Paxillin LD4 Motif Binds PAK and PIX through a Novel 95-kD Ankyrin Repeat, ARF–GAP Protein: A Role in Cytoskeletal Remodeling (original) (raw)
Abstract
Paxillin is a focal adhesion adaptor protein involved in the integration of growth factor- and adhesion-mediated signal transduction pathways. Repeats of a leucine-rich sequence named paxillin LD motifs (Brown M.C., M.S. Curtis, and C.E. Turner. 1998. Nature Struct. Biol. 5:677–678) have been implicated in paxillin binding to focal adhesion kinase (FAK) and vinculin. Here we demonstrate that the individual paxillin LD motifs function as discrete and selective protein binding interfaces. A novel scaffolding function is described for paxillin LD4 in the binding of a complex of proteins containing active p21 GTPase–activated kinase (PAK), Nck, and the guanine nucleotide exchange factor, PIX. The association of this complex with paxillin is mediated by a new 95-kD protein, p95PKL (paxillin-kinase linker), which binds directly to paxillin LD4 and PIX. This protein complex also binds to Hic-5, suggesting a conservation of LD function across the paxillin superfamily. Cloning of p95PKL revealed a multidomain protein containing an NH2-terminal ARF–GAP domain, three ankyrin-like repeats, a potential calcium-binding EF hand, calmodulin-binding IQ motifs, a myosin homology domain, and two paxillin-binding subdomains (PBS). Green fluorescent protein- (GFP-) tagged p95PKL localized to focal adhesions/complexes in CHO.K1 cells. Overexpression in neuroblastoma cells of a paxillin LD4 deletion mutant inhibited lamellipodia formation in response to insulin-like growth fac- tor-1. Microinjection of GST–LD4 into NIH3T3 cells significantly decreased cell migration into a wound. These data implicate paxillin as a mediator of p21 GTPase–regulated actin cytoskeletal reorganization through the recruitment to nascent focal adhesion structures of an active PAK/PIX complex potentially via interactions with p95PKL.
Keywords: migration, signal transduction, integrins, trafficking, vesicular transport
The Rho family of p21 GTPases plays a pivotal role in regulating changes in the actin-based cytoskeleton observed in response to growth factors and cell adhesion. A hierarchy is suggested in which activation of Cdc42 progresses through Rac to Rho (Ridley and Hall, 1992; Hall, 1994). Rho A has been implicated in the formation of focal adhesions and actin stress fibers, while Cdc42 and Rac activation stimulate the more dynamic processes of filopodial and lamellipodial extension, respectively, in association with focal complex formation (Nobes and Hall, 1995). The precise mechanism(s) by which these GTPases remodel the actin cytoskeleton remains to be determined, although direct association of serine/threonine kinases with the individual GTPase is likely a primary mediator of these effects. For example, Rho-associated protein kinase N and Rho kinase increase the activity of myosin light chain kinase (MLCK)1 and myosin, triggering the generation of tension along the actin and myosin-containing stress fibers, leading to focal adhesion formation (Burridge and Chrzanowska-Wodnicka, 1996; Leung et al., 1996; Narumiya, 1996). Conversely, p21 GTPase–activated kinase (PAK) activity has been linked to focal adhesion disassembly (Manser et al., 1997), possibly through its ability to phosphorylate and thereby inhibit MLCK (Sanders et al., 1999). PAK, a serine/threonine kinase, binds tightly to activated Cdc42 and Rac1, and has been localized to sites of primary cell–matrix interaction called focal complexes (Nobes and Hall, 1995; Lim et al., 1996). However, the role of PAK in Cdc42- and Rac-mediated filopodia and lamellipodia formation is unclear (Nobes and Hall, 1995; Lamarche et al., 1996; Manser et al., 1997; Sells et al., 1997; Westwick et al., 1997).
The focal complexes and focal adhesions formed in response to p21 GTPase activity are sites of integrin engagement with the extracellular matrix that consist of structural proteins, such as talin and vinculin, that link integrins to the actin cytoskeleton and regulatory proteins, such as focal adhesion kinase (FAK) and Src, that likely transduce integrin-, as well as growth factor-dependent signals (Clark and Brugge, 1995; Craig and Johnson, 1996). Paxillin likely serves a multidomain adaptor function at these sites (Turner et al., 1990; Tachibana et al., 1995; Turner, 1998). Indeed, the paxillin NH2 terminus contains binding sites for the nonreceptor kinases FAK, PYK2, Csk, Src, and the structural protein vinculin (Sabe et al., 1994; Turner and Miller, 1994; Schaller and Parsons, 1995; Tachibana et al., 1995; Salgia et al., 1995), whereas the COOH terminus, composed of four LIM domains, mediates paxillin targeting to focal adhesions (Brown et al., 1996, 1998a).
Paxillin has been implicated in the regulation of focal adhesion assembly and signal transduction through these elements, primarily as a result of its propensity to become tyrosine phosphorylated in response to cell adhesion to extracellular matrix and following challenge of serum-starved cells with soluble growth factors (Burridge et al., 1992; Zachary et al., 1993; Rozengurt, 1995). Recent reports indicate that serine/threonine phosphorylation of paxillin also occurs in response to adhesion of cells to extracellular matrix and that, as with FAK, the serine/threonine kinases are physically associated with paxillin (DeNichilo and Yamada, 1996; Bellis et al., 1997; Brown et al., 1998a).
We have identified a leucine-rich motif with the consensus LDXLLXXL that is repeated five times within the NH2 terminus of paxillin (Brown et al., 1996, 1998b). The presence of these motifs within the binding sites on paxillin for FAK, vinculin, and the E6 oncoprotein from papillomavirus (Tong et al., 1997; Vande Pol et al., 1998), as well as their occurrence in other diverse protein families, suggests that the LD motif represents a novel protein-binding module of general importance (Brown et al., 1998b).
In this report, we present evidence that the individual LD motifs of paxillin function as independent and selective protein binding sites. Furthermore, we show that through the LD4 motif, paxillin binds a protein complex containing PAK, the adaptor protein Nck, two members of the Cdc42/Rac1 guanine nucleotide exchange factor (PIX/COOL/p85SPR family; Woo et al., 1997; Bagrodia et al., 1998; Manser et al., 1998), and a new multidomain ARF–GAP protein, p95PKL (paxillin-kinase linker; pronounced pickle). p95PKL binds paxillin and PIX directly in vitro. The association of paxillin with this complex suggests a potential function for paxillin in recruiting these proteins to the plasma membrane and thereby directly mediating p21 GTPase reorganization of the actin-based cytoskeleton.
Materials and Methods
Materials
PAK antibody was purchased from Santa Cruz, vinculin (Vin 11-5) from Sigma Chemical Co., and Nck, p130Cas, and clathrin heavy chain antibodies were obtained from Transduction Labs. FAK antibody (BC3) was a generous gift of Dr. J.T. Parsons (University of Virginia, Charlottesville, VA), antitalin (8d4) was provided by Dr. K. Burridge (University of North Carolina, Chapel Hill, NC). p50-COOL-1 antibody, which recognizes p85/p75PIX/COOL, was as described (Bagrodia et al., 1998).
Preparation of Fusion Proteins and Binding Assays
Individual glutathione S-transferase (GST) fusion proteins spanning paxillin amino acid (aa) 54–313, or encompassing LD1 (aa 1–20), LD2 (aa 141–160), LD3 (aa 212–231), LD4 (aa 263–282), and LD5 (aa 299–317) were expressed in Escherichia coli (DH5α) and purified on glutathione agarose beads as previously described (Turner and Miller, 1994; Brown et al., 1996).
Tissue lysates (newborn rat brain, a gift of Qin He, State University of New York, Syracuse, NY, or embryonic day 17 chicken gizzard smooth muscle) were prepared by Dounce homogenization in 10 vol lysis buffer (50 mM Tris-HCl, pH 7.6, 50 mM NaCl, 1 mM EGTA, 2 mM MgCl2, 0.1% β-mercaptoethanol, 0.5% TX-100) containing protease inhibitors (Complete™ EDTA-free; Boehringer Mannheim), followed by clarification for 15 min at 15,000 g. Alternatively, cultured cells were washed twice with ice-cold Hanks' buffer and processed as for tissue lysates. Lysates (0.5–1 mg protein) were incubated with the individual fusion proteins (50 μg) in fusion protein-binding buffer (same as lysis buffer, but with the Triton X-100 adjusted to 0.1%) for 120 min at 4°C and then washed four times in binding buffer. The washed precipitates were boiled in SDS-PAGE sample buffer, followed by SDS-PAGE and Western blot analysis, or processed for in vitro kinase assay.
For in vivo coprecipitation experiments, rat brain was homogenized in 10 mM Tris-HCl, pH 7.6, 50 mM NaCl, 1% NP-40, 10% glycerol. Paxillin was precipitated with antipaxillin antibody (Transduction Labs) and the blots probed with antibodies to PIX, PAK, paxillin, and p130Cas. Mouse Ig was used as a control. To demonstrate an association in vivo between paxillin and p95PKL, green fluorescent protein– (GFP–) p95PKL was transfected into COS 7 cells. 72 h after transfection, the cells were lysed in 10 mM Tris-HCl, pH 7.6, 50 mM NaCl, 1% NP-40, 10% glycerol, and clarified by centrifugation at 15,000 g for 15 min. Paxillin was precipitated with antipaxillin antibody and the blots probed with antibodies to paxillin, GFP (CLONTECH Laboratories, Inc.), and p130Cas. Mouse IgG was used in a control precipitation.
In Vitro Kinase Assays
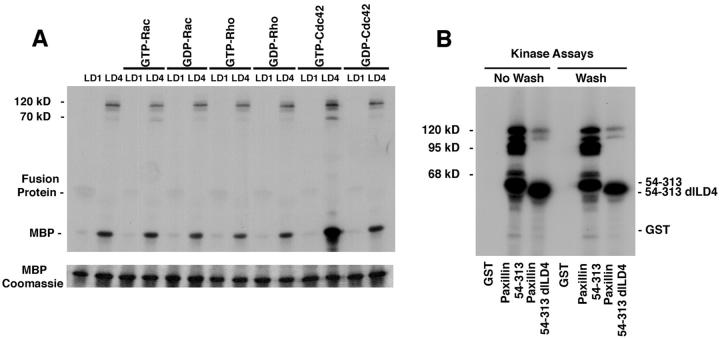
Fusion protein precipitates were washed four times in fusion protein lysis buffer and once in kinase buffer (20 mM Tris-HCl, pH 7.6, 20 mM MgCl2, 10 mM MnCl2, 1 mM EDTA, 1mM EGTA, 40 μM ATP). The pellets were resuspended in 20 μl kinase buffer and incubated for 20 min at room temperature in the presence of 5 μCi [32P]γ-ATP (>4,000 Ci/mmol; ICN Biomedicals). The effect of activated p21 GTPases on precipitated kinase activity was determined by the addition of GTPγS- or GDP-loaded GST p21 GTPases (Rho, Rac, or Cdc42) and 2.5 μg myelin basic protein, as previously described (Bagrodia et al., 1995). The reactions were terminated by boiling in SDS-PAGE sample buffer. To evaluate if phosphorylation of precipitated proteins regulated binding to the paxillin fusion protein, the kinase reaction was followed (see Fig. 8) by several washes of the immobilized GST fusion protein complex with kinase buffer before boiling in SDS-PAGE sample buffer. Results were visualized by SDS-PAGE on 10 or 15% gels followed by autoradiography.
Figure 8.
Paxillin fusion proteins precipitate kinase activity that is stimulated by activated Cdc42. (A) GST–LD1 and LD4 fusion proteins were incubated with brain lysate and the washed precipitate subjected to in vitro kinase assays in the presence of GTPγS or GDP-loaded p21 GTPases. GST–LD4 precipitated kinase activity in the absence of added p21 as judged by phosphorylation of exogenous myelin basic protein and phosphorylation of coprecipitating proteins migrating between 70 and 120 kD. The kinase activity associated with LD4 was further stimulated by the addition of GTP-loaded Cdc42. (B) GST–54-313 previously has been shown to precipitate both tyrosine kinase (FAK) and serine kinase activity, resulting in phosphorylation of the paxillin fusion protein and proteins of 70, 95, and 120 kD. Repeating the assay with GST–54-313 dl LD4 failed to generate phosphorylated proteins of 70 and 95 kD consistent with the absence of p95PKL and PAK from these precipitates. Reduction in the intensity of the 120-kD band is consistent with the partial loss of FAK binding to the LD4 deletion mutant. Washing of the precipitates following the in vitro kinase reaction failed to release any of the coprecipitating phosphoproteins.
