) incorporating diffusion (↔), directed transport (↑), and endocytosis (↓) (see Results). Model parameters are shown in red; polarized protein density at the membrane is shown schematically as a histogram.
(B and C) Example of polarized membrane protein, GFP-Cdc42Q61L cap (B) and its membrane intensity (C).
In total, the model used six physically interpretable parameters to determine the density distribution, f, of a polarized protein at the plasma membrane (Figure 1A, Table S1, Experimental Procedures): (1) the plasma membrane diffusion constant, df; (2) the directed transport window function χ, defining the region of the plasma membrane to which cytoskeletal tracks are attached; (3) the total cellular amount of the protein, FT; (4) the directed transport rate along cytoskeletal tracks, h; (5) the endocytosis rate within the directed transport window, ea; and (6) the ratio of the endocytosis rates within and away from the directed transport region, α. In the model we assumed that the total protein pool FT is constant, and that the cell is rotationally symmetric. We further assumed that the cytoplasmic pool FCyto is homogeneous, due to the fast dispersion of vesicles in the cytosol (Ozbudak et al., 2005; Wedlich-Soldner et al., 2003). For simplicity, we assumed that χ is a rectangular window, though a Gaussian window may also be used (Figure S1). Finally, we reasoned that spontaneous association of the proteins from the cytoplasm to the plasma membrane is a negligible effect for the maintenance of polarity in comparison with active transport and did not model this effect.
Polarization morphologies for different parameter values
Morphologically distinct distributions of cortically polarized proteins can arise from variations to the redistribution mechanisms, potentially leading to different morphogenetic responses. In order to explore the relationship between polarization morphology and each redistribution mechanism, we used the model to find steady-state solutions over a wide range of values for each of our six parameters (Figure 2). We solved the equation numerically for initial conditions in which the protein distribution was entirely cytoplasmic and let the system evolve to its unique steady-state solution (Supplemental Experimental Procedures).
Figure 2. Relationship of cap morphology to model parameters.
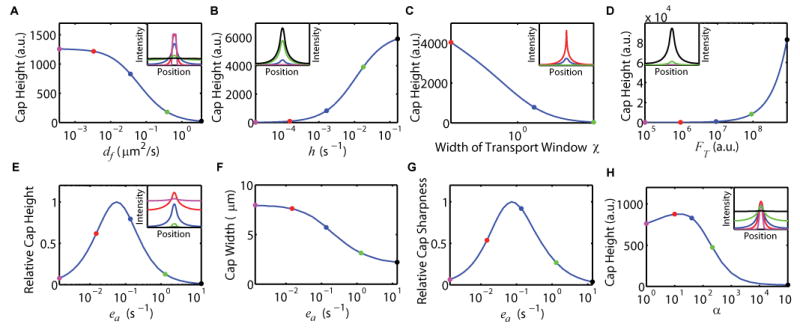
(A–D) Cap height is monotone with diffusion constant df (A), transport rate h (B), the directed transport window χ width, (C) and total protein amount FT (D).
(E–G) Non-monotone relationship of ea to relative cap height (E), cap width (F), and relative cap sharpness (G).
(H) Non-monotone relationship of endocytosis ratio α to cap height.
Non-varying parameters were fixed at estimated population means obtained from our analysis of polarized Cdc42Q61L (Figure 3). Free parameters were varied by factors as indicated on the horizontal axes. Insets show predicted solutions and are color-coded within each panel to indicate selected parameter values. Note, the blue curves in each inset use the same estimated population-mean parameter values.
Polarity regulators, such as Cdc42, need to provide precise, unambiguous signals in time and space in order to induce subsequent polarity processes, and may require that the polarity regulator itself is concentrated in a sharply defined region of the plasma membrane. Referring to a polarized distribution as a “cap,” we selected three intuitive measurements to quantitatively assess the strength of the polarization signal, namely: cap height, cap width, and cap sharpness. Cap height measures the peak density (or peak fluorescence intensity) of the polarity marker distribution f above background (i.e. max(f) − min(f)), and reflects the degree to which signal and noise can be unambiguously separated. Cap width measures the width of the cap region, defined as the smallest region in which the background subtracted density of f is at least 25% of the cap height (i.e. width of the smallest connected region {x | f(x) − min(f) > 0.25 cap height}). Finally, cap sharpness estimates the signal gradient within the cap (i.e. cap height / cap width), and reflects the spatial precision with which the polarity signal is defined.
The model predicts that cap height changes monotonically with respect to diffusion, directed transport rate, the width of the directed transport window, and total protein amount (Figures 2A–2D). Interestingly, only parameters related to endocytosis have a more complex relationship to the cap morphology (Figures 2E–2H). Both very large and very small endocytosis rates lead to distributions with low “signal” above background: large endocytosis rates remove most membrane proteins; whereas small endocytosis rates and lateral diffusion lead to homogenous steady-state distributions (Figure 2E). Importantly, cap height and cap sharpness (Figures 2E and 2G) achieve their theoretical maxima at single, optimal endocytosis rates. Cap height also reaches a maximum at a single optimal endocytosis ratio α (Figure 2H). These observations suggest that endocytosis can be a useful evolutionary and regulatory target for fine tuning the spatial distribution of polarity proteins.
Methods for estimating parameter values
We developed a general analytical approach to estimate actual parameter values from data. The methods require two sets of time-lapse microscopy images. These data sets captured the Fluorescence Recovery After Photobleaching (FRAP) of: (I) drug-treated unpolarized cells (used to estimate df; Figures 3A and 3B), and (II) polarized cells (pre-FRAP segment used to estimate χ and FT, Figure 3F and 3G; FRAP segment used to estimate ea, α, and h, Figures 3G and 3K). These methods are described in detail below using the example of Cdc42 polarization in budding yeast. The need to acquire rapid time points required us to capture images on a fixed focal plane, and to infer protein intensity from 2D-images by assuming rotational symmetry.
Figure 3. Estimation of model parameters from GFP-Cdc42Q61L intensity.
