The Type IVB Pili of Salmonella enterica Serovar Typhi Bind to the Cystic Fibrosis Transmembrane Conductance Regulator (original) (raw)
Abstract
Salmonella enterica serovar Typhi expresses type IVB pili. We show that the prePilS protein (the soluble precursor form of the structural pilin) interacts with a 15-mer peptide representing the first extracellular domain of the cystic fibrosis transmembrane conductance regulator (CFTR), a recognized human epithelial cell receptor for serovar Typhi (G. B. Pier et al., Nature 393:79-82, 1998). This indicates that after mediating bacterial self-association (C. Morris et al., Infect. Immun. 71:1141-1146, 2003), the pili then act to attach the bacterial clumps to CFTR in the membrane of gut epithelial cells. These sequential type IVB pilus-mediated events cannot be performed by (for example) S. enterica serovar Typhimurium, which may explain why only serovar Typhi causes epidemics of enteric fever in humans.
The type IVB pilus operon of Salmonella enterica serovar Typhi contains a pilS gene encoding the structural pilin (1, 5). A pilS mutant of serovar Typhi was much reduced in adhesion to and invasion of human epithelial gastrointestinal cells in vitro, and soluble purified prePilS protein (retaining the signal sequence normally cleaved when the protein is excreted to form insoluble pili based on polymerized PilS) inhibited bacterial invasion (5). While the pili mediate bacterial self-association (3), these data did not explain why purified prepilin should inhibit serovar Typhi entry to human intestinal epithelial cells. This rather suggested that the prepilin might interact with an epithelial cell receptor.
It is known that the first extracellular domain of the cystic fibrosis transmembrane conductance regulator (CFTR) is a serovar Typhi receptor domain (4). To determine if soluble prePilS protein could interact with this domain of CFTR, bacteria of serovar Typhi strain J341 (Ty2 Vi−) (5), grown in Luria broth for 14 to 16 h at 30°C to reach optical densities at 600 nm of 0.5 to 0.7, were resuspended in Eagle's basal medium, which also contained CFTR peptides, prePilS protein, or both. Then the bacteria were centrifuged for 10 min at 3,500 × g onto washed cells (bacterium/cell ratio of 1:1) of the human embryonic intestine cell line INT407 (4). Bacterium-cell interaction proceeded for 2 h at 37°C in a 5% (vol/vol) CO2 atmosphere. After incubation, cells were washed, treated with gentamicin, washed once more, and lysed for enumeration of internalized bacteria (3 to 5% of added bacteria, in the absence of an inhibitor of INT407 cell entry by serovar Typhi) (5).
The 15-mer peptides representing the first extracellular human CFTR domain (GRIIASYDPDNKEER) and a scrambled version of this domain (GKDPNYRDEAIRSIE) (4) were synthesized by Research Genetics, Huntsville, Ala.. The prePilS protein was purified from a glutathione _S_-transferase (GST) fusion protein, expressed in Escherichia coli K-12 as previously described (5). Affinity beads with bound fusion protein were washed with 100 volumes of phosphate-buffered saline (PBS) before thrombin cleavage of prePilS, and the prePilS preparation was confirmed to be free of bacterial lipopolysaccharide in assays detecting periodate-cleavable carbohydrate. Also, heat (boiling for 5 min) destroyed the ability of prePilS to inhibit eukaryotic cell entry by serovar Typhi, in that boiled prePilS at 4 μM did not detectably inhibit such entry, while ca. 75% cell entry inhibition was afforded by the same concentration of native prePilS (described below).
