Increased cerebrospinal fluid and serum levels of S100B in first‐onset schizophrenia are not related to a degenerative release of glial fibrillar acidic protein, myelin basic protein and neurone‐specific enolase from glia or neurones (original) (raw)
Abstract
Objective
To assess levels of glial fibrillar acidic protein (GFAP), myelin basic protein (MBP), neurone‐specific enolase (NSE) and S100B in patients with first‐onset schizophrenia.
Method
We investigated CSF and serum samples from 12 patients with first‐onset schizophrenia and from 17 control subjects by ELISA (GFAP, MBP) or immunoluminometric sandwich assays (NSE, S100B).
Results
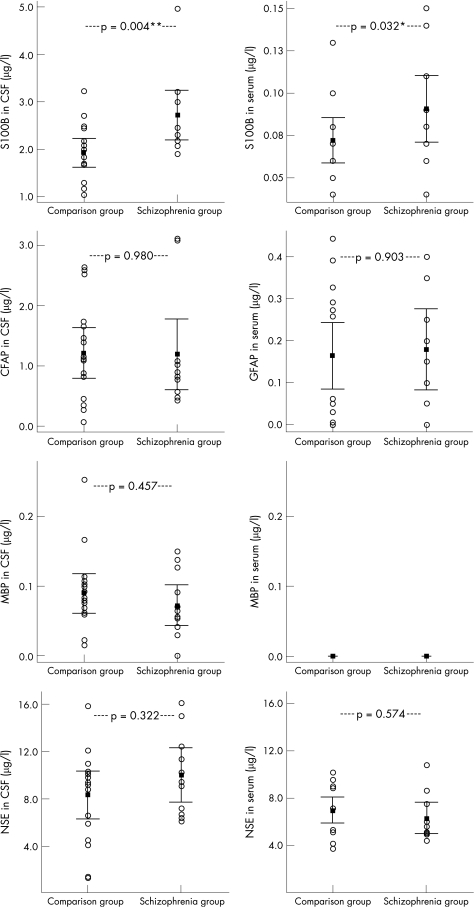
Patients with schizophrenia had significantly higher levels of S100B in CSF (p = 0.004; 2.73 (SD 0.80) v 1.92 (0.58) μg/l) and serum (p = 0.032; 0.09 (0.03) v 0.08 (0.02) μg/l) in comparison with those in the matched control group. No diagnosis‐dependent differences of protein concentration were seen for GFAP, MBP and NSE.
Discussion
Our finding of increased levels of S100B in patients with schizophrenia without an indication for significant glial (GFAP, MBP) or neuronal (NSE) damage may be interpreted as indirect evidence for increased active secretion of S100B during acute psychosis.
S100B is a calcium‐binding, growth‐regulating secretory protein that is expressed particularly in brain tissue and mediates the interaction among glial cells and between glial cells and neurones. Nanomolar levels of S100B protein stimulate neurite growth and promote neurone survival, whereas micromolar levels result in the opposite effects and can even induce neuronal apoptosis.1 S100B expression in the central nervous system has been found in astrocytes, oligodendrocytes, ependyma and a few neurones.2,3,4
Increased levels of S100B in cerebrospinal fluid (CSF) and serum have been reported in various neuropsychiatric diseases, including acute phases of schizophrenia.5,6,7,8,9,10,11 High levels of S100B were considered to be a biomarker for astrocytic damage or dysfunction in schizophrenia. However, this assumption has not been proved yet, as only one study on S100B in CSF and no histological study has been published on this topic.9
The aim of this study was to replicate the findings of Rothermundt et al9 on S100B expression in the CSF, and to identify whether there is an indication for astrocytic, oligodendrocytic or neuronal damage in acute schizophrenia, leading to increased levels of S100B in CSF and serum. We investigated non‐secretory glial and neuronal proteins in CSF and serum to clarify this question. Glial fibrillar acidic protein (GFAP), myelin basic protein (MBP) and neurone‐specific enolase (NSE) are well‐established markers to identify the brain compartment involved in neuropsychiatric disorders.12,13 We hypothesised that there would be increased CSF levels of GFAP, MBP or NSE in cases of astrocytic, oligodendrocytic or neuronal damage, whereas unchanged levels of these proteins and increased levels of S100B in the CSF would be indicative of an increased active secretion of S100B during acute schizophrenia.
