All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation (original) (raw)
Abstract
We demonstrate that all-trans retinoic acid (RA) induces FoxP3+ adaptive T regulatory cells (A-Tregs) to acquire a gut-homing phenotype (α4β7+ CC chemokine receptor 9+) and the capacity to home to the lamina propria of the small intestine. Under conditions that favor the differentiation of A-Tregs (transforming growth factor–β1 and interleukin 2) in vitro, the inclusion of RA induces nearly all activated CD4+ T cells to express FoxP3 and greatly increases the accumulation of these cells. In the absence of RA, A-Treg differentiation is abruptly impaired by proficient antigen presenting cells or through direct co-stimulation. In the presence of RA, A-Treg generation occurs even in the presence of high levels of co-stimulation, with RA attenuating co-stimulation from interfering from FoxP3 induction. The recognition that RA induces gut imprinting, together with our finding that it enhances A-Treg conversion, differentiation, and expansion, indicates that RA production in vivo may drive both the imprinting and A-Treg development in the face of overt inflammation.
T regulatory cells (T reg cells) function to temper the massive proinflammatory stimulus created by commensal bacteria within the gut. Evidence of their functional importance in suppressing gut inflammation is observed by the fact that their absence permits the onset of gut autoimmunity (1, 2). Therefore, manipulation of T reg cell function within the gut provides an attractive therapeutic approach in enforcing gut tolerance during times when the balance is shifted toward autoimmunity.
The ability of activated T cells to exit the blood and enter different tissues of the body is “imprinted” on them within the secondary lymphoid organs by DCs (3, 4). T cells homing to the small intestine lamina propria express the integrin α4β7, which binds mucosal addressin cell adhesion molecule–1 and CC chemokine receptor (CCR) 9, whose ligand is secreted within the lamina propria (5–8). A population of CD103+ DCs that primarily resides within the lamina propria, Peyer's patches, and mesenteric lymph nodes are responsible for imprinting a gut-homing capacity on T cells by an all-trans retinoic acid (RA)–dependent mechanism (1, 9–11).
It has been shown that CD4+FoxP3− naive T cells can be converted into CD4+FoxP3+ T reg cells (i.e., adaptive T reg cells [A-Tregs]) exhibiting the same suppressive and phenotypic characteristics as thymically derived, natural T reg cells (nTregs) both in vivo and in vitro (12–16). This conversion is dependent on TGF-β1 and requires IL-2 in vitro (13, 17, 18). The use of A-Tregs as a means of inducing tolerance has been demonstrated in numerous settings, in cluding inflammatory bowel disease and allo transplantation models (12, 15, 16, 19). Improving the potency of A-Treg treatments is of great clinical interest, and harnessing imprinting mechanisms to target A-Tregs to a specific organ is one means to this end.
In this report, we use RA to generate a uni que population of A-Tregs in vitro that is able to home in vivo to the small intestine lamina propria. Surprisingly, RA potently synergized with TGF-β1 in driving FoxP3 induction. In addition to increasing the frequency and durability of these A-Tregs, RA also allowed FoxP3 expression to occur in the presence of high levels of co-stimulation, levels that normally prevent FoxP3 induction. These experiments highlight the central importance of RA in serving two roles: driving gut homing and permitting the development of A-Tregs in the presence of overt inflammation. These data im plicate RA as a mediator of gut tolerance in vivo.
RESULTS AND DISCUSSION
RA synergizes with TGF-β1 to generate A-Tregs that home to the small intestine in vivo and express suppressive activity
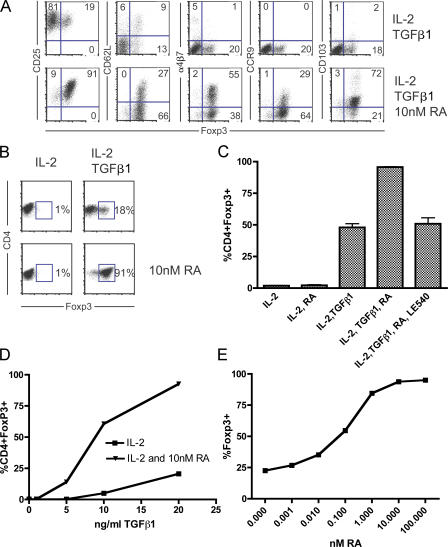
RA induces α4β7 and CCR9 expression on CD4+ T cells during in vitro activation (11). As A-Tregs can be generated from naive CD4+FoxP3− cells in vitro during stimulation in the presence of TGF-β1 and IL-2, we addressed whether CD4+FoxP3− T cells cultured with TGF-β1, RA, and IL-2 under activating conditions would generate cells with a CD4+FoxP3+α4β7+CCR9+ phenotype. We sorted CD4+FoxP3− cells from the FoxP3/GFP reporter mouse to >99.9% purity, using this approach throughout the study (20). After 5 d of activation by plate-bound αCD3/αCD28 with TGF-β1, IL-2, and RA, we observed the emergence of CD4+FoxP3+α4β7+CCR9+ T cells (RA–T reg cells; Fig. 1 A). Concordant with conversion was the induction of CD25, a marker for both T reg cells and activated T cells, and the expression of CD103, which is induced by TGF-β1 and identifies an in vivo subset of effector/memory T reg cells, which are more potent suppressors than T reg cells lacking expression of this molecule (21–23). These data show that a population bearing the phenotype of a gut-homing T reg cell population was generated. Additionally, we observed a >90% conversion rate of CD4+FoxP3− to CD4+FoxP3+ T cells in the presence of RA, compared with considerably less in its absence (Fig. 1 B). Between experiments, we observed variable conversion rates ranging between 15–50% in the culture conditions of IL-2 and TGF-β1, with the addition of RA consistently increasing FoxP3 expression to 90% or greater. This synergy between TGF-β1 and RA in inducing FoxP3 expression could be blocked by the addition of the selective RA receptor antagonist LE540 (Fig. 1 C). RA was able to enhance conversion throughout a titration of TGF-β1, although RA, by itself, did not induce conversion. The in ability of RA to induce FoxP3 expression without the addition of exogenous TGF-β1 indicates that conversion was exclusively dependent on exogenous TGF-β1 (Fig. 1 D). Any endogenous TGF-β1 potentially present within our culture medium was unable to mediate this effect. When RA was titrated against constant TGF-β1 and IL-2, RA was able to enhance TGF-β1–dependent conversion even at subnanomolar concentrations (Fig. 1 E). For future experiments within this study, unless otherwise indicated, we used 1 nM RA in our cultures. This was done for two reasons: first, at concentrations <1 nM, the percentage of T cells expressing FoxP3 begins to decrease, and second, it has been observed that RA has antiproliferative effects on T cells at high concentrations (30–100 nM RA suppresses T cell proliferation, whereas doses <5 nM do not; unpublished data) (24). Results similar to those shown in Fig. 1 (A–D) were observed using 1 nM RA. These data show that RA potently enhances TGF-β1–dependent FoxP3 induction.