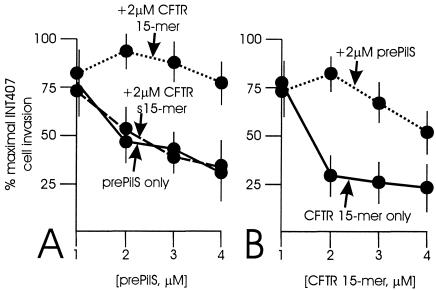
As demonstrated before (5), prePilS protein inhibited serovar Typhi entry into INT407 cells, with 50% inhibition noted at ca. 2 μM prePilS (Fig. 1A). Addition of the scrambled version of the CFTR 15-mer to assays including prePilS protein did not affect the inhibition noted. When the authentic CFTR 15-mer was present (at 2 μM) in assays in which the prePilS protein was included at 1, 2, 3, or 4 μM, the CFTR 15-mer effectively neutralized the INT407 cell entry inhibition afforded by prePilS protein (Fig. 1A). In the reciprocal experiment (Fig. 1B), the CFTR 15-mer inhibited INT407 cell entry by serovar Typhi, as expected (4), while the scrambled version of the CFTR 15-mer was without inhibitory effect (data not shown). When prePilS protein was present at 2 μM in assays in which the CFTR 15-mer was included at 1, 2, 3, or 4 μM, prePilS protein effectively neutralized the INT407 cell entry inhibition caused by the CFTR 15-mer (Fig. 1B). Maximal INT407 cell entry (ca. 75%) was seen when the 15-mer and prePilS were in equimolar proportions; an excess of either component reduced INT407 cell entry from this peak. A two-way analysis of variance with INT407 cell entry level as the dependent variable and concentrations of prePilS and CFTR 15-mer as independent variables showed that the prePilS-CFTR peptide interactions of both Fig. 1A and B were significant, with P < 0.001.
FIG. 1.
Effects of CFTR peptides and prePilS protein on INT407 cell entry by S. enterica serovar Typhi. Cell entry levels of serovar Typhi in the absence of any added CFTR peptides or prePilS protein were set at 100%. Each point represents the mean of seven tests, each run in quintuplicate; standard error bars are shown. s15-mer, scrambled CFTR 15-mer.
These data indicate that the prePilS protein and the CFTR 15-mer interact, and this in turn suggests that the PilS protein of the type IVB pili binds to CFTR in the INT407 cell membrane. It was not possible to use purified PilS protein in the tests described above, because PilS is insoluble, precipitating in the affinity matrix upon thrombin release from a GST conjugate (data not shown). Because the signal sequence of prePilS will be removed upon PilS secretion by serovar Typhi, a physiologically relevant interaction with CFTR would involve the PilS portion of prePilS.
Serovar Typhi bacteria use the type IVB pili for bacterial self-association (3). The data described here suggest that such aggregates may use the pili also to bind to the human intestinal cell membrane. The next step would logically be bacterial invasion of the intestinal mucosa, perhaps facilitated by association of bacterial lipopolysaccharide to pili-recruited CFTR (2). Because expression of the type IVB pili is confined to serovar Typhi and a few human-invasive strains of S. enterica serovars Paratyphi C and Dublin (our unpublished observations), expression of the type IVB pili may be important in the mediation of enteric fever in humans.
Acknowledgments
This work was supported by the Hong Kong Government Research Grants Council.
REFERENCES
- 1.Kim, S.-R., and T. Komano. 1997. The plasmid R64 thin pilus identified as a type IV pilus. J. Bacteriol. 179**:**3594-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyczak, J. B., and G. B. Pier. 2002. Salmonella enterica serovar Typhi modulates cell surface expression of its receptor, the cystic fibrosis transmembrane conductance regulator, on the intestinal epithelium. Infect. Immun. 70**:**6416-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris, C., C. M. C. Yip, I. S. M. Tsui, D. K.-H. Wong, and J. Hackett. 2003. The shufflon of Salmonella enterica serovar Typhi regulates type IVB pilus-mediated bacterial self-association. Infect. Immun. 71**:**1141-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pier, G. B., M. Grout, T. Zaidi, G. Meluleni, S. S. Mueschenborn, G. Banting, R. Ratcliff, M. J. Evans, and W. H. Colledge. 1998. Salmonella typhi uses CFTR to enter intestinal epithelial cells. Nature 393**:**79-82. [DOI] [PubMed] [Google Scholar]
- 5.Zhang, X.-L., I. S. M. Tsui, C. M. C. Yip, A. W. Y. Fung, D. K.-H. Wong, X. Dai, Y. Yang, J. Hackett, and C. Morris. 2000. Salmonella typhi uses type IVB pili to enter human intestinal epithelial cells. Infect. Immun. 68**:**3067-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]