CD4+CD28– costimulation-independent T cells in multiple sclerosis (original) (raw)
Abstract
Multiple lines of evidence suggest that CD4+ lymphocytes initiate autoimmune responses against myelin antigens in multiple sclerosis (MS). The increased frequency of activated myelin-specific cells in MS patients indicates that the activation of autoreactive cells represents a central event in the pathogenesis of the disease. We identified a CD4+ subpopulation that is characterized phenotypically by the persistent absence of surface CD28 expression and functionally by CD28-independent activation and Th1 cytokine secretion. Owing to their costimulation-independent activation and their expression of a full agonist signaling activation pattern, CD4+CD28– cells have the potential to initiate autoimmune responses in the central nervous system, a compartment devoid of professional antigen presenting cells. Long-term memory CD4+CD28– cells produce high amounts of IFN-γ and maximally upregulate IFN-γ and IL-12Rβ2 chain expression in the absence of costimulation. They exhibit prominent growth characteristics and increased survival after activation, likely related to their persistent lack of CTLA-4 surface expression. The CD4+CD28– population is expanded in a subgroup of MS patients. Myelin basic protein-specific cells detected in this cell subset may play an important role in the inflammatory response within the central nervous system.
Introduction
Current studies support a critical role of CD4+ myelin-specific cells in the initiation of multiple sclerosis (MS), an inflammatory demyelinating disease of the central nervous system CNS (1). However, myelin-reactive cells are represented in the normal T cell repertoire and are found in comparable frequencies in the peripheral blood of both MS patients and normal controls (2, 3). Thus, their mere presence is not sufficient to trigger a pathological autoimmune response.
It is the frequency of activated myelin-reactive cells that is increased in MS patients in comparison to healthy individuals (4), suggesting their involvement in disease development. An increased frequency of hypoxanthine-guanine phosphoribosyltransferase reporter (HPRT) gene mutations in myelin basic protein–specific (MBP-specific) and proteolipid protein–specific cells derived from MS patients suggests their active replicative history (5). A substantial fraction of autoreactive cells derived from peripheral blood and cerebrospinal fluid (CSF) in MS patients secrete IL-2, IFN-γ, TNF-α and soluble IL-2 receptor (6, 7) and have an increased surface expression of adhesion molecules VLA 3-6, LFA-1, LFA-3, CD2, CD26, and CD44 (8). Lejon and Fathman (9) recently reported that CD4+ cells upregulate CD4 expression after antigen challenge. They demonstrated that the CD4high subset of the pancreatic islet infiltrate in nonobese diabetic (NOD) mice contain autoreactive cells that can efficiently transfer disease.
Peripherally activated autoreactive lymphocytes can cross the blood brain barrier (BBB) and initiate an autoimmune response in the CNS (10), as documented in experimental autoimmune encephalomyelitis (EAE), an animal model of MS. It is therefore important to understand which factors contribute to the activation of myelin-reactive T cells.
Growing evidence indicates that functional characteristics of the autoreactive T lymphocytes determine their propensity for activation (11). In the two-signal activation paradigm, the first signal induced by T cell receptor (TCR) engagement determines the antigen specificity, whereas the second costimulatory signal determines the activation threshold and the functional outcome of the antigen-specific activation. The activation threshold is modulated by costimulatory signals, and several reports (12, 13) indicate that their dysregulation may play a critical role in the activation of autoreactive T cells. CD80/CD86–CD28/CTLA-4 is the most important and best-studied costimulatory pathway. CD80 and CD86 molecules are expressed on activated antigen-presenting cells (APCs) and bind to their ligands CD28 and CTLA-4 on T cells. CD86 is constitutively expressed on dendritic cells and monocytes and is rapidly upregulated after activation. In contrast, CD80 is slowly upregulated on APCs after stimulation and plays a more important role in chronic inflammatory responses (14). CD28 is constitutively expressed on the surface of more than 95% of CD4+ lymphocytes and is only transiently downmodulated after binding to CD80 and CD86. CD28 costimulation synergizes with TCR activation and induces IL-2, IL-4, IL-5, TNF-α, and GM-CSF cytokine production. It regulates Th1/Th2 differentiation and the proliferative capacity, including cell-cycle progression and susceptibility to apoptotic cell death (15). Upon activation and CD28 downmodulation, CD4+ cells upregulate surface expression of cytotoxic T lymphocyte–associated antigen 4 (CTLA-4), a structural homologue of CD28 that binds the same ligands with a higher affinity and delivers a negative signal with respect to T cell activation (16).
Costimulatory requirements for T cell activation are influenced by previous T cell antigen exposure: costimulation is required for the activation of naive cells, whereas previously activated memory cells do not depend on CD28-mediated costimulation (17). Factors that further affect the activation requirements are TCR avidity and antigen dose required for the activation, the context in which T cell activation is occurring, the APCs’ activation state, and the local cytokine milieu (18).
After an initial expansion, the majority of activated cells become effector cells and perish via activation-induced cell death (AICD), whereas a small portion differentiates into memory cells. Long-term memory cells can survive for months to years without repeated antigenic stimulation. Instead, they may need only occasional low-grade stimulation with cross-reactive antigens to maintain their persistence in the peripheral blood (19).
The recent demonstrations that autoantigen-specific T cells in MS are less CD28-costimulation dependent (20, 21), and the observation of a CD4+ CD28– T cell population in patients with other autoimmune diseases (22, 23), led to the following questions. Can we detect a costimulation-independent cell population in MS patients? What are the growth characteristics, phenotype, function, and activation requirements of CD4+ cells with differential CD28 surface expression? What is the antigen specificity of cells with differential costimulatory requirements? Is the costimulation-independent cell population enriched for myelin-specific T cells in MS patients?
Methods
CD4+CD28– cell separation.
