RIDDLE immunodeficiency syndrome is linked to defects in 53BP1-mediated DNA damage signaling (original) (raw)
Abstract
Cellular DNA double-strand break-repair pathways have evolved to protect the integrity of the genome from a continual barrage of potentially detrimental insults. Inherited mutations in genes that control this process result in an inability to properly repair DNA damage, ultimately leading to developmental defects and also cancer predisposition. Here, we describe a patient with a previously undescribed syndrome, which we have termed RIDDLE syndrome (radiosensitivity, immunodeficiency, dysmorphic features and learning difficulties), whose cells lack an ability to recruit 53BP1 to sites of DNA double-strand breaks. As a consequence, cells derived from this patient exhibit a hypersensitivity to ionizing radiation, cell cycle checkpoint abnormalities, and impaired end-joining in the recombined switch regions. Sequencing of TP53BP1 and other genes known to regulate ionizing radiation-induced 53BP1 foci formation in this patient failed to detect any mutations. Therefore, these data indicate the existence of a DNA double-strand break-repair protein that functions upstream of 53BP1 and contributes to the normal development of the human immune system.
Keywords: DNA repair, cell cycle checkpoint, radiosensitivity, BRCA1
DNA damage recognition and repair is a fundamental process required to maintain the fidelity of the genome. Disruption of this process is implicated in the development of human disease and tumorigenesis. The impact of defective DNA double-strand break repair (DSBR) on human development can be observed in patients with inherited disorders such as ataxia-telangiectasia (A-T), Nijmegen breakage syndrome (NBS), DNA ligase IV deficiency syndrome (LiDS), and radiosensitive severe combined immunodeficiency (RS-SCID) who have mutations in the DSBR genes ATM, NBS1, DNA Ligase IV, and Artemis and Cernunnos, respectively. These patients manifest a multitude of clinical features, which include a marked hypersensitivity to agents that induce DNA double-strand breaks (DSBs), such as ionizing radiation (IR), neurological abnormalities, mental retardation, growth defects, gonad dysfunction, and an elevated predisposition to the development of tumors. Because normal development of the immune system requires the introduction of DNA DSBs during antigen receptor gene assembly, defects in the repair of these specialized breaks can lead to profound immunodeficiency (1). This is most notable in LiDS and RS-SCID patients, although immune system abnormalities are also associated with A-T and NBS.
It is known that Artemis and DNA ligase IV, along with XRCC4, Cernunnos/XLF, and the DNA-PK holoenzyme, comprise the components of the nonhomologous DNA end-joining (NHEJ) machinery. The error-prone nature of this DNA repair pathway is invaluable for creating genetic diversity when assembling immune system gene receptors. For example, class switch recombination (CSR) results in the editing of a functional μ-Ig heavy-chain gene, so that the μ-constant region (Cμ) is replaced with an alternative constant region (e.g., α, γ, ε) to generate different Ig isotypes e.g., IgA, IgG, and IgE (2).
During the process of CSR, it has been hypothesized that two closely positioned single-stranded DNA nicks result in the production of a DNA DSB. Although the mechanism of DNA DSB generation during this process remains unclear, there is a requirement for the presence of a DNA DSB to be produced for the proper resolution of the switch recombination process, as loss of components of the DSBR repair pathway, such as H2AX, DNA-PKcs, ATM, and Nbs1, may affect the ability to efficiently switch from IgM to other Ig isotypes (2).
More recently it has been demonstrated that loss of the DSB response protein, 53BP1, in mice also results in a CSR defect (3, 4). In support of this role, it has been shown that 53BP1 rapidly relocalizes to sites of DNA damage marked by γ-H2AX after exposure of cells to IR, as well as to the Ig heavy-chain (IgH) loci that have been induced to undergo switch recombination (5).
Here, we describe the analysis of cells derived from a newly described human syndrome, termed RIDDLE syndrome whose clinical features are an increased radiosensitivity, immunodeficiency, dysmorphic features, and learning difficulties. Cells from this patient exhibit a different pattern of Sμ–Sα junctions in CSR compared with normal and 53BP1-mediated cellular responses to DNA damage, without any genetic alterations in the TP53BP1 gene. The increased sensitivity to IR is likely a result of a failure to properly relocalize 53BP1, Rif1, and BRCA1 to sites of DNA DSBs. In addition, these cells exhibit protracted ATM-dependent DNA damage signaling, characterized by elevated numbers of γ-H2AX, MDC1, and Nbs1 foci, hyperphosphorylation of Nbs1, RPA2, and H2AX and a prolonged G2/M checkpoint. These data indicate the existence of a previously uncharacterized component of the DSB repair pathway that facilitates 53BP1 recruitment to sites of DNA damage as well as promoting efficient repair of DNA DSBs that occurs after exposure to IR or during class switch recombination.
Results
A clinical description of patient 15–9BI is given in supporting information (SI) Text.
