Role of Nod2 in the Response of Macrophages to Toll-Like Receptor Agonists (original) (raw)
Abstract
Nod2 (CARD15) is a macrophage-specific protein containing two CARD domains, a large nucleotide binding domain and leucine-rich repeats. Human genetic studies have linked mutations in NOD2/CARD15 with Crohn's disease, although the mechanisms involved are unknown. However, Nod2 has been proposed to directly bind bacterial lipopolysaccharide (LPS) and subsequently act as an activator of NF-κB via the association of the CARD domains with Rip2/RICK/CARDIAK. This is hypothesized to constitute a pathogen recognition pathway distinct from Toll-like receptor 4-mediated recognition of LPS. Using targeted mutagenesis, we introduced a mutation to delete the CARD domains of mouse Nod2. Mice lacking Nod2 were indistinguishable from controls and showed no signs of intestinal pathology. Macrophages responded normally to multiple Toll-like receptor agonists in terms of NF-κB target activation, mitogen-activated protein kinase activation, and cytokine secretion. However, Nod2−/− mice were significantly protected in endotoxin challenge experiments, and Nod2−/− macrophages were refractory to muramyl dipeptide stimulation. These results argue that Nod2 does not play an essential, nonredundant role in the response of macrophages to bacterial products but rather plays unexpected roles in regulating systemic responses to pathogens.
Nod2 is a macrophage-specific protein that is a member of a larger family that contains an overall domain architecture of N-terminal CARD or Pyrin domains, a central nucleotide binding domain, and C-terminal leucine-rich repeats (LRRs) (31). Genes with a similar modular structure have recently been christened the CATERPILLER family (15). In mammals, Nod2's closest relative is Nod1, a ubiquitously expressed protein having a similar structure except for a single CARD domain, while Nod2 has two N-terminal CARD domains (31). It is the CARD domains that have most readily linked Nod function to other cellular processes. Both Nod1 and Nod2 have been shown to associate with the Rip2/RICK/CARDIAK kinase through heterotypic CARD domain interactions (6, 24, 31, 41). This association has been postulated to link Nod function to apoptotic cascades and the modulation of NF-κB activation (6, 24, 31, 41).
Interest in Nod function has accelerated because substantial numbers of familial Crohn's disease patients contain mutations in Nod2 (18, 30). Although Crohn's disease is an extremely complex disease group where multiple genetic and environmental factors conspire to initiate and perpetuate pathogenesis (34), linkage analysis unequivocally identified NOD2/CARD15 as the IBD1 locus (18). Subsequent human genetic studies have confirmed the initial linkage observations predominantly in Western populations (13, 14, 28), while others (e.g., Japanese Crohn's disease patients) lack any of the commonly observed mutant alleles found in European cohorts (22, 40). The mutations found in European populations appear, based on genetic analyses, to be loss-of-function or partial loss-of-function alleles (28). Most disease-causing mutations, as defined by genetic studies, cluster in the LRR-coding exons (28). Their roles in Crohn's disease pathogenesis are unknown (4).
Nuñez and colleagues and Girardin et al. have proposed that Nod proteins are intracellular receptors for bacterial products, in particular lipopolysaccharide (LPS) (11, 19, 20, 30). This hypothesis is correlated with two key findings. Toll-like receptors (TLRs) also contain LRRs and are linked to the recognition of pathogen-derived products, including LPS (37). Second, Nod proteins have domains similar to those of a large group of plant R proteins involved in resistance to pathogens (16). Taken together, Nod proteins are thus suspected of being responsible for the intracellular discrimination of pathogen products, analogous to the essential extracellular recognition functions mediated through the TLRs (19). The intracellular recognition of LPS by the Nods activates NF-κB and other critical innate defense mechanisms. The same groups that proposed that Nods recognize LPS have recently revised their initial observations and now propose that muramyl dipeptide (MDP) moieties from bacterial peptidoglycan, rather than LPS, are the key components selected by the Nod2 LRRs (10, 21). It is tempting to speculate that disruption of this process would be associated with Crohn's disease because of the key role that bacterial surveillance by macrophages is expected to play in gut physiology (2, 34). However, no direct evidence exists for such a role for Nod2 (2). Furthermore, a direct function of Nod2, a macrophage-specific protein, in LPS recognition is uncertain, since mice and macrophages with a complete disruption of TLR4 are nonresponsive to LPS (17), arguing that if Nod proteins were indeed unique receptors for LPS, their activity must be downstream of TLR4 function. To gain further insight into this controversial field, we constructed mice that lack functional Nod2 and examined the responses of animals and macrophages derived from them to key pathogen products that activate innate immune responses.
MATERIALS AND METHODS
Targeting vectors, ES cell culture, and mouse breeding.
PCR primers were designed to amplify the 5′ and 3′ targeting vector arms encompassing 1.5 and 5 kb, respectively, that flank the first exon encoding the first and half of the second CARD domains encoding Nod2. The arms were isolated by high-fidelity long-range PCR (Herculase; Stratagene) from C57BL/6 genomic DNA. The arms were cloned into pGT-N28-Asc I (39) to create the final targeting vector. The linearized targeting vector was electroporated into C57BL/6 Bruce4 ES cells (39), and G418-resistant clones were selected. One clone (855-13) was isolated and showed correct targeting of the NOD2 locus by Southern blotting and long-range PCR. This clone also had a correct karyotype, and fluorescence in situ hybridization (FISH) analysis using the entire targeting vector as a probe showed that the targeting vector had been integrated into chromosome 8, the known location of the NOD2 locus. Germ line transmitting chimeras were crossed to BALB/c mice to generate Nod2+/− mice. These animals were intercrossed to generate Nod2−/− mice and control animals on a BALB/c × C57BL/6 mixed background.