Metabolic Labeling
Asynchronously growing CHO.K1 fibroblasts were washed in serum-free DME and then incubated for 24 h in labeling buffer (8 ml methionine- and cysteine-free DME, 1 ml complete DME, 1 ml FBS [Summit Biotechnology], 2 mM glutamine, 200 μCi Trans35S-label™ [>1,000 Ci/mmol; ICN Biomedicals]). The cells were washed in Hanks' buffered saline and lysed in lysis buffer (50 mM Tris-HCl, pH 7.6, 50 mM NaCl, 1 mM EGTA, 2 mM MgCl2, 0.1% β-mercaptoethanol, 0.5% Triton X-100) containing protease inhibitors. The lysate was clarified in a microfuge for 15 min at 14,500 g, and the supernatant incubated with the appropriate fusion protein (50 μg) in binding buffer (lysis buffer containing 0.1% Triton X-100) for 120 min at 4°C. GST–LD-binding proteins and associated proteins were washed four times with binding buffer and analyzed on SDS-PAGE gels followed by enhanced fluorography using Amplify™ (Nycomed Amersham, Inc.).
Alternatively, metabolic labeling of individual proteins was performed by coupled transcription/translation reaction in a cell-free reticulocyte lysate system (TNT; Promega Corp.) as directed by the manufacturer. The reaction mixture containing the [35S]methionine- or [35S]cysteine- (in vitro translation grade; ICN) labeled proteins was clarified by centrifugation before incubation with GST fusion proteins in fusion protein binding buffer for 120 min at 4°C as described above.
Microsequencing and Cloning of p95PKL and p85PIX
LD4-binding proteins from chicken smooth muscle tissue lysate were separated by SDS-PAGE and transferred to PVDF membrane. Protein bands were visualized by Ponceau S staining and excised. Proteolytic digestion, HPLC fractionation, and internal peptide sequencing was performed by Dr. John Leszyk (Worcester Foundation for Biomedical Research, Worcester, MA).
Several of the peptide sequences generated from the p95 protein (p95PKL) precipitated by LD4 of paxillin were identical to the product of the KIAA0148 gene, which encodes a 50-kD protein (Nagase et al., 1995). The equivalent cDNA was isolated by reverse transcriptase-PCR from human U937 cells and a probe spanning nucleotides 527 to 999 of the KIAA0148 cDNA was used to screen a Molt-4 human leukemia cDNA library (Stratagene). Sequencing of a 2.5-kb clone revealed sequence in the 3′ end that coded for the additional peptide derived from the microsequencing of p95. A full-length clone of p95 was isolated and sequenced on both strands at the BioResource Center of Cornell University (Ithaca, NY). The sequence for p95PKL has been deposited in GenBank/EMBL/ DDBJ (accession number AF112366). Several additional p95PKL clones, representing alternative splice variants, were isolated and will be described elsewhere.
The cDNA for p85 PIX was generated by reverse transcriptase-PCR using RNA prepared from NIH3T3 cells. Forward and reverse primers were derived from the mouse PIX cDNA sequence (GenBank/EMBL/ DDBJ U96634; Woo et al., 1997). p95PKL, p85PIX, and mPAK3 were subcloned into pcDNA3 for coupled transcription/translation analyses.
Cell Culture, Transfection, and Immunofluorescence Microscopy
SH-SY5Y human neuroblastoma cells were grown in medium consisting of 1:1 mixture of DME and Ham's F-12 (GIBCO BRL) containing 15% vol/vol FBS (Summit Biotechnologies), 100 IU/ml penicillin, and 50 μg/ml streptomycin and maintained at 37°C in an atmosphere of 5% CO2. 18 h before treatment, the cells were removed to serum-free medium. IGF-1 was added to a final concentration of 10 nM for the indicated time. SH-SY5Y cells were transfected using SuperFect (QIAGEN, Inc.) according to the manufacturer's protocol. Stable transfectants were obtained by growing in media containing 200 μg/ml G418. NIH3T3 and COS 7 cells were maintained in DME (Mediatech) supplemented with 10% vol/vol FBS, 1 mM glutamine, and 1% penicillin/streptomycin. CHO.K1 cells were cultured in modified Ham's F-12 (Mediatech) supplemented with 10% vol/vol heat-inactivated, certified FBS, and 1% penicillin/streptomycin (Sigma Chemical Co.) at 37°C in a humidified chamber with 5% CO2. Lipofectamine (GIBCO BRL)-mediated transfection of CHO.K1 and COS 7 cells was as described elsewhere (Brown et al., 1996). Indirect immunofluorescence analysis was performed as previously described in Brown et al. (1996). Photographs were taken on a Zeiss Axiophot photomicroscope equipped with epifluorescence illumination using Kodak Tmax 400 film. Images were scanned using Kodak Coolscan II™ and processed using Adobe™ Photoshop 3.0.5.
Cell Migration Assay
GST fusion proteins of paxillin LD4 were purified from bacterial lysates as described previously and dialyzed against microinjection buffer (10 mM KPO4, pH 7.5, 5 mM KCl, 0.1% β-mercaptoethanol). Aliquots of dialyzed fusion protein (1 mg/ml) were stored at −70°C. Microinjection was performed using standard protocols (Gilmore and Romer, 1996) using a Narishige micromanipulator and borosilicate glass capillary needles (World Precision Instruments) pulled on a Brown-Flaming micropipette puller (Sutter Instrument).
To measure migration rates of injected cells, confluent NIH3T3 cells were wounded by clearing the cells from one side of the coverslip with a Teflon spatula. The first line of cells immediately adjacent to the wound were injected with either GST or GST–LD4. Cells were allowed to migrate for 24 h and then fixed and processed for immunofluorescence microscopy. Slides were coded, and microinjected cells scored blind for position in one of three categories: baseline, cells remaining at the edge of the original wound, i.e., at the site of injection; pack, cells which had migrated, but were not at the leading edge; and leading edge, cells at the front of the migrating pack. Four replicates were examined for each injected protein (>200 cells per treatment) and the data analyzed by t test.
Results
Differential Binding of Vinculin and FAK to Paxillin LD Motifs
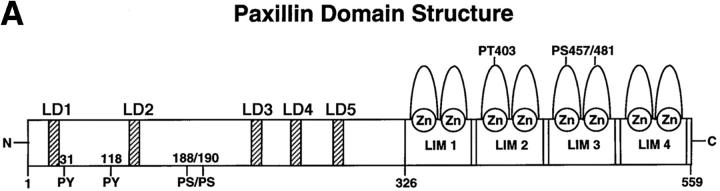
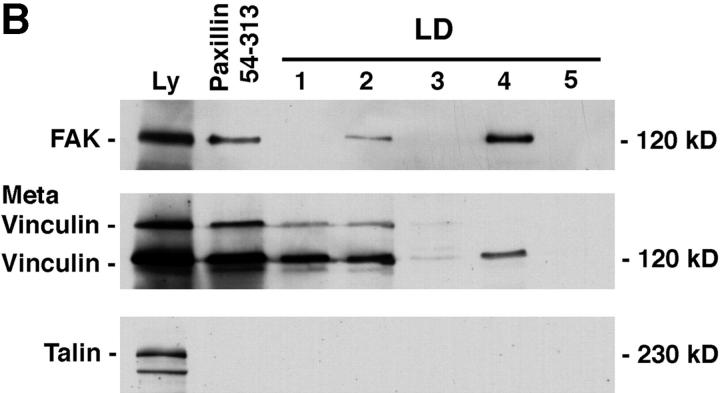
Paxillin comprises an NH2 terminus containing five LD motifs and a COOH terminus made up of four LIM domains (Fig. 1 A). Previously, we have localized the vinculin and FAK binding sites on the NH2 terminus of paxillin to extended regions encompassing LD motifs 2 and 4 (Brown et al., 1996). To determine the ability of vinculin and FAK to bind independently to the discrete paxillin LD motifs, each LD motif of paxillin was synthesized as a GST fusion protein (Fig. 1 C) and incubated with unlabeled smooth muscle cell lysate in precipitation-binding assays followed by Western blotting. Consistent with our previous findings (Brown et al., 1996), FAK was precipitated by LD4, and to a lesser extent, LD2 (Fig. 1 B). Vinculin exhibited strong binding to LD2, the domain previously identified as the principal binding site for this protein, in addition to a low binding to LD4. In contrast to FAK, vinculin also bound to LD1. Both vinculin and FAK binding to the aa 54–313 paxillin fusion protein was observed as previously shown (Brown et al., 1996). Thus, FAK and vinculin differentially associate with the individual paxillin LD motifs (Fig. 1 B). A reprobe of the blot confirmed that smooth muscle talin is unable to bind to the individual LD motifs or to aa 54–313 of paxillin (Fig. 1 B).
Figure 1.
Differential binding of vinculin and FAK to paxillin LD motifs. (A) Schematic representation of the domain structure of paxillin showing the position of the LD motifs and the LIM domains. Previously reported sites of paxillin phosphorylation are also marked (Turner, 1998). (B) Individual GST–LD domains of paxillin were incubated with smooth muscle cell lysate and LD-binding proteins were affinity-isolated on glutathione agarose beads. A GST–paxillin fusion spanning aa 54–313, previously shown to contain vinculin and FAK binding sites, was included as a positive control. The coprecipitating proteins were resolved by SDS-PAGE and transferred to nitrocellulose. The membranes were probed with antibodies to FAK or vinculin. The FAK blot was then reprobed with an antibody to talin. FAK binding was restricted to GST–LD2, LD4, and the GST–paxillin-54–313 fusion protein. Vinculin bound preferentially to paxillin 54–313, LD1, and LD2. No binding of talin was detected to any of the paxillin fusion proteins. Ly, cell lysate; C, Coomassie blue stained gel of the GST–paxillin fusion proteins; MW, molecular weight standards.
Individual Paxillin LD Motifs Exhibit Selective Protein Binding
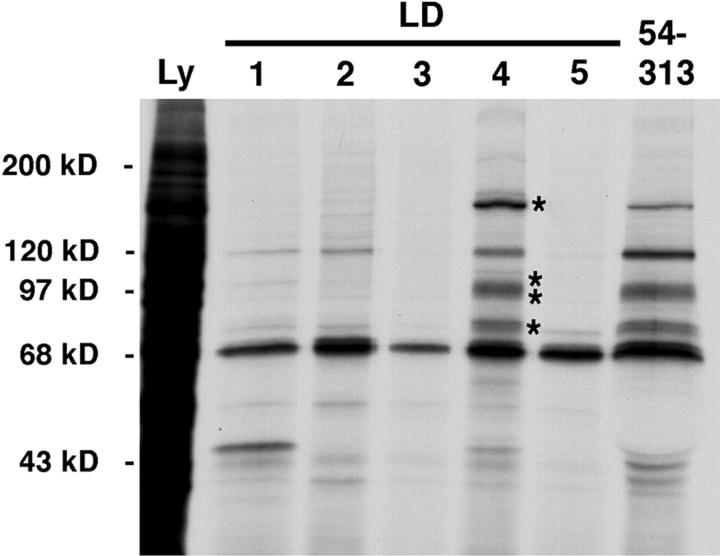
Having established that the individual LD motifs can support specific and differential binding of known focal adhesion binding partners for paxillin, we determined if the individual LD fusion proteins associate with additional cellular proteins. GST–LD fusion proteins were incubated with [35S]methionine/cysteine metabolically labeled CHO.K1 lysates. GST–LD-binding proteins were visualized by SDS-PAGE followed by fluorography (Fig. 2). Each LD motif precipitated a discrete profile of cellular proteins. LD1 precipitated a protein of 120 kD (vinculin) and a prominent band of 50 kD. LD2 precipitated protein(s) of 120 kD (vinculin and FAK). LD3 and 5 precipitated no obvious 35S-labeled binding partners in this assay. In contrast, LD4 precipitated several prominent proteins ranging from 80 to 150 kD. Importantly, a paxillin fusion protein spanning aa 54–313, and therefore containing LD2–5, precipitated a complement of proteins similar to the individual LD motifs (Fig. 2). This suggests that the binding of proteins to the individual paxillin LD motifs was comparable to their binding when presented in the context of an intact NH2 terminus. Similar results were obtained using lysates from NIH3T3 and Swiss 3T3 fibroblasts (data not shown).The 68-kD protein precipitated in approximately equal amounts by all fusion proteins also is precipitated to the same extent by GST alone (not shown), and is likely a heat shock protein.
Figure 2.
Differential binding of metabolically labeled proteins to paxillin LD motifs. CHO.K1 cells were metabolically labeled for 24 h with [35S]cysteine/methionine. Lysates were incubated with GST–paxillin LD motifs or GST–54-313 of paxillin, and binding proteins were precipitated using glutathione agarose beads. Coprecipitating proteins were resolved on SDS-PAGE gels and visualized by fluorography. Individual paxillin LD motifs precipitated a distinct set of labeled proteins. In a parallel experiment, proteins from unlabeled smooth muscle tissue specifically binding to LD4 that comigrated with the bands marked with asterisks (*) were processed for microsequence analysis. Ly, cell lysate.