PBMCs were isolated from the blood obtained by lymphocytapheresis using lymphocyte separation medium (BioWhittaker Inc., Walkersville, Maryland, USA). CD4+CD28– cells were separated using MACS beads (Miltenyi Biotec, Auburn, California, USA) according to the manufacturer’s recommendation. Briefly, PBMCs were resuspended at 107 cells/ml of MACS buffer (0.5% human serum and 2 mM EDTA in PBS) and incubated with anti-CD4 magnetic beads at 6°C for 15 minutes. The cell suspension was applied to lymphocyte separation column, and CD4+ cells were retained in the column. Beads were cleaved off using the release and stop solution and cells were subsequently incubated with anti-CD28-PE antibodies (BD PharMingen, San Diego, California, USA). Negative CD4+CD28– selection was performed with anti-PE magnetic beads, and CD28– cells were eluted from the column. Monocytes with a low CD4 and no surface CD28 expression were excluded by their adherence to plastic, and by separation with anti-CD14 magnetic beads. Separated CD4+CD28– populations had greater than 95% purity.
CD4+CD28– and CD4+CD28+ cloning.
T cell clones were generated from separated CD4+CD28– and CD4+CD28+ bulk cultures. Cells were plated at 1 cell per well with 105 allogeneic feeders, 2.5 μg/ml phytohemagglutinin (PHA), and 40 U/ml rIL-2 (National Cancer Institute, Frederick, Maryland, USA) in 96-well plates. Every 3–4 days, fresh T cell media (Iscove’s modified Dulbecco’s medium; Life Technologies Inc., Grand Island, New York, USA), 5% human serum (Sigma Chemical Co., St. Louis, Missouri, USA), and 2 mM L-glutamine containing 40 IU/ml IL-2 were added. On days 12–14, positive wells were selected for further expansion. Stable long-term clones were characterized for surface markers, with repeated monitoring of CD28 expression.
Proliferation assay.
The 96-well plates were precoated with suboptimal concentrations of αCD3 (0.01–1.0 μg/ml), αCD28 (5 μg/ml), αCTLA-4 (5 μg/ml), or isotype control anti-IgG1 (1 μg/ml) antibodies (BD PharMingen) in 100 μl PBS for 4 hours at 37°C. CD4+CD28– and CD4+CD28+ clones were plated in duplicates at 105 cells per well and cultured for 3–7 days. During the last 16 hours, 3[H]thymidine was added and plates were harvested and counted by Beta counter (Wallac, Gaithersburg, Maryland, USA). To detect the frequency of MBP-reactive cells, CD4+CD28- and CD4+CD28+ cells derived from ten relapsing remitting (RR) MS patients were plated at three concentrations (3 × 04, 1.5 × 104 , and 0.75 × 104 cells per well, 60–20 wells for each cell number), with 105 well irradiated autologous PBMCs prepulsed with human MBP (50 μg/ml). Reactivity to control influenza hemagglutinin (306–318) (PKYVKQNTLKLAT) antigen was tested in parallel using final concentration of 10 μg/ml. Antigen specificity was measured on day 14 by a modified split-well assay (24). Wells were scored as positive if counts per minute exceeded the mean control counts by at least two times (SI > 2).
FACS analysis.
The expression of surface markers was analyzed in 31 stable long-term CD4+CD28– and CD4+CD28+ clones. Staining was performed on resting cells 7–10 days after PHA stimulation, and 104 CD3+ lymphocyte gated events were acquired (FACSCalibur, Becton Dickinson Immunocytometry Systems Inc., San Jose, California, USA). The following antibodies were used: IgG1-FITC, IgG1-PE, IgG1-Cy, and CD4-FITC, CTLA-4-PE, CD3-Cy, CD96-PE, CD25-FITC, CD45RA-FITC, CD45RO-PE, CD54-PE, CD11a-FITC, CD134-FITC, CD40L-PE, CD29-FITC, CD26-PE, CD28-PE, TCR-FITC and CD69-PE (BD PharMingen). The results were analyzed with Cell Quest software (Becton Dickinson Immunocytometry Systems Inc.) after subtracting the control IgG1 staining.
Cytokine detection.
IL-2, IFN-γ, IL-4, and IL-10 production was measured by ELISA using CytoSets (Biosource International, Camarillo, California, USA) according to the manufacturer’s recommendation. Cells were plated in 48-well plates at 106/ml and stimulated with 1 μg/ml PHA and 106 irradiated allogeneic feeders. Culture supernatants were collected after 48 hours and stored at –70°C until the measurement was performed in duplicates on diluted supernatants.
Survival assay.
Apoptosis was induced according to a protocol published previously (25). Briefly, 106 cells were stimulated with PHA (2.5 μg/ml) and 106 irradiated allogeneic feeders for 48 hours. Dead cells were subsequently removed by Ficoll density gradients. Cells were plated at 106 cells/ml with 40 IU IL-2/ml and cultured for 3 days. Seven days after the second stimulation with plate-bound αCD3 mAb (2 μg/ml), cells were washed and 0.5 μg/ml of propidium iodide (PI) (Sigma Chemical Co.), was added to each sample. Non-gated events were acquired over 90 seconds, and results were expressed as a percentage of viable PI excluding cells out of total number of CD4+ cells.
TCR signal transduction.
A total of 106 T cells were stimulated with αCD3 alone or αCD3 and αCD28 for 5 minutes at 37°C. Samples were then washed with PBS and placed in lysis buffer (1% NP-40, 10 mM Tris-HCL [pH 7.2], 140 mM NaCl, 2 mM EDTA, 5 mM iodoacetamide, 1 mM Na3VO4), and complete protease inhibitor cocktail (Boehringer Mannheim Biochemicals Inc., Mannheim, Germany) for 25 minutes on ice. After removing nuclear debris, lysate supernatants were subjected to immunoprecipitation with rabbit antiserum to ZAP-70 (gift from I. Stefanova, NIH, Bethesda, Maryland, USA) at 4°C for 12 hours. Samples were analyzed by SDS-PAGE and immunoblotting with a mouse mAb to phosphotyrosine 4G10 (Upstate Biotechnology Inc., Lake Placid, New York, USA).
Real time PCR.
