AP-2/Eps15 Interaction Is Required for Receptor-mediated Endocytosis (original) (raw)
Abstract
We have previously shown that the protein Eps15 is constitutively associated with the plasma membrane adaptor complex, AP-2, suggesting its possible role in endocytosis. To explore the role of Eps15 and the function of AP-2/Eps15 association in endocytosis, the Eps15 binding domain for AP-2 was precisely delineated. The entire COOH-terminal domain of Eps15 or a mutant form lacking all the AP-2–binding sites was fused to the green fluorescent protein (GFP), and these constructs were transiently transfected in HeLa cells. Overexpression of the fusion protein containing the entire COOH-terminal domain of Eps15 strongly inhibited endocytosis of transferrin, whereas the fusion protein in which the AP-2–binding sites had been deleted had no effect. These results were confirmed in a cell-free assay that uses perforated A431 cells to follow the first steps of coated vesicle formation at the plasma membrane. Addition of Eps15-derived glutathione-S-transferase fusion proteins containing the AP-2–binding site in this assay inhibited not only constitutive endocytosis of transferrin but also ligand-induced endocytosis of epidermal growth factor. This inhibition could be ascribed to a competition between the fusion protein and endogenous Eps15 for AP-2 binding. Altogether, these results show that interaction of Eps15 with AP-2 is required for efficient receptor-mediated endocytosis and thus provide the first evidence that Eps15 is involved in the function of plasma membrane–coated pits.
Receptor-mediated endocytosis plays a major role in the regulation of cellular functions such as uptake of nutrients and downregulation of growth factor receptors (Sorkin and Waters, 1993; Mellman, 1997). Most of the known membrane receptors are internalized from the cell surface through specialized structures of the plasma membrane called coated pits, characterized by the presence of a coat on the cytosolic side of the plasma membrane. The receptors undergoing endocytosis are first recruited and concentrated into coated pits and internalized as a result of the progressive invagination of the coated pits that pinch off from the membrane to give coated vesicles (Smythe and Warren, 1991).
The AP-2 complex, one of the major identified components of the coat along with clathrin, plays a central role in both the organization and the function of plasma membrane coated pits (Kirchhausen, 1993; Robinson, 1994). AP-2 is an heterotetramer composed of two large chains, the α- and β2-adaptins (100 kD), a medium chain μ2 (50 kD), and a small chain σ2 (17 kD). α-Adaptin is necessary for the targeting of AP-2 to the plasma membrane (Robinson, 1993; Page and Robinson, 1995) and seems to modulate the functions of AP-2 through interactions with phosphoinositides (Beck and Keen, 1991; Gaidarov et al., 1996). β2-adaptin binds to clathrin and is involved in the assembly of the clathrin lattice (Gallusser and Kirchhausen, 1993; Shih et al., 1995). The μ2 subunit binds to tyrosine-based endocytic signals present within the cytosolic tail of endocytosed receptors (Boll et al., 1996; Ohno et al., 1995, 1996).
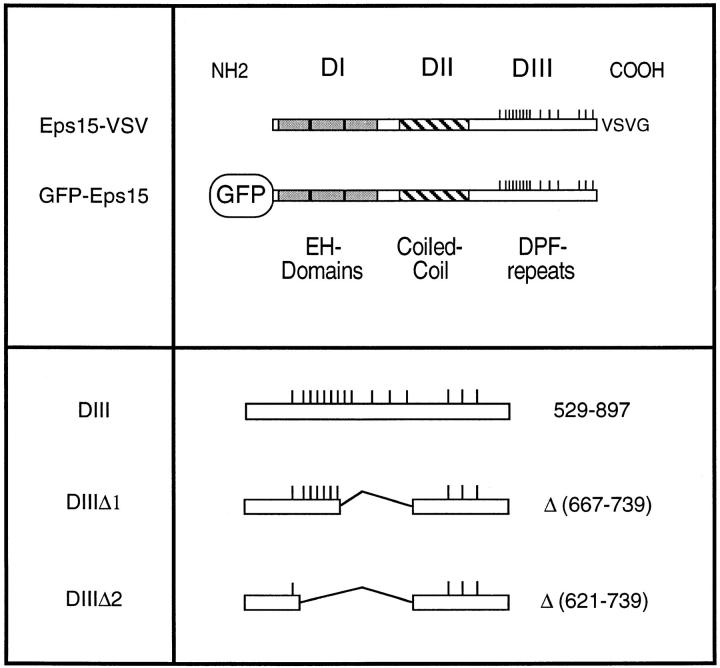
Recently, we have shown that AP-2 is constitutively associated with Eps15 (Benmerah et al., 1995, 1996), a highly conserved protein organized in three distinct structural domains (Fazioli et al., 1993; Wong et al., 1994) (Fig. 1). Its NH2-terminal domain (DI) is composed of three imperfect repeats of 70 amino acids homologous to each other and to domains found in proteins in mammals, yeast, and nematodes. These domains, called EH for Eps15 Homology (Wong et al., 1995), were recently shown to mediate interaction with other proteins via an NPF motif (Salcini et al., 1997). Moreover, two yeast proteins containing EH domains, End3p and Pan1p, have been implicated in the internalization step of endocytosis (Benedetti et al., 1994; Wendland et al., 1996). The central domain of Eps15 (DII) is characterized by the presence of heptads required for coiled-coil structures and is involved in the homodimerization of Eps15 (Tebar et al., 1997). The COOH-terminal domain (DIII) is characterized by the presence of repeats of the DPF sequence and contains the AP-2–binding site (Benmerah et al., 1996; Iannolo et al., 1997). The fact that Eps15 is associated with AP-2 and is homologous to yeast proteins involved in endocytosis suggested a possible role for Eps15 in coated pit–mediated endocytosis. This hypothesis is supported by two studies showing a colocalization of Eps15 with both clathrin and AP-2 in vivo, and the presence of Eps15 in plasma membrane coated pits and vesicles (Tebar et al., 1996; van Delft et al., 1997).
Figure 1.
Structural organization of Eps15 and Eps15 constructs.
However, direct evidence for a role for Eps15 in clathrin-mediated endocytosis has been lacking. To assess the role of Eps15 in the function of plasma membrane coated pits, we have transiently transfected wild-type and mutant constructs of Eps15 and tested their effect on endocytosis. The inhibition obtained in living cells with the mutant constructs was confirmed in a cell-free assay that also allowed us to define at which step along the endocytic pathway the Eps15/AP-2 interaction is required.
Materials and Methods
Cells, Antibodies, and Ligands
HeLa cells were grown in RPMI supplemented with 10% FCS, 2 mM L-glutamine, penicillin, and streptomycin. A431 cells were cultured in DME containing 10% defined-FCS (Hyclone, Aalst, Belgium). After trypsinization, 4 × 106 cells were seeded onto 15-cm culture dishes 20–24 h before preparing perforated cells, as previously described (Smythe et al., 1992).
