Pathological role of osteoclast costimulation in arthritis-induced bone loss (original) (raw)
Abstract
Abnormal T cell immune responses induce aberrant expression of inflammatory cytokines such as TNF-α, leading to osteoclastmediated bone erosion and osteoporosis in autoimmune arthritis. However, the mechanism underlying enhanced osteoclastogenesis in arthritis is not completely understood. Here we show that TNF-α contributes to inflammatory bone loss by enhancing the osteoclastogenic potential of osteoclast precursor cells through inducing paired Ig-like receptor-A (PIR-A), a costimulatory receptor for receptor activator of NF-κB (RANK). In fact, bone erosion and osteoporosis, but not inflammation, caused by aberrant TNF-α expression were ameliorated in mice deficient in Fc receptor common γ subunit or β2-microglobulin, in which the expression of PIR-As and PIR-A ligands is impaired, respectively. These results establish the pathological role of costimulatory receptors for RANK in bone loss in arthritis and may provide a molecular basis for the future therapy of inflammatory diseases.
Keywords: paired immunoglobulin-like receptor, rheumatoid arthritis, TNF-α, costimulatory
Osteoclasts, multinucleated cells of hematopoietic origin that degrade the bone matrix (1, 2), are regulated by immunoregulatory molecules under both physiological and pathological conditions (3). Osteoclast costimulation is an emerging concept that has recently come into acceptance based on the observation that combined deficiency of Fc receptor common γ subunit (FcRγ) and DNAX-activation protein 12 (DAP12) results in a complete lack of osteoclasts (4, 5). In addition to receptor activator of NF-κB (RANK), the receptor for RANK ligand (RANKL), the Ig-like receptors associated with FcRγ and DAP12 have been recognized as essential receptors for osteoclastogenesis (2, 3). Ig-like receptors were extensively studied in natural killer and myeloid cells, as well as in B cells, but this observation established that Ig-like receptors function as osteoclast costimulatory receptors, which are crucial for bone homeostasis under physiological conditions. The mutation in DAP12 causes Nasu-Hakola disease in humans (6, 7), but there has been no other report on the contribution of osteoclast costimulation in pathological conditions in the skeletal system.
Ig-like receptors associate with adaptors harboring an immunoreceptor tyrosine-based activation motif such as FcRγ and DAP12 in immune cells (6). In osteoclasts, Ig-like receptors that associate with FcRγ include paired Ig-like receptor-A (PIR-A) and osteoclast-associated receptor (OSCAR), whereas those that associate with DAP12 include triggering receptor expressed on myeloid cells-2 (TREM-2) and signal-regulatory protein β (SIRPβ1) (4). Although the ligands for these receptors have not been well characterized in the context of osteoclastogenesis, it is likely that the ligands for DAP12-associating receptors are expressed in the culture system of osteoclast precursor cells, but those for FcRγ-associating receptors are mainly expressed by osteoclastogenesis-supporting cells (4, 8). RANK and its costimulatory receptors synergistically initiate the activation of calcium signaling that leads to the induction and autoamplification of nuclear factor of activated T cells c1 (NFATc1) (4), the key transcription factor for osteoclastogenesis (9, 10).
Rheumatoid arthritis (RA) is an autoimmune disease characterized by inflammation of synovial joints with CD4+ T cell infiltration and synovial cell proliferation, leading to severe bone destruction mediated by osteoclasts (11). Bone loss is not only observed as erosion in the affected joints, but also in the forms of periarticular and systemic osteoporoses (12). Importantly, inflammatory cytokines directly act on osteoclast precursor cells of hematopoietic lineage and activate their differentiation into osteoclasts by cooperating with RANKL (13–15), but it is not fully understood how these cytokines exert their direct effect. Although the higher circulation level of these cytokines may contribute to enhanced osteoclastogenesis in bone tissues apart from inflammatory lesions, the mechanism of systemic osteoporosis associated with arthritis also remains unclear.
TNF-α is one of the critical cytokines in the pathogenesis of RA, as shown by many gain- and loss-of-function genetic models (16, 17), as well as by the clinical efficacy of anti-TNF-α therapy (18). It is notable that transgenic mice that express human TNF-α (hTNFtg mice) spontaneously develop destructive arthritis (16). Interestingly, the anti-TNF-α antibody has been shown to suppress bone damage in patients who had no clinical improvement in terms of pain and inflammation (19), suggesting that, under arthritic condition, TNF-α exerts an important direct action on bone, which is independent of its action on the immune system. Although there has been no in vivo evidence that TNF-α induces osteoclastogenesis in mice lacking RANKL signaling or that TNF-α rescues osteopetrosis in such mice (20–22), TNF-α clearly acts on osteoclast precursor cells and changes the responsiveness of the cells under certain conditions (13–15). However, the molecular mechanism underlying the enhanced osteoclastogenic potential of osteoclast precursor cells in the presence of TNF-α remains to be elucidated.
Here we examined the effect of TNF-α on osteoclastogenesis in a culture of osteoclast precursor cells stimulated with RANKL and M-CSF. We found that the promotive effect of TNF-α is notably observed in the late phase of differentiation characterized by NFATc1 autoamplification. Because NFATc1 autoamplification depends on calcium signaling, we explored the involvement of Ig-like receptors that initiate calcium signaling in the effect of TNF-α. We show that TNF-α specifically induces Ig-like receptors PIR-As and their ligands, MHC class I molecules (23, 24). The in vitro effect of TNF-α on osteoclastogenesis was significantly suppressed by PIR-A-Fc addition and abrogated in _FcR_γ-deficient cells. The effect was also markedly suppressed in cells deficient in β2M, an essential subunit for MHC class I molecules (25). We further demonstrate the importance of the TNF-α-mediated induction of PIR-As by using hTNFtg mice crossed with mice deficient in FcRγ (_FcR_γ−/−) or β2M (_B2m_−/−). Thus, TNF-α renders osteoclast precursor cells prone to differentiating into osteoclasts by up-regulating costimulation through PIR-As, which contribute to both local and systemic osteoporoses in arthritis.
