Selective predisposition to bacterial infections in IRAK-4–deficient children: IRAK-4–dependent TLRs are otherwise redundant in protective immunity (original) (raw)
Abstract
Human interleukin (IL) 1 receptor–associated kinase 4 (IRAK-4) deficiency is a recently discovered primary immunodeficiency that impairs Toll/IL-1R immunity, except for the Toll-like receptor (TLR) 3– and TLR4–interferon (IFN)-a/b pathways. The clinical and immunological phenotype remains largely unknown. We diagnosed up to 28 patients with IRAK-4 deficiency, tested blood TLR responses for individual leukocyte subsets, and TLR responses for multiple cytokines. The patients' peripheral blood mononuclear cells (PBMCs) did not induce the 11 non-IFN cytokines tested upon activation with TLR agonists other than the nonspecific TLR3 agonist poly(I:C). The patients' individual cell subsets from both myeloid (granulocytes, monocytes, monocyte-derived dendritic cells [MDDCs], myeloid DCs [MDCs], and plasmacytoid DCs) and lymphoid (B, T, and NK cells) lineages did not respond to the TLR agonists that stimulated control cells, with the exception of residual responses to poly(I:C) and lipopolysaccharide in MDCs and MDDCs. Most patients (22 out of 28; 79%) suffered from invasive pneumococcal disease, which was often recurrent (13 out of 22; 59%). Other infections were rare, with the exception of severe staphylococcal disease (9 out of 28; 32%). Almost half of the patients died (12 out of 28; 43%). No death and no invasive infection occurred in patients older than 8 and 14 yr, respectively. The IRAK-4–dependent TLRs and IL-1Rs are therefore vital for childhood immunity to pyogenic bacteria, particularly Streptococcus pneumoniae. Conversely, IRAK-4–dependent human TLRs appear to play a redundant role in protective immunity to most infections, at most limited to childhood immunity to some pyogenic bacteria.
Inherited IL-1R–associated kinase 4 (IRAK-4) deficiency is an autosomal recessive disorder that was first described in three unrelated children (1). IRAK-4–deficient patients' fibroblasts and/or leukocytes show an impaired response to most Toll-like receptor (TLR) and IL-1R agonists tested (1–12). Specifically, the patients' whole blood cells or PBMCs do not respond to IL-1β, in terms of IL-6 secretion (1), or to IL-18, in terms of IFN-γ production (1, 4). Moreover, agonists of TLR1/2 (Pam3CSK4), TLR2/6 (Pam2CSK4), TLR3 (poly(I:C)), TLR4 (LPS), TLR5 (flagellin), and TLR9 (CpG DNA), do not induce the production of major inflammatory cytokines (TNF-α, IL-6, and IL-12) and growth factors (G-CSF and GM-CSF) in whole blood cells and PBMCs (1–9, 11, 12). However, the patients' PBMCs do respond to the nonspecific TLR3 agonist poly(I:C) and the TLR4-specific agonist LPS by producing IFN-β mRNA (for poly(I:C) and LPS) or IFN-α protein (for poly(I:C) only) (13). Moreover, the patients' fibroblasts have been shown to respond to poly(I:C) by inducing IFN-β, IFN-λ, and IL-6 (13). The human IRAK-4–independent TLR3/4 pathway is reminiscent of the mouse MyD88-independent, Toll/IL-1 receptor (TIR) domain–containing adaptor-inducing IFN-β (TRIF)–dependent TLR3/4 pathway (14, 15), which also controls the induction of cytokines other than IFNs, at least for TLR3 (16, 17). Despite the lack of IL-6 and TNF-α induction in response to poly(I:C) in human IRAK-4–deficient whole blood cells (1), the normal induction of IFN-α, -β, and -λ in response to poly(I:C) and LPS (13) raises the possibility that IRAK-4 deficiency may not prevent the induction of other cytokines in response to these two and possibly other TLR agonists.
The lack of response of IRAK-4–deficient whole blood cells and PBMCs to TLR and IL-1R agonists also does not exclude the possibility that individual leukocyte subsets may respond to at least some agonists. Several human leukocyte subsets produce TLR mRNAs and/or proteins. In the myeloid lineage, neutrophilic granulocytes express TLR1, 2, 4, 5, 6, 7, 8, and 10, as well as TLR9 upon induction with GM-CSF (18); monocytes express TLR1, 2, 4, 5, 6, 7, 8, and 9 (19–21); myeloid DCs (MDCs) express TLR1, 2, 3, 4, 5, 6, 7, 8, and 10 (22); and plasmacytoid DCs (PDCs) express TLR1, 6, 7, 9, and 10 (19, 21–23). Monocyte-derived DCs (MDDCs) express TLR1, 2, 3, 4, 5, 6, 8, 9, and 10, but hardly any TLR7 (24, 25). In basophilic and eosinophilic granulocytes, substantial expression has been confirmed only for TLR7 in eosinophils (26). In the lymphoid lineage, blood B cells express TLR1, 6, 7, 9, and 10 (20, 23, 27); NK cells express TLR1, 2, 3, 5, 6, 7, and 8 (20); CD4 α/β T cells express at least TLR1, 2, and 5 (28); and effector α/β CD8 T cells and γ/δ T cells express TLR3 (29, 30). In healthy controls, most subsets could be activated by the corresponding TLR agonists tested. In contrast, the range of blood cells in which TLR responses are affected by IRAK-4 deficiency remains unclear.
IRAK-4 deficiency may have an even broader impact, given the well-established role of IRAK-4 downstream from multiple IL-1Rs (1, 31) and the recently proposed role of IRAK-4 in TCR signaling (32). It is thus surprising that the first three patients identified were alive and well and had experienced only a few infectious diseases (1). To date, 21 IRAK-4–deficient patients have been reported in individual case reports or small series (1, 4–13, 33–36). Most presented with peripheral (e.g., pharyngotonsillitis, sinusitis, cellulitis, and endophthalmitis) and/or invasive bacterial diseases (e.g., meningitis, arthritis, septicemia, and visceral abscess) caused mostly by Streptococcus pneumoniae and Staphylococcus aureus (1, 4–13, 33–36). Only seven patients also presented infectious disease caused by Gram-negative bacteria (Pseudomonas aeruginosa in most cases) (1, 4–6, 8, 13, 33, 36). Although IRAK-4 deficiency appears to be more severe than initially thought (1), with seven reported deaths (5, 7–9, 13, 34, 36), the condition seems to improve with age, even without prophylaxis (4, 6, 36). The apparent broad resistance of IRAK-4–deficient patients challenges the prevailing view that TLRs are the principal sentinels of innate immunity (37–39). However, it has been difficult to draw firm conclusions in the absence of a large series of patients. Moreover, the rarity of infections may reflect the TLR-dependent, yet IRAK-4–independent, induction of certain cytokines in specific leukocyte subsets. We thus investigated the contribution of human TLRs to host defense by documenting the clinical course of a large number of IRAK-4–deficient patients and testing the TLR responses of their PBMCs for multiple cytokines, as well as the TLR responses of their individual leukocyte subsets.
RESULTS
IRAK4 mutations
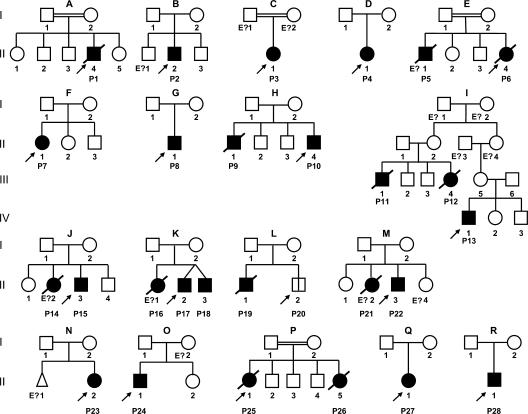
We report 28 patients with IRAK-4 deficiency. The patients originate from 18 unrelated kindreds and 11 countries (Table I and Fig. 1). All IRAK4 exons, flanking intron regions, and, when appropriate, entire introns, were sequenced in 24 patients (P1–4, 6–13, 15, 17–20, and 22–28). IRAK-4 deficiency was diagnosed on clinical grounds in four deceased relatives (P5, 14, 16, and 21) for whom no biological material was available. The patients of 13 kindreds were apparently homozygous (kindreds A–C, E, F, H–L, and P–R), and those from 5 kindreds were compound heterozygous (D, G, and M–O) for IRAK4 mutations. However, four seemingly homozygous patients from three unrelated families (P2 from kindred B, P7 from kindred F, and P11 and 12 from kindred I) had one parent who did not carry the mutant allele. Fluorescence in situ hybridization with BAC210N13, which covers the entire IRAK4 locus, and the genotyping of polymorphic markers showed that P2 was heterozygous for a large de novo deletion (designated BAC210N13del) encompassing IRAK4 (Fig. S1, top, available at http://www.jem.org/cgi/content/full/jem.20070628/DC1; and not depicted). For P7, using the same BAC as for P2, fluorescence in situ hybridization revealed two signals, consistent with homozygosity owing to segmental uniparental disomy or compound heterozygosity with an undetected deletion encompassing a fraction of IRAK4 (Fig. S1, bottom; and not depicted). Not enough material was available to explore the IRAK4 locus in the deceased patients P11 and 12 from kindred I (8). 3 out of the 14 mutant alleles identified carried nonsense mutations (Y48X, Q293X, and E402X) (1, 3, 4, 6, 8, 9, 11, 36), 3 carried large deletions (1-1096_40+23del, BAC210N13del, and 942-1481_1125+547del), 2 carried splice mutations (1188+520A>G and 1189-1G>T) (12), and 6 carried frameshift insertions and deletions (167_172insA, 573delA, 620_621delAC, 631delG, 821delT, and 1240insA) (1, 4, 7, 34) (Table I and Fig. 2 A). All mutations are predicted to be null, as they create a premature termination codon or delete a large segment of the gene. No missense mutation was found. The 14 mutations were not found in 100 healthy controls sequenced. The Q293X mutant allele was found in homozygotes from six kindreds (C, H, K, P, Q, and R) and compound heterozygotes from four kindreds (B, D, M, and possibly F). The recurrence of this mutation may reflect a mutational hotspot, a founder effect, or both (unpublished data).