The production and characterization of the 6G4 anti–Eps15 mAb has been previously reported (Benmerah et al., 1995). Mouse mAb P5D4 (IgG1) against the epitope YTDIEMNRLGK of the G protein of vesicular stomatitis virus (VSV)1 was a gift of Dr. T. Kreis (University of Geneva, Geneva, Switzerland; Kreis, 1986). Mouse mAb AC1-M11 (IgG2a) against the NH2-terminal domain of α-adaptin and mAb AP-6 (IgG2b) against the COOH-terminal domain of α-adaptin were provided by Drs. M.S. Robinson (University of Cambridge, Cambridge, UK) and F. Brodsky (University of California, San Francisco, CA), respectively (Robinson, 1987; Chin et al., 1989). mAb 100/2, against the COOH-terminal domain of α-adaptin, mAb 100/1 (IgG1), against the NH2 terminus of β-adaptin, and mAb 100/3 (IgG2A), against γ-adaptin (Ahle et al., 1988), were purchased from Sigma Immunochemicals (Sigma, Saint Quentin Fallavier, France). FITC-conjugated goat anti–mouse IgG1, Texas red–conjugated goat anti–mouse IgG2a, and anti–mouse IgG2b and anti–mouse IgG antisera, were obtained from Southern Biotechnology Associates Inc. (Birmingham, AL).
Biotinylated transferrin (B-Tf) was prepared as previously described (Lamaze et al., 1993) using sulfo-NHS-SS-biotin (6-[{6-[biotinoyl] amino} hexanoyl] amino) hexanoic acid, sulfosuccinimidyl ester, sodium salt) obtained from Molecular Probes (Eugene, OR). Biotinylated epidermal growth factor (B-EGF) was purchased from Boehringer-Mannheim S.A. (Meylan, France).
Plasmids
The cDNA of human eps15 subcloned in pBluescript II KS (Stratagene, La Jolla, CA) was obtained in the laboratory (Benmerah et al., 1995) and used as a template to generate the different cDNA fragments used in this study.
The glutathione-S-transferase (GST) fusion proteins were derived from eps15 using the GST gene fusion system and the PGEX5.1 vector (_Pharmacia_-LKB, Les Ulis, France) as previously described (Benmerah et al., 1995, 1996). Briefly, a BamHI and an XhoI site were introduced in the upper and lower primers, respectively, to allow subcloning of the PCR products in the PGEX5.1 vector in frame with the GST moiety. Production of fusion proteins in DH5α bacteria and purification were performed as described (Benmerah et al., 1995, 1996).
The green fluorescent protein (GFP) fusion proteins were derived from the corresponding Eps15-derived GST fusion protein constructs. The different domains of Eps15, or the full-length sequence of Eps15 subcloned in PGEX5.1, were excised using the BamHI/XhoI sites, purified on agarose gel, and then cloned into the BamHI/XhoI sites of the pCDNA3 vector (Invitrogen, Leek, The Netherlands). We then used the XbaI site downstream from the XhoI site to generate BamHI/XbaI fragments that were then cloned into the BamHI and XbaI of the EGFP-C2 vector (CLONTECH, Palo Alto, CA). This vector allowed us to generate Eps15-derived fusion proteins with the GFP at the NH2 terminus end in the same open reading frame as in the PGEX5.1 vector.
The VSV-tagged form of Eps15 was generated using PCR by introducing a SmaI site starting at the stop codon of Eps15 and the VSV-G epitope was introduced between the SmaI/XbaI sites. The construct was then subcloned into the BamHI/XbaI sites of the pCDNA3 vector (Invitrogen).
All the constructs were checked by nucleotide sequencing (Thermosequenase; Amersham, Les Ulis, France). Sequences of the used primers are available on request.
Biochemical Procedures
HeLa cells were lysed in 50 mM Tris-HCl, pH 8, 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, containing a mix of protease inhibitors (4 mM PMSF, 10 μg/ml aprotinin, leupeptin, pepstatin, 50 μg/ml trypsin inhibitor [_Sigma_]).
For precipitation, cell lysates were cleared with protein A–Sepharose or GST coupled to glutathione–Sepharose 4B beads (_Pharmacia_-LKB), and then incubated overnight with mAb 6G4 (10 μg/3 × 107 cells) coupled to protein A–Sepharose (_Pharmacia_-LKB) or with GST fusion proteins (5–10 μg/107 cells) coupled to glutathione–Sepharose 4B beads (20–30 μl/ 107 cells). Precipitated proteins were separated by SDS-PAGE under reducing conditions.
For Western blotting, acrylamide gels were transferred onto nitrocellulose membranes (Schleicher & Schüell, Keene, NH) in 10 mM Tris, 0.2 M glycine, and 30% methanol. Nonspecific binding sites were blocked by incubation in Tris-HCl, pH 7.6, containing 5% BSA and 0.2% Tween (Sigma). The blots were then sequentially incubated for 1 h with mouse mAbs at the indicated dilutions, followed by peroxidase-labeled sheep anti–mouse antiserum (1:20,000) (Amersham). Labeled bands were revealed using enhanced chemiluminescence (Amersham).
Transfections and Immunofluorescence
Subconfluent HeLa cells were used for transient expression of the different constructs. Transfections were performed using the CalPhos Maximizer Transfection Kit (CLONTECH).
For immunofluorescence studies, transfected HeLa cells were grown on coverslips and used 1 or 2 d after transfection. The cells were washed in PBS and fixed in 3.7% paraformaldehyde and 0.03 M sucrose for 15 min at room temperature. The cells were then washed once in PBS, and after quenching for 10 min in 50 mM NH4Cl in PBS, were washed again in PBS supplemented with 1 mg/ml BSA. The cells were then incubated with the different mAbs in permeabilization buffer (PBS with 1 mg/ml BSA and 0.05% saponin) for 45 min at room temperature. After two washes in the permeabilizing buffer, the presence of antibodies was revealed by incubating the cells for 45 min at room temperature in permeabilizing buffer containing labeled secondary antibodies. After two washes in permeabilizing buffer and one wash in PBS, the cells were mounted on microscope slides in 25 mg/ml Dabco (Sigma), 100 mg/ml Mowiol (Calbiochem-Novabiochem, La Jolla, CA), 25% glycerol (vol/vol), 100 mM Tris-HCl, pH 8.5.
The samples were examined under an epifluorescence microscope (Axiophot; Carl Zeiss, Oberkochen, Germany) attached to a cooled CCD camera (Photometrics, Tucson, AZ).
No immunofluorescence staining was ever observed when secondary antibodies were used without the first antibody or with an irrelevant first antibody.
