Emotion, olfaction, and the human amygdala: Amygdala activation during aversive olfactory stimulation (original) (raw)
Abstract
Electrophysiologic and lesion studies of animals increasingly implicate the amygdala in aspects of emotional processing. Yet, the functions of the human amygdala remain poorly understood. To examine the contributions of the amygdala and other limbic and paralimbic regions to emotional processing, we exposed healthy subjects to aversive olfactory stimuli while measuring regional cerebral blood flow (rCBF) with positron emission tomography. Exposure to a highly aversive odorant produced strong rCBF increases in both amygdalae and in the left orbitofrontal cortex. Exposure to less aversive odorants produced rCBF increases in the orbitofrontal cortex but not in the amygdala. Change of rCBF within the left amygdala and the left OFC was highly intercorrelated, indicating a strong functional interaction between these brain regions. Furthermore, the activity within the left amygdala was associated significantly with subjective ratings of perceived aversiveness. These findings provide evidence that the human amygdala participates in the hedonic or emotional processing of olfactory stimuli.
Keywords: positron emission tomography, orbitofrontal, brain blood flow, brain mapping, affect
How the human brain processes emotions is unclear. Studies using electrophysiologic and lesion techniques suggest that the amygdala plays a crucial role in emotional processing in mammals (1, 2). Amygdala lesions critically disrupt the development and expression of conditioned fear in rodents (3). Nonhuman primates with amygdala lesions demonstrate abnormal emotional responses to biologically significant stimuli (Kluver–Bucy syndrome), including marked reductions in the expression of fear and aggression (4). Single cell studies of the amygdala in nonhuman primates indicate that the activity of many amygdala cells depends on the hedonic significance of stimuli (5, 6). These cells do not respond to sensory stimuli per se but to stimuli with unconditioned or conditioned aversive (punishing) qualities. Such research suggests that the amygdala may play an important role in emotional processing and psychopathology in humans. However, an understanding of the functions of the human amygdala has proven elusive.
Data regarding the role of the amygdala in humans remain scarce and are largely limited to case studies of patients with neurological conditions. The observation that electrical stimulation and seizures focused on the human amygdala frequently produce fear or other emotional responses provides strong evidence implicating the amygdala in emotional processing in humans (7, 8). However, lesions of the amygdala in humans rarely produce the constellation of emotional abnormalities associated with lesions of the amygdala in nonhuman primates, except when amygdala damage occurs in conjunction with diffuse cerebral disease (9). Recently, several cases of selective amygdala lesions due to Urbach–Wiethe syndrome have been reported. Studies of these patients indicate that bilateral amygdala lesions cause impairments in storing or recalling emotional memories, selective impairments in the recognition of fearful (but not positive) facial expressions, and impairments in cross-modal associations of olfactory and visual stimuli (10–12).
The anatomy and behavioral features of olfactory processing suggest that hedonically valenced olfactory stimuli may act as useful probes for studying limbic regions. The perception of smell is dominated by a hedonic (pleasantness–unpleasantness) dimension, and exposure to odorants produces robust approach and withdrawal responses (13, 14). For example, the smell of smoke can evoke potently either fear and withdrawal responses or happiness and approach responses, depending on the circumstances surrounding odor perception. Such phenomena reflect the inextricable anatomical connections between the mammalian limbic and olfactory systems. The primary olfactory cortex (POC) is continuous with the anterior portion of the amygdala and projects directly to the amygdala and posterior orbitofrontal cortex (OFC) as well as perirhinal, entorhinal, and insular cortices (15, 16). Approximately 40% of the neurons in the rodent amygdala respond to olfactory stimulation (17). Despite the amygdala’s diminishing role in olfaction during evolution (18–20), primates retain direct projections from the lateral olfactory tract to the anterior cortical nucleus of the amygdala, and the medial nucleus of the amygdala remains intimately connected with the POC (15). As such, olfaction is the only exteroceptive sensory modality possessing direct bidirectional projections between the amygdala and primary sensory cortex. This anatomy suggests a high level of functional connectivity between the olfactory and limbic systems. Not surprisingly, the medial amygdala has been observed to increase its firing during the inhalation of odorants as measured electrophysiologically in conscious monkeys and humans (21, 22).
Based on evidence cited above, we hypothesized that odorants with strong hedonic qualities would activate the human amygdala and other limbic or paralimbic regions receiving olfactory input. To test this, we exposed healthy subjects to aversively valenced olfactory stimuli while regional cerebral blood flow (rCBF), a marker of neuronal activity, was measured with positron emission tomography (PET).
METHODS
Subjects.