Table I.
Genotypes, origin, and clinical phenotypes of IRAK-4–deficient patients
| Kindred | Patient | Mutation | Origin | Follow-up | Age | Pathogens causingsevere Gram-positiveinfections | Pathogens causingsevere Gram-negativeinfections | References |
|---|---|---|---|---|---|---|---|---|
| A | P1 (II-4) | 821delT | KSA | deceased | 7 yr | Sp, Sa | – | (1, 13) |
| B | P2 (II-2) | Q293X_/ BAC210N13del_ | Portugal | alive | 14 yr | Sp, Sa | – | (1, 10, 13) |
| C | P3 (II-1) | Q293X | USA | alive | 11 yr | Sp, Sa | Ec | (1, 3, 33) |
| D | P4 (II-1) | Q293X_/ 620-621delAC_ | USA | alive | 24 yr | Sp, Cs | Nm | (2, 4, 35) |
| E | P5 (II-1) | ND | Turkey | deceased | 16 mo | Sp, Spa | – | (34) |
| E | P6 (II-4) | 573delA | Turkey | deceased | 2 mo | Sp | – | (34) |
| F | P7 (II-2) | Q293X | UK | alive | 32 yr | Sp | Ss | (6, 10, 13) |
| G | P8 (II-1) | 1188+520A>G/ 1189-1G>T | Hungary | alive | 9 yr | Sp | – | (10, 12, 13) |
| H | P9 (II-1) | Q293X | Canada | deceased | 6 yr | Sp | Pa | (5, 9, 13) |
| H | P10 (II-4) | Q293X | Canada | alive | 7 yr | Sp | – | (5, 9, 13) |
| I | P11 (III-1) | E402X | Spain | deceased | 2 yr | Sa | Pa | (8, 13) |
| I | P12 (III-4) | E402X | Spain | deceased | 8 mo | Sp | Pa | (8, 13) |
| I | P13 (IV-1) | E402X | Spain | alive | 9 yr | Sp | – | (8, 10, 13) |
| J | P14 (II-2) | ND | Israel | deceased | 3 mo | Sm | – | (13) |
| J | P15 (II-3) | 1-1096_40+23del | Israel | alive | 9 yr | Sp | – | (10, 13) |
| K | P16 (II-1) | ND | Canada | deceased | 5 mo | Sa | – | (13, 36) |
| K | P17 (II-2) | Q293X | Canada | alive | 27 yr | Sp | Pa | (13, 36) |
| K | P18 (II-3) | Q293X | Canada | alive | 27 yr | Sp | – | (13, 36) |
| L | P19 (II-1) | 167_172insA | Japan | deceased | 2 yr | Sp | – | (7) |
| L | P20 (II-2) | 167_172insA | Japan | alive | 24 mo | – | – | (7) |
| M | P21 (II-2) | ND | USA | deceased | 4 mo | bacterial meningitis | – | this study |
| M | P22 (II-3) | Q293X_/ 620-621delAC_ | USA | alive | 10 yr | Sp, Sa | – | this study |
| N | P23 (II-1) | Y48X_/ 631delG_ | Canada | alive | 2 yr | Sa | – | this study |
| O | P24 (II-1) | 1240insA/ 942-1481_1125+547del | Canada | alive | 16 yr | Sp, Sa | – | this study |
| P | P25 (II-1) | Q293X | Australia | deceased | 4 mo | Sp | – | this study |
| P | P25 (II-5) | Q293X | Australia | deceased | 6 mo | Sp, Sa | – | this study |
| Q | P27 (II-2) | Q293X | USA | alive | 11 yr | Sp | – | this study |
| R | P28 (II-1) | Q293X | USA | alive | 6 yr | Sp | – | (11) |
Figure 1.
Pedigree of the 18 kindreds identified with IRAK-4 deficiency. Each kindred is designated by a capital letter (A–R), each generation is designated by a Roman numeral (I–IV), and each individual is designated by an Arabic numeral (from left to right). IRAK-4–deficient patients with a clinical phenotype are represented as closed symbols. P20, the only patient with confirmed IRAK-4 deficiency but no known clinical phenotype, is represented with an open square divided by a black line. In each family, the proband is indicated by an arrow. Individuals whose genetic status could not be evaluated are indicated by “E?”; they include four individuals (P5, 14, 16, and 21) thought to be IRAK-4 deficient based on their clinical phenotypes.
Figure 2.
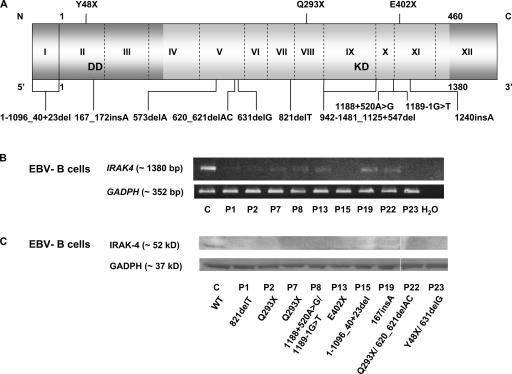
IRAK-4 deficiency. (A) Schematic representation of IRAK4 with all identified mutations. The gene is composed of 12 exons, with exon 1 and a part of exon 12 noncoding. The N-terminal death domain (DD) and C-terminal kinase domain (KD) are shown in light gray. (B) RT-PCR of the full-length IRAK4 and GAPDH genes in B-EBVs from a healthy control (C) and nine IRAK-4–deficient patients. (C) IRAK-4 and GAPDH protein levels in B-EBVs from a healthy control and nine IRAK-4–deficient patients, as shown by Western blotting. White lines indicate that intervening lanes have been spliced out.
IRAK-4 expression and function
We assessed IRAK4 mRNA levels in EBV-transformed B lymphocyte cell lines (B-EBVs; Fig. 2 B) derived from most patients and a healthy control by RT-PCR. The two patients carrying the 573delA mutation died before cell lines could be established (34). Most other patients lacked detectable full-length IRAK4 mRNAs species, presumably because of nonsense-mediated mRNA degradation. However, P7 (mutation Q293X), P8 (mutations 1188+520A>G and 1189-1G>T), P13 (mutation E402X), P19 (mutation 167_172insA), and P22 (mutation Q293X/620-621del) had low levels of detectable full-length IRAK4 mRNA. We then assessed IRAK-4 protein levels in B-EBVs (Fig. 2 C). No IRAK-4 protein was detected in any of the patients tested, even in P7, 8, 13, 19, and 22, all of whom had detectable full-length mRNAs, excluding a potential role of IRAK-4 as a scaffold protein in our patients (40, 41). Finally, we assessed the functional impact of IRAK4 mutations. B-EBVs bearing mutations 821delT (P1), Q293X (P2, 3, and 7), 1188+520A>G/1189-1G>T (P8), E402X (P13), and 1-1096_40+23del (P15) did not respond to TLR7 and 8 agonists, as measured by TNF-α production (Fig. 3 A). SV40-transformed fibroblasts (SV40-fibroblasts) bearing mutations 821delT (P1), Q293X (P2 and 3), 1188+520A>G/1189-1G>T(P8), E402X (PI3), 1-1096_40+23del (P15), Y48X/631delG (P23), and 1240insA/942-1481_1125+547del (P24) did not respond to IL-1β, as assessed by measuring IL-6 production. However, IRAK-4–deficient SV40-fibroblasts did produce IL-6 upon activation by poly(I:C) (Fig. 3 B) (13). Thus, all patients had complete IRAK-4 deficiency and a complete absence of IRAK-4–dependent TIR signaling, owing to the inheritance of two loss-of-expression, loss-of-function IRAK4 alleles.
Figure 3.
Impaired cellular responses to TIR agonists in IRAK-4–deficient cell lines. (A) TNF-α production by B-EBVs from a healthy control (C) and seven IRAK-4–deficient patients 24 h after stimulation with various TLR agonists and PMA/ionomycin. (B) IL-6 production by SV40-fibroblasts from a healthy control and eight IRAK-4–deficient patients after 24 h of stimulation with IL-1β, TNF-α, poly(I:C), and PMA/ionomycin. Mean values and SDs are shown for triplicates of a single experiment.
Development and function of blood leukocyte subsets
We analyzed blood leukocyte subsets in 12 IRAK-4–deficient patients. We previously showed that granulocytes, CD14+, CD16+, and CD14+/CD16+ monocyte subsets, and MDCs and PDCs, were present in normal numbers in three patients (13). We now report that T cell subsets, including CD4+ and CD8+, and CD45RA+ and CD45RO+ T cells, are also present in normal numbers (Table S1, available at http://www.jem.org/cgi/content/full/jem.20070628/DC1), with the possible exception of normal to low levels of T cells in P17 and 18 (36). T cells proliferated normally in response to the mitogen PHA, CD3, and recall antigens in vitro (Table S2). B cells and memory B cells (CD27+) were also present in normal numbers (Table S1). Serum Ig levels for IgA were normal in five, high in two (P8 and 11), and low in four (P1, 2, 17, and 18) patients (36). IgG levels were normal in seven and high in four (P7, 8, 11, and 17) patients, and IgM levels were normal in seven, high in three (P7, 11, and 19), and low in one (P2) patients. IgE levels were high in 8 (P1, 7, 8, 11, 13, 15, 17, and 23) out of the 11 patients evaluated (Table S2). Antibody responses to protein antigens were normal in all but two patients, who had slightly low titers (P7 and 15); however, the date of recall vaccination before serological testing was unknown. The antibody response to glycans was impaired in some (P2, 8, 17, 18, and 29) but not all patients, and in response to some but not all pneumococcal and erythrocyte AB antigens (Table S2 and unpublished data) (11, 12, 33). Finally, the surface expression of CD16 and CD56 on NK cells was normal (Table S1). IFN-γ secretion and surface expression of CD107 (degranulation) by the patients' NK cells were normal (unpublished data). Overall, there seemed to be no overt defect of leukocyte development in IRAK-4–deficient patients. Thus, antigen-specific T and B cell responses seemed to be normal, except for an impaired glycan-specific antibody response in at least some patients and against some glycans, and except for an overproduction of IgE in most of the patients tested.