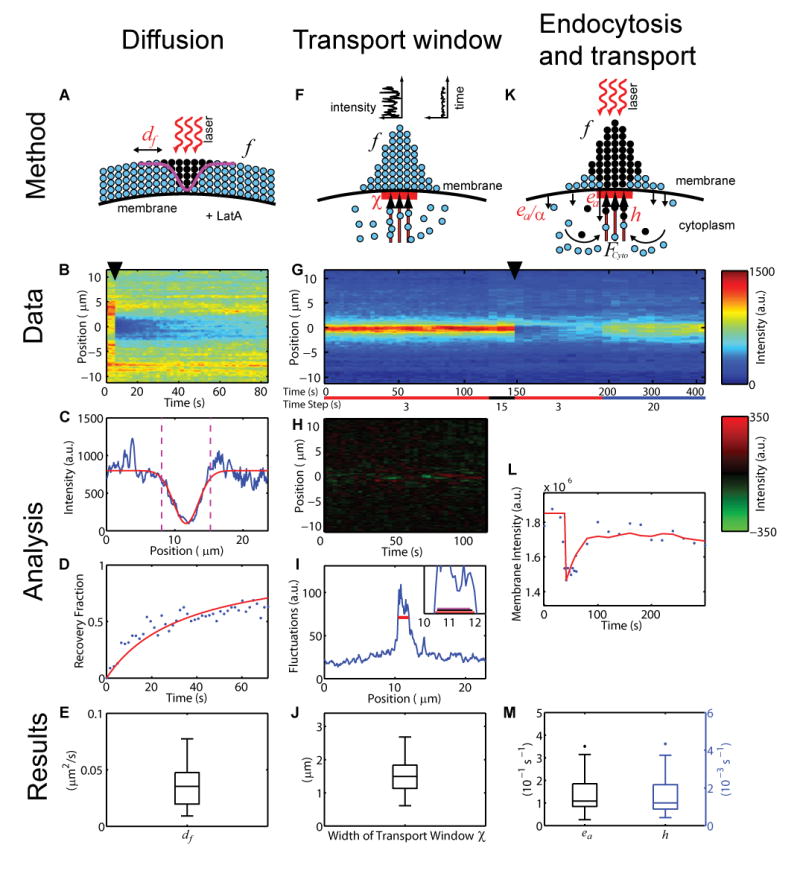
Shown is the process used to extract the diffusion constant (A–E), directed transport window (F–J) and endocytosis and transport rates (G,K–M).
(A, F and K) Schemas for parameter extraction (notation as in Figure 1A). (●) indicates photobleached Cdc42Q61L.
(B and G) Kymographs of Cdc42Q61L intensity along membrane (_y_-axis) in time (_x_-axis). (▼) indicates time of photobleaching. Time steps in (B) are ∼1.65 seconds, and for (G) are indicated below; color bar is indicated at right.
(C–D) Estimation of df. Membrane intensity after initial photobleaching (C) and subsequent recovery fraction over time (D). Experimental data (blue), regressed curves (red), and window in which recovery fraction is calculated (magenta). (H–I) Estimation of χ. (H), Kymograph of Cdc42Q61L temporal deviations from the mean along membrane (_y_-axis) in time (_x_-axis) derived from data in (G) before photobleaching. High fluctuations persist ∼50 seconds (Figure 4). Red-green colorbar is given at right.
(I) Mean temporal fluctuations from (H) with estimated transport window (red segment, threshold s = 65). Inset shows the estimated transport windows for s = 65 (red), s = 70 (black) and s = 75 (magenta).
(L) Estimation of ea and h. Observed recovery of total membrane intensity (blue) and regressed curve (red) from right half of data in (G).
(E, J and M) Quartile boxplots of estimated parameter values. Whiskers show the data within a length of 1.5 times the inter-quartile region; dots represent outliers. (n = 32 for (E), n = 25 for (J, M).).
Testing the model on a prototypical polarized membrane protein
To test our model and approach for measuring parameters, we investigated the dynamic maintenance of the polarized distribution for the constitutively activated Cdc42, Cdc42Q61L, in budding yeast (Johnson, 1999; Ziman et al., 1993). Upon expression of GFP-labeled Cdc42Q61L (hereafter referred to as Cdc42Q61L), G1-arrested budding yeast cells spontaneously establish morphologically stable Cdc42Q61L caps on the plasma membrane (Wedlich-Soldner et al., 2003) (Figures 1 and 3G).
All three of the previously mentioned redistribution mechanisms have been found to be required for the maintenance of polarity in this system: (1) actin cable-dependent transport, bringing Cdc42Q61L to the membrane (Wedlich-Soldner et al., 2003; Wedlich-Soldner et al., 2004), (2) lateral diffusion, spreading Cdc42Q61L along the membrane (Wedlich-Soldner et al., 2004), and (3) endocytosis (Irazoqui et al., 2005; Valdez-Taubas and Pelham, 2003) concentrated in the center of the cap region (Huckaba et al., 2004; Newpher et al., 2005; Toshima et al., 2006), returning Cdc42Q61L to the cytoplasm. This cytoskeletal-dependent system is independent of complicating interactions with spatial cues, signaling feedback, and nucleotide exchange mechanisms (Ozbudak et al., 2005; Wedlich-Soldner et al., 2004), and is thus an ideal starting point for quantitative studies of the dynamic maintenance of polarity.
The polarization process is nonlinear since there is a positive feedback between Cdc42Q61L and actin cable nucleation (Wedlich-Soldner et al., 2003). However, once Cdc42 has achieved a steady-state polarized distribution, the region of actin cable nucleation appears to be stably maintained (Pruyne et al., 1998). In this case, Cdc42Q61L behaves like a prototypical polarized membrane protein, whose maintenance requires continuous transport and endocytosis. Below, we first investigate principles for the maintenance of cortical polarity assuming the region of directed transport is determined independently of the polarized protein, and conclude by investigating the role of positive feedback in stabilizing the region of directed transport.
Estimation of the diffusion constant
To estimate the membrane diffusion constant df, we used the actin-depolymerizing drug Latrunculin A (LatA) to disrupt endocytosis and directed transport (Ayscough et al., 1997) and generated data set (I). We analyzed the fluorescence recovery of Cdc42Q61L in membrane regions bleached with a laser pulse (Figures 3A–3D and S2). In order to recover diffusion constants from FRAP data, we modified the existing, planar formulation (Axelrod et al., 1976) to account for the influence of spherical geometry due to the small diameter of yeast; numerical simulation showed that diffusion constants obtained on yeast assuming planar geometry can underestimate the true value by as much as 20% (Figure S3). Analysis of data set (I) using these methods showed close agreement between the model-predicted intensity recovery profiles and the experimental data, indicating high accuracy and robustness despite the presence of noise (Figure S4).
We estimated df = (0.036 ± 0.017) μm2/s (mean ± s.d., n = 32) (Figure 3E). This value is around ten times smaller than typical diffusion constants for prenylated proteins in mammalian cells (Pyenta et al., 2003) but is consistent with findings indicating that proteins in the yeast plasma membrane exhibit unusually slow diffusion constants (Valdez-Taubas and Pelham, 2003). Additionally, we found no statistically significant differences in estimates of df for (1) GFP-Cdc42D57Y, a mostly GDP-bound form of Cdc42 that should not bind effectors, (2) prenylated GFP, with and without LatA, and (3) Cdc42Q61L in a myo2-66 strain defective in actin-dependent transport (Govindan et al., 1995; Johnston et al., 1991; Pruyne et al., 1998) (Table S2 and Figure S5), suggesting that binding or scaffolding effects are not significantly interfering with Cdc42Q61L diffusion.
