Regulation of β-Catenin Levels and Localization by Overexpression of Plakoglobin and Inhibition of the Ubiquitin-Proteasome System (original) (raw)
Abstract
β-Catenin and plakoglobin (γ-catenin) are closely related molecules of the armadillo family of proteins. They are localized at the submembrane plaques of cell–cell adherens junctions where they form independent complexes with classical cadherins and α-catenin to establish the link with the actin cytoskeleton. Plakoglobin is also found in a complex with desmosomal cadherins and is involved in anchoring intermediate filaments to desmosomal plaques. In addition to their role in junctional assembly, β-catenin has been shown to play an essential role in signal transduction by the Wnt pathway that results in its translocation into the nucleus. To study the relationship between plakoglobin expression and the level of β-catenin, and the localization of these proteins in the same cell, we employed two different tumor cell lines that express N-cadherin, and α- and β-catenin, but no plakoglobin or desmosomal components. Individual clones expressing various levels of plakoglobin were established by stable transfection. Plakoglobin overexpression resulted in a dose-dependent decrease in the level of β-catenin in each clone. Induction of plakoglobin expression increased the turnover of β-catenin without affecting RNA levels, suggesting posttranslational regulation of β-catenin. In plakoglobin overexpressing cells, both β-catenin and plakoglobin were localized at cell– cell junctions. Stable transfection of mutant plakoglobin molecules showed that deletion of the N-cadherin binding domain, but not the α-catenin binding domain, abolished β-catenin downregulation. Inhibition of the ubiquitin-proteasome pathway in plakoglobin overexpressing cells blocked the decrease in β-catenin levels and resulted in accumulation of both β-catenin and plakoglobin in the nucleus. These results suggest that (a) plakoglobin substitutes effectively with β-catenin for association with N-cadherin in adherens junctions, (b) extrajunctional β-catenin is rapidly degraded by the proteasome-ubiquitin system but, (c) excess β-catenin and plakoglobin translocate into the nucleus.
Cell adhesion plays a central role in complex biological processes including motility, growth, differentiation and cell survival. The most direct effect of adhesion is on morphogenesis, i.e., the assembly of individual cells into highly ordered tissues and organs through cell–cell junctions (Takeichi, 1995; Gumbiner, 1996; Larue et al., 1996). The specific adhesive interactions between cells involve transmembrane cell adhesion receptors of the cadherin family (Takeichi, 1991, 1995; Geiger and Ayalon, 1992; Kemler, 1992), but effective adhesion and junction formation requires an association of the receptors with the cytoskeleton which is mediated by junctional plaque proteins (Kemler, 1993; Knudsen et al., 1995; Rimm et al., 1995). Cadherin-mediated cell–cell junctions are linked to either actin filaments in adherens junctions (via catenins, α- , β- , and γ-catenin, or plakoglobin; reviewed in Takeichi, 1991; Kemler, 1992; Knudsen and Wheelock, 1992; Wheelock et al., 1996), or to intermediate filaments in desmosomes (via plakoglobin, desmoplakins, plakophilins and other molecules; Schmidt et al., 1994). Plakoglobin is a common plaque component to both types of cell–cell junctions (Cowin et al., 1986; Franke et al., 1989; Cowin and Burke 1996; Wahl et al., 1996) that is essential for the sorting out of desmosomes and adherens junctions in the embryonic heart. Its elimination by gene disruption results in the collapse of this segregation in mouse embryos, and the development in the heart of extended adherens junctions that contain desmosomal proteins, which is lethal in the embryo (Bierkamp et al., 1996; Ruiz et al., 1996).
In addition to their function in cell adhesion, β-catenin and plakoglobin are highly homologous to Drosophila armadillo (Peifer and Weischaus, 1990) and belong to the armadillo family (Peifer et al., 1994_a_ ). Armadillo in Drosophila and β-catenin in Xenopus have been shown to play a role in the transduction of transmembrane signals initiated by the extracellular glycoprotein wg/Wnt that regulates cell growth, differentiation and fate (Peifer et al., 1994_b_; Peifer and Wieschans, 1990, Peifer, 1995; Gumbiner, 1995, 1996; Huber et al., 1996_a_ ; Miller and Moon, 1996). Activation of this pathway results in the elevation of β-catenin levels and its nuclear localization in a complex with the TCF/LEF family of transcription factors (Behrens et al., 1996; Huber et al., 1996_b_ ; Molenaar et al., 1996), suggesting that β-catenin may have a role in regulating gene expression (Riese et al., 1997; van de Wetering et al., 1997). In the absence of wg/Wnt signaling, β-catenin is degraded in mammalian cells by a process involving the adenomatous polyposis coli (APC)1 tumor suppressor protein (Powell et al., 1992) and the ubiquitin-proteasome degrading pathway (Aberle et al., 1997). Mutations in the APC gene that constitute the major genetic defect in inherited colon cancer and certain melanoma result in the accumulation of β-catenin (Munemitsu et al., 1995, 1996; Papkoff et al., 1996; Peifer, 1996; Rubinfeld et al., 1996; Yost et al., 1996), and most probably cause inappropriate activation of target genes by the β-catenin–LEF/TCF complex (Korinek et al., 1997; Morin et al., 1997; Rubinfeld et al., 1997). In contrast, the involvement of plakoglobin in suppressing tumorigenesis was inferred from studies showing loss of heterozygosity of the plakoglobin gene in certain types of tumors (Aberle et al., 1995), its reduction in several tumor cell types (Sommers et al., 1994; Navarro et al., 1994; Simcha et al., 1996), and by demonstrating that plakoglobin overexpression can suppress the tumorigenicity of mouse and human cells, while localized in the nuclei of such cells (Simcha et al., 1996). The regulation of β-catenin and plakoglobin level may therefore be a key element in their nuclear localization and signal transduction. In addition, plakoglobin and β-catenin bind in a mutually exclusive manner to cadherins, APC, and transcription factors (Butz and Kemler, 1994; Hülsken et al., 1994; Nathke et al., 1994; Rubinfeld et al., 1995; Huber et al., 1996_b_ ).
To study the relationship between β-catenin and plakoglobin expression and their localization in the same cell, we have employed cell lines that express β-catenin, but very low, or undetectable levels of plakoglobin, and do not form desmosomes. We present data indicating that overexpression of plakoglobin affects β-catenin stability, we identify the plakoglobin domains that are responsible for conferring β-catenin instability, and demonstrate that when the ubiquitin-proteasome protein degradation system is inhibited, plakoglobin and β-catenin translocate into the nucleus.
Materials and Methods
Cell Culture and Transfection
BALB/C 3T3, SVT2 that are BALB/C 3T3 cells transformed by SV40, MDCK, and HT1080 cells were grown in Dulbecco's modified Eagle medium plus 10% calf serum (GIBCO BRL, Gaithersburg, MD). SVT2 cells were transfected with a full-length human plakoglobin cDNA (Franke et al., 1989) cloned into the EcoRI site of the polylinker of the pJ4Ω expression vector (Rodríguez Fernández et al., 1992) that consists of the Mo-MuLV LTR promoter-enhancer sequence, the SV40 small t-antigen intron, and the SV40 large T polyadenylation signal in the pBR322 plasmid. The neomycin resistance gene (neor), which was cotransfected with the pJ4Ω construct, was subcloned into the pSVL expression vector. Transfection was carried out by the calcium phosphate precipitation method and colonies resistant to 800 μg/ml G418 (Geneticin; GIBCO BRL) were isolated. HT1080 cells were transfected with full-length or mutant plakoglobin cDNA constructs inserted into the pLKneo plasmid, driven by the dexamethasone-inducible MMTV promoter, as described (Sacco et al., 1995; Wahl et al., 1996). The construction and isolation of mutant plakoglobin cDNAs, as well as their ability to complex with either N-cadherin or α-catenin, by coimmunoprecipitation, were previously described (Sacco et al., 1995; Wahl et al., 1996). HT1080 clones transfected with plakoglobin cDNA were grown in the presence of 1 mg/ml G418 and the expression of plakoglobin was induced using 10−7 M dexamethasone. Mutant β-catenin lacking the NH2-terminal 57 amino acids (ΔN57) was prepared by isolating a 2.6-kb HincII fragment of mouse β-catenin cDNA (Butz et al., 1992) from the bluescript plasmid. This cDNA fragment lacking the first 57 NH2-terminal amino acid sequences was subcloned into the SmaI site of the pECE-Flag plasmid. The Flag epitope is localized at the NH2 terminus of the ΔN57 construct.
Protease Inhibitors
The calpain inhibitor _N_-acetyl-leu-leu-norleucinal (ALLN, used at 25 μM) and the inactive analogue _N_-acetyl-leu-leu-normethional (ALLM), as well as aprotinin, leupeptin, and pepstatin A (all used at 10 μg/ml), were purchased from Sigma Chemical Co. (St. Louis, MO). Lactacystin A (dissolved in water at 0.4 μg/ml was used at a final concentration of 4 ng/ml) and MG-132 (used at 10 μM) were purchased from Calbiochem-Novabiochem (La Jolla, CA).
