Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-γ production from activated B cells (original) (raw)
Abstract
Interleukin 18 (IL-18), originally called interferon (IFN)-γ-inducing factor, is a recently cloned cytokine of approximately 18 kDa synthesized by Kupffer cells and activated macrophages. The major activity associated with this molecule is the induction of IFN-γ production from anti-CD3-activated T helper 1 cells in the presence of IL-12. B cells produce IgG1 and IgE when stimulated with anti-CD40 and IL-4. Here we show that a combination of IL-12 and IL-18 induces anti-CD40-activated B cells to produce IFN-γ, which inhibits IL-4-dependent IgE and IgG1 production and enhances IgG2a production without inhibiting the B cell proliferative response. We also show that 24.3% of B cells became positive for cytoplasmic IFN-γ after being stimulated with IL-12 and IL-18. Furthermore, we show that, like splenic T cells stimulated with anti-CD3, IL-12, and IL-18, B cells produced high level of IFN-γ in response to anti-CD40, IL-12, and IL-18. Injection of a mixture of IL-12 and IL-18 into mice inoculated with Nippostrongylus brasiliensis or injected with anti-IgD induced IFN-γ-producing cells that inhibit IgE production in them. Furthermore, B cells obtained from normal mice could develop into IFN-γ-producing cells in IFN-γ−/− host mice in response to in vivo treatment with IL-12 and IL-18. These results indicate that IFN-γ from activated B cells differentially regulates IgG1/IgE and IgG2a responses in vitro and in vivo, indicating that B cells act as regulatory cells in the immune response. Present results suggested that injection of IL-12 and IL-18 could present a unique approach for the treatment of allergic disorders.
Keywords: helminth/cytokine
The activation, proliferation, and differentiation of B cells are highly regulated events in which the action of T cells and their soluble products plays a major role (1–3). However, a few studies have suggested that activated B cells also may regulate immune response by production of interleukin (IL)-10 that inhibits T helper 1-mediated immune response (4, 5). IL-18, originally called interferon gamma (IFN-γ)-inducing factor, is a recently cloned cytokine of approximately 18 kDa synthesized by Kupffer cells and activated macrophages (6). IL-18 acts on T helper 1-type T cells and in combination with IL-12 strongly induces them to produce IFN-γ (6). Recently a cDNA for human IL-18 has been cloned (7). It has been shown to stimulate T cells and NK cells to produce IFN-γ and enhances Fas ligand expression (6–8). In this study we sought to determine whether or not IL-18 by itself or in combination with IL-12 could stimulate B cells to produce IFN-γ and thus potentially act as regulatory cells in the immune response.
MATERIALS AND METHODS
Animals and Reagents.
Virus-free C57BL/6, BALB/c, or BALB/c nu/nu female mice, 8–12 weeks of age, were used. Homozygous IFN-γ knockout (IFN-γ−/−) mice were established and maintained at the Laboratory Animal Research Center, Institute of Medical Science, University of Tokyo. Recombinant mouse IL-12 and IL-18 were generous gifts from Hayashibara Biochemical Laboratories (Okayama, Japan). Recombinant mouse IFN-γ was purchased from PharMingen. Rat anti-mouse IFN-γ (R4–6A2) (9) and rat anti-mouse CD40 (LB429) (10) antibodies were purified in our laboratory. Goat anti-IgD antisera were kindly provided by Fred Finkelman (University of Cincinnati, Cincinnati, OH). Fluorescein isothiocyanate (FITC)-rat anti-mouse B220 (RA3–6B2), FITC-rat anti-mouse IFN-γ (XMG 1.2), and phycoerythrin-rat anti-mouse IL-2 receptor β chain (IL-2Rβ) (TMβ-1), were purchased from PharMingen. Magnetic beads coated with rat anti-mouse B220 antibody were purchased from PerSeptive Diagnostics (Cambridge, MA).
In Vivo Treatment of Mice.
IFN-γ+/+ C57BL/6 mice (5 per group) or IFN-γ−/− C57BL/6 mice (4 per group) were either not treated or treated with s.c. injection of 100 μl of goat anti-IgD antiserum or s.c. inoculation of 700 Nippostrongylus brasiliensis (Nb) third-stage larvae on the first day of experiment. Anti-IgD-injected or Nb-inoculated IFN-γ+/+ mice were injected daily with IL-12 (50–100 ng/mouse) and/or IL-18 (500 ng/mouse) for 6 and 12 days, respectively. Serum IgE levels were measured at 7 and 13 days after anti-IgD-injection and Nb-inoculation, respectively. In some experiments, IFN-γ+/+ mice, IFN-γ+/− mice or IFN-γ−/− mice administered with highly purified B cells (108/mouse) from IFN-γ+/+ C57BL/6 mice, were injected i.p. daily for 4 days with IL-12 (100 ng/mouse) and IL-18 (1,000 ng/mouse). Spleen cells, B cells, and non-B cells were obtained from such treated mice and examined for their expression of IFN-γ mRNA by reverse transcription–PCR (RT–PCR).