Results
TNF-α Enhances Late Phase of Osteoclast Differentiation and NFATc1 Autoamplification.
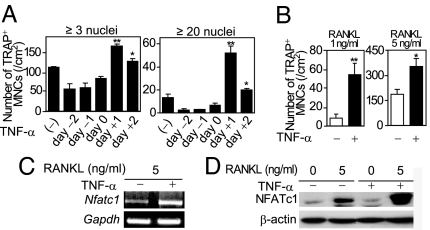
To gain insight into the molecular mechanism underlying the promotive effect of TNF-α on osteoclastogenesis, TNF-α was added at various time points to the osteoclast formation system: Bone marrow-derived monocyte/macrophage lineage cells (BMMs) were stimulated with RANKL and M-CSF, and multinucleated cells positive for tartrate-resistant acid phosphatase (TRAP+ MNCs) were counted [supporting information (SI) Fig. 7_A_]. When TNF-α was added to BMMs at the same time as RANKL or before it, TNF-α showed no promotive effect on osteoclastogenesis (Fig. 1A), consistent with a previous report (13). The promotive effect of TNF-α on TRAP+ MNC formation was prominently observed when TNF-α was added 1 day after RANKL stimulation or later (Fig. 1A and SI Fig. 7_B_), and such an effect was more distinctly observed at lower RANKL concentrations (Fig. 1B). These results suggest that the effect of TNF-α is not dependent on RANK or signaling molecules immediately activated by RANKL stimulation, such as NF-κB or TNF receptor-associated factor 6 (2). Thus, we evaluated the effect of TNF-α on RANKL-mediated NFATc1 induction, a hallmark event in the late phase of osteoclastogenesis (9). Both NFATc1 mRNA and protein levels were highly up-regulated by TNF-α (Fig. 1 C and D), suggesting that TNF-α has an effect on molecules regulating NFATc1 autoamplification.
Fig. 1.
Promotive effects of TNF-α on RANKL-induced osteoclastogenesis and NFATc1 autoamplification. (A) Effect of TNF-α on osteoclastogenesis. Number of TRAP+ MNCs (≥3 or ≥20 nuclei) was counted. See SI Fig. 7_A_ for the treatment periods. ∗, P < 0.05; ∗∗, P < 0.01. (B) Effect of TNF-α on osteoclastogenesis in the presence of RANKL (1 or 5 ng/ml). BMMs cultured in the presence of RANKL (1 or 5 ng/ml) were stimulated with TNF-α. ∗, P < 0.05; ∗∗, P < 0.01. (C) Expression of Nfatc1 mRNA in TNF-α-stimulated BMMs (RT-PCR analysis). (D) Expression of NFATc1 in TNF-α-stimulated BMMs (immunoblot analysis).
TNF-α Promotes Osteoclastogenesis Through Induction of PIR-As and Their Ligands.
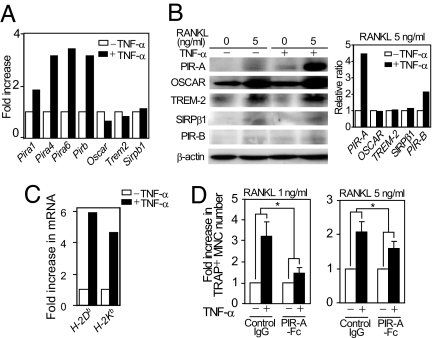
NFATc1 autoamplification requires calcium signaling, which is mediated by costimulatory receptors for RANK and immunoreceptor tyrosine-based activation motif-harboring adaptors (4, 8). GeneChip analysis showed that, among Ig-like receptors that function as costimulatory receptors, Pira expression was selectively increased by TNF-α stimulation in BMMs (Fig. 2A) together with a 2-fold increase in the expression level of _FcR_γ (data not shown). There are at least six Pira genes in the mouse chromosome 7 that encode PIR-A molecules containing a similar ectodomain with six Ig-like loops and a transmembrane domain possessing a positively charged arginine critical for the association with FcRγ (24). TNF-α-mediated induction of PIR-As was also confirmed by real-time PCR and immunoblot analyses (Fig. 2B and SI Fig. 8).
Fig. 2.
Selective induction of PIR-As and their ligands in response to TNF-α stimulation. (A) GeneChip analysis of mRNA expression of Ig-like receptors in BMMs cultured with or without TNF-α. (B) Expression of PIR-As in TNF-α-stimulated BMMs (immunoblot analysis, Left). The relative ratio of the protein level in TNF-α-treated cells to that in nontreated cells was calculated (Right). (C) Expression of H-2 mRNAs in TNF-α-stimulated BMMs (real-time PCR analysis). (D) Effect of PIR-A-Fc on osteoclastogenesis in TNF-α-stimulated BMMs. BMMs cultured in the presence of RANKL (1 or 5 ng/ml) and TNF-α were treated with control IgG or PIR-A-Fc. ∗, P < 0.05.
PIR-A-mediated signals are counterbalanced by the related inhibitory receptor PIR-B (26). Although TNF-α also increased the PIR-B expression level, the ratio of the total protein level of PIR-As to that of PIR-B was markedly increased by TNF-α (Fig. 2B), indicating that the PIR-A-mediated signal is strengthened after TNF-α stimulation. PIR-A ligands have not been fully determined, but recent reports indicate that they include MHC class I molecules (23, 24). In fact, the expression levels of MHC class I (H-2) molecules such as H-2Db and H-2Kb in BMMs were significantly increased by TNF-α (Fig. 2C).
