BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin–β-catenin interactions (original) (raw)
Abstract
Neurons of the vertebrate central nervous system have the capacity to modify synapse number, morphology, and efficacy in response to activity. Some of these functions can be attributed to activity-induced synthesis and secretion of the neurotrophin brain-derived neurotrophic factor (BDNF); however, the molecular mechanisms by which BDNF mediates these events are still not well understood. Using time-lapse confocal analysis, we show that BDNF mobilizes synaptic vesicles at existing synapses, resulting in small clusters of synaptic vesicles “splitting” away from synaptic sites. We demonstrate that BDNF's ability to mobilize synaptic vesicle clusters depends on the dissociation of cadherin–β-catenin adhesion complexes that occurs after tyrosine phosphorylation of β-catenin. Artificially maintaining cadherin–β-catenin complexes in the presence of BDNF abolishes the BDNF-mediated enhancement of synaptic vesicle mobility, as well as the longer-term BDNF-mediated increase in synapse number. Together, this data demonstrates that the disruption of cadherin–β-catenin complexes is an important molecular event through which BDNF increases synapse density in cultured hippocampal neurons.
Introduction
Synaptic plasticity, both in the short- and long-term, enables the brain to adapt in response to environmental inputs. Substantial evidence indicates that the strength, number, and morphology of synapses can be changed by neuronal activity. Indeed, stimulation of hippocampal slices under conditions that induce long-term potentiation (LTP) not only increases the efficacy of individual synapses but also increases overall synapse number, the length of active zones (for review see Lu, 2003), and the number and length of active zones (Applegate et al., 1987; Meshul and Hopkins, 1990); it also modifies spine morphology (Nikonenko et al., 2002).
It is now widely accepted that neuronal activity enhances local synthesis and secretion of neurotrophins, most notably brain-derived neurotrophic factor (BDNF), which in turn play a crucial role in synaptic transmission and plasticity (Lu, 2003; Bramham and Messaoudi, 2005). Acute application of BDNF has been found to rapidly enhance synaptic transmission and transmitter release and to mediate increased synapse sprouting, which is similar to that seen after strong activity (Jovanovic et al., 2000). Mice expressing reduced levels of BDNF exhibit a dramatic decrease in the number of docked vesicles per synapse, pronounced synaptic fatigue, and deficits in synaptic sprouting (Pozzo-Miller et al., 1999; Genoud et al., 2004). Similarly, mice lacking the BDNF receptor TrkB exhibit a decreased number of both docked and total synaptic vesicles (SVs) per synapse, as well as a decrease in overall synapse number (Martinez et al., 1998; Luikart et al., 2005; Otal et al., 2005). TrkB receptors are localized at synapses; therefore, they are well positioned to rapidly regulate synapse form and function after activation (Drake et al., 1999; Carter et al., 2002; Nagappan and Lu, 2005). Both BDNF and TrkB mutant mice exhibit impaired induction of LTP, resulting in large part from defects in presynaptic function (Zakharenko et al., 2003).
Despite a rapid growth in this field in recent years, the molecular mechanisms that mediate changes in the structure and function of synapses remain largely unknown. Evidence suggests that classic cell adhesion molecules such as cadherins, integrins, and immunoglobulin domain–containing proteins, as well as neurexins and neuroligins, play a large role in regulating synapse formation (for review see Yamagata et al., 2003). Cadherins in particular have been well studied and shown to play a role in regulating synapse formation, function, and plasticity. Cadherins are localized at synapses just adjacent to active zones (Uchida et al., 1996) and are linked to the actin cytoskeleton via β- and α-catenin (for review see Bamji, 2005). The cadherin–catenin complex is therefore well situated to coordinate pre- and postsynaptic structural changes, as well as to facilitate the formation and maintenance of synaptic junctions. Consistent with this, cadherins and catenins have been shown to be important for localizing SVs to presynaptic compartments (Bamji et al., 2003) and for modulating the shape and formation of postsynaptic spines (Murase et al., 2002; Togashi et al., 2002). Synaptic activity has been reported to modify the conformational state of N-cadherin (Tanaka et al., 2000), and N-cadherin levels are significantly elevated during late LTP when new synapses are formed (Bozdagi et al., 2000). Furthermore, impairment of cadherin function inhibits the induction, but not the maintenance of LTP (Tang et al., 1998).
Both BDNF–TrkB and cadherin–β-catenin complexes have been shown to play an important role in regulating the number of SVs at individual presynaptic compartments, as well as regulating the overall number of synapses. Therefore, we hypothesized that the synaptic effects observed after activation of BDNF–TrkB signals might be mediated by the modulation of cadherin–β-catenin interactions. In this work, we demonstrate that acute treatment of cultured hippocampal neurons with BDNF results in a transient dispersal of SVs into perisynaptic regions, followed by a relatively sustained enhancement of SV cluster splitting. Therefore, we conclude that BDNF enhances SV mobility. Long-term treatment of neurons with BDNF resulted in an increase in both the density of SV clusters along the axon and the density of synapses, which are identified by the colocalization of SV clusters with the postsynaptic marker PSD-95. We show that BDNF treatment dissolves cadherin–β-catenin complexes by promoting the phosphorylation of β-catenin on tyrosine residue 654. Interestingly, artificially maintaining cadherin–β-catenin interactions by introducing a β-catenin point mutant that cannot be phosphorylated on this tyrosine residue (β-catenin Y654F) abolished both the enhanced mobilization of SVs and the BDNF-mediated increase in the overall density of SV clusters and synapses.
Results
SVs are the morphological hallmark of all chemical synapses, and are recruited to points of cell–cell contact during presynaptic development. Two pools of SVs have previously been identified by time-lapse analysis; a relatively stable pool that remains stationary for hours and localizes primarily at presynaptic boutons, and a mobile pool that translocates along the axon in a saltatory manner at a rate of 0.1–1 μm s−1 (Ahmari et al., 2000; Krueger et al., 2003). Stable vesicle clusters are thought to form when mobile packets of SVs become “trapped” at nascent synapses. This process is now believed to depend on signals that are activated after the interaction of cell adhesion molecules (Scheiffele et al., 2000; Biederer et al., 2002; Bamji et al., 2003).
BDNF transiently disrupts synaptic vesicle localization by dissolving cadherin–β-catenin complexes
To study the real-time effects of BDNF on SV dynamics, hippocampal neurons were transfected with the integral SV protein synaptophysin fused to GFP (synaptophysin-GFP) and imaged using time-lapse microscopy. It has previously been demonstrated that the pattern of synaptophysin-GFP expression is similar to that of endogenous synaptic vesicle proteins (Bamji et al., 2003), and that these fusion proteins do not compromise the secretory physiology of the synapse (Sankaranarayanan and Ryan, 2000). Synaptophysin-GFP is known to label both the mobile and stable pools of SVs. Two studies detailing the dynamics of SV clusters have concluded that stationary puncta typically represent synaptic sites. Consequently, to look at the dynamics of SVs at extant synapses, we focused on the SV clusters that were stably localized along the axon for the duration of our imaging (Ahmari et al., 2000; Krueger et al., 2003).