Impaired production of multiple cytokines by blood mononuclear leukocytes
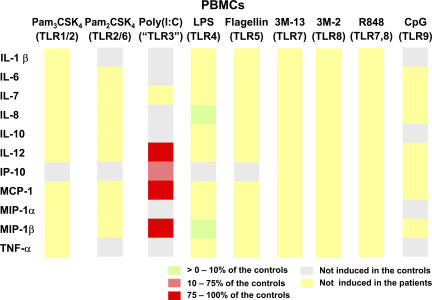
We previously reported that IRAK-4–deficient whole blood cells and PBMCs produce only very small amounts of TNF-α, IL-6, IL-12, G-CSF, GM-CSF, and IFN-γ in vitro in response to all IL-1R and TLR agonists tested (1–9, 11, 12). We wondered whether the induction of other cytokines, chemokines, IFNs, and growth factors was also dependent on IRAK-4 after TLR stimulation. We therefore activated PBMCs from IRAK-4–deficient patients with Pam3CSK4 (TLR1/2), Pam2CSK4 (TLR2/6), poly(I:C) (a nonspecific TLR3 agonist), LPS (TLR4), flagellin (TLR5), 3M-13 (TLR7), 3M-2 (TLR8), R-848 (TLR7 and 8), and CpG (TLR9) for 24 h. We did not assess TLR10 responses, as there is no known agonist for this receptor (23). Cytokine secretion into the supernatant was assessed using a multiplex cytometry-based system. 11 out of the 25 cytokines assayed were induced and detectable after TLR stimulation in healthy controls. IRAK-4–deficient cells did not respond to seven out of nine agonists for all cytokines tested (Fig. 4). Upon activation with poly(I:C), the patients' PBMCs displayed induction of IL-12, monocyte chemoattractant protein 1, and macrophage inflammatory protein 1β (MIP-1β) to levels similar to those in healthy controls, as well as some induction of IFN-inducible protein 10 (Fig. 4). However, the induction of IL-12 and MIP-1β was weak in both patients and healthy controls (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20070628/DC1). IL-7 induction was abolished in the patients, whereas other cytokines were not induced in controls. The patients' PBMCs showed detectable IL-8 and MIP-1β (an IFN-inducible cytokine) responses to LPS, but these responses were weaker than those of healthy controls (Fig. 4). The other cytokines were not induced in the patients. These data are reminiscent of our previous observation that IRAK-4–deficient PBMCs respond to poly(I:C) by producing IFN-α protein, and to poly(I:C) and LPS by producing IFN-β mRNA (13). However, whereas LPS responses can be specifically ascribed to TLR4, we recently showed, in TLR3-deficient patients, that the poly(I:C) responses of PBMCs are TLR3-independent (42). These data indicate a broad immunological impact of IRAK-4 deficiency, as the production of 11 key cytokines was completely impaired in response to all TLR agonists, with the exception of a couple of cytokines in response to poly(I:C) and LPS.
Figure 4.
Multiple cytokine secretion in IRAK-4–deficient PBMCs. PBMCs from three healthy controls and three IRAK-4–deficient patients (P17, 18, and 22) were activated with various TLR agonists for 24 h. Cytokine levels are represented as ratios of the mean secretion observed in the three IRAK-4–deficient patients to that in three healthy controls. Cytokines represented in gray are not induced upon the stimulation of control PBMCs.
TLR responses of individual myeloid subsets
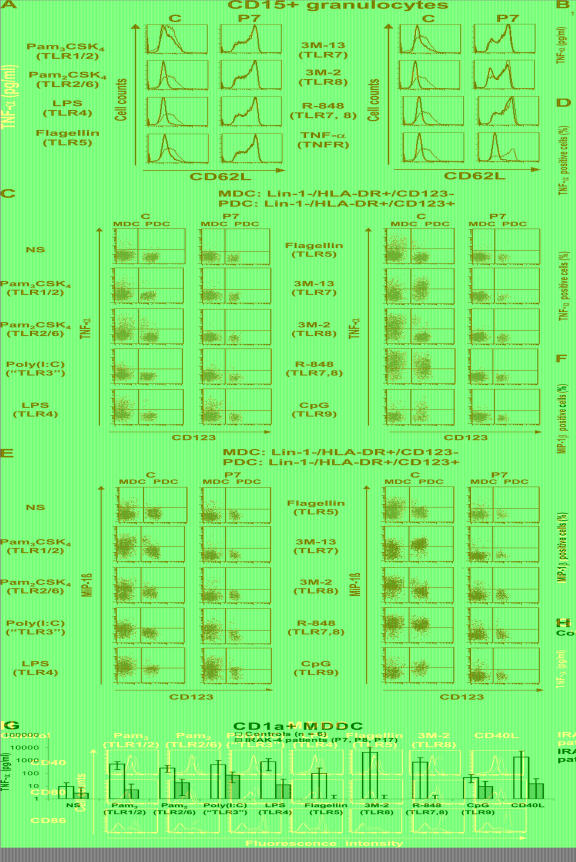
We then assessed the role of IRAK-4 in TLR signaling pathways in discrete leukocyte cell populations. Cell subsets other than granulocytes and DCs were purified by cell sorting (purity >99.5%). More than 95% of the granulocytes purified on Ficoll were CD15+. The response of DCs (MDCs and PDCs) was tested in PBMCs. We assessed the CD62L shedding of granulocytes from four healthy controls and four IRAK-4–deficient patients after activation with Pam3CSK4, Pam2CSK4, LPS, flagellin, 3M-13, 3M-2, R-848, and TNF-α (10). The response to all TLR agonists was impaired in the granulocytes of all four patients tested (Fig. 5 A). CD14+ monocytes from healthy controls responded to TLR1–8 agonists but not to TLR9 agonists. The monocytes of IRAK-4–deficient patients did not respond to these agonists, with the possible exception of very weak TNF-α production upon LPS stimulation (Fig. 5 B). Finally, we tested MDCs and PDCs by stimulating PBMCs from seven healthy donors and three IRAK-4–deficient patients with the TLR agonists Pam3CSK4, Pam2CSK4, poly(I:C), LPS, flagellin, 3M-13, 3M-2, R-848, and CpG for 3 h. We assessed TNF-α and MIP-1β production for MDCs (Lin-1−, HLA-DR+, and CD123low) and PDCs (Lin-1−, HLA-DR+, and CD123high) by intracellular staining. In healthy individuals, MDCs responded to all of the TLR agonists tested, except the TLR9 agonist, with the induction of TNF-α and MIP1-β. In contrast, only upon activation with poly(I:C) (nonspecific TLR3 agonist) and LPS (TLR4), did MDCs from the patients display normal levels of MIP1-β induction and some induction of TNF-α. PDCs from healthy individuals responded only to agonists of TLR7 and 9, whereas IRAK-4–deficient PDCs did not respond to any of the agonists tested (Fig. 5, C–F). As poly(I:C) activation in MDCs appears to be TLR3 independent (42), we further evaluated the production of TNF-α and the up-regulation of IFN-inducible surface-expressed CD40, CD80, and CD86 by in vitro MDDCs, which respond to poly(I:C) in a TLR3-dependent manner (42). MDDCs from healthy controls responded normally to the TLR agonists Pam3CSK4, Pam2CSK4, poly(I:C), LPS, flagellin, and 3M-2. In contrast, the patients' MDDCs did not respond to Pam3CSK4, Pam2CSK4, flagellin, and 3M-2. However, IRAK-4–deficient MDDCs showed a weak but not abolished TNF-α response and normal induction of CD40, CD80, and CD86 upon activation with poly(I:C) (TLR3). Normal induction of CD40, CD80, and CD86 was also observed upon activation with LPS (TLR4) (Fig. 5, G and H). These data indicate that the IRAK-4–deficient individual myeloid cell subsets tested displayed no response to most TLR agonists, with the exception of normal responses to poly(I:C) and LPS detected in MDCs for MIP-1β, an IFN type I–inducible cytokine, and in MDDCs for CD40, CD80, and CD86, which are induced by type I IFNs and TNF-α.
Figure 5.
Impaired responses to TLR agonists in IRAK-4–deficient individual myeloid subsets. (A) Cleavage of CD62 ligand (CD62L) at the surface of granulocytes from a healthy control and an IRAK-4–deficient patient (P7) after activation for 1 h with various TLR agonists and TNF-α. The black line shows CD62L expression on nonactivated granulocytes, and the red line shows CD62L expression after 1 h of activation with various agonists (induced CD62L shedding). One experiment representative of four (P7, 8, 13, and 15) is shown. (B) TNF-α secretion by CD14+ monocytes after 24 h of activation with various TLR agonists. Mean values and SDs were calculated from four healthy controls and three IRAK-4–deficient patients. (C–F) Ex vivo MDC and PDC responses. PBMCs from healthy controls and IRAK-4–deficient patients were stimulated with various TLR agonists. In both subsets, responses were measured by staining for intracellular TNF-α (C) and MIP-1β (E). Mean values and SDs were calculated from six different controls and four IRAK-4–deficient patients for TNF-α (D), and from seven different controls and three IRAK-4–deficient patients for MIP-1β (F). (G) TNF-α secretion in vitro by MDDCs after 24 h of activation. Means and SDs were calculated from six different controls and three different IRAK-4–deficient patients. (H) Induction of CD40, CD80, and CD86 surface expression on MDDCs from a control (top) and an IRAK-4–deficient patient (bottom) after 24 h of stimulation with various TLR agonists. Black and green lines indicate the expression of CD40, CD80, and CD86 without and after stimulation, respectively. The experiment shown is representative of three independent experiments (also performed on patients P15 and 18). C, control.
