The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors (original) (raw)
. Author manuscript; available in PMC: 2007 Nov 9.
Published in final edited form as: Nature. 2001 Apr 19;410(6831):948–952. doi: 10.1038/35073616
Abstract
Migration is a basic feature of many cell types in a wide range of species1. Since the 1800s, cell migration has been proposed to occur in the nervous and immune systems2,3, and distinct molecular cues for mammalian neurons and leukocytes have been identified. Here we report that Slit, a secreted protein previously known for its role of repulsion in axon guidance and neuronal migration, can also inhibit leukocyte chemotaxis induced by chemotactic factors. Slit inhibition of the chemokine-induced chemotaxis can be reconstituted by the co-expression of a chemokine receptor containing seven transmembrane domains and Roundabout (Robo), a Slit receptor containing a single transmembrane domain. Thus, there is a functional interaction between single and seven transmembrane receptors. Our results reveal the activity of a neuronal guidance cue in regulating leukocyte migration and indicate that there may be a general conservation of guidance mechanisms underlying metazoan cell migration. In addition, we have uncovered an inhibitor of leukocyte chemotaxis, and propose a new therapeutic approach to treat diseases involving leukocyte migration and chemotactic factors.
Leukocyte chemotaxis is one of the best-characterized models of cell migration in adult mammals4-12. Work in the past 20 years has demonstrated the importance of the chemokine family in leukocyte chemotaxis4-9. These structurally related small proteins regulate leukocyte migration and function4-9. Neuronal migration is an important step in neural development in the nervous system10-12; however, it is not clear whether mechanisms operating in the nervous system are conserved in the immune system. Leukocytes migrate at a rate much faster than neurons: the behaviour of leukocytes can be monitored in seconds, whereas that of the neurons is monitored in tens of minutes and hours. The cellular environment in which leukocytes migrate also seems to be different from that for axons and neurons. The morphology of migrating neurons, which have a rather long leading process, differs from that of leukocytes. At the molecular level, all neuronal guidance cues bind to receptors containing a single transmembrane domain. By contrast, G-protein-coupled receptors (GPCRs) containing seven transmembrane domains are thought to be the sole receptors mediating responses not only to all chemokines, but also to other chemotactic factors either for leukocytes5 or for the social amoebae Dictyostelium1. It is therefore not obvious whether neuronal guidance cues can have roles in the haematopoietic or immune systems.
Studies on the slit gene family13,14 have shown that secreted Slit proteins can guide both axon projection15-18 and neuronal migration19-22. We previously observed embryonic expression of Slit and Robo outside the mammalian nervous system17,22, which led us to speculate potential roles for Slit in several systems including the immune system19,20. Previous studies by us and others have focused on embryonic expression of slit and robo. We have now used RNase protection assays to examine the expression of three known slit genes in adult tissues and cell lines (Fig. 1a–c). Each slit gene has a distinct expression pattern: slit1 is specific to the brain, but slit2 and slit3 are expressed in the brain as well as other tissues. The expression level of slit2 in the kidney and the lung is comparable to that in the brain. Expression of slit3 is the highest in the lung and also detectable in the kidney, the brain, the heart, the spleen and the lymph nodes. When a number of cell lines were examined, slit2 and slit3 were found in the rat endothelial cells, mesangial cells and fibroblasts from the rat kidney. In situ hybridization of human kidneys revealed the expression of slit2 in mesangial and epithelial cells in the glomeruli; in epithelial cells in the tubules (Fig. 1e); and in endothelial cells of both arterioles and venules in the kidney (Fig. 1f).
Figure 1.