Twelve healthy women (ages 19–49 years, all right-handed) were exposed to a highly aversive odorant (a mixture of sulfide gasses) while cerebral activity was assayed through measurement of rCBF with PET. Informed consent followed procedures approved by the Veterans Affairs Medical Center Institutional Human Studies Committee and Radioactive Drug Research Committee. Two subjects were excluded from group subtraction analysis of the highly aversive condition because they failed to meet the a priori cutoff for an aversive response (rating of less than 4 on a scale described below). However, these two subjects were included in correlational analyses.
Materials and Experimental Procedure.
The sulfide cocktail (25 ppm each of dimethyl sulfide, ethanethiol, and methanethiol) was delivered from a 1-liter plastic bag with the outlet positioned ≈15 cm from the nostrils. Gas release began upon the start of radiotracer infusion and continued through the first 60 s of scan acquisition. The concentration of sulfides was below the level expected to produce trigeminal activation, and no subjects reported nasal irritation. To examine whether less aversive stimuli produce similar rCBF changes, eight of the subjects were scanned using identical imaging techniques while smelling four mild to moderately aversive stimuli. Scents were selected according to individual and normative ratings of unpleasantness from the University of Pennsylvania Smell Identification Test (UPSIT) (23). The set of four UPSIT odorants, each applied for 8 s, was presented in sequence two times during the scan. The control condition, performed first, had no odorant. Subjects were instructed for all three conditions as follows: “Close your eyes. Breathe through your nose, and see if you can smell anything.” After each scan, subjects rated the odorant for pleasantness–unpleasantness (visual analog scale of 0–10, with: 0, extremely aversive; 5, neutral; and 10, extremely pleasant) and intensity (visual analog scale of 0–10 with: 0, undetectable; and 10, extremely intense).
Imaging and Analysis.
Blood flow was estimated from the normalized (1000-count) tissue radioactivity (after correction with measured 2-dimensional attenuation) using a Siemens ECAT 953B camera (Knoxville, TN) with septae retracted; a slow bolus injection of H215O [814 MBq or 22 mCi (1 Ci = 37 GBq) initial dose infused at a constant rate over 30 s] (24), a 90-s scan acquisition beginning upon radiotracer arrival into the brain; and a 10-minute interscan interval. Images were reconstructed using a 3-dimensional reconstruction algorithm with a Hann filter (0.5 cycle/pixel) (25). Measured coincidences were corrected for randoms and electronic dead time; no corrections were made for decay or scatter. Software developed and provided by Minoshima and coworkers (26–28) enabled: normalization of global activity; coregistration within each study session; placement of the intercommissural line from image fiducials; and nonlinear warping of each subject’s scans to a reference stereotactic atlas (29). analyze (BRU, Mayo Foundation, Rochester, MN) was used for image display. Final image resolution was ≈12 mm full width at half maximum. It is important to distinguish image resolution (here approaching the size of the human amygdala) from brain mapping resolution (approaching 2–3 mm with these methods) (30). Although two activation foci separated by less than the image resolution cannot be resolved, the peak of a single activation focus can be mapped accurately well below the image resolution.
RESULTS
Analysis of psychoperceptual ratings indicated that the subjects rated the sulfides as highly aversive (mean = 1.3, SD = 1.2) and highly intense (mean = 8.6, SD = 1.4). They most frequently described the odor as smelling like rotting vegetables and reported increased muscle tension, repulsion, disgust, or fear that the gasses were dangerous. UPSIT odorants were rated significantly less aversive [mean = 2.8, SD = 1.6; _t_2-tail(16) = 2.3, P < 0.04] and less intense [mean = 5.8, SD = 1.3, _t_2-tail(16) = 4.1, P < 0.001] than the sulfide mixture.