Identification of LD4-binding Proteins
To identify LD4-binding proteins, GST–LD4 fusion protein was incubated with a smooth muscle tissue lysate. The bound proteins were subjected to SDS-PAGE, transferred to PVDF membrane, and visualized with Ponceau S staining. Individual protein bands, absent in a GST control precipitation, were excised and subjected to microsequencing. Proteins of ∼150, 97, 95, and 80 kD precipitated by LD4 were analyzed. The peptide sequencing data is summarized in Table I.
Table I.
Alignment of Internal Peptide Sequences from Paxillin LD4-binding Proteins with Previously Described Proteins
| Protein | Peptide sequence | |
|---|---|---|
| p97 | 1 | SLVDTVYALKDEVQELR 17 |
| PIX | 589 | SLVDTVYALKDEVQELR 605 |
| p80 | 1 | KPSDEEFALR 10 |
| PIX | 514 | KPSDEEFAVR 523 |
| p95 | 1 | LLSLGAQANFFHPE 14 |
| KIAA0148 | 152 | LLSLGAQANFFHPE 165 |
| p95 | 1 | VPFLPYNPEYSATR 14 |
| KIAA0148 | 314 | VPFLPYNPEYSSTR 327 |
| p95 | 1 | TPVDYAR 7 |
| KIAA0148 | 202 | TPVDYAR 208 |
| p95 | 1 | LQTLQSENTNLR 12 |
| KIAA0148 | 465 | LLGKDAN * |
| p150 | 1 | ARESYVETELIFALA 15 |
| Clathrin | 1163 | ARESYVETELIFALA 1178 |
| p150 | 1 | EEEQATETQPIVYGQ 15 |
| Clathrin | 1622 | EEEQATETQPIVYGQ 1636 |
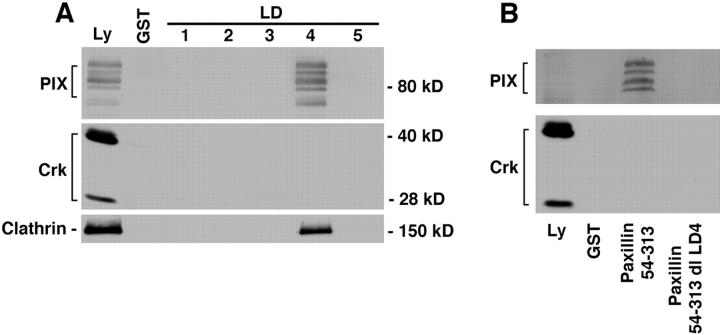
Two peptide sequences from the 150-kD protein were identical to clathrin. One peptide sequence from each of the 97- and 80-kD bands matched with two forms of the recently identified guanine nucleotide exchange factor (GEF), PAK-interacting exchange factor (PIX; Manser et al., 1998), also called COOL (Bagrodia et al., 1998), or p85SPR (Woo et al., 1997). The selective binding of PIX and clathrin to paxillin LD4 was confirmed by Western blotting (Fig. 3 A). Multiple immunoreactive bands in the PIX immunoblot are consistent with the existence of multiple isoforms (Manser et al., 1998). Importantly, deletion (dl) of LD4 from a GST–paxillin fusion protein spanning the NH2 terminus (GST–54-313 dl LD4) resulted in a complete loss of PIX binding (Fig. 3 B). It should be noted that this fusion protein retains the ability to bind FAK via LD2 (see Fig. 5 B).
Figure 3.
PIX and clathrin bind selectively to paxillin LD4. (A) Individual GST–LD domains of paxillin were incubated with rat brain lysate and binding proteins were precipitated using glutathione agarose beads. The coprecipitating proteins were resolved on SDS-PAGE and transferred to nitrocellulose membrane. The membranes were probed with an antibody raised against PIX (Materials and Methods). PIX binding was restricted to the GST–LD4 fusion protein. Note that multiple isoforms of PIX have been reported, with brain expressing the most variants. The adaptor protein Crk failed to bind to any of the GST fusion proteins. A similar experiment was performed with gizzard smooth muscle lysate and probed with anticlathrin antibody. Clathrin bound exclusively to the paxillin GST–LD4 motif. (B) To confirm a role for LD4 in PIX-binding, the experiment was repeated using a fusion protein of the NH2 terminus of paxillin containing a deletion of LD4 (54–313 dl LD4). PIX failed to bind to the LD4 deletion mutant. Crk served as a negative control.
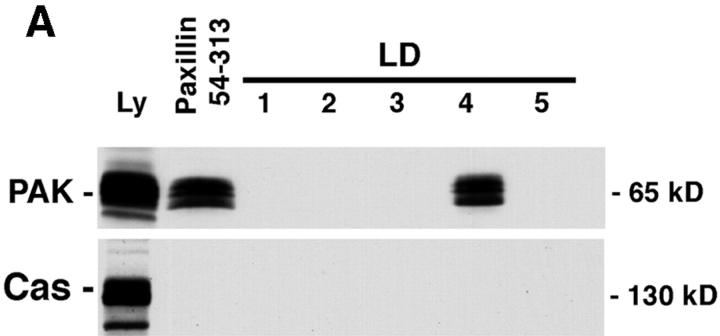
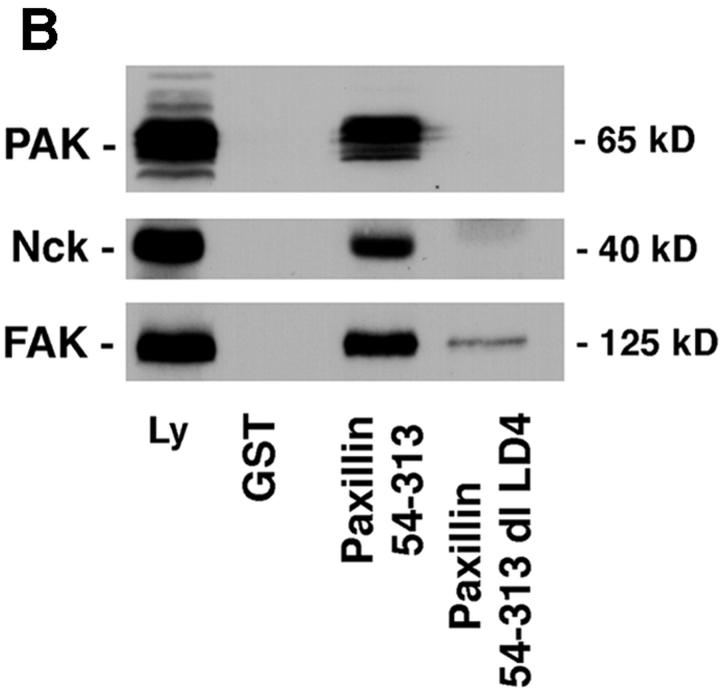
Figure 5.
PAK and the SH3-SH3-SH3-SH2-adaptor protein Nck bind to paxillin LD4. (A) Individual GST–LD motifs and paxillin GST–54-313 were incubated with rat brain lysate and precipitated on glutathione agarose beads. Coprecipitating proteins were resolved by SDS-PAGE and transferred to nitrocellulose. The membrane was probed with antibodies to PAK and p130Cas. PAK was precipitated by GST fusions comprising paxillin 54–313 and LD4. No binding of p130Cas to any of the paxillin fusion proteins was detected. (B) A specific role for the LD4 motif in binding PAK and Nck was confirmed by comparison of binding to paxillin 54–313 and the paxillin 54–313 dl LD4 mutant in precipitation assays. Deletion of LD4 eliminated the binding of PAK and Nck. FAK binding was reduced, but not completely eliminated, consistent with the ability of FAK to bind to paxillin LD2 (Fig. 1), which remains intact in the 54–313 dl LD4 mutant.
Four peptides derived from the p95 protein (p95PKL) were sequenced. Peptides one through three were 100% identical to the previously reported KIAA0148 gene (Nagase et al., 1995). However, the cDNA for KIAA0148 encodes a 50-kD protein. This cDNA was used to probe a human leukemia library to isolate a full-length p95PKL clone. The full-length p95PKL cDNA contained coding sequence for the fourth peptide derived from the original microsequencing analysis. Analysis of other cDNA clones isolated in the screen indicates that there are multiple splice variants of p95PKL. These will be described elsewhere.
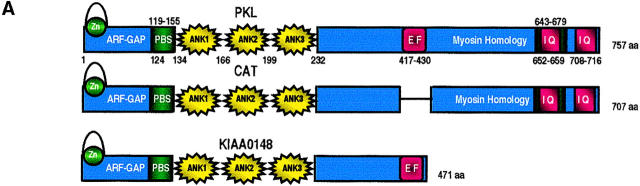
Sequence Analysis of p95PKL
The PKL gene encodes a 757-aa protein with a calculated molecular weight of ∼85 kD. It is closely related to CAT (Bagrodia et al., 1999), the 53-kD protein KIAA0148 (Nagase et al., 1995), and G protein-coupled receptor kinase-interacting protein (GIT-1), a β-adrenergic receptor kinase-binding protein (Premont et al., 1998). An alignment of PKL, CAT, and KIAA0148 is presented in Fig. 4. Although p95PKL, CAT, and KIAA0148 show significant identity within the first 471-aa, p95CAT contains a 50-aa deletion (corresponding to aa 415–464 of PKL and KIAA0148; see Fig. 4 B). PKL is more distantly related to the 130-kD ARF–GAP protein, ASAP (Brown et al., 1998c).
Figure 4.
Sequence analysis of the p95 family of proteins. (A) Schematic representation of the domain structure of the p95 family. The NH2 terminus is comprised of a GCS/SAT zinc-finger-containing ARF–GAP domain, followed by three ankyrin-like repeats. A potential EF hand and partial IQ motifs are represented in red, with potential PBS highlighted in green. A region of low myosin homology, conserved in microtubule-vesicle linker proteins, is localized on the COOH terminus of p95PKL and CAT. (B) Multialign of the p95 family is presented. Peptides obtained from microsequence analysis of LD4-bound p95PKL are underlined. Amino acids constituting functional domains are color coded to match the schematic representation. The potential PBS regions are overlined in green.
p95PKL contains several discrete structural and functional domains within the primary aa sequence. The NH2 terminus (aa 3–111) exhibits homology to a family of ARF–GAP Golgi proteins, including ASAP (aa 461–563, 34% identity, 53% conservation), that have been implicated in the regulation of secretory pathways and mitosis (Brown et al., 1998c; Zhang et al., 1998). Located within aa residues 11–44 of this domain is a GCS family zinc-finger characterized by a C2C2H2 zinc-coordinating residue scheme. This zinc-finger is contained within a consensus SAT motif (suppressor of Arf ts mutation) spanning aa residues 11–60 (Zhang et al., 1998). The fact that the p95PKL related proteins GIT-1 and ASAP have been confirmed to exhibit ARF–GAP activity (Brown et al., 1998c; Premont et al., 1998) predicts PKL, CAT, and KIAA0148 will likely share this function.
The p95 family contains three ankyrin repeats spanning aa 167–199, 200–232, and 134–166. The combined p95PKL, ARF–GAP, and ankyrin repeat region shows 26% identity to and 43% conservation with the tumor suppressor BRCA1-associated RING domain protein, BARD1 (Ayi et al., 1998). A possible role for Ca2+ calmodulin regulation of p95PKL is suggested by the presence of an EF hand (aa 417–430), and two partial IQ motifs on the COOH terminus (aa 654–671 and 710–718). The EF hand is present in p95PKL, KIAA0148, and GIT-1, but is absent from the PKL splice variant CAT (Fig. 4) and ASAP. The COOH terminus of p95PKL and p95CAT shares homology to a region of myosin maintained on clip and hook proteins such as restin/CLIP-170, giantin, EEA1, and hook-1, which are involved in vesicle and organelle linkage to microtubule networks (data not shown).
Of particular relevance to this study is the identification of two potential paxillin-binding subdomains (PBS; Wood et al. 1994, Tachibana et al., 1995; Brown et al., 1998b) within p95 family members (overlaid in green in Fig. 4). PBS1 (aa 119–155) displays 32% identity and 68% conservation with the vinculin PBS (aa 951–989; Tachibana et al., 1995). The second potential PBS sequence on p95PKL (PBS2, aa 643–679) is 19% identical and 57% conserved with the FAK PBS. The similarity of the p95 family PBS regions to those of vinculin and FAK indicate that these are candidate regions of p95PKL interaction with paxillin. The finding that both p95PKL and FAK bind to LD4, while vinculin binds to LD1 and LD2 (Fig. 1 B), favors the p95PKL PBS2 sequence as the primary paxillin-binding region.
