Force-dependent integrin–cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2 (original) (raw)
Abstract
As cells encounter new regions of the substrate, they develop bonds with new matrix molecules for migration, matrix remodeling and force generation. How cells orchestrate the assembly of adhesion sites is only partially understood. Here we show that fibroblasts deficient in the SH2 domain containing protein tyrosine phosphatase 2 (Shp2) have an increased number of immature focal complexes deficient in α-actinin. Inhibition of FAK restored α-actinin to focal complexes, whereas inhibition of RhoA did not. In correlation, adhesion site dynamics, measured by fluorescence recovery after photobleaching (FRAP) of GFP–paxillin and GFP–vinculin were dramatically increased in Shp2–/– cells and restored to normal by FAK inhibition. Shp2–/– cells failed to strengthen initial integrin–cytoskeleton linkages, as measured by optical tweezers and large bead assays, and were rescued by inhibition of FAK. In contrast, affinity modulation of adhesion receptors was unaffected. In addition, reinforcement correlated with α-actinin assembly through decreased dynamics. This shows for the first time that adhesion site dynamics are regulated during adhesion formation and that force-dependent strengthening of integrin–cytoskeleton linkages is correlated with α-actinin assembly and decreased adhesion site dynamics.
Keywords: focal adhesion kinase/focal complex/force/FRAP/Shp2
Introduction
Integrins form the link between the cytoskeleton and extracellular matrix (ECM) such as fibronectin and vitronectin. Integrins are composed of two subunits, α and β, and each αβ combination has its own binding and signaling properties (Giancotti and Ruoslahti, 1999). As cells encounter new regions of the substrate, they develop bonds with new matrix molecules for migration, matrix remodeling or traction force generation in the area of adhesion sites. The best-characterized adhesion sites are focal adhesions which are elongated structures several square microns in area near the periphery of cells that mediate strong adhesion to the substrate (Geiger et al., 2001; Geiger and Bershadsky, 2002). Another group of matrix adhesions are focal complexes, which are small adhesions present at the edges of lamella. Focal complexes are defined by their dimensions (∼1 µm2), temporal properties (precursor of FA) and signaling properties (induced by the small G-protein Rac while simultaneously inhibiting Rho). However, no differences in the molecular composition of focal adhesions versus focal complexes have been found to date. (Nobes and Hall, 1995; Rottner et al., 1999; Geiger et al., 2001). Maturation of focal complexes into focal adhesions is mediated by Rho-dependent acto-myosin contractility and requires the Rho target mDia (Riveline et al., 2001). However, regulated maturation of initial sites to focal complexes has not been described. Nevertheless, when a cell spreads on a rigid substrate, it initially forms tiny complexes that contain paxillin but not α-actinin, suggesting that a coordinated, stepwise maturation of focal complexes normally occurs (Laukaitis et al., 2001).
Physical force is a critical component in the development of cell–matrix contacts at all stages. As the rearward transport of the cytoskeleton develops force on the initial integrin–cytoskeleton linkage, the connection is reinforced in concert with the binding of focal contact proteins via force-induced signaling events (Felsenfeld et al., 1999; Galbraith et al., 2002; von Wichert et al., 2003). This strengthening of integrin–cytoskeleton linkages is blocked by a tyrosine phosphatase inhibitor, phenylarsine oxide, suggesting the involvement of tyrosine phosphatases in force-dependent signal transduction pathways (Choquet et al., 1997). It has been shown that both receptor and non-receptor protein tyrosine phosphatases influence the earliest phases of focal adhesion assembly (Petrone and Sap, 2000). The cytoplasmic phosphatase Shp2 has been described to affect integrin function (Feng, 1999; Miao et al., 2000). To become activated, Shp2 is recruited to the membrane to tyrosine-phosphorylated docking proteins such as SHPS-1/SIRP-1 (Inagaki et al., 2000). It has been speculated that Shp2 regulates integrin function by regulating FAK phosphorylation as well as RhoA activity (Yu et al., 1998; Kodama et al., 2000; Miao et al., 2000; Schoenwaelder et al., 2000). Animals deficient in Shp2 die at midgestation due to gastrulation defects (Saxton et al., 1997). Cells derived from Shp2 loss-of-function mutant embryos or expressing a Shp2 dominant-negative mutant display severe defects in spreading, haptotactic and chemotactic responses and decreased integrin-dependent activation of mitogen-activated protein kinases (Saxton et al., 1997; Yu et al., 1998; Manes et al., 1999). In addition, cells deficient in Shp2 activity show FAK hyperphosphorylation as well as increased numbers of small focal adhesions (Yu et al., 1998). Furthermore, it has been reported that Shp2 deficiency increases the formation of stress fibers via increased activation of RhoA (Yu et al., 1998; Kodama et al., 2000; Schoenwaelder et al., 2000).
Here we show that Shp2 controls a maturation cycle preceding the Rho-dependent maturation of focal complexes to focal adhesions. In particular, Shp2-dependent inhibition of FAK is required for maturation of initial adhesion sites. Moreover, Shp2-dependent inhibition of FAK dramatically decreases adhesion site dynamics, measured by fluorescence recovery after photobleaching (FRAP) of GFP–paxillin and GFP–vinculin. In addition, we demonstrate that decreased dynamics are required for force-dependent reinforcement of newly formed integrin–cytoskeleton linkages. This shows for the first time that adhesion site dynamics are actively regulated and that strengthening of integrin–cytoskeleton linkages is correlated with a decrease in adhesion site dynamics.
Results
Shp2 function is not associated with a specific integrin
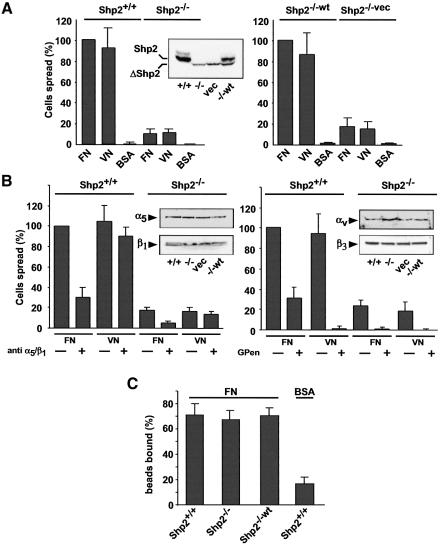
Although cells deficient for Shp2 showed decreased spreading on fibronectin (Yu et al., 1998), initial experiments suggested that they had a more general spreading defect. When fibroblasts containing a deletion of Shp2 exon 3 (Saxton et al., 1997) [referred to as Shp2–/– and Shp2–/–vec (vector control)], were plated for 15 min on fibronectin and vitronectin substrates, cells were slow in spreading compared with cells expressing wild-type Shp2 [Shp2+/+ and Shp2–/–wt (wild type reconstituted)] (Figure 1A). It has been shown recently that spreading defects can be due to signal-transduction defects downstream of subtype-specific integrins. In the case of the tyrosine kinase c-Src and the receptor-phosphatase RPTPα, spreading defects are associated with the αv/β3-integrin (Felsenfeld et al., 1999; von Wichert et al., 2003). There, inhibition of specific integrin binding equalized the rate of spreading in control and knockout cells. However, in the Shp2–/– cells, there was still less spreading after inhibition of the two major fibronectin receptors (α5/β1-integrins and αv/β3-integrins) with either an antibody to α5/β1 integrins or with a cyclic peptide [GPenGRGDSPCA(GPen)], which has been shown to be a selective, competitive inhibitor of αv/β3 integrins (Pierschbacher and Ruoslahti, 1987). Integrin expression was confirmed by western blotting (Figure 1B). Therefore, integrin signaling appeared active in both Shp2-expressing and non-expressing cell lines on both fibronectin and vitronectin. In addition, these results suggest that the spreading defect is not due to a defect associated with specific integrins and that Shp2 might play a more general role.