Cell isolation and stimulation.
Bone marrow-derived macrophages (BMDMs) were isolated and cultured as described previously (33). Macrophages were stimulated with TLR agonists, LPS (serotype 0111:B4; Sigma), Poly(I:C) dsRNA (Pharmacia), repurified LPS lacking TLR2-stimulating activity (a gift of Neal Silverman, University of Massachusetts), highly purified streptococcal peptidoglycan (a gift of Elaine Tuomanen, St. Jude Children's Research Hospital), MDP (Sigma), tumor necrosis factor alpha (TNF-α), interleukin 10 (IL-10), and gamma interferon (IFN-γ) (all from BD Biosciences), as described in the figure legends. Resident spleen dendritic cells were isolated from 8 to 10 spleens from 8-week-old mice using the method described by Aliberti et al. (1) except that anti-CD11c+ magnetic cell sorting beads (Miltenyi) were used to increase the purity of the CD11c+ fraction prior to culture. The CD11c negative fraction (∼98% spleen cells) was used to enrich T- and B-cell populations. T cells were isolated by positive selection using Thy1.2 magnetic cell sorting beads. The negative fractions were plated in 175-cm2 dishes for 2 h to remove adherent macrophages, and the resulting nonadherent fraction comprised predominantly B220+ B cells. Cell populations were lysed with Trizol, and total RNA was isolated.
RNA isolation and Northern blotting, RT-PCR analysis, immunoblotting, and ELISAs.
Total RNA was isolated from primary macrophages using Trizol and processed as described previously (27). Northern blotting was performed as described previously using probes isolated from cDNA clones (27). Reverse transcription (RT)-PCR was performed as described previously (27) using Superscript II (Life Technologies). Immunoblotting was performed as described previously (33) using the following antibodies. Anti-phospho-p42,p44 ERK, anti-phospho-p38, and anti-phospho-IκBα rabbit polyclonal antibodies were all from Cell Signaling Technology and all were used at a 1:500 final dilution. Anti-p42,p44 ERK, anti-p38, and anti-IκBα were from the same source and were used at a final concentration of 1:1,000. Cytokine-specific enzyme-linked immunosorbent assay (ELISA) capture and secondary antibodies were all from BD Biosciences. All antibodies used for flow cytometry were from BD Biosciences.
Endotoxin challenge.
Male or female mice (age 20 to 28 days in one series of experiments and 45 to 80 days in another series) were weighed and challenged intraperitoneally with 40 mg of E. coli LPS (serotype 0111:B4)/kg made to a final concentration of 2.5 mg/ml in phosphate-buffered saline. Mice were monitored every 4 to 6 h postinjection, and the experiments were terminated at 96 h. Survival data were analyzed using SAS software (Cary, N.C.), and P values were calculated using the log rank test.
Histology.
Intestines of mice were dissected and placed in buffered formalin overnight. Fecal material was removed, and the intestines were cut into fragments representative of colon, cecum, duodenum, and ileum. Paraffin-embedded material was sectioned and stained with hematoxylin and eosin.
RESULTS
Construction of mice lacking Nod2.
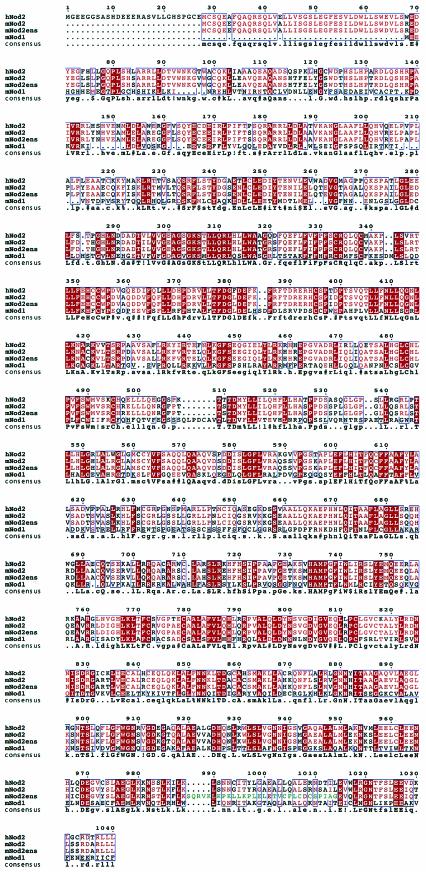
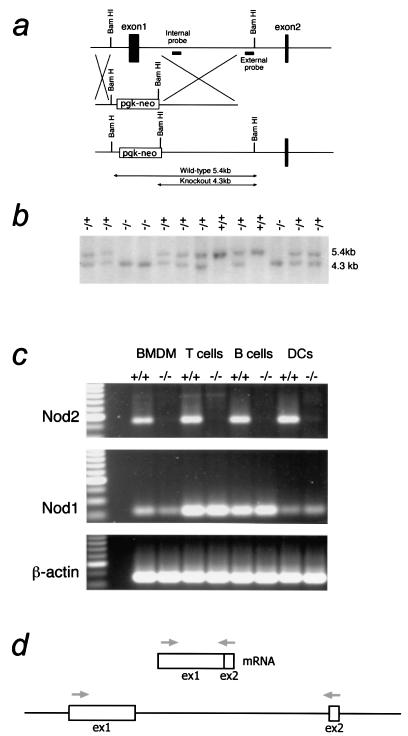
We first isolated a mouse cDNA clone from macrophages and compared it to the published human Nod1 and Nod2 sequences. The predicted mouse Nod2 sequence was highly similar to that of human Nod2 and also contained two linked CARD domains (Fig. 1). Interestingly, the macrophage mNod2 contained an alternate LRR-encoding exon (Fig. 1) compared to the predicted mouse genome sequence (Ensembl ID: ENSMUSG00000031663). The function of this exon is unknown at present. To create a null version of the NOD2 gene, a mutagenesis strategy was adopted to delete exon 1, carrying the majority of the sequence encoding the CARD domains, including the start codon (Fig. 2a). The rationale for choosing this strategy was to delete a region of Nod2 demonstrated to be necessary for its