Estimation of the directed transport window χ
Next, using the initial pre-FRAP movie segments from dataset (II), we estimated the directed transport window χ, which in our yeast system is due to delivery along actin cables. Current direct visualization techniques of actin cables, such as rhodamine phalloidin staining (Karpova et al., 1998) or GFP-tagged Abp140 (Yang and Pon, 2002), do not provide the resolution required to estimate accurately the extent of this region. As an alternative approach, we reasoned that membrane regions with a high density of actin cables would be detectable as regions of relatively high temporal fluctuations of Cdc42Q61L, possibly due to detachments and reattachments of the actin cables (Martin and Chang, 2006) or to the stochastic nature of vesicle delivery (Figures 3F–3H).
We measured temporal fluctuations using the standard deviation σ(x) of the intensities in time for each membrane position x. For threshold value s, χs is the window function defining the smallest connected region on the membrane containing {x | σ(x) > s · max(σ)/100}. Our analysis showed that regions of high temporal fluctuations (s ≈ 70) strongly overlapped with cap regions (Figures 3G–3I), consistent with our expectations for actin cable localization. A “best fit” transport window, with s in the narrow range of 65–75, was selected as explained below. We also estimated total Cdc42Q61L in each cell by summing estimated total plasma membrane and cytoplasmic intensities (Supplemental Experimental Procedures).
We tested whether the observed intensity fluctuations were due to photon-counting statistics at the detector by rescaling σ by the square root of the mean intensity in time: σ(x)I¯(x). This function should be uniform along the membrane if the fluctuations are due to shot noise of the camera detector. Figure 4A shows that the noise signal within the cap is bigger than expected due to shot noise, indicating that the observed intensity fluctuations are not dominated by detector noise.
Figure 4. Analysis of the temporal fluctuations of Cdc42 on the membrane.
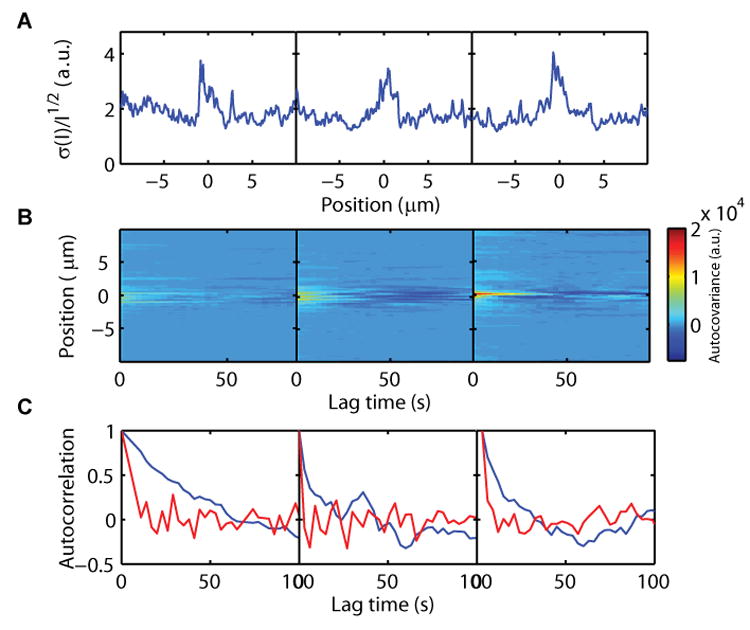
(A) Plots of the standard deviation of the intensity divided by the square root of the intensity along the membrane for three representative cells. The plots show that the noise signal is not due to shot noise from photon detection statistics.
(B) Autocovariance along the membrane (Position) as a function of lag time for the cells in (A).
(C) Autocorrelation as a function of time for a point at the center of the cap (blue) or at a point away from the cap (red). In (A), (B) and (C) each column corresponds to a representative cell. The first column corresponds to the cell in Figures 1 and 3.
Finally, we tested whether the fluctuations were due to random noise or diffusion effects by measuring the autocovariance of the intensity γk(x) for positions x along the membrane and time shift k (Experimental Procedures). Intuitively, the autocovariance measures the correlation of the intensity signal at a point with a time-shifted version of itself. Figure 4B shows that the noise at the cap region is correlated and is not random noise. The autocorrelation, given by γk(x) / γ0(x), has a characteristic time of ∼50 seconds at the cap center (Figure 4C) which is significantly bigger than expected from diffusion alone (assuming a spot radius of two pixels, r0 = 0.13 μm, and our measured diffusion constant df = 0.036 μm2/s, the characteristic time for diffusion is τD=r024df∼ 0.12 seconds (Elliot L. Elson, 1974)). Thus, it is unlikely that our observed fluctuations are predominately due to diffusion effects.
Estimation of the endocytosis and transport rates
Next, we used the FRAP movie segments of dataset (II), to estimate endocytosis and transport rates. It is experimentally difficult to independently measure ea, α and h since blocking endocytosis affects exocytosis and vice versa. However, our model allows simultaneous estimation of these values. Integration of the model equation over the membrane removes the effect of diffusion, yielding an ordinary differential equation (Experimental Procedures). Unconstrained regression of the resulting equation occasionally gave non-physiological parameter values, so in practice, with α manually estimated to be ∼50, we scanned α within the range 1–80, (Figure 5A and 5C) and used regression to estimate only ea and h per cell (Figure 3L). We selected a “best fit” value for α in this range (α = 40) as explained next.
Figure 5. Evaluation of cap morphology prediction.