TLR responses of individual lymphoid subsets
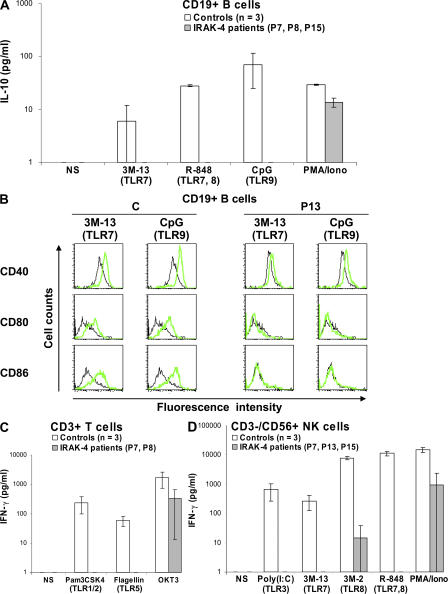
We then tested the TLR responses of the B, T, and NK lymphoid cell subsets. The subsets were purified by cell sorting (purity >99.5%). CD19+ B cells were activated by incubation with the TLR agonists Pam3CSK4, Pam2CSK4, poly(I:C), LPS, flagellin, 3M-13, 3M-2, R-848, and CpG for 24 h, and their response was measured by assessing IL-10 production. Highly purified control B cells showed a unique pattern of activation, with no response to agonists of TLR1/2, TLR2/6, TLR3, TLR4, TLR5, and TLR8, and only weak IL-10 production in response to TLR7, TLR7 and TLR8, and TLR9 agonists (Fig. 6 A and not depicted). In contrast, no response to these TLR agonists was observed in the three IRAK-4–deficient patients tested (Fig. 6 A). Moreover, the response to TLR7 and 9, as measured by cell-surface expression of CD40, CD80, and CD86 after 3 d of incubation with IL-4 and various TLR agonists, was also impaired in the patients' B cells (Fig. 6 B) (13). CD3+ T cells from healthy individuals were activated by Pam3CSK4, Pam2CSK4, poly(I:C), LPS, flagellin, 3M-13, 3M-2, R-848, and CpG. Control T cells displayed a weak but detectable response to Pam3CSK4 and flagellin in terms of IFN-γ production, whereas T cells from IRAK-4–deficient patients were not activated by any of the TLR agonists (Fig. 6 C). Finally, control NK cells were shown to respond to TLR3, 7, and TLR7 and 8 agonists in terms of IFN-γ production, but no response was observed in NK cells from IRAK-4–deficient patients (Fig. 6 D). NK cells respond to poly(I:C) through TLR3 (42), suggesting that at least some TLR3 pathways are IRAK-4 dependent. These data indicate that the three major blood lymphoid subsets require IRAK-4 for TLR responses, including TLR3 responses in NK cells.
Figure 6.
Lack of response to TLR agonists of individual IRAK-4–deficient lymphoid subsets. (A) IL-10 secretion by CD19+ B cells after 24 h of activation with various TLR agonists and PMA/ionomycin. Mean values ± SD were calculated from the data obtained for three different controls and three IRAK-4–deficient patients. (B) Induction of CD40, CD80, and CD86 surface expression on CD19+ B cells after activation for 72 h with 3M-13 and CpG. Black and green lines indicate the expression of CD40, CD80, and CD86 without and after stimulation, respectively. Data are representative of two independent experiments. (C) IFN-γ secretion by CD3+ T cells after stimulation for 24 h with various TLR agonists and anti-CD3 (50 ng/ml OKT3) antibody in the presence of 100 U/ml IL-2 for 2 d. Mean values ± SD were calculated for three different controls and two IRAK-4–deficient patients. (D) IFN-γ secretion by CD3−/CD56+ NK cells after activation for 24 h with various TLR agonists and PMA/ionomycin. Mean values and SDs were calculated for three different controls and three IRAK-4–deficient patients.
Clinical features of IRAK-4 deficiency
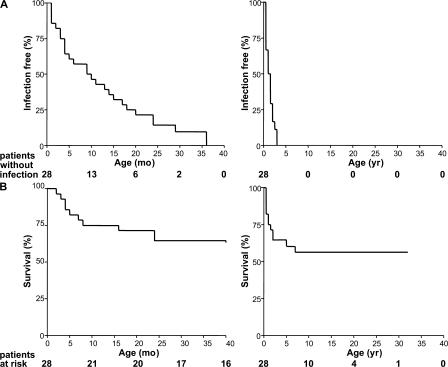
In total, 28 IRAK-4–deficient patients from 18 families were studied, including the 7 patients (P21–27) from 5 families described in this study for the first time (Table I and Fig. 1). Most IRAK-4–deficient patients had had at least one Gram-positive bacterial infection: 22 out of the 28 (79%) had had invasive disease caused by S. pneumoniae (meningitis, septicemia, or arthritis), and 9 out of the 28 (32%) had suffered severe disease caused by S. aureus (meningitis, septicemia, or liver abscess; Table I). If we also take into account peripheral staphylococcal disease (cellulitis and subcutaneous abscess), 14 patients could be considered particularly susceptible to S. aureus. One patient (P20) had had no major infectious disease. This patient is 25 mo old and was diagnosed with IRAK-4 deficiency as a neonate. He was placed on IgG substitution and antibiotic prophylaxis shortly after birth. Seven patients also suffered from severe Gram-negative bacterial infections, which were invasive in four cases (Shigella sonnei and P. aeruginosa) and peripheral in four cases (Escherichia coli, Serratia marcescens, Neisseria meningitidis, and P. aeruginosa). As previously reported in a smaller series (13), no severe viral, fungal, or parasitic infections were observed in the patients. Most patients developed their first invasive infection before the age of 2 yr (20 out of 28; 71%), often before the age of 6 mo (9 out of 28; 32%) and in the neonatal period (4 out of 28; 14%), when maternal antibodies are still present. Remarkably, no invasive infection was documented in the six patients over the age of 14 yr (P2, 14 yr; P4, 24 yr; P7, 32 yr; P17 and 18, 27 yr; and P24, 16 yr), even in the absence of prophylaxis (P2, 4, 7, 17, and 18; n = 5; Fig. 7 A) (4, 6, 36). 12 patients died of invasive Gram-positive infections, all before the age of 8 yr and most before the age of 2 yr (Fig. 7 B). IRAK-4 deficiency is thus associated with a selective predisposition to pyogenic bacterial infections, mostly caused by Gram-positive bacteria (S. pneumoniae in particular and S. aureus to a lesser extent), and clinical status and outcome both improve with age. The detailed clinical features of IRAK-4 deficiency will be reported elsewhere (unpublished data).
Figure 7.
Epidemiological features of IRAK-4 deficiency. (A) Incidence of invasive infections in IRAK-4–deficient patients during the first 40 mo of life (left) and the first 40 yr of life (right). Invasive infections included meningitis, septicemia, and arthritis. (B) Survival curve of 28 IRAK-4–deficient patients during the first 40 mo of life (left) and the first 40 yr of life (right).
DISCUSSION
The 28 patients reported in this study suffered from complete IRAK-4 deficiency. The patients had been exposed to an extremely diverse range of microorganisms, including many potential viral, bacterial, and fungal pathogens, as well as parasites (Tables S2 and S3, available at http://www.jem.org/cgi/content/full/jem.20070628/DC1). However, IRAK-4–deficient patients presented a strikingly narrow infectious phenotype (Table I), similar to the three patients initially reported (1). 27 patients suffered from invasive infectious disease, typically caused by Gram-positive S. pneumoniae (n = 22; 79%) and/or S. aureus (n = 9; 32%). Seven patients (25%) also presented severe infections with Gram-negative bacteria (P. aeruginosa, N. meningitidis, S. sonnei, and S. marcescens). 15 patients had peripheral infectious disease. When identified, the causal pathogens were S. aureus, P. aeruginosa, and Streptococcus species. The susceptibility of IRAK-4–deficient patients to S. aureus is consistent with that observed in IRAK-4– and MyD88-deficient mice (31, 43). MyD88-deficient mice are susceptible to P. aeruginosa (44) and, in some models, to S. pneumoniae (45, 46). Intriguingly, the 28 IRAK-4–deficient patients were not particularly susceptible to most other microorganisms, including common viruses (e.g., herpes viruses, enteroviruses, adenoviruses, and papillomaviruses), and widespread bacteria (e.g., Listeria, Mycobacterium, and Enterobacteriaceae), parasites (e.g., Toxoplasma), and fungi (e.g., Cryptococcus, Pneumocystis, Candida, and Aspergillus). As five of these patients have had no prophylaxis for 60 patient years (Fig. 7 B) (4, 6, 36), the resistance to most microbes observed is unlikely to be caused by the early death of some patients or to the prophylactic treatment of the survivors. Ascertainment bias cannot be excluded, but remains unlikely, as 10 affected relatives with causal mutations shared the case-definition clinical phenotype of index cases. In contrast, MyD88-deficient mice were found to be susceptible to mouse CMV (47), HSV-1(48), Listeria monocytogenes (49, 50), Mycobacterium avium (51), Toxoplasma gondii (52), Cryptococcus neoformans (53), Candida albicans, and Aspergillus fumigatus (54), among other relevant infections (37–39).