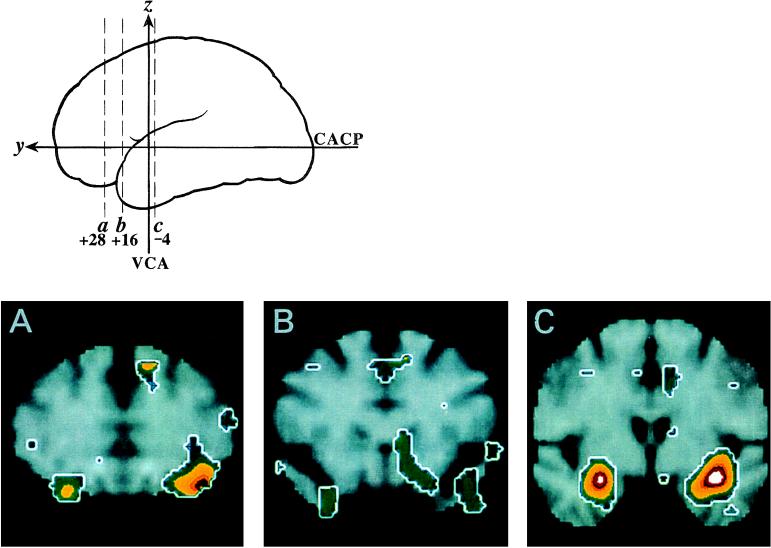
The most significant rCBF increases during the highly aversive sulfide condition relative to the no odorant condition are listed in Table 1 and are depicted in Fig. 1. The two largest responses occurred bilaterally within the amygdala (Fig. 1C). Activation within the left hemisphere included the amygdala, as well as areas lateral to the amygdala extending to the inferior insula. A significant additional focus mapped within the left posterior–lateral OFC (Fig. 1A). Fig. 1A displays the presence of a similar area of activation in the right OFC (x = 28, y = 28, _z_= −18; Z score = 3.1), but this failed to reach conservative levels of statistical significance. A weaker bilateral band of activity extended from anterior to posterior along the ventral surface of the frontal lobe to the junction of the frontal and temporal lobes (Fig. 1 A and B). This area is consistent with the localization of POC as defined by histological studies and overlaps with the location of human POC visualized previously by PET (15, 31). A post hoc analysis using a region of interest (ROI) with a 5-mm sphere placed on the coordinates of the POC identified with PET by Zatorre et al. (31) demonstrated increased rCBF [right POC, _t_2-tail(9) = 2.6, P < .05; left POC, _t_2-tail(9) = 2.3, P < .05]; POC activation did not reach the conservative threshold used to correct for multiple comparisons.
Table 1.
Locations of increased blood flow during aversive olfactory stimulation by sulfide odorants
| Area | x | y | z | Z score |
|---|---|---|---|---|
| Left amygdala | −34 | −4 | −11 | 5.2 |
| Right amygdala | 26 | −1 | −14 | 5.0 |
| Left orbitofrontal cortex (BA 11) | −42 | 35 | −14 | 4.7 |
Figure 1.
Cerebral activation during aversive olfaction. Changes in rCBF are rendered in color with white indicating the greatest magnitude (Z score > 5) of activation. The relative positions of coronal sections (A, B, and C) through the frontal (a and b) and temporal (c) lobes are shown schematically in the upper left. Maximal areas of rCBF change are displayed superimposed on a standard T-1-weighted magnetic resonance image. The rCBF maxima map to the amygdala bilaterally and the left posterior lateral OFC. The right side of this figure shows the left side of the brain. VCA, vertical line through anterior commissure; CACP, intercommissural line.
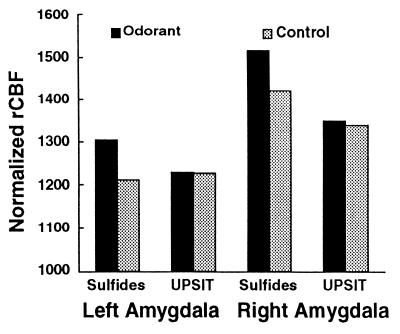
When subjects were exposed to the mildly aversive UPSIT odorants, a significant increase in rCBF again was observed in the left OFC (x = −24, y = 28, z = −11; Z score = 4.2). However, activity within the amygdala did not increase significantly over the control condition (see Fig. 2). No other areas of rCBF reached statistical significance in this condition. Fig. 2 also shows the asymmetrical pattern of rCBF within both stimulation and control conditions. Even during odor detection (in which no odorant was presented), the right amygdala showed greater activity than the left amygdala. The OFC demonstrated a similar asymmetrical pattern of activity. In both stimulation and control conditions, the right OFC focus showed ≈25% higher rCBF values than the left OFC focus (all paired _t_2-tail, P < .005). Thus, left OFC activation was greater than right OFC activation in the contrast between the conditions of aversive odorant and control although right OFC rCBF was significantly greater than left OFC rCBF across all conditions.
Figure 2.
Mean rCBF in the left and right amygdala during odorant (sulfides and UPSIT) and control (no odorant) conditions. Normalized rCBF was estimated as normalized regional tissue activity (counts). Both amygdalae showed significantly increased rCBF for the highly aversive sulfides but did not change significantly for the milder UPSIT odorants.