function. The CARD domains have been shown to play important roles in binding Rip2/RICK/CARDIAK and are likely to be essential for Nod2 binding to other CARD domain proteins (24, 31). Therefore, we anticipated that the deletion of the first exon would create a null allele. Southern blotting analysis using an external probe showed the expected pattern for the targeted allele (Fig. 2b). Mice lacking Nod2 on a mixed BALB/c × C57BL/6 background were isolated by intercrossing heterozygous mice and showed normal Mendelian ratio from >300 mice. Attempts to produce antibodies against mouse Nod2 have repeatedly failed, precluding a detailed analysis of Nod2 protein levels in tissues and cells. In addition, there is a paucity of commercially available antibodies against Nod2. Therefore, we adopted an RT-PCR approach to further characterize Nod2 mRNA expression.
FIG.1.
Alignment of the predicted amino acid sequences of Nod2 proteins. Conceptual translations of Nod proteins were aligned using the Multalign program (7). The human Nod2 (hNod2) cDNA was derived from public databases. The mouse Nod1 and Nod2 (mNod2ens) sequences were derived from genomic information obtained from the Ensembl database. These sequences were compared to cDNA sequences for Nod2 that we isolated from macrophage RNAs. Note that the Ensembl and experimentally derived Nod2 sequences differ in a region encoding approximately one LRR repeat (shown in green on the mNod2ens sequence).
FIG. 2.
Creation of Nod2−/− mice. (a) Diagram showing the targeting strategy to delete the first exon of Nod2 encoding the first CARD domain and the start codon in addition to part of the second CARD domain. (b) Southern analysis of tail DNA isolated from a litter produced by a Nod2+/− mouse cross-probed with the external probe shown in panel a. (c) RT-PCR analysis of cells isolated from Nod2−/− mice. Total RNA was isolated from BMDMs, T or B cells, or CD11c+ enriched DCs from +/+ or Nod2−/− mice. RNA was reverse transcribed and subjected to PCR analysis for Nod2 exon 1/2, Nod1, or actin (control). A diagram of the RT-PCR strategy for Nod2 is shown in panel d. A similar strategy to cross the first intron was adopted for Nod1.
To test if a putative null allele had been created, macrophages were isolated from wild-type or Nod2−/− mice and subjected to RT-PCR analysis. These results showed that the mRNA encoding the CARD domains was absent in Nod2−/− macrophages (Fig. 2c). We also measured mRNA levels in other hematopoietic cells, including T cells, B cells, and spleen dendritic cells. All these lineages showed an absence of Nod2 mRNA by RT-PCR. These experiments also show that Nod2 mRNA is detected in other cell types examined, suggesting that the initial reports of macrophage specificity in human systems probably do not extend to the mouse (23).
Macrophages lacking Nod2 have near-normal responses to TLR stimulation as well as other key macrophage-activating and -deactivating cytokines.
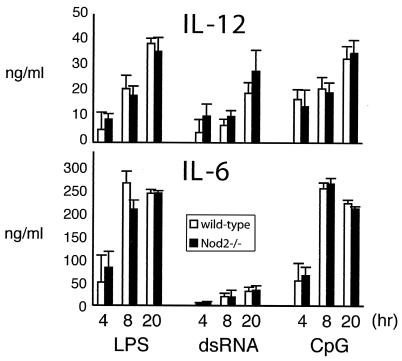
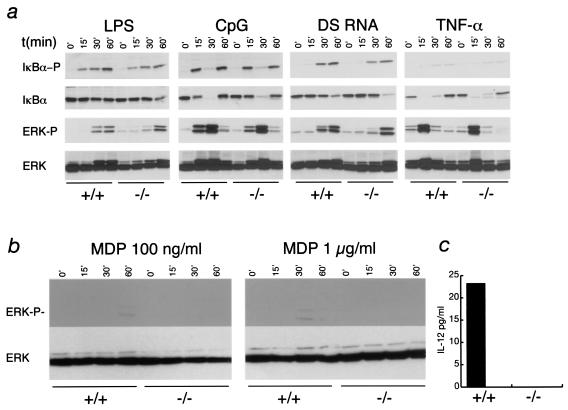
To test the role of Nod2 in regulating macrophage responses to different activating and deactivating stimuli, we challenged BMDMs with TLR agonists, LPS, repurified LPS lacking TLR2-stimulating activity, CpG DNA, double-stranded RNA (dsRNA), and gram-positive peptidoglycans. Responses were measured by the production of key inflammatory cytokines, IL-12, IL-6 (see Fig. 4), and TNF-α (data not shown). In addition, we examined whether IκBα, p44/p42 ERK, and p38 mitogen-activated protein (MAP) kinase activation was defective in Nod2−/− cells (Fig. 3). The results show that in all cases, Nod2−/− macrophages behaved similarly to wild-type cells with the exception that ERK phosphorylation was slightly delayed when macrophages were stimulated with CpG DNA or LPS (Fig. 3). However, this delay did not translate into changes in cytokine production (Fig. 4), which are dependent on the correct integration of NF-κB and MAP kinase pathways.