Immunofluorescence Microscopy
Cells were cultured on glass coverslips, fixed with 3.7% paraformaldehyde in phosphate-buffered saline and permeabilized with 0.5% Triton X-100. A mAb recognizing the COOH terminus of human plakoglobin (PG5.1; Cowin et al., 1986) was obtained from Dr. W.W. Franke. A mAb recognizing an epitope at the NH2 terminus of human plakoglobin was previously described (11E4; Sacco et al., 1995; Wahl et al., 1996). The secondary antibody was rhodamine-labeled goat anti–mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). A polyclonal antiserum against β-catenin (Sigma, Holon, Israel) was employed and the secondary antibody was FITC-labeled goat anti–rabbit IgG. The cells were examined by epifluorescence with a Zeiss Axiophot microscope.
Protein Labeling and Immunoprecipitation
Cells (on 35-mm dishes) were incubated for 30 min in methionine-free medium, followed by 30-min incubation in the presence of 250 μCi/ml of [35S]methionine (pulse). The cells were washed extensively with medium containing excess non-radioactive methionine and incubated in fresh medium (chase). At different times after initiation of the chase, the cells were harvested in RIPA buffer (50 mM Tris HCl, pH 7.5, 0.15 M NaCl, 1% Triton X-100, 0.5% deoxycholate, and 0.1% SDS). 5 × 106 cpm of radioactive total cell extract was incubated at 4°C for 1 h with 40 μl of monoclonal 11E4 anti-plakoglobin antibody, followed by incubation for 30 min with 50 μl protein A+G/agarose and then processed as described (Sacco et al., 1995). The immune complexes were recovered by boiling in Laemmli sample buffer and resolved by SDS-PAGE (Laemmli, 1970). The radioactivity associated with the plakoglobin band was determined using a Fujix Bas 1000 PhosphorImager.
Immunoblotting and Polyacrylamide Gel Electrophoresis
Equal amounts of total cell protein from the different clones were separated by SDS-PAGE, electrotransferred to nitrocellulose and incubated with the monoclonal anti-plakoglobin antibodies, or with monoclonal antibodies against human N-cadherin (13A9; Knudsen et al., 1995), α-catenin, (1G5; Johnson et al., 1993), or β-catenin, (5H10; Johnson et al., 1993). The antigens were visualized by the ECL method (Amersham, Buckinghamshire, UK), and the density of each band quantitatively determined by laser densitometry using the ImageQuant software. Affinity-purified rabbit antibodies to APC (APC2), generated against a protein fragment containing amino acid residues 1034-2130 (Rubinfeld et al., 1993), were generously provided by Dr. P. Polakis (ONYX Pharmaceuticals, Richmond, CA). For APC resolution, 6% SDS-PAGE was employed.
In some experiments, cells were fractionated into Triton X-100–soluble and –insoluble fractions as described (Rodríguez Fernández et al., 1992). Cells cultured on 35-mm dishes were incubated in 0.5 ml buffer containing 50 mM of MES, pH 6.8, 2.5 mM EGTA, 5 mM MgCl2, and 0.5% Triton-X-100, at room temperature, for 3 min. The Triton X-100–soluble fraction was removed and the insoluble fraction, enriched in membrane-cytoskeletal complexes, was scraped into 0.5 ml of the same buffer. Equal volumes of the two fractions were analyzed by SDS-PAGE followed by immunoblotting with anti-plakoglobin antibodies.
Northern Blot Hybridization
Total RNA was extracted from cells by the guanidinium thiocyanate method. Northern blots containing 20 μg, per lane, of total RNA were stained with methylene blue to determine the positions of 18S and 28S rRNA markers, and then hybridized with plakoglobin (Franke et al., 1989) or β-catenin (Butz et al., 1992) cDNAs, which were labeled with [32P]dCTP by the random priming technique, as described (Glück et al., 1993).
Results
β-Catenin Expression in Plakoglobin-transfected Cells
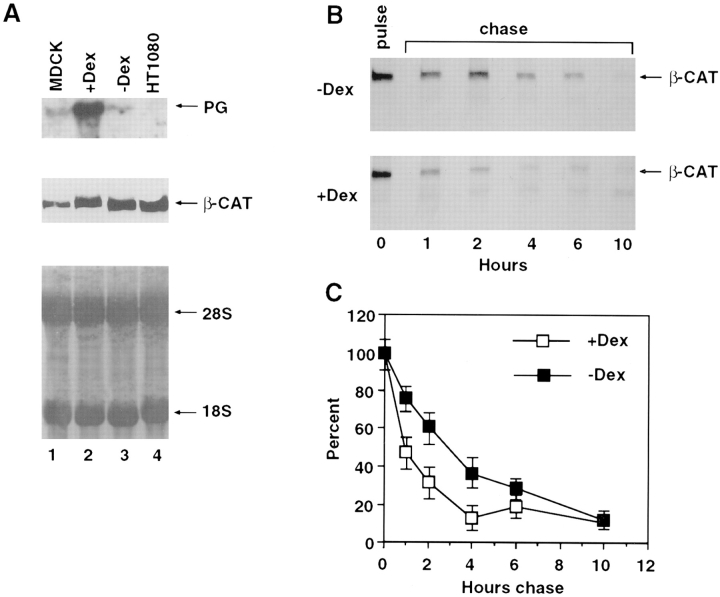
To study the effect of plakoglobin overexpression on β-catenin levels, we employed a human fibrosarcoma cell line (HT1080) and an SV40-transformed 3T3 cell line (SVT2), neither of which express detectable levels of plakoglobin (Sacco et al., 1995; Simcha et al., 1996), but express N-cadherin and α- and β-catenin (Fig. 1 A). Both cell lines were transfected with full-length plakoglobin and G418-resistant colonies were isolated that express varying levels of plakoglobin (Fig. 1, B and C). Immunoblot analysis revealed an inverse correlation between plakoglobin and β-catenin expression showing a more dramatic reduction of β-catenin in clones expressing higher levels of the transfected plakoglobin (Fig. 1, B and C). In addition, 3T3 fibroblasts that contained low levels of endogenous plakoglobin expressed high levels of β-catenin (Fig. 1 C, lane 7).
Figure 1.
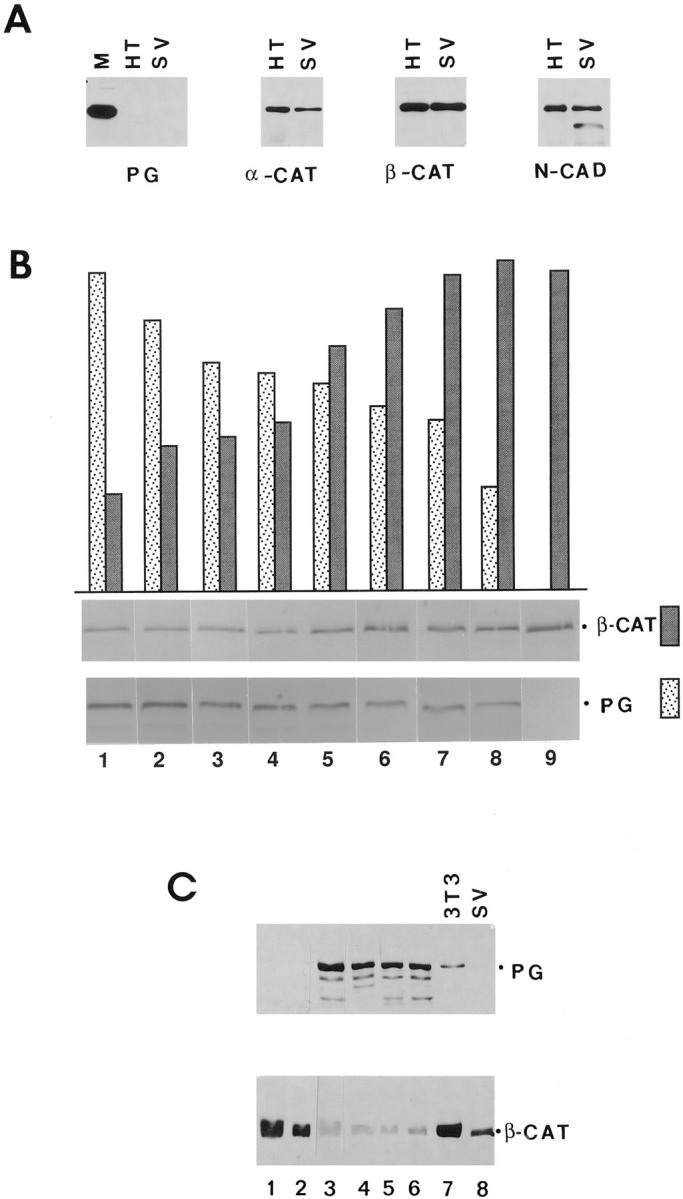
Expression of β-catenin in HT1080 and SVT2 cells overexpressing plakoglobin. (A) Equal amounts of total cell protein from HT1080 (HT), SVT2 (SV), and MDCK (M) cells were analyzed by immunoblotting with antibodies against plakoglobin (PG), α-catenin (_α_-CAT), β-catenin (_β_-CAT), and N-cadherin (N-CAD). (B) HT1080 cells were transfected with plakoglobin cDNA and equal amounts of total cell protein from individual clones stably expressing varying levels of plakoglobin and (cultured in the presence of dexamethasone) were analyzed by immunoblotting with antibodies against plakoglobin and β-catenin. The bars represent densitometer tracing of the intensity of plakoglobin (dotted bars) and β-catenin (filled bars) bands. C, SVT2 cells were stably transfected with plakoglobin and clones expressing varying levels of the transgene (lanes 1–6) were analyzed as in B. Lane 7, nontransfected 3T3 cells; lane 8, control SVT2 cells.