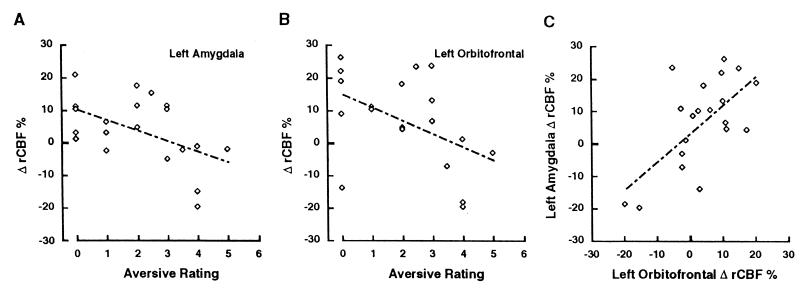
The data from both aversive conditions (sulfides and UPSIT odorants) were pooled and submitted to correlational analysis to test the hypothesis that rCBF change was related to the subjective ratings of unpleasantness. Data from both the sulfide and UPSIT stimuli (12 and 8 scan pairs, respectively) were combined for this analysis to provide a range of unpleasantness ratings and to provide a sample size with adequate statistical power. The difference in amygdala rCBF between odorant and nonodor conditions was calculated for each subject’s scan pairs by averaging the difference in rCBF in each pixel (after normalization for global activity and anatomy as above) within an ROI (sphere, 5 mm radius) centered upon the peak coordinates of the amygdala and left OFC activation from Table 1. Increases in rCBF within the left amygdala correlated significantly with decreases in numerical scores, denoting greater perceived unpleasantness (r = −0.51, P < 0.05) (Fig. 3_A_). In contrast, the correlation between rCBF change in the right amygdala and affective ratings did not reach significance (_r_ = −0.20, _P_ > 0.10). No significant association was observed between ratings of odor intensity and rCBF change in either amygdala (both P > 0.05). A methodological problem arises in these correlational analyses because eight subjects contributed more than one scan pair to the analyses, which compromises the statistical assumption of independent observations. The appropriateness of this practice remains unclear, although investigators frequently treat each scan pair as if it were an independent observation. A more conservative approach to this problem involves taking just one scan pair from each subject. To accomplish this, we used whichever odorant condition came first in the eight cases in which subjects received both the sulfide and the UPSIT conditions, resulting in a total of 12 scan pairs (8 sulfides, 4 UPSIT). rCBF change in the left amygdala remained significantly correlated with ratings of unpleasantness (r = −0.56, P < 0.05). Finally, correlational analyses of rCBF change in the left amygdala and ratings of unpleasantness were performed separately for the UPSIT and sulfide conditions to ensure that the observed correlations did not reflect an artifact of combining the different methods of stimulus presentation. These analyses produced correlations in the same direction with equal or greater magnitude to those produced by the combined data set (r = −0.50 in the sulfide condition and r = −0.65 for the UPSIT condition), but neither reached statistical significance because of the small sample sizes (n = 12 and 8, respectively). The peak activation of the left amygdala was slightly lateral to the coordinates of the amygdala in the atlas of Talairach and Tournoux (29), so a final post hoc analysis was performed with a 5-mm ROI placed on the center of the left amygdala in the atlas (x = −24, y = −3, z = −12). The results of this analysis were extremely similar to those produced by centering the ROI on the peak pixel, with the one exception that the correlation between perceived intensity and amygdala activation reached statistical significance (r = 0.62, P < 0.01). This may reflect the tight association between ratings of intensity and perceived unpleasantness that typifies psychoperceptual ratings of odorants (14).
Figure 3.
Post hoc correlational analyses of rCBF change and subjective scores (low scores indicate high aversion). Correlations are shown between subjective ratings of aversiveness and left amygdalar rCBF (A) and left OFC rCBF (B) changes and between left amygdalar and left OFC rCBF changes (C). There were no significant correlations between rCBF in these regions of interest and subjective ratings of odor intensity.
Activation of the left OFC also significantly correlated with ratings of unpleasantness (r = −0.46, P < 0.05 (Fig. 3_b_) but not with ratings of intensity (_r_ = 0.30, _P_ > 0.10). A similar pattern of results was observed when the sulfides and the UPSIT conditions were analyzed separately (r = −0.41 and r = −0.63, respectively) and when only the first scan pairs (8 sulfides, 4 UPSITs) were used to insure independence of observations (r = −0.44). However, none of these correlations reached statistical significance. Change in rCBF in the left OFC correlated significantly with rCBF change in the left amygdala (r = 0.65, P < 0.005; Fig. 3C) but did not correlate with rCBF change in the right amygdala (r = −0.07, P = 0.5). The correlation between the left OFC and left amygdala remained significant when the sulfides (r = 0.68, P < 0.05) and the UPSIT (r = 0.73, P < 0.05) conditions were examined in isolation.
DISCUSSION
The present study demonstrates large increases in amygdala activity bilaterally during exposure to aversive odorants. This represents the most statistically robust demonstration of amygdala activation observed to date using PET in humans. Several methodological issues must be considered in interpreting the current results.
First, although activation of the right amygdala centered on the expected coordinates in the Talairach atlas, increased rCBF in the left amygdala region extended laterally into the inferior insula. The peak of increased rCBF actually localized to a region slightly lateral to the Talairach boundaries of the amygdala. However, a wide range of individual variation exists in the size and exact location of the human amygdala, which is not reflected in the atlas. Also, current PET methods may not resolve independent foci in amygdala and immediately adjacent insula. Nevertheless, rCBF increased significantly in a small ROI centered upon the Talairach atlas coordinates of the amygdala, and these increases correlated with the perceived unpleasantness of the stimuli.
