The Vitronectin Receptor and its Associated CD47 Molecule Mediates Proinflammatory Cytokine Synthesis in Human Monocytes by Interaction with Soluble CD23 (original) (raw)
Abstract
The vitronectin receptor, αvβ3 integrin, plays an important role in tumor cell invasion, angiogenesis, and phagocytosis of apoptotic cells. CD47, a member of the multispan transmembrane receptor family, physically and functionally associates with vitronectin receptor (VnR). Although vitronectin (Vn) is not a ligand of CD47, anti-CD47 and β3 mAbs suppress Vn, but not fibronectin (Fn) binding and function. Here, we show that anti-CD47, anti-β3 mAb and Vn, but not Fn, inhibit sCD23-mediated proinflammatory function (TNF-α, IL-12, and IFN-γ release). Surprisingly, anti-CD47 and β3 mAbs do not block sCD23 binding to αv +β3 + T cell lines, whereas Vn and an αv mAb (clone AMF7) do inhibit sCD23 binding, suggesting the VnR complex may be a functional receptor for sCD23. sCD23 directly binds αv +β3 +/CD47− cell lines, but coexpression of CD47 increases binding. Moreover, sCD23 binds purified αv protein and a single human αv chain CHO transfectant. We conclude that the VnR and its associated CD47 molecule may function as a novel receptor for sCD23 to mediate its proinflammatory activity and, as such, may be involved in the inflammatory process of the immune response.
Keywords: CD47, CD23, vitronectin receptor, TNF-α, IFN-γ
The vitronectin receptor (αvβ3) is an ubiquitous receptor that interacts with several ligands, such as vitronectin (Vn)1, fibronectin (Fn), osteopontin, and metalloproteinase MMP-2 (for review see Felding-Habermann and Cheresh, 1993; Brooks et al., 1996). As a consequence, this integrin plays a role in diverse biologic processes such as cell migration, tumor invasion, bone resorption, angiogenesis, and immune responsiveness (Gladson and Cheresh, 1994).
During the process of inflammation, circulating human monocytes are able to leave the blood by attaching to, and migrating through, endothelial and subendothelial matrices to the site of injury. The vitronectin receptor (VnR) αvβ3, expressed on endothelial cells, is involved in the transendothelial migration process (Brown and Lindberg, 1996) together with PECAM (CD31) (Buckley et al., 1996) and CD47 (Cooper et al., 1995). When recruited at the inflammatory or infected sites, phagocytes undergo a number of physiological changes including increases in adhesiveness, production of reactive oxygen metabolites, and augmentation in phagocytosis. Extracellular matrix proteins containing RGD sequence peptides have been shown to mediate some of these functions (Hynes, 1992), especially activation of phagocytic burst (Zhou and Brown, 1993), and enhancement of ingestion of opsonized particles by monocytes (Gresham et al., 1989).
The CD47 Ag is a widely expressed 50-kD multispan transmembrane protein component of the αvβ3 and leukocyte response integrin signaling complex, since its expression was shown to enhance Vn, but not Fn, binding and function to a variety of cells (Brown et al., 1990; Rosales et al., 1992; Lindberg et al., 1993). It was reported that CD47 does not directly bind Vn, and CD47− cell lines, expressing αvβ3, failed to bind Vn-coated beads (Lindberg et al., 1996b). Moreover, CD47 deficient mice rapidly died of Escherichia coli peritonitis, a phenomenon directly correlated with a reduction in leukocyte activation in response to β3, but not β2, integrin ligation (Lindberg et al., 1996a). The αvβ3/CD47 trimolecular complex also participates in the resolution of inflammation by mediating phagocytosis of aging leukocytes undergoing apoptosis before they disgorge their potentially harmful contents (Savill et al., 1990). This process is potentiated by the synthesis of proinflammatory cytokine such as GM-CSF, TNF-α, IL-1, and IFN-γ (Ren and Savill, 1995).
CD23 has been purported to play a role in inflammation based upon its in vitro proinflammatory activity (Armant et al., 1994; Lecoanet-Henchoz et al., 1995; Bonnefoy et al., 1996; Sarfati, 1997) and the observation that soluble CD23 (sCD23) levels increased in various chronic inflammatory disorders, including rheumatoid arthritis and systemic lupus erythematosus (Ikizawa et al., 1993; Bertero et al., 1994). sCD23 (Armant et al., 1994; Leconaet-Henchoz et al., 1995) and CD23 ligation (Bonnefoy et al., 1996) can trigger monokine release by human monocytes. Our studies have demonstrated that sCD23-induced TNF-α secretion costimulates IFN-γ production by IL-2–activated T cells cocultured with syngeneic monocytes in the absence of T cell receptor ligation (Armant et al., 1995).
Here, we report a novel function for VnR and its associated CD47 molecule on monocytes, by demonstrating that this trimolecular complex mediates proinflammatory cytokine synthesis via interaction with CD23. This may contribute to the perpetuation of the inflammatory process in chronic disorders such as rheumatoid arthritis. In this disease, CD23, TNF-α, and VnR expression are found to be locally elevated in the inflamed synovium (Ashton et al., 1993; Feldman et al., 1996).
Materials and Methods
Cell Lines and Reagents
Human recombinant IL-2, kindly provided by Dr. D. Bron (Institut Bordet, Brussels, Belgium), was used at 20 U/ml; IL-15 was obtained from Immunex and used at 200 ng/ml. Endotoxin-free (<15 pg/ml, as determined by the chromogenic Limulus amebocyte lysate, QCL-1000, BioWhittaker Inc.) affinity-purified sCD23 was prepared in our laboratory from CSN of CHO cell line transfected with human cDNA encoding for aa 148–321 of the CD23 molecule. The concentration of 25 ng/ml sCD23 used throughout this study was selected on the basis of previously reported dose– response curves (Armant et al., 1994). Jurkat T (αv +β3 +), THP-1 (αv −β3 −) monocytic, Raji (αv +β3 −), and Bowes melanoma (αv +β3 +) cell lines were obtained from the American Type Culture Collection (ATCC). K562 and K562 transfected with the cDNA encoding the full-length CR2 (K562-CR2) were a generous gift from Drs. A. Masumoto and D. Fearon (Johns Hopkins University, Baltimore, MD). CD47 deficient Jurkat T cell line and 0V10 ovarian carcinoma cell line were generated in Drs. E. Brown and F. Lindberg's laboratory (Washington University, St. Louis, MO). cDNA encoding for CD51 (αv chain) was a generous gift from Dr. E. Ruoslahti (Burnham Institute, La Jolla, CA). 10G2 mAb (IgM class) was produced in our laboratory following immunization of mice with Jurkat T cells. Hybridomas producing anti-CD47 (clone B6H12) and anti-β3 (CD61) mAbs (clone AP3) were purchased at the ATCC. Anti-αvβ3 (CD51/CD61, clone LM609), and anti-αv (CD51, clone LM142) were kindly provided by Dr. Cheresh (Scripps Research Institute, La Jolla, CA). Anti-αv (CD51, clone AMF7) and anti-CD47 (clone BRIC126, CKm1) were purchased from Immunotech, Serotec Ltd., and Accurate Chemical and Scientific Corp., respectively. RGDS and RGES peptides, Vn, Fn, and thrombospondin (TSP) were obtained from GIBCO BRL.
