Tumor Suppressor PTEN Inhibits Integrin- and Growth Factor–mediated Mitogen-activated Protein (MAP) Kinase Signaling Pathways (original) (raw)
Abstract
The tumor suppressor PTEN dephosphorylates focal adhesion kinase (FAK) and inhibits integrin-mediated cell spreading and cell migration. We demonstrate here that expression of PTEN selectively inhibits activation of the extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) pathway. PTEN expression in glioblastoma cells lacking the protein resulted in inhibition of integrin-mediated MAP kinase activation. Epidermal growth factor (EGF) and platelet-derived growth factor (PDGF)- induced MAPK activation were also blocked. To determine the specific point of inhibition in the Ras/Raf/ MEK/ERK pathway, we examined these components after stimulation by fibronectin or growth factors. Shc phosphorylation and Ras activity were inhibited by expression of PTEN, whereas EGF receptor autophosphorylation was unaffected. The ability of cells to spread at normal rates was partially rescued by coexpression of constitutively activated MEK1, a downstream component of the pathway. In addition, focal contact formation was enhanced as indicated by paxillin staining. The phosphatase domain of PTEN was essential for all of these functions, because PTEN with an inactive phosphatase domain did not suppress MAP kinase or Ras activity. In contrast to its effects on ERK, PTEN expression did not affect c-Jun NH2-terminal kinase (JNK) or PDGF-stimulated Akt. Our data suggest that a general function of PTEN is to down-regulate FAK and Shc phosphorylation, Ras activity, downstream MAP kinase activation, and associated focal contact formation and cell spreading.
Keywords: PTEN, integrin, growth factor, MAP kinase, cell spreading
PTEN is a recently identified tumor suppressor gene that is a dual specificity phosphatase. The PTEN gene also has an NH2-terminal domain with extensive homology to tensin, a protein that interacts with actin filaments at focal adhesions. Deletions and mutations within the PTEN gene have been observed in multiple forms of human cancer, including brain, breast, prostate, and other advanced malignancies (Kong et al., 1997; Li et al., 1997; Steck et al., 1997). In addition, germ-line PTEN mutations have been detected in two autosomal-dominant disorders, Cowden disease, which confers an elevated risk for tumors of the breast and thyroid, and Bannayan-Zonana syndrome, which is characterized by hamartomas and increased susceptibility to breast and thyroid cancer (Liaw et al., 1997; Marsh et al., 1997).
PTEN encodes the catalytic motif of protein tyrosine phosphatases, and is able to dephosphorylate serine, threonine and tyrosine residues; it is thus considered a dual-specificity phosphatase (Myers et al., 1997). Recent data indicate that an intact catalytic phosphatase domain is essential for the known functions of PTEN. For example, wild-type PTEN has growth suppressor activity in glioma cells, but mutated PTEN with inactivated phosphatase activity does not (Furnari et al., 1997, and our own unpublished data). We recently found that PTEN can dephosphorylate focal adhesion kinase (FAK)1 in vitro and in vivo, and inhibits cell spreading and cell migration on extracellular matrix proteins such as fibronectin (FN) and vitronectin (Tamura et al., 1998). Based on these observations, we wondered whether PTEN might affect integrin-mediated signal transduction pathways.
The interaction of cells with the extracellular matrix via cell surface integrins generates a series of complex signaling events that serve to regulate several aspects of cell behavior, including growth, differentiation, adhesion, and motility (Hynes, 1992; Yamada and Miyamoto, 1995; Ruoslahti and Vaheri, 1997; Schwartz, 1997). Intensive studies have showed that integrin ligation can trigger activation of certain MAP kinases, especially extracellular signal-regulated kinase (ERK) members (Chen et al., 1994; Schlaepfer et al., 1994; Morino et al., 1995; Miyamoto et al., 1996). Subsequent apparently conflicting studies have reported that FAK is involved in integrin-triggered ERK signaling (Schlaepfer et al., 1994; Schlaepfer and Hunter, 1997), or is not involved (Lin et al., 1997; Wary et al., 1998) and that Ras is required (Clark and Hynes, 1996; Schlaepfer and Hunter, 1997) or that is not required (Chen et al., 1996) for integrin-mediated MAP kinase activation.
On the other hand, activation of growth factor receptors such as EGF receptor is well-known to stimulate nucleotide exchange on the Ras protein, which participates in activation of the Raf family, which then sequentially phosphorylates and activates the downstream components MAP or ERK kinase 1/2 (MEK1/2) and ERK1/2 (Jelinek et al., 1996; Renshaw et al., 1997). Other types of MAP kinases have distinct pathways and functions. For example, c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) is not activated by EGF receptors, but it can be activated by integrin clustering (Miyamoto et al., 1996), or by treatment of cells with inflammatory cytokines or stresses such as UV irradiation (Derijard et al., 1994).
Integrins can collaborate or synergize functionally with growth factors in a variety of biological processes, including cell growth and differentiation (Schwartz et al., 1995). Among other mechanisms, integrin-growth factor synergy or collaboration can involve the MAP kinase pathway, e.g., associated with altered EGF receptor and PDGF receptor phosphorylation (Miyamoto et al., 1996).
Since integrins and growth factors can each separately stimulate this pathway or function collaboratively, and since the MAP kinase pathway has been implicated as a regulator of such a wide variety of effects on cell growth and differentiation, in the present study we tested whether the tumor suppressor PTEN affects this central signaling pathway. It has already been shown in Meyers et al. (1997) that isolated PTEN cannot dephosphorylate ERK2 in vitro. Nevertheless, there are a variety of upstream steps necessary for MAP kinase activation that might be targets of PTEN. We have therefore searched for effects of PTEN on activities or phosphorylation levels of several components of the Ras/ERK signal transduction pathway, and have found that reconstitution of expression of PTEN in a cell line devoid of this protein due to mutation markedly inhibited Ras/ERK pathway activation in response to stimulation by fibronectin or growth factors. Furthermore, this inhibition by PTEN acted on Shc phosphorylation and Ras activation, but not at the level of EGF receptor phosphorylation induced by EGF.
To test a biological function of MAP kinase in these glioma cells, we cotransfected PTEN with constitutively activated MEK1. We found that the resultant MAP kinase activation could antagonize PTEN function and partially rescue cell spreading on fibronectin impaired by PTEN. We conclude that PTEN inhibits Ras activity, and then the Ras/ERK common MAP kinase pathway for integrin- and growth factor–mediated signaling, and finally alters cell behavior.