Figure 1.
RA synergizes with TGF-β1 in the generation of gut-homing A-Tregs in vitro. (A and B) FACS-sorted CD4+Foxp3− cells from the Foxp3/GFP reporter mouse were cultured under activating conditions with IL-2 and TGF-β1 ± 10 nM RA, and surface phenotype and Foxp3/GFP expression was analyzed by flow cytometry after 5 d of culture. (A) Representative staining showing the percentage of Foxp3/GFP expression against CD25, CD62L, α4β7, CCR9, and CD103. (B) Representative FACS plot depicting Foxp3/GFP expression by CD4+ T cells. (C) Quantification of one representative experiment showing the percentage of Foxp3/GFP+ cells among all CD4+ cells, with the selective RA receptor antagonist LE540 (1 μM) included as indicated. Mean ± SEM is shown. (D) Foxp3/GFP expression by CD4+ cells as a function of titrating concentrations of TGF-β1 ± RA. IL-2 concentrations were kept constant, ±RA, with TGF-β1 (20 ng/ml, 10 ng/ml, 5 ng/ml, 1 ng/ml, and 0) titrated. (E) TGF-β–mediated Foxp3/GFP expression as a result of titrating concentrations of RA. Keeping IL-2 and TGF-β1 constant, RA was titrated in serial dilutions (100 nM, 10 nM, 1 nM, 100 pM, 10 pM, 1 pM, and 0). All experiments shown are representative of at least n = 3 independent experiments.
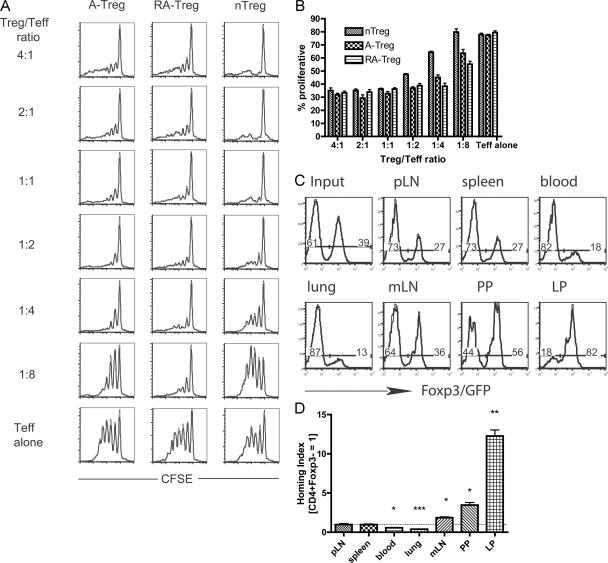
The suppressive activity of RA–T reg cells was measured relative to that of A-Tregs and freshly harvested nTregs in an in vitro suppressor assay. The data show that RA–T reg cells are potent suppressors of T effector cells in vitro (Fig. 2, A and B). It is interesting to speculate that the RA–T reg cells may even be more potent suppressors of T effector cell proliferation, as indicated by their ability to suppress at T reg cell/T effector cell ratios that nTregs and A-Tregs could not suppress.
Figure 2.
RA–T reg cells are suppressive in vitro and home to the small intestine in vivo. To examine the suppressive capacity of A-Tregs, RA–T reg cells, and nTregs, Ly5.2+Foxp3/GFP+ A-Tregs and RA–T reg cells were generated in vitro and sorted to >99% purity, with Ly5.2+ nTregs freshly isolated ex vivo from a Ly5.2+Foxp3/GFP reporter mouse. These T reg cell subsets were cultured with CFSE-labeled Ly5.1+CD4+ T effector cells at the indicated ratios under activating conditions. Representative CFSE plots of the T effector cells are shown (A), with the percentage of dividing T effector cells quantified (B). (C and D) Representative in vivo competitive homing assay. Naive CD4+Foxp3− cells were sorted from an Ly5.2+Foxp3/GFP reporter mouse and activated for 5 d in the presence of TGF-β1, IL-2, and RA to generate RA–T reg cells expressing Foxp3/GFP. Naive CD4+CD25− cells were sorted from an Ly5.2+ mouse and cultured under the same conditions, minus the RA, to generate A-Tregs lacking the Foxp3/GFP allele. These two cell populations were mixed and injected into recipient mice. (C) Organs were harvested after 18 h and stained for donor Ly5.2+CD4+ T cells, and the ratio of GFP+/− cells within the donor population was analyzed, with a representative mouse shown. Numbers show the percentage of transfered cells that are FoxP3/GFP− and FoxP3/GFP+. (D) The HI (ration of [GFP+]tissue/[GFP−]tissue to [GFP+]input/[GFP−]input) was calculated, with bar graphs showing mean ± SEM of one representative experiment using three recipient mice. Peripheral lymph node (pLN): HI = 0.96 (SEM = 0.13); spleen: HI = 0.96 (SEM = 0.08); blood: HI = 0.56 (SEM = 0.05); lung: HI = 0.39 (SEM = 0.02); mesenteric lymph node (mLN): HI = 1.86 (SEM = 0.1); Peyer's patches (PP): HI = 3.46 (SEM = 0.33); and lamina propria (LP): HI = 12.27 (SEM = 0.77). All experiments are representative of at least two independent experiments. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 compared with HI = 1 (dotted line).