Paxillin LD4 Precipitates a Protein Complex Containing PIX, PAK, and Nck
PIX was previously identified through a high affinity association with PAK complexed with the p21 GTPase, Cdc42. Both PAK and Cdc42 are involved in reorganization of the actin cytoskeleton in response to soluble growth factors, and both PAK and PIX have been colocalized with paxillin at focal complex sites formed in response to activation of Cdc42 and Rac (Manser et al., 1998). Therefore, we sought to determine if PAK was associated with the LD4 motif of paxillin also. Lysates from rat brain were incubated with the individual GST–paxillin LD motifs and binding of PAK to the precipitates was evaluated by Western blotting. PAK was precipitated by GST–LD4 and the GST–54-313 paxillin fusion containing LD2–5 (Fig. 5, A and B). PAK was not detected in individual LD1, 2, 3, or 5 precipitates. The filter was reprobed with an antibody to the focal adhesion protein p130Cas. No binding of Cas to any of the paxillin fusion proteins was detected (Fig. 5 A).
The SH3-SH3-SH3-SH2 adaptor protein Nck interacts with PAK via the second SH3 domain (Bokoch et al., 1996; Galisteo et al., 1996). The ability of paxillin to coprecipitate Nck with PAK was confirmed by blotting the GST–54-313 paxillin precipitate with anti-Nck antibody (Fig. 5 B). Importantly, deletion of LD4 from this GST– paxillin fusion protein (GST–54-313 dl LD4) resulted in complete loss of PAK and Nck binding (Fig. 5 B), whereas the binding of FAK to this fusion protein was diminished but not eliminated, due to the presence of LD2, a second FAK binding site (Fig. 1). No binding of the adaptor protein Crk was detected in any of the precipitates.
A specific association of PIX and PAK with paxillin in vivo was confirmed by coimmunoprecipitation from brain lysate (Fig. 6). The failure of the largest PIX isoform to precipitate efficiently with paxillin suggests that intact paxillin may discriminate between PIX family members for binding. No binding of p130Cas to either the paxillin or control IgG precipitate was detected.
Figure 6.
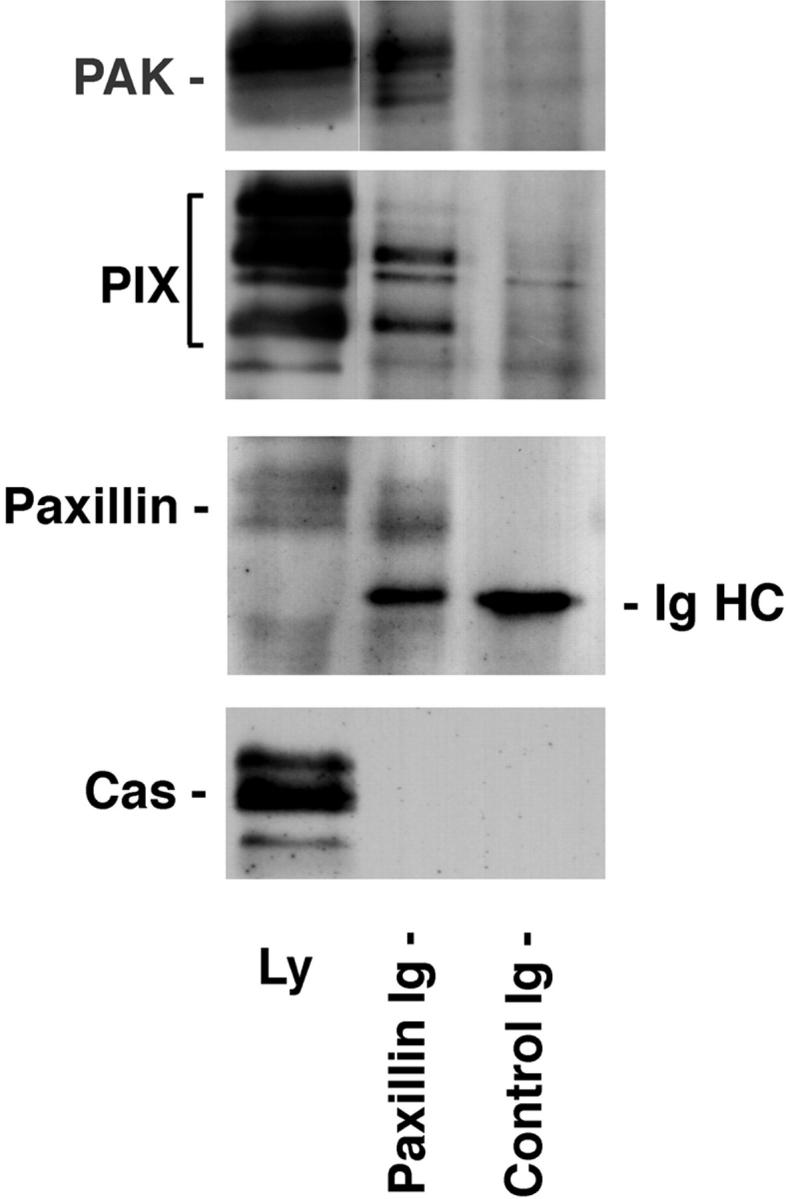
Endogenous PIX and PAK coprecipitate with paxillin. Paxillin was immunoprecipitated from a brain lysate. The paxillin precipitate and a mouse IgG control precipitate were subjected to SDS-PAGE followed by Western blotting. PIX and PAK were coprecipitated with paxillin, but not with the control IgG. The failure of the largest PIX isoform to precipitate efficiently with paxillin suggests that intact paxillin may discriminate between PIX family members for binding. No binding of p130Cas to either the paxillin or control IgG precipitate was observed. IgHC, immunoglobulin heavy chain.
The Binding of PIX and PAK to Paxillin Is Mediated by p95PKL
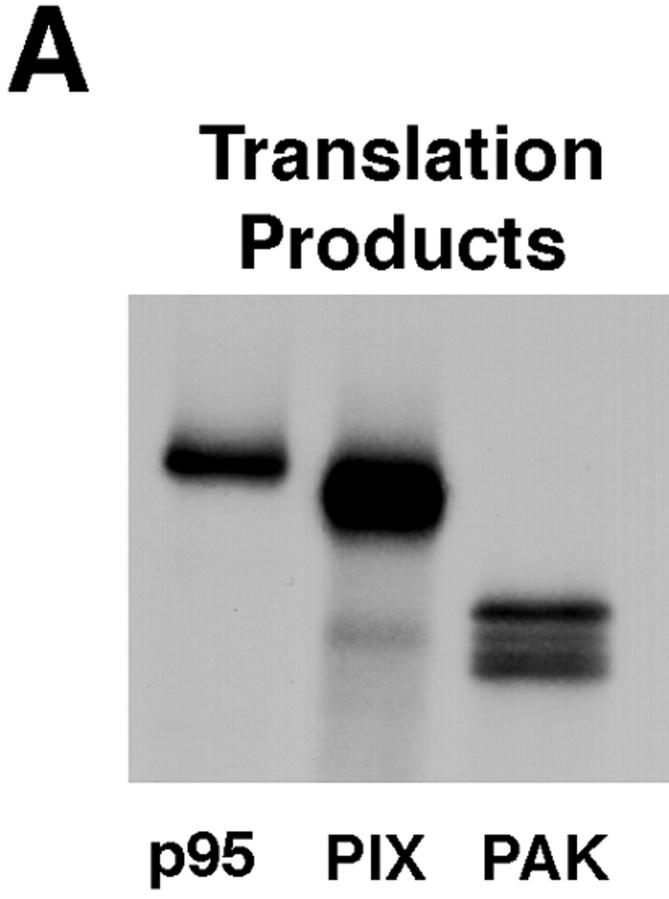
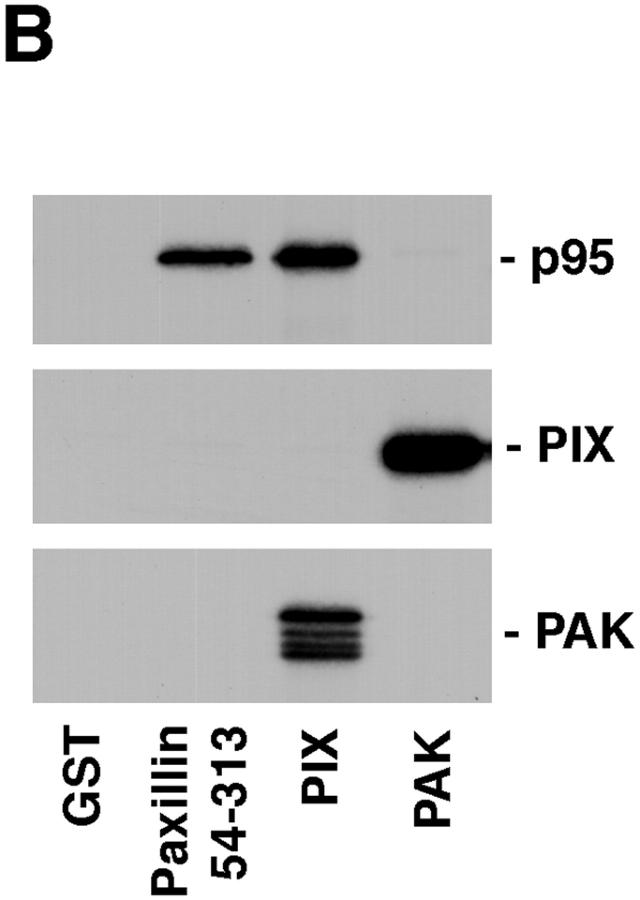
To determine whether p95PKL, PIX, or PAK interact directly with paxillin, metabolically labeled proteins were synthesized using a cell-free coupled transcription/translation system. 35S-labeled p95PKL, PIX, and PAK were incubated with GST fusion proteins of PIX, PAK, paxillin NH2 terminus (aa 54–313), or GST. Bound proteins were detected by SDS-PAGE followed by fluorography. Previously reported interactions between PAK and PIX were confirmed using this cell-free system (Fig. 7 B). However, neither of these proteins were precipitated by the paxillin NH2 terminus (Fig. 7 B). In contrast, p95PKL was enriched in both the GST paxillin NH2 terminus and GST– PIX precipitates, but was not precipitated by either GST or GST–PAK (Fig. 7 B). These data support a role for p95PKL in mediating the association of paxillin with PIX. p95PKL also bound to GST full-length paxillin but not to a GST fusion protein of the four LIM domains of paxillin (data not shown). The association of p95PKL with paxillin and PIX provides a potential mechanism for recruiting PAK to the NH2 terminus of paxillin.
Figure 7.
