Dysfunctional telomeres activate an ATM-ATR-dependent DNA damage response to suppress tumorigenesis (original) (raw)
Abstract
The POT1 (protection of telomeres) protein binds the single-stranded G-rich overhang and is essential for both telomere end protection and telomere length regulation. Telomeric binding of POT1 is enhanced by its interaction with TPP1. In this study, we demonstrate that mouse Tpp1 confers telomere end protection by recruiting Pot1a and Pot1b to telomeres. Knockdown of Tpp1 elicits a p53-dependent growth arrest and an ATM-dependent DNA damage response at telomeres. In contrast to depletion of Trf2, which activates ATM, removal of Pot1a and Pot1b from telomeres initiates an ATR-dependent DNA damage response (DDR). Finally, we show that telomere dysfunction as a result of Tpp1 depletion promotes chromosomal instability and tumorigenesis in the absence of an ATM-dependent DDR. Our results uncover a novel ATR-dependent DDR at telomeres that is normally shielded by POT1 binding to the single-stranded G-overhang. In addition, our results suggest that loss of ATM can cooperate with dysfunctional telomeres to promote cellular transformation and tumor formation in vivo.
Keywords: ATM/ATR, chromosomal instability, DNA damage, telomere, tumorigenesis
Introduction
Telomeres are specialized DNA-protein complexes that cap the ends of eukaryotic chromosomes and play crucial roles in ensuring genomic stability by providing both end protection and a mechanism for maintenance of chromosomal ends. Mammalian telomeres consist of TTAGGG repetitive duplex sequences that terminate in single-stranded 3′ G-rich overhangs that can be sequestered into lariat-like structures termed the t-loop (Griffith et al, 1999; de Lange, 2004). This structure is postulated to protect natural DNA ends from being recognized as double-strand breaks (DSBs). In most eukaryotes, telomeres are maintained by the enzyme telomerase, a specialized reverse transcriptase that adds telomeric DNA to the 3′ ends of telomeres. Telomerase is limiting in human somatic cells, leading to telomere attrition with each round of DNA replication. Failure to properly maintain telomere functions results in dysfunctional telomeres that initiate the onset of replicative senescence or fuel genomic instability to promote tumorigenesis (Maser and DePinho, 2002; Wong et al, 2006).
Telomeres are protected by a complex of six core proteins composed of TRF1, TRF2, RAP1, TIN2, TPP1 and POT1 (de Lange, 2005). This complex, termed shelterin, serves to protect telomeres from being recognized as DSBs and inappropriate repair by the non-homologous end joining (NHEJ) and homologous recombination (HR) pathways.
Three sequence-specific DNA-binding proteins are recruited to chromosomal ends: TRF1 and TRF2 bind double-stranded telomeric DNA, while POT1 binds the single-stranded 3′ overhang. POT1 homologs have been identified in most eukaryotes. All POT1 proteins examined to date contain two highly conserved oligonucleotide/oligosaccharide-binding folds (OB folds) that bind to the 3′ terminus of the single-stranded G-rich telomeric overhang. In accord with its end protective function, deletion of POT1 results in chromosomal end-to-end fusions. In addition, removal of vertebrate POT1 does not result in overhang loss, but rather elicits a DNA damage response (DDR) at telomeres. The two Pot1 proteins in mice (Pot1a and Pot1b) appear to have distinct functions—gene knockout of Pot1a elicits a DDR at telomeres, elevated chromosomal fusions and aberrant HR at telomeres (Hockemeyer et al, 2006; Wu et al, 2006), while knockout of Pot1b leads to elongated G-overhangs (Hockemeyer et al, 2006).
TPP1 forms a heterodimer with POT1, and this cooperative interaction greatly increases the affinity of POT1 for its substrate DNA (Wang et al, 2007; Xin et al, 2007). TPP1 also appears to play a crucial role in the proper assembly of the shelterin complex. TPP1 and POT1 are linked to TRF1 and TRF2 through TPP1's interaction with TIN2 (Houghtaling et al, 2004; Kim et al, 2004; Liu et al, 2004a, 2004b; Ye et al, 2004a, 2004b; Yang et al, 2005; O'Connor et al, 2006). In addition, TPP1 possesses an OB fold that is able to recruit telomerase to telomeres, providing a link between telomerase and the shelterin complex. It is likely that the shelterin complex modulates the positioning of the TPP1–POT1 heterodimer at telomeres to either negatively regulate telomerase access to inhibit telomere elongation, or to enable TPP1–POT1 to serve as a telomerase processivity factor for telomere extension (Wang et al, 2007; Xin et al, 2007).
Although the shelterin complex serves to prevent telomeres from being recognized as a DDR, paradoxically many DDR proteins localize to telomeres. Instead of recognizing telomeres as damaged DNA, these proteins appear to play a critical role for proper replication of telomeric DNA (Verdun et al, 2005; Verdun and Karlseder, 2007). Central to the DDR are ATM (ataxia-telangiectasia mutated) and ATR (ataxia-telangiectasia and Rad3 related) protein kinases (Zhou and Elledge, 2000). ATM is involved primarily in sensing and responding to DSBs (Shiloh, 2003), while ATR responds to lesions after they have been processed to single-stranded DNA intermediates (Zou and Elledge, 2003). Once activated, ATM and ATR phosphorylate a range of factors including CHK1 and CHK2, which then target various effector proteins such as p53 to affect DNA repair, transcription and cell-cycle progression (Bartek and Lukas, 2003). Recent evidence suggests that engagement of the DDR pathway is important for tumor suppression. While induction of p53-dependent apoptosis is clearly important for tumor suppression, we and others have recently shown that dysfunctional telomeres also activate a DDR response to initiate p53-dependent cellular senescence to inhibit tumorigenesis in vivo (Cosme-Blanco et al, 2007; Feldser and Greider, 2007). However, it is unclear whether telomere dysfunction-initiated DDR is capable of suppressing tumorigenesis in cells incapable of inducing cellular senescence/apoptosis due to loss of p53 function.
Although ATM is clearly involved in sensing and processing damaged signal from critically shortened telomeres or telomeres deficient in TRF2 (Karlseder et al, 1999; Herbig et al, 2004; Celli and de Lange, 2005; Verdun and Karlseder, 2006), little is known about how telomeres activate ATR. ATR is recruited to telomeres during late S-phase, suggesting that it is required for telomere replication (Verdun and Karlseder, 2006). Since POT1 specifically binds single-stranded telomeric G-overhang and is displaced during DNA replication (Verdun et al, 2005), it is likely that POT1 functions to mask an ATR-dependent DNA damage checkpoint from the single-stranded overhang.
In this study, we demonstrate that mouse Tpp1 confers telomere end protection by recruiting Pot1a and Pot1b to telomeres. Stable knockdown of Tpp1 elicits an ATM-dependent DNA damage response at telomeres. In contrast to depletion of Trf2, removal of Pot1a and Pot1b from telomeres initiates an ATR-dependent DDR. Finally, we show that telomere dysfunction promotes chromosomal instability and tumorigenesis in the absence of ATM-dependent DDR. Our results uncover an ATR-dependent DDR at telomeres that is shielded by Pot1a and Pot1b binding to the single-stranded overhang, and reveal the biological consequence of telomere dysfunction in the setting of ATM deficiency.
