Transcriptional repression by AML1 and LEF-1 is mediated by the TLE/Groucho corepressors (original) (raw)
Abstract
The mammalian AML/CBFα runt domain (RD) transcription factors regulate hematopoiesis and osteoblast differentiation. Like their_Drosophila_ counterparts, most mammalian RD proteins terminate in a common pentapeptide, VWRPY, which serves to recruit the corepressor Groucho (Gro). Using a yeast two-hybrid assay, in vitro association and pull-down experiments, we demonstrate that Gro and its mammalian homolog TLE1 specifically interact with AML1 and AML2. In addition to the VWRPY motif, other C-terminal sequences are required for these interactions with Gro/TLE1. TLE1 inhibits AML1-dependent transactivation of the T cell receptor (TCR) enhancers α and β, which contain functional AML binding sites, in transfected Jurkat T cells. LEF-1 is an additional transcription factor that mediates transactivation of TCR enhancers. LEF-1 and its_Drosophila_ homolog Pangolin (Pan) are involved in the Wnt/Wg signaling pathway through interactions with the coactivator β-catenin and its highly conserved fly homolog Armadillo (Arm). We show that TLE/Gro interacts with LEF-1 and Pan, and inhibits LEF-1:β-catenin-dependent transcription. These data indicate that, in addition to their activity as transcriptional activators, AML1 and LEF-1 can act, through recruitment of the corepressor TLE1, as transcriptional repressors in TCR regulation and Wnt/Wg signaling.
The AML genes (also termed CBFα or_PEBP2α_) are members of a gene family of heterodimeric transcription factors. Family members contain a highly conserved region of 128 aa designated the “runt domain” (RD), because of its homology to a region in the Drosophila Runt protein (1). The RD mediates both AML heterodimerization with the CBFβ protein and the binding of AML to its consensus DNA target sequence PyGPyGGT (1–4). In humans and mice, three highly conserved AML genes have been identified (2–4): AML1 on chromosome 21q22.1,AML2 on chromosome 1p36, and AML3 on chromosome 6p21 (5, 6). Homozygous disruption of AML1 and_AML3_ in mice indicated that AML1 plays a crucial role in hematopoiesis (7, 8) whereas AML3 is essential for osteogenesis (9, 10). Importantly, AML1 and_CBFβ_ genes are the most frequent targets for leukemia-associated translocations (11), further highlighting the pivotal role of these genes in hematopoiesis.
The expression of AML1 is regulated by two distinct promoters and involves complex patterns of alternative splicing (12), resulting in a diverse collection of mRNAs of different-sized coding regions (188–480 aa) (13, 14). The longer proteins contain a transactivation domain (TAD) and a nuclear matrix targeting signal (15) downstream of the RD, whereas in the shorter forms the TAD is missing (2). Of interest, we have recently shown that the short isoform AML1-d, lacking part of the TAD, was capable of suppressing _in vivo_tumor growth and differentiation (16).
Consistent with their expression patterns and knockout mouse phenotypes, AML binding sites are present in promoter regulatory regions of several hematopoietic and bone-specific genes (2, 4). Characterization of AML interactions within these promoter regions revealed that AML1 activates transcription in a context-dependent manner involving contacts with adjacently bound transcription factors (2, 4). For example, on the T cell receptor (TCR) α enhancer, AML1 associates with the adjacently bound transcription factor Ets-1, and together with LEF-1 interacts with the nonDNA-binding protein ALY, which acts as a LEF-1/AML1 coactivator in this context (17, 18). LEF-1 is also a target of the Wnt/Wg signaling pathway (19). Wnt signaling allows the translocation of cytoplasmatically tethered β-catenin into the nucleus, where it directly interacts with LEF-1, stimulating LEF-1-dependent transcriptional activation (19).
As noted before (2), most AML proteins that contain full-length TAD terminate with an identical C-terminal VWRPY motif. This pentapeptide is similar in position and sequence to the WRPW motif found in Hairy-related basic helix–loop–helix transcription factors (20). It has been shown that in the Hairy-related basic helix–loop–helix repressors, WRPW serves to recruit the corepressor Groucho (Gro) (21,22). More recently, Aronson et al. (23) showed that the VWRPY sequence is required for Gro interactions with Runt/PEBP2αB.
The Drosophila Gro and its human homologs TLE1–4 (24) are widely expressed nuclear proteins that do not bind to DNA, but include WD repeats that are implicated in protein–protein interactions (25). Gro proteins are general transcriptional corepressors that mediate repression by a variety of specific DNA-binding proteins (25). It is thought that DNA-bound transcription factors interact with and recruit Gro/TLE to target promoters, however, the mechanism by which TLE/Gro bring about transcriptional repression remains unknown.
Here we demonstrate direct interactions between Gro/TLE and the AML1, AML2 and LEF-1 proteins and show that these interactions lead to transcriptional repression of AML- and LEF-1-regulated target genes.
MATERIALS AND METHODS
Yeast Interaction Assays.
Yeast interaction trap assays were performed essentially as described (21, 26). Yeast strains were transformed with the activation domain-AML fused proteins cloned into the 2 μ TRP1 plasmid pJG4–5, which allows galactose-dependent expression. Individual transformant colonies were tested for β-galactosidase activity in a liquid assay using general procedures (27).
Plasmids.