As mentioned earlier, PIR-A ligands are mainly expressed by osteoblasts, and the PIR-A signal is not activated in the culture of BMMs under physiological conditions (4, 8). However, the TNF-α-mediated induction of PIR-As and their ligands may cooperatively strengthen the PIR-A signaling axis in the BMM culture and may contribute to the enhanced osteoclastogenesis under inflammatory conditions. Consistent with this notion, PIR-A-Fc fusion protein, but not control IgG, significantly inhibited a TNF-α-mediated enhancement of osteoclast formation from BMMs (Fig. 2D).
Requirement of FcRγ in TNF-α-Mediated Promotion of Osteoclastogenesis.
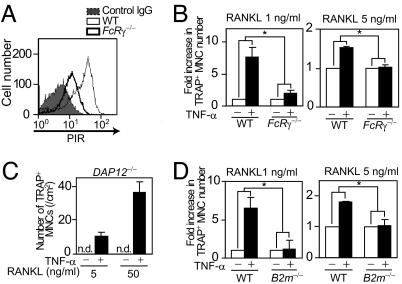
PIR-A has only a short cytoplasmic tail and requires the association with FcRγ for cell surface expression and signal transduction (26). As expected, PIR-As expression was severely impaired on the membrane of _FcR_γ−/− BMMs (Fig. 3A). Because PIR-A molecules are encoded by multiple genes and it is hardly possible to disrupt them all genetically, _FcR_γ−/− mice serve as an alternative tool for analyzing the loss of function of Pira genes despite a possible contribution of other associating receptors.
Fig. 3.
Central role of PIR-As and their adaptor FcRγ in enhanced osteoclastogenesis induced by TNF-α. (A) Cell surface expression of PIRs on BMMs. Flow-cytometric analysis of PIRs expression in WT (thin line) or _FcR_γ−/− (thick line) BMMs. The thick line shows WT BMMs stained with control IgG. (B) Effect of TNF-α on osteoclastogenesis in _FcR_γ−/− BMMs. WT or _FcR_γ−/− BMMs cultured in the presence of RANKL (1 or 5 ng/ml) were stimulated with TNF-α. ∗, P < 0.05. (C) Effect of TNF-α on osteoclastogenesis in _DAP12_−/− BMMs. _DAP12_−/− BMMs cultured in the presence of RANKL (5 or 50 ng/ml) were stimulated with TNF-α (50 ng/ml). n.d., not detected. (D) Effect of TNF-α on osteoclastogenesis in _B2m_−/− BMMs. WT or _B2m_−/− BMMs cultured in the presence of RANKL (1 or 5 ng/ml) were stimulated with TNF-α. ∗, P < 0.05.
When TNF-α was added to _FcR_γ−/− BMMs, the promotive effect of TNF-α on osteoclastogenesis was markedly suppressed compared with that on WT BMMs (Fig. 3B). These results indicate that TNF-α promotes osteoclastogenesis through FcRγ-associating receptors, including PIR-As. _DAP12_−/− BMMs are unable to differentiate into osteoclasts in the pure BMM culture, but can differentiate in the coculture with osteoblasts (which may express ligands for FcRγ-associating receptors) (4, 8). Interestingly, _DAP12_−/− BMMs could differentiate into osteoclasts with bone-resorbing activity in BMM culture if stimulated with TNF-α in addition to RANKL (Fig. 3C and SI Fig. 9). This result further supports the notion that FcRγ-associating receptors such as PIR-A function in the BMM culture under inflammatory conditions. In addition, no TNF-α-mediated promotion of osteoclastogenesis was observed in BMMs deficient in β2M, which forms the invariable light chain subunit of MHC class I molecules (25) (which represent PIR-A ligands) (Fig. 3D). Taken together, these results suggest the importance of PIR-A among Ig-like receptors in the TNF-α-mediated activation of osteoclastogenesis.
FcRγ-Dependent Enhanced Osteoclastogenic Potential of BMMs from hTNFtg Mice.
hTNFtg mice develop destructive arthritis similar to RA and thus are well suited for the analyses of TNF-α-mediated pathological processes in autoimmune arthritis. In this model, the aberrant expression of TNF-α causes an abnormal proliferation of the synovium and local inflammation, which lead to osteoclast-mediated local bone erosion and osteoporosis (12).
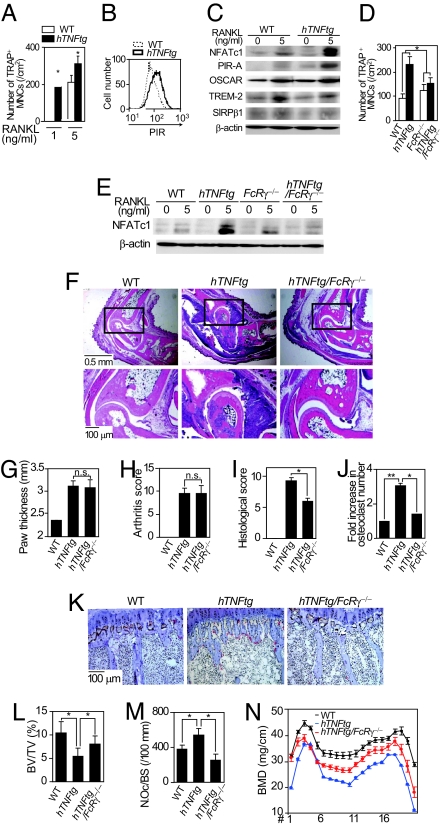
We isolated BMMs from hTNFtg mice and analyzed them by osteoclast formation assay. Interestingly, the BMMs derived from the hTNFtg mice (hTNFtg BMMs) differentiated into osteoclasts more efficiently than WT BMMs in response to RANKL (Fig. 4A). Although the mechanisms underlying the enhanced osteoclastogenesis in hTNFtg mice may include the induction of RANKL in mesenchymal cells (11, 14, 27) or the increase in the number of osteoclast precursor cells in vivo (21, 28), this result suggests a novel mechanism (i.e., the enhancement of the osteoclastogenic potential in the hTNFtg BMMs). Flow-cytometric analysis indicated that freshly isolated hTNFtg BMMs expressed a higher level of PIRs than WT BMMs, supporting the notion that the osteoclastogenic potential in the hTNFtg BMMs is enhanced (Fig. 4B). Interestingly, RT-PCR analysis revealed the expression of TNF in cultured BMMs to be undetected initially, but induced by RANKL stimulation (SI Fig. 10), suggesting that the effect of TNF-α is potentiated during the course of osteoclastogenesis in hTNFtg BMMs.