The complex responses to aversive odorants further complicate interpretation of amygdala activation. Subjects frequently reported increased muscle tension when exposed to the sulfides. Some subjects reported attempting to change their breathing to reduce inhalation of the aversive odorants. No formal measures of autonomic, visceral, or respiratory functions were assayed as part of this study. Because the amygdala receives interoceptive afferents and may play a role in autonomic functions (2, 32), a plausible alternate interpretation concerns the participation of the amygdala in respiration and autonomic regulation. Lesions of the amygdala block conditioned respiratory responses to aversive stimuli (33). Neurophysiological studies of nonhuman primates and other mammals indicate that stimulation of the amygdala can produce changes in respiration (2, 34). Cells in the amygdala fire in relation to respiration (35) although the proportion of these cells in humans appears to be smaller than in other mammals (36). However, amygdala activation has not been observed in previous neuroimaging studies in which subjects were instructed to volitionally alter their breathing (37, 38) nor has amygdala activation occurred as a primary response in other studies involving respiratory or autonomic changes (39, 40). Furthermore, preliminary experiments in our laboratory in which subjects underwent gastric dilation (which produces robust vagal stimulation, difficulty breathing, and other autonomic responses) failed to produce significant changes in amygdala rCBF (J.V.P., S. W. Kim, P. L. Faris, B. K. Hartman, and R. L. Goodale, unpublished observations). Although autonomic or visceral components cannot be ruled out absolutely, these factors alone probably do not account for the robust amygdala activity observed by exposure to aversive odorants.
The correlation between ratings of unpleasantness and changes in rCBF in the left amygdala suggests that neuronal activity in these regions is directly related to (or dependent on) the perceived hedonic valence of the stimuli. This result and its interpretation need qualification because of two methodological issues. First, the UPSIT and sulfide conditions differed in the type, intensity, and number of stimuli used in each condition (four stimuli presented twice vs. one stimulus). However, when the data were analyzed separately for these conditions, the correlations were of equal or greater magnitude. Furthermore, when subjects rated the UPSIT as highly aversive (ratings of 0–2), amygdala rCBF increased by 2–6%, indicating that increased amygdala rCBF did not result from some specific characteristic of the sulfides. Second, the observed correlations between changes in rCBF and ratings of unpleasantness do not necessarily imply a simple linear relationship between left amygdala activity and psychoperceptual ratings. Of interest, both subjects who were excluded a priori from the group analysis of the sulfide condition because they failed to perceive the sulfides as highly aversive did not show increases in left amygdala rCBF, and one actually had a strong (15%) decrease in left amygdala rCBF. Similarly, the three subjects who rated the UPSIT as only mildly unpleasant or neutral (ratings of 3.5 or higher) had either decreases or no change in left amygdala rCBF. Two different types of responses may thus occur in the left amygdala during aversive olfaction: activation for highly aversive odors and deactivation for neutral or mildly aversive odors.
To further examine whether amygdala responses to olfactory stimuli are influenced by hedonic valence, we conducted additional PET studies using pleasant odorants (fruits, spices, and florals; unpublished observations). A statistically nonsignificant increase in rCBF in the right anterior amygdala/periamygdala region occurred in response to these odorants. The increase did not localize as clearly to the amygdala and occurred inconsistently. No significant increases localized to the left amygdala during stimulation with pleasant odorants. The lack of strong amygdala activation in these pleasant conditions concurs with a previous PET study that failed to observe amygdala activation during exposure to relatively neutral and pleasant stimuli (31). These results suggest that amygdala activity (especially left amygdala activity) is not simply a consequence of olfactory perception per se. Rather, the hedonic valence of the odorant influences amygdala activity. The greater ability of aversive than neutral or positive odorants to activate the amygdala is consistent with studies of electrical stimulation and of selective lesions in humans, suggesting greater amygdala involvement in negative than positive emotions (7, 8, 10). These data also converge with studies reporting aversive olfactory hallucinations during amygdala seizures and during electrical stimulation of the amygdala (41–43).
Because of the methodological issues raised above, the potentially different roles of the left and right amygdalae require further characterization. Both amygdalae showed robust increases in rCBF during exposure to highly aversive odorants. Nevertheless, our finding that rCBF change in the left amygdala (but not the right amygdala) correlated with ratings of unpleasantness converges with a recent report by Ketter et al. (44). They observed that, despite bilateral amygdala activation in subjects experiencing procaine-induced fear, only left amygdala activation correlated with subjective ratings of fear. Similarly, PET and fMRI studies have reported increased activity in left, but not right, ROIs placed on the amygdalae of subjects exposed to negatively valenced (sad) faces (45, 46). Consistent with these neuroimaging data, subjects with left amygdala lesions rate facial expressions of disgust and sadness as slightly less intense than those with right amygdala lesions (although both ratings fell within the range produced by controls with brain damage) (47). These data also converge with evidence that clinically depressed patients scanned while resting may show elevated left amygdala rCBF, which correlates with depression severity (48). Thus, despite methodological limitations, the present correlational analyses appear quite consistent with an emerging body of evidence identifying a close relationship between left amygdala activity and negative affect.