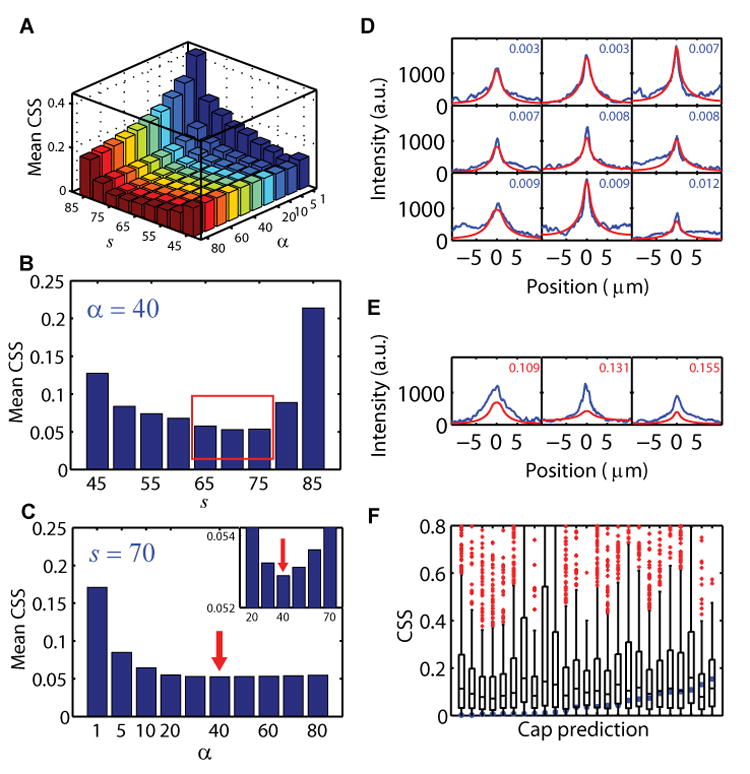
(A) Mean CSS for 25 cells obtained for different values of s and α. The minimum is obtained for s = 70 and α = 40.
(B and C) Shown are two slices through the two dimensional _α_-s plot in (A) for s with α = 40 (B) and for α with s = 70 (C). The lowest CSS values for s were between 65–75 (red box (B)); CSS showed little variation with α, with the minimum at α = 40 (red arrow, rescaled view in inset (C)).
(D and E) Comparison of observed (blue curves) and predicted caps (red curves) from estimated parameters with α = 40 (for all cells) and s between 65–75 (per cell). Cells are ordered by CSS (score in top right corner, Figure S7). Shown are in (D) the 9 best predicted cap shapes (blue scores), and in (E) the three worst predictions (red scores).
(F) Comparison of the predicted cap scores for each cell (blue dots) and the cap scores (boxplots, as in Figure 3 E, J and M) obtained in 1000 simulations for each cell. In each simulation ea, h, χ were drawn from distributions with the mean and standard deviation of their measured values. The value of α was kept at 40, df was taken as measured for Cdc42Q61L and FT was the value measured for each cell.
To choose the best values for α and the window threshold value s, we developed methods to compare predicted and observed Cdc42Q61L membrane intensities. For each cell, cap similarity scores (CSS) were computed using a normalized, mean squared difference between predicted and observed Cdc42Q61L membrane intensities (Experimental Procedures). The large mean CSS value at α = 1 (Figure 5A and 5C) strongly suggested that a single global endocytosis rate was insufficient for accurate cap morphology prediction. The minimum of the mean of CSS, over all cells, was found at α = 40 and s = 70 (Figures 5A–5C). The value found of α = 40 implies that endocytosis rates in the directed transport window χ are much bigger that in the rest of the plasma membrane. This is consistent with previous findings that endocytosis is mainly occurring through actin patches (Kaksonen et al., 2006), that are concentrated in the area around the polar Cdc42Q61L cap (Wedlich-Soldner et al., 2003). The mean CSS was fairly insensitive to the choice of α around its minimum, and a universal value of 40 was selected for all cells (Figure 5C). In contrast, the mean CSS showed more variability in s around its minimum (Figure 5B), so a best fit value for each cell was selected from among {65, 70, 75}. This final choice of parameters gave a mean value for the width of the directed transport window χ as (1.6 ± 0.6) μm (mean ± s.d., n = 25) (Figure 3J). Our estimated rates for endocytosis ea = (0.14 ± 0.09) s−1 and transport h = (1.6 ± 1.1) × 10−3 s−1 (mean ± s.d., n = 25) (Figure 3M, S6), imply that on average 13% of the Cdc42 molecules are on the membrane at steady state, and reside there ∼90 seconds before being recycled (see Supplemental Experimental Methods).
Evaluation of model predictions
As a test of the model, we used the measured parameters to predict cap morphologies in single cells. Despite the separate techniques used to obtain different model parameters, our approach produced good results for the majority of the cells (Figures 5D and S7). Though experimental difficulties (including cell movement, dim fluorescence or imperfect centering of caps within focal planes) contributed to several poor predictions (Figure 5E), overall our results were significantly better than predictions using randomized values generated from the distributions of the estimated parameter values (Figure 5F).
Large values of α, such as α = 40, dramatically improved the morphological agreement between our prediction and the observed data away from the cap region. Thus, localized endocytosis around the site of directed transport dominates the effects of global endocytosis and is essential to accurately describe polarized Cdc42Q61L. The width of the cap can be predicted using our measured diffusion constant and endocytosis rates. As previously mentioned, protein delivered at the center of the cap remains on the plasma membrane for about ∼90 seconds. The average displacement due to diffusion is estimated by 4dft (see for example (Berg, 1993)), resulting in a cap width of ∼7 μm, in close agreement with the observations (Figure S7, 6B). Taken together, the above results demonstrate that the combined redistribution mechanisms were sufficient to describe the morphology of the distribution of polarized Cdc42Q61L.
Figure 6. Relationship of cap morphology to endocytosis rates and diffusion constants.
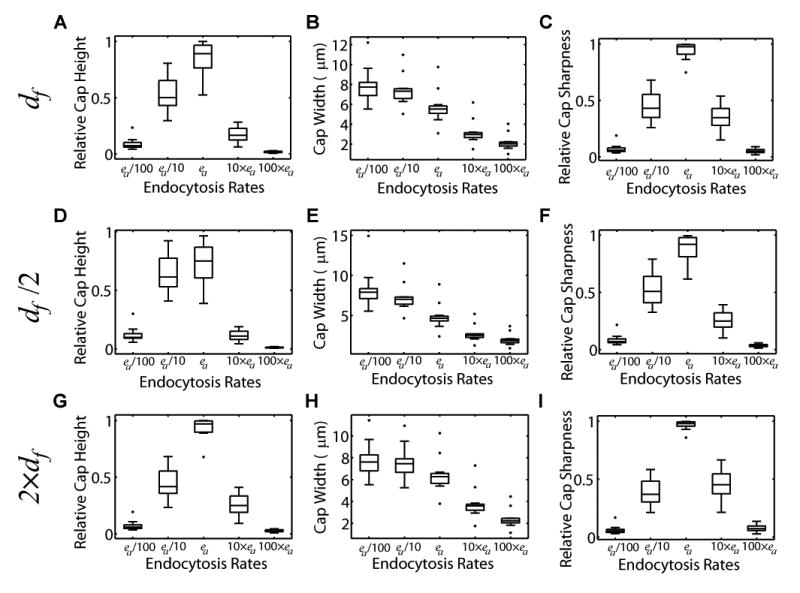
(A–C) Relationship between measured single-cell ea values and cap height, width, and sharpness. For each of the best 12 cap predictions, individual endocytosis rates were varied as in Figures 2E–G while the remaining parameters stayed at their measured, single-cell values. The distribution of cap height (A) and sharpness (C) show that the experimental values are very close to their theoretical maximum values.