Patients and methods
CSF and serum samples were obtained from 12 inpatients with first‐onset paranoid schizophrenia (_Diagnostic and statistical manual of mental disorders_—fourth edition/_International classification of diseases_—10th revision) in whom routine diagnostic lumbar and venepuncture were carried out (table 1). All patients gave written informed consent. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia was used to evaluate psychopathology. The dose of antipsychotic drugs was converted into chlorpromazine‐equivalent units for statistical purposes.14
Table 1 Clinical and cerebrospinal fluid characteristics of patients with schizophrenia and controls.
| Schizophrenia group, n = 12 (7 men, 5 women) | Control group, n = 17 (9 men, 8 women) | p Value | |
|---|---|---|---|
| Age, years | 24 (7) | 25 (8) | 0.61 |
| Duration of psychiatric disease, weeks | 21 (11) | — | — |
| PANSS total score | 87 (15) | — | — |
| PANSS positive score | 24 (6) | — | — |
| PANSS negative score | 21 (6) | — | — |
| PANSS general psychopathology | 42 (8) | — | — |
| CSF, cells/μl | 1.9 (0.8) | 1.6 (0.9) | 0.39 |
| (Albumin CSF/serum quotient)×1000 | 4.8 (1.3) | 3.9 (1.5) | 0.11 |
| CSF–GFAP, µg/l | 1.21 (0.81) | 1.19 (0.92) | 0.94 |
| Serum–GFAP, µg/l | 0.16 (0.15) | 0.18 (0.15) | 0.79 |
| CSF–MBP, µg/l | 0.89 (0.55) | 0.72 (0.46) | 0.36 |
| CSF–NSE, µg/l | 9.81 (3.28) | 10.01 (3.42) | 0.88 |
| Serum–NSE, µg/l | 7.01 (1.98) | 6.33 (1.93) | 0.39 |
| CSF–S100B, µg/l | 2.73 (0.80) | 1.92 (0.58) | <0.01* |
| Serum–S100B, µg/l | 0.09 (0.03) | 0.08 (0.02) | 0.03* |
Twenty three patients with headache (_International classification of diseases_—10th revision G44.2, G44.4) who underwent lumbar puncture to exclude infectious or autoimmune CNS disease or subarachnoidal haemorrhage served as controls. Seventeen of these controls were matched with regard to age and sex (table 1). Systemic diseases, brain injury and substance misuse were excluded. The controls had no lifetime history of psychiatric disorder.
Cellular and humoral parameters of all CSF samples were within normal range, including leucocyte cell count, albumin CSF/serum quotient, protein electropherogram, immunoglobulin G index, glucose and lactate. After investigation of these standard parameters, samples were centrifuged and stored at −80°C for later analysis.
GFAP and MBP levels were determined using sandwich ELISA (Human GFAP‐ELISA, BioVendor, Heidelberg, Germany; Active MBP‐ELISA, DSL, Webster, Texas, USA) according to the manufacturers' instructions. NSE and S100B levels were measured by immunoluminometric sandwich assays using directly coated magnetic microparticles (LIAISON, DiaSorin, Dietzenbach, Germany). The minimal measurable concentrations for these detection systems are 0.08 µg/l for GFAP, 0.03 µg/l for MBP, 0.04 µg/l for NSE and 0.02 µg/l for S100B. The reported concentrations in the serum of 95% controls are <0.3 µg/l for GFAP, <12.5 µg/l for NSE and <0.15 µg/l for S100B, whereas serum levels of MBP are below the detection limit of the assay.15,16 Normal values of GFAP, NSE and S100B in CSF are not established, whereas the reported level of MBP in the CSF of 95% controls is <0.11 µg/l.16
Statistical Analysis
Statistical analyses were carried out with SPSS V.11.0. Owing to the normal distribution of the data (Kolmogorov–Smirnov tests), the Pearson correlation coefficient was used. Age correlated positively with MBP, NSE and S100B concentrations in CSF, or GFAP and S100B concentrations in serum, and thus age was included as a covariate into the following analysis of variance (ANOVA). ANOVA with drugs or with albumin CSF/serum quotient as a second covariate showed no significant interaction of drugs or blood–brain barrier dysfunction with the results. Thus, main group effects (schizophrenia v control group) have been calculated using ANOVA with age as a single covariate. All statistical tests were two‐tailed. In cases of multiple comparisons, we applied an α‐adjustment (Bonferroni correction).