Expression Cloning of 10G2 Antigen (CD47)
COS cells were transfected using the DEAE dextran method, with an expression library derived from Jurkat cells (4 × 105 clones). Cells expressing the molecule recognized by the 10G2 mAb were immunoselected by indirect panning using 10G2 and anti–mouse IgM-coated plates. Specifically bound cells were lysed, plasmid DNA was isolated, amplified, and retransfected into COS cells. Following an additional round of immunoselection and plasmid purification, pools of 150 clones each were transfected into COS cells, which were subsequently screened for positivity by incubation with radiolabeled 10G2 mAb. Two rounds of screening resulted in the isolation of a single 10G2-reactive clone.
Establishment of Stable CHO Transfectants
CHO cells were grown at 50% confluence in 150-cm2 culture flasks in 5% heat decomplemented FCS containing DME (GIBCO BRL) supplemented with 2 mM glutamine, 100 IU penicillin, and 100 μg/ml streptomycin. Cells were harvested using versene solution (GIBCO BRL) and washed twice with pure DME. Cells were then resuspended at 11 × 106 cells/ ml, and 107 cells were incubated in a 0.4-cm electrotransfection cuvette (BioRad Laboratories) for 10 min with 20 μg of pBJαv (plasmid containing cDNA encoding the full-length αv molecule, CD51), in order to obtain CHO cells expressing the αv molecules. Cells were then pulsed at 220 V and 960 μF using a Gene Pulser (BioRad Laboratories). After another 10 min of incubation at room temperature, electroporated cells were grown for 24 h in nonselective culture medium which was then replaced by complete DME containing 500 μg/ml active G418 (GIBCO BRL). After 14 d of culture, the pool of survivors was analyzed by flow cytometry, and CHO cell line expressing αv was subsequently enriched by FACS® for the 5% highest stained cells using anti-CD51 mAb as primary antibody. After four rounds of sorting, we obtained stable cell lines, namely CHO-51, containing >99% of CD51+ cells.
Cell Separation and Culture Conditions
Monocytes.
PBMC were isolated by density gradient centrifugation of heparinized blood from healthy volunteers using Lymphoprep (Nycomed). Monocytes were prepared by cold aggregation as described in Armant et al. (1995). Monocyte purity was shown to be >95% using phycoerythrin-conjugated anti-CD14 mAb and flow cytometry (Becton Dickinson and Co.). Cellular viability was >90% using trypan blue exclusion.
T Cells.
Enriched T cell populations were obtained from the monocyte-depleted PBMC by rosetting with AET-SRBC and treatment with ammonium chloride. To obtain highly purified T cells, rosette forming cells were washed and incubated for 20 min at 37°C in Lympho-Kwik T (One Lambda). Cell purity was assessed by flow cytometry (FACScan®; Becton Dickinson and Co.) using phycoerythrin-conjugated anti-CD3 mAb (Becton Dickinson and Co.) and shown to be >98% in all cases.
Cultures were performed in complete serum-free HB101 medium (Irvine Scientific) supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 10 mM Hepes, 100 IU penicillin, and 100 μg/ml streptomycin in the presence of polymyxin B 10 μg/ml (Sigma Chemical Co.). When cultured alone, monocytes were incubated in 5 ml sterile Falcon tubes (Becton Dickinson and Co.) at 2 × 105 cells/ml for cytokine measurement. For coculture experiments, T cells (106 cells/ml) were incubated with monocytes (2 × 105 cells/ml) in 24-well Falcon plates.
Cytofluorometric Analysis
For sCD23 binding, cells or cell lines were washed in HBSS (Gibco Laboratories), resuspended in HB101 complete serum free medium containing biotinylated sCD23 (B-sCD23; 50 ng/ml) in the absence or presence of mAbs (CD47, CD51, CD51/CD61), soluble Vn or Fn (20 μg/ml), and RGDS peptides (20 μg/ml). After 4–6 h of incubation at 22°C, cells were washed with PBS containing 3% BSA, and further incubated with phycoerythrin-streptavidin (Becton Dickinson and Co.). Cell viability assessed by trypan blue exclusion was >85% before staining with fluorochrome. Indirect immunofluorescence staining of cells or cell lines with different mAbs was performed according to standard techniques (Armant et al., 1995).
Lymphokine Determinations
IFN-γ, TNF-α, IL-10, and IL-12 were measured exactly as described in Armant et al. (1994, 1995). IL-1β, IL-8, and PGE2 were measured by ELISA kits purchased from R & D Systems, Inc.
Immunoprecipitation and Western Blot Analysis
Cells were lysed in PBS containing 1% NP-40 (NP-40/PBS) supplemented with protease inhibitors. The lysate was purified on anti-β3 affinity column, and the eluate was separated by SDS-PAGE (5%) under nonreducing conditions and transferred to PDVF membrane (Millipore Corp.). Nonspecific binding sites on the membrane were blocked with PBS containing 5% milk. The membrane was incubated with milk containing B-BSA, B-sCD23, B-CD51 mAbs (clone AMF7 and LM142), or B-CD61 mAb. After overnight incubation, membrane was treated with avidin-DH and biotinylated peroxidase complex (Vectastain, ABC kit, Vector Labs, Inc.) followed by ECL detection reagent (Nycomed Amersham).