The asymmetry in OFC activation is of interest in relation to previous studies of olfaction in humans. OFC lesions in humans, especially involving the right hemisphere, produce deficits in olfactory discrimination and recognition (49, 50). Zatorre et al. (31) reported that human subjects exposed to a series of pleasant, neutral, and mildly aversive stimuli showed statistically significant activation in the right, but not in the left, OFC. As can be seen from Fig. 1A, the OFC was activated bilaterally, but only the left side reached conservative levels of statistical significance. An important difference between the present study and Zatorre’s study concerns the control condition: They told the subjects that no odor would be applied in the control condition (Zatorre, R. J., personal communication); we instructed the subjects to try to smell something, and we did not disclose the absence of odorant. Thus, subtle differences in control conditions may account for the present study’s lower level of right OFC rCBF increases. Although rCBF in the right OFC did not increase as dramatically between conditions, the rCBF in the right OFC actually exceeded the level of rCBF in the left OFC within both the control and stimulation conditions. Thus, although the left OFC responds more dramatically to aversive stimuli relative to attempting to detect an odorant, the high level of activity in the right OFC in both odor detection and aversive olfaction converges with previous lesion and PET data demonstrating the importance of the right OFC in basic aspects of olfactory processing. A similar asymmetrical involvement in different aspects of olfactory processing may also explain the consistently greater right than left amygdala rCBF within the control and stimulation conditions (see Fig. 2). Taken together, these data suggest that right-sided regions may become activated more than left-sided regions when attempting to detect an odorant, even if no odorant is present. The lack of a significant change in rCBF in the POC between the control and stimulation might similarly reflect a heightened activation of the POC during the odor detection control condition.
The consistently high correlation between left amygdala and left OFC rCBF suggests the presence of an important functional interaction between the left amygdala and OFC during the processing of aversive olfactory stimuli. This interaction is consistent with the dense anatomical connections between the regions (32, 51) and with previous observations that OFC lesions produce alterations in emotional behavior that closely resemble many of the behavioral abnormalities arising from amygdala lesions in nonhuman primates (reviewed in ref. 52). Nevertheless, these two structures likely play distinct roles during aversive olfaction given their dissimilar responses to the UPSIT stimuli. The methodological and perceptual features affecting the differential amygdala and OFC responses in the milder UPSIT condition require further investigation. Of interest, exposure to the sulfide mixture caused more fear, disgust, and desire to withdraw than exposure to UPSIT odorants. Fear or disgust might thus be necessary to induce significant rCBF increases in the amygdala but not in OFC. Experiments are planned to directly test this hypothesis.
Despite the amygdala’s diminishing role in olfactory processing during phylogeny, the current study shows that the human amygdala plays a fundamental role in olfaction. Olfactory perception robustly engages emotional processes. Although future research will be necessary to tease apart the specific factors contributing to increased rCBF in the amygdala, the current data demonstrate substantial amygdala activation during olfaction of highly aversive odorants. These findings support a critical role of the human amygdala in either the processing of aversive olfactory stimuli or the transduction of neural signals from smells into emotional responses.
Acknowledgments
We thank the technical staff of the PET Imaging Service; Michael Levitt and John Springfield of the Minneapolis Veterans Affairs Medical Center Research Service for preparing gas samples; Satoshi Minoshima (University of Michigan) for providing analysis software; Joel Lee, Humberto Temporini, and Patricia Pardo for assistance and advice; our volunteer subjects for their patience and generosity; and the anonymous reviewers for their helpful comments. This research was supported by the Department of Veterans Affairs; the Minnesota Medical Foundation; and the University of Minnesota (Grants-in-Aid of Research, Artistry, and Scholarship; Eva O. Miller Fellowship to D.H.Z.).
ABBREVIATIONS
OFC
orbitofrontal cortex
PET
positron emission tomography
POC
primary olfactory cortex
rCBF
regional cerebral blood flow
ROI
region of interest
UPSIT
University of Pennsylvania Smell Identification Test
Note Added in Proof
Since this manuscript was submitted, additional data have been reported supporting the critical role of the human amygdala for processing stimuli with negative emotional properties (53–55).