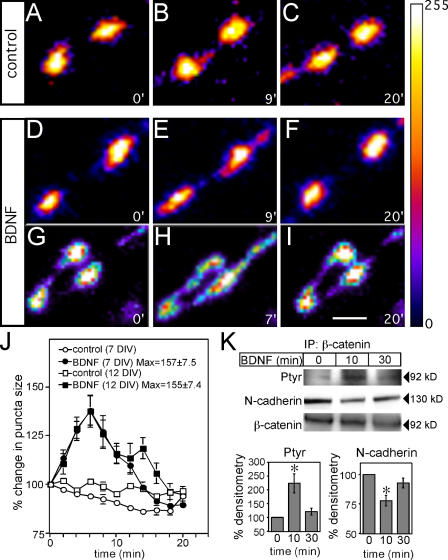
In control cultures, fluctuations in synapse size (measured as the length of the major axis of each synaptophysin-GFP puncta) were minimal (Fig. 1, A–C and J; Video 1, available at http://www.jcb.org/cgi/content/full/jcb.200601087/DC1). After acute treatment with BDNF, however, we observed a transient diffusion of SVs along the axon and into perisynaptic regions (Fig. 1, D–I and J). BDNF was added at time point 0, and left in the medium for the duration of imaging. Although the time of maximal diffusion varied from synapse to synapse, on average there was a 1.6-fold increase in the length of the major axis of stable synaptophysin-GFP puncta after BDNF treatment, both at 7 DIV, when synapses are beginning to form, and at 12 DIV, when synapses are more mature (Fig. 1 J).
Figure 1.
BDNF mediates a transient diffusion of SVs by increasing β-catenin phosphorylation and decreasing cadherin–β-catenin interactions. (A–I) Time-lapse confocal images of 12 DIV hippocampal neurons transfected with synaptophysin-GFP, pseudocolored for fluorescence intensity, and filtered in Photoshop with a Gaussian blur of 2 pixels to reduce the appearance of pixilation. Representative images show untreated cells (A–C) or cells treated with BDNF (D–I) at three time points. Numbers on bottom right of each image represent the time in minutes after the addition of media alone or 100 ng/ml BDNF to cultures. Maximal diffusion of synaptophysin-GFP fluorescence after BDNF treatment varied from puncta to puncta, and two examples are shown in E and H. (J) Quantification of the average percentage of change in the length of the major axis of each synaptophysin-GFP puncta over time ± SEM (n = at least 50 puncta). “Max” represents the average maximum length of the major axis for all puncta, irrespective of time, ± SEM. (K) BDNF induces tyrosine phosphorylation of β-catenin and decreases cadherin–β-catenin interactions. 100 ng/ml BDNF, or media alone, was added to hippocampal cultures for 10 or 30 min. Lysates were immunoprecipitated with anti–β-catenin and blotted for anti-phosphotyrosine and anti–N-cadherin. n = 6. Error bars denote ± SEM. Asterisks indicate P < 0.05. Bar, 2 μm.
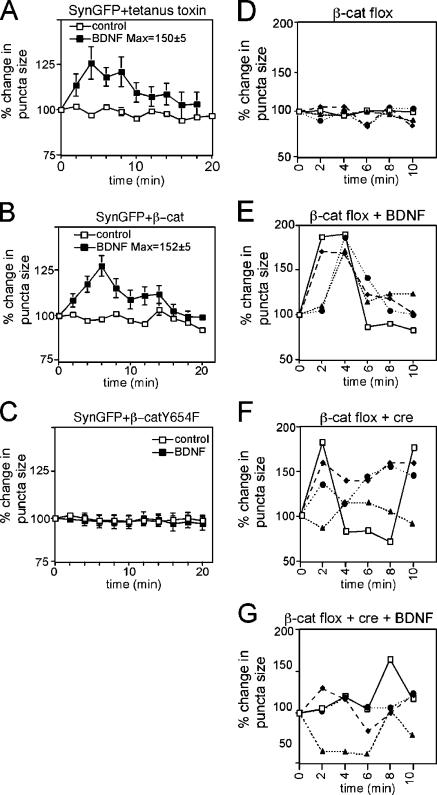
To determine whether the elongated pattern of synaptophysin-GFP expression is caused by diffusion along the axon surface after depolarization-stimulated exocytosis, which has been previously observed (Tanaka et al., 2000; Li and Murthy, 2001), cells were preincubated for 16 h with 10 nM tetanus toxin to block exocytosis. Tetanus toxin did not affect the dispersal of SVs after BDNF treatment, indicating that the elongated pattern of synaptophysin-GFP expression is caused by dispersal of SVs within the axon (Fig. 2 A).
Figure 2.
Quantitative analysis of BDNF-mediated vesicle dispersal. Graphic representation of the percentage of change in the length of the major axis of synaptophysin-GFP puncta versus time. BDNF was applied to the bath, where it is indicated at a concentration of 100 ng/ml. “Max” represents the average maximum percentage of increase in length, irrespective of time, ± SEM. (A) Treatment with tetanus toxin does not affect BDNF-induced SV cluster dispersal. Cells were preincubated in 10 nM tetanus toxin for 16 h before BDNF treatment. After treatment with BDNF, there is a transient diffusion of SVs into perisynaptic regions, as indicated by an increase in the length of synaptophysin-GFP fluorescence along the axon. n = 17 puncta over three experiments. (B and C) Phosphorylation of β-catenin at Y654 is required for BDNF-promoted vesicle dispersion. In the absence of BDNF, the size of synaptic puncta in cells expressing either wild-type β-catenin (B) or β-catenin Y654F (C) were similar to that of controls; however, expression of β-catenin Y654F abolished BDNF-induced SV dispersal (C), whereas expression of wild-type β-catenin did not significantly alter the response of SVs to BDNF (B). For A–C, n = at least 50 puncta per condition from a minimum of 10 neurons over at least three separate cultures. Error bars represent the SEM. (D–G) Dynamic regulation of synaptophysin-GFP puncta by β-catenin and BDNF. Graphic representation of the percentage of change in the length of the major axis of four representative individual synaptophysin-GFP puncta over time (representatives of a total n = 25 over three experiments were examined for each condition). Hippocampal neurons were cultured from β-catenin flox (β-catenin fl/fl) mice and transfected with synaptophysin-GFP. In the presence of β-catenin and the absence of exogenous BDNF, the sizes of each of four synaptophysin-GFP clusters were relatively stable over time (D). After treatment of β-catenin–expressing neurons with BDNF, there is a transient diffusion of SVs into perisynaptic regions as indicated by an increase in the length of synaptophysin-GFP fluorescence along the axons (E). Expression of Cre recombinase to ablate β-catenin, results in increased dynamics of SVs, as indicated by dramatic fluctuations in synaptophysin-GFP puncta length along the axon (F). Addition of BDNF to cells lacking β-catenin does not further increase the dynamics of SV cluster length (G) compared with the fluctuations observed in cells not treated with BDNF, but also lacking β-catenin (F).