Statistical Analysis
Paired t tests have been used to assess levels of significance (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
10G2 mAb, Which Inhibits sCD23 Biological Activities, Recognizes CD47 Ag
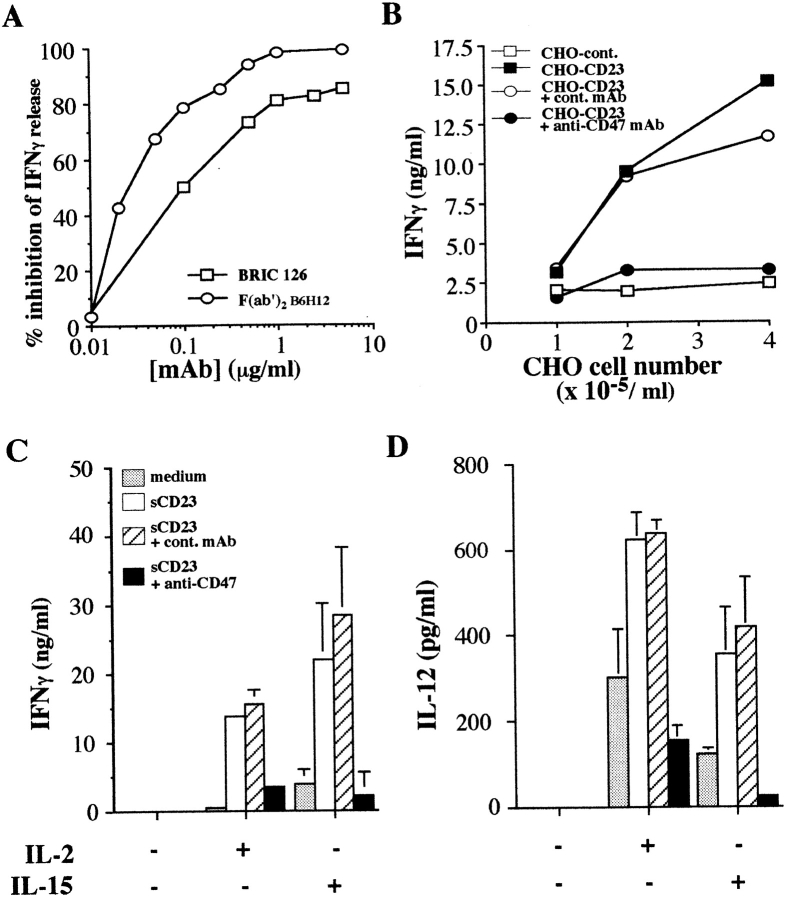
sCD23 displays potent proinflammatory activity by directly triggering monokine release from purified monocytes in the absence of costimulatory signals, such as bacterial antigens (LPS or SAC) or T cell–dependent signal (sCD40L; Armant et al., 1994, 1995; Lecoanet-Henchoz et al., 1995). Although CD21 (CR2) and CD11b (CR3) were previously described as novel CD23 counterreceptors (Aubry et al., 1992; Leconaet-Henchoz et al., 1995), we also detected binding of sCD23 to several T cell lines lacking CR2 or CR3 expression (Ishihara et al., 1995; Table I). In an effort to identify sCD23 binding component, we generated mAbs to Jurkat T cells. We identified one mAb, clone 10G2, which neutralized sCD23 biological activities (Fig. 1 A), and displayed similar cell reactivity as sCD23 (Table I). Specifically, 10G2 mAb, like sCD23, did not stain the CD11b+ (CR3) and CD11c+ (CR4) THP-1 cell line. It recognized weakly, but with similar intensity, K562 and K562-CR2 cell lines. 10G2 mAb also stained peripheral blood T, B cells, and monocytes (Table I). 10G2 mAb significantly suppressed sCD23 costimulation of IFN-γ production by IL-2–stimulated T cells cocultured with autologous monocytes (mean inhibition of 10 experiments, 66%, P < 0.03; Fig. 1 A).
Table I.
Cellular Distribution of 10G2 Antigen on Human Cell Lines
| Freshly isolated human leukocytes | Human cell lines | ||||
|---|---|---|---|---|---|
| sCD23 | 10G2 | sCD23 | 10G2 | ||
| MFI | MFI | MFI | MFI | ||
| T cells | +++ | ++++ | T cell lines (Jurkat, CEM, HUT 78) | ++ | ++++ |
| B cells | ++ | ++ | B cell lines (RPMI 8226, Raji, WIL-2, Daudi) | ++ | ++ |
| Monocytes | + | + | Monocyte cell lines (U937) | ++ | ++ |
| THP-1 | − | − | |||
| Erythrocytes | − | − | Erythroleukemia cell lines (K562/K562-CR2) | + | + |
Figure 1.
Clone 10G2 neutralizes sCD23 biological activities and recognizes CD47 Ag. (A) Inhibition of sCD23 costimulation of IFN-γ production in T cell/monocyte cocultures by anti-CD47 mAbs (clones 10G2, B6H12, C1Km1, and BRIC126). Mean ± SD for 10G2 P < 0.03 (ten experiments) and for other mAbs P < 0.001 (five independent experiments). (B) 10G2 mAb staining of untransfected (COS, dotted line) or CD47-transfected COS cell line (COS-47, bold line). Control mAb staining of both cell lines: (COS, thin line; COS-47, dashed line). (C) Fluorescence staining of Jurkat T cell line (left) and THP-1 monocyte cell line (right) by increasing concentrations of anti-CD47 mAbs (clones 10G2 and B6H12). One representative experiment out of three.
The molecule recognized by 10G2 mAb was identified by immunoaffinity panning of COS cells transfected with a cDNA library prepared from Jurkat T cells. After several rounds of selection, a single clone was selected which was found by DNA sequencing to encode CD47 Ag (data not shown). Results in Fig. 1 B demonstrate binding of 10G2 to COS cells transfected with CD47 cDNA clone.
The effects of several anti-CD47 mAbs on sCD23 biological activity were examined (Fig. 1 A). Besides the 10G2 mAb (IgM isotype), three other anti-CD47 mAbs of different Ig isotype subclasses, C1Km1 (IgG1), BRIC126 (Ig2b), and B6H12 (IgG1) inhibited IFN-γ production induced by sCD23 plus IL-2. These results support the notion that CD47 is part of the signaling complex triggered by sCD23.
However, it appears that 10G2 was preferentially binding to a particular epitope on CD47. As depicted in Fig. 1 C, THP-1 cells express CD47 Ag but are not recognized by 10G2 mAb. Clone B6H12, a well-defined anti-CD47 mAb, but not 10G2, stained THP-1 cell line in a dose-dependent manner, while Jurkat T cells were recognized with similar intensity by both anti-CD47 mAbs. There was no cross-inhibition of Jurkat staining by the two clones (data not detailed). Our unpublished data also indicated that erythrocytes which expressed CD47 in the absence of integrins (Rosales et al., 1992) were stained by B6H12 but not 10G2.
Anti-CD47 mAbs Suppress CD23 Costimulation of IL-12 and IFN-γ Production Via Fc-independent Pathways
We investigated the mechanisms underlying the suppression of IFN-γ production by anti-CD47 mAbs. In addition to 10G2 mAb of the IgM isotype, the F(ab)′2 fragments of B6H12 or intact BRIC126 mAb suppressed, in a dose-dependent manner, the ability of IL-2 and sCD23 to augment the production of IFN-γ by T cells cocultured with monocytes, demonstrating that the inhibition of IFN-γ secretion by anti-CD47 mAb was not Fc-mediated (Fig. 2 A). Interestingly, IFN-γ secretion by IL-2–stimulated T cells cocultured with monocytes and graded numbers of CD23-transfected CHO cells, was also abrogated by anti-CD47 mAbs (Fig. 2 B).
Figure 2.
