Transgenic Gαq overexpression induces cardiac contractile failure in mice (original) (raw)
Abstract
The critical cell signals that trigger cardiac hypertrophy and regulate the transition to heart failure are not known. To determine the role of Gαq-mediated signaling pathways in these events, transgenic mice were constructed that overexpressed wild-type Gαq in the heart using the α-myosin heavy chain promoter. Two-fold overexpression of Gαq showed no detectable effects, whereas 4-fold overexpression resulted in increased heart weight and myocyte size along with marked increases in atrial naturietic factor (≈55-fold), β-myosin heavy chain (≈8-fold), and α-skeletal actin (≈8-fold) expression, and decreased (≈3-fold) β-adrenergic receptor-stimulated adenylyl cyclase activity. All of these signals have been considered markers of hypertrophy or failure in other experimental systems or human heart failure. Echocardiography and in vivo cardiac hemodynamic studies indeed revealed impaired intrinsic contractility manifested as decreased fractional shortening (19 ± 2% vs. 41 ± 3%), dP/dt max, a negative force–frequency response, an altered Starling relationship, and blunted contractile responses to the β-adrenergic agonist dobutamine. At higher levels of Gαq overexpression, frank cardiac decompensation occurred in 3 of 6 animals with development of biventricular failure, pulmonary congestion, and death. The element within the pathway that appeared to be critical for these events was activation of protein kinase Cɛ. Interestingly, mitogen-activated protein kinase, which is postulated by some to be important in the hypertrophy program, was not activated. The Gαq overexpressor exhibits a biochemical and physiologic phenotype resembling both the compensated and decompensated phases of human cardiac hypertrophy and suggests a common mechanism for their pathogenesis.
Myocardial hypertrophy is the common response of the heart to a variety of conditions that increase external and/or internal work. The resulting increase in cardiac mass diminishes stress on the overloaded heart and thereby initially normalizes cardiac function. In humans, functional compensation is transient and is followed by decompensated congestive heart failure characterized by chamber dilation and diminished contractility. Due to the high mortality of idiopathic heart failure and that secondary to hypertension, cardiac valvular disease, or ischemia, there is intense interest in identifying the regulatory determinants of cardiac hypertrophy and failure. Toward this end, numerous studies have used cultured neonatal rat cardiomyocytes to demonstrate the potential for angiotensin II, the α-adrenergic receptor (αAR) agonist phenylephrine, endothelin, or prostaglandin F2α to stimulate hypertrophy (1–4). Although the relevance of such experiments to the terminally differentiated adult cardiomyocyte is unclear, these studies suggest that activation of receptors by endocrine, paracrine, or autocrine agonists may affect the adult heart. Consistent with this notion, transgenic expression of a constitutively activated mutant α1AR leads to mild cardiac hypertrophy (5).
A common feature of the cardiomyotrophic agonists described above is an interaction with receptors that activate the Gq class of GTP-binding proteins. Upon binding to an agonist-occupied receptor, the heterotrimeric Gq protein dissociates into individual Gαq and Gβγ subunits. GTP-bound Gαq activates phosphatidylinositol-specific phospholipase C and initiates a series of events, which culminate in IP3-mediated calcium release and diacylglycerol-mediated activation of protein kinase C (PKC) (6–8). Dissociated Gβγ has the potential to activate the small GTP-binding protein Ras and initiate a tyrosine kinase cascade, leading to activation of mitogen-activated protein (MAP) kinase (9). Furthermore, Gq can activate MAP kinase independent of Gβγ via a mechanism that is PKC dependent (10). Activation of PKC and MAP kinase has been implicated in stimulating cardiac growth (11–14), and homozygous transgenic expression of an activated mutant human Ras in mice causes left ventricular hypertrophy with normal systolic function (15).
In the present study we transgenically overexpressed the wild-type murine Gαq protein in mouse hearts. Expression of Gαq in cultured cells previously has been shown to activate phospholipase C (16) and to modify cell growth (17, 18). Cardiac-specific Gαq transgenesis made possible an evaluation of Gαq-mediated pathways on in vivo myocardial hypertrophy and function without interference from either noncardiac stimuli or Gβγ-mediated effects. Modest overexpression of the wild-type murine Gαq in the mouse heart resulted in cardiac hypertrophy defined as a conserved program of fetal gene expression, increased heart weight in relation to body weight, and increased cardiomyocyte size. Unlike some previously described forms of cardiac hypertrophy in transgenic mice (5, 15, 19) Gαq-stimulated myocardial hypertrophy severely compromised systolic cardiac function, and at high-expression levels resulted in a dilated cardiomyopathy with overt cardiac failure.
MATERIALS AND METHODS
Construction of Gαq Transgenic Mice.