Results
A positive correlation was found between age and protein levels in CSF for MBP (r = 0.694, p = 0), NSE (r = 0.424, p = 0.044) and S100B (r = 0.585, p = 0.003), but not for GFAP (r = 0.263, p = 0.226). An influence of age was also detected in serum for GFAP (r = 0.675, p = 0) and S100B (r = 0.645, p = 0.001), but not for NSE (r = 0.200, p = 0.385). MBP levels in serum remained below the detection limit of the assay.
Patients with schizophrenia had significantly higher levels of S100B in CSF (F = 5.25, p = 0.004; 2.73 (SD 0.80) v 1.92 (0.58) μg/l) and serum (F = 6.42, p = 0.032; 0.09 (0.03) v 0.08 (0.02) μg/l) than the control group. The statistical analysis for S100B in CSF but not in serum was confirmed after Bonferroni correction (p<0.05). No diagnosis‐dependent differences in protein concentration were seen for GFAP, MBP and NSE (table 1, fig 1).
Figure 1 Patients with schizophrenia had significantly higher levels of S100B (a calcium‐binding protein) in cerebrospinal fluid (CSF) and serum than the comparison group. This was contrary to glial fibrillar acidic protein (GFAP), myelin basic protein (MBP) and neurone‐specific enolase (NSE), where no diagnosis‐dependent differences in protein concentration were found. The error bars show 95% confidence intervals. Two‐tailed p values were calculated by analysis of variance with age as a covariate. MBP levels in serum were below the detection limit of the assay.
A significant correlation was found between the S100B CSF/serum and albumin CSF/serum quotient in controls (r = 0.458, p = 0.028). However, this effect was not seen in schizophrenia (r = −0.304, p = 0.337). ANOVA with albumin CSF/serum quotient as a second covariate along with age showed no significant influence of the albumin CSF/serum quotient on the levels of S100B in serum (F = 0.009, p = 0.925), whereas the main group effect remained significant (F = 4.356, p = 0.048). Thus, blood–brain barrier dysfunction was ruled out as a major cause of increased S100B serum levels in schizophrenia.
Sex, duration of disease, drug (cumulative dose of chlorpromazine equivalent for 1, 2, 3 and 4 weeks) and Positive And Negative Syndrome Scale—general, positive or negative scores—had no significant influence on the levels of GFAP, MBP, NSE and S100B in serum or CSF.
Discussion
Our results show a positive correlation between age and protein levels in CSF (MBP, NSE, S100B) and serum (GFAP, S100B) in accordance with van Engelen et al,17 who showed a clear age dependency of MBP, NSE and S100B in CSF. However, in contrast with Rosengren et al,18 no significant correlation between GFAP levels and age was found. A general influence on our results was ruled out by using matched controls and including age as a covariate in the statistical analysis.