Total RNA was isolated from cell cultures by phenol-chloroform extraction using Trizol Reagent (Life Technologies Inc.). RNA was reverse transcribed to cDNA with random hexamer primers using TaqMan Reverse Transcription Reagents as per manufacturer’s instructions (Perkin-Elmer Applied Biosystems, Foster City, California, USA). Quantitative RT-PCR was performed on an ABI Prism 7700 Sequence Detection System (Perkin-Elmer). Amplification of 18S rRNA was used for sample normalization. For detection of CD28, IL-12Rβ2, and SLAM transcripts, oligonucleotides were used at final concentrations of 200 nM for forward and reverse primer and 100 nM for the fluorogenic probe as follows: CD28 forward: 5′CTTCTGCAAAATTGAAGTTATGTATCCT3, reverse: 5′GGACTT-GGACAAAGGTGTTTCC3, probe: 5′FAM-CCTTACCTAGACAATGAGAAGAGCAATGGAACCA-TAMRA3, IL-12Rβ2 forward: 5′GGCATTTTCTCAACGCATTACTT3, reverse: 5′TGGATCTGGAATTTC-TCTGCTACA3, probe: 5′FAM-TTCTCCTAGCAGCC-CTCAGACCTCAGTG-TAMRA3; SLAM forward: 5′ACAGACCC-CTCAGAAACAAAACC3, reverse: 5′AACTGTAGTATTACCACCATGATGAGAATC3′ probe: 5′FAM-CCTAA-CAGCCCAGCATACACTGCCCA-TAMRA3.
For the detection of IFN-γ transcripts, TaqMan Assay Reagents (Perkin-Elmer) containing cytokine primers and probe were used. 18S rRNA was amplified using TaqMan Ribosomal RNA Control Reagents (Perkin-Elmer).
Results
The absence of surface CD28 expression is a stable phenotype of the CD4+CD28– population in MS.
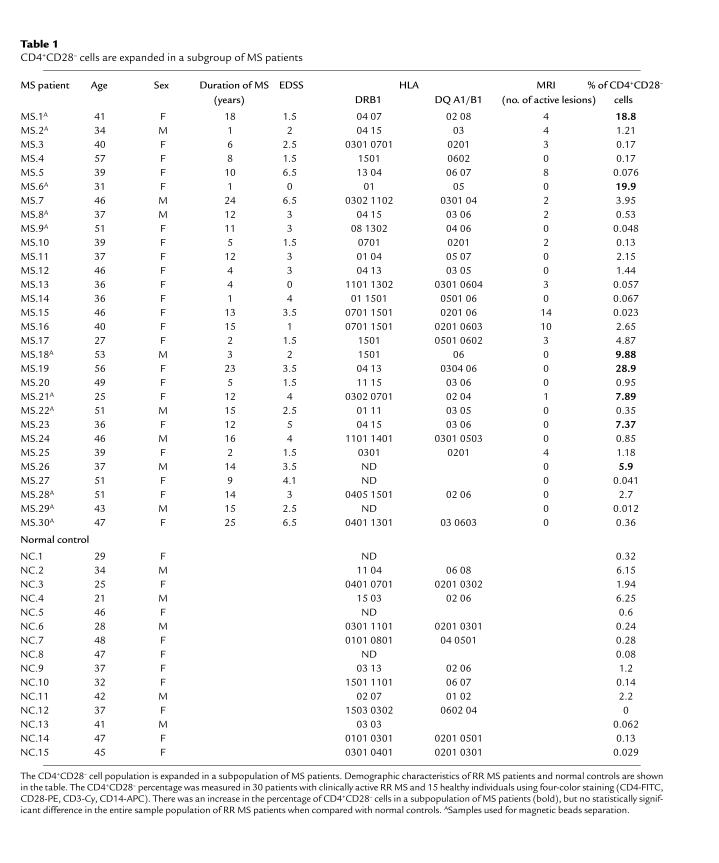
To address the CD28-costimulation requirements for the activation of autoreactive T cells in MS, we isolated subpopulations of CD4+ lymphocytes with negative and positive CD28 surface expression. CD4+CD28– and CD4+CD28+ cells were separated by MACS magnetic beads from peripheral blood of 11 nontreated patients with RR MS (Table 1). Separated CD4+CD28– cells constituted 0.012–28.9% of the CD4+ population.
Table 1.
CD4+CD28– cells are expanded in a subgroup of MS patients
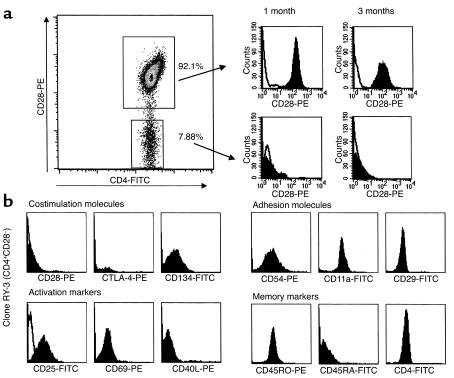
First, we assessed whether CD4+CD28– cells represent a distinct population or whether these cells only transiently downmodulate CD28 after T cell activation. Our results show that in MS patients the CD4+CD28– bulk population (Figure 1a) and subsequently generated clones have a stable CD28– surface phenotype, whereas CD4+CD28+ cells transiently downmodulate CD28 surface expression after in vitro stimulation (data not shown). In contrast, separated CD4+CD28– populations from six healthy individuals did not maintain surface CD28– phenotype upon subsequent staining.
Figure 1.
Longitudinally followed CD4+CD28– subpopulations have a stable CD28– surface expression in MS patients. (a) CD4+CD28– and CD4+CD28+ populations were separated using magnetic beads. PHA-expanded bulk cultures and subsequently generated clones continuously lacked CD28 surface expression when maintained in culture for periods up to 10 months. (b) CD4+CD28– and CD4+CD28+ clones are characterized for the expression of costimulatory activation and memory phenotype markers in the resting state, 7–10 days after PHA stimulation. CD4+CD28– clones are CD4+, CD45RO+, CD54+, CD11a+, CD29+ and differ from the CD4+CD28+ clones by the negative surface staining for CD28 and CTLA-4.
CD28 mRNA expression was detectable by real time PCR in long-term CD4+CD28– clones from RR MS patients, but at a very low level when compared with CD4+CD28+ clones (relative CD28 mRNA level 13.1 ± 4.7 vs. 732.7 ± 66.3).