To study the dynamics of the relationship between plakoglobin expression and β-catenin levels, we used HT1080 cells stably expressing plakoglobin under the dexamethasone-inducible MMTV promoter. Upon stimulation with dexamethasone, plakoglobin expression was induced reaching a peak between 6 and 8 h (Fig. 2 A). Simultaneous analysis of plakoglobin (Fig. 2 A) and β-catenin (Fig. 2 B) after induction with dexamethasone revealed a three- to fivefold decrease in β-catenin levels 8–12 h after plakoglobin induction (Fig 2 C). By 12 h after induction, β-catenin was barely detectable (Fig. 2 B).
Figure 2.
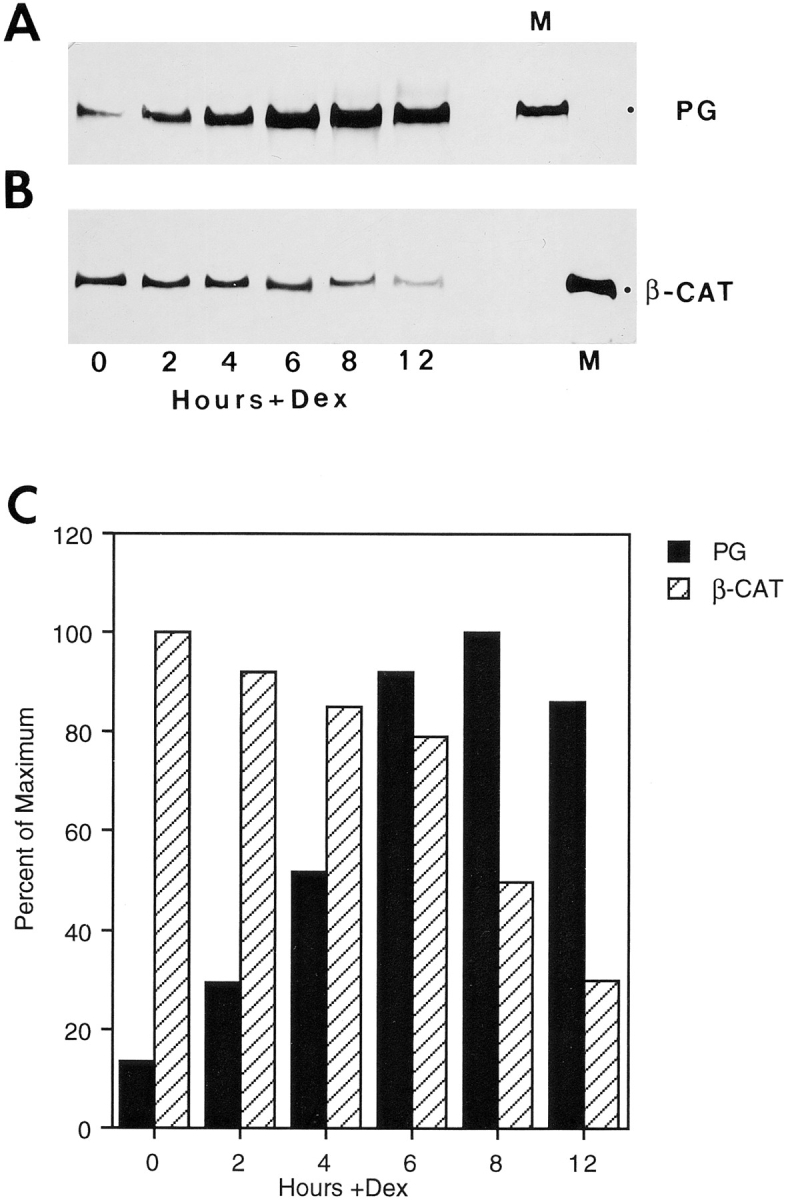
Regulation of β-catenin levels in HT1080 cells stably transfected with plakoglobin under an inducible promoter. (A) HT1080 cells expressing plakoglobin under a dexamethasone- inducible MMTV promoter were stimulated with dexamethasone, and at different time points after stimulation equal amounts of total cell protein were analyzed for plakoglobin expression by immunoblotting with antibodies against plakoglobin (PG). (B) Simultaneous analysis, on the same protein blot, of β-catenin (_β_-CAT) expression at different times after induction with dexamethasone. (C) Densitometer tracing of the levels of plakoglobin (filled bars) and β-catenin (hatched bars) expressed in A and B. M, MDCK cell lysates.
β-Catenin Localization and Degradation in Cells Overexpressing Plakoglobin
When HT1080 cells were stably transfected with plakoglobin under an inducible promoter, β-catenin (but not plakoglobin) was observed in intercellular junctions before induction with dexamethasone (Fig. 3, A and B). In cells stimulated with dexamethasone for 12 h, when β-catenin was reduced by about fourfold and plakoglobin was at its peak (Fig. 2), plakoglobin displayed very prominent junctional staining (Fig. 3 D). The β-catenin still expressed in these cells was apparently organized in the same junctions (Fig. 3 C), but displayed a much reduced intensity of staining. This suggests that the remaining β-catenin in plakoglobin overexpressing clones was not displaced from the junctions by the exogenous plakoglobin.
Figure 3.
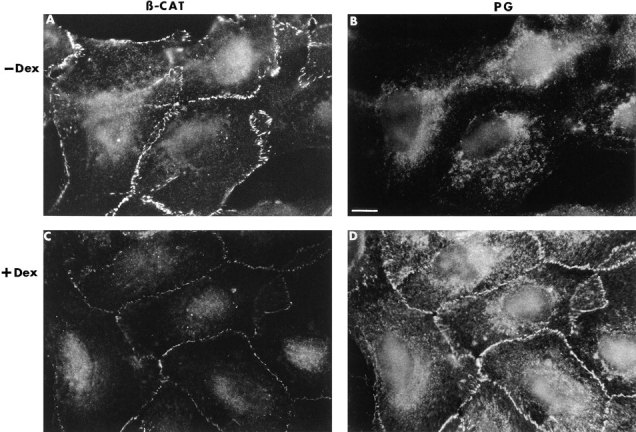
Organization of β-catenin and plakoglobin in HT1080 cells before and after induction of plakoglobin expression. HT1080 cells expressing inducible plakoglobin (as described in Fig. 2) were doubly stained with polyclonal anti–β-catenin (A and C) and monoclonal anti-plakoglobin (B and D) antibodies, before (A and B) and 12 h after (C and D) induction with dexamethasone (Dex). The secondary antibodies were FITC–anti-rabbit antibody and rhodamine anti–mouse-IgG. Bar, 10 μm.
To determine if the decrease in β-catenin levels resulted from a reduction in β-catenin RNA, Northern blots from uninduced and dexamethasone-induced HT1080 cells were hybridized with cDNAs to β-catenin and plakoglobin (Fig. 4 A). Dexamethasone stimulation resulted in a dramatic increase in plakoglobin RNA levels (Fig. 4 A, compare lanes 2 with 3), but did not effect the level of β-catenin RNA (Fig. 4 A). This suggests that the decrease in β-catenin levels of plakoglobin overexpressing cells does not result from a loss of β-catenin mRNA.
Figure 4.
Expression of β-catenin RNA and stability of β-catenin protein in cells overexpressing inducible plakoglobin. (A) RNA was extracted from MDCK cells (lane 1), untransfected HT1080 cells (lane 4), and plakoglobin-transfected HT1080 cells before (lane 3), and 12 h after stimulation with dexamethasone (lane 2). Equal amounts of total cell RNA were analyzed by Northern blot hybridization with plakoglobin (PG) and β-catenin (β_-CAT) cDNAs. The levels of 18 and 28S rRNA are shown for comparison. (B) Uninduced (−_Dex) and cells induced for 12 h (+Dex) were pulse labeled with [35S]methionine for 30 min, followed by chase with fresh medium. Equal amounts of radioactive cellular proteins were immunoprecipitated with anti–β-catenin antibody and analyzed by SDS-PAGE. (C) The radioactivity in the β-catenin band in B and in an identical independent experiment was determined by a phosphorimager, and the values ±SD are presented as percent of the values obtained after 30 min pulse labeling.