Materials and Methods
Reagents and Antibodies
EGF and platelet-derived growth factor-BB (PDGF-BB) and myelin basic protein (MBP) were purchased from Sigma Chemical Co. (St. Louis, MO). Polyethyleneimine-F (PEI-F) cellulose was obtained from VWR Scientific Products (Willard, OH). Enhanced chemiluminescence (ECL) Western blotting detection reagents and 32P-phosphate were from Amersham Life Science, Inc. (Arlington Heights, IL). Monoclonal anti-H-Ras, anti-phospho-JNK and anti-Shc, as well as polyclonal anti-EGF receptor, anti-Grb2 and anti-Shc antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-phospho-p44/p42 MAP kinase antibody and p44/42 MAP kinase assay kits, as well as polyclonal anti-phospho-MEK1, anti-phospho-Akt, and anti-Akt were purchased from New England BioLabs, Inc. (Beverly, MA). Monoclonal anti-hemagglutinin (HA) antibody was purchased from Berkeley Antibody Co. (Richmond, CA). Monoclonal anti-paxillin, anti-FAK, and anti-phosphotyrosine antibodies (RC20) were obtained from Transduction Laboratories (Lexington, KY). Cy-3–conjugated goat antibody to mouse immunoglobulin G (Jackson ImmunoResearch Laboratories, West Grove, PA) were used at 1/600 dilution. Culture medium and FBS were provided by GIBCO BRL (Gaithersburg, MD).
Plasmids
Green fluorescent protein (GFP) expression plasmid pGZ21δxZ contained GFP with a Kozak consensus sequence in a cytomegalovirus (CMV) promoter-based expression system (Tamura et al., 1998). GFP-PTEN was generated by inserting a full-length human PTEN cDNA segment digested with HindIII and Xbal (Li et al., 1997) into the pGZ21δxZ plasmid. GFP-PTEN point mutant C124A (Cys124→ Ala) was generated by site-directed mutagenesis using PCR as described (Tamura et al., 1998). Plasmids containing HA-ERK2 and HA-JNK1, as well as constitutively activated H-RasV12 pcDNA3 were gifts from Dr. J.S. Gutkind (Oral and Pharyngeal Cancer Branch, NIDR, NIH, Bethesda, MD), and three plasmids containing pMCL⊗HA-tagged MEK1 (wild-type, dominant-negative, and constitutively activated) were provided by Dr. N.G. Ahn (Department of Chemistry and Biochemistry, University of Colorado, Boulder, CO; Mansour et al., 1994). Puromycin-resistance plasmid pHA262pur was obtained from Dr. Hein te Riele (Division of Molecular Carcinogenesis, The Netherlands Cancer Institute, The Netherlands; Lacalle et al., 1989).
Cell Culture and Transfections
The PTEN mutated glioblastoma cell lines U-87MG, DBTRG-05MG and U-373MG were obtained from American Type Culture Collection (Rockville, MD). U-87MG cells were maintained in DME containing 10% FBS in a humidified atmosphere containing 10% CO2 at 37°C. DBTRG-05MG and U-373MG cells were cultured in RPMI medium 1640 and MEM, respectively, with 10% FBS in a humidified atmosphere containing 5% CO2 at 37°C. Electroporation of cells was performed as previously described (LaFlamme et al., 1994) at 170 V and 960 μF with a Gene Pulser (Bio-Rad Laboratories, Hercules, CA). To increase expression of transfected genes, 5 mM sodium butyrate was included in culture media. Equal transfection efficiencies of GFP and GFP-PTEN (e.g., 42 ± 3% and 40 ± 5%, respectively) were confirmed by fluorescence microscopy to determine percentages of GFP-positive cells using a Zeiss Axiophot (Oberkochen, Germany).
Cell Adhesion and Preparation of Cell Lysates
24 h after transfection, cells were washed three times with PBS and then cultured in media containing 0.2% FBS overnight. For growth factor or FBS stimulation experiments, EGF or PDGF (10 ng/ml final concentration) or FBS (10% final concentration) were added to the culture medium, and incubated for another 10 min. The cells were then washed once with cold PBS and homogenized with 1% Triton X-100 lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium vanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride) for analysis of protein tyrosine phosphorylation. For cell adhesion experiments, cells were washed with PBS and detached by treating with 0.05% trypsin-EDTA. Trypsin was inactivated with 1 mg/ml soybean trypsin inhibitor. The suspended cells were washed two times in DME with 1% BSA. Cell suspensions were incubated in DME without serum with 1% BSA at 37°C for 30 min on a rotator. Thereafter, cells were counted and plated on dishes or glass coverslips coated with fibronectin (10 μg/ml), and then incubated at 37°C for the indicated times in DME with 1% BSA. The adherent cells were washed and lysed as described above.
Immunoprecipitation and Immunoblotting
Cell lysates were incubated with primary antibodies (3 μg/ml) for 4 h at 4°C with gentle rocking. The mixtures were further incubated with GammaBindG Sepharose beads for 2 h at 4°C. Thereafter, beads were washed three times with lysis buffer to remove unbound proteins. Immunocomplexes were suspended in reducing sample buffer, heated to 100°C for 5 min, and then subjected to SDS-PAGE and immunoblotting for phosphotyrosine, activated ERK2, phospho-MEK1, phospho-JNK, or other epitopes. Antibody binding was detected using the ECL system and Hyperfilm x-ray film.
ERK Kinase Assay
ERK2 activity was assayed using Elk-1 fusion protein as a substrate purchased from New England Biolabs, Inc. We confirmed that Elk-1 was comparable to MBP as a substrate of ERK2 in these cells. The assay protocol recommended by the manufacturer was used, except for an additional two washes with the kinase buffer after three washes with the lysis buffer. Immunocomplex bead pellets were suspended in 50 μl kinase buffer supplemented with 200 μM ATP and 2 μg Elk-1 fusion protein. After incubation for 30 min at 30°C, the reactions were stopped by adding SDS sample buffer and heating the samples for 5 min at 100°C. Homogenates were subjected to SDS-PAGE and immunoblotting for anti-phospho-Elk-1. The phosphorylated substrate bands were quantitated by densitometry using NIH Image software. ERK2 activity was also assayed as described (Miyamoto et al., 1996) using MBP as a substrate. Radioactivity in gel slices from each sample was determined with a liquid scintillation counter.
Jun Kinase Assay
Phosphorylation of c-Jun NH2-terminal kinase (JNK) was evaluated using anti-phospho-JNK monoclonal antibodies. Subconfluent cells were washed once with PBS and were then exposed to 200 mJ/cm2 of UV light using a Stratalinker UV Crosslinker (Stratagene, La Jolla, CA) followed by incubation for 1 h at 37°C. Cells were lysed as described above.
Akt Phosphorylation Assay
For assaying Akt phosphorylation levels, we selected transfected cells using puromycin. Cotransfections of various plasmids with a puromycin-resistance plasmid were performed as described above, and at 24 h after transfection cells were subcultured at a 1:2 dilution and maintained for 3 d in culture media containing puromycin (800 ng/ml). After 3 d selection, surviving cells were detached and replated in 60-mm dishes (3 × 105 cells/ dish) and cultured overnight in the absence of puromycin in regular culture media. The subconfluent cells were then cultured in media containing 0.2% FBS overnight. After PDGF treatment as indicated, the cells were rinsed with cold PBS and homogenized in 300 μl lysis buffer. The cell homogenates were subjected to SDS-PAGE and immunoblotting for anti-phospho-Akt or total Akt.