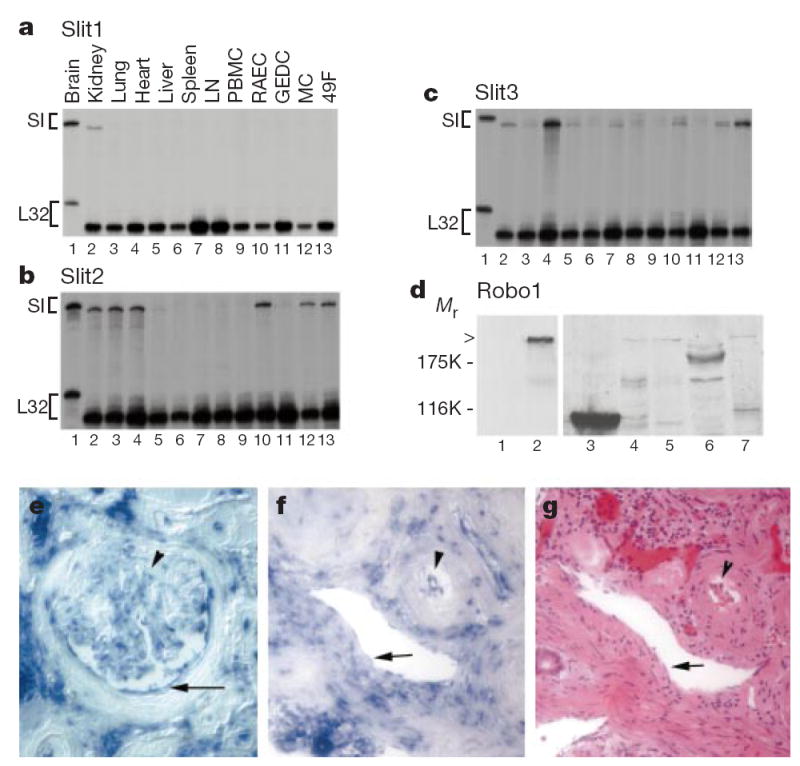
Expression of Slit and Robo in adult tissues. a–c, RNase protection assays (RPAs) were used to determine the expression of three slit genes in adult rats. Total RNA was prepared from different rat tissues and cell lines, as indicated (lanes 2–13). Total RNA (5 μg) was used in RPAs with probes specific for slit1, slit2 and slit3 genes (Sl). The rat L32 gene probe controlled for RNA input (lower band in each panel). Probes in lane 1 contain polylinker regions and are longer than the protected bands. LN, lymph node; PBMC, peripheral blood mononuclear cells; RAEC, rat endothelial cell line; GEDC, glomerular endothelial cells from the rat kidney; MC, rat kidney mesangial cells; 49F, rat kidney fibroblasts. d, Expression of Robo1 protein detected with anti-Robo1 antibodies. Arrowhead indicates full-length Robo1. Lane 1, HEK cells; lane 2, Robo1-transfected HEK cells; lane 3, a strong band with lower relative molecular mass (_M_r) than full-length Robo1 was detected reproducibly in rat thymus, indicating a crossreacting band or a proteolytically cleaved product; lane 4, HL-60 cells; lane 5, neutrophils differentiated from HL-60 cells; lane 6, rat PBMCs: no full-length Robo1 was detected but several lower _M_r bands were visible; lane 7, rat lymph node. e, slit2 messenger RNA distribution detected by in situ hybridization in the glomerulus of adult human kidney. Arrowhead indicates mesangial cells; arrow indicates epithelial cells in Bowman’s capsule. In the glomerulus, most of the positive cells are epithelial cells, but some endothelial cells can be seen. f, slit2 mRNA expression in vascular endothelial cells in the human kidney. Arrow indicates endothelial cells in a venule; arrowhead indicates endothelial cells in an arteriole. g, Haemotoxylin–eosin staining of a section of the same kidney as that shown in f. These two sections are not immediately next to each other, but corresponding regions are conveniently identified by landmarks in the sections.
Anti-Robo1 antibodies detected Robo1 in the lymph nodes, the thymus and neutrophils differentiated from HL-60 cells (Fig. 1d). By polymerase chain reaction with reverse transcription (RT–PCR), robo1 was detected in the thymus, the spleen, the lymph nodes, the liver, the kidney and the heart (data not shown), whereas robo2 was found in the spleen, the thymus, the liver, the lung and the kidney (data not shown).
To investigate whether Slit could affect chemotaxis, we used standard transwell and transfilter assays in which a chemokine was placed in the lower chamber and the leukocytes were placed in the upper chamber. We then analysed the migration of leukocytes into the lower chamber or onto the underside of the filter. Conditioned serum-free media from human embryonic kidney (HEK) cells expressing human and Xenopus Slit2 (SLIT2 and Xenopus Slit2) proteins17, as well as SLIT2 and Xenopus Slit2 proteins purified from serum-containing conditioned media, were tested.