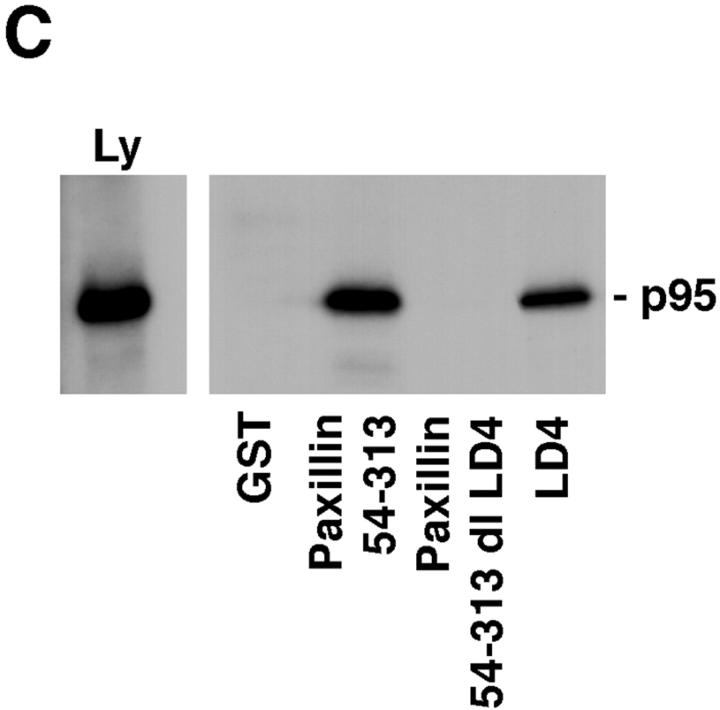
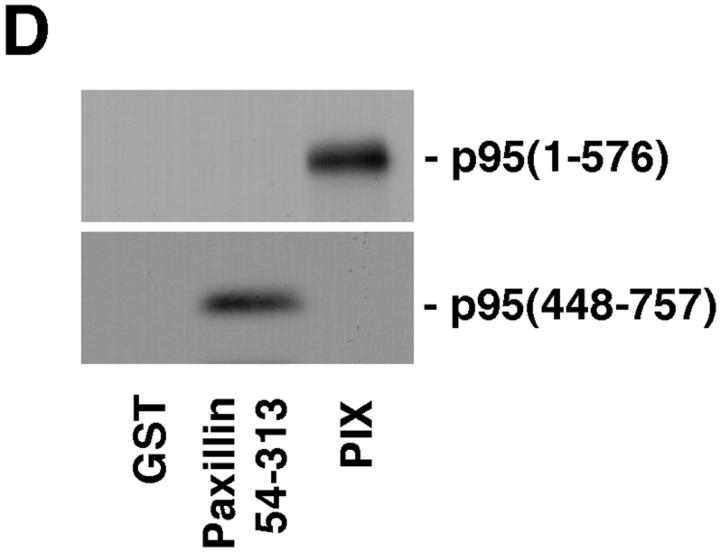
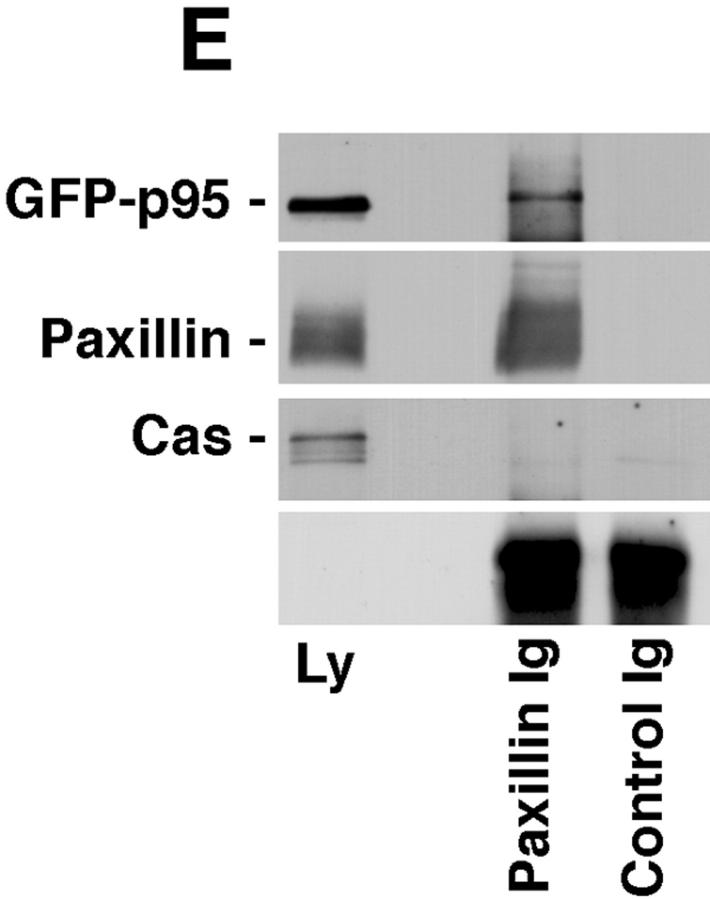
p95PKL directly binds to paxillin LD4 and PIX. To identify the components of the LD4-binding complex that bound directly to paxillin, p95PKL, p85PIX, and PAK were 35S metabolically labeled in vitro by coupled transcription/translation and tested for their ability to bind GST fusion proteins of paxillin (aa 54–313), PIX, and PAK. (A) Translation products of p95PKL, PIX, and PAK. (B) PAK bound to PIX as previously reported. However, no binding of either of these proteins to paxillin 54–313 or GST was detected. In contrast, p95PKL bound to both the paxillin and PIX fusion proteins. No binding of p95PKL to PAK or GST was detected. (C) Deletion of LD4 from the paxillin NH2 terminus fusion protein eliminated the binding to p95PKL. GST–LD4 was sufficient for p95PKL binding. These data provide evidence that p95PKL functions to link paxillin and PIX to PAK. (D) Two regions of p95PKL spanning aa 1–576 and 448–757, respectively, were generated by coupled in vitro transcription/translation and tested for paxillin and PIX binding. PIX bound selectively to the NH2 terminus of p95PKL, containing PBS1 and the ARF–GAP and ankyrin domains, while paxillin bound to the COOH-terminal region containing PBS2. (E) To demonstrate an association in vivo between paxillin and p95PKL, GFP– p95PKL was transfected into COS 7 cells. After lysis, a paxillin precipitate and a mouse IgG control precipitate were subjected to SDS-PAGE, followed by Western blotting with antibodies to GFP, paxillin, and p130Cas. GFP–p95PKL was coprecipitated with paxillin, but not with the control Ig. No binding of p130Cas to either the paxillin or control IgG precipitate was observed. IgHC, immunoglobulin heavy chain; Ly, cell lysate.
To confirm that the paxillin LD4 motif supports p95PKL binding, a precipitation assay was performed using GST–LD4 and the NH2 terminus of paxillin containing a deletion of LD4 (GST–54-313 dl LD4). p95PKL bound to the GST–LD4 fusion protein, but not the LD4 paxillin deletion mutant (Fig. 7 C). To delineate further the PIX- and paxillin binding sites on p95PKL, two in vitro translation products of p95PKL were generated encoding aa 1–576 and 448–757, respectively, and tested for binding to GST–PIX and paxillin. PIX bound only to aa 1–576 of p95PKL, whereas paxillin bound to the 448–757-aa region of p95PKL containing the PBS2 region (Fig. 7 D).
To demonstrate an in vivo association between paxillin and p95PKL, GFP–p95PKL was transfected into COS 7 cells. Western blotting of a paxillin precipitate from lysates of these cells confirmed that GFP–p95PKL, but not p130Cas, coprecipitated with paxillin (Fig. 7 E).
Kinase Activity Associated with the LD4 Motif Precipitate Is Stimulated by Activated Cdc42
PIX has been suggested to mediate PAK activation by Cdc42 (Manser et al., 1998). To determine if the PAK associated with LD4 was enzymatically active, precipitation kinase assays using rat brain lysate were performed in the presence of the exogenous substrate for PAK, myelin basic protein. Phosphorylation of myelin basic protein was detected in coprecipitates of GST–LD4 in the absence of exogenously added GTPase, but not with GST–LD1 precipitates (Fig. 8 A). The associated kinase activity was stimulated further by the addition of GTP-loaded GST– Cdc42. Enhanced phosphorylation of coprecipitating proteins of 100–120, and 70 kD was also noted (Fig. 8 A). Although Rac stimulates PAK in vivo, GST–Rac, as well as GST–Rho, were unable to activate LD4 associated PAK in this in vitro assay, consistent with previous reports (Bagrodia et al., 1995).
Previously, we have demonstrated that the GST paxillin NH2 terminus precipitates both tyrosine and serine kinase activity from tissue and cell lysates (Bellis et al., 1997). The tyrosine kinase has been identified as FAK, while the serine kinase is currently unidentified. These kinases phosphorylate the paxillin fusion protein on residues that are also phosphorylated on paxillin in vivo in response to integrin-mediated cell adhesion (Bellis et al., 1995, 1997; Schaller and Parsons, 1995), as well as a coprecipitating 95-kD protein that was heavily phosphorylated on both tyrosine and serine residues (Bellis et al., 1997). To investigate the possibility that the p95 phosphoprotein was PKL and/or PIX, the precipitation kinase assay was repeated with GST–54-313 and GST–54-313 dl LD4 (Fig. 8 B). Deletion of LD4 completely eliminated the 95- and 68-kD phosphorylated bands, consistent with the loss of binding of the p95PKL/PIX/PAK complex. In contrast, the 120-kD band that previously has been confirmed to be FAK was reduced, but not eliminated, consistent with the binding data presented in Fig. 5 B.
To determine if phosphorylation of the coprecipitating proteins caused dissociation of any of the proteins, the complex was washed extensively after the kinase reaction, before electrophoresis. The profile of precipitating phosphoproteins was identical to the unwashed sample (Fig. 8 B), indicating the paxillin/p95PKL/PIX/PAK complex is not perturbed by phosphorylation in this assay.
The Paxillin-related Protein, Hic-5, Binds the p95PKL/PIX/PAK Complex
Hic-5, a paxillin superfamily member (Shibanuma et al., 1994; Brown et al., 1998b), may act as a functional antagonist of paxillin in vivo (Fujita et al., 1998; Thomas et al., 1999). The highest level of homology between the two proteins occurs within the LD motifs and the LIM domains (Brown et al., 1996). Hic-5, like paxillin, is targeted to focal adhesions via the LIM domains, and also binds vinculin, FAK/FRNK (FAK-related nonkinase), and Pyk2 (Fujita et al., 1998; Hagmann et al., 1998; Matasuya et al., 1998; Thomas et al., 1999). To test whether Hic-5 also precipitates a p95PKL/PIX/PAK complex, the NH2 terminus of Hic-5 (aa 1–226; Thomas et al., 1999) was expressed as a GST fusion protein and used in precipitation experiments. Western blot analysis of proteins precipitated by the Hic-5 NH2 terminus confirmed the binding of FAK, PAK, Nck, and PIX (Fig. 9). p130Cas served as a negative control. Direct binding of the Hic-5 NH2 terminus to p95PKL was detected by using 35S-labeled, in vitro translated p95PKL protein (Fig. 9).
Figure 9.
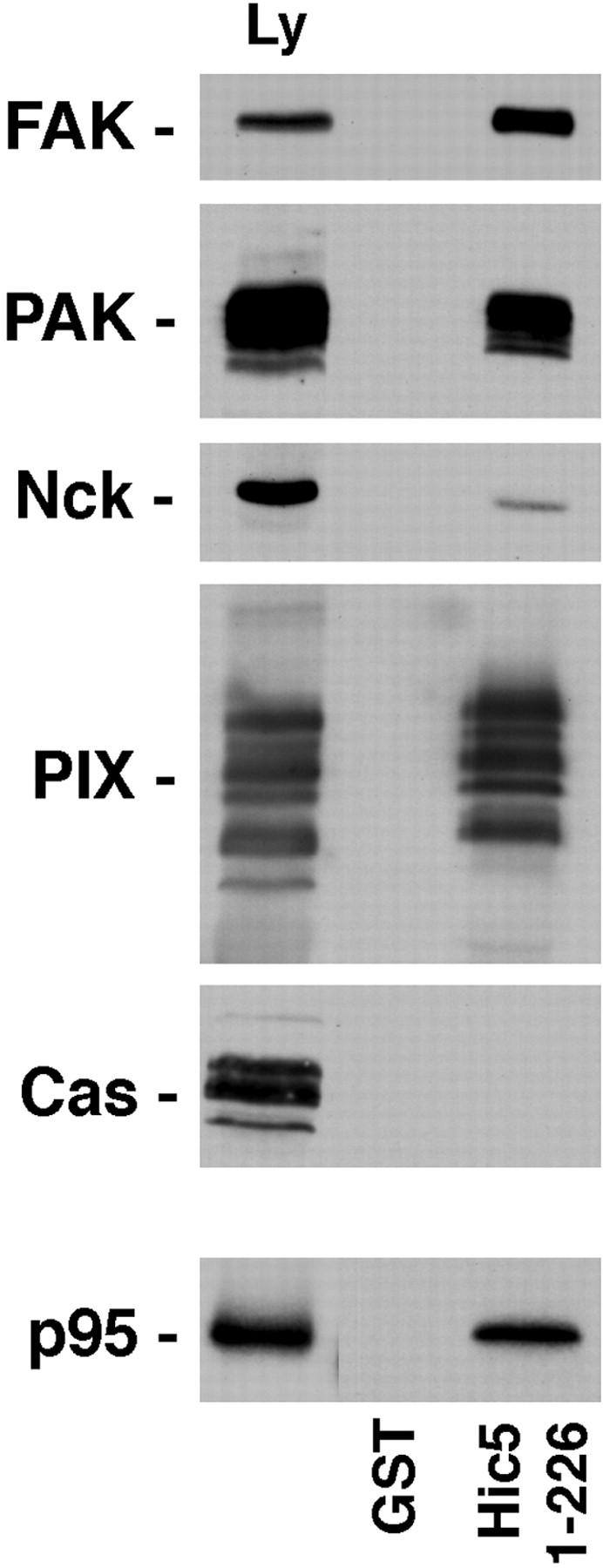
The paxillin family member, Hic-5, binds FAK, p95PKL, PIX, PAK, and Nck. The NH2 terminus of Hic-5 (1–226) was expressed as a GST fusion protein, incubated with rat brain lysate and precipitated on glutathione agarose beads. Coprecipitating proteins were resolved by SDS-PAGE and transferred to nitrocellulose. The membrane was probed with antibodies to FAK, PAK, Nck, PIX, and p130Cas. Alternatively, the GST–Hic-5 fusion protein was incubated with p95PKL that had been 35S metabolically labeled in vitro by coupled transcription/translation. As with paxillin, Hic-5 precipitated FAK, PAK, Nck, and PIX, but not p130Cas. Direct binding of Hic-5 to p95PKL was also detected.
p95PKL Subcellular Localization
To evaluate the subcellular distribution of p95PKL, the protein was expressed as a GFP fusion protein in CHO.K1 fibroblast cells. GFP–PKL localized to small focal adhesions associated with the ends of actin stress fibers (Fig. 10, A and B, arrowheads), and to paxillin-containing focal adhesions (Fig. 10, C and D). GFP–paxillin also localized to focal adhesions at the ends of actin stress fibers (Fig. 10, E and F). Some labeling of both GFP–PKL and GFP–paxillin along the stress fibers was also noted. GFP alone demonstrated a diffuse cytoplasmic and nuclear staining with no enrichment at the ends of actin stress fibers (Fig. 10, G and H).