So why are the infectious phenotypes of MyD88/IRAK-4–deficient mice and IRAK-4–deficient humans so different? An overrepresentation of MyD88 deficiency with respect to IRAK-4 deficiency in mouse studies may be involved, although IRAK-4– and MyD88-deficient mice, when infected by the same pathogens, are indistinguishable (31, 43). We provide an experimental demonstration in this paper that the occurrence of human-specific IRAK-4–independent TLR pathways is not involved. We show that IRAK-4–deficient PBMCs do not secrete any of 11 cytokines tested when stimulated with agonists of TLR1, 2, 5, 6, 7, 8, and 9. The TLR4 response was abolished for all but two cytokines, which were weakly induced. One of these two cytokines was the IFN-inducible MIP-1β, consistent with the IFN-β mRNA response to LPS in IRAK-4–deficient PBMCs (13). IRAK-4–deficient PBMCs also responded to poly(I:C), producing IFN-inducible monocyte chemoattractant protein 1 and IFN-inducible protein 10, as expected from the previously reported induction of IFN-α, -β, and -λ in IRAK-4–deficient PBMCs and fibroblasts (13). However, poly(I:C) activates PBMCs normally in patients with TLR3 deficiency (42), making it difficult to infer conclusions about TLR3 responses from the data for poly(I:C) stimulation. In any event, the MyD88- and IRAK-4–independent TLR3 and TLR4 pathways, present in mice, cannot account for humans being more resistant (13, 15). The “conventional” MyD88-dependent pathway downstream from TLRs appears to be strictly IRAK-4–dependent in humans; no detectable leakiness can apparently account for the narrow infectious phenotype. We cannot, however, exclude the possibility that other TLR-inducible genes may be IRAK-4 independent.
We further excluded the possibility that human IRAK-4 deficiency may be milder than mouse MyD88/IRAK-4 deficiency owing to the occurrence of human-specific IRAK-4–independent TLR pathways in discrete leukocyte subsets, as suggested by the normal induction of both IL-6 and IFN-β/λ in IRAK-4–deficient fibroblasts (13). We showed that IRAK-4 deficiency impaired the TLR responses of all lymphoid and myeloid leukocyte subsets tested ex vivo, including granulocytes, monocytes, PDCs, MDCs, NK, T, and B cells. With the exception of the induction of IFN-inducible MIP-1β production in MDCs in response to poly(I:C) and LPS (Fig. 5, E and F), there was no detectable TLR response in individual subsets. The LPS response is TLR4 dependent, whereas the poly(I:C) response in MDCs appears to be TLR3 independent (42). Even IRAK-4–deficient NK cells did not respond to poly(I:C), suggesting that responses to poly(I:C) in NK cells are largely TLR3- (42) and IRAK-4–dependent. Moreover, MDDCs generated in vitro did not respond to TLR agonists, with the exception of poly(I:C) and LPS. The poly(I:C)-triggered induction of TNF-α, CD40, CD80, and CD86 in MDDCs was IRAK-4 independent (Fig. 5, G and H) and seemed to be TLR3 dependent (42). These data extend previous findings (1, 13) and show that human IRAK-4 plays a nonredundant role in the conventional TLR signaling pathway in at least seven major leukocyte subsets. In contrast, IRAK-4 may be dispensable for the “alternative,” TRIF-dependent pathways downstream from TLR3 (for IFNs and other cytokines) and TLR4 (for IFNs). Obviously, we cannot formally exclude the possibility that specific leukocyte subsets in certain tissues (55) and nonleukocyte cell types (56–59) display IRAK-4–independent TLR responses involved in host defense.
There are, therefore, no overt immunological differences between MyD88/IRAK-4–deficient mice and IRAK-4–deficient patients. Nonetheless, MyD88 and IRAK-4 are critical for protective immunity to numerous pathogens in the mouse, whereas IRAK-4 is largely redundant for protective immunity in humans. Intrinsic differences between mice and humans, affecting receptors other than TLRs, may account for the observed discrepancies. There may be non-TLRs governing the innate immune recognition of pathogens in humans but not in mice. An alternative, complementary hypothesis is that immunity to infection in animals is studied in experimental conditions, whereas immunity to infection in humans operates in natural conditions, accounting for considerable differences in the hosts, microbes, and routes of infection (60, 61). The human model can be used to define the function of host genes in a natural ecosystem in which species live and undergo selection. The ecologically relevant and evolutionarily selected function of human IRAK4 appears to be narrower than predicted from experimental studies in the mouse. This is reminiscent of the narrow infectious phenotype of patients with mycobacterial disease and mutations in the IL-12–IFN-γ circuit (62), or patients with herpes simplex encephalitis and mutations in the TLR3–UNC-93B pathway (42, 63). In any event, whether owing to species differences or to the conditions of infection, our findings for this series of IRAK-4–deficient patients strongly suggest that human IRAK-4–dependent TLRs are redundant for protective immunity to most microbes.
IRAK-4 seems to be crucial for protective immunity to Gram-positive S. pneumoniae and S. aureus and a few Gram-negative bacteria. It remains unknown whether invasive bacterial disease in patients with IRAK-4 deficiency results from an upstream impairment of IL-1R and TLR signaling or a combination of both pathways, from the defective induction of one or a combination of specific target genes downstream, or a combination of upstream and downstream defects. Impaired IL-1R and TLR2 signaling may play a role in the observed infections. Indeed, studies of experimental infection models in knockout mice have indicated that defense against S. pneumoniae and S. aureus may depend on IL-1R (64, 65), TLR2 (43, 66), and, for S. pneumoniae, perhaps also TLR4 (67, 68). Interestingly, the role of TLR2 in mouse defense against S. pneumoniae has been called into question in some experimental conditions (69, 70). Impaired stimulation of TLR7, 8, and 9 is probably not involved in predisposition to pneumococcal disease, as UNC-93B–deficient patients with impaired TLR3, 7, 8, and 9 signaling do not suffer from invasive pneumococcal disease (63). The impaired production of IL-6–inducible molecules, such as C-reactive protein (CrP), may also be involved. IRAK-4–deficient cells produce small amounts of IL-6 in vitro upon activation with IL-1β and TLR agonists. Moreover, most patients have weak or delayed acute inflammatory responses in vivo (low serum CrP levels in particular) (34, 71). As CrP contributes to the clearance of S. pneumoniae (72, 73), susceptibility to S. pneumoniae may be enhanced by the delayed increase in CrP levels. The contribution of individual molecules upstream or downstream from IRAK-4 to infectious phenotypes should be clarified by the identification of new patients with mutations in the corresponding genes (74).
Despite conferring selective susceptibility to only a few bacteria, IRAK-4 deficiency is life-threatening in infancy and childhood, with a mortality rate of 43% in our series. Most, if not all, patients would have probably died in the absence of antibiotic treatment. Strikingly, although IRAK-4 is absolutely vital in childhood, infections become rarer with age, with no deaths recorded after the age of 8 yr and no invasive infection after the age of 14 yr, even in the absence of antibiotic or IgG prophylaxis for more than 60 patient years (4, 6, 36). This dramatic improvement with age may be accounted for by the modest impact, if any, of IRAK-4 deficiency on antigen-specific T and B lymphocyte responses. Human T cells do not need IRAK-4 for activation by OKT3 in vitro (Table S2), in contrast to the results obtained for mice in a previous report (32) and in accordance with a more recent study (75). Moreover, our patients displayed no detectable global defect of protein antigen–specific T and B cell responses. However, most of the patients displayed IgE overproduction, and some patients have been shown to have weak antibody responses to a subset of glycan antigens (11, 12, 33). A more thorough investigation of B cells and antibody responses in IRAK-4–deficient patients is therefore currently underway (unpublished data). Our data are consistent with the apparently intact primary and secondary antigen-specific responses in mice with MyD88 deficiency, TRIF deficiency, or both (76, 77). Adaptive immunity may therefore progressively compensate for the poor innate immunity in our patients. An alternative and complementary hypothesis, accounting for the clinical improvement of IRAK-4–deficient patients with age, is that innate immune responses may also mature with age (78, 79). As shown in this study, the TIR pathway, including TLR responses in particular, remains dependent on IRAK-4 with age, but the maturation of other innate pathways may gradually compensate for the lack of TIR–IRAK-4 signaling.
MATERIALS AND METHODS
Subjects and kindreds.
Our study was conducted according to the principles expressed in the Helsinki Declaration, with informed consent obtained from each patient or the patient's family. The study was approved by the Comité d'Éthique, CCPPRB, Hôpital Necker–Enfants Malades.
Molecular genetics.
Genomic DNA was isolated from whole blood cells or from B-EBVs. The cells were lysed by incubation overnight at 37°C in extraction buffer (10 mM Tris, 0.1 M EDTA, 0.5% SDS, 1 mg/ml proteinase K) and subjected to phenol/chloroform extraction. DNA was precipitated in ethanol. Amplified PCR products were analyzed by electrophoresis in a 1% agarose gel purified by centrifugation through superfine resin (Sephadex G-50; GE Healthcare), sequenced by dideoxynucleotide termination with the BigDye terminator kit (Applied Biosystems), and analyzed on an ABI Prism 3730 apparatus (Applied Biosystems).
RNA and protein levels.
RNA was extracted from B-EBV and SV40-fibroblasts in TRIzol (Invitrogen), and cDNA was prepared using reverse transcriptase (SuperScript II; Invitrogen) for RT-PCR, according to the manufacturer's instructions. Proteins for Western blotting were extracted from B-EBV and SV40-fibroblasts, and Western blots were probed with rabbit antibodies against IRAK−4 (Tularik) and GAPDH (Santa Cruz Biotechnology, Inc.).
TLR agonists.