As expected, the chemokine stromal-derived factor (SDF)-1 induced leukocyte chemotaxis (Fig. 2a, b). Lymphocytes isolated from the lymph nodes were chemotactic towards 10 nM of SDF-1 (Fig. 2a), but chemotaxis induced by SDF-1 was reduced by the presence of 100 pM of SLIT2 in the lower chamber (Fig. 2a). This effect was dose dependent; 10 pM of SLIT2 was not effective in reducing chemotaxis induced by SDF-1 (Fig. 2a). The effect of SLIT2 in the lower chamber might be due to either repulsion or inhibition. To test whether Slit could inhibit leukocyte chemotaxis, we added SLIT2 to either the upper or the lower chamber, and SDF-1 to the lower chamber (Fig. 2b). Slit reduced SDF-1-induced chemotaxis when it was present in the upper chamber, and even when it was present in both the upper and the lower chambers. These results indicate that Slit inhibits chemotaxis induced by SDF-1.
Figure 2.
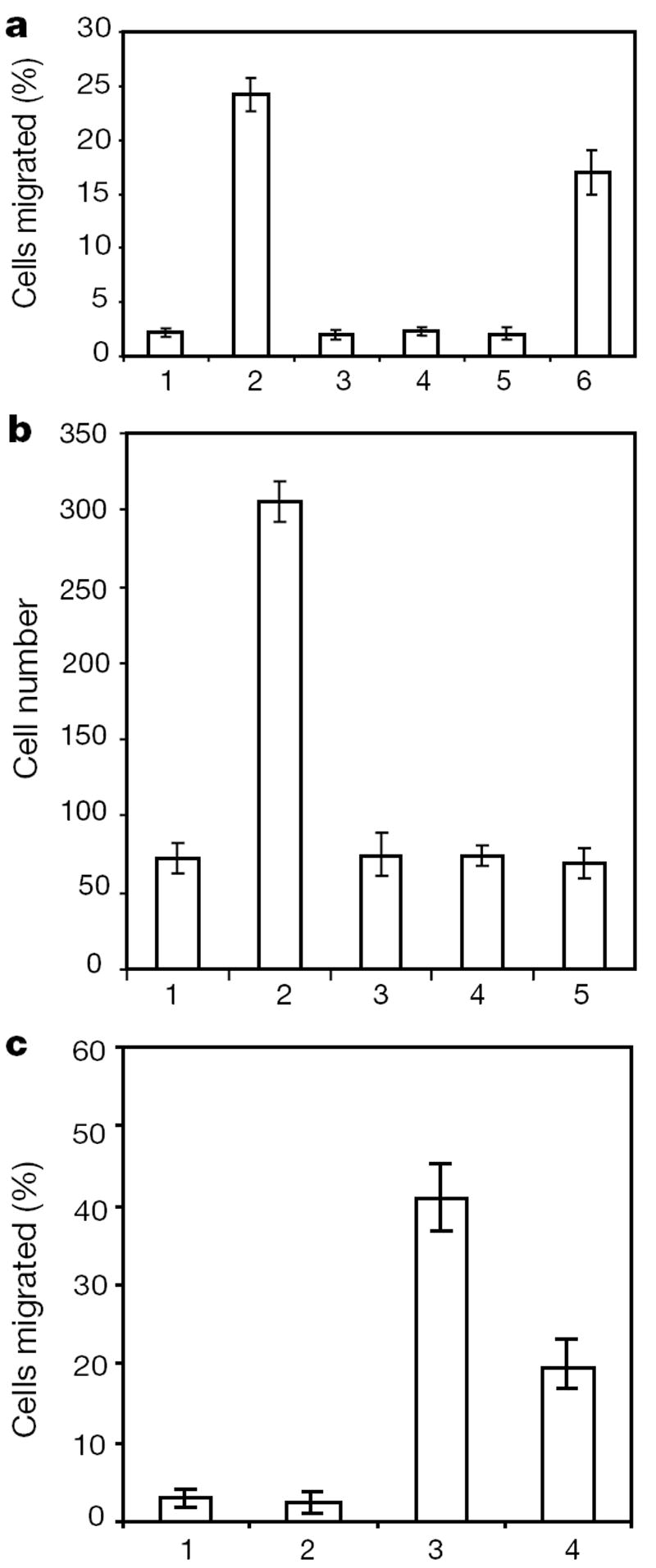
Effect of Slit on leukocyte chemotaxis induced by SDF-1α. a, Transwell migration of rat lymphocytes. Control or SDF-containing media were added to the lower chamber. Cells that migrated to the lower chamber were counted, and are expressed as a percentage of the cells added to the upper chamber. 1, control; 2, 10 nM SDF-1α; 3, 100 pM SLIT2; 4, 10 pM SLIT2; 5, 10 nM of SDF-1α and 100 pM of SLIT2; 6, 10 nM of SDF-1α and 10 pM of SLIT2. b, Rat lymphocytes were examined in transfilter assays in the presence of SDF-1α (10 nM). SLIT2 (100 pM) was added to the lower chamber, the upper chamber or both upper and lower chambers. 1, control; 2, SDF-1α; 3, both SDF-1α and SLIT2 in the lower chamber; 4, SDF-1α in the lower chamber and SLIT2 in the upper chamber; 5, SDF-1α in the lower chamber and SLIT2 in both chambers. c, Slit inhibition of chemotaxis induced by fMLP. HL-60 cells differentiated into neutrophil-like cells after treatment with DMSO. Chemotaxis was observed in transwell assays. 1, control; 2, SLIT2; 3, fMLP; 4, SLIT2 and fMLP.