Endocytosis
Endocytosis of Texas red–conjugated Tf (Molecular Probes, Eugene, OR) was performed on subconfluent HeLa cells grown on coverslips 1 or 2 d after transfection. The cells were first incubated for 30 min at 37°C in RPMI 20 mM Hepes, pH 7.2, to eliminate endogenous Tf, and then incubated in RPMI 20 mM Hepes, pH 7.2, 1 mg/ml BSA containing 100 nM Texas red–conjugated Tf. After incubation at 37°C for the indicated times, the cells were rapidly cooled to 4°C, washed twice in cold PBS, and then fixed as described above.
Cell-free Assays for Receptor-mediated Endocytosis
Perforated A431 cells were prepared and endocytosis assays were performed as previously described (Carter et al., 1993; Lamaze et al., 1993). Briefly, incubations were performed at 37°C for 30 min in 40-μl reaction volumes containing an ATP-regenerating system, 6 mg/ml cytosol (prepared from K562 human erythroleukemic cells), 2 μg/ml B-Tf, 10 ng/ml B-EGF, 0.2% BSA in KSHM assay buffer (100 mM K-acetate, 85 mM sucrose, 20 mM Hepes, pH 7.4, 1 mM Mg-acetate). Reactions were stopped on ice, cells were pelleted in a refrigerated microfuge, and the supernatants were carefully aspirated.
Measurement of Avidin Inaccessibility.
The cell pellets were resuspended in avidin (50 μg/ml) and processed as described to mask surface accessible B-Tf or B-EGF (Carter et al., 1993; Lamaze et al., 1993). The avidin reagent was quenched with biocytin, and detergent cell lysates were plated onto ELISA microtiter wells coated with anti-Tf (Boehringer-Mannheim) or anti-EGF antibodies (Upstate Biotechnology, Inc., Lake Placid, NY).
Measurement of β-Mercaptoethane Sulfonate (MesNa) Resistance.
Incubations were performed in the presence of 4 μg/ml disulfate bond–biotinylated transferrin (B-SS-Tf) for 15 min at 37°C. The cell pellets were resuspended in 50 μl of 10 mM MesNa. The tubes were agitated at 4°C. At 30 min, 12 μl of 50 mM MesNa was added to each tube, and at 60 min this was supplemented with 18 μl of 50 mM MesNa. The MesNa solutions were prepared just before each addition in 100 mM NaCl, 1 mM EDTA, 50 mM Tris, 0.2% BSA, pH 8.6. After 90 min, the MesNa was oxidized by the addition of 25 μl of 500 mM iodoacetic acid. After a 10 min agitation, the membranes were solubilized and lysates were plated onto ELISA microtiter wells coated with anti-Tf as described above.
ELISA-based Detection of Biotinylated Ligand.
Cell-associated B-Tf and B-EGF, or B-SS-Tf, which were inaccessible to avidin or MesNa, respectively were quantitated using streptavidin coupled to HRP (Boehringer-Mannheim) as described (Carter et al., 1993; Lamaze et al., 1993). Results are presented as the total cell-associated B-Tf, B-EGF, or B-SS-Tf (determined from cells not treated with avidin or MesNa) endocytosed in an ATP- and cytosol-dependent manner (i.e., backgrounds obtained from incubations in the absence of cytosol and in the presence of hexokinase and glucose, which typically were 10–20% of totals, have been subtracted). All assays were performed in duplicate and the results differed by <5%.
To block the effects of GST-DIII, purified AP-2 was added to the perforated cells at 4°C. Purified AP-2 was obtained from bovine brain–coated vesicles prepared as described previously (Smythe et al., 1992).
Results
Effect of the Overexpression of Tagged Eps15 Proteins on the Endocytosis of Transferrin in Intact HeLa Cells
To define the possible role of Eps15 in endocytosis, we have generated tagged forms of Eps15 by introducing the VSV-G epitope at the COOH-terminal end (Eps15-VSV) or the GFP at the NH2-terminal end (GFP-Eps15) (Fig. 1). The constructs were transiently transfected in HeLa cells and 2 d after transfection, the distribution of the two different constructs was analyzed by immunofluorescence: the Eps15-VSV construct was detected using the P5D4 mAb specific to the VSV-tag and GFP-Eps15 was directly visualized by the green fluorescence emitted by GFP (not shown and Fig. 2 a, respectively). Both fusion proteins displayed a similar punctate staining typical of the distribution of plasma membrane–coated pits and comparable with the staining previously described for endogenous Eps15 (Tebar et al., 1996; van Delft et al., 1997). Moreover, in cells expressing GFP-Eps15 or Eps15-VSV, as reported for the endogenous Eps15 (Tebar et al., 1996; van Delft et al., 1997), the tagged proteins colocalized with AP-2 (Fig. 2, a and b).
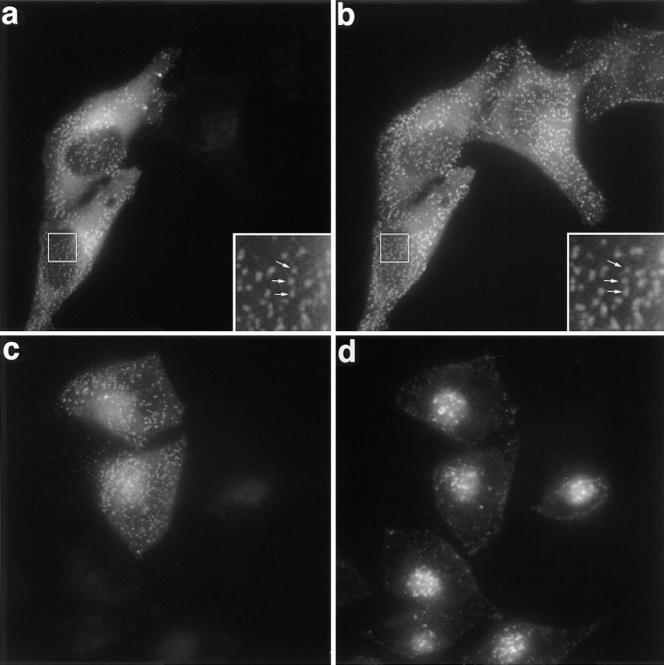
Figure 2.
Subcellular distribution of transfected GFP-Eps15 and effect on Tf endocytosis. (a and b) HeLa cells grown on coverslips were transiently transfected with GFP-Eps15, fixed, and then stained for AP-2 using the AP-6 antibody. The GFP-Eps15 fusion protein was visualized directly by the green fluorescence emitted by the GFP (a). AP-6 antibody was detected with a secondary anti-IgG2b antibody labeled with Texas red (b). The inserts show a higher magnification of the same area. Arrows point to representative spots stained in both green and red (a and b). (c and d) HeLa cells transiently transfected with GFP-Eps15 were incubated in the presence of 100 nM Texas red–conjugated Tf for 15 min at 37°C, washed twice in cold PBS, and then fixed. (c) Green fluorescence emitted by the GFP construct and (d) Texas red–conjugated Tf. a and b, and c and d represent the same fields.