We examined the possibility that β-catenin degradation was enhanced in plakoglobin overexpressing HT1080 cells, by labeling with [35S]methionine for 30 min and chasing for increasing periods of time in fresh medium with excess nonradioactive methionine, containing plakoglobin under the dexamethasone inducible MMTV promoter. Immunoprecipitation of β-catenin from equal amounts of radioactive whole cell lysates showed that the level of newly synthesized β-catenin decreased significantly faster (more than threefold) in dexamethasone induced cells than in control, uninduced cells (Fig. 4, B and C). Dexamethasone had no effect on the degradation of β-catenin in control HT1080 cells (results not shown). These results suggest that the decrease of β-catenin in plakoglobin overexpressing cells resulted from a faster turnover of β-catenin.
Expression of β-Catenin in Cells Overexpressing Mutant Plakoglobin
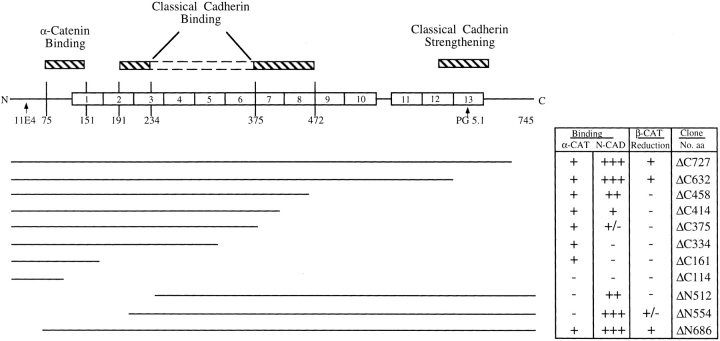
Since plakoglobin and β-catenin are localized in the submembrane plaque where they form mutually exclusive complexes with cadherins, we examined the possibility that the overexpressed plakoglobin competes with β-catenin for N-cadherin binding, and this competition leads to displacement and degradation of uncomplexed β-catenin. HT1080 cell lysates, prepared before and after plakoglobin induction, were immunoprecipitated with anti–N-cadherin antibody followed by Western blotting with anti–β-catenin and plakoglobin antibodies (Fig. 5 A). When plakoglobin expression was induced with dexamethasone, the complexes with N-cadherin contained more plakoglobin than β-catenin (Fig. 5 A, compare lanes 3 and 4 with lane 1). To examine the possibility that N-cadherin binding with plakoglobin is responsible for conferring the decrease in β-catenin levels, we employed HT1080 cells stably transfected with mutant plakoglobin constructs that included or lacked either the N-cadherin or the α-catenin binding domains (see Fig. 9; Sacco et al., 1995). Expression of full-length (FL) plakoglobin (Fig. 5 B, lanes 1 and 2), and deletions that left the armadillo repeats intact (Fig. 5 B, ΔC727, lanes 3 and 4) or removed part of the last armadillo repeat (Fig. 5 B, ΔC632, lanes 5 and 6), but were still capable of associating with N-cadherin, reduced the level of β-catenin when compared to uninduced cells (Fig. 5 B, lanes 1, 3, and 5, compare with 2, 4, and 6, respectively). In contrast, larger COOH-terminal deletions in plakoglobin that disrupt association with N-cadherin, but retain α-catenin binding (Fig. 9), leaving 458 amino acids or less (Fig. 5 C; ΔC458, ΔC414, ΔC375, ΔC161, and ΔC114), were unable to affect β-catenin levels when overexpressed (Fig. 5 C, lanes 2, 4, 6, 8, and 10, compare with 1, 3, 5, 7, and 9, respectively).
Figure 5.
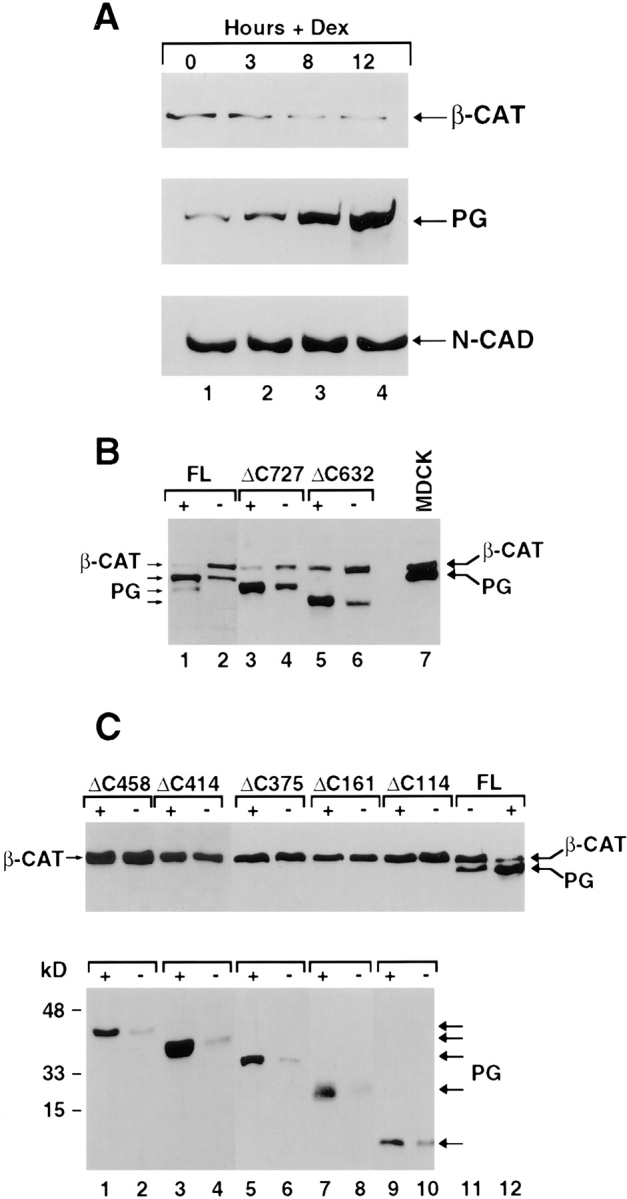
β-Catenin and plakoglobin levels in complexes with _N_-cadherin in cells transfected with COOH-terminal deletion mutant plakoglobin under control of an inducible promoter. (A) Equal amounts of cellular protein from unstimulated HT1080 cells (lane 1) and cells induced with dexamethasone to express plakoglobin driven by the MMTV promoter for: 3 (lane 2), 8 (lane 3) and 12 h (lane 4) were immunoprecipitated with anti– _N_-cadherin antibody and the protein blot was reacted with anti β-catenin (_β_-CAT), plakoglobin (PG), and anti–N-cadherin (N-CAD) antibodies. (B) Cells expressing full-length (FL), or COOH-terminal–truncated (ΔC) plakoglobin retaining 727 (ΔC727) and 632 (ΔC632) amino acids. (C) Cells expressing COOH-terminal plakoglobin deletions containing 458 (Δ458), 414 (Δ414), 375 (Δ375), 161 (Δ161), and 114 (Δ114) amino acids. Uninduced (−) and cells induced (+) with dexamethasone, to express the different plakoglobin constructs, were analyzed by immunoblotting with anti-plakoglobin (PG) and β-catenin (_β_-CAT) antibodies on the same blots.
Figure 9.
Schematic representation of plakoglobin functional domains involved in reducing β-catenin levels and in complexing with α-catenin and N-cadherin. Full-length human plakoglobin (745 amino acids) is shown with the 13 armadillo repeats and the different deletion mutants from the NH2 (ΔN) and COOH terminus (ΔC) used in this study. The binding studies to α-catenin and N-cadherin of the various plakoglobin mutants were described (Sacco et al., 1995; Wahl et al., 1996). The binding sites for the monoclonal antibodies 11E4 and PG 5.1 used in this study are also indicated.