We have recently shown that inhibiting cadherin–β-catenin interactions results in a diffuse pattern of synaptophysin-GFP localization along the axon (Bamji et al., 2003). Therefore, we hypothesized that BDNF may regulate vesicle clustering by disrupting cadherin–β-catenin complexes. Previous reports have shown that phosphorylation of β-catenin on tyrosine 654 by Src family kinases greatly decreases the affinity of β-catenin for cadherins (Roura et al., 1999; Piedra et al., 2001). Interestingly, we observed an increase in β-catenin tyrosine phosphorylation and a decrease in cadherin–β-catenin interactions 10 min after BDNF treatment (Fig. 1 K). β-catenin phosphotyrosine levels diminished and cadherin–β-catenin interactions were largely restored 30 min after BDNF treatment (Fig. 1 K). We have also shown the disruption of cadherin–β-catenin interactions by coimmunoprecipitation using a pan-cadherin antibody (unpublished data). Our results indicate that BDNF can transiently disrupt cadherin–β-catenin complexes with a time course similar to that of BDNF-induced SV diffusion. This experiment does not show that the synaptic pool of cadherin is specifically affected; however, it is well documented that TrkB is expressed at synapses (Drake et al., 1999) and that the majority of cadherin and β-catenin are localized at cell contacts, most of which form synapses in hippocampal cultures (Fannon and Colman, 1996; Benson and Tanaka, 1998; Tang et al., 1998; Bozdagi et al., 2000, 2004; Tanaka et al., 2000; Togashi et al., 2002).
To further examine the role of the cadherin–β-catenin adhesion complex in BDNF-mediated vesicle mobilization, we inhibited the BDNF-mediated dissociation of cadherin–β-catenin complexes by expressing a β-catenin point mutant that cannot be phosphorylated on tyrosine residue 654 (β-catenin Y654F). The β-catenin Y654F mutation has previously been shown to prevent Src-dependent dissociation of the cadherin–β-catenin complex (Murase et al., 2002; Lilien and Balsamo, 2005). Expression of β-catenin Y654F completely abolished the transient diffusion of vesicles seen after BDNF application, indicating that the disruption of cadherin–β-catenin complexes is required to observe significant SV dispersion (Fig. 2 C). In contrast, overexpression of wild-type β-catenin did not significantly inhibit SV dispersion (Fig. 2 B). These results indicate that BDNF disrupts synaptically localized, stable SV clusters by disrupting cadherin–β-catenin complexes, which are important for localizing SVs to specified regions underlying active zones.
We have previously shown that synaptophysin-GFP clusters are more dynamic in cells lacking β-catenin (Bamji et al., 2003). Cells lacking β-catenin were generated by culturing neurons from β-catenin flox mice (Huelsken et al., 2001) and transfecting them with a vector expressing the Cre recombinase. Changes in the length of four representative synaptophysin-GFP positive puncta in four different experimental conditions are depicted in Fig. 2 (E–G). Analysis of the length of the major axis of individual synaptophysin-GFP puncta revealed minimal fluctuations over time in control neurons, but dramatic fluctuations in length in neurons lacking β-catenin (compare Fig. 2, D and F). Moreover, although acute application of BDNF to control cultures resulted in a transient dispersal of SVs along the axon (Fig. 2 E), addition of BDNF to hippocampal neurons lacking β-catenin did not detectably increase the instability in SV localization beyond the increase resulting from the absence of β-catenin alone (Fig. 2, F and G). This is consistent with the possibility that BDNF's effects are mediated by dissolution of cadherin–β-catenin interactions.
BDNF-treatment enhances the splitting of synaptic vesicle clusters
Compared with control cultures, which displayed minimal SV dynamics (Fig. 3 A), we noted that dispersal of SV clusters after BDNF treatment often resulted in the splitting or partitioning of SV packets from preexisting stable clusters (Fig. 3 B and Videos 2 and 3, available at http://www.jcb.org/cgi/content/full/jcb.200601087/DC1). These small clusters of SVs that split away from preexisting stable clusters either rapidly translocated along the axon and left the field of view, fused with adjacent SV clusters to form larger puncta with increased fluorescence intensity (Fig. 3 B, asterisks at 8–20 min, and Video 2), or formed new, stably localized (>5 min) SV clusters (Fig. 3 B, asterisks at 15–20 min, and Video 2). Some SV clusters split into two relatively equal-sized clusters after dispersion of vesicles (Video 3). All splitting events observed in the 20-min observation period were recorded regardless of cluster size. We also observed apparent coalescence of mobile SV puncta with stable clusters, as previously described (Krueger et al., 2003). However, in this study, only the splitting of SV clusters was analyzed.
Figure 3.

BDNF enhances the splitting of stable SV clusters. Time-lapse confocal images of synaptophysin-GFP–labeled SV clusters in 12 DIV control cultures (A) or cultures treated with 100 ng/ml BDNF (B). Numbers on the bottom right of each image represent the time in minutes after the addition of media alone or BDNF to cultures. In control cultures, SV clusters are relatively stable over time. After BDNF application, SVs diffuse along the axon transiently (double-headed arrow at 6 min), resulting in the splitting of a cluster of SVs (arrow at 7 min). This splitting results in the formation of a new stable puncta (stable over 8–20 min; denoted by asterisks). SV splitting is also seen at 14 min (arrow), resulting in the formation of a new, apparently stable punctum (right, asterisks at 15 and 20 min). (C) Graphic representation of the frequency of SV cluster splitting in cells acutely treated with BDNF, or treated with BDNF for 3 d (11–14 DIV). n = at least 25–30 puncta per condition from at least 10 neurons over at least three separate cultures. Error bars denote ± SEM. Asterisks denote P < 0.05. Bar, 1 μm.
We quantified the frequency with which SV clusters split into smaller clusters in control neurons compared with those treated acutely with BDNF. We determined that application of BDNF results in a 1.7-fold increase in the average frequency of SV cluster splitting over a 20-min observation period (Fig. 3 C). The increase in splitting frequency was observed throughout the 20-min observation period (Fig. 3 B, arrow at 14 min, and Video 2). The persistence of SV splitting events suggested that there might be a long-term destabilization of presynaptic compartments. To explore this, we also quantified the frequency of splitting after 3 d of continuous BDNF treatment. Although the overall frequency of vesicle splitting was slightly lower in the older control cultures, BDNF still enhanced the frequency of SV cluster splitting (Fig. 3 C). Interestingly, after 3 d of BDNF treatment, the size of the synaptophysin-GFP clusters was similar in treated compared with untreated cultures (unpublished data). Among other possibilities, this suggests that the content of individual SV clusters may be replenished by the coalescence of mobile vesicle clusters with stable clusters, and possibly also by enhanced SV synthesis. Consistent with the latter possibility, BDNF has been shown to enhance the synthesis of some SV components (Tartaglia et al., 2001).