The constitutive presence of Eps15 in plasma membrane–coated pits strongly suggested its role in endocytosis. To test this hypothesis, we first examined whether overexpression of Eps15 could affect the endocytosis of transferrin, which has been extensively used as a marker of endocytosis via clathrin-coated pits. HeLa cells were transiently transfected with GFP-Eps15 and transfected cells were allowed to internalize Texas red–conjugated Tf for 15 min at 37°C. As shown in Fig. 2, endocytosis of Tf did not appear to be modified in cells expressing GFP-Eps15, and there were no morphological differences between the compartments loaded with Texas red–conjugated Tf in transfected and untransfected cells (compare Fig. 2, c and d). Identical results were obtained using the Eps15-VSV construct (not shown). These results show that the overexpression of wild-type Eps15 does not affect Tf endocytosis.
The Expression of the COOH-terminal Domain of Eps15 Has a Dominant-negative Effect on Tf Endocytosis in Intact HeLa Cells
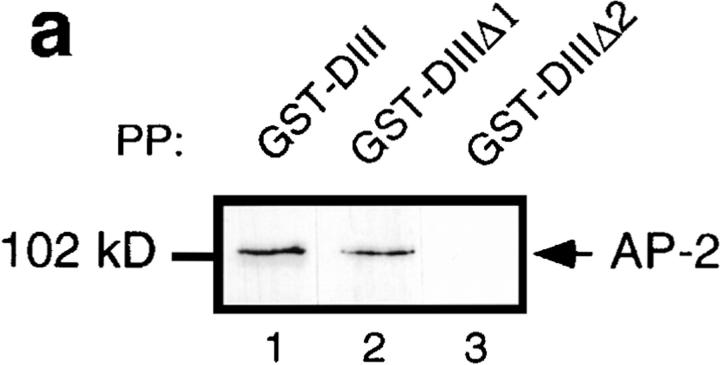
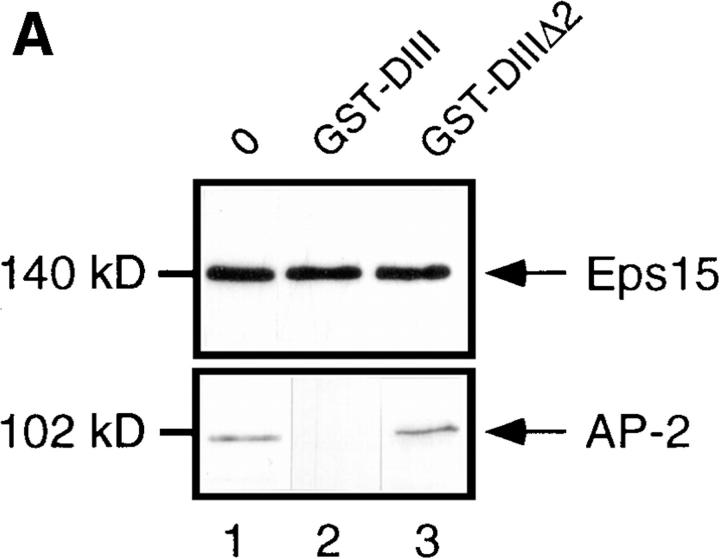
We have previously shown that residues 667–739 of the Eps15 COOH-terminal domain bind directly to the ear of α-adaptin. However, the contribution of amino acids 621– 667 could not be excluded (Benmerah et al., 1996). To assess their potential contribution to the binding of AP-2, internal deletions were introduced into GST-DIII, a GST protein containing the entire COOH-terminal domain of Eps15 (Fig. 1), and the fusion proteins were used to precipitate AP-2. As shown in Fig. 3 a, AP-2 was precipitated by GST-DIII (Fig. 3, lane 1). It was also precipitated, albeit much less efficiently, by GST-DIIIΔ1 (Fig. 3, lane 2), a construct lacking amino acids 667–739. In contrast, GST-DIIIΔ2, a construct lacking amino acids 621–739, failed to precipitate AP-2 (Fig. 3, lane 3), confirming that amino acids 621–667 present an additional binding site for AP-2, in agreement with results obtained by Iannolo and coworkers (Iannolo et al., 1997).
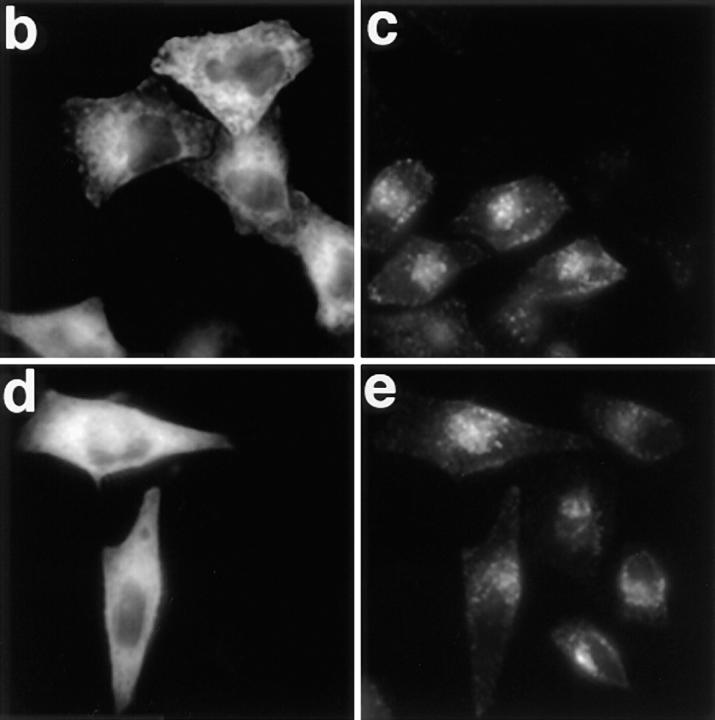
Figure 3.
Overexpression of the Eps15-binding site for AP-2 inhibits Tf endocytosis in vivo. (a) Different GST fusion proteins encoding either the COOH-terminal domain of Eps15 or deleted forms of this domain (Fig. 1) were used to precipitate cell lysates (PP). The presence of AP-2 in the precipitates was revealed by Western blotting using the anti–α-adaptin antibody 100/2. HeLa cells were transiently transfected with the GFP-DIII construct (b and c) or with the GFP-DIIIΔ2 construct lacking all the AP-2–binding sites (d and e). Transfected cells were incubated in the presence of 100 nM Texas red–conjugated Tf for 15 min at 37°C, and washed twice with cold PBS. Left panels represent the green fluorescence emitted by the GFP constructs, and the right panels represent the staining observed for Texas red–conjugated Tf. b and c, and d and e represent the same fields.