The 1.46-kb wild-type murine Gαq cDNA [pP3 Gαq (20), Genbank accession no. M55412] provided by Mel Simon (California Institute of Technology, Pasadena, CA) was released with _Sma_I and _Apa_I, blunted, and ligated into the blunted _Sal_I site of a plasmid containing the full-length (4.5 kb) murine α-myosin heavy chain (MHC) promoter provided by Jeffery Robbins (Children’s Hospital Medical Center, Cincinnati, OH) [ref. 21, Genbank accession no. U71441 (clone 26)]. The resulting recombinant plasmid, pBS-α-MHC-Gαq, was confirmed by restriction mapping and nucleotide sequencing. A linear 8-kb DNA fragment containing the entire α-MHC promoter, the complete murine Gαq cDNA ORF, and a simian virus 40 intron and polyadenylylation signal was released by digestion with _Kpn_I and _Pme_I. Approximately 1,000 copies of this fragment were microinjected into male pronuclei of fertilized FVB/N mouse oocytes and implanted into pseudopregnant females. Three-week-old mouse pups were screened for presence of the transgene by genomic Southern analysis using a 32P-labeled 900-bp _Bam_HI–_Eco_RI fragment of the murine Gαq cDNA as a probe, and three founders were identified.
Measurement of Cardiac Gene Expression.
Transgene expression was evaluated by Northern analysis of multiple tissues. RNA was extracted using Tri-Reagent (Molecular Research Center, Cincinnati) and the manufacturer’s protocol. Total RNA (10 μg/lane) was size-fractionated in formaldehyde agarose, transferred to nylon membranes, and hybridized under conditions previously described (22) using the _Bam_HI–_Eco_RI Gαq cDNA as probe.
Quantitative assessment of cardiac hypertrophy gene expression was performed by RNA dot blotting using gene-specific antisense oligonucleotides previously described (23). Radiolabeled RNA dots were quantitated with a PhosphorImager (Molecular Dynamics), and each value was related to glyceraldehyde-3-phosphate dehydrogenase expression as preliminary studies demonstrated no regulation of this gene in Gαq transgenic mice. Gαq and Gβ protein were assayed by comparative immunoblotting (24) using antibodies from Santa Cruz Biotechnology.
Physiologic Measurements.
Arterial blood pressure was determined by tail cuff measurement of unsedated 12-week-old mice using a Visitech Systems (Apex, NC) Bp-2000 system.
Closed chest invasive hemodynamic studies were performed on 12-week-old mice under ketamine/thiobutabarbital anesthesia as described (25). Atrial pacing was accomplished using a 0.010-inch floppy-tipped angioplasty guide wire placed in the right atrium via the right jugular vein. After baseline hemodynamic data were acquired, atrial pacing was instituted to overdrive the intrinsic heart rate and, unless stated otherwise maintained at a constant rate of 7.5 Hz (450 bpm). In some studies simultaneous echocardiograms were obtained (26).
Measurements of Cell Signal Activity.
MAP kinase expression was compared by immunoblot analysis (New England Biolabs anti-MAP kinase) of 10% acrylamide SDS/PAGE gels using 75 μg of ventricular homogenate per lane. MAP kinase phosphorylation was assayed by immunoblot analysis of ventricular MAP kinase immune complexes probed with anti-tyrosine-204 phosphorylated MAP kinase (New England Biolabs) or anti-threonine-202/tyrosine-204 dual-phosphorylated MAP kinase (Promega). In-gel myelin basic protein kinase assays were performed as described (27) using 75 μg of crude ventricular homogenate per lane. PKC isoform activation was assayed as the relative proportion of PKC in Triton X-100-extracted membrane versus cytosolic ventricular fractions (28). Anti-PKCɛ and PKCα antibodies were from Transduction Laboratories (Lexington, KY).
Measurements of adenylyl cyclase activity and radioligand binding with the βAR antagonist [125I]cyanopindolol or the α1AR antagonist [125I]iodophenylhydroxyethylaminomethyl tetralone were carried out as previously described (25).
Statistical Analysis.
Data are reported as means ± SEM (n). Statistical comparisons were with two-tailed Student’s t test comparing transgenic values to controls, or one-way ANOVA followed by the Bonferroni procedure for multiple group comparisons. P values of <0.05 were considered significant.
RESULTS AND DISCUSSION
Three Gαq transgenic founders were identified, bred through to F1 heterozygotes to establish germ-line transmission, and designated by transgene copy number as Gαq-9, Gαq-25, and Gαq-40. Few F1 Gαq-40 mouse pups survived to 5 weeks of age. Therefore, the current studies report results primarily from heterozygous F2–F4 Gαq-9 and Gαq-25 mice. The pattern of transgene expression as determined by Northern analysis was cardiac-specific (Fig. 1A). Myocardial Gαq protein was compared by Western analysis, which demonstrated levels of overexpression of approximately 2-fold for Gαq-9 and 4-fold for Gαq-25 (Fig. 1B, Table 1). Gβ protein also was measured by Western analysis and was not regulated in the transgenic mice (not shown). Thus, a possible mechanism whereby the organism could have counteracted the effects of Gαq overexpression, compensatory up-regulation of inhibitory Gβγ, did not occur.
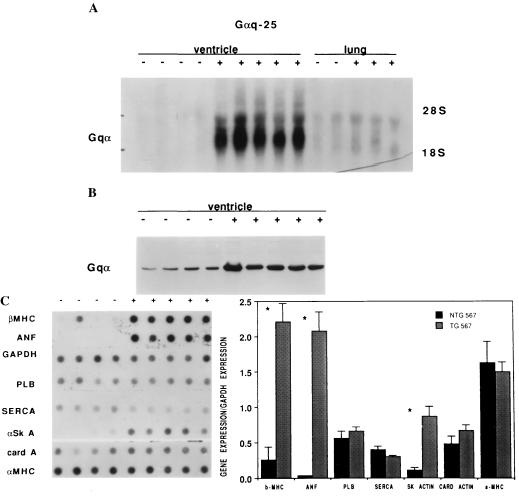
Figure 1.