Fig. 1. The Shp2-dependent spreading defect is not associated with a specific integrin and Shp2 expression does not affect ligand affinity. (A) Cells were plated and the percentage of spread cells on either fibronectin (FN), vitronectin (VN) or bovine serum albumin (BSA) was quantified 15 min after plating and normalized to control cells. Left, spreading of Shp2+/+ cells compared with Shp2–/– cells; right, Shp2–/–wt cells compared with Shp2–/–vec cells. Results shown are the mean ± SD of 3–5 independent experiments. (B) Shp2+/+ and Shp2–/– cells were plated in the absence or presence of an anti-α5/β1-integrin antibody (10 µg/ml) (left) or GPen (0.5 mM) (right), and the percentage of spread cells on fibronectin and vitronectin was quantified 15 min after plating and normalized to control cells. Results shown are the mean ± SD of three independent experiments. (C) FN-coated beads (0.91 µm diameter) bound specifically to the upper surface of Shp2+/+ and Shp2–/– cells after placement with a laser trap. Percentages of bound beads after 2 s were compared. Binding was compared with control beads coated with BSA. Results shown are the mean ± SD of three independent experiments.
Shp2 does not affect integrin–ligand interactions
The sensitivity of cell spreading on ECM substrates to Shp2 expression raises the possibility that Shp2 modulates integrin–ligand interactions. Modulation of integrin affinity and avidity for ECM substrates has been shown for several integrins (Hughes and Pfaff, 1998). To study more directly the role of Shp2 in regulating integrin–ligand interactions, we placed and held ligand-coated beads on the upper surface of spreading fibroblasts with a laser trap. This allows the quantification of changes in ligand binding and the dynamics of traction-force generation by integrins (Felsenfeld et al., 1996; Choquet et al., 1997), parameters that are essential to cell spreading and migration (Lauffenburger and Horwitz, 1996; Sheetz et al., 1998). To assess ligand–receptor interaction, beads coated with a recombinant fragment of fibronectin bearing the RGD domain (FNIII7-10) or BSA were placed with a laser trap for 3 s on the upper surface of the lamella (<0.5 µm from the leading edge). FNIII7-10 beads bound to the surface with a similar frequency in all cell lines and bound significantly better than BSA beads (Figure 1C). Thus, integrin–ligand interactions are not modulated by Shp2.
Shp2 regulates maturation of focal complexes via FAK
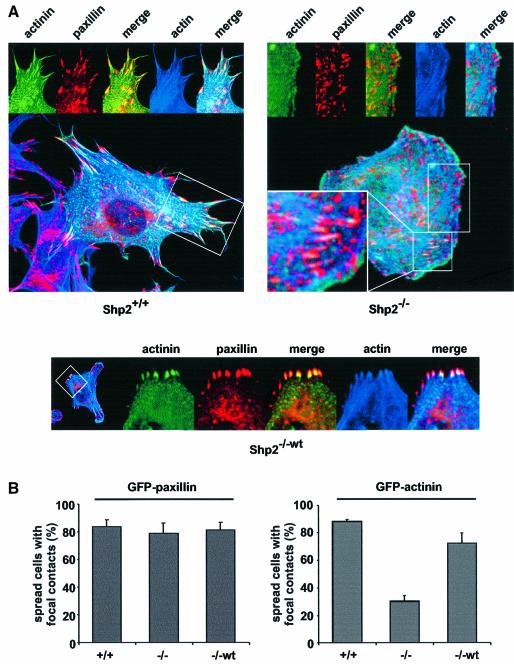
Maturation of adhesion sites involves the transformation of focal complexes to focal adhesions mediated by RhoA and acto-myosin contractility (Geiger et al., 2001). Recent data showed a coordinated, stepwise localization of integrins and focal adhesion proteins to the initial site of focal complex formation (Laukaitis et al., 2001). To address whether Shp2 might regulate the maturation of adhesion sites, Shp2+/+, Shp2–/– and Shp2–/–wt cells were transiently transfected with enhanced green fluorescent protein (EGFP)-tagged α-actinin. α-actinin has been shown to interact directly with integrins and the actin cytoskeleton as well as to be localized comparatively late to new focal complexes (Otey et al., 1993; Laukaitis et al., 2001). Cells were spread for 15 min on fibronectin, stained for both paxillin and F-actin and subsequently analyzed by confocal microscopy. In spread Shp2+/+ cells, α-actinin co-localized with paxillin and actin filaments in focal adhesions. In contrast, in spread Shp2–/– cells there was less or no accumulation of α-actinin in an increased number of small paxillin-positive adhesion sites, suggesting that these were focal complexes (Figure 2A). Quantification of the spread fraction of cells revealed that only 30% of the Shp2–/– cells displayed accumulation of α-actinin in distinct adhesion sites, whereas 80% of the control cells accumulated α-actinin. In contrast, there was no significant difference in the accumulation of GFP–paxillin in all cell lines (Figure 2B). Nevertheless, in Shp2–/– cells, some stress fibers were inserted into α-actinin-positive focal adhesions (Figure 2A). Therefore, Shp2 is not required for paxillin accumulation at adhesion sites; however, Shp2 accelerates maturation and α-actinin accumulation at focal complexes.
Fig. 2. Shp2 expression affects the incorporation of α-actinin into adhesion sites. (A) Shp2+/+, Shp2–/– and Shp2–/–wt cells were transiently transfected with α-actinin–EGFP, suspended for 30 min in serum-free medium containing 0.5% BSA and allowed to spread for 15 min on fibronectin, fixed and stained with anti-paxillin antibodies and rhodamine–phalloidin. Images shown are representative of at least three independent experiments. (B) Shp2+/+, Shp2–/– and Shp2–/–wt cells were transiently transfected with α-actinin–EGFP or GFP–paxillin, suspended for 30 min in serum-free medium containing 0.5% BSA and allowed to spread for 15 min on fibronectin, fixed and the number of spread cells with accumulation of α-actinin or paxillin in distinct adhesion sites was quantified. Results shown are the mean ± SD of three independent experiments.
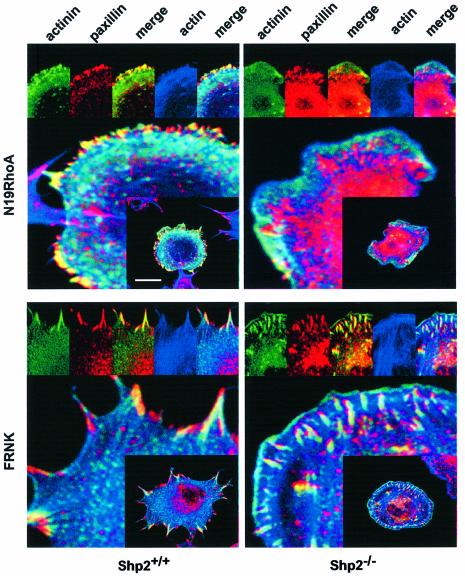
Next we wanted to test whether the Shp2 targets RhoA and FAK (Yu et al., 1998; Miao et al., 2000; Schoenwaelder et al., 2000) were possibly involved in the initial maturation of focal complexes. To address the role of RhoA, cells were co-transfected with dominant-negative RhoA (N19Rho). In Shp2+/+ cells, expression of N19Rho led to formation of small focal complex-like structures in the periphery of the cell. Although these structures were smaller than the adhesion sites in the untreated conditions and without actin stress fibers, they still stained positive for α-actinin, suggesting that the initial maturation process was undisturbed. However, in Shp2–/– cells the inhibition of RhoA further reduced the accumulation of α-actinin in adhesion sites.