Fig. 4.
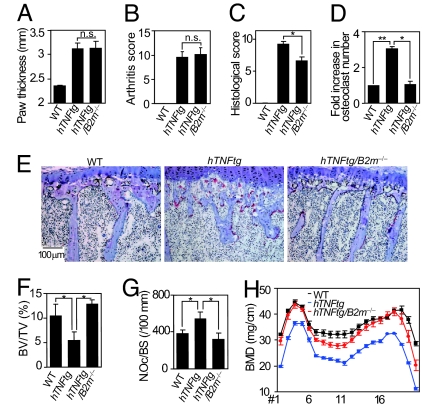
FcRγ mediates bone destruction and osteoporosis, but not inflammation in hTNFtg mice. (A) Osteoclastogenesis of hTNFtg BMMs stimulated with RANKL (1 or 5 ng/ml). ∗, P < 0.05. (B) Flow-cytometric analysis of PIRs' expression on the WT (dotted line) and hTNFtg (solid line) BMMs. (C) Expressions of NFATc1 and Ig-like receptors in hTNFtg BMMs (immunoblot analysis). (D) RANKL-induced osteoclastogenesis of WT, hTNFtg, _FcR_γ−/−, and hTNFtg/_FcR_γ−/− BMMs. ∗, P < 0.05. (E) Expression of NFATc1 in WT, hTNFtg, _FcR_γ−/−, and hTNFtg/_FcR_γ−/− BMMs (immunoblot analysis). (F) (Upper) Histological analysis of ankle joints (H&E). (Lower) Magnified view of the rectangular area. (G) Paw thickness of WT, hTNFtg, and hTNFtg/_FcR_γ−/− mice. n.s., not significant. (H–J) Arthritis score (H), histological score (I), and fold increase (J) in the number of osteoclasts in the ankles of WT, hTNFtg, and hTNFtg/_FcR_γ−/− mice. n.s., not significant; ∗, P < 0.05; ∗∗, P < 0.01. (K) Histological analysis of proximal tibiae of WT, hTNFtg, and hTNFtg/_FcR_γ−/− mice (TRAP and Toluidine blue staining). (L–N) BV/TV (L), number of osteoclasts per bone surface (N.Oc/BS) (M), and BMD (N) of tibiae of WT, hTNFtg, and hTNFtg/_FcR_γ−/− mice. ∗, P < 0.05.
NFATc1 induction by RANKL was more significant in the hTNFtg BMMs than in the WT BMMs (Fig. 4C). Therefore, we examined the expression of Ig-like receptors under the same conditions. Consistent with the observation in TNF-α-stimulated BMMs, the PIR-As' expression level was higher in the hTNFtg BMMs than in the WT BMMs, which was more obvious after RANKL stimulation (Fig. 4C). To obtain evidence of the importance of PIR-As in the enhanced osteoclastogenesis in the hTNFtg BMMs, we generated hTNFtg/_FcR_γ−/− mice in which PIR-A signaling is abrogated. Osteoclast formation and the NFATc1 induction by RANKL in the hTNFtg/_FcR_γ−/− BMMs were not so enhanced as those in the hTNFtg BMMs (Fig. 4 D and E), suggesting that enhanced osteoclastogenesis in hTNFtg mice depends on FcRγ and possibly on PIR-As.
Osteoclast Formation, but Not Joint Inflammation, Is Dependent on FcRγ in hTNFtg Mice.
Because the onset of antigen-induced arthritis is triggered by antibody recognition through Fc receptors, Fc γ receptor III and its adaptor subunit, FcRγ, are essential for the induction of antibody-mediated arthritis (29, 30), and the disease severity is enhanced in mice lacking an inhibitory Fc γ receptor IIB (31). However, adaptive immune reactions, including antibody production, are not involved in arthritis onset in hTNFtg mice (22). Therefore, we can analyze the role of FcRγ in the phase of bone destruction by crossing hTNFtg mice with _FcR_γ−/− mice. As expected, the onset and clinical course of arthritis in hTNFtg/_FcR_γ−/− mice were comparable to those in hTNFtg mice (data not shown), and there was no marked difference in inflammation severity between these mice (Fig. 4 F–H). However, bone destruction and osteoclast formation at erosive lesions in the hTNFtg/_FcR_γ−/− mice were markedly suppressed compared with those in hTNFtg mice (Fig. 4 F, I, and J).
As reported previously (12, 32), in addition to bone erosion in the affected joints, hTNFtg mice presented trabecular bone loss in the subchondral region (periarticular osteoporosis), accompanied by an increase in osteoclast number (Fig. 4 K–M). Moreover, 20-week-old hTNFtg mice showed systemic osteoporosis characterized by a decrease in bone mineral density (BMD) throughout the long bone (Fig. 4N). The decrease in periarticular trabecular bone volume [bone volume per tissue volume (BV/TV)] and the increase in osteoclast number per bone surface, as well as the reduction in BMD, in hTNFtg/_FcR_γ−/− mice were all attenuated compared with those in hTNFtg mice (Fig. 4 K–N). These results suggest that both types of inflammation-induced bone loss, namely, local bone erosion and osteoporosis, are dependent on enhanced osteoclast formation through FcRγ-associating receptors such as PIR-As.
β2M Is Required for Osteoclast Formation, but Not for Joint Inflammation, in hTNFtg Mice.