Overexpression of Gαq in Gαq-25 transgenic mice results in induction of the hypertrophy gene program. (A) Northern analysis demonstrates expression of a 1.5-kb Gαq specific transcript in ventricles from transgenic mice (+) but not in nontransgenic littermates (−). (B) Western analysis of ventricular protein from transgenic (+) and nontransgenic (−) mice demonstrated 4-fold overexpression of Gαq protein in Gαq-25 transgenic mice. (C) (Left) Gαq-25 (+) ventricular RNA dot blot analysis demonstrates increased transcript levels of β-MHC, ANF, and α-skeletal actin (α SK A), but no significant changes in levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phospholamban (PLB), sarcoplasmic reticulum ATPase (SERCA), cardiac actin (card A), and α-MHC. (Right) Quantitative grouped analysis of mRNA expression normalized to GAPDH expression.
Table 1.
Molecular analysis
| Molecule | Gene expression, fold increase | |
|---|---|---|
| Gαq-9 | Gαq-25 | |
| Gαq protein | 2× control | 4× control |
| Transcript | ||
| ANF | 1.0 | 55.0* |
| β-MHC | 1.1 | 8.3* |
| α sk actin | 0.7 | 8.2* |
| PLB | 1.2 | 1.2 |
| SERCA2 | 1.1 | 0.8 |
| Cardiac actin | 1.1 | 1.4 |
| α-MHC | 1.1 | 0.9 |
To determine if Gαq overexpression regulated myocardial hypertrophy/heart failure the pattern of myocardial gene expression was characterized in both transgenic lines. Human heart failure and physiologically relevant experimental models of murine cardiac hypertrophy exhibit increased expression of fetal genes, particularly atrial natriuretic factor (ANF), α skeletal actin, the β isoform of the MHC (29–34), and in some cases regulation of genes for the calcium cycling proteins phospholamban and sarcoplasmic reticulum ATPase (35–37). Gαq-25, but not Gαq-9, mice exhibited greatly enhanced expression of ANF, α skeletal actin, and β-MHC RNA (Fig. 1C, Table 1). The relative increases in β-MHC and ANF RNA observed in Gαq-25 mice exceed the 3-fold increase in β-MHC RNA and the 20-fold increase in ANF RNA previously observed after induction of pressure overload hypertrophy in mice by banding of the transverse aorta (33, 34). Consistent with recent reports that the cardiac MHC genes are under separate transcriptional controls (23), α-MHC was not antithetically down-regulated at the mRNA level (Fig. 1C). Interestingly, the 2-fold level of Gαq overexpression achieved in Gαq-9 mice was not sufficient to induce molecular markers of hypertrophy, indicating a gene-dosage effect with a threshold level of expression necessary for altered cardiac gene expression.
The degree of cardiac hypertrophy in Gαq transgenic mice was assessed by direct determination of cardiac weights and estimates of cardiomyocyte size. Whole heart weight corrected for body weight or tibial length averaged 30% greater in Gαq-25 mice compared with nontransgenic littermates (Table 2). In contrast, and consistent with normal expression of hypertrophy-associated genes, Gαq-9 mice had normal heart weights. There were no differences in body weight among any of the experimental groups. Importantly, the greater heart weight observed in Gαq-25 mice appeared to have occurred independently of increased hemodynamic load because there was no increase in tail cuff-measured systolic blood pressures (Table 2).
Table 2.
Characteristics of mice
| Characteristic | Gαq-9 | Gαq-25 | ||
|---|---|---|---|---|
| NTG | TG | NTG | TG | |
| Morphometry mg/g | ||||
| Ht/body wt | 6.3 ± 0.2 | 6.8 ± 0.4 | 6.6 ± 0.3 | 8.7 ± 0.3* |
| Ht wt/tibial lth, % | 0.91 ± 0.04 | 1.16 ± 0.04* | ||
| Lung/body wt | 7.2 ± 0.5 | 8.0 ± 0.7 | 7.5 ± 0.6 | 8.1 ± 0.6 |
| Liver/body wt | 54 ± 4 | 53.2 ± 2 | ||
| Myocyte area, μm2 | 196 ± 22 | 357 ± 28* | ||
| Functional analysis | ||||
| Sys BP, mmHg | 127 ± 5 | 124 ± 3 | 131 ± 4 | 119 ± 3* |
| HR, bpm | 665 ± 18 | 664 ± 41 | 690 ± 13 | 427 ± 9* |
| Paced echocardiography | ||||
| FS, % | 41 ± 3 | 19 ± 2* | ||
| HR, bpm | 450 | 450 | ||
| Unpaced echocardiography | ||||
| FS, % | 41 ± 2 | 44 ± 2 | 44 ± 1 | 36 ± 2* |
| ESD, mm | 2.1 ± 0.10 | 2.2 ± 0.11 | 1.9 ± 0.04 | 2.6 ± 0.10* |
| EDD, mm | 3.8 ± 0.04 | 3.9 ± 0.14 | 3.4 ± 0.07 | 4.0 ± 0.09* |
| SWT, mm | 0.41 ± 0.03 | 0.44 ± 0.02 | 0.42 ± 0.02 | 0.47 ± 0.03 |
| PWT, mm | 0.40 ± 0.03 | 0.39 ± 0.02 | 0.41 ± 0.01 | 0.43 ± 0.02 |
| HR, bpm | 403 ± 22 | 384 ± 15 | 403 ± 20 | 235 ± 9* |
Histological examination of Gαq-25 hearts at 12 weeks and 6 months of age revealed no evidence of necrosis, significant ventricular fibrosis, myofibrillar disarray, or increased collagen content, findings that have been reported in some other transgenic mouse models of hypertrophy (15, 19). Myocyte dimensions of fluorescein-tagged wheat germ agglutinin-labeled Gαq-25 left ventricles were significantly larger than those of nontransgenic siblings (Table 2), demonstrating hypertrophy at the level of individual cardiomyocytes.