FIG. 4.
Cytokine responses from Nod2−/− macrophages stimulated with TLR agonists. BMDM from wild-type (light bars) or Nod2−/− (black bars) mice were plated in 24-well plates at 0.5 × 106 per well and stimulated with TLR agonists as described in the legend to Fig. 3. Aliquots of cell supernatants were removed at 4, 8, or 20 h and assayed for IL-12 or IL-6 using ELISA.
FIG. 3.
Responses of Nod2−/− macrophages to TLR stimulation. (a) BMDM from wild-type or Nod2−/− mice were plated at 106 per well in 12-well plates and stimulated with LPS (100 ng/ml), CpG (2 μM), poly(I:C) dsRNA (2 μg/ml), or TNF-α (10 ng/ml) for 0, 15, 30, or 60 min and then lysed in RIPA buffer. Lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblots were probed with antibodies to the phosphorylated forms of IκBα or p42,p44 ERK. Blots were reprobed with antibodies to IκBα or ERK proteins. Results are representative of four independent experiments. (b) Response of Nod2−/− macrophages to MDP. Cells were stimulated with MDP at 100 ng/ml or 1 μg/ml for the times shown. Lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblots were probed with antibodies to the phosphorylated forms of p42,p44 ERK. Blots were reprobed with antibodies to ERK proteins. (c) IL-12 production from MDP-stimulated macrophages. Macrophages were stimulated with MDP (1 μg/ml) for 20 h, and IL-12 levels in the supernatant were measured by ELISA.
Recent data have demonstrated that in transfection assays, Nod2 can recognize MDP and activate NF-κB signaling (10, 21). To test this with Nod2−/− macrophages, we stimulated cells with chemically synthesized MDP. Nod2−/− macrophages showed a complete absence of ERK phosphorylation in response to MDP and failure to secrete IL-12. However, the response of macrophages to MDP is substantially lower than that to any TLR agonist examined. The same was true when MDP was delivered to macrophages in the presence of lipofectamine, a cationic lipid transfection agent that we used to attempt to deliver higher levels of MDP into the cells (data not shown). Thus, these data support the concept that Nod2 plays a function in the response to MDP (10, 21). Overall, however, these results suggested that Nod2 does not play a major role in the activation of proinflammatory pathways in macrophages. The slight changes evident in the activation of ERK phosphorylation suggest that Nod2 may play subtle roles in regulating this pathway that will require further analysis.
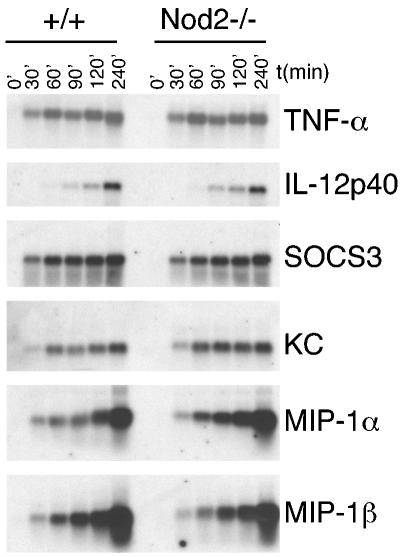
We also tested the induction of inflammatory mRNAs after macrophages were challenged with LPS. Induction of TNF-α, IL-12p40, SOCS3, and the mRNAs encoding chemokines KC, MIP1α, and MIP1β were similar in wild-type and Nod2−/− macrophages (Fig. 5). Another possibility for Nod2 function is that it affects cytokine pathways that are crucial in macrophage activation and deactivation. Accordingly, we treated Nod2−/− macrophages with a macrophage-activating cytokine (IFN-γ) or a key deactivating cytokine (IL-10) using standard readout assays, and the results from these studies (data not shown) suggest that Nod2 does not play an essential role in the signaling pathways emanating from the receptors for these cytokines.
FIG. 5.
mRNA levels of inflammatory markers from LPS-stimulated Nod2−/− macrophages. BMDM from wild-type or Nod2−/− mice were plated at 106 per well in 12-well plates and stimulated with LPS (100 ng/ml) for the times indicated at the top. Total RNA was isolated, separated in formaldehyde agarose gels, and analyzed by Northern blotting, using cDNA probes as shown on the right.
Response of Nod2−/− mice to endotoxin challenge.