Cells Derived from Patient 15–9BI Show Evidence of Normal SHM but Altered Patterns of Sμ–Sα Junctions in CSR.
There was no evidence for a defect in CSR affecting all isotypes in patient 15–9BI. Nevertheless, the absence of any gross abnormalities in B and T cell number suggested that the low serum levels of IgG observed in patient 15–9BI could have been caused by a defect in a component of class switch recombination. To address this possibility, genomic DNA was extracted from a peripheral blood sample of a healthy donor and patient 15–9BI and the Sμ–Sα and Sμ–Sγ switch junctions amplified.
In normal donors, CSR is characterized by generation of a range of IgA or IgG fragments, which exhibit little or no sequence homology across the switch junctions between Sμ and Sα or Sγ sequences respectively. Mutations or insertions around the switch break points are observed. Although the number of fragments obtained from 10 PCRs run in parallel were not different between cells from the healthy donor and patient 15–9BI, sequencing across the switch junctions revealed that cells derived from the patient utilized significantly increased levels of microhomology across the break region compared with the control; 94% (15 of 16) of Sμ–Sα switch products amplified from patient 15–9BI exhibited microhomology of 4 bp or over compared with only 17% (3 of 18) in the control; χ2, P < 0.001 (SI Table 1).
A reduced frequency of insertions and mutations around the Sμ–Sα break points was observed in the patient 15–9BI (1 of 16) compared with the control (11 of 18); χ2, P = 0.001. Analysis of a restricted number of Sμ–Sγ3 15–9BI switches revealed a defect similar in the patient to that observed for the Sμ–Sα switch sequences (data not shown).
Taken together, our analysis of the patient's switch recombination fragments suggested the presence of a defect similar, although less severe, to that observed in patients with hypomorphic DNA ligase IV mutations (6).
To determine whether the resolution of other developmentally programmed DNA DSBs was also affected in the patient, we addressed the status of somatic hypermutation. However, unlike the abnormal repair of switch junctions observed, we could not detect any gross differences in the profile of somatic hypermutation between controls and the patient (SI Fig. 5).
Cells Derived from Patient 15–9BI Exhibit a Moderately Increased Hypersensitivity to IR.
Given that many proteins involved in CSR are also involved in repair of DNA DSBs that arise as a consequence of exposure to various DNA-damaging agents such as IR, we examined the radiosensitivity of cells derived from the patient. Cells from this individual exhibited a moderate but reproducible increased hypersensitivity to IR as measured by both colony formation and chromosomal radiosensitivity (SI Table 2 and Fig. 1). This indicated that the observed CSR defect exhibited by 15–9BI was likely to be caused by an underlying inability to properly repair DNA DSBs. However, no gross defect in NHEJ, as measured by plasmid religation, could be detected in his cells (SI Fig. 6_A_), indicating that the genetic abnormality in this patient does not reside in a core component of the end-joining machinery.
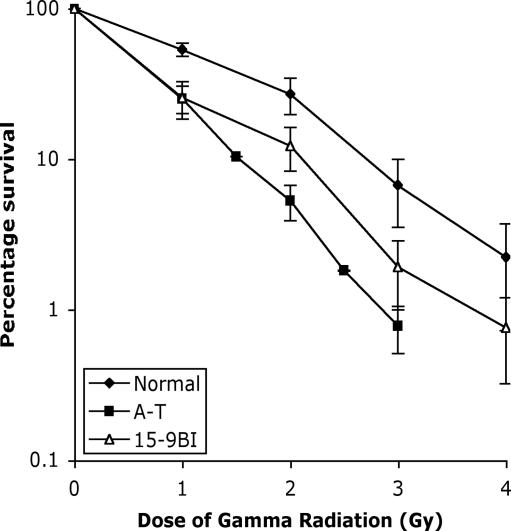
Fig. 1.
Skin fibroblasts derived from patient 15–9BI are radiosensitive, as measured by colony formation assay. Normal, classical A-T and 15–9BI primary fibroblasts were plated at low density and irradiated with the doses indicated, and the number of surviving colonies were counted.
Patient 15–9BI Cells Do Not Show Alterations in DNA DSB Repair Genes.
To investigate the genetic defect resulting in the hypersensitivity to IR, extracts of cells derived from patient 15–9BI were immunoblotted to assess the expression levels of various proteins known to be involved in the cellular response to DNA DSBs. None of the proteins analyzed in 15–9BI cells exhibited any alterations in expression level or size (SI Fig. 6_B_). Furthermore, the ATM, hMRE11, NBS1, hRAD50, DNA ligase IV, XRCC4, Cernunnos/XLF, MCPH1 (BRIT1), and H2AX genes were sequenced by using genomic DNA from patient 15–9BI cells and found to be wild type. These results indicate that the genetic defect in patient 15–9BI may lie within a previously uncharacterized DNA DSBR gene.