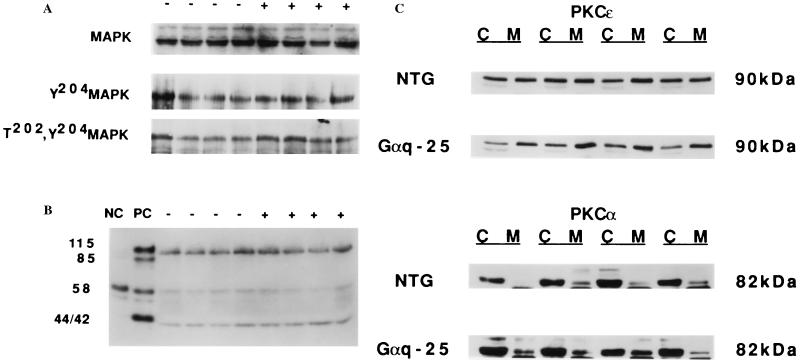
As Gαq can potentially activate both MAP kinase and PKC, and both of these signaling proteins have been implicated in the cardiotrophic actions of Gq-coupled agonists, MAP kinase and PKC activation were measured in Gαq-25 mouse hearts. There was no increase in MAP kinase phosphorylation (Fig. 2A) or in the myelin basic protein phosphorylating activity of p42/44 (Fig. 2B) in ventricular extracts from Gαq-25 mice. Furthermore, the overall amount of MAP kinase was not regulated (Fig. 2A). In contrast, the ɛ isoform of PKC was activated in Gαq-25 hearts as determined by preferential location in the particulate fraction of cardiac homogenates (Fig. 2C) and immunohistologic localization of PKCɛ in cardiomyocyte sarcolemma (not shown). PKCα however, was not activated (Fig. 2C). These findings indicate that PKCɛ activation rather than MAP kinase activation is more important in transducing the cardiac effects of Gαq overexpression.
Figure 2.
Cell signal activation in Gαq-25 mice. (A) (Top) Western analysis of total, (Middle) tyrosine-204 phosphorylated, and (Bottom) threonine-202/tyrosine-204 dual-phosphorylated MAP kinase. (B) MAP kinase activity assayed by in-gel assay. Serum-starved and 10% serum-stimulated HEK293 cells were used as negative (NC) and positive (PC) controls, respectively. By assessment of either phosphorylation or enzyme activity there is no increase in MAP kinase (p42, p44) activity in Gαq-25 compared with control. Other regulated myelin basic protein kinases (p85, p115) are also not activated in Gαq-25. (C) Western analysis of PKCɛ and PKCα in particulate and cytosolic fractions of ventricular homogenates shows preferential localization of PKCɛ, but not PKCα, to cardiomyocyte particulates in Gαq-25. The ratio of PKCɛ in cardiac membranes/cytosol was 1.2 ± 0.1 in nontransgenic (NTG) and 2.3 ± 0.3 in Gαq-25 (n = 4 pairs, P = 0.02).
The consequences of Gαq-induced myocardial hypertrophy on cardiac function were assessed in vivo using echocardiography and catheterization-derived hemodynamic measurements. Interestingly, the heart rates of Gαq-25 mice were slower than respective controls during the conscious blood pressure measurements and our initial anesthetized echocardiographic studies (Table 2). Because differences in heart rate can confound measurements of cardiac function, echocardiographic assessment also was performed in four pairs of atrially paced mice at matched heart rates of 450 bpm (Table 2). Contractility, assessed as echocardiographic fractional shortening, was depressed in Gαq-25 mice. Although left ventricular end systolic and end diastolic dimensions were enlarged in transgenic mice, wall thickness was similar to controls.