These results were used to design GFP fusion proteins containing or missing the Eps15 AP-2–binding sites. DIII, the COOH-terminal domain of Eps15, and DIIIΔ2, lacking all the AP-2–binding sites, were transferred from the PGEX5.1 vector into the EGFP-C2 vector, to generate the corresponding GFP fusion proteins. HeLa cells were transiently transfected with these constructs, and transfected cells were tested for their capacity to internalize Texas red–conjugated Tf. As shown in Fig. 3, cells expressing GFP-DIIIΔ2 internalized Tf as well as untransfected cells (Fig. 3, compare d and e) or cells transfected with GFP alone (not shown). In contrast, HeLa cells transfected with GFP-DIII failed to internalize Tf (Fig. 3, compare b and c), suggesting that the expression of the COOH-terminal domain of Eps15 containing the AP-2–binding site inhibited the internalization of Tf.
The COOH-terminal Domain of Eps15 Inhibits Receptor-mediated Endocytosis in Perforated A431 Cells
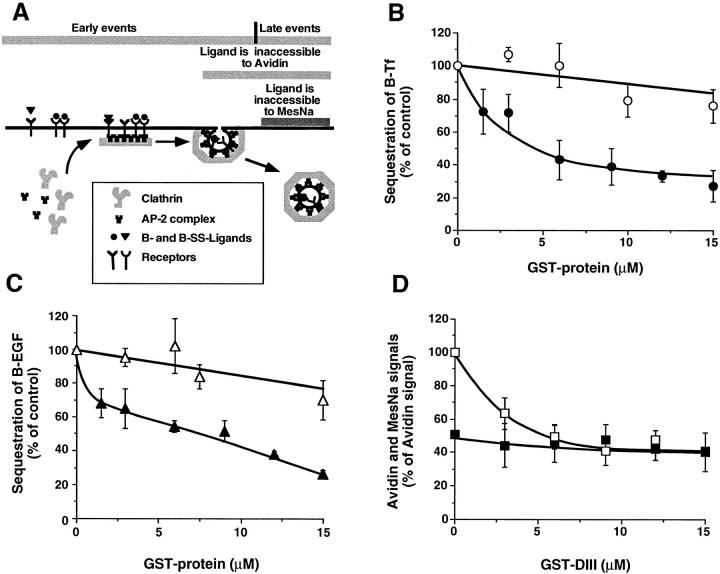
To confirm the results obtained in vivo and define which step along the endocytic pathway was blocked in cells overexpressing the COOH-terminal domain of Eps15, the fusion proteins were tested in a cell-free assay designed to dissect the formation of coated vesicles from the plasma membrane (Fig. 4 A). This assay uses human A431 cells that are perforated mechanically to wash out the endogenous cytosol. In the presence of cytosol and an ATP- regenerating system, B-Tf or B-EGF are sequestered into deeply invaginated, coated pits and become inaccessible to avidin (Lamaze et al., 1993; Schmid, 1993). Since perforated cells remain fully accessible to exogenously added reagents, Eps15-derived GST fusion proteins could be introduced to test their effect on endocytosis. The data in Fig. 4 B show that the GST-DIII fusion protein containing the AP-2–binding sites significantly inhibited B-Tf sequestration. This inhibition was dose dependent, the half-maximal inhibition occuring at ∼3 μM GST-DIII, and a plateau was reached at ∼60% inhibition in the presence of ⩾9 μM GST-DIII. The inhibitory effect of GST-DIII was specific and required the presence of the AP-2–binding site since the GST-DIIIΔ2 that does not bind AP-2 had no effect (Fig. 4 B). To further test the role of Eps15 in receptor-mediated endocytosis, we next studied the effect of DIII on B-EGF sequestration. When GST-DIII was tested on EGF endocytosis in perforated cells, we found that B-EGF endocytosis was inhibited within the same range of concentrations needed for Tf inhibition. Furthermore, no inhibition was observed at these concentrations for GST-DIIIΔ2 that lacks AP-2–binding sites (Fig. 4 C). Altogether, these results show that addition of the COOH-terminal domain of Eps15 in the in vitro assay inhibits both Tf and EGF endocytosis, two prototypes of constitutive and ligand-induced, receptor-mediated endocytosis, respectively. Furthermore, they also suggest that this domain inhibits the formation of clathrin-coated vesicles.
Figure 4.
GST-DIII inhibits both EGF and Tf endocytosis in perforated A431 cells. (A) Schematic representation of the perforated cell assay and the different steps measured by the inaccessibility to avidin and to MesNa. (B and C) The sequestration of B-Tf (B) and B-EGF (C) was measured in perforated A431 cells under standard assay conditions (see Materials and Methods) in the presence of increasing concentrations of GST-DIII (closed symbols) or GST-DIIIΔ2 (open symbols). (D) The sequestration of B-SS-Tf into constricted coated pits and coated vesicles was detected by the inaccessibility to avidin (□), whereas the sequestration of B-SS-Tf into coated vesicles only is measured by the resistance to MesNa (▪). The experiments were performed as in B and C, except that the incubation at 37°C was shortened to 15 min. The data represent averages (±SD) of three experiments.
The inaccessibility of B-Tf to avidin, i.e., sequestration, can occur as a result of its inclusion into either constricted coated pits that remain plasma membrane associated and/or coated vesicles, representing early and late events in coated vesicle formation, respectively (Fig. 4 A). The data presented in Fig. 4 B show that the inhibition of the avidin signal by GST-DIII is not complete; thus GST-DIII could either partially inhibit both early and late events of coated vesicle formation, or inhibit solely early or late events. To discriminate between early and late events of clathrin-coated vesicle formation, we used a modified version of the in vitro assay for Tf endocytosis. The late events are selectively detected when Tf, biotinylated through a cleavable disulfide bond (B-SS-Tf), becomes inaccessible to the small membrane–impermeable reducing agent, β-mercaptoethane sulfonate (MesNa) (Schmid, 1993). The effect of the GST-DIII construct was studied in the same experiment, both on the avidin and the MesNa signals. As previously described (Carter et al., 1993; Lamaze et al., 1993), the MesNa signal in perforated A431 cells is about half of the avidin signal (Fig. 4 D). This MesNa signal was not inhibited by GST-DIII at concentrations that inhibited the avidin signal (Fig. 4, B and D). Importantly, at the inhibitory concentrations of GST-DIII, the avidin signal was superimposed on the MesNa signal. Altogether, the data obtained using the in vitro assay strongly suggest that adding GST-DIII inhibits early, but not late events of coated vesicle formation.