To examine the in vivo homing capacity of RA–T reg cells, we performed an in vivo competitive homing experiment (3, 4). RA–T reg cells were generated in vitro from a Ly5.2+FoxP3/GFP reporter mouse. Sorted CD4+CD25− cells from a Ly5.2+ mouse that did not contain the FoxP3/GFP knock-in allele were used to generate a population of A-Tregs. As the A-Tregs were generated from mice lacking the FoxP3/GFP allele, it was impossible to sort them to purity after inducing FoxP3 expression in vitro, and thus, the A-Treg population used contained a substantial percentage of FoxP3− cells (unpublished data). However, this does not diminish the utility of these cells in an in vivo competitive homing experiment. RA–T reg cells and A-Tregs were equally mixed and injected intravenously into recipient Ly5.1+ mice, and various organs were analyzed for donor cells after 18 h (Fig. 2 C). After at least 18 h, we did not observe reversion of CD4+FoxP3+ RA–T reg cells to a CD4+FoxP3− pheno type in vivo (see Fig. 5 A). As expected from their phenotype, the RA–T reg cells had the capacity to home to the small intestine lamina propria and were in the minority compared with A-Tregs in the blood and lung (Fig. 2 D). There was also an accumulation of RA–T reg cells in the mesenteric lymph nodes and the Peyer's patches. Collectively, these data show that functionally suppressive T reg cells with a gut-homing phenotype can be generated from CD4+FoxP3− T cells in vitro, and this cell population has the capacity to home to the small intestine in vivo.
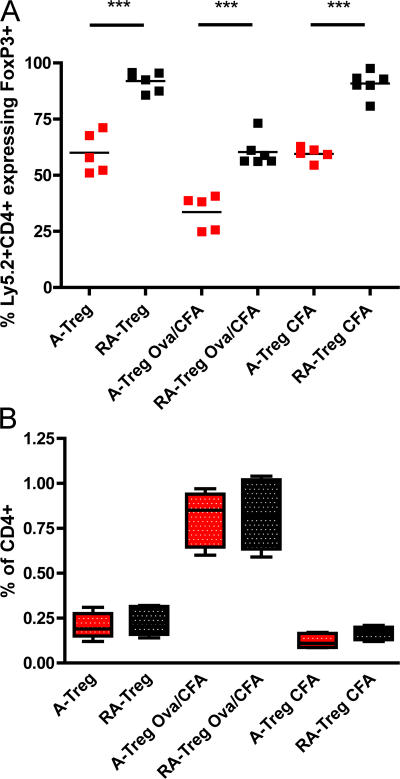
Figure 5.
RA–T reg cells are refractory to reversion in vivo. The ability of RA–T reg cells and A-Tregs to revert in vivo was analyzed by transferring 1.5–2 × 106 FACS-sorted congenically marked OTII+ RA–T reg cells or A-Tregs generated from a Ly5.2+OTII+Foxp3/GFP mice into Ly5.1+ hosts, who were either left untouched or intraperitoneally injected on the same day with either CFA or 1 mg OVA/CFA, as indicated. Donor RA–T reg cells or A-Tregs within the spleen were analyzed for Foxp3 expression on day 5 by staining for CD4+Ly5.2+ cells. RA–T reg cells are red, whereas A-Tregs are black (A). Horizontal lines represent the means. To examine relative donor T cell expansion 5 d after transfer, the percentage of donor OTII+ cells within the recipient splenic CD4+ compartment is shown in a box and whisker plot depicting the median, 25th, and 75th percentiles and range of values (B). Data are pooled from n = 3 independent experiments. ***, P < 0.001.
Co-stimulation and A-T reg cell generation: RA allows DCs to drive TGF-β1–mediated FoxP3 induction
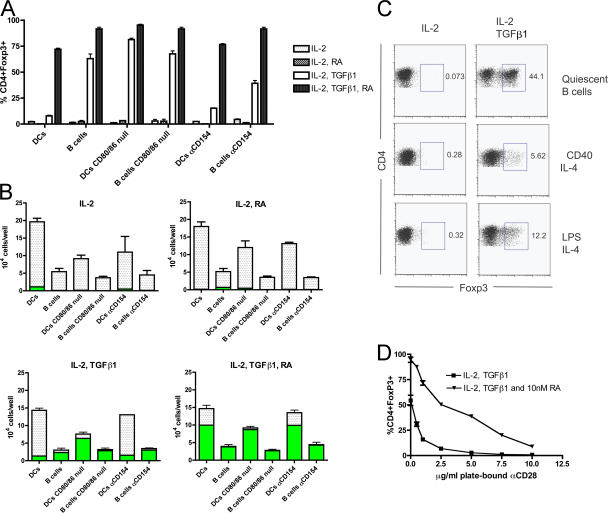
To investigate the capacity of different APC subsets to induce A-Treg generation in vitro, peptide-pulsed splenic CD19+ B cells and CD11c+ DCs were analyzed for their ability to drive TGF-β1–dependent FoxP3 induction in CD4+OTII+FoxP3− cells harvested and purified by sorting from the OTII+FoxP3/GFP mouse. A difference was observed between these two APC subsets to mediate conversion: B cells repeatedly induced conversion at rates between 40–60%, with DCs inducing conversion at substantially lower rates of 0–14% (Fig. 3 A; and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20070719/DC1), consistent with a previous study (25). The ability of B cells and DCs to mediate conversion was further analyzed by titrating exogenous TGF-β1, further sub stantiating the differential capacity of these APC subsets to medi ate T reg cell generation (Fig. S1).