Anti-CD47 mAb inhibits CD23 costimulation of IFN-γ production. (A) Dose-dependent inhibition of IFN-γ production in IL-2 plus sCD23 stimulated coculture system by mAb clone BRIC126 and F(ab′)2 fragments of mAb clone B6H12. One representative experiment out of two. (B) Anti-CD47 mAb (clone B6H12) inhibition of IFN-γ secretion by T cells cocultured with autologous monocytes and increasing numbers of untransfected or CD23-transfected CHO cells. Similar data were obtained using clone 10G2 in three independent experiments. (C and D) Anti-CD47 mAbs (clone B6H12) mediated inhibition of IFN-γ and IL-12 secretion in cocultures of IL-2– or IL-15–stimulated T cells and autologous monocytes in the absence or presence of sCD23. Mean ± SD of six experiments. Anti-CD47 mAb inhibition in IL-2– or IL-15–stimulated cocultures, for IFN-γ production P < 0.001, and P < 0.01 for IL-12 secretion.
We previously reported in the presence of IL-2 or IL-15, low levels of CD40L, expressed by unstimulated T cells, were sufficient to engage CD40 on monocytes and trigger IL-12 release. This monocyte-derived IL-12 production synergized with IL-2 or IL-15 to augment IFN-γ production by T cells (Armant et al., 1996; Avice et al., 1998). The IFN-γ response could be further amplified by sCD23-induced TNF-α release (Armant et al., 1995). Although sCD23 did not trigger IL-12 production by purified monocytes (Armant et al., 1995), it costimulated IFN-γ and IL-12 release in this coculture system, and the secretion of both cytokines was strongly inhibited by anti-CD47 mAbs (Fig. 2, C and D).
Anti-CD47 mAb Suppresses sCD23-induced Monokine Release
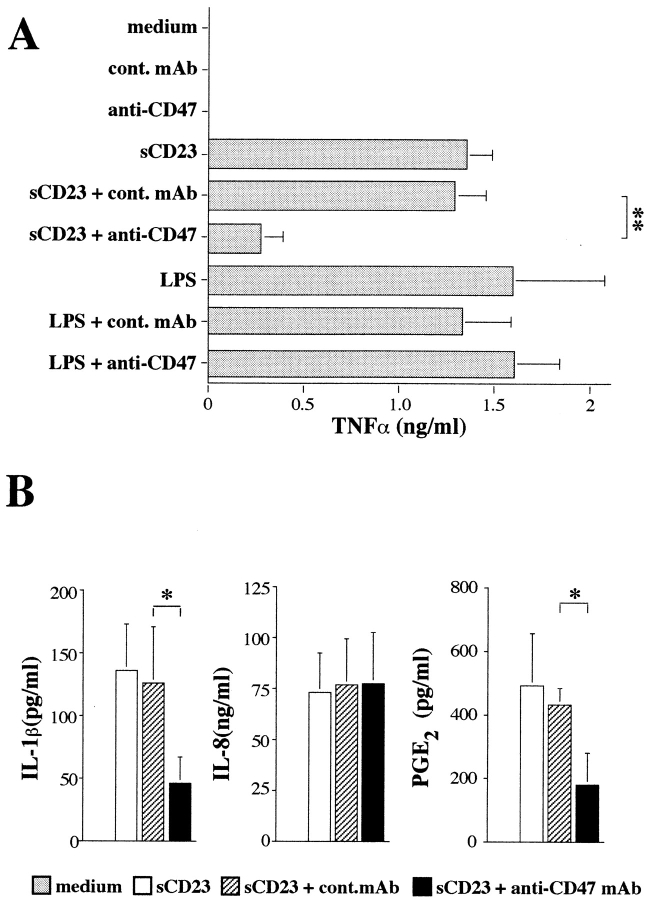
Because sCD23 directly triggers TNF-α release by purified monocytes (Armant et al., 1995), and monocytes express CD47 (Table I), we examined the effect anti-CD47 mAb had on sCD23-induced monokine release by monocytes. Anti-CD47 mAb significantly suppressed the induction by sCD23 of TNF-α, IL-1β, and PGE2 without affecting IL-8 secretion (Fig. 3). The data (i.e., inhibition of TNF-α and IL-12 production) provide a mechanism by which anti-CD47 mAb can suppress sCD23 costimulation of IFN-γ production.
Figure 3.
Anti-CD47 mAb inhibition of sCD23-induced monokine release by purified monocytes. Monocytes (106/ml) were cultured in the absence or presence of sCD23 or LPS with or without anti-CD47 mAb (clone 10G2). After overnight cultures, TNF-α (A), IL-1β, IL-8, or PGE2 (B) was measured in the culture supernatant. Mean ± SD of six experiments (*P < 0.05, **P < 0.01).
However, anti-CD47 mAb, in the absence of sCD23 or in the presence of LPS, did not modulate TNF-α production (Fig. 3 A). Our unpublished observations revealed that anti-CD47 mAb, used alone or in combination with sCD23, did not induce the production of monocyte deactivators (such as IL-10 or TGF-β), nor did it modulate SAC-induced TNF-α, IL-6, or IL-10 release. These results support the hypothesis that anti-CD47 mAbs interfere with the sCD23 signaling pathway without impairing general monocyte function.
sCD23 and Vitronectin Share the Same Receptor: VnR/CD47 Complex
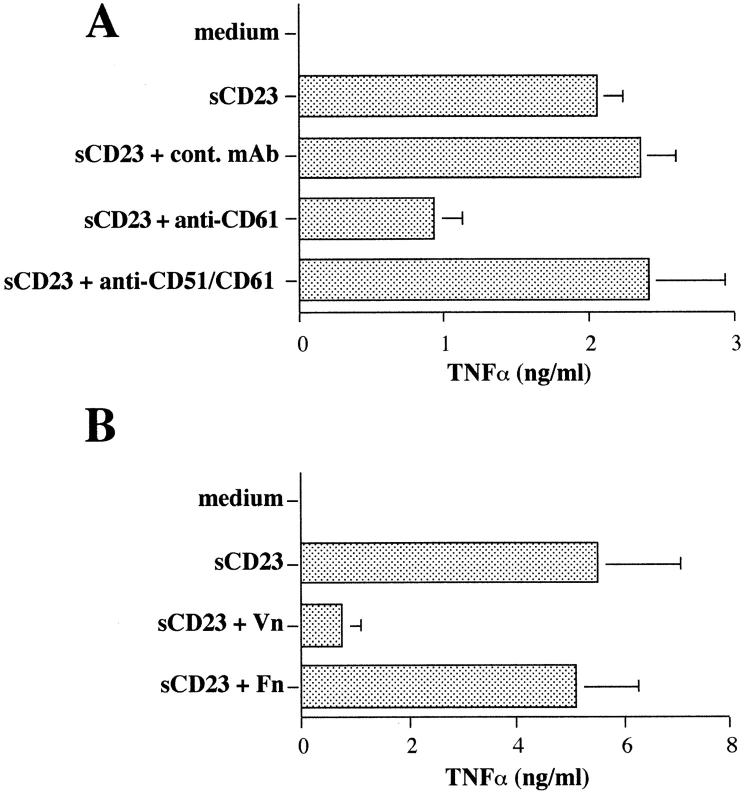
Several studies indicate that CD47 is physically and functionally associated with the vitronectin receptor, αvβ3. Both anti-CD47 and anti-β3 (CD61) mAbs can block binding of Vn, but not Fn, to αvβ3, even though Vn does not bind to CD47 (Brown et al., 1990; Lindberg et al., 1993). Therefore, we examined the biological effects of anti-β3 mAb and the natural ligands of VnR (Vn and Fn) on sCD23 function. It is important to note that all cultures were performed in HB101 serum-free medium to eliminate FCS as a source of Vn and Fn. The results (Fig. 4) suggested that Vn and sCD23 might share the same receptor, namely VnR/CD47 complex. Anti-β3 mAb suppressed sCD23 function, as defined by TNF-α secretion and IFN-γ production (Fig. 4 A, and data not shown). Furthermore, soluble Vn, but not Fn, suppressed sCD23-induced TNF-α release by purified monocytes (Fig. 4 B). sCD23 and Vn most likely bound to distinct epitopes on αvβ3, since an anti-αvβ3 mAb (clone LM609), which specifically inhibited Vn binding and function (Gao et al., 1996a), and RGDS peptide had no suppressive activity (Fig. 4 A, and data not shown).