B and T Cell Preparation.
Highly purified splenic B cells were prepared from BALB/c mice pretreated with anti-asialo- GM1, which was used to eliminate NK cells, followed by passage of spleen cells over a Sephadex G10 column and two rounds of complement-mediated lysis of T cells with monoclonal anti-Thy-1.2 and anti-Lyt-1.2 antibodies (11) This procedure routinely yields cells that are >99% surface IgM, B220, and Ia positive and <1% CD3 positive. Highly purified splenic T cells were prepared from anti-asialo-GM1-treated mice by passing their spleen cells through a nylon wool column (12), followed by treatment of resultant cells with a mixture of magnetic beads coated with monoclonal antibodies against B cells and macrophages to remove residual B cells and macrophages as detailed previously (13), yielding 99% CD3 positive cells.
Intracellular Cytokine Staining.
For analysis of intracellular IFN-γ positive B cells, we followed the modified protocol of immunofluorescent staining of intracellular cytokines for the flow cytometric analysis described by Vikingson and Muller (14). Briefly, highly purified B cells (2 × 106/ml per well) were cultured with or without anti-CD40 antibody (0.5 μg/ml) in the presence or absence of 10 ng/ml each of IL-12 and IL-18 for 84 h with a pulse of 3 μg/ml monensin during the final 12 h to inhibit IFN-γ secretion (15). Such treated B cells first were stained with phycoerythrin-conjugated rat anti-mouse B220 and followed by fixation with 4% (wt/vol) paraformaldehyde in PBS and permeabilization of cell membrane with ice-cold PBS containing 1% fetal calf serum plus 0.1% saponin. Resultant cells were further stained with 0.5 μg of FITC-conjugated rat anti-mouse IFN-γ antibody in the presence or absence of excess IFN-γ (10 μg/ml) and analyzed for their proportion of cytoplasmic IFN-γ positive B cells by two-color flow cytometrical analysis by FACScan (Becton Dickinson). The percentages shown represent the proportion of IFN-γ positive cells among B220 positive cells. Quadrants were set on the basis of stained profiles in the presence of IFN-γ.
Cell Cultures.
Purified B cells (105/0.2 ml per well), cultured with anti-CD40 (0.5 μg/ml) alone or with anti-CD40 and IL-12 and/or IL-18 (37 pg/ml to 27 ng/ml) in the presence or absence of anti-IFN-γ antibody (1.25 to 20 μg/ml) for 24 h, were followed by additional stimulation with 5,000 units/ml IL-4 for 7 days. Supernatants in triplicate cultures were collected at 4 or 8 days after the initiation of the culture, and quantitative immunoassays for secreted IFN-γ or IgE, IgG1, IgG2a and IgM, respectively, were performed by using specific two-site ELISA, with reference standard curve prepared using known amounts of rIFN-γ, or IgE, IgG1, IgG2a and IgM (13). In some experiments, highly purified B cells (2 × 106/ml per well) cultured with or without anti-CD40 antibody (0.5 μg/ml) or splenic T cells (2 × 106/ml per well) cultured in 24-well anti-CD3 (10 μg/ml) coated plates were stimulated with IL-12 (20 ng/ml) and/or IL-18 (20 ng/ml) for 72 h. Supernatants were measured for IFN-γ contents by ELISA.
Analysis of Expression of IFN-γ mRNA.
Total RNA was prepared from cells using the guanidinium method. As positive control, mRNA extracted from total spleen cells from mice treated with anti-CD3 antibody 90 min earlier were used (16). For analysis of expression of IFN-γ mRNA, mRNAs were amplified by a modified standard RT–PCR amplification procedure as described in our previous paper (16).
RESULTS
Combination of IL-12 and IL-18 Inhibits IgE and IgG1 Production from Anti-CD40 plus IL-4-Stimulated B Cells.