To address the role of FAK, we co-expressed the autonomously expressed C-terminal part of FAK, termed FAK related non-kinase (FRNK), which has been shown to interfere with both focal adhesion targeting of FAK as well as FAK activation (Richardson and Parsons, 1996; Mortier et al., 2001; Taylor et al., 2001). Co-expression of FRNK in Shp2+/+ cells led to large focal adhesions and to a less polarized morphology, as described for FAK deficiency (Ilic et al., 1995). Interestingly, in Shp2–/– cells, co-expression of FRNK led to accumulation of α-actinin in initial adhesion sites (Figure 3). However, the spreading defect was unaffected by co-expression of FRNK (data not shown) (Lacalle et al., 2002). Taken together these results indicate that Shp2-dependent downregulation of FAK activity is required for the initial maturation of focal complexes but additional steps may require FAK. Moreover, these data suggest that Rho-dependent maturation is intact, and that increased RhoA activity in Shp2–/– cells can at least in part compensate for the maturation defect (Figures 2 and 3).
Fig. 3. Shp2-dependent inhibition of FAK but not Rho affects the incorporation of α-actinin into adhesion sites. Shp2+/+ and Shp2–/– cells were transiently transfected with α-actinin–EGFP with or without co-transfection of N19RhoA or FRNK. Cells were subsequently suspended for 30 min in serum-free medium containing 0.5% BSA and allowed to spread for 15 min on fibronectin. Cells were then fixed and stained with anti-paxillin antibodies and rhodamine–phalloidin. Scale bar indicates 10 µm. Images shown are representative of at least three independent experiments.
Shp2 controls tyrosine phosphorylation of α-actinin via regulation of FAK activity
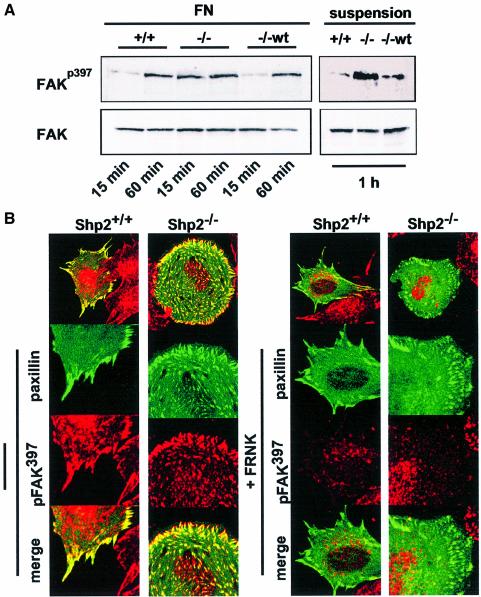
It has been shown that Shp2 controls FAK activity by dephosphorylation of specific tyrosine residues (Feng, 1999; Manes et al., 1999). Indeed, FAK was hyperphosphorylated on the autophosphorylation site (Y397) in suspension and 15 min after plating on fibronectin in Shp2–/– cells. In contrast, control cells showed low levels of FAK autophosphorylation in suspension and during spreading (Figure 4A). Since unbound cells were removed by vigorous washing before lysis these results represent the adherent fraction and are not biased by the differences in spreading. To test whether co-transfection of FRNK would lead to reduced levels of FAK autophosphorylation, GFP–paxillin and FRNK were co-transfected in Shp2+/+ and Shp2–/– cells, plated for 15 min on fibronectin and stained with an antibody specific for autophosphorylated FAK. As expected, co-transfection of FRNK prevented the accumulation of autophosphorylated FAK in adhesion sites almost completely (Figure 4B).
Fig. 4. Shp2 controls tyrosine phosphorylation of α-actinin via FAK. (A) Shp2+/+, Shp2–/– and Shp2–/–wt cells were suspended for 60 min in serum-free medium containing 0.5% BSA and were either allowed to spread for 15 or 60 min on fibronectin (FN) or kept in suspension. Cells were subsequently lysed and equal amounts of protein were analyzed by western blots. Results shown are representative of three independent experiments. (B) Shp2+/+ and Shp2–/– cells were transiently transfected with GFP–paxillin with or without co-transfection of FRNK and subsequently suspended for 30 min and allowed to spread for 15 min on fibronectin. Cells were then fixed and stained with autophosphorylation-specific anti-FAK antibodies. Images shown are representative for at least three independent experiments.
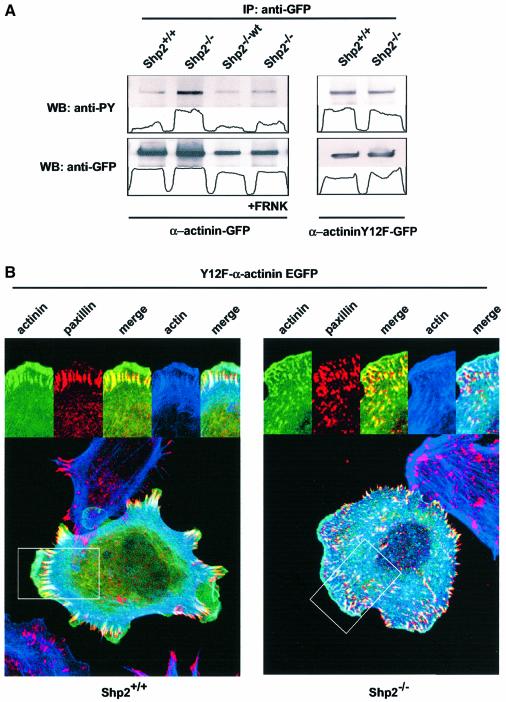
In order to address the phosphorylation status of α-actinin we transfected either α-actinin–EGFP alone or in combination with FRNK in Shp2+/+, Shp2–/– and Shp2–/–wt cells. Interestingly, tyrosine phosphorylation of α-actinin was significantly increased in anti-GFP immunoprecipitates from Shp2–/– cells compared to control cells. In addition, reintroduction of wild-type Shp2 and co-transfection of FRNK prevented the increase in tyrosine phosphorylation of α-actinin in Shp2–/– cells completely, suggesting that the increase in tyrosine phosphorylation depends on increased activity of FAK (Figure 5A). It has been shown recently that FAK-dependent tyrosine phosphorylation of α-actinin occurs on a single tyrosine residue (Y12) (Izaguirre et al., 2001). In order to address whether the increase in tyrosine phosphorylation occurs on this tyrosine residue, we transfected Shp2+/+ and Shp2–/– cells with an EGFP-tagged α-actinin construct with a Y12F mutation that could not be phosphorylated by FAK (Izaguirre et al., 2001). Interestingly, there was no difference in the amount of phosphorylation of the mutant α-actinin between Shp2+/+ and Shp2–/– cells (Figure 5A). Taken together, these results indicate that FAK is indeed hyperactivated in Shp2–/– cells in suspension and during spreading. Furthermore, co-transfection of FRNK prevents the increase in tyrosine phosphorylation of FAK as well as α-actinin. Moreover, Shp2-controled, FAK-mediated phosphorylation of α-actinin occurs on tyrosine 12.