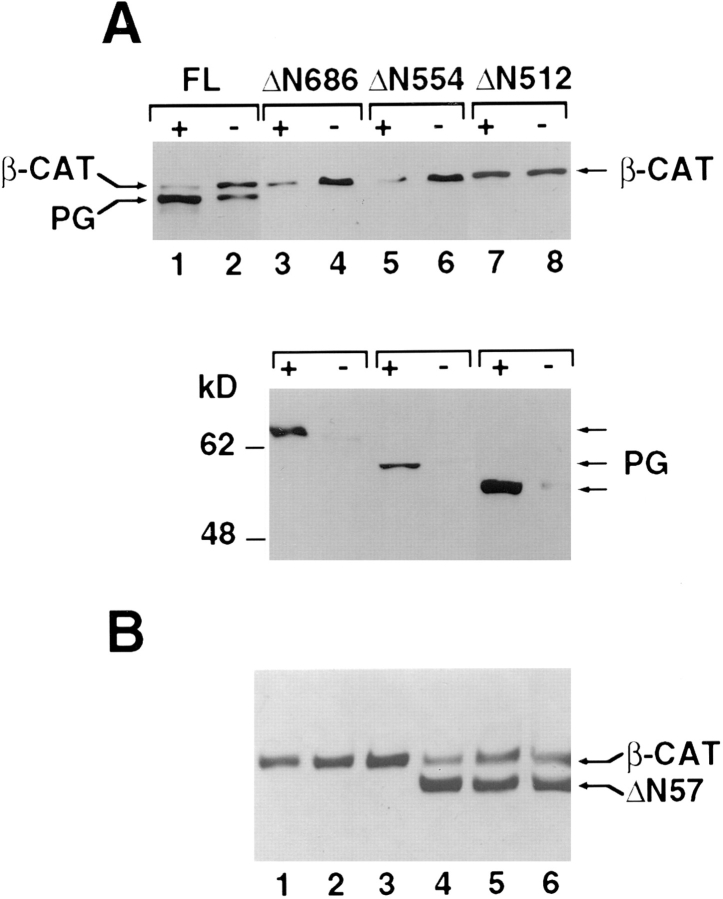
NH2-terminal deletions of plakoglobin that retained both α-catenin and N-cadherin binding sites (Fig. 9; ΔN686), or removed armadillo repeat 1 and part of armadillo repeat 2 (see Fig. 9; ΔN554), and thus deleted α-catenin binding but retained N-cadherin binding, were able to reduce β-catenin levels when overexpressed in HT1080 cells (Fig. 6 A, compare lanes 3 with 4 and 5 with 6). However, more extensive NH2-terminal truncations that removed armadillo repeat 3, which is important for N-cadherin binding (see Fig. 9, Sacco et al., 1995), did not affect β-catenin levels (Fig. 6 A, compare lane 7 with 8). The expression of other partners that associate with both β-catenin and plakoglobin, such as N-cadherin, α-catenin, and APC, was not affected by the overexpression of full-length or mutant plakoglobin (results not shown).
Figure 6.
Levels of β-catenin in cells expressing inducible NH2-terminal deleted plakoglobin and β-catenin mutants. (A) Levels of β-catenin in cells expressing dexamethasone-inducible plakoglobin mutants with NH2-terminal deletions retaining 686 (ΔN686), 554 (ΔN554), or 512 (ΔN512) amino acid, were determined as in Fig. 5. (B) Levels of β-catenin in individual clones stably expressing an NH2-terminal deleted β-catenin lacking the first 57 amino acids (ΔN57, lanes 4–6) or neor controls (lanes 1–3).
We have also analyzed the association of the various plakoglobin mutants with a Triton X-100–insoluble membrane-cytoskeletal complex. Interestingly, COOH-terminal plakoglobin mutants that were unable to confer a decrease in β-catenin levels were mostly detected in the Triton X-100–soluble fraction (Fig. 7 A, compare lanes 9 with 10, and 11 with 12; Fig. 7 B, compare lanes 3 with 4, 5 with 6 and 7 with 8), while plakoglobin deletions that conferred a decrease in β-catenin were found mostly in the Triton X-100–insoluble fraction (Fig. 7 A, compare lane 3 with 4 and 13 with 14), similar to full-length plakoglobin expressing HT1080 (Fig. 7, A–C, lanes 1 and 2) and MDCK cells (Fig. 7 A, lanes 7 and 8). Analysis of NH2-terminal deletions of plakoglobin gave similar results, with ΔN512 being completely Triton X-100–soluble (Fig. 7 C, lanes 7 and 8), in contrast to ΔN686 (Fig. 7 C, lanes 3 and 4) that was mostly in the Triton X-100–insoluble fraction, like full-length plakoglobin (Fig. 7 C, lanes 1 and 2). This distribution of the plakoglobin mutants between the Triton X-100–soluble and –insoluble fractions most likely reflects their ability or inability to associate with N-cadherin.
Figure 7.
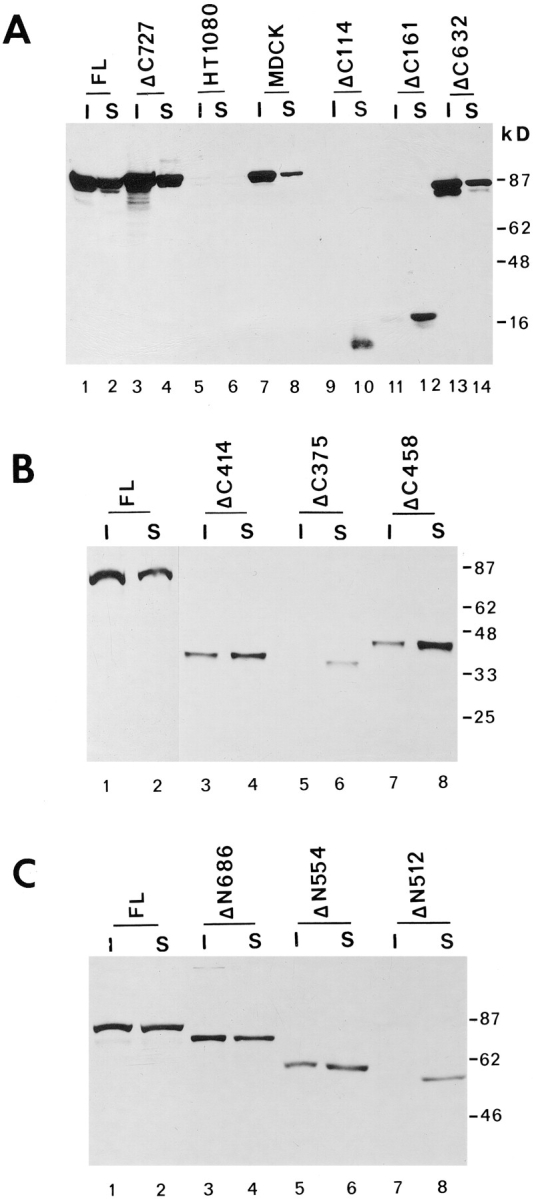
The solubility in Triton X-100 of full-length and plakoglobin mutants. (A) HT1080 cells expressing full-length plakoglobin (FL), untransfected HT1080, MDCK, and cells expressing COOH-terminal deletions of plakoglobin containing 727 (Δ727), 632 (Δ632), 161 (Δ161), and 114 (Δ114) amino acids were separated into Triton X-100–soluble (S) and –insoluble (I) fractions. Equal volumes of each fraction were analyzed for plakoglobin levels by immunoblotting. (B) HT1080 expressing COOH-terminal deletions that contain 458 (Δ458), 414 (Δ414), and 375 (Δ375) amino acids were analyzed as described in A. (C) Analysis of the Triton X-100 solubility of NH2-terminal plakoglobin deletions containing 686 (Δ686), 554 (Δ554), and 512 (Δ512) amino acids. In A and B the 11E4 antibody was used, while in C antibody PG 5.1 was used (see Fig. 9 for the plakoglobin domains recognized by these antibodies).
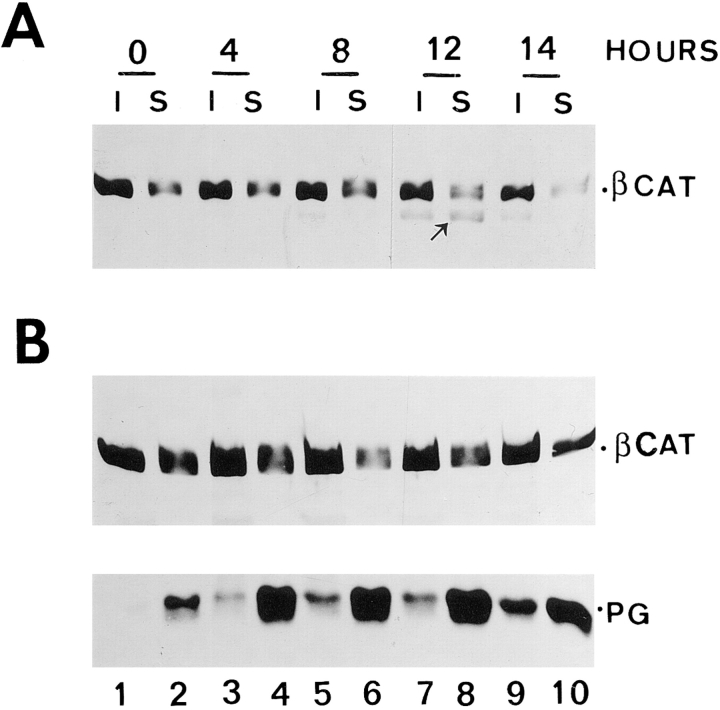
We have also determined the distribution of β-catenin between the Triton X-100–soluble and –insoluble fractions in HT1080 cells expressing full-length plakoglobin and in COOH-terminal deleted plakoglobin expressing cells (ΔC161) where the truncated plakoglobin could not confer a decrease in β-catenin levels (see Fig. 9). The results summarized in Fig. 8 show that β-catenin levels were reduced in both Triton X-100–soluble and –insoluble fractions upon plakoglobin induction, and a lower molecular mass product of β-catenin (probably a degraded form) was apparent at later times after plakoglobin induction (Fig. 8 A, lanes 7–9). In ΔC161-expressing cells, no significant changes in β-catenin levels and detergent solubility were apparent when the mutant plakoglobin (mainly found in the soluble fraction) was induced (Fig. 8 B).