Secondly, significantly higher concentrations of S100B were found in the CSF and serum of patients with schizophrenia, confirming the results of previous studies. However, there was no indication for increased release of GFAP and MBP from damaged astrocytes and oligodendrocytes or myelin, or release of NSE due to acute neuronal loss. This may be interpreted as indirect evidence for increased active secretion of S100B in the brains of people during acute psychosis in schizophrenia. Nevertheless, it remains unclear whether increased S100B secretion plays a pathogenetic role in the context of schizophrenia or whether it must be interpreted as a compensatory attempt.19
The present constellation of findings with an isolated S100B increase in CSF and serum is dissimilar to neurodegenerative disorders. In Alzheimer's disease, for example, along with increased S100B concentrations increased GFAP and NSE were also found in the CSF, which correlated with the degree of atrophy.5,20,21 The fact that CSF findings are dissimilar to neurodegenerative diseases does not exclude the early stages of a neurodegenerative disease in our patients with first‐onset schizophrenia. However, it should be noted that histological studies on schizophrenia—in contrast with those on Alzheimer's disease—found no indications of astrogliosis or neuronal loss.22,23,24
In comparison with acute relapses in multiple sclerosis, it should be mentioned that increased MBP can be detected in the CSF and serum of patients with multiple sclerosis, owing to the destruction of myelin and oligodendrocytes.12 Although disorders of myelinisation are discussed in the context of schizophrenia, there was no evidence for an acute destruction of myelin or oligodendrocytes during acute psychotic episodes.25
The present study has certain limitations that need to be taken into account: (1) Sample sizes were small; however, our finding of increased S100B in the CSF of patients with schizophrenia was confirmed after Bonferroni correction. (2) The confounding factor of drugs cannot be ruled out with this study and has to be examined by extending the study to unmedicated patients with schizophrenia. However, our study is in accordance with that by Rothermundt et al,9 who also found increased S100B levels in CSF and serum of unmedicated patients with schizophrenia. (3) An influence of blood brain–barrier changes on levels of S100B in serum cannot be completely ruled out owing to a trend towards higher albumin CSF/serum quotients in the schizophrenia group (p = 0.11, table 1). (4) A degenerative loss of immature oligodendrocytes that are MBP‐negative but S100B‐positive, or a loss of astrocytes which are GFAP‐negative but may be expressing S100B cannot be ruled out.3
In summary, our finding of increased levels of S100B in patients with schizophrenia without an indication for significant glial (GFAP, MBP) or neuronal (NSE) damage may be interpreted as indirect evidence for an increased active secretion of S100B in the brain during an acute psychotic episode in first‐onset schizophrenia.
Acknowledgements
This work was supported by Saxony‐Anhalt Ministry of Research (XN3594O/0405M, N2‐OGU) and Stanley Foundation.
Abbreviations
ANOVA - analysis of variance
CSF - cerebrospinal fluid
GFAP - glial fibrillar acidic protein
MBP - myelin basic protein
NSE - neurone‐specific enolase
S100B - calcium‐binding protein
Footnotes
Competing interests: None.
References
- 1.Van Eldik L J, Wainwright M S. The Janus face of glial‐derived S100B: beneficial and detrimental functions in the brain. Restor Neurol Neurosci 20032197–108. [PubMed] [Google Scholar]
- 2.Tiu S C, Chan W Y, Heizmann C W.et al Differential expression of S100B and S100A6 in the human fetal and aged cerebral cortex. Brain Res Dev Brain Res 2000119159–168. [DOI] [PubMed] [Google Scholar]
- 3.Deloulme J C, Raponi E, Gentil B J.et al Nuclear expression of S100B in oligodendrocyte progenitor cells correlates with differentiation toward the oligodendroglial lineage and modulates oligodendrocytes maturation. Mol Cell Neurosci 200427453–465. [DOI] [PubMed] [Google Scholar]
- 4.Rickmann M, Wolff J R. S100 protein expression in subpopulations of neurons of rat brain. Neuroscience 199567977–991. [DOI] [PubMed] [Google Scholar]