Fig. 5. Y12F α-actinin–EGFP localizes to focal complexes in Shp2+/+ and Shp2–/– cells. (A) Shp2+/+, Shp2–/– and Shp2–/–wt cells were transiently transfected with α-actinin–EGFP or Y12F α-actinin–EGFP with or without co-transfection of FRNK. Cells were plated on fibronectin for 15 min, lysed and subjected to immunoprecipitation (IP) with an anti-GFP antibody. Lysates were halved and subsequently analyzed by western blotting (WB) using anti-phosphotyrosine (anti-PY) or anti-GFP antibodies. (B) Shp2+/+ and Shp2–/– cells were transiently transfected with Y12F α-actinin–EGFP, suspended for 30 min in serum-free medium containing 0.5% BSA and allowed to spread for 15 min on fibronectin, fixed and stained with anti-paxillin antibodies and rhodamine–phalloidin. Images shown are representative of at least three independent experiments.
Shp2 controls the localization of α-actinin to adhesion sites
It has been shown previously that the FAK-dependent tyrosine phosphorylation of Y12 occurs in the actin binding domain and that this phosphorylation affects the affinity for the actin-cytoskeleton (Izaguirre et al., 2001). In addition, it has been shown that the Y12F-mutated form of α-actinin has the same binding capability as the dephosphorylated native form (Izaguirre et al., 2001). Since we have shown earlier that Shp2 controls the FAK-dependent phosphorylation of α-actinin, we wanted to test whether an EGFP-tagged Y12F α-actinin would localize to focal complexes in Shp2–/– cells. Therefore, Shp2+/+ and Shp2–/– cells were transfected with Y12F α-actinin–EGFP spread for 15 min on fibronectin and stained for paxillin and actin and subsequently analyzed by confocal microscopy. In Shp2+/+ cells, distribution of Y12F α-actinin–EGFP was the same as for native α-actinin. In contrast, in Shp2–/– cells Y12F α-actinin–EGFP localized to focal complexes while the endogenous α-actinin did not, although the number and shape of the adhesion sites was unaffected (Figure 5B). Thus, Shp2-dependent, FAK-mediated regulation of α-actinin phosphorylation regulates the localization to focal complexes.
Maturation of adhesion sites involves regulation of focal adhesion protein dynamics
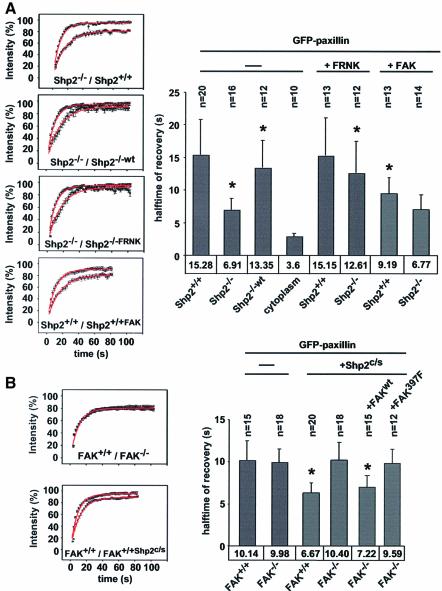
There was speculation that the exchange rates of focal adhesion proteins were differently regulated in assembling and disassembling adhesion sites (Ballestrem et al., 2001; Wehrle-Haller and Imhof, 2002). Since adhesion sites in Shp2–/– cells seemed to be more immature, we were interested in whether the exchange rates of focal adhesion proteins were differently regulated in Shp2+/+ and Shp2–/– cells. FRAP studies revealed that the halftime of recovery of the adapter-protein paxillin, a well established marker for focal complexes and contacts (Turner, 2000), was more than twice as fast in Shp2–/– cells (6.9 s) than in Shp2+/+ cells (15.3 s) (Figure 6A). Similar results were obtained with GFP–vinculin, where the halftime of recovery was 30% faster in Shp2–/– cells (6.1 s ± 0.9) than in Shp2+/+ cells (8.8 s ± 1.2) (P ≤ 0.004; data not shown). These extremely fast exchange rates were in contrast to the overall lifetimes of adhesion sites as visualized by GFP–paxillin or vinculin, which were many minutes in both Shp2+/+ and Shp2–/– cells (data not shown). Reintroduction of Shp2 into Shp2–/– cells increased the halftime of recovery of GFP–paxillin to control levels (13.3 s). Inhibition of FAK through co-expression of FRNK, also significantly reduced the turnover of paxillin in focal adhesions in Shp2–/– cells (12.6 s). Interestingly, in Shp2+/+ cells there was no reduction of turnover by expression of FRNK (15.1 s) (Figure 6A). In contrast, over-expression of wild-type FAK led to an increase in turnover in Shp2+/+ cells (9.2 s), possibly through titration of endogenous Shp2, whereas there was no change in Shp2–/– cells (6.7 s). Co-expression of N19RhoA had no effect on the halftime of recovery of GFP–paxillin in both Shp2+/+ (13.4 s ± 3.6) and Shp2–/– cells (7.8 s ± 1.8) (P ≤ 0.004; data not shown). Although the percent recovery of GFP–paxillin fluorescence was greater in Shp2–/– cells, neither re-expression of Shp2 nor co-expression of FRNK reduced it to control levels suggesting cell-type-dependent differences.
Fig. 6. Shp2 deficiency-dependent loss of FAK inhibition is associated with increased dynamics of GFP–paxillin in adhesion sites. (A) Left: Shp2+/+, Shp2–/– and Shp2–/–wt cells were transiently transfected with GFP–paxillin with or without co-transfection of FRNK or FAK and allowed to spread for 15 min on fibronectin. Fluorescence intensity recovery was measured on a confocal microscope after photobleaching. Shown are the means of recovery curves ± SEM of at least 3–5 independent experiments normalized to prebleach intensity. Curves are superimposed with the best exponential curve fit (red line). Recovery of GFP–paxillin fluorescence intensity in: top, Shp2+/+ cells compared with Shp2–/– cells; middle top, Shp2–/– cells compared with Shp2–/–wt cells; middle bottom, Shp2–/– cells compared with Shp2–/– cells co-transfected with FRNK; bottom, Shp2+/+ cells compared with Shp2+/+ cells co-transfected with FAK. Right: recovery halftimes were calculated by least-squares regression of the equation I = 1 – _A_e–t/τ*tn, where tn is the halftime of recovery, shown is the mean of halftimes ± SD of at least 3–5 independent experiments. Asterisks indicate significant statistical difference compared with wild type or untransfected controls, P < 0.002. (B) Left: FAK+/+ and FAK–/– cells were transiently transfected with GFP–paxillin with or without co-transfection of Shp2c/s, wtFAK or FAKY397F and allowed to spread for 15 min on fibronectin. Fluorescence intensity recovery was measured on a confocal microscope after photobleaching. Shown are the means of recovery curves ± SEM of at least three independent experiments normalized to prebleach intensity. Curves are superimposed with the best exponential curve fit (red line). Right: recovery halftimes of GFP–paxillin shown are the mean of halftimes ± SD of at least four independent experiments. Asterisks indicate significant statistical difference compared with wild type or untransfected controls, P ≤ 0.002.