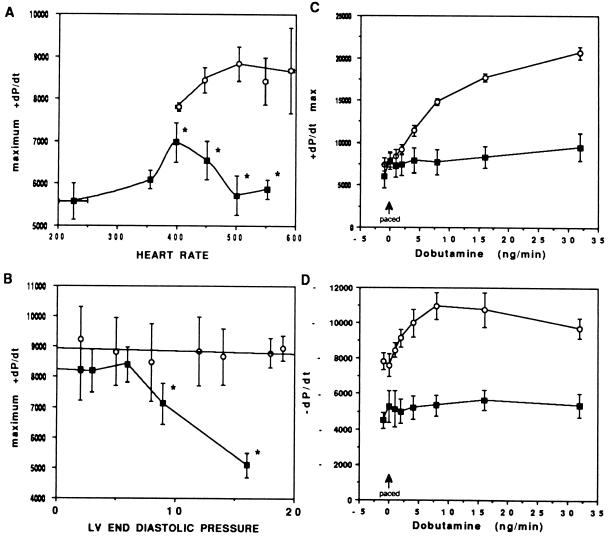
Systolic dysfunction was confirmed by invasive hemodynamic studies. As illustrated in Fig. 3A, left ventricular contractility of Gαq-25 mice was significantly lower than littermate controls. At intrinsic heart rates, dP/dt max of Gαq-25 mice was 5,576 ± 430 compared with 7,805 ± 100 in controls (n = 4 pairs, P < 0.05). At matched atrially paced heart rates of 450 bpm, dP/dt max remained depressed in the transgenic mice (Fig. 3A). Importantly, at paced heart rates of greater than 400 bpm, Gαq-25 mice exhibited a progressive decline in dP/dt max (negative force-frequency relationship). Furthermore, intravascular fluid challenge with 6% albumin revealed a progressive decrease in contractility with increased left ventricular end diastolic pressure, indicating an altered Starling relationship (Fig. 3B).
Figure 3.
Hemodynamic characteristics and left ventricular function of Gαq-25 mice. (A) Force–frequency relations in Gαq-25 mice. Control mice (n = 4, ○) exhibit enhanced left ventricular contractility with increasing heart rate to 500 bpm. Wenckebach heart block developed at rates greater than 600 bpm. ∗, P < 0.05 compared with controls. Gαq-25 mice (n = 4, ▪) showed a negative force–frequency relationship with progressive decline in dP/dt at heart rates greater than 400 bpm. (B) Starling relations in control (n = 4, ○) and Gαq-25 (n = 4, ▪) mice paced at 450 bpm. Intravascular volume expansion was accomplished by intravenous infusion of 400–700 μl of 6% albumin. The Starling relationship is essentially flat for nontransgenics, whereas the Gαq overexpressors are on the “descending limb” at left ventricular end diastolic pressure greater than 7 mmHg. ∗, P < 0.05 compared with baseline. (C and D) Hemodynamic response to βAR stimulation (n = 5 pairs). At matched heart rates of 450 bpm, there is no inotropic (C) or lusitropic (D) response to dobutamine infusion in Gαq transgenic mice, consistent with impaired βAR coupling.
In several forms of human heart failure, AR-mediated signaling is blunted. To determine whether this was also a feature of Gαq-25 mice, the hemodynamic responses to incremental doses of dobutamine were measured. As shown in Fig. 3 C and D, normal β-adrenergic augmentation of cardiac contractility and relaxation was absent in Gαq-25 mice. A biochemical mechanism for this functional observation was identified using ventricular membranes from Gαq-25 mice that exhibited significantly depressed βAR agonist (isoproterenol)-stimulated adenylyl cyclase activity (9.3 ± 1.2 pmol/min per mg vs. 30.8 ± 7.9 pmol/min per mg for nontransgenic littermates; n = 4 pairs, P < 0.01). This occurred despite similar βAR density (20.4 ± 2.9 vs. 20.5 ± 0.8 fmol/mg) and proportion of β1AR to β2AR. Such dysfunction might be due to receptor phosphorylation by the βAR kinase, which is known to alter cardiac βAR signal transduction (38) and has been found to be elevated 2- to 3-fold in human heart failure (39), or possibly due to phosphorylation of βAR by PKC. Regardless of the mechanism, a decrease in βAR number (which has been reported in heart failure, but the relevance of which was never established) is not responsible for the depressed β-agonist responsiveness observed herein. We also considered that overexpression of Gαq might result in a compensatory decrease in expression of a Gαq-coupled receptor such as α1AR. However, α1AR expression was unaltered in transgenic mice (14.8 ± 5.5 fmol/mg) as compared with nontransgenic littermates (18.9 ± 5.0 fmol/mg).