Figure 3.
DC expression of CD80/86 co-stimulatory molecules prevents Foxp3 induction by T cells, with exogenous RA overcoming this inhibition and allowing T reg cell generation. Sorted CD4+OTII+Foxp3− T cells from the OTII+Foxp3/GFP reporter mouse (100,000 T cells/well) were cultured with either purified WT or CD80/86 knockout CD19+ B cells or CD11c+ DCs (100,000 APCs/well) pulsed with ISQ peptide, or under activating conditions. Media was supplemented with 100 U IL-2, ±20 ng/ml TGF-β1, ±1 nM RA, ±10 μg/ml αCD154 (unless otherwise indicated) for 5 d. (A) WT or CD80/86 knockout splenic B cells or DCs plus T cells were cultured under the depicted culture conditions, and the percentage of CD4+ T cells expressing Foxp3/GFP was measured. (B) Same experimental conditions as in A, except with absolute CD4+ T cells per well counted, with white bars representing CD4+Foxp3− cells and green bars representing CD4+Foxp3+ cells. (C) Freshly harvested B cells (quiescent B cells) or 48-h preactivated B cells with the indicated stimulus were co-cultured with T cells, and the percentage of CD4+ cells expressing Foxp3/GFP was measured after 5 d. (D) CD4+ T cells were activated using 10 μg/ml of plate-bound αCD3 and titrating concentrations of αCD28, with media supplemented with IL-2 and TGF-β1 ±10 nM RA as indicated; Foxp3/GFP expression was plotted as a function of plate-bound αCD28 concentration. Mean ± SEM is depicted in A, B, and D.
When the total number of CD4+ T cells per well after culture was quantified, DCs generated far more CD4+ T cells per well then B cells (Fig. 3 B). This was expected, given the differing capacities of these APC subsets to activate T cells through their differential co-stimulatory molecule expression, with DCs far superior at activating CD4+ T cells than resting B cells (26). We hypothesized that the capacity of APCs to activate T cells through co-stimulatory molecule expression, such as CD80/86, is inversely correlated with their ability to support the generation of A-Tregs. By extension of this hypothesis, the greater the absolute yield of daughter T cells generated by an APC, the fewer the number of FoxP3-expressing T cells. To test this hypothesis directly, we examined the ability of CD80/86 knockout splenic DCs and B cells to mediate both conversion and T cell expansion. The CD80/86 knockout DCs were able to induce TGF-β1–dependent FoxP3 expression in CD4+ T cells at a substantially increased rate in comparison with WT DCs (81.33% [SD = 2.31] compared with 7.9% [SD = 1.14]; Fig. 3 A). There was no change in the ability of WT B cells versus CD80/86 knockout B cells to mediate conversion (63% [SD = 7.81] and 67.66% [SD = 5.03], respectively), which is expected because quiescent B cells express very low basal levels of CD80/86 (26).
The in vitro and in vivo activation of both B cells and DCs through CD40 engagement by its ligand, CD154, increases the expression of CD80/86 by these APCs. The inclusion of blocking αCD154 antibody to our co-cultures enabled DCs to have an increased ability to mediate conversion (7.94% [SD = 1.14] compared with 15.26% [SD = 0.55] with αCD154; Fig. 3 A). Similar results were observed using CD40 knockout DCs (unpublished data). Interestingly, αCD154 antibody appeared to decrease B cell–mediated conversion, possibly implying a requirement for very low levels of co-stimulation in this co-culture system (63% [SD = 7.81] compared with 39.33% [SD = 4.5] with αCD154).
We next addressed whether B cell activation and the acquisition of heightened co-stimulatory molecule expression impaired the ability to induce conversion. When B cells were preactivated for 48 h by either LPS or agonistic-αCD40 supplemented with IL-4 and then used as APCs, B cell–mediated conversion was impaired (Fig. 3 C).
When CD4+ T cells were activated using WT or CD80/86 knockout DCs in the presence of exogenous IL-2, considerably more T cells per well were generated by WT DCs in comparison with CD80/86 knockout DCs (19.64 × 104 cells [SD = 2.42 × 104] compared with 9.13 × 104 cells [SD = 2.48 × 104]; Fig. 3 B). The addition of RA or TGF-β1 or both did not affect this observed difference (Fig. 3 B). Upon the addition of TGF-β1 to these cultures, the decrease of T cell numbers between WT and CD80/86 knockout DCs inversely correlated with a substantial increase in the number of FoxP3-expressing T cells in the CD80/86 knockout group versus the WT group (6.35 × 104 cells [SD = 0.15 × 104] compared with 1.33 × 104 cells [SD = 0.25 × 104]; Fig. 3 B).
The addition of αCD154 did not lead to a considerable change in T cell numbers generated by DCs with the addition of exogenous IL-2 (19.64 × 104 [SD = 2.42 × 104] compared with 11.13 × 104 [SD = 7.75 × 104] with αCD154; Fig. 3 B). No difference in T cell number was again observed with the further addition of RA or TGF-β1, or both. Additionally, there was no considerable increase in the numbers of FoxP3-expressing cells because of the inclusion of αCD154 to our DC co-cultures supplemented with TGF-β1 (1.33 × 104 [SD = 0.25 × 104] compared with 1.56 × 104 [SD = 0.36 × 104]; Fig. 3 B). Despite the enhanced conversion imparted by αCD154 in our DC/T cell co-cultures in Fig. 3 A, this result was not entirely unexpected. First, even with a CD154/CD40 blockade, DCs will still express levels of CD80/86 that are sufficient in expanding T cells. Second, the increase in DC-mediated conversion caused by αCD154 may not be enough to net an absolute increase in T reg cell numbers in this experimental system.