Ras GDP/GTP Exchange Assay
Levels of GTP-Ras were measured by labeling selected cells for 2 h in phosphate-free media in the presence of 0.5 mCi/ml 32P-phosphate. The selected cells as described above, were then incubated in serum-free and phosphate-free medium with 0.1% BSA (lipid ultra-free) for 18 h, and then labeled with 32P-phosphate at 0.5 mCi/ml for 2 h. After EGF treatment as indicated, the cells were rinsed with cold PBS, and homogenized in 500 μl lysis buffer (50 mM Tris, pH 7.5, 20 mM MgCl2, 150 mM NaCl, 0.5% NP40, 1 mM sodium vanadate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride). The cell extracts were clarified by centrifugation at 15,000 rpm (16,000 g) for 20 min, and then incubated with 20 μl anti-H-ras coupled to Sepharose beads for 1 h. After three washes with lysis buffer containing NaCl increased to a concentration of 500 mM, and two washes with 20 mM Tris, pH 7.5, 20 mM MgCl2, 500 mM NaCl, the sedimented beads were resuspended in 10 μl of 1 M KH2PO4, pH 7.8, 5 mM EDTA plus 10 μl methanol, and then heated for 2 min at 68°C. After brief centrifugation, the supernatant solutions were analyzed by thin layer chromatography on cellulose PEI-F plates in the presence of 1 M KH2PO4, pH 3.5. The plates were air dried and exposed to x-ray film. The spots corresponding to GDP and GTP were quantitated by excising the spots and scintillation counting.
Immunofluorescence Microscopy
12-mm glass coverslips were incubated with PBS containing 10 μg/ml FN overnight at 4°C. The coverslips were blocked with 10 mg/ml BSA for an additional 1 h at 37°C. Suspended cells treated as described above were allowed to spread for 10 min in DME containing 1% BSA, fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100 in PBS for 5 min, and then incubated with 4% paraformaldehyde in PBS for an additional 20 min. Focal adhesion formation was visualized by incubating first with mouse anti-paxillin monoclonal antibody, and then with Cy-3–conjugated goat antibody to mouse immunoglobulin G.
Results
PTEN Inhibition of MAP Kinase Activation Stimulated by Fibronectin
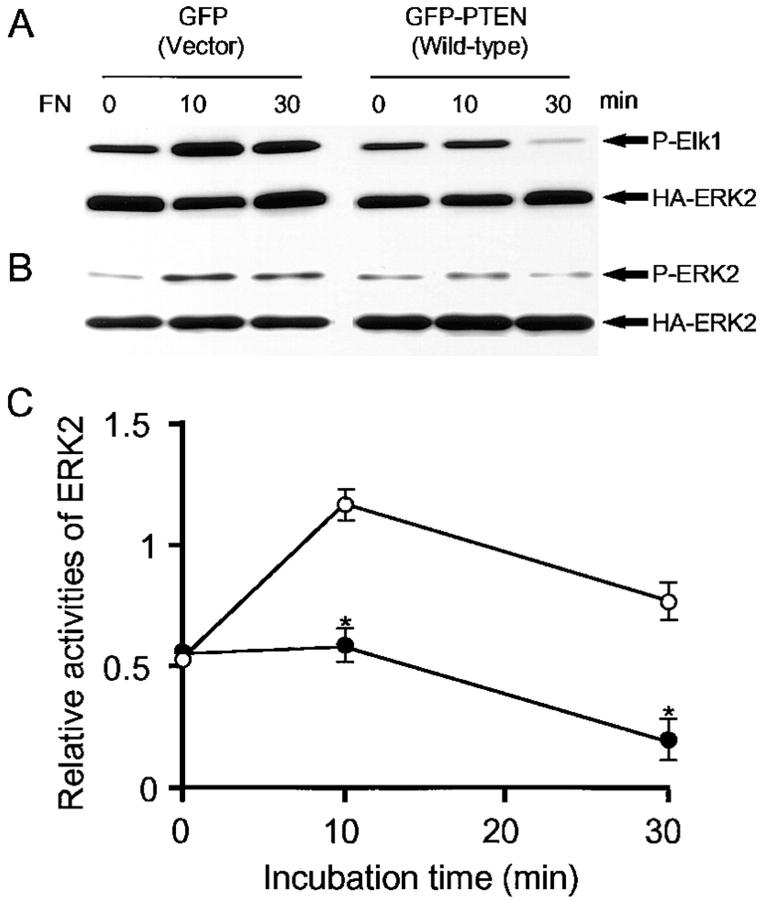
Although it is now well-established that PTEN is frequently deleted or mutated in a variety of tumors and tumor cell lines including glioblastomas, the biological functions of PTEN are poorly understood. Because we found recently that PTEN can help to regulate integrin-dependent cell spreading and focal adhesion formation (Tamura et al., 1998), we examined integrin-mediated signaling pathways. We measured the kinetics of ERK activation stimulated by adhesion to fibronectin in U-87MG, a glioblastoma cell line missing PTEN protein due to a frameshift mutation (Li et al., 1997; Steck et al., 1997). Within 10 min after plating on fibronectin, approximately twofold increases in MAP kinase activation were detected as measured by increased phosphorylation of the ERK substrate Elk-1 in nontransfected and control cells transfected with GFP plasmid without insert (Fig. 1 A).
Figure 1.
PTEN inhibition of ERK2 activation by adhesion to FN. U-87MG cells were transiently cotransfected with HA-ERK2 and either GFP or GFP-PTEN. 24 h after transfection, cells were serum-restricted overnight in 0.2% FBS. Cells were detached and incubated in serum-free medium for 30 min at 37°C as described under Materials and Methods. The cells were then either maintained in suspension (0 min) or allowed to attach to FN-coated dishes at 37°C for the indicated times. Cell lysates were prepared and ERK2 activity was assayed after immunoprecipitation as described under Materials and Methods. (A) A representative example of ERK2 substrate phosphorylation assay: upper panels show phosphorylation levels of the substrate Elk-1 stained by anti-phospho-Elk1 antibody and lower panels indicate total quantities of HA-ERK2 stained by anti-HA antibody. (B) Representative example of ERK2 phosphorylation assay: upper panels show phosphorylation levels of ERK2 stained with anti-activated ERK antibody and lower panels indicate total quantities of HA-ERK2 stained by anti-HA antibody. (C) Quantitation of ERK2 as assayed by Elk-1 phosphorylation; the data shown represent the average of triplicate samples ± SD, with normalization of P-Elk-1 levels by HA-ERK2 levels as determined by densitometric scanning of transblot bands. ○, GFP-expressing cells; •, GFP-PTEN-expressing cells. *P < 0.0001 according to Student's two-tailed t test.