Figure 4.
Suppression of sCD23-induced TNF-α release by anti-β3 (CD61) mAb and Vn. sCD23-activated monocytes were cultured with anti-β3 (CD61) clone AP3, anti-αvβ3 (CD51/CD61; clone LM609), or isotype-matched mAbs (A) and with soluble Vn or Fn (20 μg/ml, B). Mean ± SD of four experiments (P < 0.01).
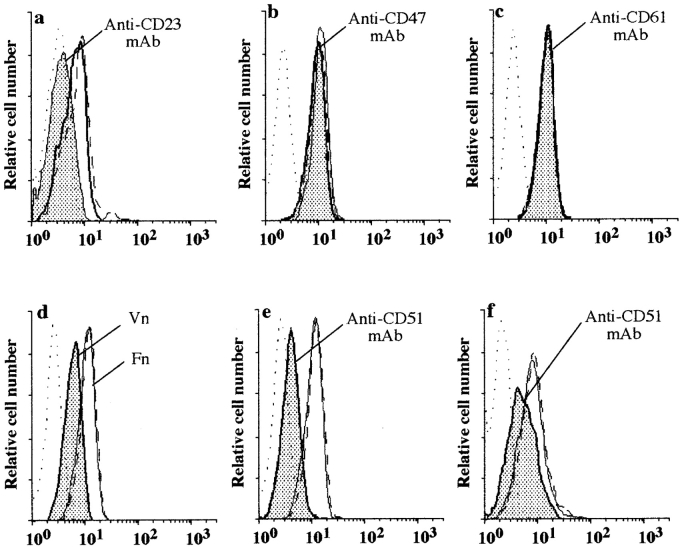
We next investigated the ability of mAbs directed to the VnR complex, anti-CD47, CD61 (β3) and αvβ3 (LM609) mAbs, and natural ligands of VnR, Vn, Fn, and RGDS peptides, to alter sCD23 binding to the Jurkat T cell line. Unexpectedly, anti-CD47 mAbs, alone or in combination (Fig. 5 b), anti-CD61 (Fig. 5 c), or anti-αvβ3 clone LM609 (not shown) did not inhibit sCD23 binding to Jurkat or monocytes (Hermann, P., unpublished observations). Note that sCD23 binding was specifically suppressed by anti-CD23 mAb (Fig. 5 a). The data suggested ligation of the CD47 or β3 chain of the trimolecular complex by mAbs could indirectly inhibit sCD23 function by providing a negative signal to the target cells, or by modifying CD47 complex configuration without displacing the sCD23 molecule. We examined whether engagement of VnR/CD47 complex by its natural ligands would modify sCD23 binding. As shown in Fig. 5 d, Vn, but not Fn or RGDS peptide (not shown), significantly inhibited sCD23 binding, strongly suggesting the VnR complex was involved in the secretion of proinflammatory cytokine via interaction with sCD23.
Figure 5.
Effect of mAbs or natural ligands to VnR/CD47 complex on sCD23 binding to Jurkat and Raji cell lines. Jurkat αv +β3 + (CD51+/CD61+) (a–e), or Raji αv +β3 − (CD51+/CD61−) (f) cell lines were stained by B-BSA (dotted line) or B-sCD23 in the absence (solid line) or presence of the following inhibitors (plain histograms): anti-CD23 mAb (a); anti-CD47 mAbs (cocktail of four anti-CD47 mAbs; b); anti-β3 (CD61) mAb (c); Vn (d); anti-αv (CD51) mAb (e and f). B-sCD23 plus isotype-matched control mAb (a–c, e, and f) or Fn (d) were shown as dashed lines. One representative experiment out of four.
The possible role of the αv chain (CD51) of VnR in sCD23 binding was explored. Three anti-CD51 mAbs were tested, and we found one anti-CD51 mAb (clone AMF7) inhibited the interaction between sCD23 and αv +β3 + Jurkat T (Fig. 5 e), as well as αv +β3 − Raji B cell lines (Fig. 5 f). Note that the Raji B cell line expresses the β5 integrin (data not shown). We postulated that sCD23 was binding to αv and not β3 chain of the VnR, whereas β3 and CD47 chains were likely to be involved in the signaling of the trimolecular complex in monocytes because anti-CD47 and anti-β3 inhibited sCD23 function, but not binding.
sCD23 Interacts with αv/CD51 and CD47 Coexpression Increases its Binding
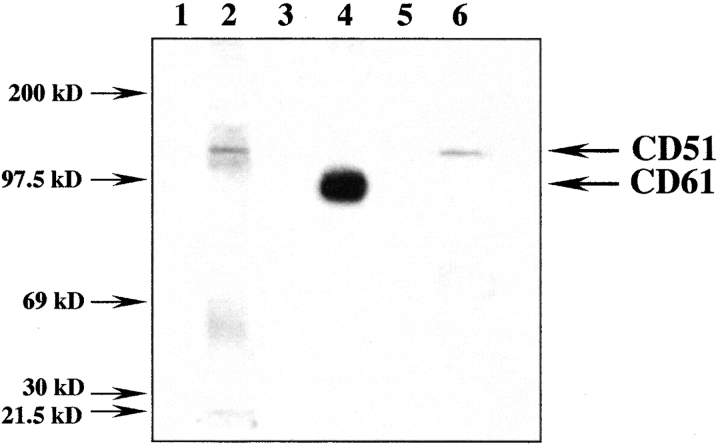
Next, we selected a melanoma cell line, strongly expressing αvβ3 to purify this integrin by affinity chromatography using anti-β3 (CD61) immobilized AFFi-gel. Western blot analysis (Fig. 6) shows sCD23 (lane 6) reacted with a single band of ∼135 kD, displaying a similar migration pattern as molecular species recognized by a cocktail of anti-CD51 mAbs (lane 2). However, sCD23 did not appear to bind purified β3 (CD61) chain which was identified by anti-CD61 mAb (lane 4), or purified recombinant CD47 (not shown).