TLR agonists and cytokines were used at the following final concentrations, unless otherwise indicated: synthetic triacylated lipopeptide (PAM3CSK4, agonist of TLR1/2; Invivogen), 100 ng/ml; synthetic diacylated lipopeptide (PAM2CSK4, agonist of TLR2/6; Invivogen), 100 ng/ml; poly(I:C) (a synthetic analogue of dsRNA, polyinosine-polycytidylic acid, and nonspecific TLR3 agonist; Invivogen), 25 μg/ml; LPS (Re 595 from Salmonella minnesota, agonist of TLR-4; Sigma-Aldrich), 100 ng/ml; flagellin (TLR5 agonist; Invivogen), 1 μg/ml; 3M-13 (TLR7 agonist) and 3M-2 (TLR8 agonist; both provided by 3M Pharmaceuticals), 3 μg/ml each; R-848, resiquimod hydrochloride (TLR7 and TLR8 agonist; provided by PharmaTech), 3 μg/ml; and unmethylated CpG DNA CpG-C (C274; 5′-TCGTCGAACGTTCGAGATGAT-3′; TLR9 agonist; provided by R. Coffman and F. Barrat, Dynavax Technologies, Berkeley, CA), 3 μg/ml. Polymyxin B was used at 10 μg/ml (Sigma-Aldrich).
B-EBV and SV40-fibroblast activation.
We suspended 106 B-EBV cells per well in RPMI 1640 (Invitrogen) supplemented with 10% FCS (Invitrogen) and activated them by incubation with 3M-13, 3M-2, R-848, and 10−7 M PMA plus 10−5 M ionomycin (Sigma-Aldrich) for 24 h. 105 SV40-fibroblast cells per well were seeded in DMEM (Invitrogen) supplemented with 10% FCS in 24-well plates. Cells were activated with 20 ng/ml TNF-α (R&D Systems), 10 ng/ml IL-1β (R&D Systems) and 10−7 M PMA plus 10−5 M ionomycin the next day. The supernatants were harvested after 24 h of activation.
Cytokine measurement.
ELISA determinations of TNF-α, IL-6, and IL-10 in cell culture supernatants were performed with a kit (PeliPair reagent set; Sanquin), according to the manufacturer's instructions. Optical density was determined by an automated ELISA reader (MR5000; Thermolab Systems). We used a fluorescence-based assay (a human cytokine 25-plex antibody bead kit) that can detect 25 cytokines (LHC0009; Biosource International) for the simultaneous determination of multiple cytokines. Fluorescence was measured with a 100 IS system (Luminex Corporation). The assay and analysis were performed according to the manufacturer's instructions.
Cell purification and activation.
Blood samples from healthy controls or patients were collected into heparin-containing tubes, and PBMCs and granulocytes were separated by Ficoll-gradient centrifugation. The patients were of different ages when the experiments were performed, ranging from 7 to 32 yr old. For granulocyte isolation, erythrocytes were lysed and washed twice in PBS. More than 95% of the granulocytes purified on Ficoll were CD15+. We did not purify granulocytes by flow cytometry, as the surface expression and TLR-induced shedding of L-selectin (CD62L) were not better detected (unpublished data).The PBMC preparation was enriched in T cells, B cells, monocytes, and NK cells by magnetic bead isolation using anti-CD3, -CD19, -CD14, and -CD56 microbeads (Miltenyi Biotec), according to the manufacturer's instructions. Purified T cells were labeled with anti-CD3–FITC (BD Biosciences), B cells with anti-CD19–PE (BD Biosciences), monocytes with anti-CD14–FITC (BD Biosciences), and NK cells with anti-CD3–FITC/anti-CD56–PE (BD Biosciences) antibodies, and sorting was performed on a flow cytometer (FACSVantage; BD Biosciences). The isolated cells were cultured in RPMI 1640 supplemented with 10% FCS, with immediate TLR agonist stimulation. We added 100 U/ml IL-2 to cultures of purified T cells. Purified B cells were suspended in RPMI 1640 supplemented with 10% FCS at a density of 106 cells/ml. Cells were stimulated with TLR agonists together with 100 U/ml IL-4 for 3 d.
Analysis of selectin (CD62L) shedding on granulocytes.
Granulocytes were isolated as described in the previous section, activated with TLR agonists, stained with anti-CD62L–FITC (BD Biosciences) antibody, and analyzed by flow cytometry, as previously described (10).
Ex vivo analysis of PDCs and MDCs.
PBMCs were suspended at a final density of 2 × 106 cells/ml in RPMI supplemented with 10% FCS. They were incubated at 37°C, under an atmosphere containing 5% CO2, and stimulated with TLR agonists. 10 μg/ml brefeldin A was added after 1 h of activation. After 3.5 h of activation, cells were washed and stained with anti-Lin1–FITC (BD Biosciences), anti-HLADR–PerCP (BD Biosciences), and anti-CD123–PE-Cy7 (e-Bioscience) antibodies. For intracellular staining, PBMCs were permeabilized with the Cytofix/Cytoperm kit (BD Biosciences), according to the manufacturer's instructions. Anti–TNF-α–allophycocyanin (BD Biosciences) and anti–MIP-1β–PE (BD Biosciences) antibodies were used to assess the response of MDCs and PDCs to TLR agonists. PBMCs were also incubated with the respective isotype controls, and cells were acquired on a three-laser flow cytometer (LSR system; BD Biosciences). MDCs were defined as Lin-1−, HLA-DR+, and CD123low, and PDCs were defined as Lin-1−, HLA-DR+, and CD123high. For analysis, the quadrant for each individual tested was set such that 98% of PBMCs incubated with the respective isotype controls were negative for nonspecific staining.
MDDCs.
MDDCs were prepared as previously described (80). In brief, PBMCs were suspended in RPMI 1640 supplemented with 10% FCS, plated in cell culture flasks, and incubated for 1 h. Monocytes attached to the bottom of the culture flask and nonadherent cells were removed with medium. Monocytes were then cultured in RPMI 1640 supplemented with 10% FCS, 25 ng/ml GM-CSF, and 100 U/ml IL-4. GM-CSF and IL-4 were added to the medium every other day to maintain their initial concentrations. On day 7 or 8, some of the MDDCs were stained for CD1a and CD14. Living cells and cell debris were distinguished by forward/side scatter. More than 95% of living cells were CD1a+, and no CD14+ cells were detected. On day 7 or 8, MDDCs were suspended in RPMI 1640 supplemented with 10% FCS at a density of 2 × 105cells/ml, and supernatants were collected after 24 h of activation. The up-regulation of surface markers was assessed by collecting MDDCs and staining them with anti-CD1a–PE (BD Biosciences), anti-CD40–FITC (BD Biosciences), anti-CD80–FITC (BD Biosciences), and anti-CD86–FITC (BD Biosciences) antibodies.
Vaccination schedules of patients.
Patients were immunized against diphtheria and tetanus in accordance with international recommendations. Nine patients received multiple injections of glycan antigens (nonconjugated [“Pneumo23”] and conjugated [“Prevenar”] antipneumococcal vaccine), and their specific antibody titers were subsequently monitored in detail.
Online supplemental material.
Fig. S1 demonstrates a deletion of the IRAK4 locus on one allele in P2 and the presence of both IRAK4 loci in P7. Fig. S2 shows the detailed results for each of the 11 cytokines for which a response to TLR agonists in healthy controls could be detected by multiplex assay. Table S1 shows blood leukocyte subsets in IRAK-4–deficient patients. Table S2 highlights T cell proliferation, Ig levels, and humoral responses to recall antigens and to glycans in IRAK-4–deficient patients. Table S3 offers the serology of patients to common viruses. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20070628/DC1.
Supplemental Material
[Supplemental Material Index]
Acknowledgments
We thank all members of the laboratory for helpful discussions, and Catherine Bidalled, Martine Courat, and Tony Leclerc for secretarial and technical assistance. We would particularly like to thank the patients and their families, whose trust, support, and cooperation were essential for collection of the data used in this study.
H. von Bernuth was supported by grants from the Deutsche Forschungsgemeinschaft (VO 995/1-1 and VO 995/1-2), from the European Ph.D. program of the San Raffaele Institute, and from the program “Legs Poix” of the Parisian Universities. L. Maródi was supported by a grant from the Hungarian Research Fund (OTKA 49017), and A. Puel was supported by a grant from the European Union (QLK2-CT-2002-00846). The Laboratory of Human Genetics of Infectious Diseases is supported by the March of Dimes, the BNP Paribas Foundation, the Dana Foundation, and the Schlumberger Foundation. J.L. Casanova is an International Scholar of the Howard Hughes Medical Institute.
The authors have no conflicting financial interests.
Abbreviations used: B-EBV, EBV-transformed B lymphocyte cell line; CrP, C-reactive protein; IRAK-4, IL-1R–associated kinase 4; MDC, myeloid DC; MDDC, monocyte-derived DC; MIP-1β, macrophage inflammatory protein 1β; PDC, plasmacytoid DC; SV-40 fibroblast, SV40-transformed fibroblast; TIR, Toll/IL-1 receptor; TLR, Toll-like receptor; TRIF, TIR domain–containing adaptor-inducing IFN-β.
C.-L. Ku and H. von Bernuth contributed equally to this work.