Figure 10.
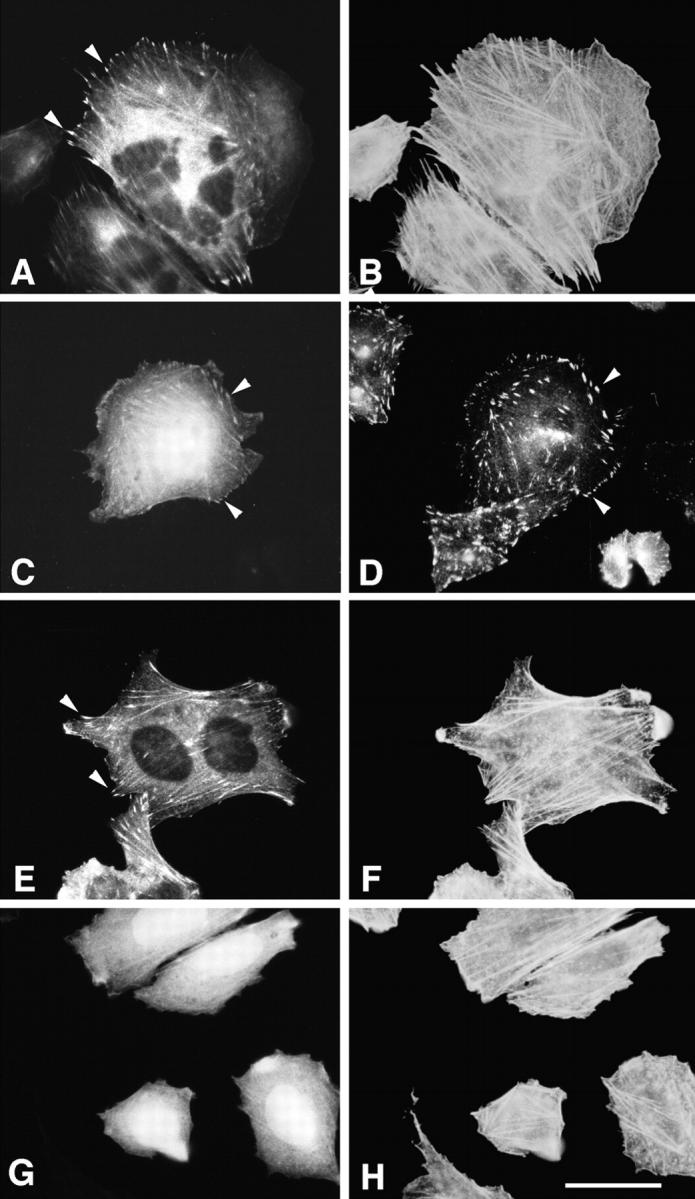
p95PKL localizes to focal adhesions. CHO.K1 cells, transfected with GFP–PKL (A and C), GFP–paxillin (E), or GFP alone (G) were double-labeled with rhodamine phalloidin (B, F, and H; to visualize actin filaments) or with antipaxillin antibody (D). GFP–PKL was enriched in small focal adhesions at the ends of actin stress fibers (A and B, arrowheads), similar to paxillin (E and F, arrowheads). In contrast, GFP alone demonstrated a diffuse cytoplasmic and nuclear distribution with no concentration at the end of actin bundles (G and H). GFP–PKL also colocalized with endogenous paxillin to focal adhesions (C and D, arrowheads). Bar, 5 μm.
A Potential Role for Paxillin LD4 in Cytoskeletal Organization and Migration
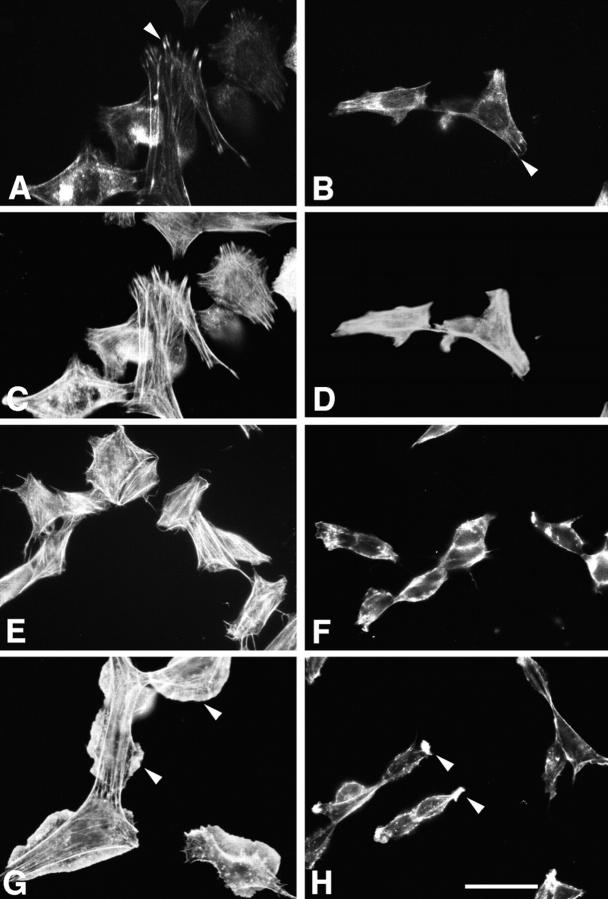
To examine a role for the LD4 motif of paxillin in p21 GTPase and PAK-dependent cytoskeletal reorganization such as lamellipodia formation (Dharmawardhane et al., 1997), SH-SY5Y neuroblastoma cells were transfected with either wild-type chicken paxillin or an LD4 deletion paxillin mutant. Cells expressing wild-type paxillin were generally more spread and contained a much more organized actin stress fiber network than cells expressing the LD4 deletion mutant, although both constructs localized to focal adhesions as determined by immunofluorescence staining with a chicken paxillin-specific antiserum (Fig. 11, A and B, arrowheads). Actin was visualized with rhodamine phalloidin (Fig. 11, C–H). Serum-starved cells (Fig. 11 E, wild-type paxillin transfectants; and F, LD4 deletion transfectants) were stimulated with insulin-like growth factor-1 (IGF-1) to induce lamellipodia (Leventhal et al., 1997). Cells expressing wild-type chicken paxillin exhibited numerous, large lamellipodia in response to 5 min exposure to IGF-1 (Fig. 11 G, arrowheads). In contrast, the cells expressing the LD4 deletion mutant formed fewer, substantially smaller lamellipodia (Fig. 11 H, arrowheads).
Figure 11.
Overexpression of a paxillin LD4 deletion mutant in neuroblastoma SH-SY5Y cells inhibits cell spreading and lamellipodia formation in response to IGF-1. SH-SY5Y cells transfected with wild-type chicken paxillin (A, C, E, and G) or an LD4 deletion mutant of paxillin (B, D, F, and H) were either fixed before serum starvation (A–D) or serum-starved for 12 h (E and F), followed by stimulation for 5 min with 10 nM IGF-1 (G and H). Paxillin distribution (shown only for asynchronously growing cells) was visualized using a chicken paxillin-specific polyclonal antibody and a fluorescein-conjugated anti–rabbit secondary antibody (A and B). Actin distribution was visualized with rhodamine phalloidin (C–H). Both wild-type and mutant paxillin localized to focal adhesions in asynchronously growing cells (A and B, arrowheads). Cells containing the LD4 deletion mutant were generally less well-spread than those expressing wild-type paxillin. The LD4 deletion mutant exhibited significantly fewer lamellipodia (H, arrowheads) in response to treatment with IGF-1, as compared with the cells expressing wild-type paxillin (G, arrowheads). Bar, 5 μm.
FAK and PAK have a role in stimulating cell migration (Cary et al., 1996, 1998; Adam et al., 1998; Klemke et al., 1998). To test whether the interaction of FAK and PAK with paxillin through the LD4 motif is potentially important for FAK- and/or PAK-mediated cell motility, GST or GST–LD4 fusion protein was microinjected into NIH3T3 cells at the edge of a wounded monolayer of cells cultured on glass coverslips. A comparison of the rate of migration of injected cells into the wound indicated a statistically significant reduction in the rate of migration of cells injected with GST–LD4, as compared with control GST-injected cells (Fig. 12). Taken together, these data support a role for the paxillin LD4 motif in coordinating actin cytoskeleton dynamics at the plasma membrane.
Figure 12.
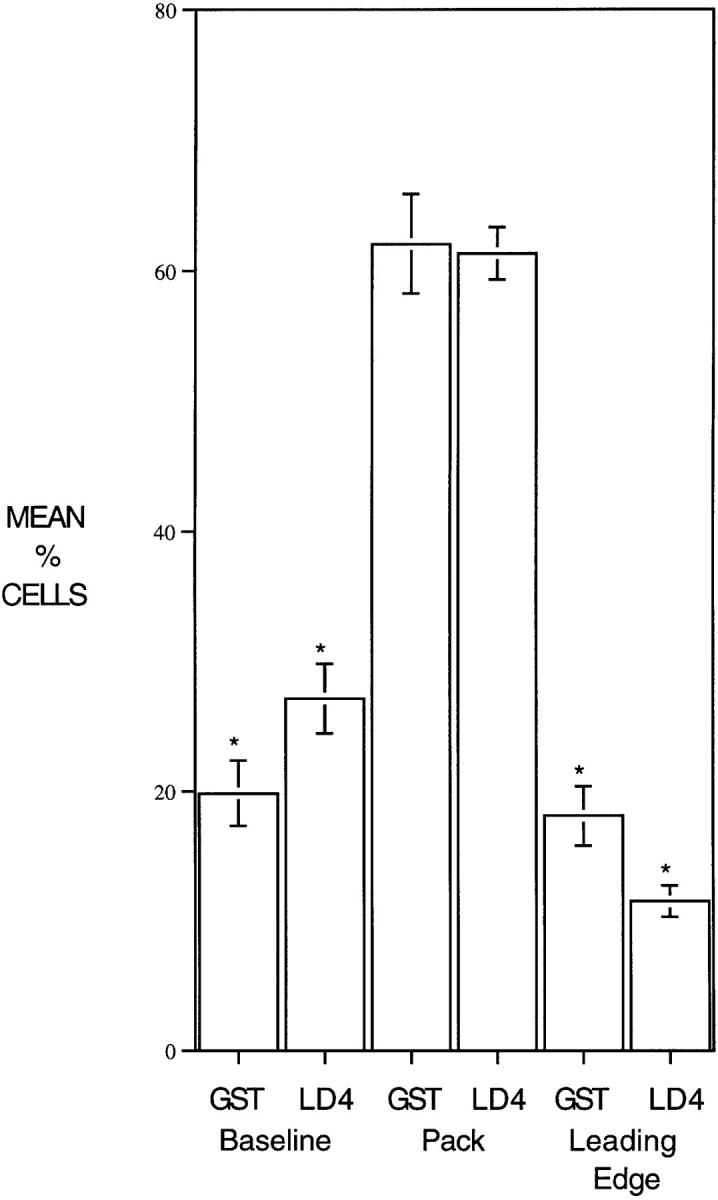
Microinjection of GST–LD4 into NIH3T3 cells retards migration. NIH3T3 cells were grown to confluence on glass coverslips and then half of the cells were removed with a teflon spatula. Cells at the edge of the wound were microinjected with either GST or GST– LD4, and then allowed to migrate into the wound for 24 h. Cells were fixed and stained with antibodies to GST. Microinjected cells (scored blind) were scored for position in one of three categories: baseline, cells remaining at the edge of the original wound; pack, cells which had migrated, but were not at the leading edge; and leading edge, cells at the front of the migrating pack. Four replicates were examined for each injected protein (>200 cells per treatment) and the data analyzed by t test.