Fifty-six long-term clones were used in the subsequent experiments. When characterized for multiple surface markers, CD4+CD28– clones consistently lacked CD28 and CTLA-4 surface expression (Figure 1b). The surface activation markers CD25, CD69, and CD40L were not continuously expressed on CD4+CD28– clones, consistent with their long-term memory phenotype (26). However, these activation markers were readily upregulated to maximal levels upon CD3 ligation (data not shown). CD54 (ICAM-1), CD11a (LFA-1), and CD29 adhesion molecules were constitutively expressed in this memory (CD45RA–CD45RO+) population, and alternative costimulatory molecules that function independently of the CD28 pathway (CD134, CD29, CD26) were expressed in the absence of CD28 expression.
CD4+CD28– clones are CD28-costimulation independent and show a full agonist signaling activation pattern in the absence of CD28 costimulation.
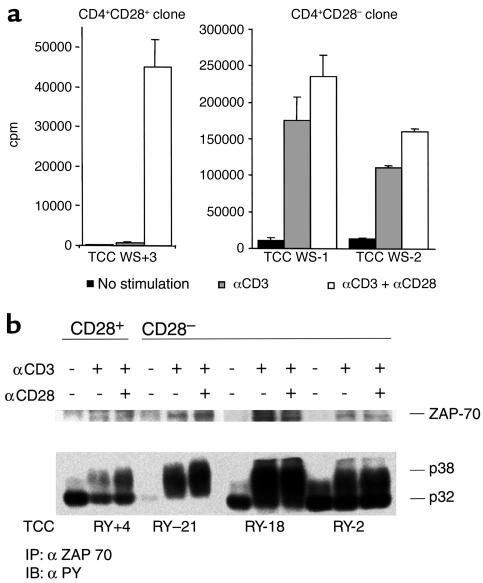
CD4+CD28– cells from RR MS patients had a prominent growth potential; they had a higher cloning efficiency in comparison to CD4+CD28+ cells. We examined the costimulation requirements for the activation of CD4+CD28– and control CD4+CD28+ clones from MS patients by measuring their proliferative response and signaling patterns after CD3 ligation alone or in the presence of CD28-mediated costimulation. The proliferation of CD4+CD28– clones was consistently independent of CD28 costimulation, and the addition of αCD28 mAb’s did not significantly increase their response. Results representative of ten independent experiments performed with 29 clones are shown in Figure 2a. Although the addition of αCD28 mAb to αCD3 mAb-stimulated CD4+CD28– clones increased the proliferative response by an average of 8%, the αCD28 mAb stimulation of CD4+CD28+ clones induced an average increase in proliferation by 1,241% compared with the response induced by αCD3 mAb only.
Figure 2.
CD4+CD28– clones are CD28 costimulation–independent. (a) CD4+CD28+ and CD4+CD28– clones were plated at 105 cells per well in plates precoated with a suboptimal concentration of αCD3 (0.1 μg/ml) or αCD3 + αCD28 (5 μg/ml). Cells were cultured in duplicates for 72 hours, and proliferation was assessed by pulsing cultures with 3[H]thymidine for the final 16 hours of culture. (b) CD4+CD28– and CD4+CD28+ clones were stimulated with αCD3 or αCD3 + αCD28 mAb’s. The signaling pattern was analyzed by immunoprecipitation with anti-ZAP mAb’s and subsequent immunoblotting with mAb to phosphotyrosine. CD4+CD28– clones displayed maximal phosphorylation of TCR-ζ chain p32 and p38 when stimulated in the absence of CD28 costimulation. TCC WS+3, T cell clone WS (CD28+) 3; TCC WS-1, T cell clone WS (CD28–)1; TCC WS-2, T cell clone WS (CD28–) 2.
Analysis of the TCR signaling pathway by Western blot permits identification of distinct patterns of phosphorylation which correspond to different degrees of T cell activation. In particular, we can differentiate a full-agonist response, characterized by phosphorylation of the TCR-ζ chain (p38>p32) and of ZAP-70, from a partial agonist response, characterized by modest TCR-ζ chain phosphorylation (only p32) and the absence of ZAP-70 phosphorylation. Consistent with the proliferative results, experiments examining TCR signal transduction in 11 clones reveal that CD4+CD28– clones display a full-agonist activation pattern after stimulation with suboptimal concentrations of αCD3 in the absence of CD28 costimulation. Measurement of ZAP-70 band intensities revealed 1.8–2.5 times higher band intensities in CD4+CD28– clones in comparison to the CD4+CD28+ clone after αCD3 stimulation. The addition of conjugated αCD28 stimulatory antibody did not change this signaling pattern (Figure 2b). In contrast, CD4+CD28+ clones achieved only a partial agonist response when stimulated with suboptimal concentrations of αCD3, requiring both αCD3 and αCD28 stimulus for a full-agonist response.
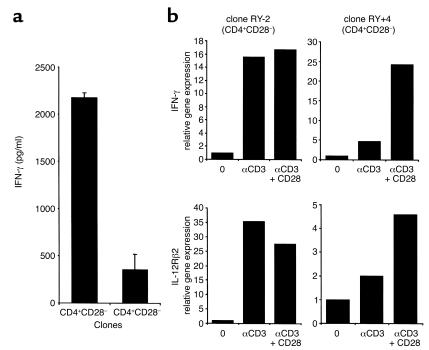
CD4+CD28– clones have a Th1 phenotype and maximally upregulate IFN-γ and IL-12Rβ2 chain gene expression in the absence of CD28 costimulation. It is well documented that CD28 costimulation plays an important role in Th1/Th2 lineage differentiation. We measured IL-2, IFN-γ, IL-4, and IL-10 cytokine production in supernatants from ten CD4+CD28– and CD4+CD28+ clones, 48 hours after PHA stimulation (Figure 3a). CD4+CD28– clones produced Th1 cytokines, with high IFN-γ production characteristic of differentiated memory cells. CD4+CD28– clones had 6.2 times higher mean production of IFN-γ than did control CD4+CD28+ clones.
Figure 3.