Abnormal Relocalization of DSBR Proteins in RIDDLE Syndrome Cells After IR Exposure.
One important aspect of the cellular response to DNA damage is the ability of the repair and checkpoint proteins to efficiently relocalize to sites of damage; this has been clearly demonstrated in mice that lack histone variant, H2AX, which fail to properly relocalize 53BP1, BRCA1, and Nbs1 to repair foci, subsequently resulting in increased genomic instability and a hypersensitivity to agents that induce DNA DSBs (7, 8). To assess whether cells from patient 15–9BI exhibited any abnormalities relocalizing DNA DSBR proteins to sites of damage, immunofluorescence was used to detect protein redistribution over time after exposure to IR. Unexpectedly, fibroblasts from patient 15–9BI formed significantly increased numbers of MDC1 (Fig. 2), γ-H2AX, Nbs1, and Rad51 (SI Fig. 7 A and B and SI Fig. 8) but not Fancd2 foci (data not shown) when compared with a control fibroblast cell line. These foci persisted at 24 h after irradiation, when the majority of lesions in the control fibroblast cell line had been repaired, as judged by γ-H2AX staining.
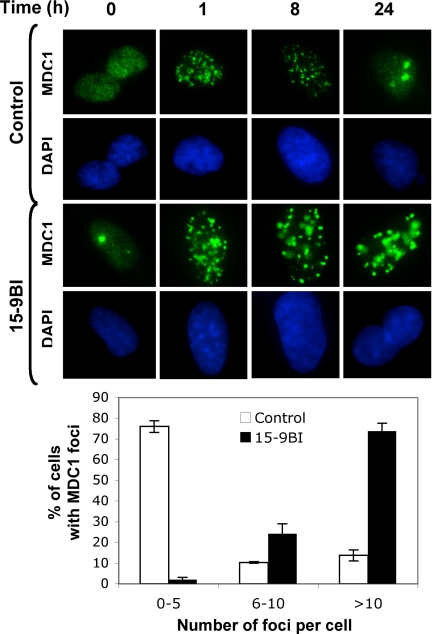
Fig. 2.
The 15–9BI cells show enhanced MDC1 recruitment to DNA breaks after irradiation. Control and 15–9BI fibroblasts were mock-irradiated or exposed to 3 Gy of IR and fixed at the times indicated after irradiation. Cells were stained with an anti-MDC1 antibody and DAPI to visualize the nuclear DNA. The histogram indicates the number of cells with MDC1 foci at 24 h after irradiation. A minimum of 300 cells was counted, and this was repeated at least three times.
Quite surprisingly, however, in stark contrast to the other DNA DSBR proteins analyzed, fibroblasts from patient 15–9BI completely failed to form any 53BP1 foci, even at late times after IR exposure (Fig. 3A). It has been reported that at early times (15–30 min) after irradiation, 53BP1 does not require H2AX to form DNA damage-induced foci but H2AX is required to stabilize 53BP1 once it has loaded on to damaged chromatin (9). A lack of 53BP1 IRIF at 15, 30, and 60 min after irradiation (Fig. 3A), indicates that the defect in 15–9BI is a global inability of 53BP1 to relocalize to damaged chromatin rather than being an abnormality in a pathway dependent on H2AX-mediated recruitment.
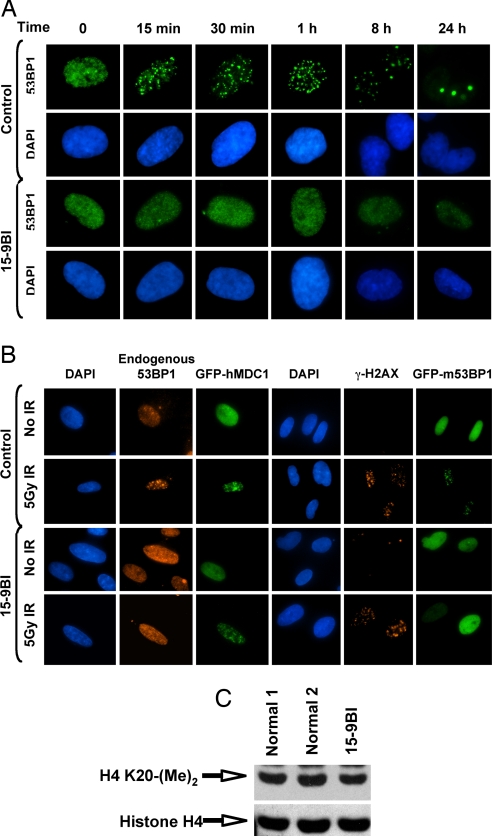
Fig. 3.