When cadherin–β-catenin complexes were maintained by expression of the β-catenin Y654F point mutant, the BDNF-mediated increase in the splitting of vesicle clusters was abolished (Fig. 3 C). This suggests that BDNF regulates the splitting of SV clusters, in part, through regulation of cadherin–β-catenin association. Cells expressing wild-type β-catenin did respond to BDNF with increased SV cluster splitting, but the magnitude of this effect was somewhat diminished compared with that observed in untransfected cells (Fig. 3 C). This intermediate effect was seen throughout the study, and it is most likely caused by an enhanced probability of cadherin–β-catenin association in cells overexpressing wild-type β-catenin. The absence of a more dramatic result probably reflects the fact that β-catenin that is not associated with cadherin is highly unstable.
Maintenance of cadherin–β-catenin interactions prevents BDNF-mediated synapse formation
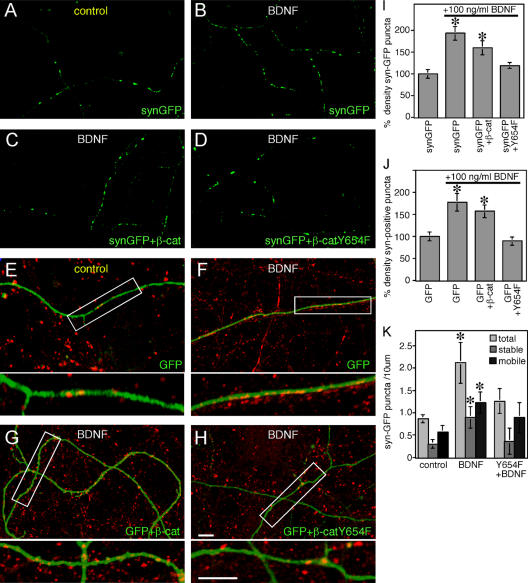
BDNF signaling through its receptor TrkB has been reported to increase synapse formation, as well as neurite branching, in hippocampal neurons (Vicario-Abejon et al., 1998; Collin et al., 2001; Danzer et al., 2002; Tolwani et al., 2002; Tyler and Pozzo-Miller, 2001, 2003). Using two approaches, we observed that the addition of 100 ng/ml BDNF for 3 d mediates an obvious increase in the density of SV clusters, and that this event is dependent on BDNF's modulation of cadherin–β-catenin interactions. Compared with untreated cultures (Fig. 4, A and I), the addition of BDNF to hippocampal cultures transfected with synaptophysin-GFP resulted in an increased density of synaptophysin-GFP puncta (Fig. 4, B and I). The BDNF-mediated increase in SV puncta density was prevented by coexpression of β-catenin Y654F (Fig. 4, D and I), but not wild-type β-catenin (Fig. 4, C and I).
Figure 4.
Maintenance of the association between cadherin and β-catenin inhibits BDNF-dependent increases in the density of SV clusters and mature synapses. (A–H) Confocal images of hippocampal neurons transfected with the indicated constructs at 10 DIV and imaged at 14 DIV after treatment of BDNF for 3 d (11–14 DIV). (E–H) Immunolabeling with anti-synaptophysin to identify clusters of endogenous synaptophysin present within GFP-filled processes. Synaptophysin-positive clusters that do not overlap with GFP-filled processes represent presynaptic clusters in untransfected cells. (I–K) Quantification of the density of SV clusters. Changes in the density of SV clusters along the axon after BDNF treatment were determined by quantifying the density of synaptophysin-GFP clusters along the axon (I), or the density of endogenous synaptophysin-immunopositive puncta along a GFP-labeled axon (J). To examine the density of stable and mobile clusters, synaptophysin-GFP dynamics were imaged over a 20-min period, and each synaptophysin-GFP puncta was scored for mobility (K). For each condition, cultures grown in the presence of BDNF were compared with neurons grown without BDNF. Results in I and J demonstrate that 3 d of BDNF treatment results in an increased density of endogenous synaptophysin and synaptophysin-GFP clusters. These increases are prevented by expression of Y654F mutant β-catenin, but not WT β-catenin. Results in K demonstrate that 3 d of BDNF increases the density of both stable and mobile synaptic vesicle clusters and that this increase is prevented by expression of the Y654F mutant of β-catenin. n = at least 13 neurons. Error bars represent ± SEM. Asterisks denote P < 0.05. Bars, 5 μm.
The density of endogenous synaptophysin puncta was also examined. Compared with untreated cultures (Fig. 4 E, red), there was an overall increase in the number of endogenous synaptophysin-immunopositive puncta in cultures treated with 100 ng/ml BDNF for three days (Fig. 4, F–H, red). This is consistent with published results and could be caused, in part, by an increase in axonal extension and branching (Vicario-Abejon et al., 1998; Collin et al., 2001; Danzer et al., 2002; Tolwani et al., 2002; Tyler and Pozzo-Miller, 2001, 2003). To more specifically examine the density of SV clusters, synaptophysin-immunopositive puncta were counted within GFP-labeled processes (Fig. 4, E–H [yellow puncta], and J). Treatment with BDNF of wild-type neurons, or of neurons overexpressing full-length β-catenin, increased the density of synaptophysin-immunopositive puncta (Fig. 4, F, G [yellow puncta], and J). In contrast, expression of β-catenin Y654F completely inhibited BDNF's effects on the density of synaptophysin-immunopositive puncta (Fig. 4, H and I). It is important to note the internal control in these cultures. The overall density of synaptophysin-immunopositive puncta was increased in cultures treated with BDNF (compare red boutons in Fig. 4 E to those in F–H). However, the number of synaptophysin-immunopositive puncta in individually labeled β-catenin Y654F–expressing cells was similar to controls (Fig. 4, E and H).
SV clusters are expected to include both the mobile pool of vesicles and the pool of vesicles stably localized at synapses. To identify the pools affected by BDNF, we imaged hippocampal neurons transfected with synaptophysin. As expected, there was a twofold increase in the total number of SV clusters after BDNF treatment for 3 d. Moreover, BDNF increased the density of both the stable and mobile SV clusters (Fig. 4 K). Neurons expressing β-catenin Y654F did not exhibit any significant increase in the density of total, stable, or mobile vesicle clusters compared with untreated cells.