To further provide in vivo evidence that PIR-A-mediated activated osteoclastogenesis contributes to bone loss in hTNFtg mice, we investigated mice with the _B2m_−/− background, in which the expression of PIR-A ligands (MHC class I molecules) is compromised. _B2m_−/− mice develop normally and exhibit no obvious bone phenotype, suggesting that MHC class I molecules do not play a crucial role in the regulation of bone metabolism under physiological conditions (25, 33). There was no significant difference in clinical course or inflammation severity between hTNFtg and hTNFtg/_B2m_−/− mice (Fig. 5 A and B). However, the degree of bone erosion and the number of osteoclasts in the affected bone lesions in hTNFtg/_B2m_−/− mice were markedly suppressed compared with those in hTNFtg mice (Fig. 5 C and D). The increase in the number of osteoclasts, the reduction in subchondral bone volume, and the decrease in BMD in hTNFtg/_B2m_−/− mice were greatly attenuated compared with those in hTNFtg mice (Fig. 5 E–H), suggesting that periarticular and systemic osteoporoses in hTNFtg mice are dependent on β2M expression. Although the role of β2M may not be limited to PIR-A activation, these results in toto suggest the importance of the PIR-A axis in enhanced osteoclastogenesis and bone loss in arthritis.
Fig. 5.
Involvement of MHC class I molecules in TNF-α-induced bone loss. (A) Paw thickness of WT, hTNFtg, and hTNFtg/_B2m_−/− mice. n.s., not significant. (B–D) Arthritis score (B), histological score (C), and fold increase (D) in the number of osteoclasts in the ankles of WT, hTNFtg, and hTNFtg/_B2m_−/− mice. n.s., not significant; ∗, P < 0.05; ∗∗, P < 0.01. (E) Histological analysis of proximal tibiae of WT, hTNFtg, and hTNFtg/_B2m_−/− mice (TRAP and Toluidine blue staining). (F–H) BV/TV (F), number of osteoclasts per bone surface (N.Oc/BS) (G), and BMD (H) of tibiae of WT, hTNFtg, and hTNFtg/_B2m_−/− mice. ∗, P < 0.05.
Anti-TNF-α Antibody Normalizes the Accelerated Osteoclastogenesis and Inhibits Bone Loss in Arthritis.
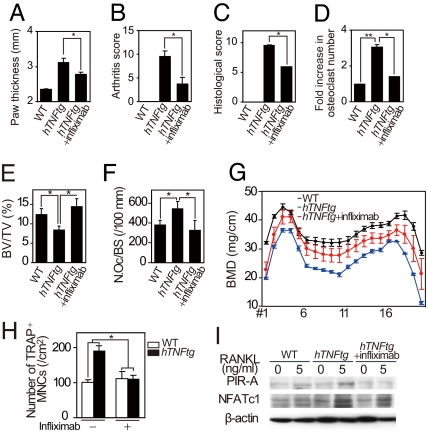
Finally, we examined the therapeutic effect of the anti-human TNF-α antibody (infliximab) on bone loss in hTNFtg mice and the enhanced osteoclastogenic potential of BMMs from these mice. Inflammatory changes and the local bone erosion in hTNFtg mice were ameliorated by the administration of infliximab (Fig. 6 A–D and SI Fig. 11_A_) to the similar extent as reported previously (34). Furthermore, we observed a marked therapeutic effect on both periarticular and systemic osteoporoses accompanied by a decrease in the number of osteoclasts in subchondral bone (Fig. 6 E–G and SI Fig. 11_B_). Consistent with this, the enhanced osteoclastogenesis in hTNFtg BMMs was normalized in the presence of infliximab (Fig. 6H). It is notable that the addition of infliximab repressed the enhanced induction of PIR-As and NFATc1 observed in the hTNFtg BMMs (Fig. 6I). These results suggest that blocking TNF-α suppresses osteoclastogenesis by inhibiting the PIR-A induction, and this mechanism may underlie, at least in part, the therapeutic effect of the anti-TNF-α antibody on bone loss in arthritis.
Fig. 6.
Infliximab normalizes enhanced osteoclastogenic potential and bone loss in hTNFtg mice. (A) Paw thickness of WT, hTNFtg, and infliximab-treated hTNFtg (+infliximab) mice. ∗, P < 0.05. (B–D) Arthritis score (B), histological score (C), and fold increase in the number of osteoclasts (D) in the ankles of WT, hTNFtg, and infliximab-treated hTNFtg mice. ∗, P < 0.05; ∗∗, P < 0.01. (E–G) BV/TV (E), number of osteoclasts per bone surface (N.Oc/BS) (F), and BMD (G) of tibiae of WT, hTNFtg, and infliximab-treated hTNFtg mice (hTNFtg+infliximab). ∗, P < 0.05. (H) Effect of infliximab on osteoclastogenesis in hTNFtg BMMs. hTNFtg BMMs cultured in the presence of RANKL were treated with saline (−infliximab) or 100 μg/ml of infliximab (+infliximab). ∗, P < 0.05. (I) Effect of infliximab on the expression of NFATc1 and PIR-As in hTNFtg BMMs (immunoblot analysis). Increased expression of PIR-As and NFATc1 in hTNFtg BMMs was inhibited by infliximab (hTNFtg + infliximab).
Discussion
Cytokine-targeted therapy is one of the greatest recent advances in the treatment of autoimmune diseases, including RA. TNF-α is an inflammatory cytokine that has a profound impact on the innate and adaptive immunities and was first adopted as a target of a biological therapy (18). Accumulating evidence indicates that the anti-TNF-α antibody has beneficial effects on not only inflammation, but also bone destruction in arthritis (19). Although it has been shown that osteoclasts are effector cells essential for bone loss in arthritis (22, 35), it has not been fully understood how TNF-α activates osteoclastic bone resorption and exerts its detrimental effects on bone.