The 15–9BI cells fail to form 53BP1 IRIF after γ-irradiation. (A) Control and 15–9BI fibroblasts were mock-irradiated or exposed to 3 Gy of IR and fixed at the times indicated after irradiation. Cells were stained with an anti-53BP1 antibody and DAPI to visualize the nuclear DNA. (B) Exogenous MDC1 and 53BP1 do not correct the defective recruitment of 53BP1 to DNA breaks in cells from patient 15–9BI. Control and 15–9BI fibroblasts were transfected with GFP-hMDC1 or GFP-m53BP1, irradiated 48 h after transfection with 5 Gy, and allowed to recover for 4 h. The cells were fixed and stained with either an anti-53BP1 antibody to detect endogenous 53BP1 or an anti-γ-H2AX antibody. DAPI was used to visualize the nuclear DNA. (C) The 15–9BI cells exhibit normal levels of histone H4 dimethylation on lysine-20. Whole-cell extract was prepared from a normal or 15–9BI LCL and separated by SDS/PAGE. Western blots were carried out by using antibodies that recognized pan-histone H4 and histone H4 dimethylated on lysine-20.
To confirm this defect, we analyzed the ability of these cells to form Rif1 foci, a recently characterized checkpoint protein that requires both ATM and 53BP1 for its ability to form IRIF (10). Consistent with the 53BP1 relocalization defect in 15–9BI cells, these cells also failed to form Rif1 IRIF (SI Fig. 9).
In addition to Rif1, 53BP1 has been reported to regulate BRCA1 relocalization after the induction of DNA DSBs (11). Therefore, we assessed whether the absence of 53BP1 IRIF observed in cells from patient 15–9BI would also affect BRCA1 recruitment. Interestingly, although BRCA1 formed elevated numbers of IRIF at later times after IR exposure in a similar way to γ-H2AX and MDC1, 15–9BI cells exhibited defective BRCA1 foci formation at the 30-min and 1-h time points (SI Fig. 10).
These data suggest that the underlying genetic defect in this patient results in a complete failure of his cells to properly target 53BP1 and 53BP1-dependent repair/checkpoint proteins, e.g., Rif1, to sites of DNA damage. In addition, it appears that this defect also has a moderate affect on BRCA1 radiation-induced relocalization at early times during DSBR.
Reconstituting 15–9BI Cells with 53BP1 or MDC1 Does Not Correct the IRIF Defect.
In an attempt to further identify the genetic defect in RIDDLE syndrome cells that results in the inability of these cells to form 53BP1 IRIF, the TP53BP1 gene was completely sequenced at the genomic level, but no mutations were identified. This analysis included sufficient amounts of intronic sequence flanking each exon to exclude possible noncoding alterations that may affect splicing. To confirm the absence of any detectable mutations in the TP53BP1 gene, full-length TP53BP1 fused to GFP was transfected into control and 15–9BI cells. After IR exposure, the control cells formed robust GFP-m53BP1 containing foci that colocalized with γ-H2AX, whereas 15–9BI cells remained unable to form GFP-m53BP1 IRIF (Fig. 3B). These data indicated that the inability of 15–9BI cells to form 53BP1 foci after the induction of DSBs did not result from mutation of the TP53BP1 gene.
It has been demonstrated that MDC1 is required for the efficient recruitment of 53BP1 to sites of DNA DSBs (12). The MDC1 gene was also sequenced at the genomic level from 15–9BI cells and was also found to be wild type. Heterozygous polymorphic variants were identified in both the MDC1 and TP53BP1 genes, indicating that both alleles from patient 15–9BI were represented equally during the analysis. Again, control and 15–9BI cells were transfected with a full-length _hMDC1_-GFP expression construct and the ability of this to correct endogenous 53BP1 foci formation after exposure to IR was assessed. Both control and 15–9BI cells formed GFP-hMDC1 IRIF. However, this was unable to complement the 53BP1 IRIF defect observed in 15–9BI cells (Fig. 3B), thereby ruling out MDC1 as the responsible gene.
More recently, it has been shown that the tudor domain of 53BP1 specifically binds dimethylated lysine-20 (K-20) on histone H4 and that this interaction was responsible for the relocalization of 53BP1 to sites of damage (13). Therefore, it is conceivable that reduced or absent histone H4 K-20 dimethylation in 15–9BI cells could account for the lack of 53BP1 foci induced by IR exposure.
However, as shown in Fig. 3C, 15–9BI cells did not exhibit any alterations in the level of histone H4 K-20 dimethylation, indicating that it was not loss of this histone modification resulting in the inability of 15–9BI cells to form 53BP1 IRIF.
These data point to the existence of an additional factor, other than MDC1, 53BP1, and PR-Set7, the histone H4 K-20 methyltransferse, that can regulate the damage-induced accumulation of 53BP1 at sites of DNA breaks.
Patient 15–9BI Cells Exhibit Aberrant DNA Damage-Induced Cell Cycle Checkpoint Activation.