Results
TPP1 confers telomere end protection by recruiting Pot1a and Pot1b to telomeres
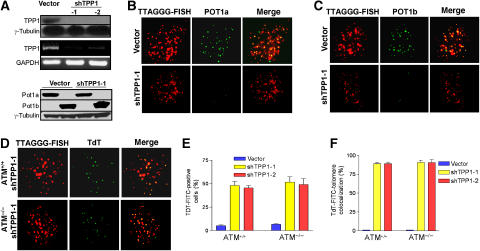
In human cells, POT1 forms a heterodimer with TPP1 to modulate telomere structure and telomerase activity (Wang et al, 2007; Xin et al, 2007). We have previously shown that murine Tpp1 interacts with the C-terminus of both Pot1a and Pot1b (He et al, 2006; Wu et al, 2006). To further elucidate the role of Tpp1 at telomeres, we utilized RNAi knockdown of Tpp1 to determine its requirement for the localization of Pot1a and Pot1b to telomeres. Two independent shRNAs targeting Tpp1 were found to effectively reduce endogenous Tpp1 mRNA levels in mouse embryo fibroblasts (MEFs) as determined by RT–PCR (Figure 1A). Both Tpp1 shRNAs also significantly diminished the protein levels of exogenously expressed HA-tagged Tpp1 (Figure 1A, and data not shown). Expression of Myc-tagged Pot1a or Flag-tagged Pot1b in MEFs showed their expected telomeric accumulation (Figure 1B and C). However, while highly expressed, both proteins failed to localize to telomeres in appreciable levels when Tpp1 is depleted (Figure 1A–C), consistent with recent reports that interactions of Pot1a/Pot1b with Tpp1 is required for proper localization to telomeres (Hockemeyer et al, 2007).
Figure 1.
Tpp1 mediates Pot1a and Pot1b telomeric localization and protection. (A) Top panel: immunoblot showing reduced expression of exogenous HA-tagged Tpp1 upon introduction of Tpp1 shRNAs-1 and -2. Middle panel: RT–PCR of MEFs infected with the control vector or Tpp1 shRNAs-encoding retroviruses was processed to detect Tpp1 and GAPDH mRNAs. Bottom panel: western blot of control vector or Tpp1-knockdown p53−/−MEFs expressing Myc-tagged Pot1a or Flag-tagged Pot1b. Tubulin serves as loading control. (B) Tpp1 knockdown reduced telomeric localization of Pot1a. p53−/− MEFs infected with control vector or shTpp1 were costained with PNA-TTAGGG to detect telomeres (red), and with anti-Myc antibody (green) to detect Myc-tagged Pot1a. Merged image is shown on right. (C) Tpp1 knockdown reduced Pot1b telomeric localization. MEFs were treated as in panel B, with PNA-TTAGGG to detect telomeres (red) and anti-Flag antibody (green) to detect Flag-tagged Pot1b. (D) Knockdown of Tpp1 induces accessible telomere ends. PNA-TTAGGG repeats (red) were used to detect telomeres, while TdT-FITC (green) was used as a marker of uncapped telomeres in ATM+/+ and ATM−/− Tpp1-knockdown MEFs. Merged signals are on the right. (E) Quantification of the percentage of TdT–FITC-positive cells in ATM+/+ and ATM−/− Tpp1-knockdown cells. (F) Quantification of the percentage of colocalization of telomeric signals with TdT–FITC signals in ATM+/+ and ATM−/− Tpp1-knockdown cells. In panels E and F, a minimum of 100 nuclei was scored, and error bars represent standard error of the mean (s.e.m).
Since both Pot1a and Pot1b play important roles in telomere end protection, knockdown of Tpp1 is predicted to reduce the accumulation of both Pot1 proteins at telomeres and render them incapable of forming protective structures. To examine this possibility, a Terminal deoxy-Transferase (TdT) assay that adds FITC-conjugated deoxy-uridine to naked telomere ends (Verdun et al, 2005) was used to probe telomeric structure in unsynchronized MEFs infected with vector or shTpp1. The TdT–FITC assay did not detect specific nuclear staining in vector-infected MEFs (Figure 1E and F). In contrast, ∼95% of TdT signals colocalized with telomeres in ∼50% of TPP1 knockdown cells examined, indicating robust telomere uncapping (Figure 1D–F). A similar quantity of TdT signals colocalized with telomeres in Tpp1 knockdown, ATM−/− MEFs, indicating that functional ATM is not required to uncap telomeres (Figure 1D–F). This result suggests that telomeres cannot efficiently form a protective structure in the absence of Tpp1.
Knockdown of Tpp1 elicits a p53-dependent growth arrest
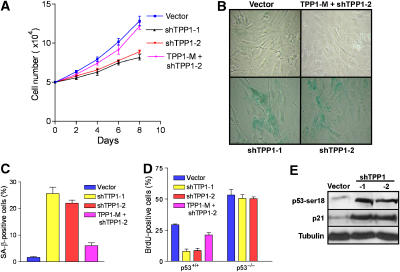
Uncapped telomeres activate a DDR that, depending on cell type, impinges upon the p53 pathway to elicit apoptosis or cellular senescence (Karlseder et al, 1999; d'Adda di Fagagna et al, 2003). To assess the effect of Tpp1 depletion on cellular proliferation, we treated early-passage primary MEFs with vector, shTpp1-1 or shTpp1-2, and monitored cell growth. Depletion of Tpp1 resulted in significant growth arrest, while little growth effect was observed in vector-infected MEFs (Figure 2A). Growth-arrested shTpp1 knockdown cells displayed a flattened morphology and a 10-fold increase in the number of senescence-associated (SA)-β-galactosidase-positive cells, a marker for cellular senescence (Figure 2B and C). Correlating with this decline in cellular proliferation was the observation of a threefold decrease in the number of 5-bromodeoxyuridine (BrdU) signals in Tpp1-depleted cells (Figure 2D). Importantly, the senescence phenotype observed in shTpp1-expressing cells was largely abrogated when a full-length Tpp1 construct mutated to be resistant to shTpp1-mediated degradation was coexpressed, indicating that the Tpp1 hairpins utilized in our experiments indeed target endogenous Tpp1 (Figure 2A–D). Transient knockdown of Tpp1 in p53−/− MEFs did not adversely affect cell growth (Figure 2D; Supplementary Figure 1A), suggesting that the presence of p53 is required to mediate growth arrest. Consistent with the observation that cells undergoing cellular senescence activate the p53–p21-mediated signaling pathway, knockdown of Tpp1 results in phosphorylation of p53 serine 18 and upregulation of the CDK inhibitor p21 (Figure 2E). Taken together, these results indicate that depletion of Tpp1 elicits the onset of p53-dependent cellular senescence.
Figure 2.
Tpp1 knockdown initiates premature cellular senescence in primary mouse fibroblasts. (A) Diminished cell proliferation upon inhibition of Tpp1. Early-passage (P2) primary MEFs were infected with control vectors, shRNA-1, -2 or RNAi-resistant mutant retroviruses, and subjected to puromycin selection. Subsequently, cells numbers were measured at the indicated time points, with day 0 representing the first day after puromycin selection. Error bars represent s.e.m. (B) MEFs with reduced Tpp1 expression display a senescence phenotype and stain for SA-β-galactosidase. (C) Quantification of SA-β-gal-positive cells. Error bars represent s.e.m. (D) Quantification of BrdU incorporation in p53+/+ or p53−/− primary MEFs expressing vector, shTpp1-1, -2 or shRNA-resistant Tpp1 mutant. Error bars represent s.e.m. (E) Induction of p53-ser18 phosphorylation and p21 upon Tpp1 inhibition. Immunoblot of extracts from cells expressing vector or shTpp1-1 and -2.