Figure 8.
Triton X-100 solubility of β-catenin in cells expressing full-length and mutant plakoglobin. HT1080 cells transfected with full-length plakoglobin (A), or with the COOH-terminal deletion mutant ΔC161 (B) were stimulated to express plakoglobin by dexamethasone, and at various times after induction the levels of plakoglobin and β-catenin were determined in the Triton X-100– soluble and –insoluble fractions as described in Fig. 7.
Expression of β-Catenin in Cells Overexpressing Mutant β-Catenin
To compare the ability of ectopically expressed β-catenin to that of plakoglobin in conferring a decrease in the level of cellular β-catenin, HT1080 cell lines stably expressing an epitope tagged NH2-terminal deleted β-catenin were isolated (ΔN57). This mutant, lacking the first 57 amino acids, but containing the α-catenin and N-cadherin binding domains (Fig. 9), was shown to be more stable than wt β-catenin (Munemitsu et al., 1996; Yost et al., 1996). Individual clones expressing this mutant β-catenin (Fig. 6 B) contained a lower level of endogenous β-catenin. The mutant β-catenin was localized at cell–cell junctions, and its distribution in the cell was indistinguishable from that of the endogenous wt protein (data not shown).
Stabilization of β-Catenin and Nuclear Translocation of Plakoglobin and β-Catenin after Inhibition of the Ubiquitin-dependent Proteasome System
A recent study has demonstrated that the turnover of β-catenin is mediated by the ubiquitin-proteasome degradation system (Aberle et al., 1997). When this proteolytic pathway is inhibited by specific inhibitors of the proteasome-mediated proteolysis such as Lactacystin, MG-132 and the peptide aldehyde ALLN, it leads to the stabilization and accumulation of ubiquitinated forms of β-catenin (Aberle et al., 1997). To examine if stabilization of β-catenin against proteasome-mediated degradation will block the decrease in β-catenin levels of HT1080 cells induced to express plakoglobin, we treated cells with MG-132 or ALLN and determined the levels of β-catenin and plakoglobin. The results summarized in Fig. 10 show that while β-catenin levels were reduced in cells treated with an inactive peptide analogue (ALLM) (Fig. 10 A, lanes 1 and 2), they were even higher than the controls in the presence of the active proteasome inhibitors ALLN and MG-132 (Fig. 10 A, lanes 3–8, compare to lanes 1, 2, and 9). Furthermore, a higher molecular mass form of β-catenin, probably representing ubiquitinated β-catenin, could be detected in the presence of these inhibitors (Fig. 10 B, lanes 3–8), in agreement with Aberle et al. (1997). These results imply that plakoglobin overexpression cannot confer a decrease in β-catenin levels when the proteasome degradation pathway is inhibited, which results in β-catenin stabilization against degradation.
Figure 10.
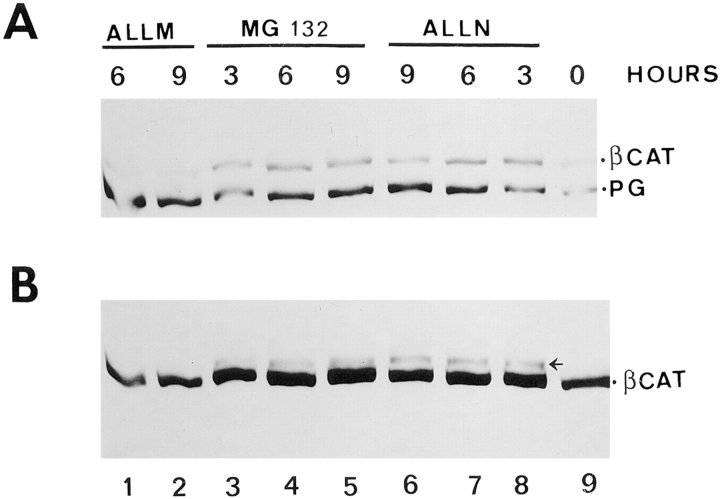
Expression of β-catenin and plakoglobin in cells treated with inhibitors of the ubiquitin-proteasome system. (A) HT1080 cells were treated for 2 h with proteasome inhibitors (MG 132 or ALLN), or with the inactive analogue ALLM, as described in Materials and Methods, and then induced to express plakoglobin with dexamethasone in the presence of the inhibitors. At different times after dexamethasone stimulation, equal amounts of total cell lysate were analyzed for β-catenin and plakoglobin expression by immunoblotting as described in Fig. 8. (B) The immunoblot for β-catenin was overexposed to reveal the higher molecular mass forms of β-catenin (arrowhead, probably ubiquitinated) formed in the presence of the proteasome inhibitors.
To determine the localization of plakoglobin and β-catenin when the ubiquitin-proteasome proteolytic pathway is inhibited, HT1080 cells were treated with ALLN or MG-132 and plakoglobin expression was induced for 9 h. The cells were immunostained with anti-plakoglobin and anti– β-catenin antibodies (Fig. 11). The results shown in Fig. 11 demonstrate that when proteasome inhibitors were applied, a significant amount of β-catenin translocated into the nuclei of the cells (Fig. 11, B and C), in contrast to cells treated with the inactive ALLM peptide that showed only cell–cell junctional staining (Fig. 11 A). Interestingly, under these conditions plakoglobin was not organized in cell–cell junctions, but was diffusely distributed in the cytoplasm with a significant accumulation in the nuclei of the cells (Fig. 11 D). Similar results were obtained using Lactacystin, but not with other protease inhibitors including pepstatin A, aprotinin and leupeptin which have no effect on this degradation pathway (results not shown).
Figure 11.
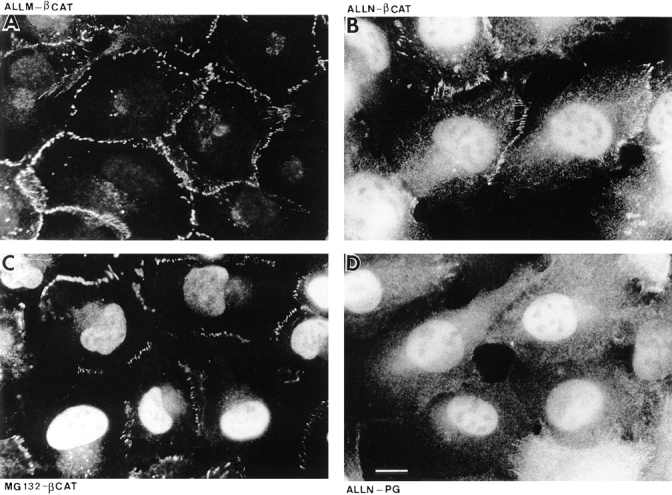
The localization of β-catenin and plakoglobin in cells treated with inhibitors of the ubiquitin-proteasome system. HT1080 cells were pretreated with the inactive analogue ALLM (A), with the proteasome inhibitors ALLN (B and D), or with MG-132 in the presence of dexamethasone to induce plakoglobin expression (C). After 9 h, the cells were immunostained for β-catenin (A–C) or plakoglobin (D). Bar, 10 μm.
Discussion
Regulation of the expression of β-catenin and plakoglobin are important in morphogenetic events during embryonic development (McCrea et al., 1993; Funayama et al., 1995; Karnovsky and Klymkowsky, 1995; Miller and Moon, 1996; Orsulic and Peifer, 1996; Peifer, 1996; Schneider et al., 1996; Rubenstein et al., 1997), and in the process of tumorigenesis (Aberle et al., 1994; Inomata et al., 1996; Simcha et al., 1996; Ben-Ze'ev, 1997; Korinek et al., 1997; Morin et al., 1997; Rubinfeld et al., 1997). In this study, we have addressed the regulation of β-catenin levels and localization by the overexpression of plakoglobin, and by the inhibition of the ubiquitin-proteasome proteolytic pathway.