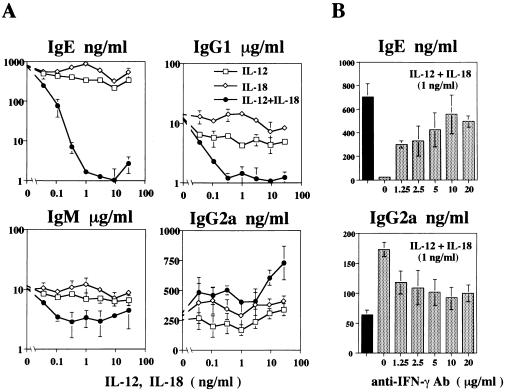
Highly purified B cells (≈99% surface IgM+ and B220+) were prepared from BALB/c mice and cultured with anti-CD40 antibody and IL-4, which induce B cells to produce IgE/IgG1 and IgM (17). NK cells were depleted by previous injection of the mice with anti-asialo-GM 1 (11). In Fig. 1A, we show the effect of IL-12 and/or IL-18 addition on this IL-4-regulated isotype switching. We find that IL-12 stimulation partially inhibited IL-4-induced Ig synthesis, whereas IL-18 stimulation did not affect this Ig response. However, the combination of 500 pg to 1 ng per ml each of IL-12 and IL-18 inhibited IgE and IgG1 production almost completely without inhibiting anti-CD40 plus IL-4-induced B cell growth responses (data not shown). This combination also inhibited IgM production markedly. Moreover, IL-18 with IL-12 induced a 3-fold enhancement in IgG2a synthesis, especially when IL-12 and IL-18 were used at high concentrations.
Figure 1.
Effects of IL-12 and/or IL-18 on Ig production by anti-CD40 and IL-4 stimulated B cells in the absence (A) or presence (B) of anti-IFN-γ antibody. (A) Purified B cells (105/0.2 ml per well) were cultured with anti-CD40 antibody (LB429, IgG2a; 0.5 μg/ml) in the presence of recombinant murine IL-12 and/or IL-18 (37 pg/ml to 27 ng/ml) for 24 h, followed by additional stimulation with 5,000 units/ml IL-4 for 7 days. (B) B cells (105/0.2 ml per well) cultured with anti-CD40 antibody (0.5 μg/ml) or anti-CD40 plus IL-12/IL-18 (1 ng/ml) at various concentrations of anti-IFNγ antibodies (R4–6A2; 1.25 to 20 μg/ml) for 24 h were further incubated with IL-4 (5,000 units/ml) for 7 days. Quantitative immunoassays for secreted IgE, IgG1, IgG2a, and IgM were performed by using an avidin-biotin microtiter ELISA. Results are mean ± 1 SD.
Because IgG1/IgE and IgG2a responses depend upon IL-4 and IFN-γ (18, 19), respectively, IL-18 with IL-12 might have differentially regulated these Ig responses by inducing IFN-γ from anti-CD40-activated B cells. As shown in Fig. 1B, we tested this possibility by adding an anti-IFN-γ antibody and found that it enhances IgE production but inhibits IgG2a production, indicating that endogenous IFN-γ reciprocally regulates these Ig responses in a dose-dependent manner.
Combination of IL-12 and IL-18 Induces IFN-γ Production from B Cells in Vitro.
Even though the B cell preparations were very highly purified by cell surface marker analysis (see above), we sought to assess any possible T cell and NK cell contamination by hybridizing Northern blots with T-cell receptor Cβ probe (86T5) and CD16 probe, respectively. We found no hybridization (data not shown), consistent with the cell surface marker analysis. Furthermore, the resultant cells had no detectable B220−IL-2Rβ+ cells (NK cells) or Mac-1+ cells (macrophages) on FACS analysis. Therefore, it appears that anti-CD40-stimulated B cells produce IFN-γ in response to IL-12 plus IL-18.