CD4+CD28– clones secrete Th1 type cytokines and maximally upregulate IFN-γ and IL-12Rβ chain gene expression in the absence of costimulation. (a) IFN-γ production was measured in supernatants of ten CD4+CD28– and CD4+CD28+ clones 48 hours after PHA stimulation. (b) IFN-γ and IL-12Rβ2 chain gene expression was measured by real time PCR in CD4+CD28– and CD4+CD28+ clones after stimulation with immobilized control IgG1, αCD3, or αCD3 + αCD28 mAb’s. Quantitative RT-PCR was performed and gene expression levels normalized to 18S RNA. CD4+CD28– clones maximally upregulate IFN-γ and IL-12Rβ2 chain, critical molecules in Th1 lineage differentiation, in the absence of CD28 costimulation.
To characterize the Th1 differentiation of CD28-costimulation–independent clones, we studied gene expression patterns after differential stimuli using quantitative real time PCR (Figure 3b). CD4+CD28– clones maximally upregulate IFN-γ mRNA after αCD3 stimulation. CD4+CD28+ clones have only minimal IFN-γ gene upregulation with αCD3 alone and require both the αCD3 and αCD28 stimulus for maximal IFN-γ expression, which is significantly lower than in CD4+CD28– clones in all tested clones (data not shown).
The IL-12Rβ2 subunit, a critical molecule in Th1 lineage differentiation, is maximally upregulated in CD4+CD28– clones in the absence of CD28 costimulation. CD4+CD28+ clones, on the other hand, require both TCR- and CD28-mediated stimulus for a maximal IL-12Rβ2 chain expression. Results in Figure 3b are expressed as changes relative to gene expression levels in the nonstimulated clones and are representative of six experiments.
To address whether the upregulation of the IL-12Rβ2 chain gene occurs as a consequence of autocrine IFN-γ secretion, we blocked IFN-γ with αIFN-γ mAb before measurement of activation-induced IL-12Rβ2 expression. Given that IL-12Rβ2 chain expression continued to be maximal in the absence of CD28 costimulation, we propose that direct TCR signaling with a subsequent CD40L upregulation might be a mechanism for IL-12Rβ2 chain upregulation in CD4+CD28– clones (data not shown). Alternative costimulatory pathways and cytokines may also play a role in the induction of IL-12Rβ2 expression.
Signaling lymphocyte activation marker (SLAM), a CD28-independent costimulatory molecule that selectively increases IFN-γ production upon TCR activation, may provide an additional stimulus for IFN-γ production. In six independent experiments, we observed increased SLAM gene expression in CD+CD28– clones after αCD3 stimulation. This finding further supports the CD28-costimulation–independent activation of CD4+CD28– clones (data not shown) and points to the possible compensatory role of the CD28-independent costimulatory pathways.
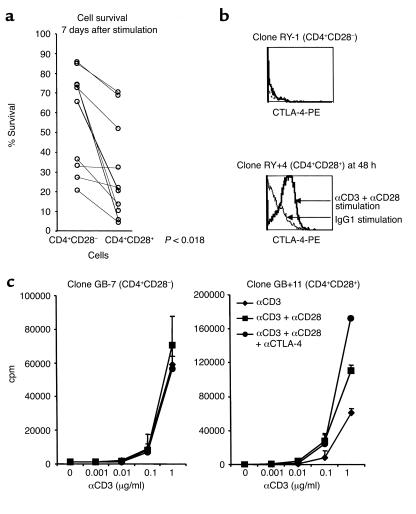
CD4+CD28– clones escape mechanisms that control clonal expansion. The CD4+CD28– population shows a prolonged proliferative response after mitogenic stimulation. To examine whether CD+CD28– cells escape mechanisms that normally downregulate clonal expansion, AICD was induced by αCD3 apoptotic stimuli in bulk population of CD4+CD28– cells, and their survival was compared with the survival of CD4+CD28+ cells from the same RR MS patient. In ten independent experiments (Figure 4a), we detected a significantly higher survival in CD4+CD28– cells, 7 days after activation and subsequent IL-2 withdrawal, in comparison to the CD4+CD28+ population (Wilcoxon signed rank test, P = 0.0018).
Figure 4.
CD4+CD28– cells exhibit prolonged survival after activation. (a) CD4+CD28– and CD4+CD28+ populations from the same individuals were exposed to apoptotic stimuli, and the percentage of surviving cells was measured by PI exclusion 7 days after stimulation. (b) CTLA-4 surface expression was measured in CD4+CD28– and CD28+ clones at resting state and at multiple time points over the course of 72 hours after stimulation. CD4+CD28+ clones maximally upregulated CTLA-4 surface expression 48 hours after the stimulation, whereas CD4+CD28– clones did not have detectable CTLA-4 surface expression at any time point after activation. (c) The effect of CTLA-4 blockade (anti–CTLA-4 mAb, 5 μg/ml) was assessed in CD4+CD28– and CD4+CD28+ clones stimulated with indicated concentration of αCD3 or αCD3 + αCD28. The proliferative response measured 72 hours after stimulation was not changed in CD4+CD28– clones with the addition of anti–CTLA-4 blockade, whereas CD4+CD28+ clones exhibited an increased proliferation upon CTLA-4 blocking.
A second mechanism of controlling clonal expansion by the inhibitory effect of CTLA-4 is particularly interesting in this system, as CD28 signaling is required for CTLA-4 expression. Eight clones with CD4+CD28– and CD4+CD28+ phenotype were tested for CTLA-4 expression in time course experiments from 3 to 72 hours after αCD3 or αCD3+αCD28 stimulation. There was neither CTLA-4 expression on resting CD4+CD28– clones nor upon their activation, whereas CD4+CD28+ clones upregulated CTLA-4 48 hours after αCD3+αCD28 stimulation (Figure 4b).
Furthermore, we tested the effect of CTLA-4 blockade by anti–CTLA-4 mAb in proliferation assays. The CD4+CD28+ clones showed an increased proliferative response upon blockade of the inhibitory CTLA-4 pathway. In parallel experiments, maximal proliferation of CD4+CD28– clones induced by αCD3 antibodies was not exceeded upon addition of either αCD28 or anti–CTLA-4 mAb, consistent with the absence of both CD28 and CTLA-4 surface expression. Results presented in Figure 4c are representative of seven similar experiments.