References
- 1.Aggleton J P. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley–Liss; 1992. [Google Scholar]
- 2.LeDoux J E. In: Handbook of Physiology. Plum F, Mountcastle V B, editors. Vol. 5. Bethesda, MD: Am. Physiol. Soc.; 1987. pp. 419–459. [Google Scholar]
- 3.Davis M. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 4.Kluver H, Bucy P C. Am J Physiol. 1937;119:352–353. [Google Scholar]
- 5.Ono T, Nishijo H. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton J P, editor. New York: Wiley–Liss; 1992. pp. 167–190. [Google Scholar]
- 6.Rolls E T. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton J P, editor. New York: Wiley–Liss; 1992. pp. 143–166. [Google Scholar]
- 7.Gloor P. Brain. 1990;113:1673–1694. doi: 10.1093/brain/113.6.1673. [DOI] [PubMed] [Google Scholar]
- 8.Halgren E. Hum Neurobiol. 1982;1:251–260. [PubMed] [Google Scholar]
- 9.Marlowe W B, Mancall E L, Thomas J J. Cortex. 1975;11:53–59. doi: 10.1016/s0010-9452(75)80020-7. [DOI] [PubMed] [Google Scholar]
- 10.Adolphs R, Tranel D, Damasio H, Damasio A R. Nature (London) 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 11.Cahill L, Babinsky R, Markowitsch H J, McGaugh J L. Nature (London) 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 12.Markowitsch H J, Calabrese P, Wurker M, Durwn H F, Kessler J, Babinsky R, Brechtelsbauer D, Heuser L, Gehlen W. NeuroReport. 1994;5:1349–1352. [PubMed] [Google Scholar]
- 13.Engen T. Am Sci. 1987;75:497–503. [Google Scholar]
- 14.Richardson J E, Zucco G M. Psychol Bull. 1989;105:352–360. doi: 10.1037/0033-2909.105.3.352. [DOI] [PubMed] [Google Scholar]
- 15.Price J L. In: The Human Nervous System. Paxinos G, editor. New York: Academic; 1991. pp. 979–998. [Google Scholar]
- 16.Carmichael S T, Clugnet M C, Price J L. J Comp Neurol. 1994;346:403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- 17.Cain D P, Bindra D. Exp Neurol. 1972;35:98–110. doi: 10.1016/0014-4886(72)90062-3. [DOI] [PubMed] [Google Scholar]
- 18.Cragg B G. Exp Neurol. 1960;2:547–572. doi: 10.1016/0014-4886(60)90031-5. [DOI] [PubMed] [Google Scholar]
- 19.Stephan H, Frahm H D, Baron G. J Hirnforsch. 1987;28:571–584. [PubMed] [Google Scholar]
- 20.Halgren E, Babb T L, Rausch R, Crandall P H. Neurosci Lett. 1977;4:331. doi: 10.1016/0304-3940(77)90179-3. [DOI] [PubMed] [Google Scholar]
- 21.Narabayashi, H. (1980) Acta. Neurochir. 30, Suppl., 75–81. [DOI] [PubMed]
- 22.Tanabe T, Iino M, Takagi S F. J Neurophysiol. 1975;38:1284–1296. doi: 10.1152/jn.1975.38.5.1284. [DOI] [PubMed] [Google Scholar]
- 23.Doty R L, Shaman P, Dann M. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 24.Silbersweig D A, Stern E, Frith C D, Cahill C, Schnor L, Grootoonk S, Spinks T, Clark J, Frackowiak R S, Jones T. J Cereb Blood Flow Metab. 1993;13:617–629. doi: 10.1038/jcbfm.1993.80. [DOI] [PubMed] [Google Scholar]
- 25.Kinahan P E, Rogers J G. IEEE Trans Nucl Sci. 1988;36:964–986. [Google Scholar]
- 26.Minoshima S, Koeppe R A, Frey K A, Kuhl D E. J Nucl Med. 1994;35:1528–1537. [PubMed] [Google Scholar]
- 27.Minoshima S, Koeppe R A, Mintun M A, Berger K L, Taylor S F, Frey K A, Kuhl D E. J Nucl Med. 1993;34:322–329. [PubMed] [Google Scholar]
- 28.Minoshima S, Berger K L, Lee K S, Mintun M A. J Nucl Med. 1992;33:1579–1585. [PubMed] [Google Scholar]
- 29.Talairach J, Tournoux P. Coplanar Stereotactic Atlas of the Human Brain. New York: Theime; 1988. [Google Scholar]
- 30.Fox P T, Mintun M A, Raichle M E, Miezin F M, Allman J M, Van Essen D C. Nature (London) 1986;323:806–809. doi: 10.1038/323806a0. [DOI] [PubMed] [Google Scholar]