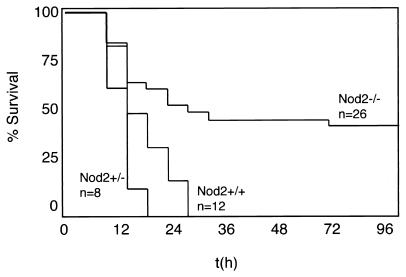
Preliminary experiments established that Nod2−/− mice behaved similarly to wild-type littermates at lower LPS challenge doses (32 mg/kg; data not shown). However, when weanling mice were challenged with a higher dose determined to induce >50% lethality in wild-type mice at 24 h, approximately half the Nod2−/− mice survived the entire length of two experiments (Fig. 6) through 96 h. Surviving Nod2−/− mice exhibited signs of endotoxin shock, including lethargy, dehydration, and ruffled fur. The surviving fraction of Nod2−/− mice, however, had completely recovered by 96 h. The same experiments were repeated with older mice (45 to 80 days), but in these experiments, the dramatic differences in the survival curves were less apparent but still statistically different (data not shown). These experiments indicate that the absence of Nod2 enhances survival in endotoxic shock under some conditions.
FIG. 6.
Nod2−/− mice can withstand endotoxin challenge. Twenty- to twenty-eight-day-old wild-type, Nod2+/−, or Nod2−/− mice were weighed and challenged intraperitoneally with 40 mg of E. coli LPS/kg of body weight made to a final concentration of 2.5 mg/ml in phosphate-buffered saline. Mice were monitored every 4 to 6 h postinjection, and the experiments were terminated at 96 h. Statistical analysis demonstrated that Nod2−/− mice behaved significantly differently from +/+ or +/− mice (P = 0.0069 and 0.0015, respectively) when analyzed using the log rank sum test. The data shown are the combination of results from two independent experiments using the same batch of LPS and identical conditions. In one experiment, +/+ mice were compared to −/− mice, and in the other, +/− and −/− mice were compared. Accordingly, the difference between +/+ and +/− mice was not analyzed.
Normal dendritic cell maturation in response to TLR ligands.
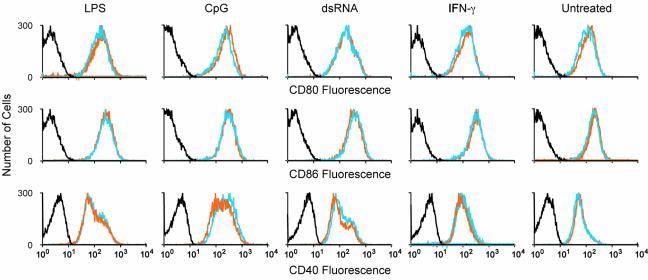
Dendritic cells (DCs) play an obligate role in the early response to pathogens and the subsequent development of adaptive immunity. Nod2 mRNA expression was detected in CD11c+ splenic DCs, suggesting a possible role in DC biology. Accordingly, we tested multiple markers of TLR-stimulated DC maturation. DCs were isolated in equivalent numbers from wild-type and Nod2−/− mice (∼1 to 2% of the total cell population), and major histocompatibility class II expression was uniformly high for both populations. We matured the DCs in vitro with LPS, CpG, dsRNA, or IFN-γ and examined the cell surface levels of three key costimulatory molecules, CD80, CD86, and CD40. Nod2−/− DCs exhibited identical levels of each marker (Fig. 7), suggesting that DC maturation is unaffected in the absence of Nod2.
FIG. 7.
DC maturation in response to TLR stimulation. Spleen CD11c+ enriched DCs from wild-type (orange lines) or Nod2−/− (blue lines) mice were isolated as described in Materials and Methods. Cell were placed in culture (4 × 106 cells per well in 12-well plates) and stimulated overnight as described in the legend to Fig. 3. Cell surface levels of CD80, CD86, and CD40 were analyzed by flow cytometry. Data from isotype controls is shown in the black line. In these experiments, cells were labeled with fluorescein isothiocyanate-conjugated anti-CD11c and phycoerythrin- or antigen-presenting-cell-labeled antibodies against the costimulatory markers. Cells present within the fluorescein isothiocyanate gate only are included in the data shown. This experiment is representative of two independent experiments, each including 8 to 10 mice per genotype for DC enrichment.
Lack of intestinal pathology in Nod2−/− mice.
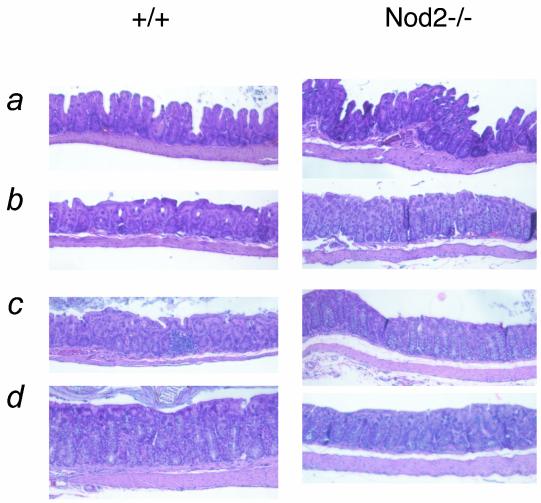
To test if Nod2−/− mice manifested any symptoms consistent with human Crohn's disease, we examined the intestines from cohorts of mice aged 1 to 7 months (Fig. 8). No Nod2−/− mice had any obvious histological abnormalities in the colon, cecum, duodenum, or ileum.