In contrast, reconstitution of full-length wild-type PTEN- GFP to levels 1–1.5 times the level in human fibroblasts (confirmed by immunoblotting using polyclonal anti-PTEN antibody; data not shown), resulted in suppression of MAP kinase activation, such that there was no increase in Elk-1 phosphorylation at 10 min, and sharply decreased MAP kinase activity within 30 min as compared with controls (Fig. 1, A and C). The differences were significant at the P < 0.0001 level. Conversely, reduction of endogenous PTEN levels by anti-sense RNA expression in NIH 3T3 cells (Tamura et al., 1998) resulted in a 1.4 ± 0.2-fold increase in MAP kinase activation (difference significant at the P < 0.05 level). This result supports the notion that PTEN normally acts as a brake or suppressor of MAP kinase activation. These findings were confirmed by direct examination of ERK2 phosphorylation by immunoblotting with phospho-ERK2 antibody, which identifies the characteristic phosphorylation of activated ERK2 (Fig. 1 B). The inhibition of MAP kinase activation after PTEN expression was not accompanied by any change in cell viability (∼95% viability by trypan blue assay with or without PTEN expression).
Effects of PTEN on Activation of MAP Kinase Kinase, JNK, or Akt
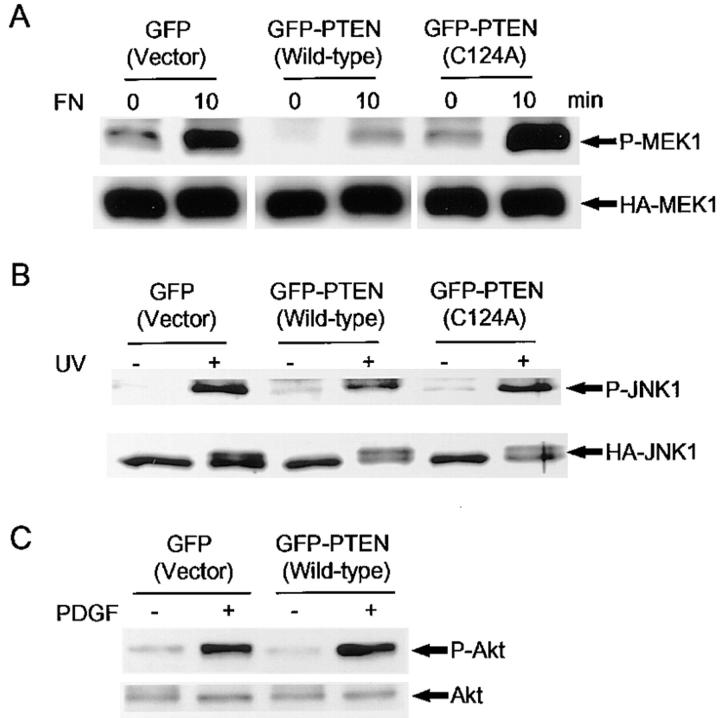
It has been reported that PTEN cannot dephosphorylate isolated, activated ERK2 in vitro (Myers et al., 1997). We examined whether PTEN affects the FN-stimulated phosphorylation level of MAP kinase kinase 1 (MEK1), which is the component immediately upstream of ERK in the Ras/ERK classic signaling pathway. We performed cotransfections of GFP or GFP-PTEN with wild-type HA-MEK1, immunoprecipitated homogenates with anti-HA antibody, and then immunoblotted with anti-phospho-MEK1, which recognizes only activated MEK1/2. The increased phosphorylation of MEK1 stimulated by FN was significantly inhibited in GFP-PTEN expressing cells as compared with cells expressed GFP tag only (Fig. 2 A). Previous experiments have indicated that growth suppression and inhibition of integrin-mediated spreading and focal adhesion formation in glioma cells requires a functional PTEN phosphatase catalytic domain (Furnari et al., 1997; Tamura et al., 1998). As shown in Fig. 2 A, the pattern of induction of MEK1 phosphorylation in cells transfected with the PTEN phosphatase-inactive mutant C124A was the same as controls without insert. This result demonstrates a similar requirement for the PTEN phosphatase domain in its downmodulation of MAP kinase activation.
Figure 2.
Effects of PTEN on phosphorylation of MEK1, JNK1, and Akt. U-87MG cells were transiently cotransfected with GFP, wild-type GFP-PTEN or C124A mutant GFP-PTEN plus either HA-MEK1 or HA-JNK1. MEK1 phosphorylation was assayed using anti-phospho-MEK antibody as described for HA-ERK2 in the legend to Fig. 1. For assaying JNK1 phosphorylation, transfectants were washed with PBS and then exposed to 200 mJ/cm2 of UV irradiation followed by incubation in complete culture medium for 1 h at 37°C. Immunoprecipitations for HA-MEK1 and HA-JNK1 were performed as described under Materials and Methods. For assaying Akt phosphorylation, U-87MG cells were cotransfected with GFP vector or GFP-PTEN with a puromycin resistance plasmid, and selected using puromycin for 3 d. After PDGF treatment, the cells were homogenated in lysis buffer as described under Materials and Methods. Total homogenates were subjected to SDS-PAGE and immunoblotting with anti-phospho-Akt. (A) Immunoprecipitated MEK1 phosphorylation stained by anti-phospho-MEK antibody (top) and total HA-MEK1 stained by anti-HA antibody (bottom). Note suppression of phospho-MEK1 phosphorylation in wild-type PTEN-transfected cells. (B) JNK1 phosphorylation stained by anti-phospho-JNK (top) and total HA-JNK1 stained by anti-HA antibody (bottom). (C) Akt phosphorylation stained by anti-phospho-Akt (top) and total Akt stained by anti-Akt (bottom). Note lack of effects of PTEN cotransfection.
In contrast to the effects of PTEN on ERK activation, there were no significant effects on JNK activation after stimulation by UV irradiation (Fig. 2 B) or Akt phosphorylation (a target of phosphatidylinositol 3′-kinase) stimulated by PDGF (Fig. 2 C). Identical induced patterns of JNK phosphorylation were observed in all three transient transfectants, including wild-type PTEN and the C124A mutant (Fig. 2 B). Under all conditions, an additional high- molecular mass band was observed by anti-HA-JNK1 staining, which may represent additional phosphorylation independent of the sites evaluated by the anti-phospho-JNK antibody (which recognizes phosphorylated Ser 63 and Ser 73); however, again no differences levels of this band due to PTEN were observed (Fig. 2 B). For examining Akt phosphorylation levels, we performed cotransfection of GFP or GFP-PTEN with a puromycin resistance plasmid. U-87MG cells were cotransfected with GFP vector or GFP-PTEN and a puromycin resistance plasmid, and selected using puromycin for 3 d. This selection for transient transfectants resulted in ∼90% positive cells expressing GFP or GFP-PTEN as determined by fluorescence microscopy. As shown in Fig. 2 C, PTEN expression had no detectable effect on Akt phosphorylation stimulated by PDGF.