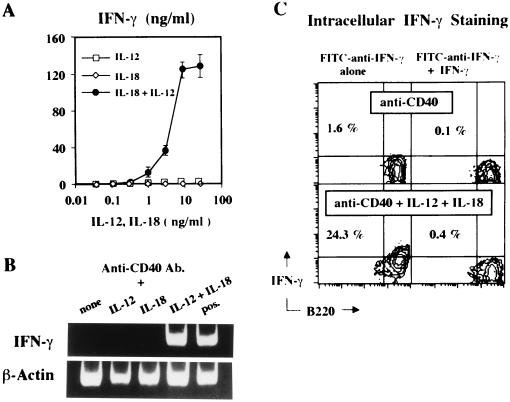
To show that these activated B cells produce IFN-γ and to determine whether or not this IFN-γ production requires stimulation with anti-CD40, we incubated B cells with or without anti-CD40 in the presence of various doses of IL-12, IL-18, or IL-12/IL-18 for 3 or 4 days. While stimulation of B cells with anti-CD40 alone or with anti-CD40 and IL-12 or IL-18 produced little or no IFN-γ mRNA and protein, stimulation with anti-CD40 plus IL-12/IL-18 strikingly induced B cells to synthesize IFN-γ mRNA as well as biologically active IFN-γ (Fig. 2A and B). Measurement of IFN-γ mRNA expression by RT–PCR in B220+ cells purified from anti-CD40 plus IL-12/IL-18-stimulated B cells by using magnetic beads coated with rat anti-mouse B220 antibody clearly revealed that such activated B cells also strongly expressed IFN-γ mRNA (data not shown). Levels of IFN-γ in the culture supernatants of B cells stimulated with IL-12/IL-18 in the presence and absence of anti-CD40 revealed that anti-CD40 stimulation induced ≈3-fold increase in the level of secreted IFN-γ (Table 1). Similar results were obtained when B cells prepared from anti-asialo-GM1-treated BALB/c nu/nu mice were stimulated with IL-12 and IL-18 in the presence or absence of anti-CD40 (Table 1). We also compared the capacity of T cells and B cells to produce IFN-γ. Like T cells incubated with anti-CD3, IL-12, and IL-18, B cells produced high levels of IFN-γ when they were similarly stimulated (Table 1).
Figure 2.
IFN-γ production from purified B cells in response to anti-CD40, IL-12, and IL-18. (A) Highly purified B cells were cultured at 105 in flat-bottomed 96-well plates in 200 μl of culture medium with anti-CD40 antibody (0.5 μg/ml) in the presence of various concentrations of recombinant murine IL-12 (□), IL-18 (⋄), or a combination of IL-12/IL-18 (•) (37 pg/ml to 27 ng/ml) for 96 h. Supernatants in triplicate culture were harvested and tested for their concentrations of IFN-γ by ELISA. Results are mean ± 1 SD. (B) One microgram of total RNAs from B cells stimulated with anti-CD40 antibody (0.5 μg/ml) alone, or anti-CD40 plus 10 ng/ml each of IL-18 or IL-12, or its combination for 72 h were amplified by modified standard RT–PCR amplification procedure. (C) Intracellular IFN-γ staining of B cells stimulated with anti-CD40 antibody alone or with anti-CD40, IL-12, and IL-18 for 96 h were performed and analyzed as described in Materials and Methods.
Table 1.
IFN-γ production by B cells stimulated with IL-12 and/or IL-18
| Stimulus | IFN-γ production, ng/ml | ||||
|---|---|---|---|---|---|
| B cells | T cells | ||||
| Without anti-CD40 | With anti-CD40 | With anti-CD3 | |||
| BALB/c | nu/nu | BALB/c | nu/nu | BALB/c | |
| Medium | <0.1 | <0.1 | <0.1 | <0.1 | 2.50 |
| IL-12 | 0.42 | <0.1 | 0.55 | <0.1 | 16.7 |
| IL-18 | <0.1 | <0.1 | <0.1 | <0.1 | 3.64 |
| IL-12 + IL-18 | 45.7 | 33.7 | 112 | 86.7 | 122 |
To examine the proportion of IFN-γ-producing cells, we stained B cells for cytoplasmic IFN-γ and analyzed them by FACS analysis (14). B cells were cultured with or without anti-CD40 antibody in the presence or absence of 10 ng/ml each of IL-12 and IL-18 for 84 h with a pulse of 3 μg/ml monensin during the final 12 h to inhibit IFN-γ secretion (15). As shown in Fig. 2C, 24.3% of B cells cultured with anti-CD40 plus IL-12/IL-18 are cytoplasmic IFN-γ positive, whereas only 1.6% of B cells cultured with anti-CD40 alone are positive for IFN-γ. That the intracellular IFN-γ staining is specific is indicated by the fact that it is completely blocked by the preincubation of the conjugated antibody with excess recombinant mouse IFN-γ (Fig. 2C, Right). Failure to stain cytoplasmic IFN-γ in B cells with FITC-conjugated rat Ig class-matched control antibody further substantiated this specificity of the intracellular IFN-γ staining (data not shown). Obtained results (data not shown) concerning the contribution of anti-CD40 stimulation in induction of IFN-γ-producing cells revealed that stimulation with anti-CD40 enhanced IFN-γ production by causing an increase in the number of IFN-γ-producing cells without affecting the proportion of IFN-γ-producing cells. Thus, a substantial fraction of B cells expresses cytoplasmic IFN-γ after stimulation with IL-12 and IL-18.