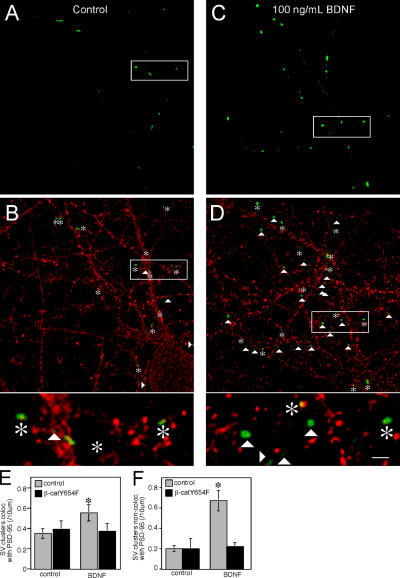
To determine if the stably localized SV clusters represented synaptic sites, we immunostained control and BDNF-treated cultures with an antibody specific for the excitatory postsynaptic marker PSD-95. The density of SV clusters apposed to PSD-95 (Fig. 5 E) was similar to the density of stable SV clusters (Fig. 4 K). As expected, treatment of neurons with BDNF for 3 d resulted in an overall increase in the density of synaptophysin-GFP–positive puncta compared with controls (compare Fig. 5, A and C). The increase in the number of PSD-95–positive puncta that colocalized with synaptophysin-GFP (Fig. 5 E) was very similar to the increase in the number of stable SV clusters (Fig. 4 K). Interestingly, BDNF also increased quite dramatically the density of synaptophysin-GFP–positive puncta that did not colocalize with PSD-95 puncta (Fig. 5 F). These may represent synaptophysin-GFP–positive puncta at inhibitory synapses, mobile SV clusters or both. The BDNF-promoted increases in synaptophysin-GFP puncta apposed and not apposed to PSD-95 were each blocked by expression of β-catenin Y654F.
Figure 5.
Increased density of puncta in excitatory synapses in neurons treated with BDNF. Compared with untreated cells (A), there is an overall increase in the number of synaptophysin-GFP–positive puncta in cells treated with BDNF (C). (B and D) Immunolabeling for PSD-95. Synaptophysin-GFP puncta that were colocalized with PSD-95 (asterisks) and synaptophysin-GFP puncta not colocalized with PSD-95 (arrowheads) were identified. Bar, 10 μm. All green and yellow puncta observed at 63×, 4× zoom were counted. Higher magnification images illustrating examples of synaptophysin-GFP puncta that colocalized or did not colocalize with PSD-95 are shown at bottom of B and D. Bar, 3.3 μm. BDNF treatment increases the density of synaptophysin-GFP puncta that colocalize with PSD-95 (E) and do not colocalize with PSD-95 (F). This increase was abolished in cells expressing the β-catenin Y654F mutant. Error bars depict ± SEM. Asterisks denote P < 0.05.
Discussion
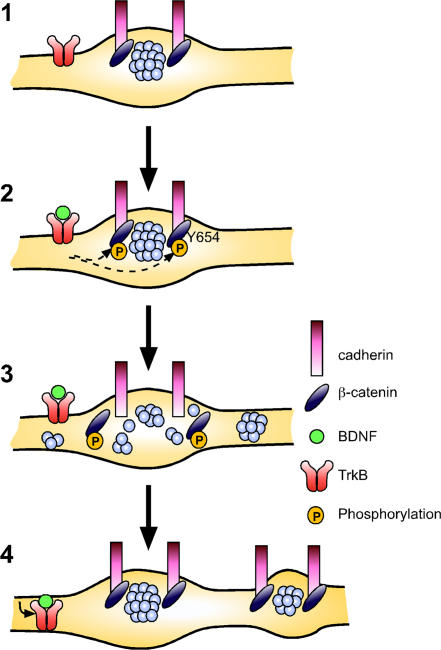
Understanding the molecular events that control synapse formation and modification is an important objective in neuroscience. BDNF is synthesized and secreted in response to neuronal activity, and it has been shown to enhance synaptic efficacy and promote the formation of new synapses both in vitro and in vivo (Bramham and Messaoudi, 2005). Despite the great interest in understanding how secreted factors regulate synapse form and function, the mechanisms underlying BDNF's actions are still not well understood. In this study, we have shown that BDNF induces the morphological rearrangement of individual presynaptic compartments and promotes the formation of new synapses, in part, by disrupting cadherin–β-catenin complexes. Specifically, we have shown that (a) BDNF signaling reduces the interaction between cadherins and β-catenin; (b) BDNF treatment results in an acute dispersion of SVs, which is inhibited by artificially maintaining cadherin–β-catenin interactions by expressing a β-catenin mutant unable to be phosphorylated at Y654; and (c) BDNF enhances the formation of new synapses, which is inhibited by artificially maintaining cadherin–β-catenin interactions. A model illustrating our observations is presented in Fig. 6. As both the “splitting” of synaptically localized SV clusters and the increase in synapse density can be inhibited by inhibiting the dissociation of cadherin–β-catenin interactions, we have speculated that SV cluster splitting contributes to the formation of new synapses. This model has yet to be rigorously tested in vivo.
Figure 6.
Model for the role of cadherin–β-catenin interactions in BDNF-mediated presynaptic plasticity. (1) In the absence of TrkB-mediated signaling, β-catenin is not phosphorylated at Y654 and is associated with cadherins at the synapse, providing a scaffold through the β-catenin PDZ domain interaction motif for recruitment of scaffold proteins and synaptic vesicle to the synapse. (2) Activation of TrkB receptor tyrosine kinase by BDNF results in the phosphorylation of β-catenin on Y654. This causes the dissociation of β-catenin from cadherins, and disruption of the signals responsible for localizing SVs to the presynaptic compartment. (3) Subsequently, SVs disperse along the axon into perisynaptic regions. (4) β-catenin dephosphorylation and reassociation with cadherin may occur after the internalization of TrkB that can occur within 5 min of BDNF treatment. As a result, SVs recluster at synaptic zones; however, presynaptic compartments are altered and there is a persistent increase in the rate of small SV clusters splitting away from the SV cluster at the active zone. We hypothesize that the increased rate of SV cluster splitting may lead to an increase in the number of mobile SV clusters, and help to promote an increase in the overall density of synapses along the axon as well.
BDNF and cadherin–β-catenin complexes enhance the motility of SV clusters
We have observed a transient dispersal of SVs into perisynaptic regions after BDNF treatment, which correlates with the temporal profile for β-catenin phosphorylation and cadherin–β-catenin dissociation. The short duration of cadherin–β-catenin disruption and SV dispersal is most likely caused by the internalization of TrkB receptors, which has been shown to occur as early as 5 min after BDNF binding (Haapasalo et al., 2002), and/or the rapid deactivation of TrkB receptors by tyrosine phosphatases (Widmer et al., 1993; Lilien et al., 2002).
Our results using tetanus toxin indicate that this transient dispersal of SVs into the perisynaptic regions is caused by mobilization of SVs within the axon and not by diffusion in the surface membrane of synaptic vesicle proteins after exocytosis. Although a similar elongation of VAMP-GFP signals is observed after fusion of vesicles with the membrane (Li and Murthy, 2001; Tanaka et al., 2000), BDNF alone does not induce exocytosis at such levels (Jovanovic et al., 2000). In addition, our observed time course for vesicle diffusion is very different from that seen after vesicle exocytosis and reuptake. Li and Murthy (2001) demonstrate that the onset of VAMP-GFP or synaptophysin-GFP diffusion occurs only seconds after depolarization, and reclustering occurs with a time constant of 118.2 s. In contrast, our observations show a maximal diffusion of synaptophysin-GFP fluorescence after 6 min of BDNF treatment. Second, the splitting of vesicle clusters that often occurs after diffusion (Fig. 2 and Videos 2 and 3) is best explained by enhanced SV mobility within the axon.