Discussion
We previously identified a novel leucine-rich repeating sequence within the NH2 terminus of paxillin, termed paxillin LD motifs (Brown et al., 1996). These motifs are implicated in the binding of paxillin to vinculin, FAK, and the E6 oncoprotein from papillomavirus (Brown et al., 1996; Tong et al., 1997; Vande Pol et al., 1998). In this study, we have demonstrated that individual LD motifs can function independently to support the differential binding of the focal adhesion proteins vinculin and FAK. Several proteins were identified that were precipitated by paxillin LD4. These include clathrin and a new 95-kD, ARF–GAP domain- and ankyrin repeat-containing protein. PAK, Nck, and members of the Cdc42/Rac1 guanine nucleotide-exchange factor family PIX/COOL (Bagrodia et al., 1998; Manser et al., 1998) also associated with LD4. In vitro binding experiments using isolated proteins demonstrated that p95PKL binds directly to paxillin and PIX. Since direct binding of PAK to paxillin was not observed, the precipitation of PAK by paxillin LD4 from cell and tissue lysates likely occurs as a result of its association with PIX (Fig. 7; Bagrodia et al., 1998; Manser et al., 1998). Similarly, it is expected that LD4 precipitation of Nck is a result of its stable association with PAK (Bokoch et al., 1996; Galisteo et al., 1996). The coprecipitation of endogenous PIX family members and PAK with paxillin from brain lysate (Fig. 6), as well as previous reports detailing coprecipitation of PIX and PAK (Bagrodia et al., 1998; Manser et al., 1998), provides evidence for the existence of these interactions in vivo.
P95PKL is a member of a family of ARF–GAP proteins that include the closely related GIT-1, CAT, and KIAA0148, as well as the more distantly related ASAP1. Both GIT-1 and ASAP1 exhibit ARF–GAP activity and have been implicated in membrane remodeling events associated with Arf function. GIT-1 binds the β-adrenergic receptor kinase and is involved in coordinating receptor endocytosis (Premont et al., 1998), while ASAP, the largest family member, which also binds Src and Crk, has been localized to the plasma membrane where it is suggested to coordinate membrane trafficking associated with cytoskeletal remodeling or cell growth (Brown et al., 1998c). KIAA0148 is a smaller (50 kD) protein, containing highly conserved ARF–GAP and ankyrin-repeat regions, but lacking the more diverse COOH terminus domain, which in the larger proteins may be important in conferring binding specificity for accessory proteins. In this regard, it is of note that the paxillin binding site on p95PKL is within aa 577–757, and therefore not shared with KIAA0148. In contrast, PIX, which binds to aa 1–576 of PKL but not 448–757, potentially binds both p95PKL and KIAA0148. Thus, KIAA0148 may be a functional antagonist for some, or all, of the larger ARF–GAP family members. Such a mechanism has been demonstrated with FAK and FRNK, which comprises only the COOH-terminal, noncatalytic domain of FAK (Richardson and Parson, 1996), and more recently, with the regulation of PAK activity by the PIX family of GEFs (Bagrodia et al., 1998).
Paxillin itself is a member of a larger family that is characterized primarily by the conservation of NH2-terminal LD motifs and COOH-terminal LIM domains (Brown et al., 1998b). One family member, Hic-5, shares several additional features with paxillin, including focal adhesion targeting via the LIM domains and binding to FAK, FRNK, vinculin, and CSK (Fujita et al., 1998; Thomas et al., 1999). The p95PKL/PIX/PAK/Nck protein complex also bound to the NH2 terminus of Hic-5, suggesting conservation of function of the LD motifs of these two proteins. In contrast, unlike paxillin, Hic-5 is not tyrosine phosphorylated during cell adhesion and consequently is unable to bind the SH2 domain-containing adaptor, Crk (Thomas et al., 1999). This suggests that Hic-5 and paxillin may act as functional antagonists with regards to Crk-dependent signaling events, such as cell migration (Klemke et al., 1998) and activation of the Ras–MAP kinase pathway via C3G. It will be important to determine the relative contribution of each of these multifamily members to particular cellular events and signal transduction pathways.
What is the functional significance of a paxillin, p95PKL ARF–GAP protein association? A role for ARF1 and ARF6 proteins in cytoskeletal reorganization, including cortical actin reorganization during cell spreading is apparent (Van Aelst and D'Souza-Schorey, 1997; Frank et al., 1998; Sasaski and Takai, 1998; Song et al., 1998). The capacity of paxillin to assemble a PAK-containing complex through the ARF–GAP proteins PKL and CAT provides a possibility for the integration of ARF and Rho family signal transduction at the cytoskeleton. In this regard, we and others recently have determined that paxillin cycles in an ARF-dependent manner, between the trans-Golgi/endosomal network and focal adhesions (Norman et al., 1998; Brown, M.C., and C.E. Turner, unpublished observations). One possible model would predict that regulation of different ARF family members by ARF–GAPs such as p95PKL determines the subcellular localization of paxillin and other focal complex/adhesion components, including PAK, PIX Cdc42, and vinculin (Erickson et al., 1996; Norman et al., 1998; our unpublished observations). Thus, cell adhesion or growth factor stimulation would promote the release of paxillin and associated proteins from a perinuclear vesicle compartment, permitting targeting to sites of cell matrix interaction, and thereby contributing to the Cdc42/Rac/Rho paradigm. Interestingly, clathrin was also precipitated specifically by paxillin LD4. Although clathrin has not been observed in focal adhesions, α-actinin and the vitronectin receptor have been reported to bind clathrin (Merisko et al., 1988; De Deyne et al., 1998). It is unknown at this time whether the binding of clathrin to paxillin is direct or indirect. However, clathrin does contain a well conserved PBS (Brown et al., 1998b). The functional consequence of clathrin binding to paxillin remains to be determined, but it also may be associated with a role for p95PKL ARF–GAP activity and PAK in vesicular transport (Dharmawardhane et al., 1997), endocytosis (Brown et al., 1998c), and membrane trafficking to the leading edge during cell motility or in response to growth factors (Bretscher and Aguado-Velasco, 1998a,b). It is also a potential factor in the redistribution of paxillin to the trans-Golgi/endosomal network in epithelial cells, where colocalization of paxillin and clathrin has been observed (Brown, M.C., and C.E. Turner, unpublished observations).
Stimulation of p21 GTPase activity in response to integrin-mediated cell adhesion (Clark et al., 1998) or growth factors results in reorganization of the actin cytoskeleton and the formation of distinct sites of cell attachment with the extracellular matrix. Rac and Cdc42 activation leads to the formation of small focal complexes at the base of lamellipodia and filopodia, respectively, while larger focal adhesions associated with robust actin stress fibers are the hallmark of Rho activity (Ridley and Hall, 1992; Ridley et al., 1992). Focal complexes and focal adhesions contain several common components including transmembrane integrin receptors and intracellular proteins, such as paxillin, vinculin, and FAK (Nobes and Hall, 1995). PIX has been suggested as the protein that recruits PAK to focal complexes (Manser et al., 1998), but the protein responsible for PIX targeting has not been identified. It has been proposed that paxillin serves an adaptor function at these sites of adhesion, using the LIM domains for focal adhesion targeting (Brown et al., 1996; Thomas et al., 1999) and the NH2 terminus in a scaffolding capacity, recruiting multiple signaling components such as the tyrosine kinases FAK, PYK2, Src, and Csk (Sabe et al., 1994; Turner and Miller, 1994; Schaller and Parsons, 1995; Tachibana et al., 1995; Salgia et al., 1995). In view of the binding of a p95PKL/ PIX/PAK complex to the NH2 terminus of paxillin, and the localization of a pool of PKL to focal complexes/adhesions in CHO.K1 cells, future experiments will be directed towards evaluating a potential role for p95PKL in recruiting PIX and PAK to the paxillin-rich sites of cell adhesion.
In support of a role for paxillin in p21 GTPase- and PAK-dependent cytoskeletal reorganization, we found that overexpression in neuroblastoma SH-SY5Y cells of a paxillin mutant lacking the LD4 motif caused an overall reduction in actin cytoskeletal organization in comparison to wild-type paxillin-expressing cells, and substantially reduced lamellipodia formation in these cells in response to IGF-1. Overexpression of PAK in neuronal PC12 cells also stimulates lamellipodia in the absence of IGF-1 (Obermeier et al., 1998). This response, which requires structural elements within both the NH2- and COOH-terminal region of PAK, but is not dependent on PAK kinase activity, is inhibited by coexpression of dominant-negative Rac or by interfering with the interaction between PAK and PIX (Obermeier et al., 1998). Thus, the ability of the paxillin LD4 deletion mutant to inhibit lamellipodia formation in response to IGF-1 likely is due in part to the failure of a PAK/PIX complex to target effectively to the plasma membrane through an association with paxillin. Similarly, the well-spread phenotype of SH-SY5Y cells overexpressing wild-type paxillin, as compared with the LD4 deletion mutant, provides further evidence for a role for paxillin in Cdc42 and Rac-dependent regulation of cell spreading (Price et al., 1998). Finally, the absence of stress fibers in the paxillin LD4 deletion-expressing cells suggests that this mutant may also affect Rho-dependent signaling.
Regulated attachment and detachment of integrin receptors to the extracellular matrix, as well as reorganization of the actomyosin cytoskeleton is required for cell movement (Burridge and Chrzanowska-Wodnicka, 1996). Recent reports have indicated a role for both PAK and FAK in cell migration (Ilic et al., 1995; Cary et al., 1996; Gilmore and Romer, 1996; Adam et al., 1998). A paxillin/ FAK and/or a paxillin/PAK association at the plasma membrane may be required for the cytoskeletal changes necessary for efficient cell motility. Importantly, PAK has been shown to phosphorylate and inhibit MLCK (Sanders et al., 1999), thereby providing a potential mechanism by which PAK can promote a motile Cdc42/Rac phenotype while inhibiting the less motile morphology characterized by robust stress fibers resulting from Rho-dependent activation of MLCK (Burridge and Chrzanowska-Wodnicka, 1996). Thus, microinjection of the nontargeting GST–LD4 fusion protein into NIH3T3 cells may be exerting an inhibitory effect on cell migration by sequestering sufficient FAK, PAK, and Cdc42 or Rac1 in the cytoplasm, preventing efficient lamellipodia and filopodia formation and extension at the leading edge of the cells.
Cell spreading and cell migration also have been associated with increased kinase activity of FAK (Ilic et al., 1995; Cary et al., 1996). We previously have shown that the NH2 terminus of paxillin, when expressed as a GST fusion protein, precipitates FAK and serine kinase activities. The paxillin residues phosphorylated by these kinases in vitro are also major sites of paxillin phosphorylation during cell spreading (Bellis et al., 1997). Conversely, inhibition of paxillin tyrosine phosphorylation blocks cell spreading (Richardson et al., 1997). Since p95PKL is phosphorylated on both tyrosine and serine residues in in vitro paxillin precipitation kinase assays (Bellis et al., 1997; Fig. 8), it will be important to determine whether p95PKL is a target for FAK and/or PAK during cell adhesion/migration. These data are also consistent with the observation that p95CAT, (a PKL family member) is tyrosine phosphorylated during cell adhesion (Bagrodia et al., 1999).
In conclusion, we have demonstrated that the LD motifs of paxillin exhibit differential binding to several molecules important in the regulation of actin cytoskeleton dynamics. This includes the identification and cloning of a novel protein, p95PKL, that links paxillin indirectly to PAK and shares homology with ARF–GAP proteins involved in vesicular transport. Future experiments will examine the role of paxillin in coordinating the potential cross-talk between these paxillin-binding proteins
Acknowledgments
Supported by National Institutes of Health grant GM47607 and a Grant-in-Aid from the American Heart Association, both to C.E. Turner. C.E. Turner is an Established Investigator of the American Heart Association. A.R. McDonald and M.C. Riedy are American Heart Association Postdoctoral Fellows.