Figure 6.
Western blot analysis of αv +β3 + (CD51+/CD61+) melanoma cell line. Cell lysate was purified on anti-β3 (CD61) mAb-affinity column. The eluate was separated by SDS-PAGE (5%) and transferred to membrane. Staining by anti-αv (CD51) mAb (clone AMF7 and LM142; lane 2), anti-β3 (CD61, clone AP3; lane 4), isotype-control matched mAbs (lanes 1 and 3), B-BSA (lane 5), and B-sCD23 (lane 6) is shown.
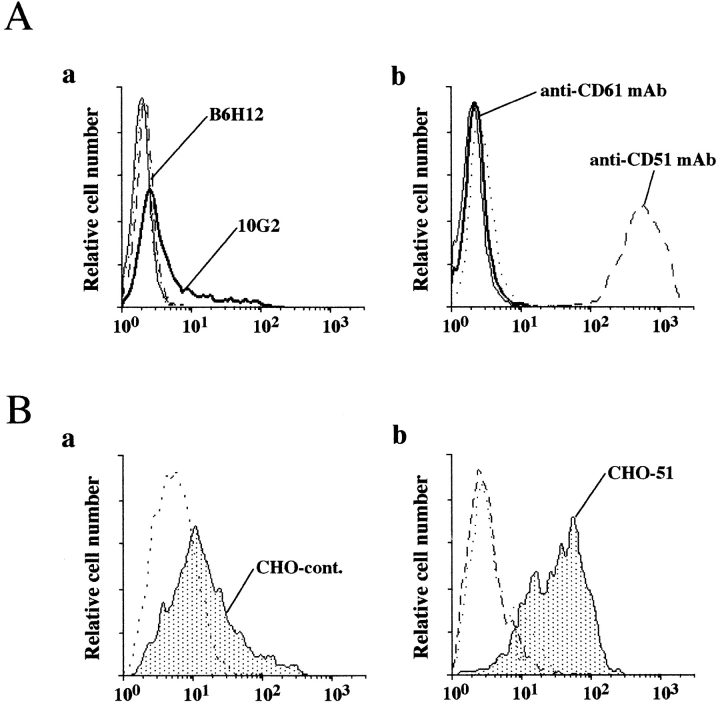
To further support the hypothesis that sCD23 may directly interact with αv chain of the trimolecular complex, we prepared CHO transfectants singly expressing human αv chain (CHO-CD51). The results in Fig. 7 demonstrated sCD23 strongly bound to CHO-CD51 compared to untransfected cell line. Although CHO-CD51 transfectant did not express human β3 (CD61) chain, CHO cell lines were reported to express rodent β chain integrins (Lindberg et al., 1993) which likely associated with the human αv chain underlying the successful stable expression of a single human integrin chain. Nevertheless, CHO cells also expressed hamster CD47 which might contribute to sCD23 binding to αv/CD51 on live cells as reported for Vn binding to untransfected CHO cells (Lindberg et al., 1993). To directly assess whether CD47 expression was required for sCD23 interaction with αv/CD51, we examined sCD23 binding to human CD47 deficient cell lines. As shown in Fig. 8, sCD23 bound to OV10 ovarian carcinoma VnR+/CD47− cell line demonstrating that CD47 was dispensable for sCD23 binding. Coexpression of CD47 further increased its binding. A similar effect was seen on transfection of CD47− Jurkat with CD47 (data not shown).
Figure 7.
Binding of sCD23 to CHO-51 (CHO αv) transfected cell line. (A) αv (CD51)-transfected CHO cell line was stained with (a) anti-CD47 mAbs (clones 10G2, solid line and B6H12, dotted line) or isotype-matched control mAbs or with (b) anti-αv (CD51) mAb (clone AMF7, dashed line) and anti-β3 (CD61) mAb (clone AP3, solid line), or isotype-matched cont mAbs. (B) Untransfected (a), and αv (CD51)-transfected CHO cell lines (b) were stained with B-sCD23 (plain histograms) or B-BSA (dotted line). One representative experiment out of three.
Figure 8.
Binding of sCD23 to CD47+ and CD47− cell lines. CD47+ and CD47− cell lines were stained with two anti-CD47 mAbs (clone 10G2 and B6H12), anti-αv (CD51) and anti-β3 (CD61) mAbs, or B-sCD23 as described in Materials and Methods. OV10 carcinoma and Jurkat cell lines express different levels of αv (CD51) chain which correlate with sCD23 binding.
Given that sCD23 was not binding to CD47+/αv − (THP-1 cell line; Fig. 8), but reacted with αv +β3 − (Raji cell line; Fig. 5 f), we concluded that sCD23 ligated αv/CD51. The VnR complex (αvβ3/CD47 and/or αvβx/CD47) was used as a functional receptor for sCD23 to mediate its proinflammatory activity and, as such, may be involved in the inflammatory process of the immune response.
Discussion
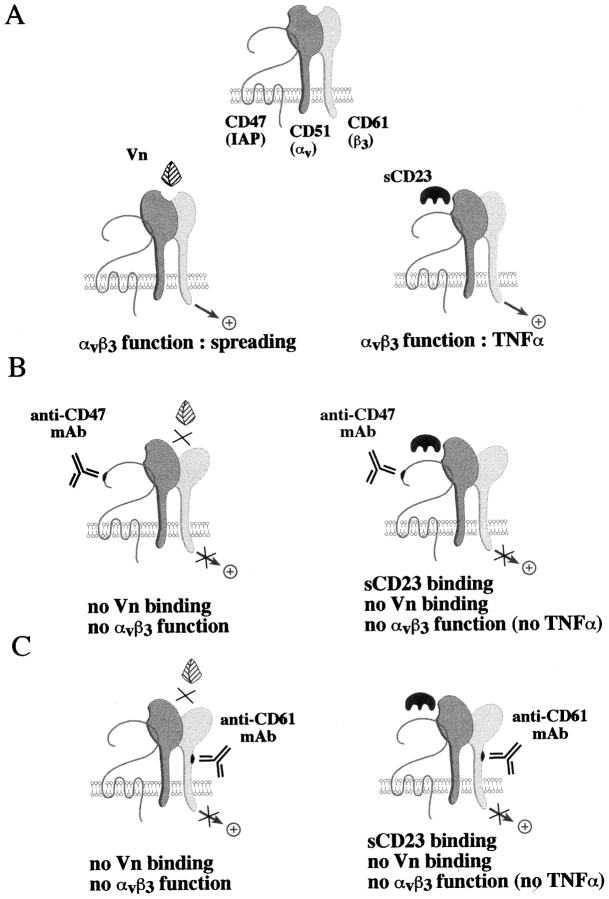
Our results can be summarized in the schematic model presented in Fig. 9. We first postulate (Fig. 9 A) that sCD23 and Vn have distinct recognition sites on the αvβ3/ CD47 complex. sCD23 binds to αv (CD51) and Vn to αvβ3 (CD51/CD61) conformational site. Binding of Vn may induce structural changes in the integrin complex leading to the masking of sCD23 binding sites, and vice versa. This hypothesis is based on observations that Vn, anti-αv mAb (clone AMF7), but not clone LM609 (which specifically recognizes Vn binding site), nor RGDS peptide inhibited sCD23 binding and function (Figs. 4 and 5), and sCD23 directly bound to αv chain (Figs. 6–8). However, CD47 was not a direct ligand for sCD23 while its coexpression improved sCD23 binding to αv/CD51. sCD23 bound CD47 deficient cell lines, but failed to bind CD47 in the absence of αv/CD51 integrin (THP-1 cell line, erythrocytes and recombinant CD47 protein; Fig. 8, and data not shown).