The CD4+CD28– population contains MBP-specific T cells in MS patients.
CD4+CD28– cells comprise a distinct population in MS patients. To evaluate whether this population expands in RR MS, we measured the percentage of CD28– cells out of total CD4+ cells in 30 patients with active RR MS (at least one clinical exacerbation in the past 6 months) and 15 healthy individuals (Table 1). Owing to the low frequency of CD4+CD28– cells, particular care was taken to select cells by gating on CD3+, CD4+, CD14– lymphocytes, and to acquire high numbers (5 × 104) of gated events. We observed the expansion of CD4+CD28– cells in a subgroup of MS patients (Table 1, bold characters: mean normal controls ± 2 SD), but not in all tested patients when compared with normal controls (Wilcoxon rank sum test, P = 0.21). The proportion of CD4+CD28– cells showed a significant variability in both normal individuals (0–6.15%) and MS patients (0.023–28.90%). However, the percentage of CD4+CD28– cells appeared rather stable in the individual MS patient, as observed in two patients followed up monthly for 12 months (data not shown).
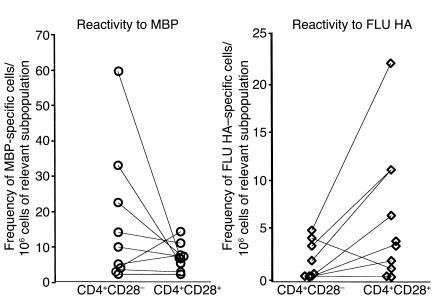
In an attempt to address the antigen specificity of CD4+CD28– population, we measured the frequency of cells reactive to MBP, one candidate autoantigen in MS, in CD4+CD28– and CD4+CD28+ populations in ten RR MS patients. A large number of PBMCs (6 × 108) obtained by lymphapheresis was needed to isolate the rare CD4+CD28– population and to determine the precursor frequency of MBP-reactive cells in both CD4+CD28– and CD4+CD28+ subpopulations. Our results confirm the presence of MBP-reactive cells in the CD4+CD28– cell subset. However, in comparison to the frequency of MBP-specific cells in the CD4+CD28+ population, there is no statistically significant difference (Wilcoxon signed rank test, P = 0.3) (Figure 5). In addition, we tested the reactivity to the control FLU HA 306-318 antigen in both cell subsets. Because FLU-HA-specific cells were more frequent in the CD4+CD28+ subpopulation, we excluded the possibility that the MBP reactivity detected in CD4+CD28– cell subset simply reflects a general increase in the memory cells response.
Figure 5.
MBP-specific cells are detected in the CD4+CD28– population. Reactivity to MBP was measured in CD4+CD28– and CD4+CD28+ populations separated from ten RR MS patients by limiting dilution assays using three cell concentrations: 0.7, 1.5, and 3 × 104/well. The mean frequency of MBP-reactive cells for each patient was expressed as the number of MBP-reactive cells per 106 cells of the relevant CD4+CD28– or CD4+CD28+ cell subpopulation. Reactivity against the control FLU HA (308–319) antigen was tested in ten RR MS patients using the same experimental approach.
Discussion
Previous studies of the autoimmune response in MS have primarily focused on the antigen specificity of myelin-reactive CD4+ cells. Comparable precursor frequencies of myelin-reactive CD4+ cells in MS patients and normal individuals support the findings from studies on MBP-specific TCR-transgenic mice (27), that the presence of even high numbers of myelin-specific T cells is not sufficient to trigger an autoimmune disease.
In human studies of the costimulatory requirements of autoreactive T cells, two groups have recently demonstrated that MBP-specific T cells exhibit decreased CD28-costimulatory requirements in MS patients when compared with normal controls. Scholz et al. (20), using CD80- and CD86-transfected Chinese hamster ovary (CHO) cells as APCs, found that MBP-reactive T cell clones from normal individuals require B7 costimulation, whereas MBP-reactive clones from MS patients proliferate in the absence of B7 costimulation. Lovett-Racke et al. (21) reported that the addition of anti-CD28 or CTLA-4 Ig prevented MBP-specific proliferation in normal controls, but not in MS patients. The authors concluded that MBP-reactive CD4+ cells expand in the absence of CD28/B7-mediated costimulation, due to their previous in vivo activation and memory phenotype.
Studies in several other autoimmune diseases identified a specific costimulation-independent CD4+ population that can be detected in the patients’ blood over long periods. In RA, CD4+CD7–CD28– cells were identified as expanded T cell clonotypes that persist in vivo for several years (22). The longevity and clonal expansion of this population are related to their resistance to activation induced cell death. Expanded CD11b+CD28–CD4+ clonotypes are also identified in IDDM (23), chronic inflammatory bowel disease (28), and Wegener granulomatosis (29). However, the antigen specificities of those populations have not been identified, and autoreactivity is only presumed owing to their proliferation in the autologous mixed lymphocyte reaction. Expanded CD4+ and CD8+ clones with persistent lack of CD28 surface expression are also detected in the elderly population. Their significance is unknown, and it is speculated that it may be related to the decline in protective immunity and an increased risk of developing malignancies and autoimmune diseases (30) in the aging population.
In this study, we characterized a subpopulation of CD4+ cells with a long-term stable CD28– surface phenotype in RR MS patients. CD4+CD28– clones exhibited CD28-costimulation-independent activation, prominent Th1 cytokine production, and a prolonged proliferative response in vitro. In CD4+CD28– clones, signal transduction studies reveal a full agonist signaling activation pattern in the absence of CD28-costimulation. In contrast, CD4+CD28+ clones derived from the same MS patients required αCD28 costimulation in order to obtain a full agonist signal. Rearrangements of the topology of the signaling machinery in memory T cells were recently described for CD8+ cells (31), and further studies are needed to address whether similar mechanisms underlie the facilitated activation of costimulation-independent CD4+CD28– clones in MS.
The dysregulation of costimulatory pathways plays a role in the activation of autoreactive T cells in several autoimmune disease animal models (32, 33). Mice deficient in Cbl-b, an adaptor molecule that regulates the requirement for CD28 costimulation for proliferation and IL-2 production, develop spontaneous systemic autoimmune disease. B cell and T cell activation is uncoupled from the requirement for CD28 costimulation, and the resulting auto-antibody production, proliferation, and infiltration of multiple organs by activated B and T lymphocytes leads to autoimmune disease.