MAP Kinase Activation by Growth Factors Is Also Blocked by PTEN Expression
Both integrins and growth factors can stimulate ERK activation. DTBRG-05MG glioma cells expressing EGF but not PDGF receptors displayed ERK activation in response to EGF, e.g., in control cells expressing GFP without PTEN insert (Fig. 3 A). This activation was not blocked by 50 nM wortmannin, a phosphatidylinositol 3′-kinase inhibitor (data not shown). However, EGF stimulation of ERK activation was suppressed in PTEN-expressing cells (Fig. 3 A). ERK2 activity was assayed using MBP as a substrate (Fig. 3 B, a). The differences between effects of EGF in cells that expressed wild-type PTEN versus mutant PTEN were significant at the P < 0.0001 level (Fig. 3 B, b). PTEN also inhibited MEK1 phosphorylation stimulated by either PDGF or FBS in U-87MG cells (Fig. 3 C). Similarly, PTEN was found to inhibit MEK1 phosphorylation in DTBRG-05MG cells, as well as ERK activation after EGF stimulation in U-87MG cells (data not shown). Taken together, these results suggested that PTEN might block the MAP kinase pathway at some point upstream of MEK that might be common to both integrin- and growth factor–mediated signaling pathways.
Figure 3.

Effects of PTEN on MAP kinase activation stimulated by growth factors. Cells were cotransfected with GFP or GFP-PTEN with HA-ERK2 (A and B) or HA-MEK1 (C), then were switched to culture in 0.2% serum at 24 h after transfection. The serum-restricted cells were then incubated 10 min with EGF (A, DBTRG-05MG cells; B, U-87MG) or with PDGF or 10% FBS (C, U-87MG cells). Cell lysates were prepared and equal protein concentrations were analyzed by immunoblotting as described under Materials and Methods. Upper panels show phosphorylation levels using anti-phospho-ERK (A) or anti-phospho-MEK (C). Lower panels show relative quantities of total protein as determined by anti-HA immunoblotting (A, B a, C). ERK2 activity after EGF stimulation was examined using MBP as a substrate (B a). (B b) Quantitation of ERK2 activity by scintillation counting of incorporated radioactivity; the data are shown as fold increases in 32P incorporation into MBP compared with the unstimulated control, which is taken as 1.0. Data represent means ± SD of three experiments. *P < 0.0001 compared with EGF stimulation in cells transfected with GFP or GFP-PTEN with the C124A mutation.
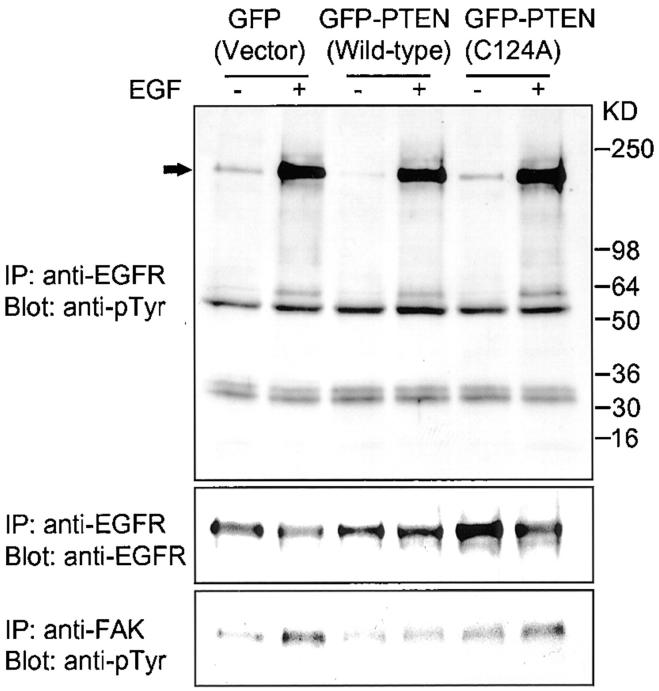
PTEN Does Not Inhibit EGF Receptor Phosphorylation
Integrin cooperation with growth factors can include marked changes in the state of growth factor receptor autophosphorylation (Cybulsky et al., 1994; Miyamoto et al., 1996). Therefore, we examined for PTEN regulation of EGF receptor phosphorylation levels. Interestingly, PTEN expression had no detectable effect on EGF receptor autophosphorylation stimulated by EGF in the three transfectant glioma cell lines. As shown in Fig. 4, EGF stimulation in U-87MG cells expressing wild-type PTEN had the same levels of receptor phosphorylation as in control cells or in those transfected with mutant PTEN. We compared the effects of PTEN on FAK phosphorylation levels in these same cell lines, since we recently found that PTEN dephosphorylates FAK in vivo and in vitro and inhibits FAK phosphorylation stimulated by FN (Tamura et al., 1998). In this study, we observed that wild-type PTEN inhibited FAK phosphorylation stimulated by EGF, whereas phosphatase-inactive mutant PTEN permitted the induction of FAK phosphorylation (Fig. 4).
Figure 4.
Effects of PTEN on EGF receptor and FAK phosphorylation. U-87MG cells were cotransfected with GFP, wild-type GFP-PTEN, or phosphatase-inactivated mutant GFP-PTEN and a puromycin-resistance plasmid. Transfectant cells were selected for 3 d using puromycin as described under Materials and Methods. Immunoprecipitations were carried out with anti-EGFR and anti-FAK. Upper panel shows phosphorylation of EGF receptor stained by anti-phosphotyrosine (monoclonal antibody RC20) and the middle panel indicates total EGF receptor. Lower panel shows FAK phosphorylation levels.
PTEN Inhibition of Ras Activation
Ras has been suggested as an initial component in common between integrin- and growth factor-mediated MAP kinase signaling pathways. Therefore, we speculated that the ability of PTEN inhibit both integrin- and growth factor–mediated MAP kinase signaling pathways might be due to PTEN inhibiting Ras activation. We tested for effects on activation in the EGF pathway by examining Ras GTP loading in EGF-stimulated cells. As shown in Fig. 5, transfection with GFP tag or mutant GFP-PTEN still permitted substantial increases in GTP loading of Ras by a factor of 2.1–2.6-fold over nonstimulated controls. However, cells transfected with wild-type GFP-PTEN were unable to generate significant increases in GTP loading of Ras over controls after EGF stimulation (Fig. 5). The differences between effects of EGF in cells that expressed wild-type GFP-PTEN and GFP tag or mutant GFP-PTEN were significant at the P < 0.0001 level. We also performed cotransfection of wild-type GFP-PTEN with constitutively activated H-Ras, and found that PTEN could not block its ability to induce ERK2 phosphorylation (data not shown). These results identify the initial Ras activation step as an upstream source of PTEN inhibition of MAP kinase activation.