To test whether Slit could affect chemotaxis induced by other chemotactic factors, we used the bacterial product _N_-formyl peptide f-Met-Leu-Phe (fMLP). fMLP attracts neutrophil-like cells differentiated from HL-60 cells23. We found that SLIT2 could inhibit the migration of differentiated HL-60 cells induced by fMLP (Fig. 2c).
Robo is a protein with a single transmembrane domain24,25, serving as a Slit receptor in axon guidance15-17 and neuronal migration19,21. To investigate whether Robo mediates leukocyte responses to Slit, we used RoboN, a fragment of Robo that contains only the extracellular part of the Robo protein19. The addition of RoboN abolished the inhibitory effect of Slit on leukocyte chemotaxis (Fig. 3a). Together with the expression of Robo in the haematopoietic system, these results provide evidence for the involvement of Robo in mediating Slit responses in leukocytes, supporting a conserved guidance mechanism for cell migration.
Figure 3.
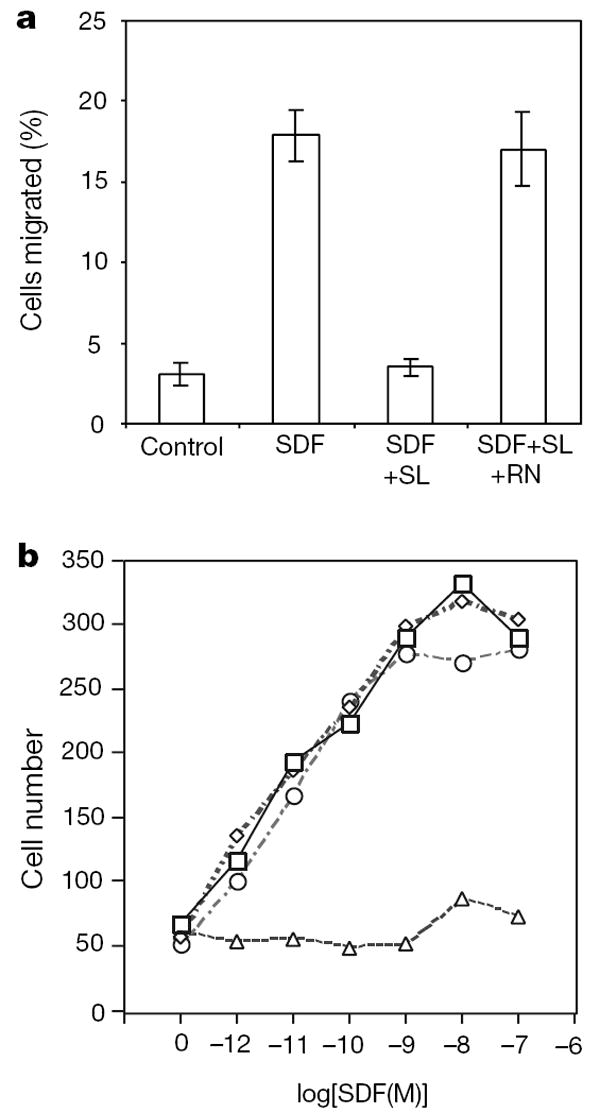
Robo is involved in mediating Slit inhibition of chemotaxis. a, The effect of Slit is inhibited by RoboN. Chemotaxis was analysed by using rat lymph node cells in transwell assays. SLIT2 (SL) and RoboN (RN) were added to the upper well and SDF-1α was added to the lower well. b, Expression of CXCR4 and Robo in HEK cells can reconstitute Slit inhibition of SDF-1α-induced chemotaxis. HEK migration was measured with the transfilter assay in microchemotaxis chambers using HEK cells expressing CXCR4, or both CXCR4 and rat Robo1 in the presence of different concentrations of SDF-1α. Control vehicle or 100 pM of purified Slit protein was added. Squares, migration of