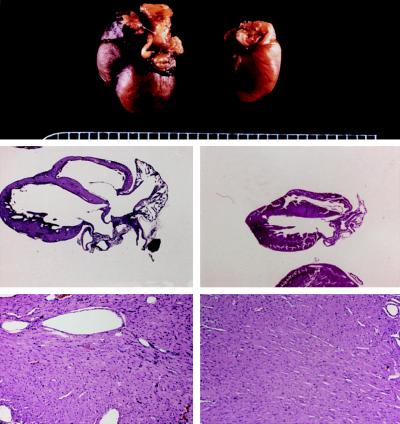
Based on the early mortality of the first-generation Gαq-40 mice, we felt that Gαq protein expression obtained in these animals might be nearing levels that provoked frank cardiac decompensation. Thus, surviving Gαq-40 males were mated with Gαq-25 females. In two separate litters, spontaneous cardiac decompensation was observed in 3 of 6 Gαq-25/Gαq-40 dual heterozygotes, which, between ages of 11 and 14 weeks became tachypneic and developed severe respiratory distress. Postmortem examination of these mice showed large bilateral pleural effusions and lung/body weight ratios averaging 40% greater than controls, indicating left ventricular failure. Pathological examination of these hearts showed massive enlargement and dilation of all four cardiac chambers (Fig. 4). Histologic examination of the ventricles (Fig. 4) showed mild to moderate cellular edema with little or no myocyte loss. Increased size of myocyte nuclei, some of which demonstrated a vesicular staining pattern, also was observed. Importantly, myocyte necrosis, fibrosis, and myofibrillar disarray were absent. Thus, in exploring the transgene dose-dependent effects of Gαq in the heart we observed that Gαq overexpression predisposes to contractile dysfunction, which, at levels of expression obtained by crossing Gαq-25 and Gαq-40 mice causes a dilated cardiomyopathy, the consequence of which is lethal congestive heart failure.
Figure 4.
Pathologic examination of a Gαq-25/Gαq-40 dual-heterozygote that developed congestive heart failure (Left) and a nontransgenic sibling (Right). (Top) Gross examination revealed massive cardiomegaly of transgenic heart (×1.8). (Middle) Four-chamber section of hearts (×1.8) demonstrates massive enlargement of both ventricles and atria with atrial filling by organized thrombus. (Bottom) Cardiac histology (left ventricular free wall, ×18) shows mild edema and pale, hypertrophied myocytes without significant inflammation in transgenic heart. Results are representative of three dual-heterozygous mice that exhibited spontaneous cardiac decompensation.
These studies show that 4-fold overexpression of wild-type Gαq protein in the mouse heart causes increased expression of hypertrophy-associated genes, increased cardiac mass, and enlarged cardiomyocytes. A noteworthy feature of this form of cardiac hypertrophy is severely depressed systolic function, which, in the highest expressing dual-heterozygote animals, predisposed to the development of a dilated cardiomyopathy and overt heart failure. The cardiac phenotype observed is clearly the direct result of cardiac Gαq signaling because there was no evidence of any systemic effects. Furthermore, as 2-fold overexpression in Gαq protein had no effect on cardiac gene expression, function, or hypertrophy, a threshold level of Gαq expression appears necessary to transduce these effects.
To identify the cell signaling pathway transducing Gαq-mediated cardiac effects we assayed the activities of MAP kinase and PKC and found that PKCɛ was activated in Gαq-25 mouse hearts. Studies in cultured neonatal cardiomyocytes, isolated perfused rat hearts, and pressure-overloaded rat hearts also have demonstrated activation of PKCɛ, but not PKCα, in the heart (40–43). However, it was unexpected that MAP kinase was not activated. It is widely accepted, based primarily on the observation that agonists that stimulate hypertrophy of cultured neonatal cardiomyocytes also activate MAP kinase, that this is an important signaling pathway for agonist-stimulated hypertrophy (12–14, 44, 45). MAP kinase could have been activated in the Gαq transgenic mice via the actions of PKC on Ras-GTPase activating protein (46). However, in Gαq-25 mouse hearts significant MAP kinase activation was not observed using two independent methodologies. This indicates that measurable MAP kinase activation is not required either for hypertrophy-associated expression of fetal cardiac genes or for development of cardiac hypertrophy itself when these responses are stimulated via Gαq. In the context of a previous study demonstrating cardiomyocyte MAP kinase activation by agents that clearly did not stimulate hypertrophy (47), these results indicate that other cell signaling events are more important transducers of this form of myocardial hypertrophy.
The substantial physiologic alterations observed with relatively modest overexpression of wild-type Gαq signaling protein contrast to previous findings in a transgenic mouse overexpressing an activated mutant form of oncogenic human Ras in the heart (15). These important studies used a Ras transgene that was constitutively activated. Heterozygous animals were phenotypically normal, but homozygous Ras transgenics showed increases in ANF expression and a histologic picture resembling hypertrophic cardiomyopathy. Interestingly, homozygous Ras transgenics had normal systolic function, and the hypertrophy was restricted to the left ventricle, although the transgene was clearly expressed in both ventricles. The hypertrophy with contractile dysfunction exhibited by heterozygous wild-type Gαq transgenic mice is clearly distinct from the hypertrophic cardiomyopathy observed in homozygous Ras or mutant α-MHC transgenic mice (15, 19) and more closely resembles reactive processes observed in response to increased hemodynamic stress (33, 34).
An interesting facet of the Gαq hypertrophy/contractile dysfunction phenotype is the transgene dose-dependent progression from hypertrophy with subnormal systolic function to, in the case of the Gαq-25/Gαq-40 dual heterozygotes, a congestive cardiomyopathy. In humans, reactive hypertrophy from systemic hypertension or aortic valvular stenosis is characterized by a compensated phase, which can last for decades, although the natural history of these conditions is that decompensation ultimately occurs if the hemodynamic stress is not relieved. An intriguing possibility is that the cardiac hypertrophic response to increased hemodynamic load in these conditions is mediated by autocrine or paracrine stimulation of receptors coupled to Gq, but that the increase in wall thickness of the hypertrophied heart normalizes ventricular wall stress and thus attenuates the stimulus, allowing only sufficient activation of the pathway to maintain hypertrophy without progression to failure. In contrast, constant high-level overexpression of Gαq in dual-heterozygote transgenic mice results in chronic activation of downstream pathways and decompensation, even in the absence of increased hemodynamic load.