Figure 5.

Inhibition of Ras activation by wild-type PTEN. Puromycin-selected cells were prelabeled with 32P-phosphate, then exposed to 10 ng/ml EGF for 10 min. Cell extracts were immunoprecipitated with specific anti-H-Ras antibody, and bound GTP and GDP were resolved by thin layer chromatography as described under Materials and Methods. (A) A representative example of separation patterns of GTP and GDP on a PEI-F cellulose plate for cells transfected by GFP (vector control), wild-type GFP-PTEN, or GFP-PTEN with the C124A mutation. (B) Quantitative evaluation of Ras activation presented as the fold increase in GTP/GDP ratio compared with unstimulated control, which is taken as 1.0. Data represent the means ± SD of three experiments. *P < 0.0001 compared with its control using Student's t test.
PTEN Inhibits Shc phosphorylation and Grb2 Recruitment
The adapter protein Shc has been implicated in the Ras signaling pathway in growth factor-stimulated cells by virtue of its association with the Grb2 adapter molecule. In stimulated cells, Shc binds to the activated growth factor receptor and becomes phosphorylated. Upon phosphorylation, Shc interacts with the SH2 domain of Grb2 and functions as an alternative docking site for the Grb2-Sos complex (Rozakis-Adcock et al., 1992; Pawson, 1995). As shown in Fig. 6, Shc has three isoforms of 66, 52, and 46 kD, which derive from differential translation initiation at three ATG sites (Migliaccio et al., 1997). U-87MG cells expressing wild-type PTEN showed substantial suppression of Shc phosphorylation after EGF stimulation (especially the 52-kD isoform, which showed a 61 ± 9% reduction compared with control Shc phosphorylation; Fig. 6, upper middle). Consistent with this effect, recruitment of Grb2 was also downregulated by PTEN expression (Fig. 6, lower middle). However, quantities of EGF receptor associated with Shc (Fig. 6, top) showed no detectable differences between control and PTEN-expressing cells, consistent with the finding that PTEN does not inhibit EGF receptor phosphorylation. PTEN also inhibited Shc phosphorylation stimulated by FN binding to integrins (data not shown).
Figure 6.
Inhibition of Shc phosphorylation by PTEN. Puromycin-selected transfectant cells were exposed to 10 ng/ml EGF for 10 min. Cell extracts were immunoprecipitated with monoclonal anti-Shc antibody. Phosphorylation of Shc was detected by anti-phosphotyrosine (monoclonal antibody RC 20; upper middle panel), and other proteins associated with Shc were detected using polyclonal anti-EGF receptor (top) or Grb2 (lower middle panel) antibodies. Total Shc levels were evaluated by immunoblotting using polyclonal anti-Shc antibody (bottom).
Experimental Activation of MAP Kinase Partially Rescues Cell Spreading Impaired by PTEN Expression
To investigate the role of MAP kinase activation in a putative cell biological function of PTEN, we used a transient cotransfection assay that permitted restoration of MAP kinase activation impaired by PTEN expression using constitutively activated MEK1, a downstream component of the Ras/ERK signaling pathway. Our recent studies had indicated that PTEN affects cell spreading on FN by human fibroblasts, as well as by U-87MG and DBTRG-05MG glioma cell lines, though the duration of PTEN effects differed. In human foreskin fibroblasts, the effect on spreading was maximal at 20–30 min after adhesion to fibronectin, but diminished after 2 h. However, clear inhibition of cell spreading could be observed even after 18 h in DBTRG-05MG cells (Tamura et al., 1998). These cell-type differences may be explained by the fact that human fibroblasts express endogenous PTEN, whereas U-87MG and DBTRG-05MG cells do not express the protein due to mutations. The morphology of PTEN-expressing U-87MG cells includes thin cell processes somewhat resembling these of oligodendroglial cells (Tamura et al., 1998). In this study, we compared cell spreading at 10 min in three glioma cell lines: U-87MG, DBTRG-05MG and U-373MG; FN stimulation of ERK activation is maximal at this time point in these cells. Overexpression of constitutively activated MEK1 was accompanied by two- to fivefold increases in ERK activity compared with wild-type MEK1 stimulated by FN. Of particular interest, cotransfection of constitutively activated MEK1 with wild-type PTEN partially rescued cell spreading and focal contact formation. As shown in Fig. 7 A, the enhancement of focal contact formation was apparent by paxillin staining. The proportion of cells capable of spreading increased by >65%, rising from a value of 31% of total cells spread with PTEN alone to 52% in cells with PTEN plus activated MEK1 (Fig. 7 B). The differences in cell spreading were significant at the P < 0.01 level. In contrast, parallel cooverexpression of constitutively activated MEK1 with the PTEN controls of GFP alone or mutant GFP-PTEN had no effect on cell spreading and focal contact formation (data not shown). Because we had previously shown that FAK overexpression could also partially rescue the cell spreading response, we performed a triple transfection experiment combining PTEN, FAK, and activated MEK1. The results showed no further rescue of cell spreading, with the percentages of cell spreading when cotransfected with PTEN as follows: active MEK1 = 50 ± 3%; HA-FAK = 52 ± 5%; and active MEK1 + HA-FAK = 49 ± 6%. This result strongly suggests a linear rather than parallel biological pathway, consistent with a downstream effect of PTEN on MEK and ERK through FAK for cell spreading.
Figure 7.

Effects of constitutively activated MEK1 on PTEN- expressing cells. U-87MG cells were cotransfected with GFP, wild-type GFP-PTEN or phosphatase mutant GFP-PTEN, with or without constitutively activated MEK1 or dominant-negative MEK1, and then allowed to attach to FN-coated coverslips for 10 min as described under Materials and Methods. (A) A representative example of paxillin staining indicated by arrowheads; the green fluorescence-expressing cells are marked by open arrows; (a) GFP tag; (b) C124A mutant GFP-PTEN; (c) wild-type GFP-PTEN; (d) GFP-PTEN with dominant negative MEK1; (e) GFP-PTEN with constitutively activated MEK1. Localization in the thick central portion of poorly spread cells does not represent specific staining; this region is so much thicker than spreading cytoplasm that normal low-level, nonspecific staining appears brighter. The same immunofluorescence artifact is seen with nonimmune antibody in poorly spread cells (data not shown). Bar, 10 μm. (B) Cell spreading assay showing the percentage of cells that had spread at 10 min as determined by paxillin staining; the data are means ± SD of four experiments; 100 cells were counted for each experiment. *P < 0.01 versus cells transfected with GFP-PTEN only, or versus GFP-PTEN plus dominant negative MEK1. DN, dominant negative; Act, constitutively activated MEK1.