Stable Tpp1 depletion and expression of Tpp1 mutants initiates an ATM-dependent DDR at the telomeres
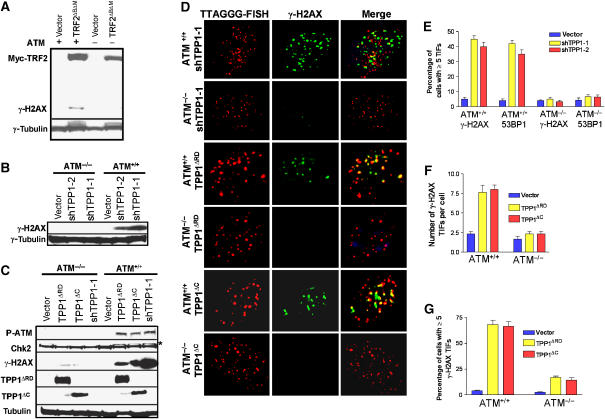
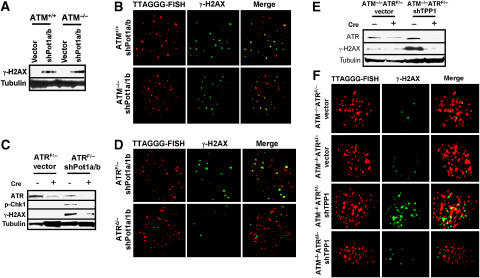
Removal of the double-stranded telomere-binding protein TRF2 from telomeres initiates an acute telomere deprotection phenotype, resulting in the activation of the ATM kinase and a DDR manifested in part as increased phosphorylation of DNA damage proteins such as γ-H2AX (Figure 3A; Karlseder et al, 1999). To test whether depletion of Tpp1 initiates an ATM-dependent DDR, we monitored γ-H2AX levels in ATM+/+ and ATM−/− cells stably expressing vector or shTPP1. Phosphorylated γ-H2AX and Chk2 accumulated in ATM+/+ but not in ATM−/− cells in which Tpp1 is depleted (Figure 3B and C). To ascertain whether the DDR originated at telomeres, we utilized the telomere-dysfunction-induced foci (TIF) assay, which monitors the telomeric association of DNA damage proteins γ-H2AX and 53BP1 (Takai et al, 2003). Approximately 50% of Tpp1-depleted ATM+/+ cells exhibited a minimum of five TIFs, whereas TIFs were observed in only ∼5% of the Tpp1-depleted ATM−/− cells (Figure 3D and E; Supplementary Figure 1B). This result indicates that long-term depletion of Tpp1 elicits a robust ATM-dependent DDR at telomeres.
Figure 3.
Stable knockdown of Tpp1 or overexpression of Tpp1 mutants initiates an ATM-dependent DDR at telomeres. (A) Western blot showing overexpression of myc-TRF2ΔBΔM-induced γ-H2AX phosphorylation in ATM+/+ but not in ATM−/− MEFs. Trf2ΔBΔM was detected by anti-myc antibody and tubulin serves as a loading control. (B) Western analysis revealing that knockdown of Tpp1 with shTpp1-1 and -2 induced γ-H2AX phosphorylation in ATM+/+ but not in ATM−/− MEFs. Tubulin serves as loading control. (C) Knockdown of Tpp1 or overexpression of HA-Tpp1ΔRD or HA-Tpp1ΔC mutants induced phosphorylation of ATM, γ-H2AX and Chk 2 in ATM+/+ but not in ATM−/− MEFs. Tpp1ΔRD and Tpp1ΔC were determined by anti-HA antibody and tubulin serves as loading control. *, phospho-Chk2. (D) Expression of shTpp1-1 or Tpp1 mutants induced ATM-dependent phosphorylation of γ-H2AX at telomeres. ATM+/+ or ATM−/−MEFs infected by the indicated retroviral constructs were analyzed by telomere PNA-FISH (red) and antibody to γ-H2AX (green). Representative images are shown. (E) Percentage of cells containing five or more γ-H2AX or 53BP1 TIFs in ATM+/+ or ATM−/−, Tpp1-depleted MEFs. Error bars represent s.e.m. (F) Quantitation of the number of γ-H2AX positive TIFs per cell expressing Tpp1ΔRD or Tpp1ΔC mutants. Error bars represent s.e.m. (G) Percentage of cells containing five or more γ-H2AX-positive TIFs in ATM+/+ or ATM−/−MEFs stably expressing Tpp1ΔRD or TPP1ΔC mutants. Error bars represent s.e.m.
Among the six core proteins that bind telomeres, multiple pairwise interactions have been demonstrated to form a high-order telomeric complex that mediates telomere end -capping and length control. Previous observations indicated that TPP1 interacts not only with POT1 to form a complex that caps telomeres, but also with TIN2 to mediate proper six-protein complex assembly (O'Connor et al, 2006; Xin et al, 2007). Therefore, disruption of either TPP1–POT1 or TPP1–TIN2 interactions may result in deprotected telomeric ends to elicit a DDR at telomeres. To further probe which Tpp1 subcomplex is important for telomere end capping, we generated two Tpp1 mutants containing-domain specific mutations. The Tpp1ΔRD mutant lacks the Pot1 recruitment domain and is predicted to disrupt only Tpp1–Pot1 interactions, while the Tpp1ΔC mutant lacks the C-terminal half of Tpp1 and is able to eliminate the binding of Tpp1 to TIN2 but not to Pot1 (Xin et al, 2007; Supplementary Figure 2A–D, and data not shown; O'Connor et al, 2006). Western analysis revealed that overexpression of Tpp1ΔRD or Tpp1ΔC induced robust phosphorylation of γ-H2AX in ATM+/+ but not in ATM−/− cells (Figure 3C). TIF assays showed that expression of Tpp1ΔRD and Tpp1ΔC elicited a potent damage response at telomeres, with more than five TIFs in 70% ATM+/+ cells, while the TIF signal was significantly reduced in ATM−/− cells (Figure 3D–G). These results reinforce the notion that interaction of Tpp1with both Pot1 and Tin2 is essential to prevent telomere ends from being inappropriately recognized as DSBs to activate an ATM-dependent DDR.
Expression of mutant Tpp1 results in a DDR at telomeres that is independent of ATR
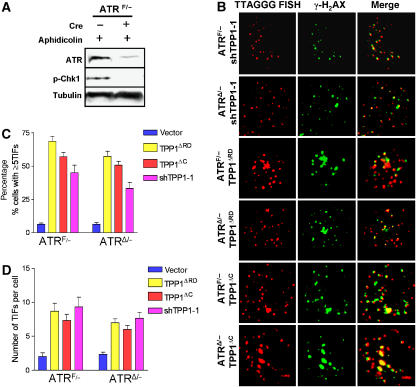
Although our data suggest that ATM is required for the DDR following stable Tpp1 depletion or expression of Tpp1 mutants, it is possible that the ATR kinase is also involved in this damage response. Depletion of Tpp1 resulted in a threefold increase in single-stranded telomeric DNA, which is independent of ATM status (Supplementary Figure 3), and may therefore activate an ATR-dependent response. To test this possibility, we utilized E1-transformed ATRF/− MEFs (Brown and Baltimore, 2003). In control experiments with aphidicolin treatment, Chk1 phosphorylation, a hallmark of ATR signaling, was markedly reduced when the floxed allele of ATR was deleted by adenoviral Cre-recombinase (AdCre; Figure 4A). ATRF/− MEFs were infected with shTpp1, and then treated with either the empty adenoviral vector (AdE) or AdCre to generate Tpp1-depleted, ATRÄ/− MEFs (Figure 4A, and data not shown). TIF analysis revealed that ∼40% ATRF/− cells and ∼35% ATRÄ/− cells in which Tpp1 was stably depleted have a minimum five TIFs (Figure 4B–D). TIF formation with approximately equal frequency was observed in both ATRF/−and ATRÄ/− cells expressing Tpp1ΔRD mutant (∼65 versus 60%) or Tpp1ΔC mutant (55 versus 50%) (Figure 4B–D). Since there is no significant differences in TIF formation between ATRF/−and ATRÄ/− MEFs in which Tpp1 levels were perturbed by stable shRNA knockdown or overexpression of Tpp1 mutants, these results suggest that an ATR-dependent DDR is not induced by inactivation of endogenous Tpp1. These data further strengthen the notion that ATM plays a dominant function in sensing dysfunctional telomeres when Tpp1 containing protein complexes are disrupted.