Figure 9.
sCD23 and Vn share the same functional receptor, VnR/CD47 complex. (A) sCD23 binds to αv/CD51 and Vn to αvβ3 (CD51/CD61) conformational site while neither bind to CD47. Binding of Vn to αvβ3 (CD51/CD61) obliterates sCD23 binding site, and vice versa. (B and C) Anti-CD47 and anti-β3 (CD61) mAbs suppress Vn binding and function (left) while they inhibit sCD23 function, but not binding (right).
Secondly, (Fig. 9, B and C) CD47 or CD61 engagement by mAbs prevents Vn binding (Lindberg et al., 1993) without displacing sCD23 molecule (Fig. 5) while these mAbs inhibit both sCD23 (Figs. 2–4) and Vn function perhaps by modifying β3 conformation or signaling pathway. Lindberg et al. (1993) indicated that anti-CD47 and anti-β3 (CD61) mAbs (directed against an epitope of β3 located outside the Vn binding site) inhibited Vn-opsonized particle binding and function. They also proposed the CD47 molecule participated in the appropriate folding of αvβ3, and modulated the affinity of αvβ3 for Vn (Brown et al., 1990; Lindberg et al., 1993). Vn-coated particles failed to bind to a CD47 deficient cell line (Lindberg et al., 1996b) or to cells isolated from CD47 deficient mice (Lindberg et al., 1996a), while these cells expressed αvβ3, demonstrating that CD47 is dispensable for VnR expression, but required for Vn binding and function. Using truncated forms of CD47, they also reported the interaction between the extracellular domain of CD47 (IgV) and αv integrins was sufficient for Vn binding (Lindberg et al., 1996b).
It has been reported that adhesion of integrins to their counterreceptors is a dynamic phenomenon regulated by intracellular signal transduction pathways (Diamond and Springer, 1994). Structural changes in the extracellular domains of integrins following mAb ligation may modify ligand adhesiveness in inducing or inhibiting ligand binding (inside-out signaling), or may directly provide a negative signal to the cell (outside-in signaling), as proposed here for anti-CD47 and anti-CD61 mAb-mediated inhibition of sCD23 function.
In agreement with this hypothesis, it was reported that antibodies recognizing CD81, another member of multispan transmembrane receptors family, inhibited FcεRI-mediated mast cell degranulation without affecting IgE binding, receptor-mediated Ca2+ release, or tyrosine phosphorylation (Fleming et al., 1997).
Therefore, we propose a novel function for VnR/CD47 complex in the regulation of inflammatory response. In the absence of pathogen, ligation of VnR by sCD23 mediates monokine release such as TNF-α which enhances the inflammatory process by triggering the cascade of proinflammatory cytokine secretion (IL-1, IL-6, GM-CSF . . .; Feldman et al., 1996), and facilitates the elimination of apoptotic cells (Ren and Savill, 1995). Both effects are negatively regulated by the presence of Vn (Fig. 4 B and Savill et al., 1990). Vn is a glycoprotein which is synthesized in the liver and circulates in plasma at high concentration (200–400 μg/ml). The insoluble form is localized extravascularly and is associated with granulation tissue areas in rheumatoid arthritis synovia (Seiffert et al., 1993).
It has been reported that β3 complex signaling via CD47 affected β2 (CD18/CD11b and CD18/CD11c) integrins binding to their ligands (Van Strijp et al., 1993; Ishibashi et al., 1994). Previous studies identified CD11b and CD11c as novel ligands for sCD23 (Lecoanet-Henchoz et al., 1995). Our study shows that sCD23 bound CD11− cell lines (Jurkat and CHO cells; Figs. 1 and 8) failed to stain the THP-1 (CD11+) cell line (Fig. 8), and anti-CD47 mAb did not alter CD11b expression, or sCD23 binding on monocytes (data not shown), indicating that sCD23 does not interact with β2 integrins. We currently have no explanation for these contradictory results.
Interestingly, our unpublished data indicating TSP, a newly discovered CD47 ligand (Gao et al., 1996b), also suppressed sCD23 function, without directly triggering monokine release, further supporting our present model. The absence of VnR-mediated monokine secretion following engagement by Vn, Fn, or TSP does not exclude the possibility that other ligands (see review Felding-Habermann and Cheresh, 1993; Gladson and Cheresh, 1994) would share, with sCD23, its proinflammatory activity. Finally, the ability of the anti-CD47 mAbs examined to inhibit the function of sCD23 without interfering with the phagocytosis of senescent cells (Savill et al., 1990) may help in the design of novel therapeutic strategies for chronic inflammatory disorders, such as rheumatoid arthritis, in which CD23 and TNF-α are implicated (Plater-Zyberk and Bonnefoy, 1995; Feldman et al., 1996).
Acknowledgments
We thank Dr. Y. Ohshima and Dr. C.E. Demeure for their help and criticism of the manuscript. The secretarial assistance of Norma Del Bosco is greatly appreciated.
This work was supported by a grant from the Medical Research Council of Canada (MRC). Dr. M. Sarfati is supported by a MRC Scientist Scholarship.