Another well-studied autoimmune disease, IDDM, is exacerbated in CD80/CD86 and CD28-deficient NOD mice. This finding, in marked contrast with the resistance of B7- and CD28-deficient mice to the induction of EAE, illustrates the different role of B7/CD28 costimulation in the setting of acute and chronic autoantigen exposure (34).
The most dramatic phenotype is observed in CTLA-4–deficient mice, which develop spontaneous autoimmune disease with massive lymphoproliferation, organ infiltration, and death within 4–5 weeks. The costimulatory deficit here leads to the loss of CTLA-4–mediated inhibitory signals that control lymphocytic proliferation (35).
The origin of CD4+CD28– cells in MS patients remains to be determined. They might originate from a distinct lineage of CD4+ cells, as suggested by their reported presence in normal individuals, or from CD4+CD28+ effector cells, which undergo functional and phenotypic changes after activation. Consistent with the reports in other autoimmune diseases, we demonstrated this population in all MS patients, whereas in the six tested healthy individuals, the initially CD28– population subsequently regained CD28 surface expression. This is an important finding, and further studies examining the regulation of costimulatory pathways in autoimmune diseases are under way in our laboratory.
It is postulated that costimulation-independent autoreactive CD4+ cells undergo activation in the periphery by the mechanism of molecular mimicry or bystander activation. Subsequently, activated CD4+CD28– cells may migrate through the BBB and initiate an inflammatory response within the CNS.
Costimulation-independent activation is particularly important for antigen recognition within the CNS, where competent APCs are sparse. Microglia can effectively present antigens, whereas astrocytes lack the expression of CD80 and CD86 and upregulate MHC class II molecules only after the activation by IFN-γ (10).
CD4+CD28– clones derived from RR MS patients have a Th1 phenotype with a particularly high IFN-γ secretion. They maximally upregulate the expression of IL-12Rβ2 subunit gene, which plays a role in the Th1 differentiation, maintaining IL-12 responsiveness and continuous Th1 lineage commitment (36), in the absence of CD28 costimulation.
CD4+CD28– clones exhibit increased survival after apoptotic stimuli, a finding consistent with studies in rheumatoid arthritis patients. These studies reported a resistance to apoptosis in CD4+CD28– cells due to an elevated expression of antiapoptotic protein Bcl-2 (37) and Fas-associated death domain-like IL-1–converting enzyme inhibitory protein (FLIP) (38). The absence of CTLA-4 surface expression on CD4+CD28– cells may also play a role in their prolonged proliferative response and resistance to activation-induced cell death (16).
In this study, we detected the expansion of the CD4+CD28– cell population in a subgroup, but not in all tested RR MS patients, when compared with normal controls. Our results show a substantial variability in the percentage of CD4+CD28- cells in both MS patients and normal controls and are consistent with the results of Chapman et al. (23). They found a significant variability of the percentage of CD4+CD28– cells in normal individuals and reported a higher percentages of CD4+CD28– cells in individuals with HLA-DRB1*0401 and DR1, the alleles associated with an increased risk for RA. A larger number of RR MS patients and healthy individuals are currently being evaluated in our laboratory in an attempt to relate the age, HLA type, disease duration, and activity with the size of CD4+CD28– population.
To address the involvement of CD4+CD28– cells in the formation of CNS inflammatory lesions, we tested their reactivity to MBP, a candidate autoantigen in MS. MBP-specific T cells were detected in the CD4+CD28– population in all tested MS patients. We intend to focus on this population of rare but potentially important cells in our future studies.
New insights into the costimulatory requirements of autoreactive T cells may help us design specific therapies targeting costimulatory pathways. The results of a phase I clinical trial of CTLA-4 Ig costimulation blockade in patients with psoriasis vulgaris were encouraging: there was a sustained improvement in clinical disease activity in 46% of treated patients; namely, reduction in epidermal hyperplasia and skin infiltrating T cells (39). Costimulatory blockade is a promising therapeutic approach that may prevent effective priming of naive cells in the early phases of MS (40, 41). However, the studies on EAE suggest that it may not be an effective treatment for already established immune response. In most of the reported studies, treatment with anti-B7 antibodies and CTLA-4 Ig reduced clinical EAE activity and the production of inflammatory cytokines, but did not completely abrogate the disease, suggesting that costimulation-independent populations may continue to perpetuate the disease (42). The clonally expanded populations, identified in RA, IDDM, Wegener granulomatosis, systemic lupus erythematosus, chronic inflammatory bowel disease, and now in MS patients, require further functional characterization that should provide us with a better understanding of the events involved in the development of autoimmune response. Our results have significant therapeutic implications, indicating that CD28/B7 costimulatory blockade may not be an effective treatment for MS, due to the potential of CD28-costimulation–independent CD4+CD28– cells to escape this therapy and mediate future relapses of MS.
Acknowledgments
We thank L. Kopylev for help with the statistical analysis, and J. Eaton for superb patient care. K.P. Wandinger was supported by a grant of the Deutsche Forschungsgemeinschaft (Wa 1343/1-1).