Combination of IL-12 and IL-18 Inhibits IgE Production by Induction of IFN-γ-Producing Cells in Vivo.
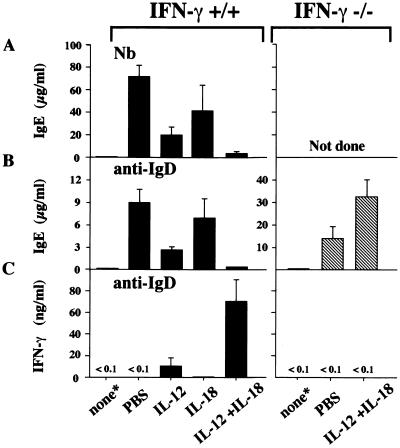
Next we examined whether daily i.p. injections of IL-12 and/or IL-18 could inhibit IgE production in mice inoculated with Nb or injected with anti-IgD antibody (20) by the induction of IFN-γ-producing B cells. We used IFN-γ+/+ and IFN-γ−/− C57BL/6 mice. As shown in Fig. 3A and B and consistent with the previous report (20, 21), injection of IL-12 (50 ng/mouse) into Nb-inoculated- or anti-IgD-injected-IFN-γ+/+ mice markedly inhibited IgE production but significantly enhanced IgG2a production (data not shown), whereas injection of IL-18 (500 ng/mouse) only modestly inhibited IgE production in these mice. However, daily i.p. injection of both IL-12 and IL-18 into Nb-inoculated- or anti-IgD-injected-IFN-γ+/+ mice almost completely inhibited IgE production (Fig. 3 A and B, Left) but markedly enhanced IgG2a production (data not shown). Like anti-IgD-injected-IFN-γ+/+ mice, IFN-γ−/− mice produced IgE in response to anti-IgD. However, injection of a mixture of IL-12 and IL-18 failed to inhibit IgE production in such anti-IgD-treated IFN-γ−/− mice (Fig. 3B, Right). To our surprise, such injection rather enhanced IgE production in anti-IgD-treated IFN-γ−/− mice (Fig. 3B, Right). Furthermore injection of anti-IFN-γ antibody reversed the IL-12 and IL-18-induced IgE inhibition in the normal mice (data not shown). These results strongly indicate that IFN-γ from IL-12/IL-18-stimulated cells can suppress IgE production. Indeed as shown in Fig. 3C, spleen cells from anti-IgD and IL-12-treated mice produced low levels of IFN-γ, whereas anti-IgD, IL-12, and IL-18-treated mice produced high levels.
Figure 3.
Inhibition of IgE production by IL-12/IL-18 in mice. (A and B) IFN-γ+/+ C57BL/6 mice (5 per group) were either not treated (*) or treated with s.c. inoculation of 700 Nb third-stage larvae or s.c. injection of 100 μl of goat anti-IgD antiserum on the first day of experiment. Nb-inoculated or anti-IgD injected IFN-γ+/+ mice were treated with daily injections of IL-12 (50 ng/mouse) and/or IL-18 (500 ng/mouse) for 12 and 6 days, respectively. IFN-γ−/− C57BL/6 mice (4 per group) also were either not treated or treated with s.c. injection of 100 μl of goat anti-IgD antiserum followed by daily injection of PBS or IL-12 (100 ng/ml) and IL-18 (500 ng/ml). Serum IgE levels were measured at 13 and 7 days after Nb-inoculation and anti-IgD injection, respectively. (C) Spleen cells (2 × 106) prepared from the IFN-γ+/+ mice (5 per group) or IFN-γ−/− mice (4 per group) injected with ant-IgD 7 days previously followed by daily injection of PBS, IL-12, IL-18, or IL-12 and IL-18, were cultured for 48 h. Supernatants were examined for their IFN-γ activity. Results are mean + 1 SD.
Combination of IL-12 and IL-18 Induces IFN-γ-mRNA-Expressing B Cells in Vivo.