Abbreviations used in this paper
B
biotinylated
Fn
fibronectin
sCD23
soluble CD23
TSP
thrombospondin
Vn
vitronectin
VnR
vitronectin receptor
Footnotes
P. Hermann and M. Armant contributed equally to this study.
References
- Armant M, Ishihara H, Rubio M, Delespesse G, Sarfati M. Regulation of cytokine production by soluble CD23: Costimulation of IFN-γ secretion and triggering of TNF-α release. J Exp Med. 1994;180:1005–1011. doi: 10.1084/jem.180.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armant M, Rubio M, Delespesse G, Sarfati M. Soluble CD23 directly activates monocytes to contribute to the Ag-independent stimulation of resting T cells. J Immunol. 1995;155:4868–4875. [PubMed] [Google Scholar]
- Armant M, Armitage R, Boiani N, Delespesse G, Sarfati M. Functional CD40 L expression on T lymphocytes in the absence of TCR engagement: involvement in IL-2-induced IL-12 and IFNγ production. Eur J Immunol. 1996;26:1430–1434. doi: 10.1002/eji.1830260705. [DOI] [PubMed] [Google Scholar]
- Ashton BA, Ashton IK, Marshall MJ, Butler RC. Localization of vitronectin receptor immunoreactivity and tartrate resistant acid phosphatase activity in synovium from patients with inflammatory or degenerative arthritis. Ann Rheum Dis. 1993;52:133–137. doi: 10.1136/ard.52.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry J-P, Pochon S, Graber P, Jansen KU, Bonnefoy J-Y. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992;358:505–507. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- Avice M-N, Demeure CE, Delespesse G, Rubio M, Armant M, Sarfati M. IL-15 promotes IL-12 production by human monocytes via T cell-dependent contact and may contribute to IL-12–mediated IFN-γ secretion by CD4+T cells in the absence of TCR ligation. J Immunol. 1998;161:3409–3415. [PubMed] [Google Scholar]
- Bertero MT, Converso M, Aimo G, Merico F. Serum levels of cytokines and soluble (s) CD23 in systemic lupus erythematosus. Fund Clin Immunol. 1994;2:37–43. [Google Scholar]
- Bonnefoy J-Y, Plater-Zyberk C, Lecoanet-Henchoz S, Gauchat J-F, Aubry J-P, Graber P. A new role for CD23 in inflammation. Immunol Today. 1996;17:418–420. doi: 10.1016/0167-5699(96)10054-2. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Strömblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Brown E, Hooper L, Ho T, Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990;6:2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Lindberg FP. Leucocyte adhesion molecules in host defense against infection. Ann Med. 1996;28:201–208. doi: 10.3109/07853899609033121. [DOI] [PubMed] [Google Scholar]
- Buckley CD, Doyonnas R, Newton JP, Blystone SD, Brown EJ, Watt SM, Simmons DL. Identification of αvβ3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci. 1996;109:437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- Cooper D, Lindberg FP, Gamble JR, Brown EJ, Vadas MA. Transendothelial migration of neutrophils involves integrin-associated protein (CD47) Proc Natl Acad Sci USA. 1995;92:3978–3982. doi: 10.1073/pnas.92.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Springer TA. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994;4:506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B, Cheresh DA. Vitronectin and its receptors. Curr Opin Cell Biol. 1993;5:864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Feldman M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Fleming TJ, Donnadieu E, Song CH, Van Laethem F, Galli SJ, Kinet J-P. Negative regulation of FcεRI-mediated degranulation by CD81. J Exp Med. 1997;186:1307–1314. doi: 10.1084/jem.186.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A-G, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA. Thrombospondin modulates αvβ3function through integrin-associated protein. J Cell Biol. 1996a;135:533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A-G, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996b;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- Gladson, C.L., and D.A. Cheresh. 1994. The αv integrins. In Integrins: The Biological Problems. Y. Takoda, editor. CRC Press, Boca Raton, FL. 83–99.
- Gresham HD, Goodwin JL, Allen PM, Anderson DC, Brown EJ. A novel member of the integrin receptor family mediates Arg-Gly-Asp-stimulated neutrophil phagocytosis. J Cell Biol. 1989;108:1935–1943. doi: 10.1083/jcb.108.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ikizawa K, Yanagihara K, Kajiwara T, Koshio T, Shida T, Yamada A. Possible role of CD5+B cells expressing CD23 in mediating the elevation of serum soluble CD23 in patients with rheumatoid arthritis. Int Arch Allergy Immunol. 1993;101:416–424. doi: 10.1159/000236485. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Claus S, Relman DA. Bordetella pertussisfilamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18) J Exp Med. 1994;180:1225–1233. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara, H., M. Rubio, G. Delespesse, and M. Sarfati. 1995. Soluble CD23 does not react with complement receptor (CR2) antigen. In Leukocyte Typing V. Shlossman, editors. Oxford University Press, New York. 536–538.
- Lecoanet-Henchoz S, Gauchat J-F, Aubry J-P, Graber P, Life P, Paul-Eugene N, Ferrua B, Corbi AL, Dugas B, Plater-Zyberk C, et al. CD23 regulates monocyte activation through a novel interaction with the adhesion molecules CD11b-CD18 and CD11c-CD18. Immunity. 1995;3:119–125. doi: 10.1016/1074-7613(95)90164-7. [DOI] [PubMed] [Google Scholar]
- Lindberg FP, Gresham HD, Schwarz E, Brown EJ. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in αvβ3-dependent ligand binding. J Cell Biol. 1993;123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996a;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- Lindberg FP, Gresham HD, Reinhold MI, Brown EJ. Integrin-associated protein immunoglobulin domain is necessary for efficient vitronectin bead binding. J Cell Biol. 1996b;134:1313–1322. doi: 10.1083/jcb.134.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plater-Zyberk C, Bonnefoy J-Y. Marked amelioration of established collagen-induced arthritis by treatment with antibodies to CD23 in vivo. . Nat Med. 1995;1:781–785. doi: 10.1038/nm0895-781. [DOI] [PubMed] [Google Scholar]
- Ren Y, Savill J. Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J Immunol. 1995;154:2366–2374. [PubMed] [Google Scholar]
- Rosales C, Gresham HD, Brown EJ. Expression of the 50-kD integrin-associated protein on myeloid cells and erythrocytes. J Immunol. 1992;149:2759–2764. [PubMed] [Google Scholar]
- Sarfati, M. 1997. CD23 workshop panel report. B7/608. In Leukocyte Typing VI. T. Kishimoto, H. Kikutani, A. von dem Borne, S.M. Goyert, D.Y. Mason, M. Miyasaka, L. Moretta, K. Okumura, S. Shaw, T.A. Springer, et al., editors. Garland Publishing, Inc., New York. 144–147.
- Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Seiffert, D., T.J. Podor, and D.J. Loskutoff. 1993. Distribution of vitronectin. In Biology of Vitronectins and their Receptors. K.T. Preissner, S. Rosenblatt, C. Kost, H. Wegerhoff, and D.F. Mosher, editors. Excerta Medica, Amsterdam/New York/London/Tokyo, 75.
- Van Strijp JAG, Russell DG, Tuomanen E, Brown EJ, Wright SD. Ligand specificity of purified complement receptor type three (CD11b/ CD18, αmβ2, Mac-1) J Immunol. 1993;151:3324–3336. [PubMed] [Google Scholar]
- Zhou M, Brown EJ. Leukocyte response integrin and integrin-associated protein act as a signal transduction unit in generation of a phagocyte respiratory burst. J Exp Med. 1993;178:1165–1174. doi: 10.1084/jem.178.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]