We have shown for the first time that stable or inducible overexpression of plakoglobin leads to a decrease in β-catenin level that is proportional to the level of plakoglobin expression, and results in increased degradation of β-catenin. Since the cells used in this study lack desmosomal cadherins, it is conceivable that the exogenous plakoglobin competes with β-catenin for N-cadherin binding, directing the displaced β-catenin molecules for degradation by the ubiquitin-proteasome system (Aberle et al., 1997). This notion is supported by our results showing that when plakoglobin expression was induced, the level of β-catenin in complex with N-cadherin decreased, and the level of plakoglobin in complexes with N-cadherin increased (Fig. 5 A). In addition, deletion of the N-cadherin binding domain of plakoglobin abolished the ability of the mutant plakoglobin molecules to downregulate β-catenin levels. In contrast, the binding of plakoglobin to α-catenin was neither necessary nor sufficient to influence β-catenin levels, and deletion of this domain of plakoglobin did not affect β-catenin levels in the cell. Expression of a more stable, NH2-terminal deletion mutant of β-catenin (ΔN57), also conferred a decrease in the level of the endogenous β-catenin (Fig. 6 B). This mutant β-catenin, which retained the cadherin and α-catenin binding sites, was localized at cell–cell junctions, displacing endogenous β-catenin and leading to its degradation. The mutant plakoglobin molecules that could not confer β-catenin degradation were mostly soluble in Triton X-100, and thus were unable to compete with the membrane-cytoskeleton–associated N-cadherin adhesion complex that contained the junctional β-catenin.
Inhibition of the ubiquitin-proteasome protein degradation system resulted in the stabilization of β-catenin (in agreement with Aberle et al., 1997), thus blocking the ability of plakoglobin to confer a decrease in the level of β-catenin (Fig. 10). In such cells, higher molecular weight forms of β-catenin, probably representing ubiquitinated β-catenin molecules, were apparent.
Immunofluorescence staining revealed that in wt plakoglobin overexpressing cells the remaining β-catenin was associated with cell–cell junctions (where it was probably protected from degradation). These junctions also contained the majority of the plakoglobin. In contrast, when β-catenin degradation by the proteasome system was inhibited, β-catenin accumulated in the cell, and a significant amount of the protein translocated into the nucleus. Plakoglobin was unable to displace β-catenin from cell–cell junctions under these conditions. It was diffusely distributed in the cytoplasm, and a significant level of plakoglobin was also localized in the nucleus (Fig. 11 D). This implies that β-catenin may have higher affinity for binding to N-cadherin than plakoglobin, and when its level in the cell is increased, plakoglobin is no more capable of effectively competing and replacing the junctional β-catenin. The regulation of β-catenin and plakoglobin contents in the cell therefore determines, to a large extent, their cellular localization, and an additional site where these junctional molecules can accumulate is the nucleus of the cell.
Our results reinforce the view that nuclear translocation of β-catenin during signaling, conceivably results from increases in its level in the cell. This increase could be achieved by influencing the regulated degradation of β-catenin by the ubiquitin-proteasome pathway (as shown in this study), or by artificially overexpressing very high levels of β-catenin that can saturate the degradation system, as is the case in transiently transfected cells that often express abnormally high levels of the protein, which is mainly localized in the nucleus (Simcha, I., B. Geiger, and A. Ben-Ze'ev, unpublished results).
Another well documented posttranscriptional regulation of β-catenin level is obtained by binding/sequestration with cadherins, as was demonstrated in studies overexpressing N- or E-cadherin, that leads to increased β-catenin levels in these cells (Kowalczyk et al., 1994; Nagafuchi et al., 1994; Simcha et al., 1996). Similarly, stabilization of plakoglobin was documented in CHO cells transfected with desmosomal cadherins (Kowalczyk et al., 1994). An increase in β-catenin and plakoglobin levels, without an effect on their mRNA levels, was also seen in Wnt-transfected mammalian cells (Bradley et al., 1993; Hinck et al., 1994; Papkoff et al., 1996). Thus, modulating the degradation of β-catenin by the formation of complexes between β-catenin and cadherins may constitute an important means to regulate β-catenin levels and consequently, its extrajunctional function(s) in the cell.
Wnt-induced signaling that involves β-catenin during development also correlates with changes in β-catenin levels in the cell, and artificially elevated cadherin expression in Xenopus can antagonize the propagation of the Wnt signal, by sequestering free pools of β-catenin into a complex with cadherin, and thus limiting its function in extra-junctional signaling (Heasman et al., 1994; Fagotto et al., 1996; Yost et al., 1996). The current results suggest that plakoglobin can serve as an additional regulator of β-catenin level acting upstream of the APC-GSK-3β step, by competing on the cadherin binding site, and thus releasing β-catenin and exposing it to the degradation fate.
The accumulation of β-catenin and its nuclear translocation in complex with transcription factors, its aberrant effect on the transcription of genes during development of colon cancer and melanoma (Korinek et al., 1997; Morin et al., 1997; Rubinfeld et al., 1997), as well as the ability of plakoglobin to influence the tumorigenicity of cells when overexpressed and localized in the nucleus (Simcha et al., 1996), highlight the importance of mechanisms that regulate the level of β-catenin in the cell, as shown in this study. Interestingly, in tumor cells where plakoglobin overexpression resulted in suppression of the tumorigenic ability (Simcha et al., 1996), the level of β-catenin was reduced (this study). This may indicate that plakoglobin confers a tumor suppressive phenotype on these cells by decreasing the level of β-catenin, whose abnormally increased level can be oncogenic (Korinek et al., 1997; Morin et al., 1997; Peifer, 1997; Rubinfeld et al., 1997).
The challenge for future studies is to determine whether elevated β-catenin can confer tumorigenicity on nontransformed cells, the physiological conditions that are associated with the regulated expression and translocation of β-catenin and plakoglobin into the nuclei of mammalian cells, and the target genes whose expression is modulated by transactivation involving complexes that contain these junctional plaque proteins.
Acknowledgments
We thank Dr. Kemler for communicating results prior to their publication and B. Geiger for useful comments.
These studies were supported in part by grants from the USA-Israel Binational Foundation, the Forchheimer Center for Molecular Genetics, the Pasteur-Weizmann Research Program, the German-Israeli Foundation for Scientific Research and Development to A. Ben-Ze'ev, and by National Institutes of Health GM 51188 to M.J. Wheelock and K.R. Johnson.
Abbreviations used in this paper
ALLM
_N_-acetyl-leu-leu-normethional
ALLN
_N_-acetyl-leu-leu-norleucinal
APC
adenomatous polyposis coli
References
- Aberle H, Bierkamp C, Torchard D, Serova O, Wagener T, Natt E, Wirsching J, Heidkämper C, Montagna M, Lynch HT, et al. The human plakoglobin gene localizes on chromosome 17q21 and is subject to loss of heterozygosity in breast and ovarian cancer. Proc Natl Acad Sci USA. 1995;92:6384–6388. doi: 10.1073/pnas.92.14.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO (Eur Mol Biol Organ) J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bradley RS, Cowin P, Brown AM. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J Cell Biol. 1993;123:1857–1865. doi: 10.1083/jcb.123.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol. 1997;9:99–108. doi: 10.1016/s0955-0674(97)80158-5. [DOI] [PubMed] [Google Scholar]
- Bierkamp C, Mclaughlin KJ, Schwartz H, Huber O, Kemler R. Embryonic heart and skin defects in mice lacking plakoglobin. Dev Biol. 1996;180:780–785. doi: 10.1006/dbio.1996.0346. [DOI] [PubMed] [Google Scholar]
- Butz S, Kemler R. Distinct cadherin-catenin complexes in Ca2+- dependent cell–cell adhesion. FEBS Lett. 1994;355:195–200. doi: 10.1016/0014-5793(94)01205-9. [DOI] [PubMed] [Google Scholar]
- Butz S, Stappert J, Weissig H, Kemler R. Plakoglobin and β-catenin: distinct but closely related. Science. 1992;257:1142–1144. doi: 10.1126/science.257.5073.1142-a. [DOI] [PubMed] [Google Scholar]
- Cowin P, Kapprell HP, Franke WW, Tamkun J, Hynes RO. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell. 1986;46:1063–1073. doi: 10.1016/0092-8674(86)90706-3. [DOI] [PubMed] [Google Scholar]