In conclusion, we explored the effects of activation of the Gαq signaling pathway in the development of cardiac hypertrophy and heart failure. Four-fold overexpression of Gαq in the heart resulted in a striking recapitulation of gene expression and signaling defects observed in model systems and human heart failure. Furthermore, frank failure was observed with higher levels of expression, suggesting a common mechanism for development and decompensation of cardiac hypertrophy. As such, these mice represent the first animal model to achieve such characteristics of human failure. Attempts to rescue this failing phenotype by genetic manipulations may provide insight into novel therapeutic options.
Acknowledgments
We thank M. Simon for plasmid containing murine Gαq cDNA, J. Robbins for cardiac-specific oligonucleotides and clone 26 containing the murine α-MHC promoter, and J. Neumann, M. Davis, J. Mathews, S. McCluskey, and R. Cantwell for technical and secretarial assistance. This work was supported in part by grants from the National Institutes of Health HL49267 (G.W.D.), HL45967 and HL41496 (S.B.L.), and HL52318 (G.W.D., R.A.W., and S.B.L.), the Veterans Administration (G.W.D.), and an endowment from Marion-Merrell Dow. G.W.D. is an Established Investigator of the American Heart Association.
ABBREVIATIONS
PKC
protein kinase C
AR
adrenergic receptor
MAP
mitogen-activated protein
MHC
myosin heavy chain
ANF
atrial natriuretic factor
References
- 1.Shubeita H E, McDonough P M, Harris A N, Knowlton K U, Glembotski C C, Brown J H, Chien K R. J Biol Chem. 1990;265:20555–20562. [PubMed] [Google Scholar]
- 2.Sadoshima J-I, Xu Y, Slayter H S, Izumo S. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 3.Knowlton K U, Michel M C, Itani M, Shubeita H E, Ishihara K, Brown J H, Chien K R. J Biol Chem. 1993;268:15374–15380. [PubMed] [Google Scholar]
- 4.Adams J W, Migita D S, Yu M K, Young R, Hellickson M S, Castro-Vargas F E, Domingo J D, Lee P H, Bui J S, Henderson S A. J Biol Chem. 1996;271:1179–1186. doi: 10.1074/jbc.271.2.1179. [DOI] [PubMed] [Google Scholar]
- 5.Milano C A, Dolber P C, Rockman H A, Bond R A, Venable M E, Allen L F, Lefkowitz R J. Proc Natl Acad Sci USA. 1994;91:10109–10113. doi: 10.1073/pnas.91.21.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishizuka Y. Science. 1984;225:1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- 7.Nishizuka Y. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 8.Hug H, Sarre T F. Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biesen T V, Hawes B E, Luttrell D K, Krueger K M, Touhara K, Porfiri E, Sakaue M, Luttrell L M, Lefkowitz R J. Nature (London) 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- 10.Hawes B E, Biesen T V, Koch W J, Luttrell L M, Lefkowitz R J. J Biol Chem. 1995;270:17148–17153. doi: 10.1074/jbc.270.29.17148. [DOI] [PubMed] [Google Scholar]
- 11.Allo S N, McDermott P J, Carl L L, Morgan H E. J Biol Chem. 1991;266:22003–22009. [PubMed] [Google Scholar]
- 12.Clerk A, Bogoyevitch M A, Andersson M B, Sugden P H. J Biol Chem. 1994;269:32848–32857. [PubMed] [Google Scholar]
- 13.Bogoyevitch M A, Glennon P E, Andersson M B, Clerk A, Lazou A, Marshall C J, Parker P J, Sugden P H. J Biol Chem. 1994;269:1110–1119. [PubMed] [Google Scholar]
- 14.Gillespie-Brown J, Fuller S J, Bogoyevitch M A, Cowley S, Sugden P H. J Biol Chem. 1995;270:28092–28096. doi: 10.1074/jbc.270.47.28092. [DOI] [PubMed] [Google Scholar]
- 15.Hunter J J, Tanaka N, Rockman H A, Ross J, Jr, Chien K R. J Biol Chem. 1995;270:23173–23178. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 16.Qian N-X, Winitz S, Johnson G L. Proc Natl Acad Sci USA. 1993;90:4077–4081. doi: 10.1073/pnas.90.9.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalinec G, Nazarali A J, Hermouet S, Xu N, Gutkind J S. Mol Cell Biol. 1992;12:4687–4693. doi: 10.1128/mcb.12.10.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaMorte V J, Thorburn J, Absher D, Spiegel A, Brown J H, Chien K R, Feramisco J R, Knowlton K U. J Biol Chem. 1994;269:13490–13496. [PubMed] [Google Scholar]