The COOH-terminal Domain of Eps15 Competes with Endogenous Eps15 for AP-2 Binding
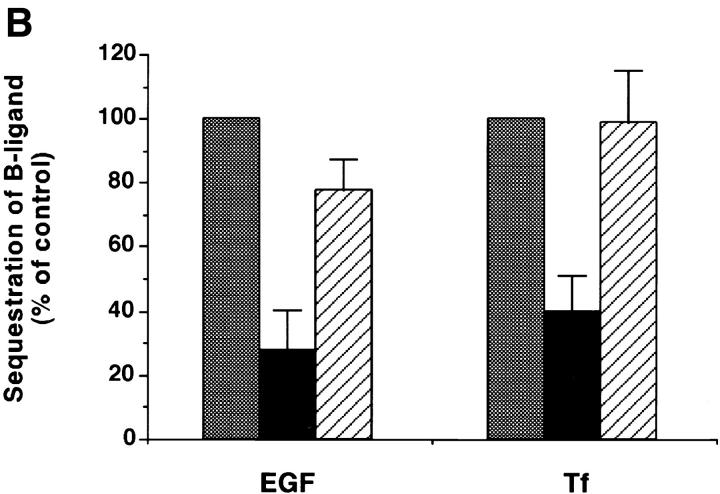
To test whether the inhibitory effect of the GST-DIII construct resulted from a competition between fusion proteins and endogenous Eps15 for AP2-binding, cell lysates were incubated in the presence of GST fusion proteins before and during precipitation with the anti-Eps15 antibody, 6G4. As previously shown, 6G4 coimmunoprecipitated AP-2 with endogenous Eps15 from untreated lysates and from lysates treated with 10 μM GST-DIIIΔ2 (Fig. 5 A, lanes 1 and 3). In contrast, the amount of AP-2 coimmunoprecipitated with endogenous Eps15 in lysates treated with 10 μM GST-DIII was markedly reduced (Fig. 5 A, lane 2), indicating that GST-DIII at a concentration that inhibits endocytosis in vitro (Fig. 4 B) competed with endogenous Eps15 for AP-2 binding. Further evidence that inhibition resulted from specific competition between GST-DIII and endogenous Eps15 for AP-2 binding is shown in Fig. 5 B. In this experiment, perforated A431 cells were incubated with inhibitory concentrations of GST-DIII in the presence of excess purified AP-2. As can be seen, the addition of purified AP-2 complexes blocked the inhibition of B-Tf and B-EGF endocytosis by GST-DIII. These results provide strong evidence that the DIII construct of Eps15 acts as a dominant-negative inhibitor of constitutive endocytosis by preventing Eps15 from binding to AP-2 complexes.
Figure 5.
GST-DIII competes with endogenous Eps15 for AP-2 binding. (A) Cell lysates were preincubated for 30 min on ice with no GST fusion protein (lane 1), or with 10 μM GST-DIII (lane 2), or GST-DIIIΔ2 (lane 3), and then immunoprecipitated with the anti-Eps15 antibody 6G4, specific for an epitope located in the NH2-terminal domain of Eps15, which does not recognize the GST-DIII construct (Benmerah et al., 1996). The presence of Eps15 and AP-2 in the precipitates was analyzed by Western Blotting using the 6G4 (top panel) or the 100/2 (bottom panel) antibody. (B) Sequestration assays for B-Tf and B-EGF were performed as described in Fig. 4 with 12 μM GST-DIII in the absence (black bars) or presence (striped bars) of purified AP-2 (0.3 mg/ml). Controls (gray bars) were done in the absence or presence of purified AP-2, to match the experimental conditions, in perforated A431 cells incubated without GST-DIII.
Discussion
Using two complementary approaches, we provide the first direct functional evidence that Eps15 is involved in constitutive receptor-mediated endocytosis of Tf, as well as ligand-induced endocytosis of the EGF, two receptors endocytosed via clathrin-coated pits. This finding is consistent with our previous discovery that Eps15 is constitutively associated with AP-2 (Benmerah et al., 1995) and the work of others showing that Eps15 is localized in coated pits (Tebar et al., 1996).
The possible role of Eps15 in coated pit–mediated endocytosis was first assessed by testing the effect of overexpressed wild-type constructs on Tf endocytosis; little or no difference in Tf endocytosis was detected between transfected and untransfected cells. The distribution of the transfected tagged forms of the full-length Eps15 was similar to that of the endogenous protein (Tebar et al., 1996; van Delft et al., 1997) and both GFP- and VSV-tagged Eps15 colocalized with AP-2. This suggests both that AP-2–binding sites for Eps15 were not saturated and that AP-2–independent membrane binding sites for Eps15 (van Delft et al., 1997) were also not saturated. The latter finding is consistent with our previous data showing that not all AP-2 complexes are associated with endogenous Eps15 (Benmerah et al., 1995).
Eps15 is associated with AP-2 in the various cell types and species that we have tested (Benmerah et al., 1995), suggesting that the association of Eps15 with AP-2 may play an important role in its function. This led us to generate mutant forms of Eps15 able to interfere with endogenous Eps15/AP-2 association. With this goal in mind, we first more precisely defined the AP-2–binding site of Eps15. Our previous data obtained with GST fusion proteins showed that the 621–739 region of Eps15 bound AP-2 very efficiently. Further trimming of this segment to amino acids 667–739 reduced AP-2 binding. We therefore concluded that amino acids 667–739 contain an AP-2–binding site, whereas amino acids 621–666 are required for the correct presentation and/or conformation of the 667–739 region (Benmerah et al., 1996). However, a GST fusion protein containing the COOH-terminal domain of Eps15 but lacking amino acids 667–739 was still able to precipitate AP-2. This result suggested the presence of an additional binding site in the 621–666 region. Accordingly, deletion of the entire 621–739 region completely abolished AP-2 binding. These results indicate that efficient AP-2 binding is not because of a unique binding site, but instead results from several elementary interacting sites located within the 621–739 region. A similar conclusion was reached by Iannolo and coworkers who identified three distinct AP-2–binding sites located around residues 650–660, 680–690, and 720–730 of the COOH-terminal domain of Eps15 (Iannolo et al., 1997).
To test the effect of the Eps15-binding site for AP-2 in live cells, the Eps15 moieties of the GST-Eps15 constructs used to precipitate AP-2 were fused to the GFP and transiently expressed in HeLa cells. Endocytosis of Tf was strongly inhibited by overexpression of the entire COOH-terminal domain of Eps15 but not by constructs in which all the AP-2–binding sites were deleted. These results strongly suggest that disruption of the Eps15/AP-2 association inhibits the function of plasma membrane–coated pits. However, a possible inhibitory effect on a later step along the Tf receptor trafficking pathway could not be excluded from these experiments.