To confirm that FAK activity did not contribute to focal adhesion dynamics in control cells, we performed these experiments in FAK-deficient cells (Ilic et al., 1995). Interestingly, halftimes of recovery of GFP–paxillin were the same in FAK+/+ and FAK–/– cells, although slightly faster than in Shp2+/+ cells (both 10 s) (Figure 6B). Since we have shown earlier that Shp2 regulates turnover via FAK, we wanted to test whether co- expression of a dominant-negative Shp2 (Shp2c/s) construct would alter halftimes of recovery of GFP–paxillin in FAK+/+ and FAK–/– cells. Interestingly, co-expression of GFP–paxillin and Shp2c/s decreased halftimes of recovery to 6.7 s in FAK+/+ cells, whereas there was no change in FAK–/– cells (10.4 s). Moreover, co-expression of wild-type FAK and Shp2c/s in FAK–/– cells led to decreased halftimes of recovery (7.2 s), whereas co-expression of autophosphorylation-defective FAK (FAKY397F) did not (9.6 s).
Taken together, these data suggest that the increased focal adhesion dynamics in Shp2–/– cells reflect the maturation defect. In addition, these data show that the increased focal adhesion dynamics in Shp2–/– cells are due to high levels of FAK activity and that FAK activity is tightly regulated during the assembly of initial adhesion sites.
Shp2 regulates strengthening of integrin–cytoskeleton linkages
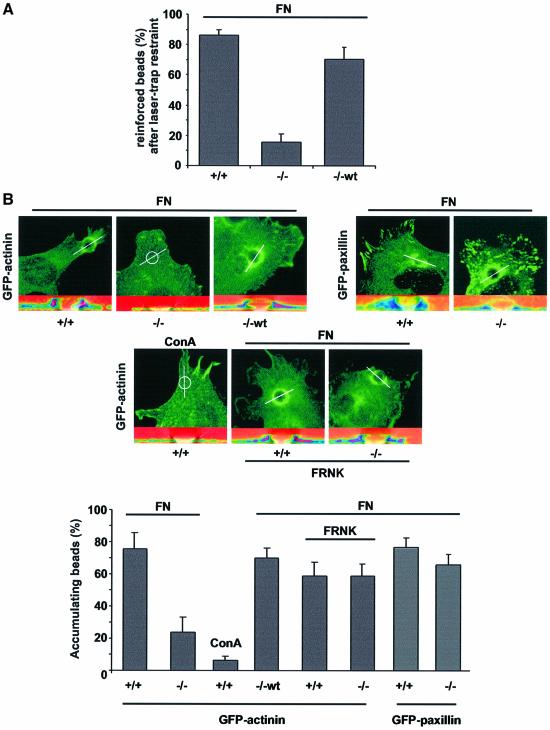
To evaluate whether the altered dynamics affect the strength of integrin–cytoskeleton bonds, we placed and held beads (0.91 µm diameter) on the cell surface using a laser trap. We have shown previously that fibronectin-coated beads bind to cell surface integrins and are drawn out of the trap in association with the retrograde moving actin-cytoskeleton and that this linkage is reinforced in response to the force exerted on the bead (Choquet et al., 1997). As expected, the restraint of FNIII7-10 beads led to the reinforcement of integrin–cytoskeleton linkages in Shp2+/+ cells. In sharp contrast, only 17% of the beads placed on Shp2–/– cells were reinforced. Reintroduction of Shp2 into Shp2–/– cells led to a full rescue in the ability to stabilize initial integrin–cytoskeleton linkages (Figure 7A). These data clearly indicate that Shp2 is required for the force-dependent reinforcement of integrin–cytoskeleton linkages.
Fig. 7. Shp2 deficiency leads to weak integrin–cytoskeleton linkages. (A) Beads (0.91 µm diameter) coated with fibronectin (FN) were placed on the upper surface of Shp2+/+ (+/+), Shp2–/– (–/–) and Shp2–/–wt (–/–wt) cells and beads were allowed to escape the trap field (500 nm). Shown is the percentage of reinforced beads (resistant to re-trapping). Results are the mean ± SD of three independent experiments. (B) Top: Shp2+/+ (+/+), Shp2–/– (–/–) and Shp2–/–wt (–/–wt) cells were transiently transfected with α-actinin–EGFP or GFP–paxillin with or without co-transfection of FRNK and allowed to spread for 15 min. Large beads (5.9 µm diameter) coated with either FN or concanavalin A (ConA) were spun onto the cells and incubated for 15 min. The percentage of bound beads causing accumulation (fluorescence intensity >2× surrounding) of α-actinin–GFP was determined. Confocal stacks were resliced along the indicated line over the beads position (bottom of each panel); shown are overlays of α-actinin–GFP fluorescence intensity in pseudo-colors and the differential interference contrast image. Bottom: percentage of FN- or ConA-coated beads causing accumulation of α-actinin–EGFP with or without co-transfection of FRNK. Results shown are the mean ± SD of three independent experiments
To further analyze the role of Shp2 in the reinforcement process we centrifuged (50 g/5 min) large FNIII7-10 beads (5.9 µm) onto cells. In contrast to the small beads, large beads (>3 µm diameter) stimulated adhesion-site assembly and reinforcement by cellular contractions independent of laser trap restraint and independent of RhoA (Galbraith et al., 2002). For Shp2+/+, Shp2–/– and Shp2–/–wt cells transiently transfected with α-actinin–EGFP, there was assembly of α-actinin at the large beads in a Shp2-dependent manner. In the Shp2–/– cells, the co-expression of FRNK with α-actinin–EGFP caused a dramatic increase in the number of accumula ting beads in the Shp2–/– cells, whereas there was no change in Shp2+/+ cells (Figure 7B). In contrast, there was no significant difference in the percentage of Shp2+/+ and Shp2–/– cells having contacts that stained with GFP–paxillin. Application of beads coated with concanavalin A confirmed the need for integrin binding to cause reinforcement. Thus, the Shp2-dependent downregulation of FAK is required for the force- dependent reinforcement of initial integrin–cytoskeleton linkages.