(D–F) Results as in (A–C), but obtained by decreasing df by a factor of 2. df = 0.018 μm2/s.
(G–I) Results as in (A–C), but obtained by increasing df by a factor of 2. df = 0.072 μm2/s.
(A), (D) and (G) show the distribution of cap heights relative to the maximum cap height when using the measured ea values, or when varying by two orders of magnitude. (B), (E) and (H) show the distribution of cap widths. (C), (F) and (I) show the distribution of cap sharpness relative to the maximum cap sharpness when using the measured ea values, or when varying by two orders of magnitude. Boxplots are as in Figure 3.
Optimality of cap height and sharpness with respect to endocytosis rates
To test how well yeast endocytosis rates for Cdc42Q61L optimize cap height and sharpness, we selected the top 50% of the cells as measured by CSS. For each cell, we perturbed the theoretical solution by varying its endocytosis rate four orders of magnitude around its estimated values, while fixing all other model parameters at their experimentally measured values (Figure 6). The measured endocytosis rates provided cap heights close to, but slightly lower than the theoretical maximum (Figure 6A). Furthermore, cap sharpness was also close to its maximum value for our measured parameters (Figures 2G and 6C). Importantly, the optimality of cap height and cap sharpness is independent of the total amount FT of Cdc42Q61L (see Supplemental Experimental Procedures), suggesting that our results are not affected by cell-to-cell variations in the expression level of Cdc42Q61L. Furthermore, the attainment of cap sharpness optimality was robust to a 4-fold range of df (Figures 3E and 6D–6I), suggesting relative insensitivity to scaffolding effects, where polarity regulators could be immobilized through interactions with proteins or lipids. Together, these results suggest that yeast cells establish polarized states optimized for the strength and sharpness of the polarized Cdc42Q61L signal.
The role of positive feedback in the dynamic maintenance of cortical polarity
The model presented above addresses the effects of varying transport rates and diffusion on the morphology of the polar domain assuming a steady-state window of directed transport. However, it is known that actin cables undergo constant turnover (Ayscough et al., 1997; Yang and Pon, 2002) and are affected by external perturbations (Delley and Hall, 1999; Desrivieres et al., 1998; Lillie and Brown, 1994). To understand the consequences of fluctuations and perturbations to the transport window, we incorporated dynamic actin cable attachment and detachment via Poisson processes into our model (Experimental Procedures).
Furthermore, we previously demonstrated that the establishment of polarity in the yeast system is driven by feedback between Cdc42-stimulated actin cable assembly and actin-dependent transport of Cdc42 to the plasma membrane (Wedlich-Soldner et al., 2003). Therefore, we additionally incorporated feedback into the model of actin cable dynamics by relating increased plasma membrane concentrations of Cdc42 to: 1) increased probabilities of actin cable attachment; and 2) decreased probability of actin cable detachment (Experimental Procedures). We chose to use simple linear and inverse exponential relationships between the concentration of f and the probability of actin cable attachment and detachment (respectively). The directed transport window χ previously used in the evolution of f was then replaced by the collection of actin cable attachment sites.
Starting from initial steady-state solutions, we explored the ability of the Cdc42 and actin cable distributions to remain highly polarized for varying detachment feedback strengths (Figure 7, first 500 seconds). We found that small detachment feedback strengths resulted in rapid depolarization, while large detachment feedback strengths resulted in dynamically maintained stable polarization morphologies. (Alternative heuristic models yielded analogous results (data not shown; see author website)). These results suggest that as long as actin cables undergo stochastic turnover, positive feedback is critical for the dynamic maintenance of a stable transport window. Next, we simulated the response of the system to a catastrophic loss of actin cables by randomly detaching 50% of the nucleated actin cables at one time (Figure 7, last 100 seconds). Remarkably, strong feedback was sufficient for a rapid and precise recovery to nearly identically polarized Cdc42 and actin cable distributions (Figure 7, right column). Relatively high rates of actin attachment in regions of high Cdc42 concentration, and detachment in regions of low Cdc42 concentration, were essential for this robust response, allowing rapid correction of misplaced nucleation sites before the polarization morphology of Cdc42 was affected.
Figure 7. The role of positive feedback in dynamically maintaining cortical polarity.
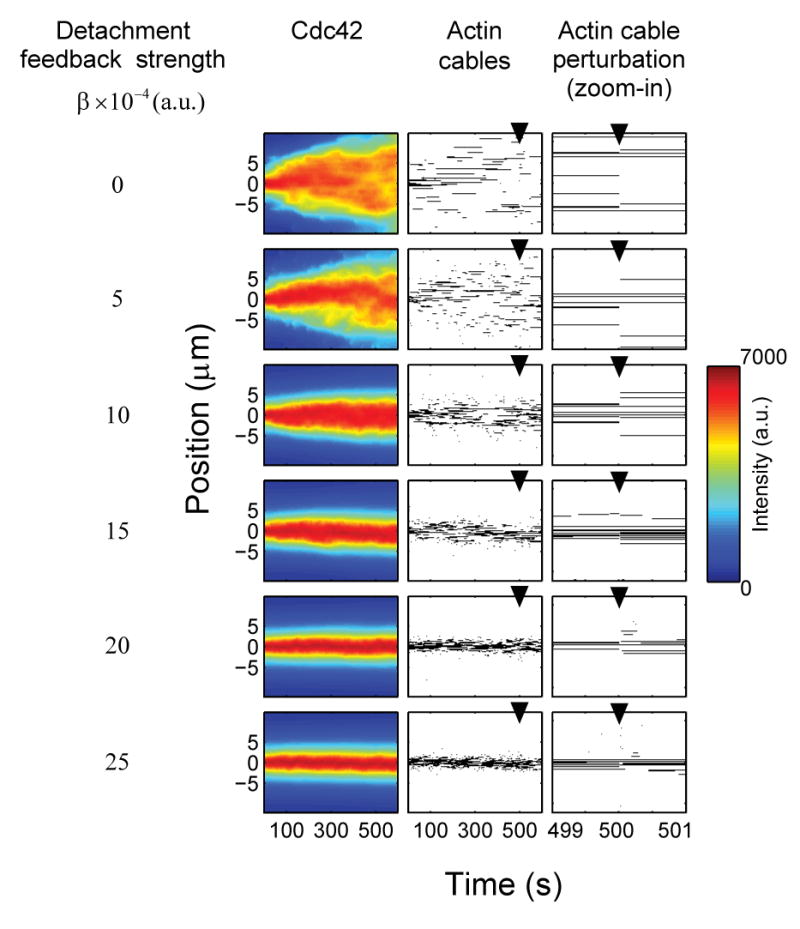
Solutions for the distributions of Cdc42 (first column) and actin cable attachment sites (middle column) for the positive feedback model are computed for a range of detachment feedback parameter β (rows) (see Equation (9)). At 500 seconds (arrowhead), half of the actin cables were randomly detached (right column: zoom of middle column around 500 seconds). Results are computed in 1-D for simplicity. Model parameter values are the mean for those previously estimated in Equation 1, and the steady-state solution was used as initial condition for Equations 8–10. aon was fixed at 10−4 (a.u.) for all simulations; for each β, aoff was chosen so that at _t_=0 cables had the same probability of detachment at the cap center. The allowable number of actin attachment sites was limited to ∼7% (=20/300) of the total membrane, in rough agreement with experimental observations.