References
- 1.Bielekova B, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 2.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 3.Ota K, et al. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, et al. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994;179:973–984. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allegretta M, Nicklas JA, Sriram S, Albertini RJ. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990;247:718–721. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- 6.Sharief MK, Thompson EJ. Correlation of interleukin-2 and soluble interleukin-2 receptor with clinical activity of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1993;56:169–174. doi: 10.1136/jnnp.56.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strunk T, et al. Increased numbers of CCR5+ interferon-gamma- and tumor necrosis factor-alpha-secreting T lymphocytes in multiple sclerosis patients. Ann Neurol. 2000;47:269–273. [PubMed] [Google Scholar]
- 8.Svenningsson A, et al. Adhesion molecule expression on cerebrospinal fluid T lymphocytes: evidence for common recruitment mechanisms in multiple sclerosis, aseptic meningitis, and normal controls. Ann Neurol. 1993;34:155–161. doi: 10.1002/ana.410340210. [DOI] [PubMed] [Google Scholar]
- 9.Lejon K, Fathman CG. Isolation of self antigen-reactive cells from inflamed islets of nonobese diabetic mice using CD4high expression as a marker. J Immunol. 1999;163:5708–5714. [PubMed] [Google Scholar]
- 10.Williams KC, Ulvestad E, Hickey WF. Immunology of multiple sclerosis. Clin Neurosci. 1994;2:229–245. [PubMed] [Google Scholar]
- 11.Anderson DE, et al. Paradoxical inhibition of T-cell function in response to CTLA-4 blockade; heterogeneity within the human T-cell population. Nat Med. 2000;6:211–214. doi: 10.1038/72323. [DOI] [PubMed] [Google Scholar]
- 12.Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187:427–432. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA. CTLA-4: a negative regulator of autoimmune disease. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson DE, Sharpe AH, Hafler DA. The B7-CD28/CTLA-4 costimulatory pathways in autoimmune disease of the central nervous system. Curr Opin Immunol. 1999;11:677–683. doi: 10.1016/s0952-7915(99)00036-9. [DOI] [PubMed] [Google Scholar]
- 15.Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 16.Linsley PS, et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 17.Garcia S, DiSanto J, Stockinger B. Following the development of a CD4 T cell response in vivo: from activation to memory formation. Immunity. 1999;11:163–171. doi: 10.1016/s1074-7613(00)80091-6. [DOI] [PubMed] [Google Scholar]
- 18.Fasso M, et al. T cell receptor (TCR)-mediated repertoire selection and loss of TCR vbeta diversity during the initiation of a CD4(+) T cell response in vivo. J Exp Med. 2000;192:1719–1730. doi: 10.1084/jem.192.12.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 20.Scholz C, Patton KT, Anderson DE, Freeman GJ, Hafler DA. Expansion of autoreactive T cells in multiple sclerosis is independent of exogenous B7 costimulation. J Immunol. 1998;160:1532–1538. [PubMed] [Google Scholar]
- 21.Lovett-Racke AE, et al. Decreased dependence of myelin basic protein-reactive T cells on CD28- mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells. J Clin Invest. 1998;101:725–730. doi: 10.1172/JCI1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman A, et al. CD11b+CD28-CD4+ human T cells: activation requirements and association with HLA-DR alleles. J Immunol. 1996;157:4771–4780. [PubMed] [Google Scholar]
- 24.Muraro PA, Pette M, Bielekova B, McFarland HF, Martin R. Human autoreactive CD4+ T cells from naive CD45RA+ and memory CD45RO+ subsets differ with respect to epitope specificity and functional antigen avidity. J Immunol. 2000;164:5474–5481. doi: 10.4049/jimmunol.164.10.5474. [DOI] [PubMed] [Google Scholar]
- 25.Zheng L, Trageser CL, Willerford DM, Lenardo MJ. T cell growth cytokines cause the superinduction of molecules mediating antigen-induced T lymphocyte death. J Immunol. 1998;160:763–769. [PubMed] [Google Scholar]
- 26.Sprent J. Immunological memory. Curr Opin Immunol. 1997;9:371–379. doi: 10.1016/s0952-7915(97)80084-2. [DOI] [PubMed] [Google Scholar]
- 27.Goverman J, et al. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 28.Probert CS, et al. Persistent clonal expansions of peripheral blood CD4+ lymphocytes in chronic inflammatory bowel disease. J Immunol. 1996;157:3183–3191. [PubMed] [Google Scholar]
- 29.Moosig F, Csernok E, Wang G, Gross WL. Costimulatory molecules in Wegener’s granulomatosis (WG): lack of expression of CD28 and preferential up-regulation of its ligands B7-1 (CD80) and B7-2 (CD86) on T cells. Clin Exp Immunol. 1998;114:113–118. doi: 10.1046/j.1365-2249.1998.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallejo AN, Nestel AR, Schirmer M, Weyand CM, Goronzy JJ. Aging-related deficiency of CD28 expression in CD4+ T cells is associated with the loss of gene-specific nuclear factor binding activity. J Biol Chem. 1998;273:8119–8129. doi: 10.1074/jbc.273.14.8119. [DOI] [PubMed] [Google Scholar]
- 31.Bachmann MF, et al. Developmental regulation of Lck targeting to the CD8 coreceptor controls signaling in naive and memory T cells. J Exp Med. 1999;189:1521–1530. doi: 10.1084/jem.189.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachmaier K, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 33.Chiang YJ, et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 34.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 35.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 36.Chang JT, Shevach EM, Segal BM. Regulation of interleukin (IL)-12 receptor beta2 subunit expression by endogenous IL-12: a critical step in the differentiation of pathogenic autoreactive T cells. J Exp Med. 1999;189:969–978. doi: 10.1084/jem.189.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schirmer M, Vallejo AN, Weyand CM, Goronzy JJ. Resistance to apoptosis and elevated expression of Bcl-2 in clonally expanded CD4+CD28- T cells from rheumatoid arthritis patients. J Immunol. 1998;161:1018–1025. [PubMed] [Google Scholar]
- 38.Vallejo AN, Schirmer M, Weyand CM, Goronzy JJ. Clonality and longevity of CD4(+)CD28(null) T cells are associated with defects in apoptotic pathways. J Immunol. 2000;165:6301–6307. doi: 10.4049/jimmunol.165.11.6301. [DOI] [PubMed] [Google Scholar]
- 39.Abrams JR, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–1252. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perrin PJ, June CH, Maldonado JH, Ratts RB, Racke MK. Blockade of CD28 during in vitro activation of encephalitogenic T cells or after disease onset ameliorates experimental autoimmune encephalomyelitis. J Immunol. 1999;163:1704–1710. [PubMed] [Google Scholar]
- 41.Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 42.Neville KL, Dal Canto MC, Bluestone JA, Miller SD. CD28 costimulatory blockade exacerbates disease severity and accelerates epitope spreading in a virus-induced autoimmune disease. J Virol. 2000;74:8349–8357. doi: 10.1128/jvi.74.18.8349-8357.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]