Discussion
The loss of Shp2 causes dramatic changes in the α-actinin assembly in adhesion sites and in the force-dependent reinforcement of integrin–cytoskeleton linkages. Our findings indicate that these changes can be explained as an alteration in focal complex protein dynamics caused by hyperactivation of FAK. Interestingly, this correlates with the inability to develop mature focal complexes. In contrast to previous studies that describe maturation of adhesion sites as the development of focal complexes into focal adhesions, our data imply that focal complexes also undergo a regulated maturation cycle. Recent observations suggested that α-actinin incorporation into newly formed adhesion sites is a late event during the formation of focal complexes (Laukaitis et al., 2001). In addition, it has been shown that aggregation of integrins by non-inhibitory monoclonal antibodies on beads induced intracellular accumulations of FAK and tensin, whereas combined antibody-mediated clustering and monovalent ligand occupancy was necessary to induce accumulation of more proteins, including α-actinin, paxillin, vinculin, talin and F-actin to the site (Miyamoto et al., 1995; Galbraith et al., 2002). This again suggests that formation of new adhesions is a regulated stepwise process. It has been shown that Rho-mediated acto-myosin contractility promotes the maturation of focal complexes to focal adhesions (Rottner et al., 1999) and overexpression of active Rho increases the lifetime of focal adhesions (Ren et al., 2000). In contrast, formation of focal complexes does not depend on Rho, but on Rac (Nobes and Hall, 1995; Galbraith et al., 2002). Indeed, in control cells the inhibition of RhoA does not diminish α-actinin assembly into focal complexes. The importance of mechanical stimuli in the initiation of focal complex formation has been shown recently (Galbraith et al., 2002; von Wichert et al., 2003), as well as the requirement for mechanical strain to mature from a focal complex to a focal adhesion (Balaban et al., 2001; Riveline et al., 2001). However, in Shp2–/– cells, inhibition of RhoA activity decreases focal complex maturation, suggesting that Rho partially compensates for the increased dynamics, possibly through increased contractility. Thus, Rho-dependent maturation cycles are functional in Shp2–/– cells. Nevertheless, both the assembly of α-actinin in focal complexes and the force-dependent reinforcement can be restored by the inhibition of FAK activity. Although there are other consequences of FAK inhibition, which preclude normal cell behavior, elements of focal complex maturation and reinforcement are restored. FAK has major roles in promoting turnover and disassembly of focal adhesions (Fincham and Frame, 1998; Sieg et al., 1999). Interestingly, Shp2-dependent inhibition of FAK activity addresses the apparent paradox that although FAK is one of the first proteins localized to new adhesion sites (Miyamoto et al., 1995), its disassembly function does not disturb growth and maturation of new adhesion sites. Moreover, the assembly of α-actinin in focal complexes could be directly dependent on Shp2-dependent inhibition of FAK activity, since FAK-dependent phosphorylation of a tyrosine at position 12 in the actin-binding domain of α-actinin decreases actin binding (Izaguirre et al., 2001). Furthermore, a direct role for α-actinin in reinforcement is possible, since α-actinin binds both integrins and the cytoskeleton (Pavalko et al., 1991; Geiger et al., 2001). In addition, strain hardening of F-actin networks depends on the amount of bound α-actinin (Xu et al., 1998) and the concentration of α-actinin controls the physical properties of actin–α-actinin gels (Wachsstock et al., 1994). Although it has been suggested that the highest forces are applied to the ECM via small focal complexes (Beningo et al., 2001), our data suggest that focal complexes have to undergo a distinct maturation process in order to be able to apply these forces to the ECM. Moreover, localization of α-actinin to newly formed adhesion sites is an indicator of their ability to generate force. Regulated force generation is essential for cell spreading and migration. For spreading to proceed, adhesion sites must be continually remodeled (Sheetz et al., 1999). Our data raise the possibility that the inability of focal complexes to mature leads to weak linkages and results in an impaired progression through the normal cycle at the integrin–cytoskeleton linkage needed for spreading and migration. Moreover, it suggests that FAK activity is tightly regulated spatially within forming adhesion sites. Thus, we suggest that hyperactive FAK in Shp2–/– cells locally increases the dynamics and prevents complex maturation through force-dependent reinforcement.
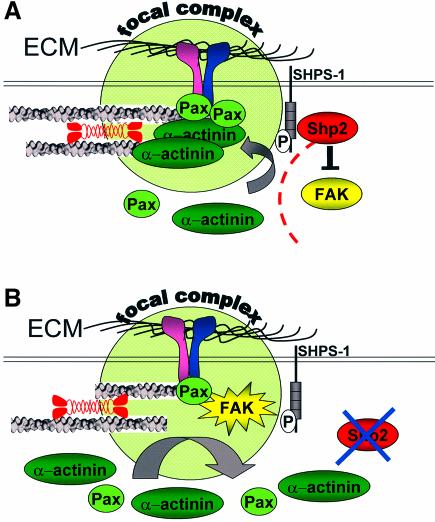
It is now well established that focal adhesions show movement in stationary and migrating cells (Smilenov et al., 1999; Zamir et al., 2000) and that lifetimes of focal adhesions are in the order of tens of minutes (Ren et al., 2000). Surprisingly, the exchange rates of structural components (i.e. β3-integrins and α-actinin) of focal adhesions determined so far are in the order of minutes (Ballestrem et al., 2001; Edlund et al., 2001; Tsuruta et al., 2002). These components are either bound to the ECM or bind both integrins and the cytoskeleton. In contrast, paxillin and vinculin only bind to the actin cytoskeleton and are thought to have a scaffolding function for other focal adhesion proteins (Critchley, 2000; Schaller, 2001). This correlates with the exchange rates in the order of seconds that we find for paxillin and vinculin, and favors models in which the stabilization of paxillin and/or vinculin complexes catalyzes the further maturation of focal complexes. In addition, it has been shown recently that integrin dynamics are different between cell adhesion sites formed at the front, and those that move in the retracting rear of migrating cells (Ballestrem et al., 2001). Our data suggest that exchange rates directly affect adhesion site function. Since focal adhesions can respond quickly to changes in the mechanical environment (Balaban et al., 2001; Riveline et al., 2001; Galbraith et al., 2002; Sawada and Sheetz, 2002), we speculate that adhesion sites actively regulate their dynamics in order to control their size and strength (Figure 8). Thus, Shp2-dependent regulation of FAK activity and subsequent changes in the exchange rates of focal adhesion proteins provide a mechanism by which Shp2 may influence cell adhesion and migration by spatially regulating adhesion site maturation.
Fig. 8. Model for the effects of Shp2 and FAK during the formation of integrin–cytoskeleton linkages. (A) New adhesion sites are protected by Shp2 during adhesion site formation. Shp2 dependent inhibition of FAK increases the residency times of adhesion site-associated structural and adapter proteins and enables strengthening and maturation of integrin–cytoskeleton linkages. Therefore, new focal complexes can withstand applied forces. (B) Loss of Shp2 leads to increased activity of FAK. Subsequently decreased residency times of adhesion site-associated structural and adapter proteins prevent the strengthening and maturation of integrin–cytoskeleton linkages. Therefore, new focal complexes cannot withstand applied forces.
Materials and methods
Cell culture
Cell lines were described elsewhere (Ilic et al., 1995; Saxton et al., 1997). All cells were maintained in Dulbecco’s modified Eagles medium (DMEM) supplemented with 10% (v/v) fetal bovine serum in a humidified atmosphere of 5% CO2/95% air at 37°C.
Spreading assays
Vitronectin (5 µg/ml) and the 120 kb chymotryptic fragment of fibronectin (6.6 µg/ml) were adsorbed onto tissue culture plastic. Cells were suspended and incubated for 30 min with or without GPenGRGDSPCA (GPen; 0.5 mM) (Sigma, St Louis, MO) or anti-α5/β1-integrin antibody (10 µg/ml) (Chemicon, Temecula). Cells were plated for 15 min, fixed and 20 serial non-overlapping fields were counted (×20 magnification).