Abbreviations used in this paper
aa
amino acid
FAK
focal adhesion kinase
FRNK
FAK-related nonkinase
GFP
green fluorescent protein
GIT
G protein-coupled receptor kinase-interacting protein
MLCK
myosin light chain kinase
PAK
p21 GTPase–activated kinase
PBS
paxillin binding subdomain
PIX
PAK-interacting exchange factor
PKL
paxillin-kinase linker
References
- Adam L, Vadlamuda R, Kondapaka B, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- Ayi TC, Tsan JT, Hwang LY, Bowcock AM, Baer R. Conservation of function and primary structure in the BRCA1-associated RING domain (BARD1) protein. Oncogene. 1998;17:2143–2148. doi: 10.1038/sj.onc.1202123. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Creasy CL, Chernoff J, Cerione RA. Identification of a mouse p21Cdc42/Racactivated kinase. J Biol Chem. 1995;270:22731–22737. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Jordan KA, Van Aelst L, Cerione RA. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- Bagrodia, S., D. Bailey, Z. Leonard, M. Hart, J.-L. Guan, R.T. Premont, S.J. Taylor, and R.A. Cerione. 1999. A tyrosine phosphorylated protein that binds to an important regulatory region on the Cool family of Pak-binding proteins. J. Biol. Chem. In press. [DOI] [PubMed]
- Bellis SL, Miller JT, Turner CE. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J Biol Chem. 1995;270:17437–17441. doi: 10.1074/jbc.270.29.17437. [DOI] [PubMed] [Google Scholar]
- Bellis SL, Perrotta JA, Curtis MS, Turner CE. Adhesion of fibroblasts to fibronectin stimulates both serine and tyrosine phosphorylation of paxillin. Biochem J. 1997;325:375–381. doi: 10.1042/bj3250375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM, Wang Y, Bohl BP, Sells MA, Quilliam LA, Knaus UG. Interaction of the Nck adapter protein with p21-activated kinase (PAK1) J Biol Chem. 1996;271:25746–25749. doi: 10.1074/jbc.271.42.25746. [DOI] [PubMed] [Google Scholar]
- Bretscher MS, Aguado-Velasco C. Membrane traffic during cell locomotion. Curr Opin Cell Biol. 1998a;10:537–541. doi: 10.1016/s0955-0674(98)80070-7. [DOI] [PubMed] [Google Scholar]
- Bretscher MS, Aguado-Velasco C. EGF induces recycling membrane to form ruffles. Curr Biol. 1998b;8:721–724. doi: 10.1016/s0960-9822(98)70281-7. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perrotta JA, Turner CE. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and FAK binding. J Cell Biol. 1996;135:1109–1124. doi: 10.1083/jcb.135.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Perrotta JA, Turner CE. Serine and threonine phosphorylation of the paxillin LIM domains regulates paxillin focal adhesion localization and cell adhesion to fibronectin. Mol Biol Cell. 1998a;9:1803–1816. doi: 10.1091/mbc.9.7.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Curtis MS, Turner CE. Paxillin LD motifs may define a new family of protein recognition domains. Nature Struct Biol. 1998b;5:677–678. doi: 10.1038/1370. [DOI] [PubMed] [Google Scholar]
- Brown MT, Andrad J, Radhakrishna H, Donaldson JG, Cooper JA, Randazzo PA. ASAP1, a phospholipid-dependent Arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol Cell Biol. 1998c;18:7038–7051. doi: 10.1128/mcb.18.12.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility and signaling. Ann Rev Cell Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAKaccompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary LA, Chang JF, Guan J-L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with src and fyn. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- Cary LA, Han DC, Polte TR, Hanks SK, Guan J-L. Identification of p130Casas a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the roads taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the Rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig SW, Johnson RP. Assembly of focal adhesions: progress, paradigms and portents. Curr Opin Cell Biol. 1996;8:74–85. doi: 10.1016/s0955-0674(96)80051-2. [DOI] [PubMed] [Google Scholar]
- De Deyne PG, O'Neill A, Resneck WG, Dmytrenko GM, Pumplin DW, Bloch RJ. The vitronectin receptor associates with clathrin-coated membrane domains via the cytoplasmic domain of its beta 5 subunit. J Cell Sci. 1998;111:2729–2740. doi: 10.1242/jcs.111.18.2729. [DOI] [PubMed] [Google Scholar]
- DeNichilo MO, Yamada KM. Integrin αvβ3-dependent serine phosphorylation of paxillin in macrophages adherent to vitronectin. J Biol Chem. 1996;271:11016–11022. doi: 10.1074/jbc.271.18.11016. [DOI] [PubMed] [Google Scholar]
- Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JW, Zhang CJ, Kahn RA, Evans T, Cerione RA. Mammalian Cdc42 is a brefeldin A-sensitive component of the Golgi apparatus. J Biol Chem. 1996;271:26850–26854. doi: 10.1074/jbc.271.43.26850. [DOI] [PubMed] [Google Scholar]
- Frank SR, Hatfield JC, Casanova JE. Remodeling of the actin cytoskeleton is coordinately regulated by protein kinase C and the ADP-ribosylation factor nucleotide exchange factor ARNO. Mol Biol Cell. 1998;9:3133–3146. doi: 10.1091/mbc.9.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Kamiguchi K, Cho D, Shibanuma M, Morimoto C, Tachibana K. Interaction of Hic-5, a senescence-related protein, with focal adhesion kinase. J Biol Chem. 1998;273:26516–26521. doi: 10.1074/jbc.273.41.26516. [DOI] [PubMed] [Google Scholar]
- Galisteo ML, Chernoff J, Su Y-C, Skolnik EY, Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann J, Grob M, Welman A, van Willigen G, Burger MM. Recruitment of the LIM protein hic-5 to focal contacts of human platelets. J Cell Sci. 1998;111:2181–2188. doi: 10.1242/jcs.111.15.2181. [DOI] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Ann Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuat Y, Kanazawa S, Taked N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a molecular switch for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal PS, Sheldon EA, Kim B, Feldman EL. Tyrosine phosphorylation of paxillin and focal adhesion kinase during insulin-like growth factor-I–stimulated lamellipodial advance. J Biol Chem. 1997;272:5214–5218. doi: 10.1074/jbc.272.8.5214. [DOI] [PubMed] [Google Scholar]
- Lim L, Manser E, Leung T, Hall C. Regulation of phosphorylation pathways by p21 GTPases, the p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways. Eur J Biochem. 1996;242:171–185. doi: 10.1111/j.1432-1033.1996.0171r.x. [DOI] [PubMed] [Google Scholar]
- Manser E, Huang H, Loo T, Chen X, Dong J, Leung T, Lim L. Expression of constitutively active α-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Matasuya M, Sasaki H, Aoto H, Mitaka T, Nagura K, Ohba T, Ishino M, Takahashi S, Suzuki R, Sasaki T. Cell adhesion kinase β forms a complex with a new member, hic-5, of proteins localized at focal adhesions. J Biol Chem. 1998;273:1003–1014. doi: 10.1074/jbc.273.2.1003. [DOI] [PubMed] [Google Scholar]
- Merisko EM, Welch JK, Chen TY, Chen M. Alpha-actinin and calmodulin interact with distinct sites on the arms of the clathrin trimer. J Biol Chem. 1988;263:15705–15712. [PubMed] [Google Scholar]
- Nagase T, Seki N, Tanaka A, Ishikawa K, Nomura N. Prediction of the coding sequences of unidentified human genes. IV. The coding sequences of 40 new genes (KIAA0121-KIAA0160) deduced by analysis of cones from human cell line KG-1. DNA Res. 1995;2:167–174. doi: 10.1093/dnares/2.4.167. [DOI] [PubMed] [Google Scholar]
- Narumiya S. The small GTPase rho: cellular functions and signal transduction. J Biochem. 1996;120:215–228. doi: 10.1093/oxfordjournals.jbchem.a021401. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fiber, lamellapodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Norman JC, Jones D, Barry ST, Holt MR, Cockcroft S, Critchley DR. ARF1 mediates paxillin recruitment to focal adhesions and potentiates Rho-stimulated stress fiber formation in intact and permeabilized Swiss 3T3 fibroblasts. J Cell Biol. 1998;143:1981–1995. doi: 10.1083/jcb.143.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier A, Ahmed S, Manser E, Yen SC, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO (Eur Mol Biol Organ) J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Claing A, Vitale N, Freeman JLR, Pitcher JA, Patton WA, Moss J, Vaughan M, Lefkowitz RJ. β2-adrenergic receptor regulation by GIT1, a G protein coupled receptor kinase-associated ADP ribosylation factor GTPase-activation protein. Proc Natl Acad Sci USA. 1998;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Parsons JT. A mechanism for regulation of the focal adhesion-associated protein tyrosine kinase pp125FAK . Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- Richardson A, Malik RK, Hildebrand JD, Parsons JT. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann J, Hall A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Convergent signalling in the action of integrins, neuropeptides, growth factors and hormones. Cancer Surv. 1995;24:81–96. [PubMed] [Google Scholar]
- Sabe H, Hata A, Okada M, Nakagawa H, Hanafusa H. Analysis of the binding of the Src homology 2 domain of Csk to tyrosine-phosphorylated proteins in the suppression and mitotic activation of c-Src. Proc Natl Acad Sci USA. 1994;91:3984–3988. doi: 10.1073/pnas.91.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgia R, Li JL, Lo SH, Brunkhorst B, Kansas GS, Sobhany ES, Sun Y, Pisick E, Hallek M, Ernst T, et al. Molecular cloning of human paxillin, a focal adhesion protein phosphorylated by p210BCR/ABL. J Biol Chem. 1995;270:5039–5047. doi: 10.1074/jbc.270.10.5039. [DOI] [PubMed] [Google Scholar]
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Takai Y. The Rho small G protein family-Rho GDI system as a temporal and spatial determinant for cytoskeletal control. Biochem Biophys Res Commun. 1998;245:641–645. doi: 10.1006/bbrc.1998.8253. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Parsons JT. pp125FAK-dependent phosphorylation of paxillin creates a high affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Ambrose D, Bagrodia S, Bokoch GM, Chernoff J. Human p21 activated kinases regulate actin reorganization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Shibanuma M, Mashimo J, Kuroki T, Nose K. Characterization of the TGF beta 1-inducible hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence. J Biol Chem. 1994;269:26767–26774. [PubMed] [Google Scholar]
- Song J, Khachikian Z, Radhakrishna H, Donaldson JG. Localization of endogenous ARF6 to sites of cortical actin rearrangement and involvement of ARF6 in cell spreading. J Cell Sci. 1998;111:2257–2267. doi: 10.1242/jcs.111.15.2257. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Sato T, D'Airro N, Morimoto C. Direct association of pp125FAK with paxillin, the focal adhesion-targeting mechanism of pp125FAK. J Exp Med. 1995;182:1089–1100. doi: 10.1084/jem.182.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Hagel M, Turner CE. Characterization of Hic-5, a focal adhesion protein that shares extensive homology with paxillin. J Cell Sci. 1999;112:181–190. doi: 10.1242/jcs.112.2.181. [DOI] [PubMed] [Google Scholar]
- Tong X, Salgia R, Li JL, Griffin JD, Howley PM. The bovine papillomavirus E6 protein binds to the LD motif repeats of paxillin and blocks its interaction with vinculin and the focal adhesion kinase. J Biol Chem. 1997;272:33373–33376. doi: 10.1074/jbc.272.52.33373. [DOI] [PubMed] [Google Scholar]
- Turner CE. Molecules in focus: paxillin. Int J Biochem Cell Biol. 1998;30:955–959. doi: 10.1016/s1357-2725(98)00062-4. [DOI] [PubMed] [Google Scholar]
- Turner CE, Miller JT. Primary sequence of paxillin contains putative SH2 and SH3 domain binding motifs and multiple LIM domains: identification of a vinculin and pp125FAK binding. J Cell Sci. 1994;107:1583–1591. doi: 10.1242/jcs.107.6.1583. [DOI] [PubMed] [Google Scholar]
- Turner CE, Glenney JR, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990;111:1059–1068. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Vande Pol, S.B., M.C. Brown, and C.E. Turner. Association of papillomavirus E6 oncoproteins with the focal adhesion protein paxillin through a conserved protein interaction motif. Oncogene. 1998;16:43–52. doi: 10.1038/sj.onc.1201504. [DOI] [PubMed] [Google Scholar]
- Westwick JK, Lambert QT, Clark GJ, Symons M, Van Aelst L, Pestell RG, Der CJ. Rac regulation of transformation, gene expression, actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo KO, Yoo JC, Jo D, Song YH, Kim MG, Park D. Cloning of a SH3 domain-containing proline-rich protein p85SPR, and its localization in focal adhesions. Biochem Biophys Res Comm. 1997;235:794–798. doi: 10.1006/bbrc.1997.6875. [DOI] [PubMed] [Google Scholar]
- Wood CK, Turner CE, Jackson P, Critchley DR. Characterization of the paxillin-binding site and the C-terminal focal adhesion targeting sequence in vinculin. J Cell Sci. 1994;107:709–717. [PubMed] [Google Scholar]
- Zachary I, Sinnett-Smith J, Turner CE, Rozengurt E. Bombesin, vasopressin and endothelin rapidly stimulate tyrosine phosphorylation of the focal adhesion associated protein paxillin in Swiss 3T3 cells. J Biol Chem. 1993;268:22060–22065. [PubMed] [Google Scholar]
- Zhang CJ, Cavenagh MM, Kahn RA. A family of Arf effectors defined as suppressors of the loss of Arf function in the yeast Saccharomyces cerevisiae. . J Biol Chem. 1998;273:19792–19796. doi: 10.1074/jbc.273.31.19792. [DOI] [PubMed] [Google Scholar]