Figure 4.
Expression of Tpp1 mutants does not engage an ATR-dependent DDR at telomeres. (A) ATR is required for Chk1 phosphorylation. ATRF/− MEFs were infected with the AdE empty vector (−) or AdCre (+) at an MOI of 200, and treated with aphidicolin to induce Chk1 phosphorylation at Serine 345. Tubulin served as loading control. (B) Stable shRNA-mediated depletion of Tpp1 or expression of Tpp1 mutants induced ATR-independent phosphorylation of γ-H2AX at telomeres. ATRF/− MEFs were treated with either AdE, or with AdCre at an MOI of 200 to generate ATRΔ/− MEFs. Cells were infected with the indicated retroviral constructs and analyzed by telomere PNA-FISH (red) and with antibody to γ-H2AX (green) to detect TIF formation. The images were merged to evaluate colocalization. Representative images are shown. (C) Quantitation of percentage of cells with ⩾5 γ-H2AX-positive TIFs in ATRF/− or ATRΔ/−MEFs expressing shTpp1, Tpp1ΔRD or Tpp1ΔC mutants. Error bars represent s.e.m. (D) Quantitation of the number of γ-H2AX-positive TIFs per cell expressing shTPP1, TPP1ΔRD or TPP1ΔC mutants. Error bars represent s.e.m.
Removal of Pot1a and Pot1b from telomeres initiates an ATR-dependent DDR
Pot1a and Pot1b specifically bind to single-stranded telomeric G-rich DNA, and deletion of both proteins results in a robust DDR at telomeres (He et al, 2006; Hockemeyer et al, 2006, 2007; Wu et al, 2006). We have previously shown that deletion of Pot1a initiates an ATM-dependent DDR (Wu et al, 2006). However, this activation of ATM could be an indirect result of dicentric chromosome formation, which when resolved by the breakage–fusion–bridge (BFB) cycle could lead to the production of DNA DSBs that are sensed by ATM. Indeed, shRNA-mediated depletion of both Pot1a and Pot1b in ATM−/− MEFs still resulted in robust phosphorylation of γ-H2AX (Figure 5A). The TIF assay showed that ∼40% Pot1a/Pot1b-depleted ATM+/+ cells exhibited a minimum of five TIFs, with no significant difference in TIF formation observed when Pot1a and Pot1b were depleted in ATM−/− MEFs (Figure 5B; Supplementary Figure 4A and B). This result suggests that ATM is not the primary sensor of a Pot1a/Pot1b-dependent DDR at telomeres.
Figure 5.
Removal of Pot1a and Pot1b from telomeres initiates an ATR-dependent, ATM-independent DDR. (A) ATM+/+ and ATM−/− MEFs were infected with control vector or shRNAs targeting both Pot1a and Pot1b. Pot1a and Pot1b knockdown induced γ-H2AX phosphorylation. Tubulin served as loading control. (B) Depletion of Pot1a and Pot1b induced the formation of γ-H2AX-positive TIFs in the absence of ATM. TIF formation in ATM+/+ or ATM−/−MEFs were analyzed following shPot1a and shPot1b retroviral infection with telomere PNA-FISH (red) and an antibody to γ-H2AX (green). (C) Lysates from ATRF/− or ATRΔ/−MEFs treated with vector or shPot1a and shPot1b were probed for ATR, Chk1 and γ-H2AX. Tubulin served as a loading control. (D) ATR is required for TIF formation in Pot1a- and Pot1b-knockdown MEFs. ATRF/− or ATRΔ/− MEFs were infected with shPot1a and shPot1b and analyzed by telomere PNA-FISH (red) and with an antibody to γ-H2AX (green). (E) Transient knockdown of Tpp1 elicits an ATR-dependent DDR at telomeres. ATR and γ-H2AX phosphorylation levels were monitored by western blotting in ATM−/−ATRF/− and ATM−/−ATRΔ/− MEFs transiently treated with vector or shTPP1. Tubulin served as a loading control. (F) ATR is required for TIF formation in Tpp1-depleted MEFs. ATM−/−ATRF/− or ATM−/−ATRΔ/− MEFs was transiently infected with vector or shTpp1-1 and analyzed by telomere PNA-FISH (red) and with an antibody to γ-H2AX (green) for the presence of TIFs (merge).
We postulated that removal of both Pot1a and Pot1b from telomeres would unmask the single-stranded G-overhang and activate an ATR-dependent DDR at telomeres. Previously, it has been shown that stalled DNA replication in ATR-knockout cells leads to increased γ-H2AX phosphorylation and chromosome breaks (Brown and Baltimore, 2003). This increase in γ-H2AX phosphorylation is presumably the consequence of increased replication fork collapse in the absence of ATR and subsequent ATM/DNA-PK-dependent phosphorylation of γ-H2AX (Rogakou et al, 1998; Burma et al, 2001). However, single-stranded DNA generated as a consequence of Pot1a/Pot1b reduction would not involve replication fork stalling; thus, the dependence of γ-H2AX phosphorylation on ATR in response to Pot1a/Pot1b reduction could be assessed. To test whether depletion of Pot1a/Pot1b results in ATR-dependent γ-H2AX phosphorylation, we knocked down both Pot1a and Pot1b in ATRF/− MEFs and treated these cells with either AdCre to generate ATRÄ/− MEFs, or with AdE to generate control cells. RT–PCR and Western analysis demonstrated that the floxed allele of ATR was efficiently deleted by AdCre treatment, concomitant with a reduction in the level of phospho-Chk1 induced after knockdown of Pot1a/Pot1b (Figure 5C; Supplementary Figure 5A). Compared with ATRF/− MEFs, depletion of Pot1a/Pot1b in ATRÄ/− MEFs did not result in appreciable γ-H2AX and Chk1 phosphorylation (Figure 5C). Furthermore, γ-H2AX-positive TIF formation was substantially reduced in the absence of ATR (from 48% of cells with at least 5 TIFs to 8% and a reduction of 12 to three TIFs observed per cell; Figure 5D; Supplementary Figure 4C and D). In addition, we generated Pot1aF/FATRF/F and Pot1aF/FATRF/F MEFs to further confirm that ATR signaling is required for TIF formation following Pot1a deletion by AdCre (Supplementary Figure 5A and B). These results suggest that loss of Pot1a is sufficient to unmask a novel ATR-dependent DDR at telomeres.