Discussion
Diffusion, transport and endocytosis have been shown individually, in different model organisms and different systems, to be necessary for the dynamic maintenance of polarized membrane proteins. We took a systems-level approach to study these three mechanisms for membrane protein redistribution within the framework of a single mathematical model. We were able to determine the influence of each mechanism on the polarization morphology of a polarity marker. The ability to vary parameters outside of physiological regimes allowed us to investigate whether biological systems that dynamically maintain polarized states exhibit non-obvious design principles. Our combined approach using non-intrusive experimental methods with mathematical modeling is particularly useful when: 1) it is experimentally difficult to simultaneously label the polarized protein and markers related to mechanisms of transport in live cells; and 2) transport mechanisms cannot easily be experimentally decoupled.
Dynamically polarized Cdc42Q61L in budding yeast is an ideal system on which to evaluate our general model and parameter estimation approaches. From a biological perspective, Cdc42 is a highly conserved, essential regulator of cell polarity whose localized accumulation controls most of the downstream events of polarization. It is therefore particularly meaningful, in the context of understanding polarized morphogenesis, to examine how individual dynamic processes influence the distribution of active Cdc42. From a model-system perspective, polarization of Cdc42Q61L is dependent on cytoskeleton-based transport but independent of complicating interactions with spatial cues, nucleotide exchange mechanisms and cell cycle regulation. Furthermore, Cdc42Q61L is ideal for studying cortical polarization as it has been shown to be stably associated with membranes (Wedlich-Soldner et al., 2003). Finally, from an experimental perspective, the resulting polarized state is stable for a longer period than that achieved under the normal physiological condition, allowing greater success in capturing sufficient data for analysis. These properties provide advantages for examining a relatively simple polarity system in isolation as a first step toward understanding the dynamic processes governing cell polarization.
We note that a more complex model is required to additionally account for the consequences of the rapid GTPase cycle in wild-type Cdc42 (Brandman et al., 2005). Under physiological conditions Cdc42 polarization has been shown to be much faster than spontaneous polarization of Cdc42Q61L and to involve rapid cycling of Cdc42 on and of the membrane (Wedlich-Soldner et al., 2004). However, the biological processes described in this study have all been shown to contribute to the dynamic maintenance of polarized wild-type Cdc42 (Wedlich-Soldner et al., 2004; Irazoqui et al., 2005) and are likely strong determinants of the spatial precision and persistence of wild-type polarity.
The model allowed us to uncover a central role of endocytosis in defining the spatial precision and morphology of the polarized state. Our results corroborate the assumption that colocalization of endocytosis with actin patches in the polarized region is important for cell polarity. Of particular significance is the theoretical result that endocytosis rates can regulate dynamically balanced systems to optimize the asymmetric localization of membrane protein distributions. Increasing cap height has the consequences of increasing “signal above noise” for downstream effectors, while increasing cap sharpness has the additional consequence of concentrating the signal in a smaller region. Surprisingly, we found that the endocytosis rate of the model yeast system we studied was nearly at the optima of these two morphological properties. Since enzymes and regulators required for polarized growth are concentrated in the polar cap, optimality in the localized concentration of these proteins could be important for achieving high rates in bud growth (Slaughter and Li, 2006), potentially giving yeast cells a selective advantage. Interestingly, several endocytosis mutants in Drosophila lead to loss of polarization, accompanied by overproliferation of mutant cells (Lu and Bilder, 2005; Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005), consistent with our finding that the endocytosis rates can profoundly regulate the morphogenesis and function of polarized cell types.
Finally, the maintenance of a steady-state distribution of polarized proteins requires a stable directed transport window that is robust to perturbations. Our model demonstrates that the feedback loop between polarity regulators, such as Cdc42, and the formation of actin cables plays an important role in the maintenance of the transport window. The model predicts that feedback, leading to relatively high rates of actin cable attachment in regions of high Cdc42 concentration, and detachment in regions of low Cdc42, can stabilize the transport window. As long as the transport window is stabilized through such mechanisms, other membrane proteins that are recruited passively to the polar cortical domain (i.e. those that do not play any regulatory role) can maintain their distribution through balanced directed transport, endocytosis and diffusion. This property is likely to be important for the sustained maintenance of a stable and unique axis of polarity – a defining property of polarity in many cell types.
In conclusion, our approach involving tightly coupled mathematical models and live-cell measurements provides a basis for future quantitative studies of cell polarity systems, including understanding the molecular basis of the model parameters and describing the contributions of additional dynamic mechanisms in polarity, such as nucleotide exchange and signaling feedback. The model and parameter estimation methods can also potentially be extended to include effects such as general membrane on-rate terms, partial membrane scaffolding and non-uniform diffusion rates, non-constant total pools of proteins, cytosolic diffusion, and interacting systems of polarized proteins. These advances should provide a framework for investigating more complex systems with multiple, and partially redundant polarization pathways.
Experimental Procedures
Yeast strains and growth conditions
Yeast strains are described in Table S3. For imaging of G1 cells, logarithmically growing cells (in SC–Met / 2% Glucose medium) were arrested in G1 by growth for 3 h in YP / 2% raffinose medium supplemented with 2 mM methionine. To induce expression of Cdc42 constructs under the Gal1/10 promoter, galactose was added to 2% and cells grown for another 2–3 h. Latrunculin A (LatA) was added at 100 μM for 30 min before imaging. To assay for effects of the temperature-sensitive myo2-66 allele cells were grown and arrested at the permissive temperature and then shifted to the restrictive temperature (35 °C) for 1h before imaging.