The ability to reproduce the inhibitory effect of the DIII construct in perforated A431 cells allowed us to confirm the specific role of the Eps15/AP-2 interaction during the formation of clathrin-coated vesicles. In this in vitro assay, endocytosis of both Tf and EGF were strongly inhibited by GST fusion proteins containing intact DIII, but not by fusion proteins lacking the AP-2–binding site. This effect resulted from a competition between fusion proteins and endogenous Eps15 for AP-2 binding. Significantly, inhibition of Tf sequestration reached a plateau at ∼60%, suggesting that late events in coated vesicle formation might not be inhibited by GST-DIII. This hypothesis was confirmed by measuring the inaccessibility of B-SS-Tf to MesNa, i.e., its sequestration into coated vesicles (Schmid, 1993). Altogether these results show that Eps15 is involved in the early steps of coated vesicle formation, including coated pit formation and invagination.
A previous study using a kinase-deficient mutant of the EGF-receptor (EGF-R) and a constitutively activated, soluble form of the EGF-R tyrosine kinase has shown that the recruitment of activated EGF-R into coated pits in perforated cells depends on the presence of a cytosolic substrate(s) of the EGF-R kinase other than the receptor itself (Lamaze and Schmid, 1995). The fact that Eps15 was initially cloned as a substrate of the EGF-R kinase makes it an attractive candidate as an EGF-R recruiter. This possibility was strengthened by the recent finding that Eps15 was coimmunoprecipitated with EGF-R after the specific stimulation by EGF, and that this association was not observed with an endocytosis-deficient mutant of the EGF-R (van Delft et al., 1997). However, several lines of evidence make such a role for Eps15 unlikely. First, Eps15 is expressed and associated with AP-2 in cells such as lymphocytes lacking EGF-R (Benmerah et al., 1995). Second, EGF treatment does not modify the distribution of Eps15 into coated pits nor Eps15/AP-2 interaction (Tebar et al., 1996; van Delft et al., 1997). Therefore, the coimmunoprecipitation of Eps15 with activated EGF-R could be an indirect consequence of their independent binding to AP-2 (Sorkin and Carpenter, 1993; Benmerah et al., 1995). Finally, our findings showing that endocytosis of EGF and Tf are both inhibited by DIII suggests instead that Eps15 functions as a general effector in the plasma membrane– coated pit machinery.
Altogether, our previous results showing constitutive and ubiquitous association of Eps15 with AP-2 and the data presented in this study strongly suggest that the functions of AP-2 and Eps15 are closely linked. The fact that Eps15 appears to bind to the plasma membrane in the absence of AP-2 (van Delft et al., 1997) may point to a role for Eps15 in the recruitment of AP-2 to the plasma membrane. This is in apparent contradiction with the fact that Eps15 binds AP-2 through the ear of α-adaptin (Benmerah et al., 1996; Iannolo et al., 1997), which seems to have only a minor role in targeting AP-2 to the plasma membrane (Robinson, 1993; Page and Robinson, 1995). Alternatively, Eps15 might regulate the interaction of AP-2 with other proteins capable of binding the ear of α-adaptin, such as amphiphysin (David et al., 1996), which in turn regulates the localization of dynamin to the neck of the invaginated coated pit (Shupliakov et al., 1997).
In addition to AP-2, Eps15 binds to other NPF motif– containing proteins through its NH2-terminal EH domains (Salcini et al., 1997). Data from yeast have shown that the EH domains play a critical role in the function of End3p (Benedetti et al., 1994), and are also present in pan1p, which is also involved in endocytosis (Wendland et al., 1996; Tang et al., 1997), suggesting an important functional role for EH domains in endocytosis. Our preliminary results indicate that the introduction of the Eps15 EH domains in perforated cells strongly inhibit Tf endocytosis and that they are required for the correct recruitment of Eps15 to the plasma membrane (Benmerah, A., C. Lamaze, N. Cerf-Bensussan, A. Dautry-Varsat, manuscript in preparation), suggesting that EH domains and their binding proteins may also play a critical role in endocytosis in mammals. The results presented in this study show that preventing the AP-2/Eps15 interaction by the presence of DIII inhibits endocytosis, whereas overexpression of the whole Eps15 protein does not modify endocytosis. In inhibiting the Eps15/AP-2 interaction, the COOH-terminal domain of Eps15 would disrupt the bridge formed by Eps15 between EH domain–interacting proteins and AP-2, resulting in an inhibition of coated vesicle formation. Therefore, Eps15 is likely to organize a multimolecular complex, including AP-2, necessary for proper coated vesicle formation.
Acknowledgments
We thank F. Brodsky, M. Robinson, and T. Kreis for the gift of the AP-6, AC1-M11, and P5D4 mAbs, respectively; E. Morelon and F. Niedergang for helpful discussions; K. Thébaud, V. Mallardé, and V. Collin for expert technical assistance; and D. Ojcius for critical reading of the manuscript.
This work was supported by HFSP grant number RG404/96 (to A. Dautry-Varsat and S.L. Schmid), by the Association pour la Recherche sur le Cancer (N. Cerf-Bensussan) and the CNRS (Programme Biologie Cellulaire). We are grateful to the Ligue Nationale Contre le Cancer, Comité de Paris, for support to purchase a CCD camera. A. Benmerah was supported by a fellowship from the Association pour la Recherche sur le Cancer (Villejuif, France) and C. Lamaze by the USAMRMC grant No. DAMD17-94-J-4031.