- 31.Zatorre R J, Jones-Gotman M, Evans A C, Meyer E. Nature (London) 1992;360:339–340. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]
- 32.Amaral D G, Price J L, Pitkänen A, Carmichael S T. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton J P, editor. New York: Wiley–Liss; 1992. pp. 1–66. [Google Scholar]
- 33.Zhang J X, Harper R M, Ni H F. Brain Res. 1986;386:136–145. doi: 10.1016/0006-8993(86)90150-2. [DOI] [PubMed] [Google Scholar]
- 34.Harper R M, Frysinger R C, Trelase R B, Marks J D. Brain Res. 1984;306:1–8. doi: 10.1016/0006-8993(84)90350-0. [DOI] [PubMed] [Google Scholar]
- 35.Radna R J, MacLean P D. Brain Res. 1981;213:45–61. doi: 10.1016/0006-8993(81)91247-6. [DOI] [PubMed] [Google Scholar]
- 36.Frysinger R C, Harper R M. Electroencephalogr Clin Neurophysiol. 1989;72:463–470. doi: 10.1016/0013-4694(89)90222-8. [DOI] [PubMed] [Google Scholar]
- 37.Colebatch J G, Adams L, Murphy K, Martin A J, Lammertsma A A, Tochon-Danguy H J, Clark J C, Friston K J, Guz A. J Physiol (London) 1991;443:91–103. doi: 10.1113/jphysiol.1991.sp018824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramsay S C, Adams L, Murphy K, Corfield D R, Grootoonk S, Bailey D L, Frackowiak R S, Guz A. J Physiol (London) 1993;461:85–101. doi: 10.1113/jphysiol.1993.sp019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rauch S L, Savage R, Alpert N, Euripedes C M, Baer L, Breiter H C, Fischman A J, Manzo P A, Moretti C, Jenike M A. Arch Gen Psychiatry. 1995;52:20–28. doi: 10.1001/archpsyc.1995.03950130020003. [DOI] [PubMed] [Google Scholar]
- 40.Minoshima, S. Morrow, T. J., Koeppe, R. A. & Casey, K. L. (1995) Hum. Brain Mapp. 1, Suppl., 166.
- 41.Andy O J. Electroencephalogr Clin Neurophysiol. 1967;23:292. [PubMed] [Google Scholar]
- 42.Andy O J, Jurko M F, Hughes J R. Confin Neurol. 1975;37:215–222. doi: 10.1159/000102743. [DOI] [PubMed] [Google Scholar]
- 43.Chitanodh H. Confin Neurol. 1966;27:181–196. doi: 10.1159/000103952. [DOI] [PubMed] [Google Scholar]
- 44.Ketter T A, Andreason P J, George M S, Lee C, Gill D S, Parekh P I, Willis M W, Herscovitch P, Post R M. Arch Gen Psychiatry. 1996;53:59–69. doi: 10.1001/archpsyc.1996.01830010061009. [DOI] [PubMed] [Google Scholar]
- 45.Schneider F, Gur R E, Mozley L H, Smith R J, Mozley P D, Censits D M, Alavi A, Gur R C. Psychiatry Res Neuroimaging. 1995;6:265–283. doi: 10.1016/0925-4927(95)02678-q. [DOI] [PubMed] [Google Scholar]
- 46.Grodd W, Schneider F, Klose U, Nagele T. Radiologe. 1995;35:283–289. [PubMed] [Google Scholar]
- 47.Adolphs R, Tranel D, Damasio H, Damasio A R. J Neurosci. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drevets W C, Videen T O, Preskorn S H, Price J L, Carmichael S T, Raichle M E. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zatorre R J, Jones-Gotman M. Brain. 1991;114:71–84. [PubMed] [Google Scholar]
- 50.Jones-Gotman M, Zatorre R J. Brain Cognit. 1993;22:182–198. doi: 10.1006/brcg.1993.1033. [DOI] [PubMed] [Google Scholar]
- 51.Zald D H, Kim S W. J Neuropsychiatry Clin Neurosci. 1996;8:125–138. doi: 10.1176/jnp.8.2.125. [DOI] [PubMed] [Google Scholar]
- 52.Zald D H, Kim S W. J Neuropsychiatry Clin Neurosci. 1996;8:249–261. doi: 10.1176/jnp.8.3.249. [DOI] [PubMed] [Google Scholar]
- 53.Morris J S, Frith C D, Perret D I, Rowland D, Young A W, Calder A J, Dolan R J. Nature (London) 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 54.Breitner H C, Etcoff N L, Whalen P J, Kennedy W A, Rauch S L, Buckner R L, Strauss M M, Hyman S E, Rosen B R. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 55.Scott S K, Young A W, Calder A J, Hellawell D J, Aggleton J P, Johnson M. Nature (London) 1997;385:254–257. doi: 10.1038/385254a0. [DOI] [PubMed] [Google Scholar]