FIG. 8.
Histological examination of the intestines of Nod2−/− mice. Representative micrographs from the colons of wild-type or Nod2−/− mice. Mice of various ages were included in the analysis (a, 6 to 7 months; b, 3 to 4 months; c, 2 months; d, 1 to 2 months). A total of 12 mice per genotype were examined.
DISCUSSION
The results of our analysis of Nod2−/− mice and of macrophages and other cells from these animals suggest that Nod2 does not play an overt, critical role in regulating any of the known, essential macrophage-activating or -deactivating pathways. Responses to a variety of TLR agonists, signaling through TLR2 (peptidoglycan), TLR4 (LPS), TLR3 (dsRNA), and TLR9 (CpG DNA), are intact in terms of the activation of NF-κB and MAP kinase pathways, measured through phosphorylation of IκBα and activation of ERK and p38 phosphorylation, respectively. While we did observe slight differences in the patterns and kinetics of the accumulation of phosphorylated species in these assays, these discrepancies were not translated into alterations in cytokine production. However, when challenged with MDP, a component of peptidoglycan, Nod2−/− macrophages were unresponsive in terms of ERK phosphorylation and IL-12 production, consistent with recently published data using transfection systems (10, 21). MDP, however, is a weak agonist of macrophages: it stimulates 4 to 5 orders of magnitude less IL-12 than LPS when both are used at 100 ng/ml. This raises the question of the biological significance of this finding. Because Nod2 is expressed in cells other than macrophages (4, 23, 26, 32), MDP recognition could, however, be crucial in these cell systems.
Responses to key macrophage-regulatory cytokines IFN-γ and IL-10 were also normal in Nod2−/− macrophages. Furthermore, Nod2−/− mice displayed overtly normal intestinal pathology (mice aged 1 to 7 months) when maintained under specific-pathogen-free conditions. It may be necessary to challenge Nod2−/− mice with a variety of gut flora in order to reveal any intestinal pathology. Taken together, these results suggest that Nod2 is not an essential mediator of macrophage function in response to microbial challenge using a variety of known, established in vitro assays. Future studies will be required to address the in vivo role of Nod2 in the response to a variety of pathogens, including extracellular and intracellular bacteria.
The most surprising result from these studies demonstrated that the absence of Nod2 had a substantial protective effect in systemic endotoxin challenge experiments in younger mice. Therefore, Nod2 function is involved in systemic inflammatory responses, but it is unclear at present how Nod2 would influence this complex response. The phenotype observed is reminiscent of the phenotype of ICE/Caspase-1−/− mice that can withstand a high-dose endotoxin challenge (29). These results are not directly comparable with the present study, since they used a fixed dose of LPS (800 μg/mouse) while we used a dose dependent upon the weight of each animal. ICE/Caspase-1 associates with Rip2/Rick/CARDIAK, a known Nod2 and Nod1 binding partner (38, 41). ICE/Caspase-1 is essential for the proteolytic processing of IL-1β and IL-18, initiating cytokine-mediated pathology in sepsis (12). Intriguingly, Rip2/Rick/CARDIAK-deficient mice also survive systemic LPS challenge with a low dose (28 mg/kg) (6). Finally, multiple lines of evidence have linked ICE/Caspase-1-mediated generation of IL-1 and IL-18 with human inflammatory bowel diseases (35). The link between Nod2 and ICE/Caspase-1 enzymatic activity will be an important future direction of these studies.
At present we have little indication of the true physiological role(s) of the Nod proteins. The initial suggestion that Nod2 is required for the intracellular recognition of bacterial LPS, MDP, or any other TLR agonist was not tested here for the reason that these experiments are difficult to perform in a definitive manner. Delivery of TLR agonists within macrophages without any concomitant stimulation through a TLR would seem a complex technical challenge. Furthermore, it remains to be determined why macrophages would need to express an intracellular receptor for LPS or any other pathogen patterns, given that they already have a highly efficient and potent recognition system in place with the TLRs. Perhaps the Nod proteins have nothing at all to do with direct pathogen recognition and instead are involved in other cellular processes (8).
The modular nature of the Nods, and other CATERPILLER proteins, may enable them to regulate numerous signaling pathways that could be distinct from the heterotypic CARD interaction with Rip2/RIK/CARDIAK, since this interaction has not been directly demonstrated in macrophages to date. The fact that Nod proteins contain LRRs may have little to do with pathogen recognition, since many families of proteins contain LRRs and they are used in a spectrum of cellular processes (25). Finally, the connection, either at the cellular or molecular level, between Nod2 and Crohn's diseases remains to be established (2). The generation of Nod2−/− mice will enable the detailed examination of intestinal pathogenesis in the absence of Nod2 function using the many established animal models of inflammatory bowel disease (3, 36). Finally, the recent description of mice lacking Nod1 (5, 9) suggests that mice lacking both Nod1 and Nod2 could be readily examined for redundant or overlapping effects mediated by either protein.