Abbreviations used in this paper
B-EGF
biotinylated epidermal growth factor
B-SS-Tf
disulfide bond–biotinylated transferrin
B-Tf
biotinylated transferrin
EGF and EGF-R
epidermal growth factor and receptor
GFP
green fluorescent protein
GST
glutathione-S-transferase
Tf
transferrin
Tf-R
transferrin receptor
VSV
vesicular stomatitis virus
Footnotes
A. Benmerah and C. Lamaze contributed equally to this work.
Address all correspondence to Dr. N. Cerf-Bensussan, Institut National de la Santé et de la Recherche Médicale, U 429. Hôpital Necker-Enfants Malades, 149, Rue de Sèvres, 75743 Paris Cedex 15, France. Tel.: 33-1-44-49-50-82. Fax: 33-1-40-61-32-38. E-mail: cerf@necker.fr
References
- Ahle S, Mann A, Eichelsbacher U, Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO (Eur Mol Biol Organ) J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KA, Keen JH. Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J Biol Chem. 1991;266:4442–4447. [PubMed] [Google Scholar]
- Benedetti H, Raths S, Crausaz F, Riezman H. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol Biol Cell. 1994;5:1023–1037. doi: 10.1091/mbc.5.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A, Gagnon J, Bègue B, Mégarbané B, Dautry-Varsat A, Cerf-Bensussan N. The tyrosine kinase substrate Eps15 is constitutively associated with the plasma membrane adaptor AP-2. J Cell Biol. 1995;131:1831–1838. doi: 10.1083/jcb.131.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A, Bègue B, Dautry-Varsat A, Cerf-Bensussan N. The ear of α-adaptin interacts with the COOH-terminal domain of the Eps15 protein. J Biol Chem. 1996;271:12111–12116. doi: 10.1074/jbc.271.20.12111. [DOI] [PubMed] [Google Scholar]
- Boll W, Ohno H, Zhou SY, Rapoport I, Cantley LC, Bonifacino JS, Kirchhausen T. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin Ap-2 complexes. EMBO (Eur Mol Biol Organ) J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- Carter LL, Redelmeier TE, Woollenweber LA, Schmid SL. Multiple GTP-binding proteins participate in clathrin-coated vesicle-mediated endocytosis. J Cell Biol. 1993;120:37–45. doi: 10.1083/jcb.120.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin DJ, Straubinger RM, Acton S, Nathke I, Brodsky FM. 100-kDa polypeptides in peripheral clathrin-coated vesicles are required for receptor-mediated endocytosis. Proc Natl Acad Sci USA. 1989;86:9289–9293. doi: 10.1073/pnas.86.23.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C, McPherson PS, Munndigl O, De Camilli P. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci USA. 1996;93:331–335. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazioli F, Minichiello L, Matoskova B, Wong WT, Di Fiore PP. eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol Cell Biol. 1993;13:5814–5828. doi: 10.1128/mcb.13.9.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov I, Chen Q, Falck JR, Reddy KK, Keen JH. A functional phosphatidylinositol 3,4,5-trisphosphate/phosphoinositide binding domain in the clathrin adaptor Ap-2 α subunit - implications for the endocytic pathway. J Biol Chem. 1996;271:20922–20929. doi: 10.1074/jbc.271.34.20922. [DOI] [PubMed] [Google Scholar]
- Gallusser A, Kirchhausen T. The β1 and β2 subunits of the AP complexes are the clathrin coat assembly components. EMBO (Eur Mol Biol Organ) J. 1993;12:5237–5244. doi: 10.1002/j.1460-2075.1993.tb06219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannolo C, Salcini AE, Gaidarov I, Goodman OB, Baulida J, Carpenter G, Pelicci PG, Di Fiore PP, Keen JH. Mapping of the molecular determinants involved in the interaction between Eps15 and AP-2. Cancer Res. 1997;57:240–245. [PubMed] [Google Scholar]
- Kirchhausen T. Coated pits and coated vesicles—sorting it all out. Curr Opin Struct Biol. 1993;3:182–188. [Google Scholar]
- Kreis T. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO (Eur Mol Biol Organ) J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C, Schmid SL. Recruitment of epidermal growth factor receptors into coated pits requires their activated tyrosine kinase. J Cell Biol. 1995;129:47–54. doi: 10.1083/jcb.129.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C, Baba T, Redelmeier TE, Schmid SL. Recruitment of epidermal growth factor and transferrin receptors into coated pits in vitro: differing biochemical requirements. Mol Biol Cell. 1993;4:715–727. doi: 10.1091/mbc.4.7.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1997;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Page LJ, Robinson MS. Targeting signals and subunit interactions in coated vesicle adaptor complexes. J Cell Biol. 1995;131:619–630. doi: 10.1083/jcb.131.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. 100-kD coated vesicle proteins: Molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J Cell Biol. 1987;104:887–895. doi: 10.1083/jcb.104.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Assembly and targeting of adaptin chimeras in transfected cells. J Cell Biol. 1993;123:67–77. doi: 10.1083/jcb.123.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Salcini AE, Confalonieri S, Doria M, Santolini E, Tassi E, Minenkova O, Cesareni G, Pelicci PG, Difiore PP. Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes Dev. 1997;11:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL. Coated vesicle formation in vitro: conflicting results using different assays. Trends Cell Biol. 1993;3:145–148. doi: 10.1016/0962-8924(93)90129-o. [DOI] [PubMed] [Google Scholar]
- Shih W, Gallusser A, Kirchhausen T. A clathrin-binding site in the hinge of the β2 chain of mammalian AP-2 complexes. J Biol Chem. 1995;270:31083–31090. doi: 10.1074/jbc.270.52.31083. [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- Smythe E, Warren G. The mechanism of receptor-mediated endocytosis. Eur J Biochem. 1991;202:689–699. doi: 10.1111/j.1432-1033.1991.tb16424.x. [DOI] [PubMed] [Google Scholar]
- Smythe E, Carter LL, Schmid SL. Cytosol- and clathrin-dependent stimulation of endocytosis in vitro by purified adaptors. J Cell Biol. 1992;119:1163–1171. doi: 10.1083/jcb.119.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, Carpenter G. Interaction of activated EGF receptors with coated pit adaptins. Science. 1993;261:612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Waters CM. Endocytosis of growth factor receptors. Bioessays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- Tang HY, Munn A, Cai MJ. EH domain proteins Pan1p and End3p are components of a complex that plays a dual role in organization of the cortical actin cytoskeleton and endocytosis in saccharomyces cerevisiae. Mol Cell Biol. 1997;17:4294–4304. doi: 10.1128/mcb.17.8.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebar F, Sorkina T, Sorkin A, Kirchhausen T. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J Biol Chem. 1996;271:28727–28730. doi: 10.1074/jbc.271.46.28727. [DOI] [PubMed] [Google Scholar]
- Tebar F, Confalonieri S, Carter RE, Di Fiore PP, Sorkin A. Eps15 is constitutively oligomerized due to homophilic interaction of its coiled-coil region. J Biol Chem. 1997;272:15413–15418. doi: 10.1074/jbc.272.24.15413. [DOI] [PubMed] [Google Scholar]
- van Delft S, Schumacher C, Hage W, Verkleij AJ, Henegouwen P. Association and colocalization of Eps15 with adaptor protein-2 and clathrin. J Cell Biol. 1997;136:811–821. doi: 10.1083/jcb.136.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B, McCaffery JM, Xiao Q, Emr SD. A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homolog of mammalian Eps15. J Cell Biol. 1996;135:1485–1500. doi: 10.1083/jcb.135.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WT, Kraus MH, Carlomagno F, Zelano A, Druck T, Croce CM, Huebner K, Di Fiore PP. The human eps15 gene, encoding a tyrosine kinase substrate, is conserved in evolution and maps to 1p31-p32. Oncogene. 1994;9:1591–1597. [PubMed] [Google Scholar]
- Wong WT, Schumacher C, Salcini AE, Romano A, Castagnino P, Pelicci PG, Di Fiore PP. A protein-binding domain, EH, identified in the receptor tyrosine kinase substrate Eps15 and conserved in evolution. Proc Natl Acad Sci USA. 1995;92:9530–9534. doi: 10.1073/pnas.92.21.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]