As a possible mechanism for TNF-α-mediated enhancement of osteoclastogenesis, it has been shown that the population of osteoclast precursor cells is increased in the peripheral blood of hTNFtg mice (21, 28). However, we observed that more osteoclasts were formed from hTNFtg BMMs even when the same number of BMMs was cultured. TNF-α stimulates the expression of osteoclast differentiation factor RANKL by acting on the osteoclastogenesis-supporting mesenchymal cells (11, 14, 27), but it also acts directly on osteoclast precursor cells and promotes RANKL-induced osteoclastogenesis (13, 14). In fact, the direct effect plays a critical role under certain conditions in vivo (14). To explain the direct effect of TNF-α on osteoclast precursor cells at the molecular level, it has been reported that TNF-α up-regulates or activates molecules in proximal RANK signaling such as RANK, TNF receptor-associated factor 6, and NF-κB (13, 36–38). We found that no promotive effect of TNF-α on osteoclastogenesis is observed in the early phase of differentiation in which these molecules are activated. Instead, the effect of TNF-α is more prominently observed in the late phase, characterized by NFATc1 autoamplification. NFATc1 is an essential and integral transcription factor for osteoclastogenesis, and its essential role is determined by its specific gene regulatory mechanism of autoamplification (i.e., NFATc1 binds to its own promoter and amplifies its own expression) (9). The nuclear translocation of NFATc1 is regulated by phosphatase calcineurin, the activation of which depends on calcium signaling (10). Therefore, we inferred that TNF-α targets molecules that regulate calcium signaling.
Recently, Ig-like receptors and immunoreceptor tyrosine-based activation motif-harboring adaptors such as FcRγ and DAP12 have emerged as critical initiators of calcium signaling in osteoclastogenesis (costimulatory signal for RANK) (4, 5). Among the Ig-like receptors involved in osteoclastogenesis, we found the expression of PIR-As (and their ligands MHC class I molecules) was selectively induced by TNF-α. The series of experiments using _FcR_γ−/− and _B2m_−/− mice, as well as PIR-A-Fc collectively shows that TNF-α activates the PIR-A signaling axis and enhances the ability of osteoclast precursor cells to differentiate into osteoclasts, thus establishing an additional mechanism underlying the effect of TNF-α on bone loss in arthritis. Although the increase in osteoclast number was markedly suppressed in hTNFtg/_FcR_γ−/− or hTNFtg/_B2m_−/− mice compared with hTNFtg mice, the BMD in hTNFtg/_FcR_γ−/− mice or hTNFtg/_B2m_−/− was not completely normalized. Further studies are necessary to clearly explain this discrepancy, but it is possible that decreased BMD results from the TNF-α-mediated decrease in bone formation (39, 40), which was not cancelled even in the absence of FcRγ or β2M. Interestingly, the effect of TNF-α is antiosteoclastogenic if BMMs are exposed to TNF-α before RANKL possibly because TNF-α-stimulated BMMs are prone to commit themselves to activated macrophages (13, 41). It is likely that this mechanism is inhibited by unknown mechanism(s) in vivo because we observed that the hTNFtg BMMs have a higher osteoclastogenic potential. Consistent with this, the inhibitory effect of TNF-α is less observed in the coculture system of BMMs and osteoblasts (data not shown), suggesting that bone marrow microenvironments prevent the BMMs from differentiating into activated macrophages even in the abundance of TNF-α.
β2M is a crucial subunit for MHC class I molecules and is essential for the positive selection of CD8+ T cells (25). This study showed that it plays an important role in the TNF-α-mediated acceleration of osteoclastogenesis, but β2M is also involved in other pathological conditions in bones and joints by different mechanisms. For example, β2M is related to dialysis-associated amyloid osteoarthropathy (42). In addition, _B2m_−/− mice crossed with HLA-B27 transgenic mice or _B2m_−/− mice of susceptible backgrounds develop arthritis spontaneously (43, 44). Therefore, β2M is required for suppressing the onset of autoimmune diseases under certain conditions. However, because the onset of arthritis in hTNFtg mice in the _B2m_−/− background was not altered in this study, β2M is not involved in the onset of this type of arthritis and exclusively functions in the bone-destruction phase in this model.
A defect in osteoclast costimulation (caused by loss-of-function mutation in DAP12 or TREM-2) is associated with an autosomal recessive condition with bone cysts and presenile dementia called Nasu-Hakola disease (6, 7, 45). Another osteoclast costimulatory receptor system, PIR, is involved in graft-versus-host diseases (23), but has never been linked to bone diseases. This study reports on the role of the costimulatory receptor for RANK in the context of pathological activation of osteoclastogenesis in inflammation-related bone diseases. Note that the expression level of human orthologues for PIRs, leukocyte Ig-like receptors, in synovial cells in RA is increased (46), but further studies are necessary to determine the functional significance of this finding. Currently, neither PIR-A-Fc appropriate for the in vivo administration nor the genetically modified mice lacking all PIR-A molecules are available, but it will be an important issue in the future to develop a method to specifically and completely disrupt PIR-A function in vivo. Because _FcR_γ−/− mice show no obvious defect in the bone homeostasis (4), PIR-As and their adaptor, FcRγ, play an important role specifically in the pathological activation of osteoclastogenesis. Therefore, targeting the PIR-A signaling axis will be an auspicious therapeutic strategy in inflammation-induced bone loss.
Methods
In Vitro Osteoclast Formation.
In vitro osteoclast differentiation was performed as described previously (47). See SI Methods for details.
Immunoblot and Flow-Cytometric Analyses.
Cell lysates were subjected to immunoblot analysis with the following specific antibodies: anti-PIR, which recognizes the common domain of PIR-As and PIR-Bs (band size, 90 kDa and 110 kDa, respectively) (48); anti-SIRPβ1 (49); anti-OSCAR (R&D Systems, Minneapolis, MN); anti-NFATc1 (Santa Cruz Technologies, Santa Cruz, CA); anti-TREM-2 (R&D Systems); and anti-β-actin (Sigma–Aldrich, St. Louis, MO) antibodies. Expression level was measured by using Scion Image (Scion Corporation, Frederick, MD). After the expression level of costimulatory receptors was normalized by that of β-actin, the relative ratio of the protein level in TNF-α-treated cells to that in nontreated cells was calculated in Fig. 2B. For flow-cytometric analysis, BMMs were incubated with the anti-PIR antibody or control rat polyclonal IgG (BD Biosciences Pharmingen, San Diego, CA) for 30 min, followed by staining with PE-conjugated anti-rat IgG. Cells were analyzed by FACScan using CellQuest software (Becton Dickinson, San Jose, CA).