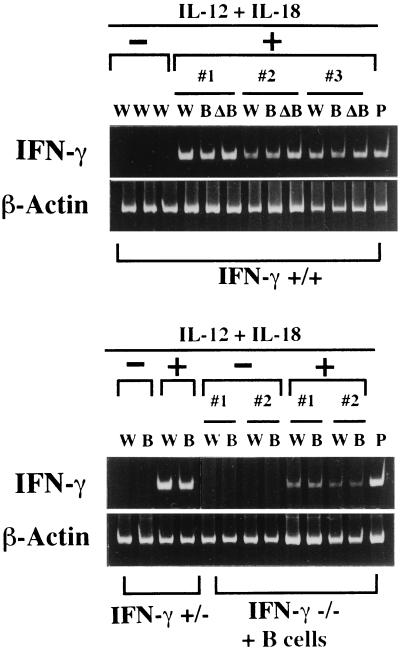
To show whether IL-12 and IL-18 stimulate B cells to produce IFN-γ in vivo, we injected IL-12 (100 ng/mouse) and IL-18 (1,000 ng/mouse) into IFN-γ+/+ or IFN-γ+/− mice or IFN-γ−/− mice transferred with IFN-γ+/+ B cells (108/mouse). As shown in Fig. 4, daily i.p. injection of IL-12 and IL-18 induced IFN-γ mRNA almost equally in whole spleen cells, positively selected B cells by using anti-B220 antibody coated magnetic beads and B cell-depleted (ΔB) spleen cells in IFN-γ+/+ (Nos. 1–3) or IFN-γ+/− mice. Furthermore, such treatment also induced IFN-γ mRNA expression in whole spleen cells as well as positively selected B cells from IFN-γ−/− mice transferred with B cells from normal mice (Nos. 1, 2), although the level of IFN-γ mRNA expression in these cells was low. We also examined the capacity of IL-12 (100 ng/mouse) and IL-18 (1,000 ng/mouse) injected into IFN-γ−/− mice either transferred with IFN-γ+/+ B cells or not to inhibit anti-IgD-induced IgE production. We found that injection of IL-12 and IL-18 inhibited anti-IgD-induced IgE production (60% inhibition) only in IFN-γ−/− mice transferred with IFN-γ+/+ B cells. These results taken together indicate that IL-12 and IL-18 activate B cells to produce IFN-γ that may differentially regulate IgG1/IgE and IgG2a responses in vivo in an autocrine or paracrine manner.
Figure 4.
Induction of spleen cells, B cells, and non-B cells to develop into cells expressing IFN-γ mRNA by injection of IL-12 and IL-18. Highly purified B cells (108) from IFN-γ+/+ C57BL/6 mice were transferred into IFN-γ−/− mice. IL-12 (100 ng/mouse) and IL-18 (1,000 ng/mouse) were injected i.p. every day for 4 days into IFN-γ+/+ mice (Nos. 1–3), IFN-γ+/− mice or IFN-γ−/− mice (Nos. 1, 2) administered with IFN-γ+/+ B cells. mRNAs extracted from whole spleen cells (W), B220+ cells (B), and B220− cells (ΔB) fractionated from whole spleen cells by using magnetic beads coated with rat anti-mouse B220 antibody were amplified by RT–PCR for analysis of expression of IFN-γ mRNA. Flow cytometric analysis revealed that more than 95% of beads bound cells (B) and less than 0.5% of beads-unbound cells (ΔB) were surface IgM positive.
DISCUSSION
B cells have been recognized as cells that produce Ig after being stimulated with antigen and T helper cells. However, several reports have demonstrated that activated B cells also produce cytokines such as IL-1 (22), IL-6 (23, 24), and IL-10 (4, 5, 24). IL-18 has been originally described as a factor that enhances IFN-γ production from T helper 1 cells, particularly in the presence of IL-12 (6). IL-18 also markedly stimulates IFN-γ production in nylon wool-purified splenic T cells in the presence of immobilized anti-CD3 antibody and IL-12 (Table 1) (6). As noted in Figs. 1 and 2, anti-CD40, IL-12, and IL-18-stimulated-B cells produce significant quantities of IFN-γ, and this inhibits IL-4-dependent IgG1 and IgE production in vitro. We also show that B cell-derived-IFN-γ can enhance IgG2a production in vitro (Fig. 1). Thus IL-12 and IL-18-stimulated B cells act as regulatory cells that differentially regulate IgG1/IgE and IgG2a responses by production of IFN-γ in vitro.