Discussion
Many recent studies have established that the PTEN gene is deleted or mutated in a wide variety of tumor tissues and tumor cell lines, as well as in two genetic disorders with predisposition to cancer (Kong et al., 1997; Li et al., 1997; Liaw et al., 1997; Marsh et al., 1997; Nelen et al., 1997; Steck et al., 1997). Furthermore, transfected PTEN suppresses cell growth and tumorigenicity in glioblastoma cell lines (Furnari et al., 1997; Cheney et al., 1998). Therefore, PTEN is considered a major new tumor suppressor.
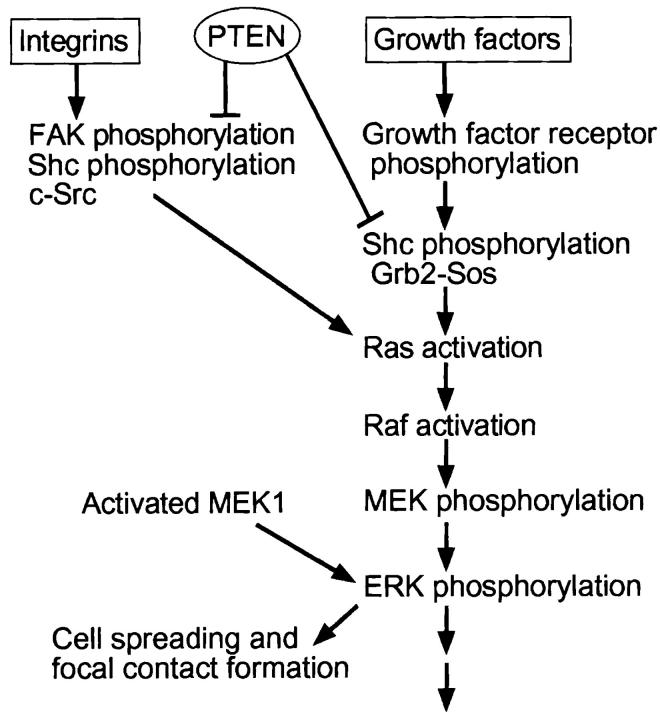
One theoretically attractive site of action of PTEN might be on the MAP kinases, which comprise central cellular regulatory pathways. In this report, we tested for effects of PTEN on integrin- and growth factor-mediated MAP kinase signaling. Our major findings are summarized in Fig. 8, and include the following: (a) activation of the ERK type of MAP kinase is inhibited by PTEN expression in cells lacking the protein, whether stimulated by integrin ligation or a growth factor, yet the JNK/SAPK MAP kinase pathway and Akt are unaffected; (b) expression of anti-sense PTEN to reduce PTEN in cells that normally express this protein results in ERK activation; (c) upstream MEK and Ras activation and Shc phosphorylation are inhibited, but EGF receptor phosphorylation is not affected; (d) the inhibition of Ras can be overcome by expression of activated Ras; and (e) overexpression of an activated downstream component of the pathway, MEK1, can antagonize the biological effects of PTEN on cell spreading and focal contact formation. Our results strongly suggest that down-regulation of the Ras/MAP kinase pathway is an important function of the PTEN tumor suppressor gene in its cell biological functions.
Figure 8.
Working model of the PTEN regulatory network. Integrins and growth factors can separately stimulate the Ras/ERK/MAP kinase pathway or function collaboratively. Integrin receptor engagement with FN stimulates FAK, Shc and c-Src, and they activate the pathway as described (Schlaepfer et al., 1998). Growth factors can activate the pathway through autophosphorylation of growth factor receptors. PTEN reduces Shc phosphorylation and its interaction with Grb2, as well as the downstream Ras/ ERK common MAP kinase pathway for integrin- and growth factor–mediated signaling, and then affects cell spreading and focal contact formation. Activated MEK1 can partially reverse the latter effects of PTEN.
Many studies have established the essential role of the MAP kinase pathway in cellular transformation and cell cycle regulation. Mutant oncogenic forms of upstream signaling components such as Ras, Raf-1, and G proteins have been found in malignant cells (Bishop, 1991). Conversely, inhibition of the MAP kinase pathway blocks c-Ras– and v-Mos–dependent cell transformation, and inhibits cell growth, cell adhesion, and spreading (Pages et al., 1993; Cowley et al., 1994; Okazaki and Sagata, 1995; Hughes et al., 1997; Whalen et al., 1997). Consistent with some of these observations, in this study increased MAP kinase activation induced by constitutively activated MEK1 could partially rescue cell spreading; it had, however, no significant effects on cell growth (data not shown). These findings indicate that the MAP kinase pathway is not the only pathway affected by PTEN.
Two substrates of PTEN have been identified recently, focal adhesion kinase (FAK) and certain phosphoinositol lipids (Maehama and Dixon, 1998; Tamura et al., 1998). The role of FAK in integrin-mediated MAP kinase activation is controversial (Schlaepfer et al., 1994; Richardson and Parsons, 1995; Wary et al., 1996; Lin et al., 1997; Schlaepfer and Hunter, 1997; Wary et al., 1998), but it appears to be one of several possible pathways for enhancing the binding of Grb2, leading to the formation of signaling complexes that promote activation of the Ras signaling pathway (Schlaepfer et al., 1994). FAK is probably not, however, an intermediary in EGF growth factor receptor stimulation. In contrast to FAK, Shc is a more plausible effector for growth factor-mediated MAP kinase activation. Several lines of evidence suggest that Shc-Grb2 association after growth factor or integrin stimulation is involved in Ras activation (Rozakis-Adcock et al., 1992; Pronk et al., 1994; Wary et al., 1998). It will be interesting to investigate the mechanisms by which PTEN inhibits Shc phosphorylation.
Recently, it has been reported that PTEN dephosphorylates phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3), which is a product of phosphoinositide 3′-kinase (PI 3-kinase), specifically at position 3 on the inositol ring (Maehama and Dixon, 1998). However, EGF receptor signaling does not appear to require PI 3-kinase activation (King et al., 1997 and data not shown), making it unlikely that the effects of PTEN on MAP kinase activation by this pathway is via effects on phosphoinositides. Moreover, even though PTEN inhibits MAP kinase activation by PDGF, it does not affect PDGF-induced phosphorylation of Akt, a target of PI 3-kinase. Integrin activation of inositol lipid signaling appears to feed into the Ras/MAP kinase pathway at Raf (King et al., 1997). In the present study, however, expression of an upstream component, activated Ras, was sufficient to overcome the effect of PTEN on MAP kinase activation. Taken collectively, these findings suggest that the effects of PTEN on MAP kinase signaling in these cells is not via effects on phosphoinositides, but is instead through effects on Ras signaling.