RT-PCR Analysis.
Total RNA was extracted by using Sepazol (Nacalai Tesque, Kyoto, Japan) from BMMs and subjected to RT-PCR analysis. PCR was performed with primers for Pir-b as described previously (50). The sequences of the primers used for Nfatc1, GAPDH, Pir-a1, Pir-a4, Pir-a6, TNF, and consensus sequences of H-2Db and H-2Kb are described in SI Methods.
GeneChip Analysis.
GeneChip analysis was performed as previously described (10). Briefly, BMMs were stimulated with RANKL (50 ng/ml) in the presence of M-CSF for 3 days. TNF-α (50 ng/ml) was added 1 day after RANKL stimulation. Total RNA was extracted by Sepazol and used for cDNA synthesis by RT, followed by the synthesis of biotinylated cRNA through in vitro transcription. After cRNA fragmentation, hybridization with an MG430v2 GeneChip (Affymetrix, Santa Clara, CA) was performed.
PIR-A-Fc Fusion Protein.
cDNA fragments encoding the common extracellular domain of PIR-As were cloned by using the specific primers (see SI Methods for details). The extracellular domain was fused to cDNA for the Fc portion of human IgG1a in frame. PIR-A-Fc fusion protein was prepared as described previously (51). Because the sequences used for the protein are commonly found in PIR-A1, PIR-A2, PIR-A3, PIR-A4, PIR-A6, and PIR-A7, this fusion protein inhibits all of these PIR-A isoforms. The fusion protein or control human polyclonal IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) was added at 0.1 mM on the same day as RANKL stimulation.
Treatment with Infliximab.
Sixteen-week-old hTNFtg mice and their littermate controls were injected i.p. with 5 mg/kg body weight infliximab (Tanabe Seiyaku, Osaka, Japan) or saline three times per week for 4 weeks. Mice were killed and analyzed at the age of 20 weeks.
Mice and Clinical/Histological Analyses.
See SI Methods.
Statistical Analyses.
See SI Methods.
Supplementary Material
Supporting Information
Acknowledgments
We thank H. Kubagawa, T. Matozaki, L.L. Lanier, and Tanabe Seiyaku Co., Ltd., for invaluable reagents; and M. Isobe-Ohba, Y. Kim, K. Okamoto, K. Arai, Y. Suzuki, N. Kumazaki, T. Nakashima, M. Asagiri, K. Nishikawa, A. Suematsu, S. Kamano, M. Hayashi, H. Murayama, I. Takayanagi, and K. Takayanagi for helpful discussion and assistance. This work was supported, in part, by a Grant-in-Aid for Creative Scientific Research (to H.T.); Postdoctoral Fellowships for Foreign Researchers from the Japan Society for the Promotion of Science (to H.-J.G.); Genome Network Project from Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) grants (to H.T.); grants from MEXT for 21st Century Center of Excellence (COE) program (to H.T., S.O., and N.M.); the Solution Oriented Research for Science and Technology program of the Japan Science and Technology Agency (to H.T.); Health Sciences Research Grants from the Ministry of Health, Labour, and Welfare of Japan (to H.T.); and grants from Inamori Foundation (to H.T.), Kanae Foundation for Life & Socio-Medical Science (to H.T.), Tokyo Biochemical Research Foundation and Yokoyama Foundation for Clinical Pharmacology (to H.T.), Nakajima Foundation (to T. Koga), and Hayashi Memorial Foundation for Female Natural Scientists (to T. Koga).
Abbreviations
β2M
β2-microglobulin
BMD
bone mineral density
BMM
bone marrow-derived monocyte/macrophage lineage cell
BV/TV
bone volume per tissue volume
DAP12
DNAX-activation protein 12
FcRγ
Fc receptor common γ subunit
NFATc1
nuclear factor of activated T cells c1
OSCAR
osteoclast-associated receptor
PIR
paired Ig-like receptor
RA
rheumatoid arthritis
RANKL
receptor activator of nuclear factor-κB ligand
TRAP
tartrate-resistant acid phosphatase
TRAP+ MNCs
multinucleated cells positive for TRAP
TREM-2
triggering receptor expressed on myeloid cells-2
SIRPβ1
signal-regulatory protein β1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Teitelbaum SL, Ross FP. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 2.Asagiri M, Takayanagi H. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Takayanagi H. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 4.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, et al. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 5.Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC. Proc Natl Acad Sci USA. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphrey MB, Lanier LL, Nakamura MC. Immunol Rev. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 7.Paloneva J, Kestila M, Wu J, Salminen A, Bohling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, et al. Nat Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 8.Takayanagi H. J Mol Med. 2005;83:170–179. doi: 10.1007/s00109-004-0612-6. [DOI] [PubMed] [Google Scholar]
- 9.Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 11.Sato K, Takayanagi H. Curr Opin Rheumatol. 2006;18:419–426. doi: 10.1097/01.bor.0000231912.24740.a5. [DOI] [PubMed] [Google Scholar]
- 12.Schett G, Hayer S, Zwerina J, Redlich K, Smolen JS. Nat Clin Pract Rheumatol. 2005;1:47–54. doi: 10.1038/ncprheum0036. [DOI] [PubMed] [Google Scholar]