Since localization of Pot1a and Pot1b to telomeres depends critically upon Tpp1, we were surprised to find that stable depletion of Tpp1 initiated primarily an ATM-dependent DDR. We reasoned that long-term depletion of Tpp1 results in the formation of fused chromosomes, which when broken could initiate the BFB cycle to generate double-strand DNA breaks and subsequent induction of ATM (Hockemeyer et al, 2007). To resolve this paradox, we treated ATM−/−ATRF/− MEFs transiently with shTPP1-1 and observed a robust DDR at telomeres, with ∼40% of cells displaying ⩾5 γ-H2AX-positive TIFs per cell (Figure 5E and F; Supplementary Figure 4E and F). This ATR-dependent DDR was abolished following Cre-mediated deletion of ATR (Figure 5E and F; Supplementary Figure 4E and F), further confirming that transient removal of Tpp1 from telomeres potently induces an ATR-dependent DDR at telomeres.
Telomere dysfunction promotes chromosomal instability and tumorigenesis in the absence of ATM-dependent DDR
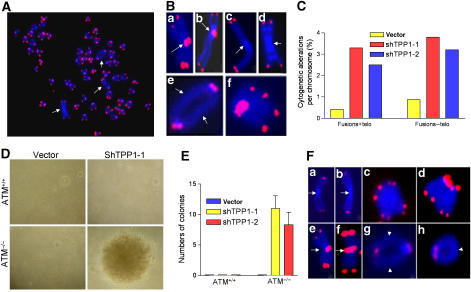
Activation of DDR genes such as ATM and ATR engage p53 to enforce cellular senescence, thereby preventing the proliferation of genetically damaged cells to suppress tumorigenesis (Bartkova et al, 2005). However, would-be tumor cells that lose p53 function cannot engage apoptosis/cellular senescence pathways and therefore must be culled by other means. The prominent γ-H2AX-positive TIFs observed in p53-null, ATM+/+ MEFs in which Tpp1 is stably depleted, prompted us to speculate that long-term depletion of Tpp1 activates a persistent ATM-dependent DDR checkpoint to inhibit tumorigenesis in the absence of p53. To examine this possibility, we stably knocked down Tpp1 in ATM+/+, p53−/− and ATM−/−, p53−/− cells. Compared with the minimum level of chromosomal aberrations present in shTPP1-1-infected, ATM+/+, p53−/− cells, approximately 55% of the metaphases generated from shTpp1-1-infected, ATM−/−, p53−/− cells (henceforth termed ‘triple deleted' cells) contained at least two aberrant chromosomes (Figure 6A, and data not shown). Telomere-FISH revealed a ∼5-fold increase in the number of chromosomal aberrations, including p–p arm fusions with telomeric DNA at the site of fusion, isochromatid rings characteristic of Pot1a deletion and tetraploid metaphases with diplochromosomes reminiscent of those observed in Pot1a/Pot1b double knockout cells (Figure 6B; Wu et al, 2006; Hockemeyer et al, 2006). In addition to p–p arm telomere fusions, triple deleted cells contained a significant number of chromosome fusions without detectable telomeric DNA at the fusion site, which could be the consequence of engagement of the BFB cycle (Figure 6B and C). Consistent with this possibility, anaphase bridges were observed with chromatin bridges containing telomeric signals (Supplementary Figure 6A and B). In sharp contrast, no anaphase bridges and a marked reduction in the number of chromosomal abnormalities were observed in shTpp1-infected, ATM+/+, p53−/−cells (Figure 6C, and data not shown). These data suggest that loss of ATM promotes chromosomal instability in the setting of telomere dysfunction and p53 deficiency.
Figure 6.
Knockdown of Tpp1 in ATM−/− cells induces chromosome instability, cellular transformation and tumorigenesis in vivo. (A) Telomeric FISH on metaphases derived from ATM−/− cells expressing shTpp1-1. Telomeric hybridization signal is shown in red and DAPI-counterstained chromosomes in blue. Arrows indicate chromosomal aberrations. (B) Knockdown of Tpp1 induced multiple chromosomal aberrations, including p–p arm fusions with TTAGGG repeats at the fusion sites (a, b); p–p arm fusions without TTAGGG repeats at the fusion sites (c, d); q–q arm chromosomal fusions without TTAGGG repeats at the fusion sites (e) and diplochromosomes (f). In all panels, arrowheads point to the site of fusion. (C) The frequency of cytogenetic aberrations is quantitated; fusions+telo, fusions with telomere signal at the site of fusion; fusions–telo, fusions without telomere signals at the site of fusion. (D) ATM−/−, p53−/− MEFs stably expressing shTpp1-1 readily formed colonies in anchorage-independent assay. Representative images are shown. (E) Quantitation of the number of colonies formed when ATM−/−, p53−/− MEFs were infected with vector and shTpp1-1 and -2 siRNAs. Error bars represents s.e.m. (F) Tumor cells from Tpp1-knockdown ATM−/− MEFs possess multiple chromosomal aberrations, including p–p arm fusions without TTAGGG repeats at the fusion sites (a, b); p–p arm fusions with TTAGGG repeats at fusion sites (e, f); diplochromosomes (c, d); q–q arm fusion without TTAGGG repeats at the sites of fusion (g) and isochromatid rings without TTAGGG repeats at the sites of fusion (h).
To investigate whether the elevated genomic instability in the triple deleted cells is able to initiate cellular transformation, we subjected triple deleted and shTpp1-1-infected, ATM+/+, p53−/− cells to a soft-agar assay. Only triple deleted cells were able to grow in an anchorage-independent manner, and ∼10 foci were obtained in the two independent triple deleted cell lines (Figure 6D and E). Next, we evaluated their ability to form solid tumor in vivo. Two independent triple deleted cell lines were injected subcutaneously into SCID mice and within 12 weeks; both cell lines gave rise to tumors (Supplementary Figure 6C and D; Supplementary Table 1). In contrast, none of the three Tpp1 depleted, ATM+/+, p53−/− cell lines formed tumor in SCID mice. Furthermore, triple deleted tumors possess elevated genomic instability, with end-to-end chromosomal fusions, diplochromosomes and isochromatid rings resembling aberrations observed in Pot1a/Pot1b double knockout cells (Figure 6F; Supplementary Figure 6E and F; Hockemeyer et al, 2006). Taken together, these data suggest that loss of ATM can cooperate with telomere dysfunction initiated by Tpp1 loss to promote cellular transformation and tumor formation in vivo in the setting of p53 deficiency.
Discussion
We have previously shown that Pot1a is required to protect telomeres from initiating a DDR and that the C-terminal domain of both Pot1a and Pot1b interacts with Tpp1 (He et al, 2006; Wu et al, 2006). In this report, we show that Pot1a and Pot1b require Tpp1 for localization to telomeres. Depletion of Tpp1 prevented Pot1a and Pot1b from localizing to telomeric DNA. In addition, shRNA-mediated knockdown of Tpp1 activates a robust DDR at telomeres, suggesting that Tpp1 plays a crucial role in preventing telomere ends from being recognized as damaged DNA. These results are consistent with recent observations that the interaction of POT1 with TPP1 is essential for complete telomere end protection (Hockemeyer et al, 2007; Xin et al, 2007), and that POT1 and TPP1 are evolutionary conserved homologues of the Oxytricha nova TEBP-á and TEBP-β heterodimer (Wang et al, 2007; Xin et al, 2007).