We have previously demonstrated that β-catenin acts as a scaffold to tether unknown PDZ proteins to cadherin clusters, thereby establishing nucleation sites for the discrete localization of SVs at the synapse in vivo and in vitro (Bamji et al., 2003). Other proteins known to affect the localization of SVs at synaptic sites include synapsin I and F-actin. Synapsin I has been shown to tether SVs to the actin cytoskeleton, and the phosphorylation of synapsin I disrupts this association (Hilfiker et al., 1999). Interestingly, both synapsin I phosphorylation and F-actin disassembly result in the dispersion of SV clusters within hippocampal growth cones (Bonanomi et al., 2005). Cadherin-mediated interactions have been shown to regulate actin dynamics, and it will be interesting to determine whether cadherin–β-catenin interactions regulate SV localization via modulation of synapsin I phosphorylation and/or F-actin organization.
BDNF and cadherin–β-catenin complexes enhance synapse formation
Although the exact mechanisms underlying BDNF-induced synapse formation are unclear, it is possible that BDNF promotes the formation of new synapses, in part, by increasing the density of mobile SV clusters, which are subsequently localized at new points of contact. The BDNF-mediated increase in mobile vesicle cluster density requires the dissociation of cadherin–β-catenin clusters and may be caused, in part, by the splitting of preexisting SV clusters. Transcriptional up-regulation of SV-associated proteins may also be involved, as both BDNF and β-catenin can regulate transcriptional events. Studies using time-lapse imaging have revealed that the overwhelming majority of contacts made between dendritic filopodia and axons are not stabilized, but are retracted within minutes of their formation (Jontes et al., 2000). The increased number of mobile vesicle clusters may increase the probability of contact stabilization and, ultimately, the formation of new synapses. Indeed, many of these mobile SV clusters are exocytosis competent (Krueger et al., 2003), and filopodia are known to be stabilized in the presence of local calcium transients (Lohmann et al., 2005).
It has recently been demonstrated using conditional _TrkB_-null mice that BDNF and TrkB signal in a cell-autonomous manner to promote excitatory synapse formation in the hippocampus (Luikart et al., 2005). Specific deletion of TrkB from presynaptic CA3 pyramidal neurons resulted in a decreased number of presynaptic terminals, with multiple postsynaptic compartments forming contacts with individual boutons (Luikart et al., 2005). Although controversial, it has been suggested that one mode of synapse formation is the splitting of individual synapses into two synapses, with perforated synapses being an intermediary (Nikonenko et al., 2002). In these mice, deletion of presynaptic TrkB may have prevented the mobilization of synaptic components such as SVs and thereby prevented the formation of new presynaptic boutons. It will be interesting to see whether artificial maintenance of cadherin–β-catenin clusters in vivo results in a phenotype similar to that seen after TrkB deletion. BDNF has also been shown to promote the formation of inhibitory, GABAergic synapses in vivo, as well as in vitro (Vicario-Abejon et al., 1998; Collin et al., 2001; Rico et al., 2002).
Interestingly, previous reports have shown that decreasing cell–cell adhesion increases the formation of new synapses. For example, the treatment of Aplysia californica sensory neurons with serotonin (5-HT) enhances the formation of new synaptic varicosities by down-regulating the Aplysia californica cell adhesion molecule (Han et al., 2004). Similarly, a decrease in the Drosophila melanogaster cell adhesion molecule fasciclin II is necessary for synapse sprouting at the neuromuscular junction in response to increased neuronal activity and cAMP concentration (Schuster et al., 1996a,b). Our demonstration that the dissolution of cadherin–β-catenin adhesive complexes at synapses promotes synaptic remodeling in response to BDNF further supports the hypothesis that synapse formation and plasticity are regulated, in part, by control of cell adhesion molecule function.
Materials and methods
Neuronal cultures
Rat hippocampi from E18 fetal rats were prepared as previously described (Xie et al., 2000) and plated at a density of 130 cells/mm2. For time-lapse studies, neurons were transfected using Effectene (QIAGEN) transfection at 10 days in vitro (DIV), or as indicated, and examined 2 d later. For long-term BDNF studies, cultures were transfected at 10 DIV, treated with 100 ng/ml BDNF at 11 DIV, and examined at 14 DIV.
Immunoblot analysis and immunohistochemistry
Protein extracts were prepared from 12 DIV primary hippocampal cultures treated with either media alone or 100 ng/ml BDNF for 10 or 30 min. Extracts were immunoprecipitated with monoclonal anti–β-catenin (Zymed Laboratories) and immunoblots were probed with anti-phosphotyrosine (4G10; Cell Signaling Solutions) and anti–N-cadherin (a gift from D. Colman, McGill University, Montreal, Quebec, Canada). Proteins were visualized using enhanced chemiluminescence. Exposed film was scanned and the brightness and contrast of entire images was moderately adjusted using Photoshop (Adobe) after recommended, scientifically acceptable procedures, and no information was obscured or eliminated from the original (Rossner and Yamada, 2004).
For immunohistochemistry, neuronal cultures were fixed in 4% paraformaldehyde/4% sucrose for 10 min, permeabilized in 0.1% Triton X-100 for 10 min, and then blocked in 10% goat serum for 1 h at room temperature. Primary antibodies were applied in 1% goat serum overnight at 4°C, and secondary antibodies were applied in 1% goat serum for 1 h at room temperature. The primary antibodies used were mouse anti-synaptophysin (Sigma-Aldrich) and mouse anti–PSD-95 (Affinity BioReagents, Inc.); the secondary antibodies used were Alexa Fluor 488 and Texas red–conjugated goat anti–mouse or goat anti–rabbit IgGs (Invitrogen). n = at least 10 neurons per condition from at least three separate cultures.
Image analysis and quantification
Dispersion of SVs along the axon.
Neurons transfected with synaptophysin-GFP were imaged every minute using a microscope (LSM 5 Pascal; Carl Zeiss MicroImaging, Inc.; 63×, 1.4 NA, oil Plan-Apochromat objective) and the corresponding LSM 5 Pascal Software. All images were captured with the same exposure time. Quantification of major axis length for each punctum over time was analyzed using LSM 5 Pascal Software. In brief, a short line was drawn along the axon and through the major axis of the puncta and analyzed at time point 0. The profile of the fluorescence intensity along the fixed line was then obtained for each time point using the “profile” function. For the analysis of each puncta, the length of the major axis at each time point was compared with the length of the major axis at time point 0. All puncta that remained localized in the same spot along the axon during the 20–30 min imaging period were quantified. t tests were used for all statistical analyses.
Density of synaptophysin-GFP puncta.