Acknowledgments
We thank Ana Varela for help with picking ES clones, Philippe Bois for discussions, Julio Aliberti for the DC isolation protocol, Jerry Shenep and Aditya Guar for statistical analysis of the endotoxin challenge experiments, Elaine Tuomanen for peptidoglycan, and Neal Silverman for the purified LPS. The technical expertise of the St. Jude Flow Cytometry Facility is acknowledged.
This work was supported by AI53478-01 and American Heart Association grant 344901 to P.J.M., Cancer Center CORE grant P30 CA 21765, and by ALSAC.
REFERENCES
- 1.Aliberti, J., S. Hieny, C. Reis e Sousa, C. N. Serhan, and A. Sher. 2002. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat. Immunol. 3**:**76-82. [DOI] [PubMed] [Google Scholar]
- 2.Beutler, B. 2001. Autoimmunity and apoptosis: the Crohn's connection. Immunity 15**:**5-14. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg, R. S., L. J. Saubermann, and W. Strober. 1999. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr. Opin. Immunol. 11**:**648-656. [DOI] [PubMed] [Google Scholar]
- 4.Bouma, G., and W. Strober. 2003. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 3**:**521-533. [DOI] [PubMed] [Google Scholar]
- 5.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4**:**702-707. [DOI] [PubMed] [Google Scholar]
- 6.Chin, A. I., P. W. Dempsey, K. Bruhn, J. F. Miller, Y. Xu, and G. Cheng. 2002. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature 416**:**190-194. [DOI] [PubMed] [Google Scholar]
- 7.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16**:**10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dangl, J. L., and J. D. Jones. 2001. Plant pathogens and integrated defence responses to infection. Nature 411**:**826-833. [DOI] [PubMed] [Google Scholar]
- 9.Girardin, S. E., I. G. Boneca, L. A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M. K. Taha, A. Labigne, U. Zathringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300**:**1584-1587. [DOI] [PubMed] [Google Scholar]
- 10.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278**:**8869-8872. [DOI] [PubMed] [Google Scholar]
- 11.Girardin, S. E., R. Tournebize, M. Mavris, A. L. Page, X. Li, G. R. Stark, J. Bertin, P. S. DiStefano, M. Yaniv, P. J. Sansonetti, and D. J. Philpott. 2001. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2**:**736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu, Y., K. Kuida, H. Tsutsui, G. Ku, K. Hsiao, M. A. Fleming, N. Hayashi, K. Higashino, H. Okamura, K. Nakanishi, M. Kurimoto, T. Tanimoto, R. A. Flavell, V. Sato, M. W. Harding, D. J. Livingston, and M. S. Su. 1997. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 275**:**206-209. [DOI] [PubMed] [Google Scholar]
- 13.Hampe, J., A. Cuthbert, P. J. Croucher, M. M. Mirza, S. Mascheretti, S. Fisher, H. Frenzel, K. King, A. Hasselmeyer, A. J. MacPherson, S. Bridger, S. van Deventer, A. Forbes, S. Nikolaus, J. E. Lennard-Jones, U. R. Foelsch, M. Krawczak, C. Lewis, S. Schreiber, and C. G. Mathew. 2001. Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet 357**:**1925-1928. [DOI] [PubMed] [Google Scholar]
- 14.Hampe, J., J. Grebe, S. Nikolaus, C. Solberg, P. J. Croucher, S. Mascheretti, J. Jahnsen, B. Moum, B. Klump, M. Krawczak, M. M. Mirza, U. R. Foelsch, M. Vatn, and S. Schreiber. 2002. Association of NOD2 (CARD 15) genotype with clinical course of Crohn's disease: a cohort study. Lancet 359**:**1661-1665. [DOI] [PubMed] [Google Scholar]
- 15.Harton, J. A., M. W. Linhoff, J. Zhang, and J. P. Ting. 2002. Cutting edge: CATERPILLER: a large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine-rich repeat domains. J. Immunol. 169**:**4088-4093. [DOI] [PubMed] [Google Scholar]
- 16.Holt, B. F., D. A. Hubert, and J. L. Dangl. 2003. Resistance gene signaling in plants—complex similarities to animal innate immunity. Curr. Opin. Immunol. 15**:**20-25. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162**:**3749-3752. [PubMed] [Google Scholar]
- 18.Hugot, J. P., M. Chamaillard, H. Zouali, S. Lesage, J. P. Cezard, J. Belaiche, S. Almer, C. Tysk, C. A. O'Morain, M. Gassull, V. Binder, Y. Finkel, A. Cortot, R. Modigliani, P. Laurent-Puig, C. Gower-Rousseau, J. Macry, J. F. Colombel, M. Sahbatou, and G. Thomas. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411**:**599-603. [DOI] [PubMed] [Google Scholar]
- 19.Inohara, N., and G. Nunez. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3**:**371-382. [DOI] [PubMed] [Google Scholar]
- 20.Inohara, N., Y. Ogura, F. F. Chen, A. Muto, and G. Nunez. 2001. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J. Biol. Chem. 276**:**2551-2554. [DOI] [PubMed] [Google Scholar]