Western blotting
Cells were lysed and equal amounts of protein were analyzed by SDS–PAGE followed by western blotting using polyclonal anti-Shp2, anti-α5, anti-β5 (Santa Cruz, Santa Cruz, CA), anti-αv-, anti-β3-, (Chemicon), phosphospecific anti-FAK antibody (Biosource, Camarillo, CA) and monoclonal FAK (Transduction Laboratories, San Diego, CA) antibodies. For immunoprecipitations, cells were treated as described in the figure legends, plated on fibronectin for 15 min as described above and lysed on ice in 50 mM Tris–HCl, 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 1% NP-40, 1 mM PMSF, 10 µg/ml aprotinin, 10 µg/ml leupeptin pH 7.6 (lysis buffer). The lysates were incubated with polyclonal anti-GFP antibody (Clontech, Palo Alto, CA) for 2 h at 4°C on a rotating wheel with protein A–Sepharose beads (Amersham/Pharmacia, Piscataway, NJ) added for the second hour. Beads were washed twice in lysis buffer and twice in wash buffer (50 mM Tris–HCl pH 8.0) and resuspended in 2× SDS–PAGE sample buffer. Western blotting was performed using monoclonal anti-phosphotyrosine (Upstate, Lake Placid) or anti-GFP (Clontech) antibodies. Immunoreactive bands were visualized by enhanced chemoluminescence (ECL) detection (Amersham/Pharmacia).
Immunofluorescence staining
Fibroblasts were transiently transfected with EGFP–α-actinin (gift of C.Otey, University of North Carolina), Y12F-α-actinin (Izaguirre et al., 2001) cloned in EGFP–N1 (Clontech), pUSERhoAT19N (Upstate) and pcDNA-FRNK (gift of M.Schaller, University of North Carolina) (each 0.3 µg/ml) using Fugene 6 (Roche Diagnostics, Indianapolis, IA) plated on fibronectin, fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Cells were incubated with either monoclonal anti-paxillin antibody (Transduction Laboratories, San Diego, CA) and rhodamine–phalloidin (Molecular Probes, Eugene, OR) or polyclonal phosphospecific anti-FAK antibody (Biosource) for 1 h followed by detection with Alexa-labeled (647 nm) secondary antibody (Molecular Probes). Samples were further analyzed by confocal microscopy (Olympus Fluoview 300).
Bead assays and laser-trapping
Beads of 0.91 and 5.9 µm (Polyscience, Niles) were coated with fibronectin (FNIII7-10) or concanavalin A (Sigma) as previously described (Felsenfeld et al., 1996). For bead binding assays, cells were plated on laminin-coated coverslips. Beads (0.91 µm) were then held for 3 s on the cell surface, 0.2–0.5 µm from the leading edge, using a 100 mW (40 pN/µm) optical-gradient laser trap setup (Zeiss Axiovert 100TV) that was calibrated as described previously (Felsenfeld et al., 1996). For reinforcement assays, beads were re-trapped after they had left the trap field and the number of reinforced beads (resistant to re-trapping) was counted. For reinforcement assays using large beads (5.9 µm), cells were transiently transfected with EGFP–α-actinin with or without co-transfection of FRNK. Bead binding was assessed by confocal microscopy and images were analyzed using ImageJ (1.28z) (available at: http://rsb.info.nih.gov/ij/). Beads were scored as accumulating if the GFP–actinin signal intensity was greater than twice that of the surrounding area.
Fluorescence recovery after photobleaching (FRAP)
Cells transiently transfected with pRK5GFP–paxillin or pRK5GFP–vinculin (both gifts of K.M.Yamada, National Institute of Dental and Craniofacial Research) with or without co-transfection of FRNK, wtFAK and FAKY397F (both kind gifts of S.Hanks, Vanderbilt University) or Shp2c/s (kind gift of S.Mañes, Universidad Autonoma de Madrid) were plated on fibronectin-coated coverslips for 15 min. FRAP experiments were done on an Olympus Fluoview 500 confocal system at 37°C. Measurements and analysis was performed as described elsewhere (Salmon and Wadsworth, 1986). Briefly, fluorescence intensity was measured at low laser power before bleach, photobleached with full laser power. Recovery was followed with low laser power at 1 s intervals until the intensity had reached a steady plateau. Curves were normalized to prebleach intensity. Recovery halftimes were calculated by least-squares regression of the equation I = 1 – _A_e–t/τ*tn, where tn is the halftime of recovery. Statistical analysis was performed using ANOVA followed by a Student–Newman–Keuls post-hoc test.
Acknowledgments
Acknowledgements
We are grateful to J.Sable for expert technical assistance and support. We thank also T.Baer, G.Giannone, K.Miller and Guoying Jiang for comments on the manuscript. This work was supported by NIH (M.P.S.) and the Deutsche Forschungsgemeinschaft (G.von W.).
References
- Balaban N.Q. et al. (2001) Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol., 3, 466–472. [DOI] [PubMed] [Google Scholar]
- Ballestrem C., Hinz,B., Imhof,B.A. and Wehrle-Haller,B. (2001) Marching at the front and dragging behind: differential αVβ3-integrin turnover regulates focal adhesion behavior. J. Cell Biol., 155, 1319–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo K.A., Dembo,M., Kaverina,I., Small,J.V. and Wang,Y.L. (2001) Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol., 153, 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D., Felsenfeld,D.P. and Sheetz,M.P. (1997) Extracellular matrix rigidity causes strengthening of integrin–cytoskeleton linkages. Cell, 88, 39–48. [DOI] [PubMed] [Google Scholar]
- Critchley D.R. (2000) Focal adhesions—the cytoskeletal connection. Curr. Opin. Cell Biol., 12, 133–139. [DOI] [PubMed] [Google Scholar]
- Edlund M., Lotano,M.A. and Otey,C.A. (2001) Dynamics of α-actinin in focal adhesions and stress fibers visualized with α-actinin-green fluorescent protein. Cell Motil. Cytoskeleton, 48, 190–200. [DOI] [PubMed] [Google Scholar]
- Felsenfeld D.P., Choquet,D. and Sheetz,M.P. (1996) Ligand binding regulates the directed movement of β1 integrins on fibroblasts. Nature, 383, 438–440. [DOI] [PubMed] [Google Scholar]
- Felsenfeld D.P., Schwartzberg,P.L., Venegas,A., Tse,R. and Sheetz,M.P. (1999) Selective regulation of integrin–cytoskeleton interactions by the tyrosine kinase Src. Nat. Cell Biol., 1, 200–206. [DOI] [PubMed] [Google Scholar]
- Feng G.S. (1999) Shp-2 tyrosine phosphatase: signaling one cell or many. Exp. Cell Res., 253, 47–54. [DOI] [PubMed] [Google Scholar]
- Fincham V.J. and Frame,M.C. (1998) The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J., 17, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith C.G., Yamada,K.M. and Sheetz,M.P. (2002) The relationship between force and focal complex development. J. Cell Biol., 159, 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. and Bershadsky,A. (2002) Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell, 110, 139–142. [DOI] [PubMed] [Google Scholar]
- Geiger B., Bershadsky,A., Pankov,R. and Yamada,K.M. (2001) Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol., 2, 793–805. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G. and Ruoslahti,E. (1999) Integrin signaling. Science, 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Hughes P.E. and Pfaff,M. (1998) Integrin affinity modulation. Trends Cell Biol., 8, 359–364. [DOI] [PubMed] [Google Scholar]
- Ilic D. et al. (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature, 377, 539–544. [DOI] [PubMed] [Google Scholar]