To quantify the density of synaptophysin-GFP puncta per axon length, images were imported into Image (National Institutes of Health), where puncta were identified and analyzed at a threshold of 55 and a minimum pixel size of 10. At least a 2,500-μm axon length from 10–30 cells from at least three separate cultures was analyzed per condition. To determine the density of synaptophysin and PSD-95 clusters, immunopositive puncta along at least 1,500 μm of GFP-labeled axon length from 10–30 cells from at least three separate cultures were counted per condition. In brief, neurons were transfected with GFP or synaptophysin-GFP at 10 DIV and treated with BDNF at 11 DIV. 3 d later, cells were fixed and immunostained for synaptophysin or PSD-95, respectively. All images were captured on the LSM 5 Pascal scanning confocal microscope using constant settings. GFP-labeled axons were then measured, and all visible synaptophysin-positive puncta that colocalized with GFP-labeled axons were counted. t tests were used for all statistical analyses.
Online supplemental material
Video 1 shows that there is minimal variability in stably localized synaptophysin-GFP puncta over time in untreated cells. Video 2 shows that BDNF induces SV dispersal and increased splitting of SV clusters from stably localized synaptophysin-GFP–labeled puncta. Video 3 shows that BDNF induces SV diffusion and the splitting of SV clusters. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200601087/DC1.
Supplementary Material
[Supplemental Material Index]
Acknowledgments
We thank A.K. McAllister for helpful suggestions for this study; D. Rogers, S.H. Lee, and J. Arikkath for comments on the manuscript; and W. Walantus for technical assistance.
This work was supported by National Institutes of Health grant P01 NS16033. L.F. Reichardt is an investigator and S.X. Bamji was an associate of the Howard Hughes Medical Institute.
S.X. Bamji's present address is Dept. of Cellular and Physiological Sciences, Brain Research Centre, University of British Columbia, Vancouver, BC V6T-1Z3.
B. Rico's present address is the Institute of Neuroscience, Consejo Superior de Investigaciones Cientificas-University Miguel Hernández, 03550 San Juan, Alicante, Spain.
N. Kimes's present address is Dept. of Pathology and Laboratory Medicine, Medical University of South Carolina, Charleston, SC 29425.
Abbreviations used in this paper: BDNF, brain-derived neurotrophic factor; DIV, day in vitro; LTP, long-term potentiation; SV, synaptic vesicle.
References
- Ahmari, S.E., J. Buchanan, and S.J. Smith. 2000. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 3:445–451. [DOI] [PubMed] [Google Scholar]
- Applegate, M.D., D.S. Kerr, and P.W. Landfield. 1987. Redistribution of synaptic vesicles during long-term potentiation in the hippocampus. Brain Res. 401:401–406. [DOI] [PubMed] [Google Scholar]
- Bamji, S.X. 2005. Cadherins: actin with the cytoskeleton to form synapses. Neuron. 47:175–178. [DOI] [PubMed] [Google Scholar]
- Bamji, S.X., K. Shimazu, N. Kimes, J. Huelsken, W. Birchmeier, B. Lu, and L.F. Reichardt. 2003. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 40:719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson, D.L., and H. Tanaka. 1998. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J. Neurosci. 18:6892–6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer, T., Y. Sara, M. Mozhayeva, D. Atasoy, X. Liu, E.T. Kavalali, and T.C. Sudhof. 2002. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 297:1525–1531. [DOI] [PubMed] [Google Scholar]
- Bonanomi, D., A. Menegon, A. Miccio, G. Ferrari, A. Corradi, H.T. Kao, F. Benfenati, and F. Valtorta. 2005. Phosphorylation of synapsin I by cAMP-dependent protein kinase controls synaptic vesicle dynamics in developing neurons. J. Neurosci. 25:7299–7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi, O., W. Shan, H. Tanaka, D.L. Benson, and G.W. Huntley. 2000. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 28:245–259. [DOI] [PubMed] [Google Scholar]
- Bozdagi, O., M. Valcin, K. Poskanzer, H. Tanaka, and D.L. Benson. 2004. Temporally distinct demands for classic cadherins in synapse formation and maturation. Mol. Cell. Neurosci. 27:509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham, C.R., and E. Messaoudi. 2005. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog. Neurobiol. 76:99–125. [DOI] [PubMed] [Google Scholar]
- Carter, A.R., C. Chen, P.M. Schwartz, and R.A. Segal. 2002. Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure. J. Neurosci. 22:1316–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin, C., C. Vicario-Abejon, M.E. Rubio, R.J. Wenthold, R.D. McKay, and M. Segal. 2001. Neurotrophins act at presynaptic terminals to activate synapses among cultured hippocampal neurons. Eur. J. Neurosci. 13:1273–1282. [DOI] [PubMed] [Google Scholar]
- Danzer, S.C., K.R. Crooks, D.C. Lo, and J.O. McNamara. 2002. Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant cultures. J. Neurosci. 22:9754–9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake, C.T., T.A. Milner, and S.L. Patterson. 1999. Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J. Neurosci. 19:8009–8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannon, A.M., and D.R. Colman. 1996. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 17:423–434. [DOI] [PubMed] [Google Scholar]
- Genoud, C., G.W. Knott, K. Sakata, B. Lu, and E. Welker. 2004. Altered synapse formation in the adult somatosensory cortex of brain-derived neurotrophic factor heterozygote mice. J. Neurosci. 24:2394–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo, A., I. Sipola, K. Larsson, K.E. Akerman, P. Stoilov, S. Stamm, G. Wong, and E. Castren. 2002. Regulation of TRKB surface expression by brain-derived neurotrophic factor and truncated TRKB isoforms. J. Biol. Chem. 277:43160–43167. [DOI] [PubMed] [Google Scholar]
- Han, J.H., C.S. Lim, Y.S. Lee, E.R. Kandel, and B.K. Kaang. 2004. Role of Aplysia cell adhesion molecules during 5-HT-induced long-term functional and structural changes. Learn. Mem. 11:421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker, S., V.A. Pieribone, A.J. Czernik, H.T. Kao, G.J. Augustine, and P. Greengard. 1999. Synapsins as regulators of neurotransmitter release. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken, J., R. Vogel, B. Erdmann, G. Cotsarelis, and W. Birchmeier. 2001. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 105:533–545. [DOI] [PubMed] [Google Scholar]
- Jontes, J.D., J. Buchanan, and S.J. Smith. 2000. Growth cone and dendrite dynamics in zebrafish embryos: early events in synaptogenesis imaged in vivo. Nat. Neurosci. 3:231–237. [DOI] [PubMed] [Google Scholar]
- Jovanovic, J.N., A.J. Czernik, A.A. Fienberg, P. Greengard, and T.S. Sihra. 2000. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat. Neurosci. 3:323–329. [DOI] [PubMed] [Google Scholar]