Microscopy
Imaging was performed in the Nikon-Harvard imaging facility on a spinning disk confocal laser (PerkinElmer, UltraView) and an inverted microscope (Nikon, TE2000U). Z-resolution obtained was around 0.5 μm. Photobleaching experiments were carried out with the MicroPoint Laser system from Photonic Instruments. Pulse frequency and laser intensity was set manually or in Metamorph and was at 10 pulses and 3–10% output, respectively. Bleaching was performed for 0.4–2 s per spot.
Imaging
The methods used for parameter extraction require two sets of time-lapse microscopy images. Data set I: LatA treated cells were imaged at 1.7–2 second time steps and 1000 ms exposure times. After three initial frames, cells were photobleached with a laser and subsequently imaged for (at least) an additional 60 seconds. Data set II: For each of the polarized cells, a single movie was taken with 500 ms exposure time consisting of several consecutive portions: 100 frames (3 seconds apart), 3 frames (15 seconds apart), photobleaching at the cap region, 9 frames (3 seconds apart), and an additional ∼14 frames (20 seconds apart).
Mathematical model for the dynamic redistribution of polarized membrane proteins
The steady-state distribution of a cortically polarized protein, f, is modeled using a balance of three redistribution mechanisms: (1) lateral diffusion of f along the plasma membrane (df_Δ_Membf), (2) endocytosis of f off the membrane at and away from the actin cables (eaχ f and eaχ̄ f / α), and (3) direct transport from an cytoplasmic pool of protein, Fcyto, to the membrane (hFCytoχ/∫χ). Here, df, ea, h, α, χ are described as in Table S1, main text and Figure 1A, and Δ_Memb_ refers to the two-dimensional diffusion operator acting on the plasma membrane. Then, the equation describing the distribution of f may be expressed mathematically as:
| ∂f∂t=dfΔMembf−[eaχ+eaαχ¯]f+hFCytoχ∫χ | (1) |
|---|
where χ̄ = 1 − χ. In equilibrium, equation (1) is identical to zero.
Estimation of df
If FWindow(t) is the integral of the membrane intensity of Cdc42Q61L in a window, then the recovery fraction of FWindow(t) after photobleaching (t > 0) is given by:
| RWindow(t)=FWindow(t)−FWindow(0)FWindow(∞)−FWindow(0) | (2) |
|---|
The relationship between the diffusion constant and FWindow(t) is given by the heat kernel on the membrane of a round two-dimensional cell with radius R:
| FWindow(t)−FWindow(0)=−∫windowΣl=0∞B0R22l+14πPl(cossR)e−l(l+1)R2(K+dft)ds , | (3) |
|---|
where {Pl} are Legendre polynomials, and K and _B_0 are related to the width and depth of the initial bleached profile. Then df is estimated from the experimentally determined recovery curve (2) using regression (Figure 3C and 3D).
Estimation of autocovariance
The autocovariance of the fluorescent intensity I(x,t) (Supplemental Experimental Procedures) for time lag k, membrane positions x, and times ti is computed by:
| γk(x)=1Nt−kΣj=1j=Nt−k[I(x,tj)−I¯(x)] [I(x,tj+k)−I¯(x)] . | (4) |
|---|
Here, Ī is the temporal mean of I (x,ti) over the Nt time points.
Estimation of ea, α and h
Integration of (1) along the membrane gives:
| dFMembdt=−eaFMemb,χs−eaαFMemb,χs¯+hFCyto , | (5) |
|---|
where FMemb,χs and FMemb,χ̄s (resp.) are the total membrane intensities of Cdc42Q61L in the non-zero regions of χs and χ̄s (resp.). Equation (5) was then integrated numerically between time points using experimentally estimated values for FMemb and FCyto, giving the vector equation:
| (⋮FMemb(tj)−FMemb(0)⋮)=−ea(⋮∫0tjFMemb,χsdt⋮)+eaα(⋮∫0tjFMemb,χs¯dt⋮)+h(⋮∫0tjFCytodt⋮) . | (6) |
|---|
Given α and χs, regression of the numerically integrated model determined the rates for ea and h.
Computation of Cap Similarity Score (CSS)
To compare the difference between steady-state predicted, _I_model (x), and time-averaged observed, Īobs (x), Cdc42Q61L membrane intensities, we define the score:
| CSS=1maxy∈R [I¯obs(y)]21NΣx∈R[I¯obs(x)−Imodel(x)]2 , | (7) |
|---|
where R is a membrane region centered around the cap covering 20% of the total contour of the membrane (∼ 4 μm wide, compared with typical actin window widths of ∼2 μm, Figure 3J) and N is the number of points in R. The restriction of comparison to the region R is to avoid overweighting of the cap “tails” when comparing peaks.
Positive feedback model of dynamically polarized membrane proteins
This model couples directed transport with the evolution of f. The probability of nucleating an actin cable at a membrane location x within a time interval dt, is proportional to a rate constant aon, the number of potential actin cables that can be nucleated AC (reflecting a postulated limiting pool of actin monomers (Karpova et al., 1995)), and the density of the polarized protein f:
| Pon(x,t,dt)=aon AC f(x,t)dt | (8) |
|---|
The probability for detachment of an actin cable is assumed to be:
| Poff(x,t,dt)=aoff e−βfdt | (9) |
|---|
for rate constant aoff and feedback parameter β. The evolution for f is given by:
| ∂f∂t=dfΔMembf−[eaχ+eaαχ¯]f+hcFCytoχ , | (10) |
|---|
Where χ is the collection of actin attachment sites, updated continuously with the processes described by equations (8) and (9), and hc is the transport rate for a single cable.