- 19.Geisterfer-Lowrance A A T, Christe M, Conner D A, Ingwall J S, Schoen F J, Seidman C E, Seidman J G. Science. 1996;272:731–734. doi: 10.1126/science.272.5262.731. [DOI] [PubMed] [Google Scholar]
- 20.Strathmann M, Simon M I. Proc Natl Acad Sci USA. 1990;87:9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramaniam A, Jones W K, Gulick J, Wert S, Neumann J, Robbins J. J Biol Chem. 1991;266:24613–24620. [PubMed] [Google Scholar]
- 22.Dorn G W, II, Becker M W, Davis M G. J Biol Chem. 1992;267:24897–24905. [PubMed] [Google Scholar]
- 23.Jones W K, Grupp I L, Doetschman T, Grupp G, Osinska H, Hewett T E, Boivin G, Gulick J, Ng W A, Robbins J. J Clin Invest. 1996;98:1906–1917. doi: 10.1172/JCI118992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali S, Becker M W, Davis M G, Dorn G W, II. Circ Res. 1994;75:836–843. doi: 10.1161/01.res.75.5.836. [DOI] [PubMed] [Google Scholar]
- 25.Turki J, Lorenz J N, Green S A, Donnelly E T, Jacinto M, Liggett S B. Proc Natl Acad Sci USA. 1996;93:10483–10488. doi: 10.1073/pnas.93.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoit B D, Khoury S F, Kranias E G, Ball N, Walsh R A. Circ Res. 1995;77:632–637. doi: 10.1161/01.res.77.3.632. [DOI] [PubMed] [Google Scholar]
- 27.Chen R-H, Sarnecki C, Blenis J. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epand R M. Anal Biochem. 1994;218:241–247. doi: 10.1006/abio.1994.1173. [DOI] [PubMed] [Google Scholar]
- 29.Bishopric N H, Simpson P C, Ordahl C P. J Clin Invest. 1987;80:1194–1199. doi: 10.1172/JCI113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izumo S, Nadal-Ginard B, Mahdavi V. Proc Natl Acad Sci USA. 1988;85:339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waspe L E, Ordahl C P, Simpson P C. J Clin Invest. 1990;85:1206–1214. doi: 10.1172/JCI114554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chien K R, Knowlton K U, Zhu H, Chien S. FASEB J. 1991;5:3037–3046. doi: 10.1096/fasebj.5.15.1835945. [DOI] [PubMed] [Google Scholar]
- 33.Rockman H A, Ross R S, Harris A N, Knowlton K U, Steinhelper M E, Field L J, Ross J, Jr, Chien K R. Proc Natl Acad Sci USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorn G W, II, Robbins J, Ball N, Walsh R A. Am J Physiol. 1994;267:H400–H405. doi: 10.1152/ajpheart.1994.267.1.H400. [DOI] [PubMed] [Google Scholar]
- 35.Arai M, Alpert N R, MacLennan D H, Barton P, Periasamy M. Circ Res. 1993;72:463–469. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- 36.Rockman H A, Ono S, Ross R S, Jones L R, Karimi M, Bhargava V, Ross J, Jr, Chien K R. Proc Natl Acad Sci USA. 1994;91:2694–2698. doi: 10.1073/pnas.91.7.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiss E, Ball N A, Kranias E G, Walsh R A. Circ Res. 1995;77:759–764. doi: 10.1161/01.res.77.4.759. [DOI] [PubMed] [Google Scholar]
- 38.Koch W J, Rockman H A, Samama P, Hamilton R, Bond R A, Milano C A, Lefkowitz R J. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 39.Ungerer M, Parruti G, Bohm M, Puzicha M, DeBlasi A, Erdmann E, Lohse M J. Circ Res. 1994;74:206–213. doi: 10.1161/01.res.74.2.206. [DOI] [PubMed] [Google Scholar]
- 40.Bogoyevitch M A, Parker P J, Sugden P H. Circ Res. 1993;72:757–767. doi: 10.1161/01.res.72.4.757. [DOI] [PubMed] [Google Scholar]
- 41.Gu X, Bishop S P. Circ Res. 1994;75:926–931. doi: 10.1161/01.res.75.5.926. [DOI] [PubMed] [Google Scholar]
- 42.Puceat M, Hilal-Dandan R, Strulovici B, Brunton L L, Brown J H. J Biol Chem. 1994;269:16938–16944. [PubMed] [Google Scholar]
- 43.Schunkert H, Sadoshima J-I, Cornelius T, Kagaya Y, Weinberg E O, Izumo S, Riegger G, Lorell B H. Circ Res. 1995;76:489–497. doi: 10.1161/01.res.76.3.489. [DOI] [PubMed] [Google Scholar]
- 44.Lazou A, Bogoyevitch M A, Clerk A, Fuller S J, Marshall C J, Sugden P H. Circ Res. 1994;75:932–941. doi: 10.1161/01.res.75.5.932. [DOI] [PubMed] [Google Scholar]
- 45.Sadoshima J, Qiu Z, Morgan J P, Izumo S. Circ Res. 1995;76:1–15. doi: 10.1161/01.res.76.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Blenis J. Proc Natl Acad Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Post G R, Goldstein D, Thuerauf D J, Glembotski C C, Brown J H. J Biol Chem. 1996;271:8452–8457. doi: 10.1074/jbc.271.14.8452. [DOI] [PubMed] [Google Scholar]