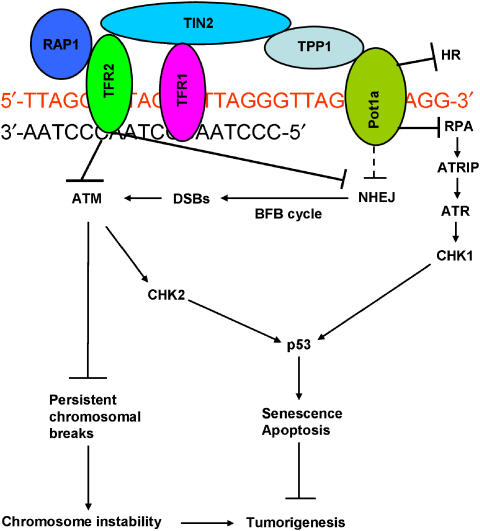
With respect to DNA damage-signaling pathway at telomeres, deletion of Pot1a is not functionally equivalent to loss of Trf2. While elimination of Trf2 initiates primarily an ATM-dependent, ATR-independent DDR, removal of Pot1a results in a novel ATR-mediated DDR at telomeres. Since Pot1a and Pot1b both specifically bind single-stranded telomeric G-overhangs, and deletion of Pot1b results in rapid overhang elongation (He et al, 2006; Hockemeyer et al, 2006; Wu et al, 2006), it is likely that Pot1a and Pot1b cooperate to mask an ATR-dependent DNA damage checkpoint emanating from the single-stranded overhang. We speculate that a primary function of Pot1a and/or Pot1b is to prevent RPA from binding to telomeric overhangs to activate an ATR-dependent DNA damage response (Figure 7). Several lines of evidence support this hypothesis. The OB fold of hRPA70 is predicted to bind tightly to telomeres, and therefore might compete with POT1 for binding to single-stranded telomeric DNA (Theobald et al, 2003). RPA is also recruited to telomeres during DNA replication when POT1 is transiently released from telomeric DNA, suggesting that the two proteins could compete for the same DNA substrate (Verdun et al, 2005; Verdun and Karlseder, 2007). Finally, ATR is potently activated by 5′ ds/ssDNA junctions that resemble the telomere overhang (MacDougall et al, 2007). We envisage a scenario in which loss of POT1 results in the coating of the single-stranded telomeric overhang by RPA, recruiting ATR/ATRIP and additional factors such as Rad 17 and the 9-1-1 complex to telomeres (Figure 7; Zou, 2007). Indeed, a role for the 9-1-1 complex has been found at telomeres, since deletion of Hus1, an integral component of this complex, results in severe telomere shortening (Francia et al, 2006). Subsequent activation of ATR-ATRIP by the addition of TopBP1 and Claspin activates the kinase activity of ATR to phosphorylate Chk1 and the transduction of the damage signal to p53, where it initiates cellular senescence or apoptosis to prevent the proliferation of genetically damaged cells. While our manuscript was under review, similar results were reported by the de Lange laboratory, in which Trf2 and Pot1a function to independently repress the activation of ATM and ATR at telomeres (Denchi and de Lange, 2007).
Figure 7.
Speculative model of how the shelterin complex represses ATM/ATR-dependent DDR at telomeres. We envision Pot1a as the main repressor of ATR, while ATM is mainly repressed by Trf2. Repression of ATR prevents the activation of Chk1 and p53, while ATM is required to prevent the accumulation of chromosomal breaks and subsequent genomic instability. NHEJ at telomeres is repressed mainly by Trf2, while aberrant telomere HR is repressed mainly by Pot1a/Pot1b.
Removal of TPP1 results in rapid telomere elongation, presumably due to inability of POT1 to protect telomeric termini, allowing telomerase access (Kelleher et al, 2005; Lei et al, 2005; Wang et al, 2007; Xin et al, 2007). Our results further show that loss of Tpp1 significantly reduced the accumulation of Pot1a and Pot1b at telomeres. While transient knockdown of Tpp1 initiates a robust ATR-dependent DDR, as was observed when Pot1a and Pot1b were depleted, long-term Tpp1 knockdown elicited primarily an ATM-dependent DDR. Although the molecular mechanism contributing to this difference in damage signaling is not yet clear, biochemical analysis suggest that TPP1 not only binds POT1, but also interacts with TIN2 to stabilize TRF1–TIN2–TRF2 interaction (O'Connor et al, 2006). ChIP data further suggest that telomeric accumulation of TPP1, TIN2 and POT1 is dependent on TRF2 (Hockemeyer et al, 2007). Long-term depletion of Tpp1 is thus predicted to disrupt not only Pot1a/1b interactions with the telomeric overhang, but also perturb Trf2 subcomplex assembly, eventually leading to the activation of an ATM-dependent DDR at telomeres. Since Tpp1ÄRD primarily activate an ATM-dependent DDR, it likely disrupts not only Tpp1–Pot1a/Pot1b interactions but also affects other components of the shelterin complex such as Trf2.
Emerging data suggest that components of the DDR pathway are either mutated or deregulated during tumorigenesis. DDR factors including ATM, ATR, γ-H2AX, 53BP1 and Chk2 are frequently activated in early precancerous lesions, but are often abrogated in malignant tumors (Khanna and Jackson, 2001; Bartkova et al, 2005; Gorgoulis et al, 2005; Nuciforo et al, 2007). In oncogene-mediated cellular transformation, DNA replication stress including the accumulation of aberrant replication forks impinges upon ATM/ATR to activate p53-dependent cellular senescence to suppress tumorigenesis (Bartkova et al, 2005; Di Micco et al, 2006). Elimination of ATM results in tumor progression, suggesting that ATM-dependent DDR checkpoint constrains malignant progression by activating a senescence barrier. Selection of genome altering events, such as inactivation of p53 and other participants in the DDR pathway, could potentially overcome this barrier. This notion is supported in mice with combined DDR and p53 deficiencies, in which increased chromosomal translocations cause accelerated tumor onset (Bassing et al, 2003; Celeste et al, 2003).
We observed that reduction of Tpp1 initiates a persistent ATM-dependent DDR at telomeres in cells that bypassed cellular senescence due to p53 deficiency. Therefore, we propose that the ATM-dependent DDR plays important roles in preventing telomere dysfunction-initiated tumorigenesis independent of p53 function. Our data revealed that dysfunctional telomeres generated by depletion of Tpp1 potently induced tumor formation in the setting of both ATM and p53 deficiency. Tpp1-depleted ATM−/− and p53−/− tumors possess elevated chromosomal aberrations, suggesting that random gains and/or losses of chromosomal segments may allow for the stepwise accumulation of genetic changes in favor of tumor progression (O'Hagan et al, 2002). Loss of ATM appears to favor the formation of chromosomal translocations, since broken chromosomes persist when ATM is deleted (Callen et al, 2007). This reservoir of translocation-competent chromosomes would be predicted to fuel rampant genomic instability and the selection of cancer-relevant pathways, as is evident in lymphomas derived from ATM−/− and p53−/− mice with dysfunctional telomeres (Maser et al, 2007).
Our results support a speculative model in which telomere dysfunction induced by loss Pot1a elicits primarily an ATR-dependent DDR, whereas long-term depletion of Tpp1 elicits primarily an ATM-dependent DDR to activate p53-mediated cell senescence or apoptosis to inhibit tumorigenesis (Figure 7). In the setting of p53 deficiency, the ATM-dependent DDR prevents tumor initiation by suppressing the generation of persistent chromosomal breaks (Callen et al, 2007). Together, these findings highlight a plausible role for ATM/ATR-mediated DDR in suppressing the genesis of chromosomal instability-driven carcinomas, and suggest that tumors initiated by dysfunctional telomeres might be refractory to therapeutic strategies based on inhibition of the DNA damage pathways.