References
- 1.Picard, C., A. Puel, M. Bonnet, C.L. Ku, J. Bustamante, K. Yang, C. Soudais, S. Dupuis, J. Feinberg, C. Fieschi, et al. 2003. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 299:2076–2079. [DOI] [PubMed] [Google Scholar]
- 2.Kuhns, D.B., D.A. Long Priel, and J.I. Gallin. 1997. Endotoxin and IL-1 hyporesponsiveness in a patient with recurrent bacterial infections. J. Immunol. 158:3959–3964. [PubMed] [Google Scholar]
- 3.Haraguchi, S., N.K. Day, R.P. Nelson Jr., P. Emmanuel, J.E. Duplantier, C.S. Christodoulou, and R.A. Good. 1998. Interleukin 12 deficiency associated with recurrent infections. Proc. Natl. Acad. Sci. USA. 95:13125–13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medvedev, A.E., A. Lentschat, D.B. Kuhns, J.C. Blanco, C. Salkowski, S. Zhang, M. Arditi, J.I. Gallin, and S.N. Vogel. 2003. Distinct mutations in IRAK-4 confer hyporesponsiveness to lipopolysaccharide and interleukin 1 in a patient with recurrent bacterial infections. J. Exp. Med. 198:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Currie, A.J., D.J. Davidson, G.S. Reid, S. Bharya, K.L. MacDonald, R.S. Devon, and D.P. Speert. 2004. Primary immunodeficiency to pneumococcal infection due to a defect in Toll-like receptor signaling. J. Pediatr. 144:512–518. [DOI] [PubMed] [Google Scholar]
- 6.Chapel, H., A. Puel, H. von Bernuth, C. Picard, and J.L. Casanova. 2005. Shigella sonnei meningitis due to interleukin-1 receptor-associated kinase-4 deficiency: first association with a primary immune deficiency. Clin. Infect. Dis. 40:1227–1231. [DOI] [PubMed] [Google Scholar]
- 7.Takada, H., H. Yoshikawa, M. Imaizumi, T. Kitamura, J. Takeyama, S. Kumaki, A. Nomura, and T. Hara. 2006. Delayed separation of the umbilical cord in two siblings with interleukin-1 receptor-associated kinase 4 deficiency: rapid screening by flow cytometer. J. Pediatr. 148:546–548. [DOI] [PubMed] [Google Scholar]
- 8.Cardenes, M., H. von Bernuth, A. Garcia-Saavedra, E. Santiago, A. Puel, C.L. Ku, J.F. Emile, C. Picard, J.L. Casanova, E. Colino, et al. 2006. Autosomal recessive interleukin-1 receptor-associated kinase 4 deficiency in fourth-degree relatives. J. Pediatr. 148:549–551. [DOI] [PubMed] [Google Scholar]
- 9.Davidson, D.J., A.J. Currie, D.M. Bowdish, K.L. Brown, C.M. Rosenberger, R.C. Ma, J. Bylund, P.A. Campsall, A. Puel, C. Picard, et al. 2006. IRAK-4 mutation (Q293X): rapid detection and characterization of defective post-transcriptional TLR/IL-1R responses in human myeloid and non-myeloid cells. J. Immunol. 177:8202–8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Bernuth, H., C.L. Ku, C. Rodriguez-Gallego, S. Zhang, B.Z. Garty, L. Marodi, H. Chapel, M. Chrabieh, R.L. Miller, C. Picard, et al. 2006. A fast procedure for the detection of defects in Toll-like receptor signaling. Pediatrics. 118:2498–2503. [DOI] [PubMed] [Google Scholar]
- 11.McDonald, D.R., D. Brown, F.A. Bonilla, and R.S. Geha. 2006. Interleukin receptor-associated kinase-4 deficiency impairs Toll-like receptor-dependent innate antiviral immune responses. J. Allergy Clin. Immunol. 118:1357–1362. [DOI] [PubMed] [Google Scholar]
- 12.Ku, C.L., C. Picard, M. Erdos, A. Jeurissen, J. Bustamante, A. Puel, H. von Bernuth, O. Filipe-Santos, H.H. Chang, T. Lawrence, et al. 2007. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. J. Med. Genet. 44:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang, K., A. Puel, S. Zhang, C. Eidenschenk, C.L. Ku, A. Casrouge, C. Picard, H. von Bernuth, B. Senechal, S. Plancoulaine, et al. 2005. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 23:465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 301:640–643. [DOI] [PubMed] [Google Scholar]
- 15.Hoebe, K., X. Du, P. Georgel, E. Janssen, K. Tabeta, S.O. Kim, J. Goode, P. Lin, N. Mann, S. Mudd, et al. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 424:743–748. [DOI] [PubMed] [Google Scholar]
- 16.Kawai, T., O. Takeuchi, T. Fujita, J. Inoue, P.F. Muhlradt, S. Sato, K. Hoshino, and S. Akira. 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887–5894. [DOI] [PubMed] [Google Scholar]
- 17.Oshiumi, H., M. Matsumoto, K. Funami, T. Akazawa, and T. Seya. 2003. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 4:161–167. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi, F., T.K. Means, and A.D. Luster. 2003. Toll-like receptors stimulate human neutrophil function. Blood. 102:2660–2669. [DOI] [PubMed] [Google Scholar]
- 19.Kadowaki, N., S. Ho, S. Antonenko, R.W. Malefyt, R.A. Kastelein, F. Bazan, and Y.J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of Toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531–4537. [DOI] [PubMed] [Google Scholar]
- 21.Ito, T., R. Amakawa, T. Kaisho, H. Hemmi, K. Tajima, K. Uehira, Y. Ozaki, H. Tomizawa, S. Akira, and S. Fukuhara. 2002. Interferon α and interleukin 12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J. Exp. Med. 195:1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krug, A., S. Rothenfusser, V. Hornung, B. Jahrsdorfer, S. Blackwell, Z.K. Ballas, S. Endres, A.M. Krieg, and G. Hartmann. 2001. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur. J. Immunol. 31:2154–2163. [DOI] [PubMed] [Google Scholar]
- 23.Hasan, U., C. Chaffois, C. Gaillard, V. Saulnier, E. Merck, S. Tancredi, C. Guiet, F. Briere, J. Vlach, S. Lebecque, et al. 2005. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J. Immunol. 174:2942–2950. [DOI] [PubMed] [Google Scholar]
- 24.Gorden, K.B., K.S. Gorski, S.J. Gibson, R.M. Kedl, W.C. Kieper, X. Qiu, M.A. Tomai, S.S. Alkan, and J.P. Vasilakos. 2005. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J. Immunol. 174:1259–1268. [DOI] [PubMed] [Google Scholar]
- 25.Renn, C.N., D.J. Sanchez, M.T. Ochoa, A.J. Legaspi, C.K. Oh, P.T. Liu, S.R. Krutzik, P.A. Sieling, G. Cheng, and R.L. Modlin. 2006. TLR activation of Langerhans cell-like dendritic cells triggers an antiviral immune response. J. Immunol. 177:298–305. [DOI] [PubMed] [Google Scholar]
- 26.Nagase, H., S. Okugawa, Y. Ota, M. Yamaguchi, H. Tomizawa, K. Matsushima, K. Ohta, K. Yamamoto, and K. Hirai. 2003. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J. Immunol. 171:3977–3982. [DOI] [PubMed] [Google Scholar]
- 27.Dasari, P., I.C. Nicholson, G. Hodge, G.W. Dandie, and H. Zola. 2005. Expression of Toll-like receptors on B lymphocytes. Cell. Immunol. 236:140–145. [DOI] [PubMed] [Google Scholar]
- 28.Caron, G., D. Duluc, I. Fremaux, P. Jeannin, C. David, H. Gascan, and Y. Delneste. 2005. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J. Immunol. 175:1551–1557. [DOI] [PubMed] [Google Scholar]
- 29.Tabiasco, J., E. Devevre, N. Rufer, B. Salaun, J.C. Cerottini, D. Speiser, and P. Romero. 2006. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J. Immunol. 177:8708–8713. [DOI] [PubMed] [Google Scholar]
- 30.Wesch, D., S. Beetz, H.H. Oberg, M. Marget, K. Krengel, and D. Kabelitz. 2006. Direct costimulatory effect of TLR3 ligand poly(I:C) on human gamma delta T lymphocytes. J. Immunol. 176:1348–1354. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki, N., S. Suzuki, G.S. Duncan, D.G. Millar, T. Wada, C. Mirtsos, H. Takada, A. Wakeham, A. Itie, S. Li, et al. 2002. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 416:750–756. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki, N., S. Suzuki, D.G. Millar, M. Unno, H. Hara, T. Calzascia, S. Yamasaki, T. Yokosuka, N.J. Chen, A.R. Elford, et al. 2006. A critical role for the innate immune signaling molecule IRAK-4 in T cell activation. Science. 311:1927–1932. [DOI] [PubMed] [Google Scholar]
- 33.Day, N., N. Tangsinmankong, H. Ochs, R. Rucker, C. Picard, J.L. Casanova, S. Haraguchi, and R. Good. 2004. Interleukin receptor-associated kinase (IRAK-4) deficiency associated with bacterial infections and failure to sustain antibody responses. J. Pediatr. 144:524–526. [DOI] [PubMed] [Google Scholar]
- 34.Enders, A., U. Pannicke, R. Berner, P. Henneke, K. Radlinger, K. Schwarz, and S. Ehl. 2004. Two siblings with lethal pneumococcal meningitis in a family with a mutation in interleukin-1 receptor-associated kinase 4. J. Pediatr. 145:698–700. [DOI] [PubMed] [Google Scholar]
- 35.Medvedev, A.E., K. Thomas, A. Awomoyi, D.B. Kuhns, J.I. Gallin, X. Li, and S.N. Vogel. 2005. Cutting edge: expression of IL-1 receptor-associated kinase-4 (IRAK-4) proteins with mutations identified in a patient with recurrent bacterial infections alters normal IRAK-4 interaction with components of the IL-1 receptor complex. J. Immunol. 174:6587–6591. [DOI] [PubMed] [Google Scholar]
- 36.Lavine, E., R. Somech, J.Y. Zhang, A. Puel, X. Bossuyt, C. Picard, J.L. Casanova, and C.M. Roifman. 2007. Cellular and humoral aberrations in a kindred with IL-1 receptor-associated kinase 4 deficiency. J. Allergy Clin. Immunol. DOI: 10.1016/j.jaci.2007.04.038. [DOI] [PubMed]
- 37.Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. [DOI] [PubMed] [Google Scholar]
- 38.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376. [DOI] [PubMed] [Google Scholar]
- 39.Beutler, B., Z. Jiang, P. Georgel, K. Crozat, B. Croker, S. Rutschmann, X. Du, and K. Hoebe. 2006. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu. Rev. Immunol. 24:353–389. [DOI] [PubMed] [Google Scholar]