B cells, CD80/86 knockout B cells, and B cells cultured with αCD154 all generated similar total numbers of T cells in all culture conditions. This was expected and is likely caused by the low levels of co-stimulatory molecule expression on the surface of resting B cells (26). Collectively, these data support our hypothesis that under conditions favoring conversion, the greater the absolute yield of T cells generated by an APC and the fewer the number of T cells expressing FoxP3. Further substantiating this is our finding that increasing levels of plate-bound co-stimulation inversely correlates with the absolute number of A-Tregs generated (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20070719/DC1).
To directly implicate CD28 as a major mediator affecting FoxP3 induction, we varied the magnitude of αCD28 co-stimulation in an in vitro assay in which plate-bound agonistic αCD28 was titrated against saturating concentrations of plate-bound αCD3. It was observed that αCD28 concentration had an inverse relationship with A-Treg generation, with optimal conversion occurring at αCD28 concentrations of 1 μg/ml and below. At saturating concentrations (10 μg/ml αCD28), no conversion was observed (Fig. 3 D). Collectively, these data show that high levels of CD80/86 co-stimulation, such as those observed on splenic CD11c+ DCs, impairs TGF-β1–driven FoxP3 induction and A-Treg generation while inducing maximal expansion of T cells. In the context of minimal to no CD80/86 co-stimulation, such as levels present on resting B cells and CD80/86 knockout DCs, little to no expansion occurs, with TGF-β1–dependent FoxP3 expression and T reg cell generation the result.
The addition of RA to our assays greatly enhanced both the percentage and total numbers of T cells expressing FoxP3 in a TGF-β1–dependent manner (Fig. 3, A and B; Fig. S1; and Fig. S2). Notably, the addition of RA to our DC/T cell co-cultures increased the percentage of T cells expressing FoxP3 from 7.94% (SD = 1.14) to 72% (SD = 2; Fig. 3 A). Additionally, the combination of RA with TGF-β1 resulted in a net increase in the numbers of FoxP3-expressing cells in all DC cultures in comparison with TGF-β1 alone (WT DCs: from 1.33 × 104 [SD = 0.25 × 104] to 9.96 × 104 [SD = 0.62 × 104]; CD80/86 knockout DCs: from 6.35 × 104 [SD = 0.15 × 104] to 8.7 × 104 [SD = 0.19 × 104]; DCs with αCD154: from 1.56 × 104 [SD = 0.36 × 104] to 9.9 × 104 [SD = 0.19 × 104]; Fig. 3 B and Fig. S1). Furthermore, RA reversed co-stimulation from suppressing, in dose-dependent manner, the net yield of FoxP3+ T cells, as concentrations of plate-bound αCD28 now positively correlate with FoxP3+ T cell numbers (Fig. S2). Across all concentrations of plate-bound αCD28, as well as in the presence of only a TCR signal with no αCD28, RA greatly enhanced the percentage of T cells expressing FoxP3 (Fig. 3 D). In this experiment, co-stimulation still affected FoxP3 induction in a dose-dependent manner, albeit to a substantially attenuated degree, as a result of the inclusion of RA. This suggests that RA lowers an as yet undefined threshold within the T cell, with this threshold determining whether the T cell can convert into a T reg cell. Thus, the presence of RA during antigen presentation allows TGF-β1–dependent FoxP3 induction to occur by T cells, even in the face of overt CD80/86 co-stimulation afforded by the DC.
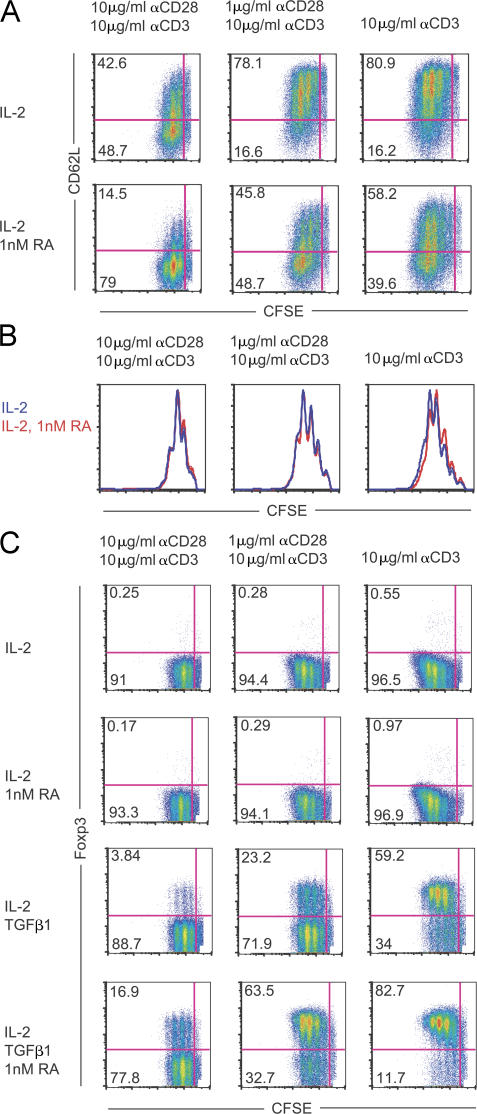
RA enhances FoxP3 expression without impeding T cell activation and proliferation
The observation that RA–T reg cells can be induced in the presence of high levels of co-stimulation led us the question whether this occurs by RA functioning to interrupt T cell activation and proliferation. To visualize this, CFSE-labeled CD4+CD25− cells were stimulated in the presence and absence of 1 nM RA, with T cell activation determined by CD62L expression and proliferation by CFSE dye dilution. The data shows that 1 nM RA does not suppress T cell activation, as indicated by CD62L expression. Instead, RA appears to enhance T cell activation, as indicated by the decreased expression of CD62L across the three levels of activating conditions tested (Fig. 4 A). Additionally, when CFSE histograms between different culture conditions are overlaid, equivalent profiles indicate that the addition of RA does not impede T cell proliferation (Fig. 4 B). The addition of TGF-β1 to these cultures generated FoxP3+ cells at expected frequencies throughout all peaks of cell division, with no impediment in cell division observed because of the presence of RA (Fig. 4 C). Importantly, RA enhances TGF-β1–mediated FoxP3 expression in the absence of any αCD28 signal and just the presence of αCD3 (Fig. 4 C and Fig. 3 D). Collectively, these data show that RA enhances FoxP3 induction and attenuates co-stimulation from interfering with T reg cell generation through a mechanism independent of dampening T cell activation and proliferation. Fur thermore, co-stimulation is not needed for RA to enhance FoxP3 induction.