HEK cells expressing CXCR4 in response to SDF-1; diamonds, migration of HEK cells expressing CXCR4 in response to SDF-1α in the presence of Slit; circles, migration of HEK cells expressing Robo and CXCR4 in response to SDF-1α; triangles, migration of HEK cells expressing Robo and CXCR4 in response to SDF-1α in the presence of Slit.
The receptor for SDF-1 is CXCR4, a GPCR with seven transmembrane domains. To determine whether Robo and CXCR4 are sufficient to mediate the functional interaction between Slit and SDF-1, we introduced Robo and CXCR4 separately or together into HEK cells (Fig. 3b). When transfected with CXCR4, HEK cells migrated towards SDF-1. However, Slit significantly reduced SDF-1-induced chemotaxis of HEK cells expressing both robo1 and CXCR4, showing that the functional interaction between Slit and the chemokine SDF-1 could be reconstituted in HEK cells by the expression of both Robo and CXCR4.
We next considered whether the inhibitory effect of Slit on migratory responses was due to a general inhibitory or toxic effect on leukocytes. We tested whether Slit could inhibit two activities of fMLP that are not related to cell migration. fMLP activates the extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2) by causing their phosphorylation (Fig. 4a)26,27; however, Slit did not inhibit fMLP-induced ERK phosphorylation (Fig. 4a). fMLP also causes the oxidative burst of neutrophils28 (Fig. 4b); Slit did not cause oxidative burst, nor did it inhibit the oxidative burst induced by fMLP (Fig. 4b). In fact, Slit increased the oxidative burst induced by fMLP, which is consistent with the previous observation that regulation of the actin cytoskeleton can prime cells for oxidative burst. These results indicate that Slit inhibits chemotaxis, but not all other functions of leukocytes.
Figure 4.