Supplementary Material
01
Acknowledgments
We would like to thank Sigurd Angenent and Guocheng Yuan for helpful mathematical discussions, Rama Ranganathan, Michael Rosen, Orion Weiner, and Zena Werb for helpful comments on the manuscript, The Nikon Imaging Center at Harvard Medical School for assistance with microscopy and FRAP, and P. Crews for Latrunculin A. E.M. gratefully acknowledges Peter Sorger for his encouragement. This research was supported by an NIH grant (RO1 GM071794-03), the Bauer Center for Genomics Research at Harvard (EM, SJA, LFW), the Endowed Scholars program at UT Southwestern Medical Center Welch Foundation (I-1619 SJA, I-1644 LFW), and a long-term fellowship from the Human Frontier Science Program (EM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Affolter M, Weijer CJ. Signaling to cytoskeletal dynamics during chemotaxis. Dev Cell. 2005;9:19–34. doi: 10.1016/j.devcel.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976;16:1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg HC. Random walks in biology. Expanded edn. Princeton, NJ: Princeton University Press; 1993. [Google Scholar]
- Brandman O, Ferrell JE, Jr, Li R, Meyer T. Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science. 2005;310:496–498. doi: 10.1126/science.1113834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS, Thomson JN. Distribution of ferritin receptors and coated pits on giant HeLa cells. Embo J. 1983;2:599–603. doi: 10.1002/j.1460-2075.1983.tb01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeks RJ, Canman JC, Gabriel WN, Meyer N, Strome S, Goldstein B. C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr Biol. 2004;14:851–862. doi: 10.1016/j.cub.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Chen K, Csikasz-Nagy A, Gyorffy B, Val J, Novak B, Tyson J. Kinetic Analysis of a Molecular Model of the Budding Yeast Cell Cycle. Molecular Biology of the Cell. 2000;11:369–391. doi: 10.1091/mbc.11.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delley PA, Hall MN. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J Cell Biol. 1999;147:163–174. doi: 10.1083/jcb.147.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrivieres S, Cooke FT, Parker PJ, Hall MN. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J Biol Chem. 1998;273:15787–15793. doi: 10.1074/jbc.273.25.15787. [DOI] [PubMed] [Google Scholar]
- Elson Elliot L., DM Fluorescence correlation spectroscopy. I. Conceptual basis and theory. Biopolymers. 1974;13:1–27. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ML, Axelrod D. Anomalously slow mobility of fluorescent lipid probes in the plasma membrane of the yeast Saccharomyces cerevisiae. J Membr Biol. 1993;131:115–127. doi: 10.1007/BF02791320. [DOI] [PubMed] [Google Scholar]
- Huckaba TM, Gay AC, Pantalena LF, Yang HC, Pon LA. Live cell imaging of the assembly, disassembly, and actin cable-dependent movement of endosomes and actin patches in the budding yeast, Saccharomyces cerevisiae. J Cell Biol. 2004;167:519–530. doi: 10.1083/jcb.200404173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazoqui JE, Howell AS, Theesfeld CL, Lew DJ. Opposing roles for actin in Cdc42p polarization. Mol Biol Cell. 2005;16:1296–1304. doi: 10.1091/mbc.E04-05-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DI. Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev. 1999;63:54–105. doi: 10.1128/mmbr.63.1.54-105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GC, Prendergast JA, Singer RA. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- Karpova TS, McNally JG, Moltz SL, Cooper JA. Assembly and function of the actin cytoskeleton of yeast: relationships between cables and patches. J Cell Biol. 1998;142:1501–1517. doi: 10.1083/jcb.142.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova TS, Tatchell K, Cooper JA. Actin filaments in yeast are unstable in the absence of capping protein or fimbrin. J Cell Biol. 1995;131:1483–1493. doi: 10.1083/jcb.131.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA, Jan LY, Jan YN. The N terminus of the Drosophila Numb protein directs membrane association and actin-dependent asymmetric localization. Proc Natl Acad Sci U S A. 1997;94:13005–13010. doi: 10.1073/pnas.94.24.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner M. The roles of APC and axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1 doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie SH, Brown SS. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–1239. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- Martin SG, Chang F. Dynamics of the formin for3p in actin cable assembly. Curr Biol. 2006;16:1161–1170. doi: 10.1016/j.cub.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Mayer B, Emery G, Berdnik D, Wirtz-Peitz F, Knoblich JA. Quantitative analysis of protein dynamics during asymmetric cell division. Curr Biol. 2005;15:1847–1854. doi: 10.1016/j.cub.2005.08.067. [DOI] [PubMed] [Google Scholar]
- Moberg KH, Schelble S, Burdick SK, Hariharan IK. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell. 2005;9:699–710. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Smith RP, Lemmon V, Lemmon SK. In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actin-based endocytic machinery in yeast. Dev Cell. 2005;9:87–98. doi: 10.1016/j.devcel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Ozbudak EM, Becskei A, van Oudenaarden A. A system of counteracting feedback loops regulates Cdc42p activity during spontaneous cell polarization. Dev Cell. 2005;9:565–571. doi: 10.1016/j.devcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Petritsch C, Tavosanis G, Turck CW, Jan LY, Jan YN. The Drosophila myosin VI Jaguar is required for basal protein targeting and correct spindle orientation in mitotic neuroblasts. Dev Cell. 2003;4:273–281. doi: 10.1016/s1534-5807(03)00020-0. [DOI] [PubMed] [Google Scholar]
- Pruyne D, Bretscher A. Polarization of cell growth in yeast. J Cell Sci. 2000;113(Pt 4):571–585. doi: 10.1242/jcs.113.4.571. [DOI] [PubMed] [Google Scholar]
- Pruyne D, Gao L, Bi E, Bretscher A. Stable and dynamic axes of polarity use distinct formin isoforms in budding yeast. Mol Biol Cell. 2004;15:4971–4989. doi: 10.1091/mbc.E04-04-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne DW, Schott DH, Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J Cell Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- Pyenta PS, Schwille P, Webb WW, Holowka D, Baird B. Lateral Diffusion of Membrane Lipid-Anchored Probes before and after Aggregation of Cell Surface IgE-Receptors. J Phys Chem A. 2003;107:8310–8318. [Google Scholar]
- Severson AF, Bowerman B. Myosin and the PAR proteins polarize microfilament-dependent forces that shape and position mitotic spindles in Caenorhabditis elegans. J Cell Biol. 2003;161:21–26. doi: 10.1083/jcb.200210171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter B, Li R. Toward a molecular interpretation of the surface stress theory for yeast morphogenesis. Curr Opin Cell Biol. 2006;18:47–53. doi: 10.1016/j.ceb.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Snaith HA, Sawin KE. Fission yeast mod5p regulates polarized growth through anchoring of tea1p at cell tips. Nature. 2003;423:647–651. doi: 10.1038/nature01672. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Mathieu J, Sung HH, Loeser E, Rorth P, Cohen SM. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell. 2005;9:711–720. doi: 10.1016/j.devcel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Toshima JY, Toshima J, Kaksonen M, Martin AC, King DS, Drubin DG. Spatial dynamics of receptor-mediated endocytic trafficking in budding yeast revealed by using fluorescent alpha-factor derivatives. Proc Natl Acad Sci U S A. 2006;103:5793–5798. doi: 10.1073/pnas.0601042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell. 2005;9:687–698. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Valdez-Taubas J, Pelham HR. Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr Biol. 2003;13:1636–1640. doi: 10.1016/j.cub.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Wai SC, Schmidt T, Li R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J Cell Biol. 2004;166:889–900. doi: 10.1083/jcb.200405061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HC, Pon LA. Actin cable dynamics in budding yeast. Proc Natl Acad Sci U S A. 2002;99:751–756. doi: 10.1073/pnas.022462899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M, Preuss D, Mulholland J, O'Brien JM, Botstein D, Johnson DI. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol Biol Cell. 1993;4:1307–1316. doi: 10.1091/mbc.4.12.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01