Figure 4.
RA enhances Foxp3 expression without impeding T cell activation and proliferation. Purified CFSE-labeled CD4+CD25− T cells from a WT B6 mouse were cultured with IL-2, TGF-β1, and 1 nM RA, as indicated, under activating conditions for 4 d, after which CD62L and Foxp3 expression was measured. (A) CFSE dye dilution versus CD62L expression is shown, with T cells cultured with IL-2 or IL-2 plus 1 nM RA under different activating conditions. The percentage of T cells within each quadrant is depicted. (B) Histograms depicting CFSE dye dilution profiles of the data shown in A (IL-2, blue; IL-2 with 1 nM RA, red). (C) CFSE dye dilution versus Foxp3 expression is shown with the indicated activating and culture conditions, with the percentage of cells in each quadrant shown. Representative experiments of at least n = 3 mice are shown.
RA–T reg cells are refractory to reversion in vivo
RA is known to induce differentiation in a variety of primary and tumor cell types. As such, we addressed whether RA–T reg cells were more committed to the T reg cell lineage and less prone to revert to FoxP3− T cells then A-Tregs in vivo. To examine the propensity of A-Tregs and RA–T reg cells to revert under different in vivo conditions, these cells were generated from Ly5.2+OTII+FoxP3/GFP reporter mice, sorted to >99.9% FoxP3+, and transferred into Ly5.1+ recipients. Hosts were either unmanipulated, or immunized with OVA/CFA or PBS/CFA (Fig. 5 A). After 5 d, the transferred cells were analyzed for FoxP3 expression. Very few (9%) RA–T reg cells lost FoxP3 expression in both the unmanipulated or CFA only mice, indicating stability in RA–T reg cell differentiation even in the presence of inflammation. Surprisingly, a much larger percentage of A-Tregs lost FoxP3 expression in both unmanipulated and CFA only treated mice (40%) when compared with RA–T reg cells in similarly treated mice (9%). When injected with OVA/CFA, the frequency of FoxP3+ cells was reduced in both RA–T reg cell and A-Treg populations at similar rates. As expected, these CD4+OTII+ cells expanded within the recipients upon antigen injection (Fig. 5 B). In conclusion, RA–T reg cells were more refractory to reversion then A-Tregs under unmanipulated and inflammatory conditions, yet antigen encounter with inflammation drove partial reversion in both groups.
The data presented in this report shows that RA is a powerful inducer of FoxP3 expression and enhances commit ment to the T reg cell lineage. The inclusion of RA during the generation of A-Tregs (a) imprints a gut-homing capacity, (b) allows FoxP3 induction in the presence of high levels of co-stimulation by attenuating the inhibitory impact of co-stimulation on FoxP3 induction, (c) increases the frequency and total numbers of FoxP3+ T cells, and (d) induces T reg cells that are more refractory to reversion in vivo. Additionally, we implicate CD80/86 expression by APCs as a major mediator of A-Treg generation, as graded ligation of CD28 on the CD4+ T cell surface has an inverse relationship with the capacity of that T cell to express FoxP3 in a TGF-β1–dependent manner. Collectively, these data underscore the potentially overwhelming impact of DCs producing RA within the gut on the fate of activated T cells. Despite their propensity to mediate A-Treg generation, it is unlikely that B cells contribute to dual gut imprinting and A-Treg generation. Unlike gut-derived DCs, B cells harvested from the spleen, Peyer's patches, or mesenteric lymph nodes do not imprint a gut-homing phenotype on CD4+ T cells (unpublished data). Because of this, B cells are not likely to secrete RA in vivo.
The implications of these data are important. First, Powrie et al. and Agace et al. jointly described CD103+CD11c+ DCs present within the mesenteric lymph nodes, Peyer's Patches, and small and large bowel lamina propria as composing the DC subset responsible for mediating gut imprinting of T cells through CCR9 and α4β7 induction. Second, these CD103+ DCs likely imprint through an RA-dependent mechanism, although this has not yet been directly shown (9, 10). Therefore, our data suggest a new role for RA in mediating not only gut imprinting but also in driving gut tolerance through RA–T reg cell induction. Finally, with the use of A-Tregs in cellular adoptive therapies on the horizon, the use of RA in vitro to facilitate the production of fully committed, gut-homing A-Tregs is an attractive technology to meet the needs for high numbers of these cells in the therapeutic treatment of autoimmune disorders such as inflammatory bowel disease.
MATERIALS AND METHODS
Mice and immunizations.
This study was approved by the Institutional Animal Care and Use Committee of Dartmouth College. C57BL/6 and C57BL/6 CD45.1 (Ly5.2+) mice were purchased from the National Cancer Institute. C56BL/6 CD40−/− mice were purchased from the Jackson Laboratory. FoxP3/GFP reporter mice were previously described and were provided by A. Rudensky (University of Washington School of Medicine, Seattle, WA) (20). FoxP3/GFP mice were bred onto OTII TCR-Tg mice purchased from the Jackson Laboratory. CD80/86 double knockout mice on the C57BL/6 background were a gift from E. Usherwood (Dartmouth Medical School, Lebanon, NH). All animals were maintained in a pathogen-free facility at Dartmouth Medical School. For immunizations, OVA (Sigma-Aldrich) resuspended in PBS or PBS alone was emulsified in CFA (Sigma-Aldrich) at a 1:1 ratio and intraperitoneally injected in a volume of 200 μl.