- 21.Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, J. Crespo, K. Fukase, S. Inamura, S. Kusumoto, M. Hashimoto, S. J. Foster, A. P. Moran, J. L. Fernandez-Luna, and G. Nunez. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278**:**5509-5512. [DOI] [PubMed] [Google Scholar]
- 22.Inoue, N., K. Tamura, Y. Kinouchi, Y. Fukuda, S. Takahashi, Y. Ogura, N. Inohara, G. Nunez, Y. Kishi, Y. Koike, T. Shimosegawa, T. Shimoyama, and T. Hibi. 2002. Lack of common NOD2 variants in Japanese patients with Crohn's disease. Gastroenterology 123**:**86-91. [DOI] [PubMed] [Google Scholar]
- 23.Iwanaga, Y., M. P. Davey, T. M. Martin, S. R. Planck, M. L. DePriest, M. M. Baugh, C. M. Suing, and J. T. Rosenbaum. 2003. Cloning, sequencing and expression analysis of the mouse NOD2/CARD15 gene. Inflamm. Res. 52**:**272-276. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi, K., N. Inohara, L. D. Hernandez, J. E. Galan, G. Nunez, C. A. Janeway, R. Medzhitov, and R. A. Flavell. 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416**:**194-199. [DOI] [PubMed] [Google Scholar]
- 25.Kobe, B., and A. V. Kajava. 2001. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 11**:**725-732. [DOI] [PubMed] [Google Scholar]
- 26.Lala, S., Y. Ogura, C. Osborne, S. Y. Hor, A. Bromfield, S. Davies, O. Ogunbiyi, G. Nunez, and S. Keshav. 2003. Crohn's disease and the NOD2 gene: a role for paneth cells. Gastroenterology 125**:**47-57. [DOI] [PubMed] [Google Scholar]
- 27.Lang, R., D. Patel, J. J. Morris, R. L. Rutschman, and P. J. Murray. 2002. Shaping gene expression in activated and resting primary macrophages by IL-10. J. Immunol. 169**:**2253-2263. [DOI] [PubMed] [Google Scholar]
- 28.Lesage, S., H. Zouali, J. P. Cezard, J. F. Colombel, J. Belaiche, S. Almer, C. Tysk, C. O'Morain, M. Gassull, V. Binder, Y. Finkel, R. Modigliani, C. Gower-Rousseau, J. Macry, F. Merlin, M. Chamaillard, A. S. Jannot, G. Thomas, and J. P. Hugot. 2002. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am. J. Hum. Genet. 70**:**845-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, P., H. Allen, S. Banerjee, S. Franklin, L. Herzog, C. Johnston, J. McDowell, M. Paskind, L. Rodman, J. Salfeld, et al. 1995. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80**:**401-411. [DOI] [PubMed] [Google Scholar]
- 30.Ogura, Y., D. K. Bonen, N. Inohara, D. L. Nicolae, F. F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J. P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nunez, and J. H. Cho. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411**:**603-606. [DOI] [PubMed] [Google Scholar]
- 31.Ogura, Y., N. Inohara, A. Benito, F. F. Chen, S. Yamaoka, and G. Nunez. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 276**:**4812-4818. [DOI] [PubMed] [Google Scholar]
- 32.Rosenstiel, P., M. Fantini, K. Brautigam, T. Kuhbacher, G. H. Waetzig, D. Seegert, and S. Schreiber. 2003. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology 124**:**1001-1009. [DOI] [PubMed] [Google Scholar]
- 33.Rutschman, R., R. Lang, M. Hesse, J. N. Ihle, T. A. Wynn, and P. J. Murray. 2001. Cutting edge: Stat6-dependent substrate depletion regulates nitric oxide production. J. Immunol. 166**:**2173-2177. [DOI] [PubMed] [Google Scholar]
- 34.Shanahan, F. 2002. Crohn's disease. Lancet 359**:**62-69. [DOI] [PubMed] [Google Scholar]
- 35.Siegmund, B. 2002. Interleukin-1beta converting enzyme (caspase-1) in intestinal inflammation. Biochem. Pharmacol. 64**:**1-8. [DOI] [PubMed] [Google Scholar]
- 36.Strober, W., I. J. Fuss, and R. S. Blumberg. 2002. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 20**:**495-549. [DOI] [PubMed] [Google Scholar]
- 37.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21**:**335-376. [DOI] [PubMed] [Google Scholar]
- 38.Thome, M., K. Hofmann, K. Burns, F. Martinon, J. L. Bodmer, C. Mattmann, and J. Tschopp. 1998. Identification of CARDIAK, a RIP-like kinase that associates with caspase-1. Curr. Biol. 8**:**885-888. [DOI] [PubMed] [Google Scholar]
- 39.Wilson, C. J., C. Guglielmo, N. D. Moua, M. Tudor, G. Grosveld, R. A. Young, and P. J. Murray. 2001. Yeast artificial chromosome targeting technology: an approach for the deletion of genes in the C57BL/6 mouse. Anal. Biochem. 296**:**270-278. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki, K., M. Takazoe, T. Tanaka, T. Kazumori, and Y. Nakamura. 2002. Absence of mutation in the NOD2/CARD15 gene among 483 Japanese patients with Crohn's disease. J. Hum. Genet. 47**:**469-472. [DOI] [PubMed] [Google Scholar]
- 41.Yoo, N. J., W. S. Park, S. Y. Kim, J. C. Reed, S. G. Son, J. Y. Lee, and S. H. Lee. 2002. Nod1, a CARD protein, enhances pro-interleukin-1beta processing through the interaction with pro-caspase-1. Biochem. Biophys. Res. Commun. 299**:**652-658. [DOI] [PubMed] [Google Scholar]