PTEN consequently has complex but important effects on signaling and cell biological processes. It can downmodulate Ras-mediated MAP kinase activation from integrins and growth factors (this study), phosphoinositide signaling, FAK and p130Cas tyrosine phosphorylation, Shc tyrosine phosphorylation and Grb2 recruitment (this study), focal adhesion and actin cytoskeletal organization, cell proliferation, and cell migration (Furnari et al., 1997; Cheney et al., 1998; Maehama and Dixon, 1998; Tamura et al., 1998). These pleiotropic but selective effects are probably due to specific actions of its phosphatase, since the biological actions examined required an intact phosphatase domain. PTEN is thus a new regulator of a specific subset of important cell biological functions. Overall, our results suggest key sites of action of PTEN on (a) Shc and its interaction with the adapter protein Grb2 and (b) focal adhesion kinase. PTEN expression results in dephosphorylation of these two molecules, accompanied by inhibition of Ras activation, followed by further downstream effects targeting the ERK pathway of MAP kinase signaling, but without affecting JNK or Akt signaling.
Abbreviations used in this paper
ERK
extracellular signal-related kinase
FAK
focal adhesion kinase
FN
fibronectin
GFP
green fluorescent protein
HA
hemagglutinin
JNK/SAPK
c-jun NH2-terminal kinase/ stress-activated protein kinase
MAP
mitogen-activated protein
MEK
MAP or ERK kinase
References
- Bishop JM. Molecular themes in oncogenesis. Cell. 1991;64:235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- Chen Q, Lin TH, Der CJ, Juliano RL. Integrin-mediated activation of MEK and mitogen-activated protein kinase is independent of Ras. J Biol Chem. 1996;271:18122–18127. doi: 10.1074/jbc.271.30.18122. [DOI] [PubMed] [Google Scholar]
- Cheney IW, Johnson DE, Vaillancourt MT, Avanzini J, Morimoto A, Demers GW, Wills KN, Shabram PW, Bolen JB, Tavtigian SV, Bookstein R. Suppression of tumorigenicity of glioblastoma cells by adenovirus-mediated MMAC1/PTEN gene transfer. Cancer Res. 1998;58:2331–2334. [PubMed] [Google Scholar]
- Clark EA, Hynes RO. Ras activation is necessary for integrin-mediated activation of extracellular signal-regulated kinase 2 and cytosolic phospholipase A2 but not for cytoskeletal organization. J Biol Chem. 1996;271:14814–14818. doi: 10.1074/jbc.271.25.14814. [DOI] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Cybulsky AV, McTavish AJ, Cyr MD. Extracellular matrix modulates epidermal growth factor receptor activation in rat glomerular epithelial cells. J Clin Invest. 1994;94:68–78. doi: 10.1172/JCI117350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Furnari FB, Lin H, Huang HS, Cavenee WK. Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc Natl Acad Sci USA. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PE, Renshaw MW, Pfaff M, Forsyth J, Keivens VM, Schwartz MA, Ginsberg MH. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jelinek T, Dent P, Sturgill TW, Weber MJ. Ras-induced activation of Raf-1 is dependent on tyrosine phosphorylation. Mol Cell Biol. 1996;16:1027–1034. doi: 10.1128/mcb.16.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Suzuki A, Zou TT, Sakurada A, Kemp LW, Wakatsuki S, Yokoyama T, Yamakawa H, Furukawa T, Sato M, et al. PTEN1 is frequently mutated in primary endometrial carcinomas. Nat Genet. 1997;17:143–144. doi: 10.1038/ng1097-143. [DOI] [PubMed] [Google Scholar]
- Lacalle RA, Pulido D, Vara J, Zalacain M, Jimenez A. Molecular analysis of the pac gene encoding a puromycin N-acetyl transferase from Streptomyces alboniger. Gene. 1989;79:375–380. doi: 10.1016/0378-1119(89)90220-5. [DOI] [PubMed] [Google Scholar]
- LaFlamme SE, Thomas LA, Yamada SS, Yamada KM. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol. 1994;126:1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- Lin TH, Aplin AE, Shen Y, Chen Q, Schaller M, Romer L, Aukhil I, Juliano RL. Integrin-mediated activation of MAP kinase is independent of FAK: evidence for dual integrin signaling pathways in fibroblasts. J Cell Biol. 1997;136:1385–1395. doi: 10.1083/jcb.136.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande GF, Woude, Ahn NG. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Dahia PL, Zheng Z, Liaw D, Parsons R, Gorlin RJ, Eng C. Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat Genet. 1997;16:333–334. doi: 10.1038/ng0897-333. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti-Furga G, Pawson T, Di Fiore PP, Lanfrancone L, Pelicci PG. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signaling pathway. EMBO (Eur Mol Biol Organ) J. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino N, Mimura T, Hamasaki K, Tobe K, Ueki K, Kikuchi K, Takehara K, Kadowaki T, Yazaki Y, Nojima Y. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44erk-1 and p42erk-2. J Biol Chem. 1995;270:269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelen MR, van Staveren WC, Peeters EA, Hassel MB, Gorlin RJ, Hamm H, Lindboe CF, Fryns JP, Sijmons RH, Woods DG, et al. Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum Mol Genet. 1997;6:1383–1387. doi: 10.1093/hmg/6.8.1383. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Sagata N. MAP kinase activation is essential for oncogenic transformation of NIH3T3 cells by Mos. Oncogene. 1995;10:1149–1157. [PubMed] [Google Scholar]
- Pages G, Lenormand P, L'Allemain G, Chambard JC, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T. protein modules and signaling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Pronk GJ, de Vries-Smits AM, Buday L, Downward J, Maassen JA, Medema RH, Bos JL. Involvement of Shc in insulin- and epidermal growth factor-induced activation of p21ras. Mol Cell Biol. 1994;14:1575–1581. doi: 10.1128/mcb.14.3.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO (Eur Mol Biol Organ) J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Parsons JT. Signal transduction through integrins: a central role for focal adhesion kinase? . Bioessays. 1995;17:229–236. doi: 10.1002/bies.950170309. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W, Batzer A, Thomas S, Brugge J, Pelicci PG, et al. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinase. Nature. 1992;360:689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Vaheri A. Cell-to-cell contact and extracellular matrix. Curr Opin Cell Biol. 1997;9:605–607. doi: 10.1016/s0955-0674(97)80112-3. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Jones KC, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- Whalen AM, Galasinski SC, Shapiro PS, Nahreini TS, Ahn NG. Megakaryocytic differentiation induced by constitutive activation of mitogen-activated protein kinase kinase. Mol Cell Biol. 1997;17:1947–1958. doi: 10.1128/mcb.17.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol. 1995;7:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]