Cell preparation.
B cells and DCs were harvested from the spleens, and CD4+FoxP3− cells were isolated from spleens and peripheral and mesenteric lymph nodes. For B cell and T cells, single-cell suspensions were generated by crushing organs by sterile slides and were purified by positive selection using either αCD4- or αCD19-labeled magnetic beads (Miltenyi Biotec). T cells were further purified by FACS sorting (FACSAria; BD Biosciences) of the CD4FoxP3/GFP− fraction, with purity always >99.9%. For CD11c+ selection, spleens were harvested and incubated at 37°C in RPMI 1640 with 50 μg/ml DNase I (Sigma-Aldrich) and 250 μg/ml Liberase (Roche) for 30 min, pushed through a 100-μM filter to create a single-cell suspension, and purified using αCD11c magnetic beads (Miltenyi Biotec). Cell preparations were always >98% in purity. For small intestine lamina propria lymphocyte preparations, intestines were removed, and Peyer's Patches were excised and used for further analysis. The intestines were washed with cold PBS, split open, and cut into 1-cm pieces. After 30 min of incubation in unsupplemented RPMI to release the intestinal epithelial lymphocytes, intestines were vortexed, filtered using a 100-μM filter, and extensively washed. Intestines were digested for 2 h using 50 μg/ml DNase I and 250 μg/ml Liberase, after which they were pushed through a 100-μM filter. The cellular suspension was centrifuged and suspended in 40% Percoll, and overlaid on 60% Percoll. The Percoll gradient was spun at 400 g at room temperature for 25 min with no brake, with the buffy (lymphocyte) coat removed for further use. As indicated in the figures, APCs were pulsed with ISQ peptide at 10 μg/ml for 1 h in cRPMI in sixwell plates at a concentration of 10 × 106 cells/ml, and were washed, counted, and used.
Cell culture/reagents.
Cells were cultured in RPMI 1640 media supplemented with 10% FBS (Atlanta Biologicals), Hepes, 50 μM β-ME, and penicillin/streptomycin/l-glutamine. LPS was purchased from Sigma-Aldrich. αCD40 (clone FGK-45), αCD154 (clone MR1), αCD28 (clone PV-1), and αCD3 (clone 2C11) were purchased from ISC BioExpress. LE540 was purchased from Wako Chemicals. For 96-well plate cultures, 200,000 cells in APC/T cell co-cultures (1:1 ratio) in round-bottom plates or 100,000 T cells in flat-bottom plates were cultured in 200 μl of media. In each experiment, triplicate wells of each experimental condition were set up. For the bulk RA–T reg cell and A-Treg cultures in Fig. 2 and Fig. 5, 24-well flat-well plates were used with 100,000 CD4+FoxP3− cells/well in 1 ml of media. For all cultures, unless indicated otherwise in the figures, T cells were activated with 1 μg/ml αCD28 and 10 μg/ml αCD3 plate-bound antibody in the presence of 20 ng/ml hTGF-β1 (PeproTech), 100 U hIL-2 (PeproTech), and RA (Sigma-Aldrich). For the in vitro suppressor assay, 50,000 CFSE-labeled CD4+ T cells were co-cultured with 100,000 irradiated T cell–depleted splenocytes, 5 μg/ml αCD3, and the numbers of T reg cells indicated in the figures for 4 d, as previously described (27, 28). For Fig. 3 and Fig. S2, cells were counted by Guava according to instructions provided by the manufacturer (Guava Technologies)
Flow cytometry.
The following antibodies were used: CD11c (clone N418), CD25 (clone PC61), and CD62L (clone MEL-14; all obtained from BioLegend); B220 (clone 6B2), CD4 (clone L3T4), and α4β7 (clone DATK32; all obtained from BD Biosciences); CCR9 (clone 242503; obtained from R&D Systems); and CD103 (clone 2E7) and the FoxP3 intracellular staining kit (obtained from eBioscience). For CFSE dye dilution, cells were labeled with 5 μM CFSE (Invitrogen). Flow cytometric analysis was performed on a refurbished machine (FACScan; Becton Dickinson) running CellQuest software (BD Biosciences), and data analysis was performed using FlowJo software (TreeStar, Inc.).
Homing assay.
Competitive homing experiments of RA–T reg cells and A-Tregs were performed as previously described, with modifications (3). In brief, 10 × 106 RA–T reg cells generated from sorted CD4+FoxP3− cells purified from a Ly5.2+FoxP3/GFP reporter mouse were mixed with an approximately equal number of A-Tregs generated from FACS-sorted CD4+CD25− cells harvested from a Ly5.2+ mouse (determined to be ∼20% FoxP3+, as gauged by intracellular FoxP3 staining) and intravenously injected into Ly5.1+ C57BL/6 mice. An aliquot was saved to determine the input ratio: IR = (GFP+)input/(GFP−)input). The homing index (HI) was calculated as the ratio of (GFP+)tissue/(GFP−)tissue to IR. Homing indices were tested versus HI = 1 using a one-sample Wilcoxon signed-rank test. Significance was set at P < 0.05.
Online supplemental material.
Fig. S1 shows that DCs, with the addition of exogenous RA, can induce FoxP3 expression in T cells in a TGF-β1–dependent manner and over a dose titration of TGF-β1. Fig. S2 shows that the addition of exogenous RA to TGF-β1 and IL-2 induces a net increase in the total number of T reg cells generated under various levels of plate-bound co-stimulation. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20070719/DC1.
Supplemental Material
[Supplemental Material Index]
Acknowledgments
We would like to thank Dr. David Gondek for reading and commenting on the manuscript and Dr. Ethan Dmitrovsky for scientific advice.