As T cells require costimulation with IL-12 to respond to IL-18 by striking production of IFN-γ (Table 1) (6), B cells also require stimulation with IL-12 to become responsive to IL-18. Furthermore, as splenic T cells stimulated with immobilized anti-CD3, IL-12 and IL-18 produce high levels of IFN-γ (122 ng/ml), B cells stimulated with IL-12/IL-18 produce a comparable level of IFN-γ (112 ng/ml) (Table 1). Thus, both B cells and T cells seem to require IL-12 to be responsive to IL-18 that strikingly enhances IFN-γ production from IL-12-stimulated B cells and T cells. We also demonstrated that anti-CD40 is not prerequisite for inducing IFN-γ-producing B cells, although stimulation with anti-CD40 enhances IFN-γ production from B cells (Table 1).
Finkelman et al. (21) reported that IL-12 inhibits IgE production in vivo via endogenous IFN-γ, although the property of IFN-γ producing cells is not elucidated. Li et al. (25) recently have reported IL-12 stimulates human B cells to produce IFN-γ in vitro. Here we show that IL-12 and IL-18 strongly activates B cells to produce IFN-γ in vitro (Fig. 2) and in vivo (Fig. 4). We have described that anti-CD40-activated B cells have the capacity to produce IFN-γ in response to IL-12 and IL-18, and this IFN-γ inhibits IL-4-dependent IgG1 and IgE responses in vitro (Figs. 1, 2). Furthermore, to reveal the proportion of IFN-γ producing B cells, we performed intracellular IFN-γ staining. 24.3% of B cells became positive for cytoplasmic IFN-γ after being stimulated with anti-CD40, IL-12, and IL-18 (Fig. 2C). We used anti-B220 to detect B cells. Similar results also were obtained when B cells incubated with IL-12 and IL-18 were stained with a combination of phycoerythrin-anti-IgM and FITC-anti-IFN-γ (data not shown). We also demonstrated injection of a mixture of IL-12 and IL-18 induces IFN-γ that inhibits IgE production in Nb-inoculated or anti-IgD-injected mice (Fig. 3).
To reveal the relative contribution of B cell-derived IFN-γ in this IL-12 and IL-18-induced IgE suppression in vivo, we tested whether B cells prepared from nontreated IFN-γ+/+ mice could develop into IFN-γ-producing cells after being stimulated with IL-12 and IL-18 in IFN-γ−/− mice and inhibited anti-IgD-induced IgE production. As expected, enriched B cells from IFN-γ−/− mice treated with IL-12/IL-18 did not express IFN-γ-mRNA (data not shown), whereas enriched B cells from IFN-γ−/− mice administered with IFN-γ+/+ B cells and treated with IL-12/IL-18 expressed IFN-γ mRNA (Fig. 4). Furthermore, injection of IL-12/IL-18 into IFN-γ−/− mice injected with anti-IgD did not inhibit IgE (Fig. 3), whereas the same treatment of IFN-γ−/− mice administered with IFN-γ+/+ B cells and injected with anti-IgD showed partial but significant inhibition of IgE production (60% suppression). These results indicate that B cells can develop into IFN-γ-producing cells after being stimulated with IL-12 and IL-18 in vivo and regulate isotype switching.
Injection of IL-12/IL-18 into anti-IgD-injected IFN-γ+/+ mice inhibited IgE production almost completely (Fig. 3). However, same treatment of IFN-γ−/− mice administered with IFN-γ+/+ B cells and injected with anti-IgD inhibited partially. Two reasons may account for this partial inhibition. First, the transferred B cells failed to develop fully into IFN-γ-producing cells. We suspect that injected B cells were widely distributed and activated poorly compared with far more abundant host B cells that are activated with CD40 ligand in peripheral lymphoid organs. Second, paracrine IFN-γ production possibly from IL-12 and IL-18-stimulated T and/or NK cells also regulate patterns of Ig isotype produced from B cells. Indeed, as shown in Fig. 4, injection of IL-12/IL-18 into normal mice induces strong IFN-γ-mRNA in both B cells and non-B cells. Thus, we conclude that, like T helper 1 cells, B cells also can act as regulator cells that play a physiologic role in Ig isotype regulation.
As IgE responses are an important factor in allergic responses, we also would suggest that IL-12/IL-18 treatment could present a unique approach for the treatment of allergic disease. The potentiality that B cells produce IFN-γ also may have enormous implications concerning understanding the regulation of immune response against infection as well as the traditional division of cell-mediated and humoral immunity.
Acknowledgments
We thank Dr. Fred Finkelman for his generous gift of anti-IgD antiserum. We are grateful to Hayashibara Biochemical Laboratories Inc. for providing recombinant murine IL-12 and IL-18. We acknowledge very helpful discussions with Dr. William E. Paul and Dr. Mark Davis in the course of this work.