- Cowin P, Burke B. Cytoskeleton-membrane interactions. Curr Opin Cell Biol. 1996;8:56–65. doi: 10.1016/s0955-0674(96)80049-4. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Funayama N, Glück U, Gumbiner BM. Binding to cadherins antagonizes the signaling activity of β-catenin during axis formation in Xenopus. J Cell Biol. 1996;132:1105–1114. doi: 10.1083/jcb.132.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Goldschmidt MD, Zimbelmann R, Mueller HM, Schiller DL, Cowin P. Molecular cloning and amino acid sequence of human plakoglobin, the common junctional plaque protein. Proc Natl Acad Sci USA. 1989;86:4027–4031. doi: 10.1073/pnas.86.11.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- Glück U, Kwiatkowski DJ, Ben-Ze'ev A. Suppression of tumorigenicity in Simian virus 40-transformed cells transfected with α-actinin cDNA. Proc Natl Acad Sci USA. 1993;90:383–387. doi: 10.1073/pnas.90.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Signal transduction by β-catenin. Curr Opin Cell Biol. 1995;7:634–640. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of β-catenin inhibits dorsal mesoderm induction in early Xenopus. . Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Hinck L, Nelson WJ, Papkoff J. Wnt-1 modulates cell–cell adhesion in mammalian cells by stabilizing β-catenin binding to the cell adhesion protein cadherin. J Cell Biol. 1994;124:729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber O, Bierkamp C, Kemler R. Cadherins and catenins in development. Curr Opin Cell Biol. 1996a;8:685–691. doi: 10.1016/s0955-0674(96)80110-4. [DOI] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996b;59:3–11. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Hülsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with β-catenin and the cytoskeleton. J Cell Biol. 1994;127:2061–2069. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata M, Ochiai A, Akimoto S, Kitano S, Hirohashi S. Alteration of β-catenin expression in colonic epithelial cells of familial adenomatous polyposis patients. Cancer Res. 1996;56:2213–2217. [PubMed] [Google Scholar]
- Johnson KR, Lewis JE, Li D, Wahl J, Soler AP, Knudsen KA, Wheelock MJ. P- and E-cadherin are in separate complexes in cells expressing both cadherins. Exp Cell Res. 1993;207:252–260. doi: 10.1006/excr.1993.1191. [DOI] [PubMed] [Google Scholar]
- Karnovsky A, Klymkowsky MW. Over-expression of plakoglobin leads to dorsalization and axis duplication in Xenopus. . Proc Natl Acad Sci USA. 1995;92:4522–4526. doi: 10.1073/pnas.92.10.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R. Classical cadherins. Semin Cell Biol. 1992;3:149–155. doi: 10.1016/s1043-4682(10)80011-x. [DOI] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genetics. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Wheelock MJ. Plakoglobin, or an 83-kD homologue distinct from β-catenin, interacts with E-cadherin and N-cadherin. J Cell Biol. 1992;118:671–679. doi: 10.1083/jcb.118.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of α-actinin with the cadherin-catenin cell–cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Backer N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/−colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kowalczyk AP, Palka HL, Luu HH, Nilles LA, Anderson JE, Wheelock MJ, Green KJ. Posttranslational regulation of plakoglobin expression: influence of desmosomal cadherins on plakoglobin metabolic stability. J Biol Chem. 1994;269:31214–31223. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larue L, Antos C, Butz S, Huber O, Delmas V, Dominis M, Kemler R. A role for cadherins in tissue formation. Development (Camb) 1996;122:3185–3194. doi: 10.1242/dev.122.10.3185. [DOI] [PubMed] [Google Scholar]
- McCrea PD, Brieher WM, Gumbiner BM. Induction of a secondary body axis in Xenopusby antibodies to β-catenin. J Cell Biol. 1993;123:477–484. doi: 10.1083/jcb.123.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Moon RT. Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopusembryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Morin, P.J., A.B. Sparks, Korinek, V., N. Barker, H. Clevers, B. Vogelstein, and K.W. Kinzler. 1997. Activation of β-catenin Tcf-signaling in colon cancer by mutations in β-catenin or APC. Science. 275:1787–1790. [DOI] [PubMed]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Rubinfeld B, Polakis P. Deletion of an amino-terminal sequence stabilizes β-catenin in vivo and promotes hyperphosphorylation of the adenomatous polyposis coli tumor suppressor protein. Mol Cell Biol. 1996;16:4088–4094. doi: 10.1128/mcb.16.8.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cells adhesion: functional analysis of E-cadherin-α-catenin fusion molecules. J Cell Biol. 1994;127:235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathke SI, Hinck L, Swedlow JR, Papkoff J, Nelson WJ. Defining interactions and distributions of cadherin and catenin complexes in polarized cells. J Cell Biol. 1994;125:1341–1352. doi: 10.1083/jcb.125.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro P, Lozano E, Cano A. Expression of E- or P-cadherin is not sufficient to modify the morphology and the tumorigenic behavior of murine spindle carcinoma cells: possible involvement of plakoglobin. J Cell Sci. 1993;105:923–934. doi: 10.1242/jcs.105.4.923. [DOI] [PubMed] [Google Scholar]
- Orsulic S, Peifer M. An in vivo structure-function study of armadillo, the β-catenin homologue, reveals both separate and overlapping regions of the protein required for cell adhesion and for wingless signalling. J Cell Biol. 1996;134:1283–1300. doi: 10.1083/jcb.134.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J, Rubinfeld B, Schryver B, Polakis P. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol Cell Biol. 1996;16:2128–2134. doi: 10.1128/mcb.16.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M. Cell adhesion and signal transduction: the armadilloconnection. Trends Cell Biol. 1995;5:224–229. doi: 10.1016/s0962-8924(00)89015-7. [DOI] [PubMed] [Google Scholar]
- Peifer M. Regulating cell proliferation: as easy as APC. Science. 1996;272:974–975. doi: 10.1126/science.272.5264.974. [DOI] [PubMed] [Google Scholar]
- Peifer M. β-Catenin as oncogene: the smoking gun. Science. 1997;275:1752–1753. doi: 10.1126/science.275.5307.1752. [DOI] [PubMed] [Google Scholar]
- Peifer M, Wieschaus E. The segment polarity gene armadillo encodes a functionally modular protein that is the Drosophilahomolog of human plakoglobin. Cell. 1990;63:1167–1176. doi: 10.1016/0092-8674(90)90413-9. [DOI] [PubMed] [Google Scholar]
- Peifer M, Berg S, Reynolds AB. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994a;76:789–791. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Peifer M, Sweeton D, Casey M, Wieschaus E. Wingless signal and zeste white 3 kinase trigger opposing changes in the intracellular distribution of armadillo. . Development (Camb) 1994b;120:369–380. doi: 10.1242/dev.120.2.369. [DOI] [PubMed] [Google Scholar]
- Powell S M, Zilz N, Beizer-Barclay Y, Bryan T, Hamilton S, Thibodean S, Vogelstein B, Kinzler K. APC mutations occur during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Riese J, Yu X, Munnerlyn A, Eresh S, Hsu S-C, Grosschedl R, Bienz M. LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. . Cell. 1997;88:777–787. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. α1(E)-catenin is a novel actin binding and bundling protein mediating the attachment of F-actin to the membrane adhesive complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez Fernández, J.L., B. Geiger, D. Salomon, I. Sabanay, M. Zöller, and A. Ben-Ze'ev. Suppression of tumorigenicity in transformed cells after transfection with vinculin cDNA. J Cell Biol. 1992;119:427–438. doi: 10.1083/jcb.119.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A, Merriam J, Klymkowsky MW. Localizing the adhesive and signaling functions of plakoglobin. Dev Genet. 1997;20:91–102. doi: 10.1002/(SICI)1520-6408(1997)20:2<91::AID-DVG2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Munemitsu S, Polakis P. The APC protein and E-cadherin form similar but independent complexes with α-catenin, β-catenin, and plakoglobin. J Biol Chem. 1995;270:5549–5555. doi: 10.1074/jbc.270.10.5549. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK-3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Ruiz P, Brinkmann V, Ledermann B, Behrend M, Grund C, Thalhammer C, Vogel F, Birchmeier C, Günthert U, Franke WW, Birchmeier W. Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J Cell Biol. 1996;135:215–225. doi: 10.1083/jcb.135.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco P, McGranahan TM, Wheelock MJ, Johnson KR. Identification of plakoglobin domains required for association with N-cadherin and α-catenin. J Biol Chem. 1995;270:20201–20206. doi: 10.1074/jbc.270.34.20201. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Heid HW, Schäfer S, Nuber UA, Zimbelman R, Franke WW. Desmosomes and cytoskeletal architecture in epithelial differentiation: cell type specific plaque components and intermediate filament anchorage. Eur J Cell Biol. 1994;65:229–245. [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. β-Catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- Simcha I, Geiger B, Yehuda-Levenberg S, Salomon D, Ben-Ze'ev A. Suppression of tumorigenicity by plakoglobin: an augmenting effect of N-cadherin. J Cell Biol. 1996;133:199–209. doi: 10.1083/jcb.133.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers CL, Gelmann EP, Kemler R, Cowin P, Byers SW. Alterations in β-catenin phosphorylation and plakoglobin expression in human breast cancer cells. Cancer Res. 1994;54:3544–3552. [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. . Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Wahl JK, Sacco PA, McGranahan T M, Sauppé L, Wheelock MJ, Johnson KR. Plakoglobin domains that define its association with the desmosomal cadherins and the classical cadherins: identification of unique and shared domains. J Cell Sci. 1996;109:1043–1054. doi: 10.1242/jcs.109.5.1143. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Knudsen KA, Johnson KR. Membrane-cytoskeleton interactions with cadherin cell adhesion proteins: roles of catenins as linker proteins. Curr Topics Membr. 1996;43:169–185. [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopusembryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]