- 5.Petzold A, Jenkins R, Watt H C.et al Cerebrospinal fluid S100B correlates with brain atrophy in Alzheimer's disease. Neurosci Lett 2003336167–170. [DOI] [PubMed] [Google Scholar]
- 6.Lara D R, Gama C S, Belmonte‐de‐Abreu P.et al Increased serum S100B protein in schizophrenia: a study in medication‐free patients. J Psychiatr Res 20013511–14. [DOI] [PubMed] [Google Scholar]
- 7.Missler U, Wandinger K P, Wiesmann M.et al Acute exacerbation of multiple sclerosis increases plasma levels of S‐100 protein. Acta Neurol Scand 199796142–144. [DOI] [PubMed] [Google Scholar]
- 8.Wunderlich M T, Wallesch C W, Goertler M. Release of neurobiochemical markers of brain damage is related to the neurovascular status on admission and the site of arterial occlusion in acute ischemic stroke. J Neurol Sci 200422749–53. [DOI] [PubMed] [Google Scholar]
- 9.Rothermundt M, Falkai P, Ponath G.et al Glial cell dysfunction in schizophrenia indicated by increased S100B in the CSF. Mol Psychiatry 20049897–899. [DOI] [PubMed] [Google Scholar]
- 10.Schroeter M L, Abdul‐Khaliq H, Fruhauf S.et al Serum S100B is increased during early treatment with antipsychotics and in deficit schizophrenia. Schizophr Res 200362231–236. [DOI] [PubMed] [Google Scholar]
- 11.Wiesmann M, Wandinger K P, Missler U.et al Elevated plasma levels of S‐100b protein in schizophrenic patients. Biol Psychiatry 1999451508–1511. [DOI] [PubMed] [Google Scholar]
- 12.Lamers K J, Vos P, Verbeek M M.et al Protein S‐100B, neuron‐specific enolase (NSE), myelin basic protein (MBP) and glial fibrillary acidic protein (GFAP) in cerebrospinal fluid (CSF) and blood of neurological patients. Brain Res Bull 200361261–264. [DOI] [PubMed] [Google Scholar]
- 13.Wunderlich M T, Lins H, Skalej M.et al Neuron‐specific enolase and tau protein as neurobiochemical markers of neuronal damage are related to early clinical course and long‐term outcome in acute ischemic stroke. Clin Neurol Neurosurg 2006108558–563. [DOI] [PubMed] [Google Scholar]
- 14.Woods S W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 200364663–667. [DOI] [PubMed] [Google Scholar]
- 15.Missler U, Wiesmann M, Wittmann G.et al Measurement of glial fibrillary acidic protein in human blood: analytical method and preliminary clinical results. Clin Chem 199945138–141. [PubMed] [Google Scholar]
- 16.Ohta M, Ohta K, Ma J.et al Clinical and analytical evaluation of an enzyme immunoassay for myelin basic protein in cerebrospinal fluid. Clin Chem 2000461326–1330. [PubMed] [Google Scholar]
- 17.van Engelen B G, Lamers K J, Gabreels F J.et al Age‐related changes of neuron‐specific enolase, S‐100 protein, and myelin basic protein concentrations in cerebrospinal fluid. Clin Chem 199238813–816. [PubMed] [Google Scholar]
- 18.Rosengren L E, Wikkelso C, Hagberg L. A sensitive ELISA for glial fibrillary acidic protein: application in CSF of adults. J Neurosci Methods 199451197–204. [DOI] [PubMed] [Google Scholar]
- 19.Kleindienst A, Harvey H B, Rice A C.et al Intraventricular infusion of the neurotrophic protein S100B improves cognitive recovery after fluid percussion injury in the rat. J Neurotrauma 200421541–547. [DOI] [PubMed] [Google Scholar]
- 20.Petzold A, Keir G, Green A J.et al An ELISA for glial fibrillary acidic protein. J Immunol Methods 2004287169–177. [DOI] [PubMed] [Google Scholar]
- 21.Blennow K, Wallin A, Ekman R. Neuron specific enolase in cerebrospinal fluid: a biochemical marker for neuronal degeneration in dementia disorders? J Neural Transm Park Dis Dement Sect 19948183–191. [DOI] [PubMed] [Google Scholar]
- 22.Falkai P, Honer W G, David S.et al No evidence for astrogliosis in brains of schizophrenic patients. A post‐mortem study. Neuropathol Appl Neurobiol 19992548–53. [DOI] [PubMed] [Google Scholar]
- 23.Arnold S E, Trojanowski J Q, Gur R E.et al Absence of neurodegeneration and neural injury in the cerebral cortex in a sample of elderly patients with schizophrenia. Arch Gen Psychiatry 199855225–232. [DOI] [PubMed] [Google Scholar]
- 24.Danos P, Schmidt A, Baumann B.et al Volume and neuron number of the mediodorsal thalamic nucleus in schizophrenia: a replication study. Psychiatry Res 2005140281–289. [DOI] [PubMed] [Google Scholar]
- 25.Tkachev D, Mimmack M L, Ryan M M.et al Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 2003362798–805. [DOI] [PubMed] [Google Scholar]