ABBREVIATIONS
IL
interleukin
IFN
interferon
IFN-γ−/−
IFN-γ knockout, FITC, fluorescein isothiocyanate
Nb
Nippostrongylus brasiliensis
RT–PCR
reverse transcription–PCR
References
- 1.Nakanishi K, Malek T R, Smith K A, Hamaoka T, Shevach E M, Paul W E. J Exp Med. 1984;160:1605–1621. doi: 10.1084/jem.160.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kishimoto T. Annu Rev Immunol. 1985;3:133–157. doi: 10.1146/annurev.iy.03.040185.001025. [DOI] [PubMed] [Google Scholar]
- 3.Howard M, Nakanishi K, Paul W E. Immunol Rev. 1984;78:185–210. doi: 10.1111/j.1600-065x.1984.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 4.Gerard C, Bruyns C, Marchant A, Abramowicz D, Vanderabeele P, Delvaux A, Fiers W, Goldmann M, Velu T. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velupillai P, Harn D A. Proc Natl Acad Sci USA. 1994;91:18–22. doi: 10.1073/pnas.91.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Nature (London) 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 7.Ushio S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, Tanabe F, Konishi K, Micallef M, Fujii M, Torigoe K, Tanimoto T, Fukuda S, Ikeda M, Okamura H, Kurimoto M. J Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]
- 8.Tsutsui H, Nakanishi K, Matsui K, Higashino K, Okamura H, Miyazawa Y, Kaneda K. J Immunol. 1996;157:3967–3973. [PubMed] [Google Scholar]
- 9.Spitalny G L, Havell E A. J Exp Med. 1984;159:1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nomura J, Inui S, Yamasaki T, Kataoka S, Maeda K, Nakanishi K, Sakaguchi N. Immunol Lett. 1995;45:195–203. doi: 10.1016/0165-2478(95)00006-q. [DOI] [PubMed] [Google Scholar]
- 11.Nakanishi K, Hirose S, Yoshimoto T, Ishizashi H, Hiroishi K, Tanaka T, Kono T, Miyasaka M, Taniguchi T, Higashino K. Proc Natl Acad Sci USA. 1992;89:3551–3555. doi: 10.1073/pnas.89.8.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julius M H, Simpson E, Herzenberg L A. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimoto T, Bendelac A, Hu-Li J, Paul W E. Proc Natl Acad Sci USA. 1995;92:11931–11934. doi: 10.1073/pnas.92.25.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vikingson A, Pederson K, Muller D. J Immunol Methods. 1994;173:219–228. doi: 10.1016/0022-1759(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 15.Lee C L Y, Lee S H S, Jay F T, Rozee K R. Immunology. 1990;70:94–99. [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimoto T, Paul W E. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakanishi K, Matsui K, Kashiwamura S-I, Nishioka Y, Noma J, Nishimura Y, Sakaguchi N, Yonehara S, Higashino K, Shinka S. Int Immunol. 1996;8:791–798. doi: 10.1093/intimm/8.5.791. [DOI] [PubMed] [Google Scholar]
- 18.Coffman R L, Ohara J, Bond M W, Carty J, Zlotnik A, Paul W E. J Immunol. 1986;136:4538–4541. [PubMed] [Google Scholar]
- 19.Snapper C M, Paul W E. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 20.Finkelman F D, Katona I M, Urban J F, Jr, Snapper C M, Ohara J, Paul W E. Proc Natl Acad Sci USA. 1986;83:9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkelman F D, Madden K B, Cheever A W, Katona I M, Morris S C, Gately M K, Hubbard B R, Gause W C, Urban J F., Jr J Exp Med. 1994;179:1563–1572. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pistoia V, Cozzolino F, Rubartelli A, Torcia M, Roncella S, Ferrarini M. J Immunol. 1986;136:1688–1692. [PubMed] [Google Scholar]
- 23.Smeland E B, Blomhoff H K, Funderud S, Shalaby M R, Espevik T. J Exp Med. 1989;170:1463–1468. doi: 10.1084/jem.170.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burdin N, Kooten C V, Galibert L, Abrams J S, Wijdenes J, Banchereau J, Rousset F. J Immunol. 1995;154:2533–2544. [PubMed] [Google Scholar]
- 25.Li L, Young D, Wolf S F, Choi Y S. Cell Immunol. 1996;168:133–140. doi: 10.1006/cimm.1996.0059. [DOI] [PubMed] [Google Scholar]