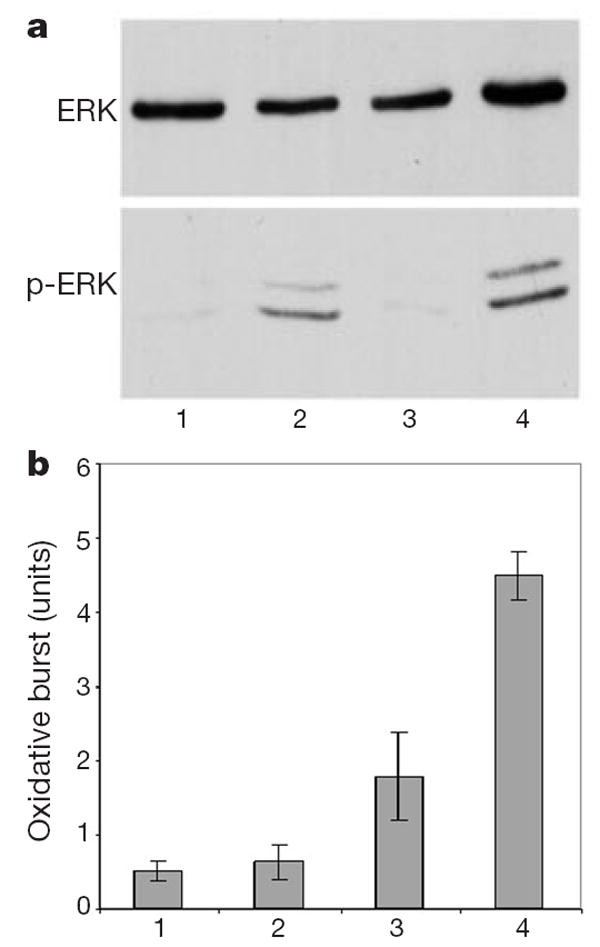
Lack of general inhibition by Slit. a, Neutrophils differentiated from HL-60 were treated with fMLP, or fMLP and SLIT2. Phosphorylation of ERK was examined by an anti-phospho-ERK antibody. Top, anti-ERK staining; bottom, anti-phospho-ERK staining. fMLP could induce ERK phosphorylation, which was not inhibited by the addition of SLIT2. Lane 1, control; lane 2, fMLP; lane 3, SLIT2; lane 4, fMLP and SLIT2. b, Oxidative burst was shown by SOD-inhibitable production of superoxide, and 1 unit was defined as nmol per 2 × 106 cells per 15 min. 1, control; 2, SLIT2; 3, fMLP; 4, SLIT 2 and fMLP. Results shown are representative of three experiments, each with triplicate samples.
Our findings that Slit can inhibit chemotaxis of leukocytes induced by chemotactic factors have implications in the conservation of molecular mechanisms of cell migration between species, and in biological and therapeutic regulations of immune responses and other physiological or pathological processes involving chemotactic factors. We suggest that neuronal guidance cues such as netrins, semaphorins and ephrins may also guide leukocyte migration and regulate biological processes involving chemotactic factors. Other molecules involved in neuronal positioning, such as Reelin12, should also be tested for their potential roles in leukocyte migration. Our results in HEK cells indicate a functional interaction between the single transmembrane receptor Robo and the GPCRs, suggesting a mechanism for regulating signal transduction pathways mediated by GPCRs and heterotrimeric G proteins.
Past research in leukocyte chemotaxis has been focused mainly on chemoattractants and positive regulation. Although there were reports of virally produced inhibitors of chemokines29,30, there is no known endogenous negative factors. Our results reveal that there are negative regulators of leukocyte chemotaxis available endogenously, which may be important in physiological and pathological situations. This expands the scope and the perspective of research in leukocyte biology. Chemokines have several roles, including inflammatory responses, leukocyte activation, lymphocyte trafficking and lymphoid organ homeostasis, tissue injury, haematopoiesis, atherosclerosis, allogeneic transplant rejection, angiogenesis, virally induced vascular diseases, cardic morphogenesis, and tumour development. In all of these situations except tumoriogenesis, inhibition of chemokine signalling is therapeutically beneficial, and much effort has been directed towards obtaining reagents that can block chemokines or their receptors. Potential applications of Slit should be tested.
Our studies of Slit broaden the search for chemokine inhibitors. Because the inhibition of receptor signalling can block HIV infection, Slit inhibition of chemokine receptors including CXCR4 (and CCR5; data not shown) suggests that Slit cannot be ruled out as a useful reagent in inhibiting HIV infection; thus, the therapeutic potentials for Slit and other negative factors are an attractive area of further research. Our unpublished data indicate that in vivo application of Slit protein attenuates crescentic glomerulonephritis in an animal model involving chemokine-induced leukocyte infiltration. In summary, our findings of Slit inhibition of leukocyte chemotaxis induced by chemotactic factors not only shed light on the fundamental conservation of mechanisms guiding cell migration, but also open up new areas for future investigations.