It has been demonstrated by using siRNA knockdown, that 53BP1 functionally regulates both the intraS- and G2/M-phase DNA damage-activated cell cycle checkpoints after low-dose irradiation (11, 14). However, mouse embryo fibroblasts (MEFs) derived from the 53BP1 knockout mouse exhibited no dramatic abnormalities in regulation of either damage-activated checkpoints (15), although these discrepancies may, in part, be attributed to differences in irradiation dose and cell types used.
To determine whether 15–9BI cells exhibited any defects in the ability to activate the G2/M checkpoint, fibroblasts were irradiated with 3 Gy of IR and harvested over a period of 24 h. Cells were fixed and stained with an antibody directed against phosphorylated serine-10 of histone H3, a well documented marker of mitotic cells, and subjected to flow cytometric analysis (16).
A clear decrease in the percentage of mitotic cells was observed in 15–9BI cells within the first hour after irradiation, which is indistinguishable from normal, indicating that these cells can clearly activate the G2/M checkpoint normally. Analysis at later times after irradiation, however, revealed an inability of 15–9BI cells to recover properly from the checkpoint, as indicated by the lower percentage of cells entering mitosis at 8 and 24 h after irradiation (Fig. 4A).
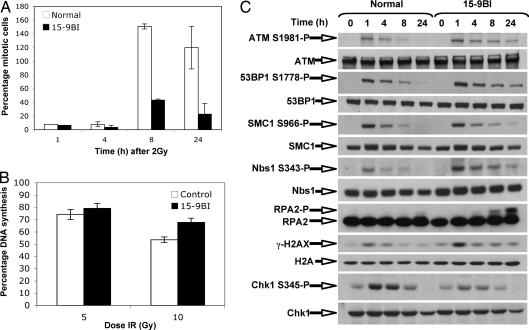
Fig. 4.
After irradiation, cells from patient 15–9BI exhibit abnormal cell cycle checkpoint response and hyperactive ATM-dependent DNA damage signaling. (A) Control and 15–9BI fibroblasts were mock-treated or irradiated with 3 Gy of γ-irradiation and then fixed at the times indicated. Cells were labeled with an anti-phospho histone H3 serine-10 antibody and propidium iodide and then subjected to FACS analysis. (B) Control and 15–9BI fibroblasts were labeled with 14[C]thymidine for 24 h, irradiated with the doses indicated, and then chased with 3[H]thymidine for 1 h. Cells were fixed and the 3[H]/14[C] calculated by using a scintillation counter. (C) Control and 15–9BI fibroblasts were mock-irradiated or exposed to 3 Gy of IR and fixed at the times indicated after irradiation. Whole-cell extract was prepared and subjected to SDS/PAGE/Western blot analysis using the antibodies indicated.
Radioresistant DNA synthesis (RDS) is a characteristic phenotype exhibited by cells that cannot appropriately regulate the intraS phase checkpoint in the presence of DNA damage induced by IR (17). To ascertain whether cells derived from patient 15–9BI also displayed this trait, control and 15–9BI cells were irradiated with 5 or 10 Gy of IR, incubated for 1 h, and then DNA synthesis was determined by thymidine incorporation. In a similar fashion to the 53BP1 knockout MEFs (15), 15–9BI cells exhibited a mild intraS phase checkpoint defect but only when irradiated with a high dose of IR (10 Gy) (Fig. 4B). This indicated that the gene defective in this patient does not contribute significantly to the regulation of the DNA damage-activated intraS-phase checkpoint.
RIDDLE Syndrome Cells Show Hyperactivation of ATM-Dependent Signaling Pathways.
It is known that ATM and ATM-dependent signaling pathways are primarily responsible for controlling the activation and maintenance of DNA damage-induced cell cycle checkpoints after exposure to IR (18). To investigate the status of these pathways in 15–9BI cells with regard to the abnormal checkpoint responses observed, a time course after exposure to 2 Gy of IR was carried out over a period of 24 h. Interestingly, an antibody to phosphorylated serine-1981 of ATM, which is believed to represent a marker of ATM activation (19), showed an increased signal in 15–9BI cells compared with a control cell line at the same time point, and was sustained over a 24-h time period. The hyperresponsive activation of ATM was mirrored by the phosphorylation status of various downstream ATM-dependent targets (Fig. 4C). In contrast however, the relocalization of proteins recognized by the phospho-ATM serine-1981 antibody to IRIF (SI Fig. 11) was impaired in 15–9BI cells compared with control cells. This observation suggested that the gene product, which is defective in patient 15–9BI, contributes to the regulation of ATM recruitment to DNA DSBs but not its activation, although it must be taken into account that phospho-dependent antibodies, when used for immunofluorescence, are likely to detect multiple epitopes in a number of different phospho-proteins. Interestingly however, this defect in the recruitment of phosphorylated ATM was not observed in the 53BP1 knockout MEFs, rather the recruitment was elevated over and above that seen in the wild-type parental MEFs (SI Fig. 12), which mirrored the γ-H2AX/MDC1 response in these cells (SI Fig. 13 A and B).