Materials and methods
Vectors, cell lines and antibodies
Retrovirus expression constructs for Pot1a and Pot1b were described (He et al, 2006). Based on cDNA gi:22823923 (Liu et al, 2004a), we used RT–PCR strategy to clone mouse Tpp1cDNA in frame with a 5′ HA epitope tag at the N-terminal, and the fragment was inserted into the _Bam_HI and _Eco_RI sites of retroviral vector pQCXIP (Novex). To obtain retrovirus expression construct of Tpp1ΔRD (residues 1–161 plus 255–416), an _Nhe_I mutation site was introduced when residues 162–254 of Tpp1 were deleted. The retroviral expression vector pQCXIP-Tpp1ΔC (residues 1–255) was constructed by removing the C-terminal residues 256–416 of Tpp1. The sequences of primers for all constructs described are available upon request. All constructs were verified by DNA sequencing. Primary ATM+/+, ATM−/−, p53+/+, ATRF/+, ATRF/− and p53−/− MEFs were generated as described (Brown and Baltimore, 2003; Wu et al, 2006). To generate ATRΔ/−MEF, ATRF/− MEFs were infected with AdCre at an MOI of 200. Deletion of ATR was confirmed by RT–PCR and Western analyses. All the primary MEFs were maintained in DMEM supplemented with 10% fetal bovine serum and cultured under 3% oxygen to minimize premature entry into senescence (Parrinello et al, 2003). Antibodies used are as follows: phospho-p53 ser15 (no. 9284), phospho-ATM Ser 1981(no. 4526) and phospho-CHK1 (no. 2341) from Cell Signaling; Chk2 (no. 611570) from BD Biosciences; p21(Sc-6246) from Santa Cruz; γ-tubulin, HA, Flag and Myc from Sigma; ATR (PC538) from Calbiochem; γH2AX (no. 05-636) from Upstate; anti-53BP1 was a kind gift from Phil Carpenter, UTHMB; and antibodies against mouse TRF1 and TRF2 were kind gifts from Dr Karlseder, Salk Institute.
RNA isolation, RT–PCR and shRNA interference
RNA was isolated from approximately 106 cells with the Qiagen RNAeasy kit. RT–PCR was performed with the oligo-dT RT–PCR system according to the protocol provided by Invitrogen. Two shRNA against Tpp1s were generated in pSuper as described (Deng et al, 2003). To generate pRetro-Super constructs, _Eco_RI- and _Xho_I-digested insert from pSuper was subcloned into the same site into pRetro-Super vector (Brummelkamp et al, 2002). The shRNA target sequences for mouse Tpp1 is shRNA–Tpp1-1, 5′-TCAGGATTCAGATGTGCAG-3′; shRNA-Tpp1-2 and 5′-GCTGTGTTCACTGTGTCTG-3′. The target sequence of shRNA-Tpp1-2 was changed to 5′GCTGTCTTCACGGTCT CTG-3′ by standard site-directed mutagenesis to create shRNA-TPP1-2-resistant construct pRetro-Super shTPP1-2-M, according to the manufacturer's recommended protocol (Stratagene).
Retroviral infections and shTPP1-knockdown cell lines
To generate retroviruses, 10-cm dishes of 293 Phoenix packaging cells were transfected with 5 μg each of the Tpp1 mutants (Tpp1ΔRD or Tpp1ΔC), pSuper-Retro-shTpp1 or retroviral control vector using Lipofectamine (Invitrogene). Viral supernatants were harvested 24 h after transfection and used to infect fibroblasts with 8 μg/ml polybrene. Fresh medium was added to the packaging cells and 12–18 h later viral supernatants were collected a second time to super-infect the fibroblasts with 8 μg/ml polybrene. To obtain shPot1a- and shPot1b-knockdown cells, cells were first infected by shPot1a viral supernatants; after 6 h the cells were infected again by shPot1b viral supernatant. To obtain shTpp1-knockdown cells, drug selection of retrovirally transduced cells was started 48 h after the second infection by supplementing the culture medium with puromycin at 2.5 μg/ml final concentration. All cells were deemed transiently knocked down when selected for 4 days, and stably knocked down when selected for 3 weeks.
Supplementary Material
Supplementary Information
Acknowledgments
We are grateful to Jan Karlseder for providing Trf1 antibody, and Philip Carpenter for the 53BP1 antibody. SC acknowledges generous financial support from the NIA (RO1 AG028888), the NCI (RO1 CA129037), the Welch Foundation, the Elsa U Pardee Foundation, the Sidney Kimmel Foundation for Cancer Research, the Abraham and Phyllis Katz Foundation and the Michael Kadoorie Cancer Genetic Research Program. YD was supported by an NCI Howard Temin Award (1K01CA124461).
References
- Bartek J, Lukas J (2003) Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3: 421–429 [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Orntoft T, Lukas J, Bartek J (2005) DNA damage response as a candidate anticancer barrier in early human tumorigenesis. Nature 434: 864–870 [DOI] [PubMed] [Google Scholar]
- Bassing CH, Suh H, Ferguson DO, Chua KF, Manis J, Eckersdorff M, Gleason M, Bronson R, Lee C, Alt FW (2003) Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell 114: 359–370 [DOI] [PubMed] [Google Scholar]
- Brown EJ, Baltimore D (2003) Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev 17: 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 [DOI] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276: 42462–42467 [DOI] [PubMed] [Google Scholar]
- Callen E, Jankovic M, Difilippantonio S, Daniel JA, Chen HT, Celeste A, Pellegrini M, McBride K, Wangsa D, Bredemeyer AL, Sleckman BP, Ried T, Nussenzweig M, Nussenzweig A (2007) ATM prevents the persistence and propagation of chromosome breaks in lymphocytes. Cell 130: 63–75 [DOI] [PubMed] [Google Scholar]
- Celeste A, Difilippantonio S, Difilippantonio MJ, Fernandez-Capetillo O, Pilch DR, Sedelnikova OA, Eckhaus M, Ried T, Bonner WM, Nussenzweig A (2003) H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell 114: 371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli GB, de Lange T (2005) DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol 7: 712–718 [DOI] [PubMed] [Google Scholar]
- Cosme-Blanco W, Shen MF, Lazar AJ, Pathak S, Lozano G, Multani AS, Chang S (2007) Telomere dysfunction suppresses spontaneous tumorigenesis in vivo by initiating p53-dependent cellular senescence. EMBO Rep 8: 497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Adda di Fagagna FD, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198 [DOI] [PubMed] [Google Scholar]
- de Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19: 2100–2110 [DOI] [PubMed] [Google Scholar]
- de Lange T (2004) T-loops and the origin of telomeres. Nat Rev Mol Cell Biol 5: 323–329 [DOI] [PubMed] [Google Scholar]
- Denchi EL, de Lange T (2007) Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448: 1068–10671 [DOI] [PubMed] [Google Scholar]
- Deng Y, Ren X, Yang L, Lin Y, Wu X (2003) A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell 115: 61–70 [DOI] [PubMed] [Google Scholar]
- Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre' M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, d'Adda di Fagagna F (2006) Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444: 638–642 [DOI] [PubMed] [Google Scholar]
- Feldser DM, Greider CW (2007) Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell 11: 461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia S, Weiss RS, Hande MP, Freire R, d'Adda di Fagagna F (2006) Telomere and telomerase modulation by the mammalian Rad9/Rad1/Hus1 DNA-damage-checkpoint complex. Curr Biol 16: 1551–1558 [DOI] [PubMed] [Google Scholar]
- Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA Jr, Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD (2005) Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434: 907–913 [DOI] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T (1999) Mammalian telomeres end in a large duplex loop. Cell 97: 503–514 [DOI] [PubMed] [Google Scholar]
- He H, Multani AS, Cosme-Blanco W, Tahara H, Ma J, Pathak S, Deng Y, Chang S (2006) POT1b protects telomeres from end-to-end chromosomal fusions and aberrant homologous recombination. EMBO J 25: 5180–5190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 14: 501–513 [DOI] [PubMed] [Google Scholar]
- Hockemeyer D, Daniels JP, Takai H, de Lange T (2006) Recent expansion of the telomeric complex in rodents: two distinct POT1 proteins protect mouse telomeres. Cell 126: 63–77 [DOI] [PubMed] [Google Scholar]
- Hockemeyer D, Palm W, Else T, Daniels JP, Takai KK, Ye JZ, Keegan CE, de Lange T, Hammer GD (2007) Telomere protection by mammalian Pot1 requires interaction with Tpp1. Nat Struct Mol Biol 14: 754–761 [DOI] [PubMed] [Google Scholar]
- Houghtaling BR, Cuttonaro L, Chang W, Smith S (2004) A dynamic molecular link between the telomere length regulator TRF1 and the chromosome end protector TRF2. Curr Biol 14: 1621–1631 [DOI] [PubMed] [Google Scholar]
- Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T (1999) p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283: 1321–1325 [DOI] [PubMed] [Google Scholar]
- Kelleher C, Kurth I, Lingner J (2005) Human protection of telomeres 1 (POT1) is a negative regulator of telomerase activity in vitro. Mol Cell Biol 25: 808–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna KK, Jackson SP (2001) DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet 27: 247–254 [DOI] [PubMed] [Google Scholar]
- Kim S, Beausejour C, Davalos AR, Kaminker P, Heo SJ, Campisi J (2004) TIN2 mediates functions of TRF2 at human telomeres. J Biol Chem 279: 43799–43804 [DOI] [PubMed] [Google Scholar]
- Lei M, Zaug AJ, Podell ER, Cech TR (2005) Switching human telomerase on and off with hPOT1 protein in vitro. J Biol Chem 280: 20449–20456 [DOI] [PubMed] [Google Scholar]
- Liu D, O'Connor MS, Qin J, Songyang Z (2004b) Telosome, a mammalian telomere-associated complex formed by multiple telomeric proteins. J Biol Chem 279: 51338–51342 [DOI] [PubMed] [Google Scholar]
- Liu D, Safari A, O'Connor MS, Chan DW, Laegeler A, Qin J, Songyang Z (2004a) PTOP interacts with POT1 and regulates its localization to telomeres. Nat Cell Biol 6: 673–680 [DOI] [PubMed] [Google Scholar]
- MacDougall CA, Byun TS, Van C, Yee MC, Cimprich KA (2007) The structural determinants of checkpoint activation. Genes Dev 21: 898–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, Choudhury B, Campbell PJ, Feng B, Wong KK, Protopopov A, O'Neil J, Gutierrez A, Ivanova E, Perna I, Lin E, Mani V, Jiang S, McNamara K, Zaghlul S, Edkins S, Stevens C, Brennan C, Martin ES, Wiedemeyer R, et al. (2007) Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature 447: 966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, DePinho RA (2002) Connecting chromosomes, crisis, and cancer. Science 297: 565–569 [DOI] [PubMed] [Google Scholar]
- Nuciforo PG, Luise C, Capra M, Pelosi G, di Fagagna FD (2007) Complex engagement of DNA-damage response pathways in human cancer and in lung tumor progression. Carcinogenesis, May 22; e-pub ahead of print [DOI] [PubMed] [Google Scholar]
- O'Connor MS, Safari A, Xin H, Liu D, Songyang Z (2006) A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proc Natl Acad Sci USA 103: 11874–11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hagan RC, Chang S, Maser RS, Mohan R, Artandi SE, Chin L, DePinho RA (2002) Telomere dysfunction provokes regional amplification and deletion in cancer genomes. Cancer Cell 2: 149–155 [DOI] [PubMed] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J (2003) Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol 25: 741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM (1998) Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem 275: 9390–9395 [DOI] [PubMed] [Google Scholar]
- Shiloh Y (2003) ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3: 155–168 [DOI] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, de Lange T (2003) DNA damage foci at dysfunctional telomeres. Curr Bio 13: 1549–1556 [DOI] [PubMed] [Google Scholar]
- Theobald DL, Cervantes RB, Lundblad V, Wuttke DS (2003) Homology among telomeric end-protection proteins. Structure 11: 1049–1050 [DOI] [PubMed] [Google Scholar]
- Verdun RE, Crabbe L, Haggblom C, Karlseder J (2005) Functional human telomeres are recognized as DNA damage in G2 of the cell cycle. Mol Cell 20: 551–561 [DOI] [PubMed] [Google Scholar]
- Verdun RE, Karlseder J (2006) The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell 127: 709–720 [DOI] [PubMed] [Google Scholar]
- Verdun RE, Karlseder J (2007) Replication and protection of telomeres. Nature 447: 924–931 [DOI] [PubMed] [Google Scholar]
- Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, Lei M (2007) The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 445: 506–510 [DOI] [PubMed] [Google Scholar]
- Wong KK, Chang S, Depinho RA (2006) Modeling cancer and aging in the telomerase-deficient mouse. In Telomeres, de Lange T, Lundblad V, Blackburn E (eds), pp 109–138. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Wu L, Multani AS, He H, Cosme-Blanco W, Deng Y, Deng JM, Bachilo O, Pathak S, Tahara H, Bailey SM, Deng Y, Behringer RR, Chang S (2006) Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell 126: 49–62 [DOI] [PubMed] [Google Scholar]
- Xin H, Liu D, Wan M, Safari A, Kim H, Sun W, O'Connor MS, Songyang Z (2007) TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase. Nature 445: 559–562 [DOI] [PubMed] [Google Scholar]
- Yang Q, Zheng YL, Harris CC (2005) POT1 and TRF2 cooperate to maintain telomeric integrity. Mol Cell Biol 25: 1070–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JZ, Donigian JR, van Overbeek M, Loayza D, Luo Y, Krutchinsky AN, Chait BT, de Lange T (2004b) TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. J Biol Chem 279: 47264–47271 [DOI] [PubMed] [Google Scholar]
- Ye JZ, Hockemeyer D, Krutchinsky AN, Loayza D, Hooper SM, Chait BT, de Lange T (2004a) POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev 18: 1649–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ (2000) The DNA damage response: putting checkpoints in perspective. Nature 408: 433–439 [DOI] [PubMed] [Google Scholar]
- Zou L (2007) Single- and double-stranded DNA: building a trigger of ATR-mediated DNA damage response. Genes Dev 21: 879–885 [DOI] [PubMed] [Google Scholar]
- Zou L, Elledge SJ (2003) Sensing DNA damage through ATRIP recognition of RPA–ssDNA complexes. Science 300: 1542–1548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information