This work was supported by National Institutes of Health grants to CA123079 and AI048667 (to R.J. Noelle).
The authors have no conflicting financial interests.
References
- 1.Izcue, A., J.L. Coombes, and F. Powrie. 2006. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 212:256–271. [DOI] [PubMed] [Google Scholar]
- 2.Bouma, G., and W. Strober. 2003. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 3:521–533. [DOI] [PubMed] [Google Scholar]
- 3.Mora, J.R., M.R. Bono, N. Manjunath, W. Weninger, L.L. Cavanagh, M. Rosemblatt, and U.H. von Andrian. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 424:88–93. [DOI] [PubMed] [Google Scholar]
- 4.Mora, J.R., G. Cheng, D. Picarella, M. Briskin, N. Buchanan, and U.H. von Andrian. 2005. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 201:303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlin, C., E.L. Berg, M.J. Briskin, D.P. Andrew, P.J. Kilshaw, B. Holzmann, I.L. Weissman, A. Hamann, and E.C. Butcher. 1993. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 74:185–195. [DOI] [PubMed] [Google Scholar]
- 6.Wagner, N., J. Lohler, E.J. Kunkel, K. Ley, E. Leung, G. Krissansen, K. Rajewsky, and W. Muller. 1996. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 382:366–370. [DOI] [PubMed] [Google Scholar]
- 7.Zabel, B.A., W.W. Agace, J.J. Campbell, H.M. Heath, D. Parent, A.I. Roberts, E.C. Ebert, N. Kassam, S. Qin, M. Zovko, et al. 1999. Human G protein–coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190:1241–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mora, J.R., and U.H. von Andrian. 2006. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 27:235–243. [DOI] [PubMed] [Google Scholar]
- 9.Annacker, O., J.L. Coombes, V. Malmstrom, H.H. Uhlig, T. Bourne, B. Johansson-Lindbom, W.W. Agace, C.M. Parker, and F. Powrie. 2005. Essential role for CD103 in the T cell–mediated regulation of experimental colitis. J. Exp. Med. 202:1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson-Lindbom, B., M. Svensson, O. Pabst, C. Palmqvist, G. Marquez, R. Forster, and W.W. Agace. 2005. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202:1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwata, M., A. Hirakiyama, Y. Eshima, H. Kagechika, C. Kato, and S.Y. Song. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 21:527–538. [DOI] [PubMed] [Google Scholar]
- 12.Cobbold, S.P., R. Castejon, E. Adams, D. Zelenika, L. Graca, S. Humm, and H. Waldmann. 2004. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J. Immunol. 172:6003–6010. [DOI] [PubMed] [Google Scholar]
- 13.Chen, W., W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park, H.B., D.J. Paik, E. Jang, S. Hong, and J. Youn. 2004. Acquisition of anergic and suppressive activities in transforming growth factor-beta-costimulated CD4+CD25− T cells. Int. Immunol. 16:1203–1213. [DOI] [PubMed] [Google Scholar]
- 15.Fantini, M.C., C. Becker, I. Tubbe, A. Nikolaev, H.A. Lehr, P. Galle, and M.F. Neurath. 2006. Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 55:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochando, J.C., C. Homma, Y. Yang, A. Hidalgo, A. Garin, F. Tacke, V. Angeli, Y. Li, P. Boros, Y. Ding, et al. 2006. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 7:652–662. [DOI] [PubMed] [Google Scholar]
- 17.Zheng, S.G., J.H. Wang, J.D. Gray, H. Soucier, and D.A. Horwitz. 2004. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J. Immunol. 172:5213–5221. [DOI] [PubMed] [Google Scholar]
- 18.Fantini, M.C., C. Becker, G. Monteleone, F. Pallone, P.R. Galle, and M.F. Neurath. 2004. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172:5149–5153. [DOI] [PubMed] [Google Scholar]
- 19.Karim, M., C.I. Kingsley, A.R. Bushell, B.S. Sawitzki, and K.J. Wood. 2004. Alloantigen-induced CD25+CD4+ regulatory T cells can develop in vivo from CD25−CD4+ precursors in a thymus-independent process. J. Immunol. 172:923–928. [DOI] [PubMed] [Google Scholar]
- 20.Fontenot, J.D., J.P. Rasmussen, L.M. Williams, J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341. [DOI] [PubMed] [Google Scholar]
- 21.Smith, T.J., L.A. Ducharme, S.K. Shaw, C.M. Parker, M.B. Brenner, P.J. Kilshaw, and J.H. Weis. 1994. Murine M290 integrin expression modulated by mast cell activation. Immunity. 1:393–403. [DOI] [PubMed] [Google Scholar]
- 22.Cepek, K.L., C.M. Parker, J.L. Madara, and M.B. Brenner. 1993. Integrin alpha E beta 7 mediates adhesion of T lymphocytes to epithelial cells. J. Immunol. 150:3459–3470. [PubMed] [Google Scholar]
- 23.Huehn, J., K. Siegmund, J.C. Lehmann, C. Siewert, U. Haubold, M. Feuerer, G.F. Debes, J. Lauber, O. Frey, G.K. Przybylski, et al. 2004. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 199:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Racke, M.K., D. Burnett, S.H. Pak, P.S. Albert, B. Cannella, C.S. Raine, D.E. McFarlin, and D.E. Scott. 1995. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J. Immunol. 154:450–458. [PubMed] [Google Scholar]
- 25.Kim, J.M., and A. Rudensky. 2006. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol. Rev. 212:86–98. [DOI] [PubMed] [Google Scholar]
- 26.Ho, W.Y., M.P. Cooke, C.C. Goodnow, and M.M. Davis. 1994. Resting and anergic B cells are defective in CD28-dependent co-stimulation of naive CD4+ T cells. J. Exp. Med. 179:1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton, A.M., and E.M. Shevach. 1998. CD4+CD25+ immunoregu latory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969–1980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Material Index]