Methods
RNase protection assay
We used 5 μg total RNA for each sample prepared from tissues or cultured cells in RNase protection assays. Riboprobes specific for rat slit1, slit2 and slit3 genes were prepared, and RNase protection assay was performed using a kit (Torrey Pines Biologicals) according to the manufacturer’s protocols with corresponding probes labelled with [32P]UTP.
Isolation of leukocytes and assays with HL-60 cells
Leukocytes were isolated from rat lymph nodes, spleen or peripheral blood according to standard protocols. Cells were kept at 4 °C in media containing 5% fetal calf serum (FCS) until use; and cells were used within 8 h after isolation.
We grew the HL-60 cell line under standard conditions with RPMI-1640 media supplemented with 10% heat-inactivated FCS23. Cells induced with 1.2% dimethyl sulphoxide (DMSO) were obtained by seeding HL-60 cells at 3 × 106 ml−1 in growth media and culturing for 4–6 days (ref. 23). ERK Phosphorylation induced by 1 min of fMLP stimulation was examined with anti-phospho ERK antibody26. In Fig. 4a, serum-free conditioned medium from control HEK cells was used as control in lane 1; 100 nM of fMLP in the presence of serum-free conditioned medium from control HEK cells was used in lane 2; serum-free conditioned medium from SLIT2-secreting HEK cells was used in lane 3; 100 nM of fMLP in the presence of serum-free conditioned medium from SLIT2 secreting HEK cells was used in lane 4. In Fig. 4b, oxidative burst was quantified according as described28 by measuring the superoxide dismutase (SOD)-inhibitable reduction of cytochrome c. Briefly, differentiated HL-60 cells (2 × 106) containing 75 μM cytochrome c with or without SOD (30 μg) were incubated with fMLP (100 nM) for 15 min at 37 °C. The reaction was stopped by submerging the tubes in ice. The tubes were centrifuged, and the absorbency of the supernatant at 550 nM was measured in a spectrophotometer.
Assays for leukocyte chemotaxis
Leukocyte chemotaxis was measured by transwell and transfilter assays. Isolated leukocytes were resuspended at a concentration of 4 × 106 cells per ml in 50% DMEM, 50% M199, 5% heat-inactivated fetal bovine serum (FBS). Unless otherwise specified, 10 nM chemokines were placed at lower wells of chemotaxis chambers. Chemotaxis results are representative of at least three independent experiments performed in triplicates.
In the transwell assay, cells (100 μl) were put in inserts (5 μm in pore size) (Costar) placed in 24-well dish containing 600 μl of culture media per well with a chemokine or the Slit protein. After incubation at 37 °C for 1.5–3 h, cells migrated through the insert filter into the lower well were collected and counted.
In the transfilter assay, we placed different chemokines and Slit in the lower wells of a 48-well chemotaxis chamber (Neuroprobe, Cabin John, MD) and separated them by a polyvinylpyrolidone-free polycarbonate filter (5 or 8 μm in pore size). Isolated leukocytes or transfected HEK cells (50 μl) were put in the upper wells. After incubating at 37 °C for 2 h, cells on the top surface of the filter were removed and cells that had migrated through the filter onto the undersurface were fixed in methanol and stained. We expressed the transfilter migration of cells as cell number per five high-power (×400) fields.
HEK cell culture
We grew HEK cells in DMEM supplemented with 10% FBS. An HEK cell line expressing the extracellular domain of rat Robo1 protein tagged at the carboxy terminus with an haemagglutinin A (HA) tag (RoboN)19 was cultured in DMEM and 5% FBS. The RoboN-containing media was collected 3–4 d after cells became confluent. The RoboN-containing media was diluted to roughly 1 nM and used in cell migration assay. We also established a stable HEK cell line expressing rat CXCR4.
Acknowledgments
The amount of data presented and the number papers cited have been limited by space constraints. We are grateful to X. He for help with FACS; to W. Smith for providing us with the HL60 cell line; to the NIH for grant support (to J.Y.W., L.F. and Y.R.); to the John Merck fund, the Klingenstein foundation, and the Leukemia Society of America for scholar awards (to Y.R. and J.Y.W.).