- Inagaki K., Yamao,T., Noguchi,T., Matozaki,T., Fukunaga,K., Takada,T., Hosooka,T., Akira,S. and Kasuga,M. (2000) SHPS-1 regulates integrin-mediated cytoskeletal reorganization and cell motility. EMBO J., 19, 6721–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre G., Aguirre,L., Hu,Y.P., Lee,H.Y., Schlaepfer,D.D., Aneskievich,B.J. and Haimovich,B. (2001) The cytoskeletal/non-muscle isoform of α-actinin is phosphorylated on its actin-binding domain by the focal adhesion kinase. J. Biol. Chem., 276, 28676–28685. [DOI] [PubMed] [Google Scholar]
- Kodama A., Matozaki,T., Fukuhara,A., Kikyo,M., Ichihashi,M. and Takai,Y. (2000) Involvement of an SHP-2-Rho small G protein pathway in hepatocyte growth factor/scatter factor-induced cell scattering. Mol. Biol. Cell, 11, 2565–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacalle R.A., Mira,E., Gomez-Mouton,C., Jimenez-Baranda,S., Martinez,A.C. and Manes,S. (2002) Specific SHP-2 partitioning in raft domains triggers integrin-mediated signaling via Rho activation. J. Cell Biol., 157, 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger D.A. and Horwitz,A.F. (1996) Cell migration: a physically integrated molecular process. Cell, 84, 359–369. [DOI] [PubMed] [Google Scholar]
- Laukaitis C.M., Webb,D.J., Donais,K. and Horwitz,A.F. (2001) Differential dynamics of α5 integrin, paxillin and α-actinin during formation and disassembly of adhesions in migrating cells. J. Cell Biol., 153, 1427–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes S., Mira,E., Gomez-Mouton,C., Zhao,Z.J., Lacalle,R.A. and Martinez,A.C. (1999) Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol. Cell. Biol., 19, 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Burnett,E., Kinch,M., Simon,E. and Wang,B. (2000) Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat. Cell Biol., 2, 62–69. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Akiyama,S.K. and Yamada,K.M. (1995) Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science, 267, 883–885. [DOI] [PubMed] [Google Scholar]
- Mortier E., Cornelissen,F., van Hove,C., Dillen,L. and Richardson,A. (2001) The focal adhesion targeting sequence is the major inhibitory moiety of Fak-related non-kinase. Cell Signal, 13, 901–909. [DOI] [PubMed] [Google Scholar]
- Nobes C.D. and Hall,A. (1995) Rho, rac and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell, 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Otey C., Vasquez,G.B., Burridge,K. and Erickson,B.W. (1993) Mapping of the α-actinin binding site within the β1 integrin cytoplasmic domain. J. Biol. Chem., 268, 21193–21197. [PubMed] [Google Scholar]
- Pavalko F.M., Otey,C.A., Simon,K.O. and Burridge,K. (1991) α-actinin: a direct link between actin and integrins. Biochem. Soc. Trans., 19, 1065–1069. [DOI] [PubMed] [Google Scholar]
- Petrone A. and Sap,J. (2000) Emerging issues in receptor protein tyrosine phosphatase function: lifting fog or simply shifting? J. Cell Sci., 113, 2345–2354. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M.D. and Ruoslahti,E. (1987) Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J. Biol. Chem., 262, 17294–17298. [PubMed] [Google Scholar]
- Ren X.D., Kiosses,W.B., Sieg,D.J., Otey,C.A., Schlaepfer,D.D. and Schwartz,M.A. (2000) Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci., 113, 3673–3678. [DOI] [PubMed] [Google Scholar]
- Richardson A. and Parsons,T. (1996) A mechanism for regulation of the adhesion-associated proteintyrosine kinase pp125FAK. Nature, 380, 538–540. [DOI] [PubMed] [Google Scholar]
- Riveline D., Zamir,E., Balaban,N.Q., Schwarz,U.S., Ishizaki,T., Narumiya,S., Kam,Z., Geiger,B. and Bershadsky,A.D. (2001) Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol., 153, 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner K., Hall,A. and Small,J.V. (1999) Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol., 9, 640–648. [DOI] [PubMed] [Google Scholar]
- Salmon E.D. and Wadsworth,P. (1986) Applications of Fluorescence in the Biomedical Sciences. Alan R.Liss, New York, NY. [Google Scholar]
- Sawada Y. and Sheetz,M.P. (2002) Force transduction by Triton cytoskeletons. J. Cell Biol., 156, 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton T.M., Henkemeyer,M., Gasca,S., Shen,R., Rossi,D.J., Shalaby,F., Feng,G.S. and Pawson,T. (1997) Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J., 16, 2352–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M.D. (2001) Paxillin: a focal adhesion-associated adaptor protein. Oncogene, 20, 6459–6472. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder S.M., Petch,L.A., Williamson,D., Shen,R., Feng,G.S. and Burridge,K. (2000) The protein tyrosine phosphatase Shp-2 regulates RhoA activity. Curr. Biol., 10, 1523–1526. [DOI] [PubMed] [Google Scholar]
- Sheetz M.P., Felsenfeld,D.P. and Galbraith,C.G. (1998) Cell migration: regulation of force on extracellular-matrix–integrin complexes. Trends Cell Biol., 8, 51–54. [DOI] [PubMed] [Google Scholar]
- Sheetz M.P., Felsenfeld,D., Galbraith,C.G. and Choquet,D. (1999) Cell migration as a five-step cycle. Biochem. Soc. Symp., 65, 233–243. [PubMed] [Google Scholar]
- Sieg D.J., Hauck,C.R. and Schlaepfer,D.D. (1999) Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci., 112, 2677–2691. [DOI] [PubMed] [Google Scholar]
- Smilenov L.B., Mikhailov,A., Pelham,R.J., Marcantonio,E.E. and Gundersen,G.G. (1999) Focal adhesion motility revealed in stationary fibroblasts. Science, 286, 1172–1174. [DOI] [PubMed] [Google Scholar]
- Taylor J.M., Mack,C.P., Nolan,K., Regan,C.P., Owens,G.K. and Parsons,J.T. (2001) Selective expression of an endogenous inhibitor of FAK regulates proliferation and migration of vascular smooth muscle cells. Mol. Cell. Biol., 21, 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta D., Gonzales,M., Hopkinson,S.B., Otey,C., Khuon,S., Goldman,R.D. and Jones,J.C. (2002) Microfilament-dependent movement of the β3 integrin subunit within focal contacts of endothelial cells. FASEB J., 16, 866–868. [DOI] [PubMed] [Google Scholar]
- Turner C.E. (2000) Paxillin and focal adhesion signalling. Nat. Cell Biol., 2, E231–E236. [DOI] [PubMed] [Google Scholar]
- von Wichert G., Jiang,G., Kostic,A., De Vos,K., Sap,J., Sheetz,M.P. (2003) RPTP-α acts as a transducer of mechanical force on αv/β3-integrin–cytoskeleton linkages. J. Cell Biol., 161, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsstock D.H., Schwarz,W.H. and Pollard,T.D. (1994) Cross-linker dynamics determine the mechanical properties of actin gels. Biophys. J., 66, 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle-Haller B. and Imhof,B. (2002) The inner lives of focal adhesions. Trends Cell Biol., 12, 382–389. [DOI] [PubMed] [Google Scholar]
- Xu J., Wirtz,D. and Pollard,T.D. (1998) Dynamic cross-linking by α-actinin determines the mechanical properties of actin filament networks. J. Biol. Chem., 273, 9570–9576. [DOI] [PubMed] [Google Scholar]
- Yu D.H., Qu,C.K., Henegariu,O., Lu,X. and Feng,G.S. (1998) Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration and focal adhesion. J. Biol. Chem., 273, 21125–21131. [DOI] [PubMed] [Google Scholar]
- Zamir E. et al. (2000) Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat. Cell Biol., 2, 191–196. [DOI] [PubMed] [Google Scholar]