- 40.Qin, J., Z. Jiang, Y. Qian, J.L. Casanova, and X. Li. 2004. IRAK4 kinase activity is redundant for interleukin-1 (IL-1) receptor-associated kinase phosphorylation and IL-1 responsiveness. J. Biol. Chem. 279:26748–26753. [DOI] [PubMed] [Google Scholar]
- 41.Kim, T.W., K. Staschke, K. Bulek, J. Yao, K. Peters, K.H. Oh, Y. Vandenburg, H. Xiao, W. Qian, T. Hamilton, et al. 2007. A critical role for IRAK4 kinase activity in Toll-like receptor–mediated innate immunity. J. Exp. Med. 204:1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, S.Y., E. Jouanguy, S. Ugolini, A. Smahi, G. Elain, P. Romero, D. Segal, V. Sancho-Shimizu, L. Lorenzo, A. Puel, et al. 2007. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 317:1522–1527. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392–5396. [DOI] [PubMed] [Google Scholar]
- 44.Skerrett, S.J., H.D. Liggitt, A.M. Hajjar, and C.B. Wilson. 2004. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J. Immunol. 172:3377–3381. [DOI] [PubMed] [Google Scholar]
- 45.Albiger, B., A. Sandgren, H. Katsuragi, U. Meyer-Hoffert, K. Beiter, F. Wartha, M. Hornef, S. Normark, and B.H. Normark. 2005. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell. Microbiol. 7:1603–1615. [DOI] [PubMed] [Google Scholar]
- 46.Khan, A.Q., Q. Chen, Z.Q. Wu, J.C. Paton, and C.M. Snapper. 2005. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on Toll-like receptor 2. Infect. Immun. 73:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delale, T., A. Paquin, C. Asselin-Paturel, M. Dalod, G. Brizard, E.E. Bates, P. Kastner, S. Chan, S. Akira, A. Vicari, et al. 2005. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J. Immunol. 175:6723–6732. [DOI] [PubMed] [Google Scholar]
- 48.Mansur, D.S., E.G. Kroon, M.L. Nogueira, R.M.E. Arantes, S.C.O. Rodrigues, S. Akira, R.T. Gazzinelli, and M.A. Campos. 2005. Lethal encephalitis in myeloid differentiation factor 88-deficient mice infected with herpes simplex virus 1. Am. J. Pathol. 166:1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edelson, B.T., and E.R. Unanue. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 169:3869–3875. [DOI] [PubMed] [Google Scholar]
- 50.Seki, E., H. Tsutsui, N.M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, et al. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 169:3863–3868. [DOI] [PubMed] [Google Scholar]
- 51.Feng, C.G., C.A. Scanga, C.M. Collazo-Custodio, A.W. Cheever, S. Hieny, P. Caspar, and A. Sher. 2003. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J. Immunol. 171:4758–4764. [DOI] [PubMed] [Google Scholar]
- 52.Scanga, C.A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E.Y. Denkers, R. Medzhitov, and A. Sher. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997–6001. [DOI] [PubMed] [Google Scholar]
- 53.Yauch, L.E., M.K. Mansour, S. Shoham, J.B. Rottman, and S.M. Levitz. 2004. Involvement of CD14, Toll-like receptors 2 and 4, and MyD88 in the host response to the fungal pathogen Cryptococcus neoformans in vivo. Infect. Immun. 72:5373–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellocchio, S., C. Montagnoli, S. Bozza, R. Gaziano, G. Rossi, S.S. Mambula, A. Vecchi, A. Mantovani, S.M. Levitz, and L. Romani. 2004. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 172:3059–3069. [DOI] [PubMed] [Google Scholar]
- 55.van der Aar, A.M., R.M. Sylva-Steenland, J.D. Bos, M.L. Kapsenberg, E.C. de Jong, and M.B. Teunissen. 2007. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J. Immunol. 178:1986–1990. [DOI] [PubMed] [Google Scholar]
- 56.Faure, E., O. Equils, P.A. Sieling, L. Thomas, F.X. Zhang, C.J. Kirschning, N. Polentarutti, M. Muzio, and M. Arditi. 2000. Bacterial lipopolysaccharide activates NF-kappaB through Toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J. Biol. Chem. 275:11058–11063. [DOI] [PubMed] [Google Scholar]
- 57.Spachidou, M.P., E. Bourazopoulou, C.I. Maratheftis, E.K. Kapsogeorgou, H.M. Moutsopoulos, A.G. Tzioufas, and M.N. Manoussakis. 2007. Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjogren's syndrome. Clin. Exp. Immunol. 147:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greene, C.M., and N.G. McElvaney. 2005. Toll-like receptor expression and function in airway epithelial cells. Arch. Immunol. Ther. Exp. (Warsz.). 53:418–427. [PubMed] [Google Scholar]
- 59.Bozza, S., F. Bistoni, R. Gaziano, L. Pitzurra, T. Zelante, P. Bonifazi, K. Perruccio, S. Bellocchio, M. Neri, A.M. Iorio, et al. 2006. Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood. 108:3387–3396. [DOI] [PubMed] [Google Scholar]
- 60.Casanova, J.L., and L. Abel. 2004. The human model: a genetic dissection of immunity to infection in natural conditions. Nat. Rev. Immunol. 4:55–66. [DOI] [PubMed] [Google Scholar]
- 61.Casanova, J.-L., and L. Abel. 2007. Human genetics of infectious diseases: a unified theory. EMBO J. 26:915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casanova, J.L., and L. Abel. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20:581–620. [DOI] [PubMed] [Google Scholar]
- 63.Casrouge, A., S.Y. Zhang, C. Eidenschenk, E. Jouanguy, A. Puel, K. Yang, A. Alcais, C. Picard, N. Mahfoufi, N. Nicolas, et al. 2006. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 314:308–312. [DOI] [PubMed] [Google Scholar]
- 64.Hultgren, O.H., L. Svensson, and A. Tarkowski. 2002. Critical role of signaling through IL-1 receptor for development of arthritis and sepsis during Staphylococcus aureus infection. J. Immunol. 168:5207–5212. [DOI] [PubMed] [Google Scholar]
- 65.Zwijnenburg, P.J., T. van der Poll, S. Florquin, J.J. Roord, and A.M. Van Furth. 2003. IL-1 receptor type 1 gene-deficient mice demonstrate an impaired host defense against pneumococcal meningitis. J. Immunol. 170:4724–4730. [DOI] [PubMed] [Google Scholar]
- 66.Koedel, U., B. Angele, T. The Rockefeller University Pressprecht, H. Wagner, A. Roggenkamp, H.W. Pfister, and C.J. Kirschning. 2003. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 170:438–444. [DOI] [PubMed] [Google Scholar]
- 67.Malley, R., P. Henneke, S.C. Morse, M.J. Cieslewicz, M. Lipsitch, C.M. Thompson, E. Kurt-Jones, J.C. Paton, M.R. Wessels, and D.T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA. 100:1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Branger, J., S. Knapp, S. Weijer, J.C. Leemans, J.M. Pater, P. Speelman, S. Florquin, and T. van der Poll. 2004. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect. Immun. 72:788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knapp, S., C.W. Wieland, C. van 't Veer, O. Takeuchi, S. Akira, S. Florquin, and T. van der Poll. 2004. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J. Immunol. 172:3132–3138. [DOI] [PubMed] [Google Scholar]
- 70.Dessing, M.C., K.F. van der Sluijs, S. Florquin, S. Akira, and T. van der Poll. 2007. Toll-like receptor 2 does not contribute to host response during postinfluenza pneumococcal pneumonia. Am. J. Respir. Cell Mol. Biol. 36:609–614. [DOI] [PubMed] [Google Scholar]
- 71.von Bernuth, H., A. Puel, C.L. Ku, K. Yang, J. Bustamante, H.H. Chang, C. Picard, and J.L. Casanova. 2005. Septicemia without sepsis: inherited disorders of nuclear factor-kappa B-mediated inflammation. Clin. Infect. Dis. 41(Suppl. 7):S436–S439. [DOI] [PubMed] [Google Scholar]
- 72.Suresh, M.V., S.K. Singh, D.A. Ferguson Jr., and A. Agrawal. 2007. Human C-reactive protein protects mice from Streptococcus pneumoniae infection without binding to pneumococcal C-polysaccharide. J. Immunol. 178:1158–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suresh, M.V., S.K. Singh, D.A. Ferguson Jr., and A. Agrawal. 2006. Role of the property of C-reactive protein to activate the classical pathway of complement in protecting mice from pneumococcal infection. J. Immunol. 176:4369–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Casanova, J.L., and L. Abel. 2005. Inborn errors of immunity to infection: the rule rather than the exception. J. Exp. Med. 202:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawagoe, T., S. Sato, A. Jung, M. Yamamoto, K. Matsui, H. Kato, S. Uematsu, O. Takeuchi, and S. Akira. 2007. Essential role of IRAK-4 protein and its kinase activity in Toll-like receptor–mediated immune responses but not in TCR signaling. J. Exp. Med. 204:1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janssen, E., K. Tabeta, M.J. Barnes, S. Rutschmann, S. McBride, K.S. Bahjat, S.P. Schoenberger, A.N. Theofilopoulos, B. Beutler, and K. Hoebe. 2006. Efficient T cell activation via a Toll-interleukin 1 receptor-independent pathway. Immunity. 24:787–799. [DOI] [PubMed] [Google Scholar]
- 77.Gavin, A.L., K. Hoebe, B. Duong, T. Ota, C. Martin, B. Beutler, and D. Nemazee. 2006. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science. 314:1936–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirsch, M.S., B. Zisman, and A.C. Allison. 1970. Macrophages and age-dependent resistance to herpes simplex virus in mice. J. Immunol. 104:1160–1165. [PubMed] [Google Scholar]
- 79.Pham, L.N., M.S. Dionne, M. Shirasu-Hiza, and D.S. Schneider. 2007. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 3:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Filipe-Santos, O., J. Bustamante, M.H. Haverkamp, E. Vinolo, C.L. Ku, A. Puel, D.M. Frucht, K. Christel, H. von Bernuth, E. Jouanguy, et al. 2006. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J. Exp. Med. 203:1745–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Material Index]