References
- 1.Devreotes PN, Zigmond SH. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- 2.McCutcheon M. Chemotaxis in leukocytes. Physiol Rev. 1946;26:319–336. doi: 10.1152/physrev.1946.26.3.319. [DOI] [PubMed] [Google Scholar]
- 3.Bentivoglio M, Mazzarello P. The history of radial glia. Brain Res Bull. 1999;49:305–315. doi: 10.1016/s0361-9230(99)00065-9. [DOI] [PubMed] [Google Scholar]
- 4.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 5.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 6.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 7.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 8.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 9.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–341. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 10.Rakic P. Principles of neural cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- 11.Hatten ME, Heintz N. In: Fundamentals of Neuroscience. Zigmond M, editor. Academic; New York: 1998. pp. 451–479. [Google Scholar]
- 12.Rice DS, Curran T. Mutant mice with scrambled brains: understanding the signaling pathways that control cell positioning in the CNS. Genes Dev. 1999;13:2758–2773. doi: 10.1101/gad.13.21.2758. [DOI] [PubMed] [Google Scholar]
- 13.Nusslein-Volhard C, Wieschaus E, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Roux’s Arch Dev Biol. 1984;193:267–282. doi: 10.1007/BF00848156. [DOI] [PubMed] [Google Scholar]
- 14.Rothberg JM, Hartley DA, Walther Z, Artavanis-Tsakonas S. slit: an EGF-homologous locus of D. melanogaster involved in the development of the embryonic central nervous system. Cell. 1988;55:1047–1059. doi: 10.1016/0092-8674(88)90249-8. [DOI] [PubMed] [Google Scholar]
- 15.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the Robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 16.Brose K, et al. Evolutionary conservation of the repulsive axon guidance function of Slit proteins and of their interactions with Robo receptors. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 17.Li HS, et al. Vertebrate Slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 18.Wang KH, et al. Purification of an axon elongation- and branch-promoting activity from brain identifies a mammalian Slit protein as a positive regulator of sensory axon growth. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 19.Wu W, et al. Directional guidance of neuronal migration in the olfactory system by the secreted protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu H. Chemorepulsion of neuronal migration by Slit2 in the developing mammalian forebrain. Neuron. 1999;23:703–711. doi: 10.1016/s0896-6273(01)80029-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Li HS, Zhou L, Wu JY, Rao Y. Cellular and molecular guidance of GABAergic neuronal migration from the striatum to the neocortex. Neuron. 1999;23:473–485. doi: 10.1016/s0896-6273(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 22.Yuan W, et al. The mouse Slit family: secreted ligands for Robo expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 1999;212:290–306. doi: 10.1006/dbio.1999.9371. [DOI] [PubMed] [Google Scholar]
- 23.Collins SJ, Fuscetti FW, Gallagher RE, Gallo RC. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979;149:969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidd T, et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- 25.Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell. 1998;92:217–227. doi: 10.1016/s0092-8674(00)80916-2. [DOI] [PubMed] [Google Scholar]
- 26.Krump E, Sanghera JS, Pelech SL, Furuya W, Grinstein S. Chemotactic peptide N-formyl-Met-Leu-Phe activation of p38 mitogen-activated protein kinase (MAPK) and MAPK-activated protein kinase-2 in human neutrophils. J Biol Chem. 1997;272:937–944. doi: 10.1074/jbc.272.2.937. [DOI] [PubMed] [Google Scholar]
- 27.Downey GP, et al. Importance of Mek in neutrophil microbicidal responsiveness. J Immunol. 1998;160:434–443. [PubMed] [Google Scholar]
- 28.O’Brien PJ. Superoxide production. Methods Enzymol. 1984;105:370–379. doi: 10.1016/s0076-6879(84)05050-3. [DOI] [PubMed] [Google Scholar]
- 29.Smith CA, et al. Poxvirus genomes encode a secreted, soluble protein that preferentially inhibits beta chemokine activity yet lacks sequence homology to known chemokine receptors. Virology. 1997;236:316–327. doi: 10.1006/viro.1997.8730. [DOI] [PubMed] [Google Scholar]
- 30.Kledal TN, et al. A broad-spectrum chemokine antagonist encoded by Kaposi’s sarcoma-associated herpes virus. Science. 1997;277:1656–1659. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]