- Krueger, S.R., A. Kolar, and R.M. Fitzsimonds. 2003. The presynaptic release apparatus is functional in the absence of dendritic contact and highly mobile within isolated axons. Neuron. 40:945–957. [DOI] [PubMed] [Google Scholar]
- Li, Z., and V.N. Murthy. 2001. Visualizing postendocytic traffic of synaptic vesicles at hippocampal synapses. Neuron. 31:593–605. [DOI] [PubMed] [Google Scholar]
- Lilien, J., and J. Balsamo. 2005. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr. Opin. Cell Biol. 17:459–465. [DOI] [PubMed] [Google Scholar]
- Lilien, J., J. Balsamo, C. Arregui, and G. Xu. 2002. Turn-off, drop-out: functional state switching of cadherins. Dev. Dyn. 224:18–29. [DOI] [PubMed] [Google Scholar]
- Lohmann, C., A. Finski, and T. Bonhoeffer. 2005. Local calcium transients regulate the spontaneous motility of dendritic filopodia. Nat. Neurosci. 8:305–312. [DOI] [PubMed] [Google Scholar]
- Lu, B. 2003. BDNF and activity-dependent synaptic modulation. Learn. Mem. 10:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart, B.W., S. Nef, T. Virmani, M.E. Lush, Y. Liu, E.T. Kavalali, and L.F. Parada. 2005. TrkB has a cell-autonomous role in the establishment of hippocampal Schaffer collateral synapses. J. Neurosci. 25:3774–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, A., S. Alcantara, V. Borrell, J.A. Del Rio, J. Blasi, R. Otal, N. Campos, A. Boronat, M. Barbacid, I. Silos-Santiago, and E. Soriano. 1998. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J. Neurosci. 18:7336–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshul, C.K., and W.F. Hopkins. 1990. Presynaptic ultrastructural correlates of long-term potentiation in the CA1 subfield of the hippocampus. Brain Res. 514:310–319. [DOI] [PubMed] [Google Scholar]
- Murase, S., E. Mosser, and E.M. Schuman. 2002. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 35:91–105. [DOI] [PubMed] [Google Scholar]
- Nagappan, G., and B. Lu. 2005. Activity-dependent modulation of the BDNF receptor TrkB: mechanisms and implications. Trends Neurosci. 28:464–471. [DOI] [PubMed] [Google Scholar]
- Nikonenko, I., P. Jourdain, S. Alberi, N. Toni, and D. Muller. 2002. Activity-induced changes of spine morphology. Hippocampus. 12:585–591. [DOI] [PubMed] [Google Scholar]
- Otal, R., A. Martinez, and E. Soriano. 2005. Lack of TrkB and TrkC signaling alters the synaptogenesis and maturation of mossy fiber terminals in the hippocampus. Cell Tissue Res. 319:349–358. [DOI] [PubMed] [Google Scholar]
- Piedra, J., D. Martinez, J. Castano, S. Miravet, M. Dunach, and A.G. de Herreros. 2001. Regulation of beta-catenin structure and activity by tyrosine phosphorylation. J. Biol. Chem. 276:20436–20443. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller, L.D., W. Gottschalk, L. Zhang, K. McDermott, J. Du, R. Gopalakrishnan, C. Oho, Z.H. Sheng, and B. Lu. 1999. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J. Neurosci. 19:4972–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico, B., B. Xu, and L.F. Reichardt. 2002. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat. Neurosci. 5:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossner, M., and K.M. Yamada. 2004. What's in a picture? The temptation of image manipulation. J. Cell Biol. 166:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura, S., S. Miravet, J. Piedra, A. Garcia de Herreros, and M. Dunach. 1999. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J. Biol. Chem. 274:36734–36740. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan, S., and T.A. Ryan. 2000. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat. Cell Biol. 2:197–204. [DOI] [PubMed] [Google Scholar]
- Scheiffele, P., J. Fan, J. Choih, R. Fetter, and T. Serafini. 2000. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 101:657–669. [DOI] [PubMed] [Google Scholar]
- Schuster, C.M., G.W. Davis, R.D. Fetter, and C.S. Goodman. 1996. a. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 17:641–654. [DOI] [PubMed] [Google Scholar]
- Schuster, C.M., G.W. Davis, R.D. Fetter, and C.S. Goodman. 1996. b. Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron. 17:655–667. [DOI] [PubMed] [Google Scholar]
- Tanaka, H., W. Shan, G.R. Phillips, K. Arndt, O. Bozdagi, L. Shapiro, G.W. Huntley, D.L. Benson, and D.R. Colman. 2000. Molecular modification of N-cadherin in response to synaptic activity. Neuron. 25:93–107. [DOI] [PubMed] [Google Scholar]
- Tang, L., C.P. Hung, and E.M. Schuman. 1998. A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron. 20:1165–1175. [DOI] [PubMed] [Google Scholar]
- Tartaglia, N., J. Du, W.J. Tyler, E. Neale, L. Pozzo-Miller, and B. Lu. 2001. Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. J. Biol. Chem. 276:37585–37593. [DOI] [PubMed] [Google Scholar]
- Togashi, H., K. Abe, A. Mizoguchi, K. Takaoka, O. Chisaka, and M. Takeichi. 2002. Cadherin regulates dendritic spine morphogenesis. Neuron. 35:77–89. [DOI] [PubMed] [Google Scholar]
- Tolwani, R.J., P.S. Buckmaster, S. Varma, J.M. Cosgaya, Y. Wu, C. Suri, and E.M. Shooter. 2002. BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience. 114:795–805. [DOI] [PubMed] [Google Scholar]
- Tyler, W.J., and L.D. Pozzo-Miller. 2001. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J. Neurosci. 21:4249–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, W.J., and L. Pozzo-Miller. 2003. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J. Physiol. 553:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida, N., Y. Honjo, K.R. Johnson, M.J. Wheelock, and M. Takeichi. 1996. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J. Cell Biol. 135:767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario-Abejon, C., C. Collin, R.D. McKay, and M. Segal. 1998. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J. Neurosci. 18:7256–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer, H.R., D.R. Kaplan, S.J. Rabin, K.D. Beck, F. Hefti, and B. Knusel. 1993. Rapid phosphorylation of phospholipase C gamma 1 by brain-derived neurotrophic factor and neurotrophin-3 in cultures of embryonic rat cortical neurons. J. Neurochem. 60:2111–2123. [DOI] [PubMed] [Google Scholar]
- Xie, C., W.R. Markesbery, and M.A. Lovell. 2000. Survival of hippocampal and cortical neurons in a mixture of MEM+ and B27-supplemented neurobasal medium. Free Radic. Biol. Med. 28:665–672. [DOI] [PubMed] [Google Scholar]
- Yamagata, M., J.R. Sanes, and J.A. Weiner. 2003. Synaptic adhesion molecules. Curr. Opin. Cell Biol. 15:621–632. [DOI] [PubMed] [Google Scholar]
- Zakharenko, S.S., S.L. Patterson, I. Dragatsis, S.O. Zeitlin, S.A. Siegelbaum, E.R. Kandel, and A. Morozov. 2003. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 39:975–990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Material Index]