In contrast to the other markers of DNA damage-induced signaling assessed in 15–9BI cells, the activation of the checkpoint kinase Chk1 (20), as measured by phosphorylation on serine-345, was not as efficient as that observed in the control cell line (Fig. 4C). This may, in part, account for the observed mild intraS-phase checkpoint defect.
Collectively, these data demonstrate that the factor required for 53BP1 relocalization after IR exposure may also play a role in suppressing many signaling pathways controlled by ATM.
Discussion
Here, we describe a patient with a newly described disorder that we have termed RIDDLE syndrome. This patient exhibits an increased radiosensitivity, mild immunodeficiency, dysmorphic features, and learning difficulties. Cells derived from this patient primarily fail to properly relocalize the DNA DSBR protein, 53BP1, to sites of DNA damage. The phenotype of cells derived from this patient recapitulated many of those previously documented in the 53BP1 knockout mouse (3, 4, 15) although no mutations could be found in the TP53BP1 gene itself nor in any additional genes that encode proteins, e.g., MDC1, H2AX, and PR-Set7 that are known to be involved in regulating 53BP1 relocalization to DNA breaks. This observation supports the existence of an as yet unknown protein that functions upstream of 53BP1 during the cellular response to DNA DSBs.
At present, the cause of the IgG deficiency and elevated level of IgM is not known, although the DNA DSBR defect leading to increased sensitivity to IR may also cause a subtle defect of end-joining in class switch recombination. The normal level of somatic hypermutation may suggest that the gene responsible for the altered pattern of switch junctions in this patient lies downstream of AID and UNG1 in this process. Moreover, the lack of an obvious NHEJ repair defect implies that the deficient factor is not part of the core NHEJ machinery. The observation that cells from patient 15–9BI do not form any 53BP1 IRIF at early or late stages of DNA DSBR suggests that the defective gene product is required for both the histone dimethylation-mediated and the γ-H2AX-mediated components of the signaling pathway responsible for 53BP1 relocalization to DNA breaks. Given that the levels of the dimethylated histone H4 do not change after induction of DNA damage, it has been hypothesized that radiation-induced changes in the surrounding chromatin is the signal that allows the methylated lysine on histone H4 to become visible, which can subsequently facilitate 53BP1 binding through its tudor domain (13). It is quite possible that the defect in patient 15–9BI resulting in loss of 53BP1 foci formation is due to a lack of an ability to remodel the nucleosome surrounding the DNA break that allows the histone methylation to be exposed. However it is unclear whether a lack of the histone H4-dependent pathway completely abolishes all 53BP1 foci formation or whether at later times during DNA DSBR, the H2AX-dependent pathway is sufficient to take over.
Interestingly, the genetic abnormality in 15–9BI cells also appears to affect BRCA1 foci formation albeit to a lesser extent than 53BP1. Consistent with these observations, 53BP1 has been shown to regulate BRCA1 foci formation after IR exposure (11), although this has not been assessed in any of the mouse 53BP1 knockout models. Furthermore, from the BRCA1 foci kinetics in 15–9BI cells, it would appear that like 53BP1, BRCA1, also has different determinants for its relocalization at early versus late times after irradiation.
The checkpoint defects in cells derived from patient 15–9BI are subtle. Taken at face value, this implies that the mutant gene product does not play a significant role in the activation of DNA damage-induced cell cycle checkpoints, although we cannot rule out the possibility that this patient carries a hypomorphic mutant allele that allows activation of the cell cycle checkpoint after DNA damage to near normal levels. The sustained G2/M arrest observed in these cells may be accounted for by the hyperactivation of ATM-dependent signaling and/or the persistence of unrepaired DNA breaks. However, the possibility that this may also arise as a consequence of an inability to inhibit the checkpoint signaling response even though the DNA damage has been repaired cannot be discounted. The mild RDS phenotype is suggestive of a checkpoint defect, and this is consistent with the abnormal activation of Chk1 observed in these cells. However, the severity of the checkpoint defect observed maybe ameliorated somewhat by the heightened MDC1-dependent DNA damage response, a pathway that is known to modulate Chk1 activation and the intraS-phase checkpoint (12).
It is tempting to speculate that cells with an inability to relocalize 53BP1 to sites of damage compensate by up-regulating the MRN/MDC1 pathway. In support of this, it has been suggested (21) by using 53BP1-directed siRNA, that this is indeed the case. Although it appears that 53BP1 and MDC1 differ in their dependency for specific DNA damage-induced histone modifications for their initial recruitment to breaks, 53BP1 still requires H2AX phosphorylation to stabilize its binding to chromatin surrounding DNA breaks at later times during DNA repair. Therefore, in the absence of 53BP1, chromatin/DNA breaks normally recognized by 53BP1 will eventually switch, during later stages of repair, to an H2AX-dependency, thus elevating the cells requirement for the H2AX/MDC1/Nbs1 pathway.