- 13.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitaura H, Sands MS, Aya K, Zhou P, Hirayama T, Uthgenannt B, Wei S, Takeshita S, Novack DV, Silva MJ, et al. J Immunol. 2004;173:4838–4846. doi: 10.4049/jimmunol.173.8.4838. [DOI] [PubMed] [Google Scholar]
- 15.Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, et al. J Exp Med. 2005;202:589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori L, Iselin S, De Libero G, Lesslauer W. J Immunol. 1996;157:3178–3182. [PubMed] [Google Scholar]
- 18.Feldmann M. Nat Rev Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 19.Smolen JS, Han C, Bala M, Maini RN, Kalden JR, van der Heijde D, Breedveld FC, Furst DE, Lipsky PE. Arthritis Rheum. 2005;52:1020–1030. doi: 10.1002/art.20982. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, et al. Proc Natl Acad Sci USA. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li P, Schwarz EM, O'Keefe RJ, Ma L, Boyce BF, Xing L. J Bone Miner Res. 2004;19:207–213. doi: 10.1359/JBMR.0301233. [DOI] [PubMed] [Google Scholar]
- 22.Redlich K, Hayer S, Ricci R, David JP, Tohidast-Akrad M, Kollias G, Steiner G, Smolen JS, Wagner EF, Schett G. J Clin Invest. 2002;110:1419–1427. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura A, Kobayashi E, Takai T. Nat Immunol. 2004;5:623–629. doi: 10.1038/ni1074. [DOI] [PubMed] [Google Scholar]
- 24.Takai T. Immunology. 2005;115:433–440. doi: 10.1111/j.1365-2567.2005.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenisch R. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 26.Takai T, Ono M. Immunol Rev. 2001;181:215–222. doi: 10.1034/j.1600-065x.2001.1810118.x. [DOI] [PubMed] [Google Scholar]
- 27.Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Bone. 1999;25:255–259. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Schwarz EM, O'Keefe RJ, Ma L, Looney RJ, Ritchlin CT, Boyce BF, Xing L. Arthritis Rheum. 2004;50:265–276. doi: 10.1002/art.11419. [DOI] [PubMed] [Google Scholar]
- 29.Kleinau S, Martinsson P, Heyman B. J Exp Med. 2000;191:1611–1616. doi: 10.1084/jem.191.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, et al. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 31.Yuasa T, Kubo S, Yoshino T, Ujike A, Matsumura K, Ono M, Ravetch JV, Takai T. J Exp Med. 1999;189:187–194. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrak P, Gortz B, Hayer S, Redlich K, Reiter E, Gasser J, Bergmeister H, Kollias G, Smolen JS, Schett G. Arthritis Rheum. 2004;50:2044–2047. doi: 10.1002/art.20384. [DOI] [PubMed] [Google Scholar]
- 33.Marusic A, Katavic V, Stimac D, Kusec V, Jonjic S. Eur J Clin Chem Clin Biochem. 1995;33:915–918. doi: 10.1515/cclm.1995.33.12.915. [DOI] [PubMed] [Google Scholar]
- 34.Redlich K, Gortz B, Hayer S, Zwerina J, Doerr N, Kostenuik P, Bergmeister H, Kollias G, Steiner G, Smolen JS, Schett G. Am J Pathol. 2004;164:543–555. doi: 10.1016/S0002-9440(10)63144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettit AR, Ji H, von Stechow D, Muller R, Goldring SR, Choi Y, Benoist C, Gravallese EM. Am J Pathol. 2001;159:1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komine M, Kukita A, Kukita T, Ogata Y, Hotokebuchi T, Kohashi O. Bone. 2001;28:474–483. doi: 10.1016/s8756-3282(01)00420-3. [DOI] [PubMed] [Google Scholar]
- 37.Kaji K, Katogi R, Azuma Y, Naito A, Inoue JI, Kudo A. J Bone Miner Res. 2001;16:1593–1599. doi: 10.1359/jbmr.2001.16.9.1593. [DOI] [PubMed] [Google Scholar]
- 38.Abu-Amer Y, Erdmann J, Alexopoulou L, Kollias G, Ross FP, Teitelbaum SL. J Biol Chem. 2000;275:27307–27310. doi: 10.1074/jbc.M003886200. [DOI] [PubMed] [Google Scholar]
- 39.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 40.Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 41.Heidenreich S, Weyers M, Gong JH, Sprenger H, Nain M, Gemsa D. J Immunol. 1988;140:1511–1518. [PubMed] [Google Scholar]
- 42.Bardin T. J Rheumatol. 1987;14:647–649. [PubMed] [Google Scholar]
- 43.Khare SD, Luthra HS, David CS. J Exp Med. 1995;182:1153–1158. doi: 10.1084/jem.182.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kingsbury DJ, Mear JP, Witte DP, Taurog JD, Roopenian DC, Colbert RA. Arthritis Rheum. 2000;43:2290–2296. doi: 10.1002/1529-0131(200010)43:10<2290::AID-ANR17>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, et al. Am J Hum Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tedla N, Gibson K, McNeil HP, Cosman D, Borges L, Arm JP. Am J Pathol. 2002;160:425–431. doi: 10.1016/S0002-9440(10)64861-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, et al. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 48.Kubagawa H, Chen CC, Ho LH, Shimada TS, Gartland L, Mashburn C, Uehara T, Ravetch JV, Cooper MD. J Exp Med. 1999;189:309–318. doi: 10.1084/jem.189.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayashi A, Ohnishi H, Okazawa H, Nakazawa S, Ikeda H, Motegi S, Aoki N, Kimura S, Mikuni M, Matozaki T. J Biol Chem. 2004;279:29450–29460. doi: 10.1074/jbc.M400950200. [DOI] [PubMed] [Google Scholar]
- 50.Kubagawa H, Burrows PD, Cooper MD. Proc Natl Acad Sci USA. 1997;94:5261–5266. doi: 10.1073/pnas.94.10.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim Y, Sato K, Asagiri M, Morita I, Soma K, Takayanagi H. J Biol Chem. 2005;280:32905–32913. doi: 10.1074/jbc.M505820200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information