Taken together, although the gene mutated in RIDDLE syndrome has yet to be identified, our initial observations characterizing cells derived from this patient serve to highlight several points, the most important being that a human disease associated with defects in 53BP1-mediated DNA damage signaling has not before been documented. Furthermore, a link between the 53BP1 pathway and immune system development in humans has not been previously identified. Second, we demonstrate the existence of an additional unknown biochemical event, other than the DNA damage-induced chromatin modification, which is required for 53BP1 relocalization to sites of DNA breaks and which also has an impact on BRCA1 recruitment. Lastly, cells with abnormalities in DNA damage-induced 53BP1 cellular signaling may partly compensate for this defect by up-regulating the γ-H2AX/MDC1/MRN pathway.
Materials and Methods
Cell Lines.
Fibroblast cell lines were maintained in DMEM supplemented with 10% FCS, glutamine, and penicillin/streptomycin (pen/strep). Lymphoblastoid cell lines (LCLs) were routinely maintained in RPMI medium 1640 supplemented with 10% FCS, glutamine, and pen/strep. The 53BP1 +/+ and −/− (MEFs) were obtained from Junjie Chen and Irene Ward (Yale University School of Medicine, New Haven, CT) and were also maintained in DMEM supplemented with 10% FCS, glutamine, and pen/strep. All experiments were carried out by using hTert-immortalized skin fibroblasts derived from patient 15–9BI and a normal donor under the same culture conditions, unless otherwise stated. Where indicated, phenotypic differences between 15–9BI and normal control fibroblasts were verified in an LCL derived from patient 15–9BI. A variety of normal LCLs derived from laboratory donors were used as controls.
Mutation Analysis.
Candidate genes were sequenced from cDNA or genomic DNA isolated from patient 15–9BI cells. The TP53BP1 and MDC1 genes were sequenced at the genomic level and compared with reference sequences (AC018924.9 and AB088099.1, respectively) deposited in the National Center for Biotechnology Information database.
Immunoblot Analysis.
Cell extracts were prepared as described (12). The generation of antibodies to MDC1 and ATM has been described (12, 22). The antibodies were used according to manufacturer's instructions; anti-phospho-H2AX, phospho-ATM, di-methyl histone H4 lysine-20 and H2A antibodies (Upstate Biotechnology, Lake Placid, NY), anti-BRCA1, Ku70, and Chk1 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), anti-53BP1, DNA-PK, and RPA1 antibodies (Merck, Nottingham, U.K.), anti-phospho-53BP1, phospho-Nbs1, phospho-SQ/TQ, phospho-Chk1 and histone H4 antibodies (Cell Signaling Technology, Beverly, MA), anti-RPA2, Rad51, Rad50, Mre11, and Nbs1 antibodies (Novus, Littleton, CO), anti-phospho-SMC1 antibody (Bethyl, Montgomery, TX). Antibodies to XRCC4 and DNA ligase IV were a gift from Penny Jeggo (University of Sussex, Brighton, U.K.).
Immunofluorescence.
Cells seeded on to glass slides were permeabilized and then fixed in extraction buffer (12). Slides were incubated for 1 h at room temperature with the primary antibody diluted in 2% FCS, washed three times in PBS, and then incubated for an additional 1 h with the secondary antibody. Slides were washed again three times in PBS and then sealed with a coverslip and Vectashield containing DAPI (Vector Laboratories, Burlingame, CA). Primary antibodies used are described above. All secondary antibodies were either Alexa Fluor 488 or Alexa Fluor 596 coupled and purchased from Invitrogen (Carlsbad, CA).
Colony Formation and Radioresistant DNA Synthesis.
These were carried out as described (12).
Nonhomologous End-Joining Assay.
Whole-cell extract was prepared from 6 liters of LCLs, and the assay was carried out as described (23).
Transfections.
Fibroblasts were transfected by using the Amaxa nucleofector (Amaxa, Gaithersburg, MD) according to the manufacturer's instructions with 3 μg of plasmid DNA. Cells were seeded on to slides 24 h after transfection and harvested after an additional 24 h.
Supplementary Material
Supporting Information
Acknowledgments
We thank Drs. Claudia Lukas (Institute of Cancer Biology and Centre for Genotoxic Stress Research, Copenhagen, Denmark) and Yasuhisa Adachi (University of Edinburgh, Edinburgh, U.K.) for providing the m53BP1-GFP expression construct, Drs. Phang-Lang Chen (University of Texas, San Antonio, TX) and Matthew Weitzman (Salk Institute for Biological Studies, La Jolla, CA) for hMDC1-GFP expression constructs, Dr. Elizabeth Blackburn (University of California, San Francisco, CA) for providing the Rif1 antibody, Drs. Irene Ward and Junjie Chen for the 53BP1 knockout MEFs, Dr. Zhenkun Lou (Mayo Clinic College of Medicine, Rochester, MN) for providing the anti-mouse MDC1 antibody, and Dr. Jean-Pierre de Villartay's laboratory for sequencing the Cernunnos gene in patient 15–9BI before it was published. This work was supported by Cancer Research UK (G.S.S., P.J.B., and A.M.R.T.) and the Leukaemia Research Fund (T.S.).
Abbreviations
DSBs
DNA double strand breaks
DSBR
DSB repair
CSR
class switch recombination
IR
ionizing radiation
IRIF
IR-induced foci
MEF
mouse embryo fibroblast.
Footnotes
The authors declare no conflict of interest.
References
- 1.Gennery AR. Br Med Bull. 2006;77–78:71–85. doi: 10.1093/bmb/ldl006. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhuri J, Alt FW. Nat Rev Immunol. 2004;4:541–552. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- 3.Manis JP, Morales JC, Xia Z, Kutok JL, Alt FW, Carpenter PB. Nat Immunol. 2004;5:481–487. doi: 10.1038/ni1067. [DOI] [PubMed] [Google Scholar]
- 4.Ward IM, Reina-San-Martin B, Olaru A, Minn K, Tamada K, Lau JS, Cascalho M, Chen L, Nussenzweig A, Livak F, et al. J Cell Biol. 2004;165:459–464. doi: 10.1083/jcb.200403021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen S, Casellas R, Reina-San-Martin B, Chen HT, Difilippantonio MJ, Wilson PC, Hanitsch L, Celeste A, Muramatsu M, Pilch DR, et al. Nature. 2001;414:660–665. doi: 10.1038/414660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan-Hammarstrom Q, Jones AM, Lahdesmaki A, Zhou W, Gatti RA, Hammarstrom L, Gennery AR, Ehrenstein MR. J Exp Med. 2005;201:189–194. doi: 10.1084/jem.20040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassing CH, Chua KF, Sekiguchi J, Suh H, Whitlow SR, Fleming JC, Monroe BC, Ciccone DN, Yan C, Vlasakova K, et al. Proc Natl Acad Sci USA. 2002;99:8173–8178. doi: 10.1073/pnas.122228699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, et al. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner WM, Nussenzweig A. Nat Cell Biol. 2003;5:675–679. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- 10.Silverman J, Takai H, Buonomo SB, Eisenhaber F, de Lange T. Genes Dev. 2004;18:2108–2119. doi: 10.1101/gad.1216004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, Matsuoka S, Carpenter PB, Elledge SJ. Science. 2002;298:1435–1438. doi: 10.1126/science.1076182. [DOI] [PubMed] [Google Scholar]
- 12.Stewart GS, Wang B, Bignell CR, Taylor AMR, Elledge SJ. Nature. 2003;421:961–966. doi: 10.1038/nature01446. [DOI] [PubMed] [Google Scholar]
- 13.Botuyan MV, Lee J, Ward IM, Kim JE, Thompson JR, Chen J, Mer G. Cell. 2006;127:1361–1373. doi: 10.1016/j.cell.2006.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiTullio RA, Jr, Mochan TA, Venere M, Bartkova J, Sehested M, Bartek J, Halazonetis TD. Nat Cell Biol. 2000;4:998–1002. doi: 10.1038/ncb892. [DOI] [PubMed] [Google Scholar]
- 15.Ward IM, Minn K, van Deursen J, Chen J. Mol Cell Biol. 2003;23:2556–2563. doi: 10.1128/MCB.23.7.2556-2563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu B, Kim ST, Lim DS, Kastan MB. Mol Cell Biol. 2002;22:1049–1059. doi: 10.1128/MCB.22.4.1049-1059.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartek J, Lukas C, Lukas J. Nat Rev Mol Cell Biol. 2004;5:792–804. doi: 10.1038/nrm1493. [DOI] [PubMed] [Google Scholar]
- 18.Kurz EU, Lees-Miller SP. DNA Repair (Amsterdam) 2004;3:889–900. doi: 10.1016/j.dnarep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Bakkenist CJ, Kastan MB. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 20.Zhao H, Watkins JL, Piwnica-Worms H. Proc Natl Acad Sci USA. 2002;99:14795–14800. doi: 10.1073/pnas.182557299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mochan TA, Venere M, DiTullio RA, Jr, Halazonetis TD. Cancer Res. 2003;63:8586–8591. [PubMed] [Google Scholar]
- 22.Starczynski J, Simmons W, Flavell JR, Byrd PJ, Stewart GS, Kullar HS, Groom A, Crocker J, Moss PA, Reynolds GM, et al. Am J Pathol. 2003;163:423–432. doi: 10.1016/S0002-9440(10)63672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumann P, West SC. Proc Natl Acad Sci USA. 1998;95:14066–14070. doi: 10.1073/pnas.95.24.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information