Plasmids encoding LexA fusions were constructed in frame by insertion of the corresponding coding regions in an_Eco_RI site at the 3′ end of LexA(202+pl) plasmid (26). LexA-Cbfβ contained amino acids 2–182 of mouse Cbfβ2 (28), LexA-TLE1 contained amino acids 32–770 (24). LexA Gro, LexA-t-Gro (amino acids 251–719 of Gro), and LexA-Dmcdc2 have been described (21). The following coding regions were cloned in-frame, through an_Eco_RI site, into pJG4–5 plasmid (26): RD (amino acids 51–181 of AML1a), t-AML1a, t-AML1b, AML1a, AML1b, and AML2 (Fig.1) (6, 14). pGEX2T-Pan, pGEX2T-PanS25, pZEX-E(spl)m7, pZEX-E(spl)m7ΔWRPW, pET-Gro, and pGEX-Gro have been described (21, 29, 30). TCRα and TCRβ reporters contain the tk promoter linked to luciferase coding region. The 98-bp human TCRα enhancer fragment was generated as described (31). The mouse minimal TCRβ enhancer with two AML binding sites [nucleotides 607–746,ref. 32; Fig. 3] was used. TCRβ enhancer with mutated AML binding sites and TCRα enhancer with mutated LEF-1 site were generated as described (17, 33). The HA-LEF-1 plasmid, TOPFLASH/FOPFLASH vectors and pCGN-β-catenin were kindly provided by Rolf Kemler (Max-Planck Institute for Immunobiology, Freiburg, Germany), Marc van de Wetering and Hans Clevers (University Hospital, Utrecht, The Netherlands) and by Avri Ben-Zeev (The Weizmann Institute of Science, Rehovot, Israel), respectively. TLE1 was cloned into pCDNA3 expression vector.
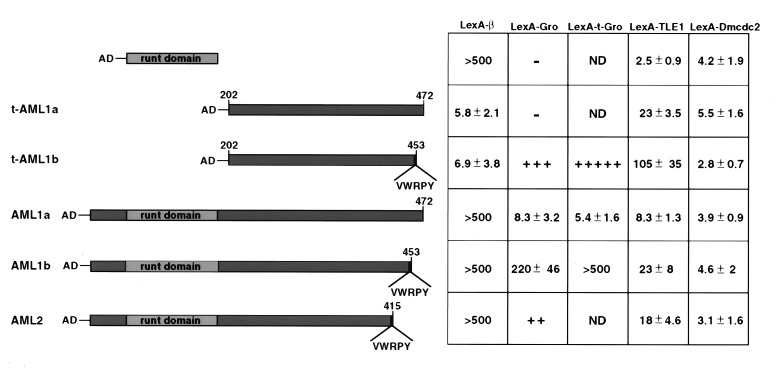
Figure 1.
Gro/TLE1 interacts specifically with AML1 and AML2 in the yeast interaction trap assay. Interactions between the AML coding regions fused to an activation domain (AD) and Gro/TLE1 proteins fused to LexA-DNA binding domain. LexA-β delineates LexA fused to Cbfβ. LexA-Dmcdc2 served as a negative control. The numbers indicate β-galactosidase activity resulting from reporter activation. + designates relative strength of blueness on X-gal indicator plates based on analyses of at least 8–10 colonies; ++ indicates >20 β-galactosidase units. − designates white colonies, indicative of absolute failure to activate the lacZ reporter gene. The assay configuration was dictated by the observation that LexA-AML constructs alone activated the reporter.
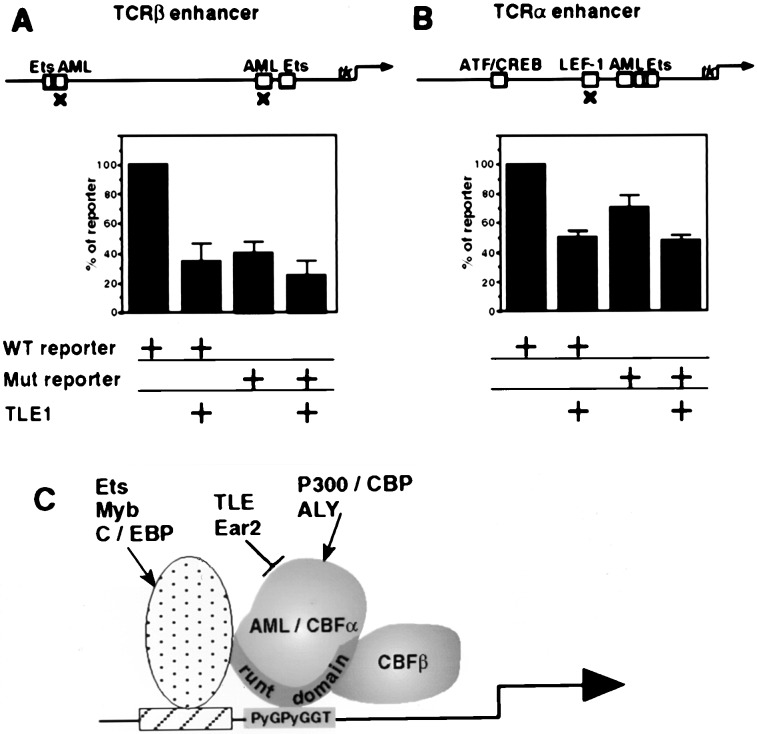
Figure 3.
TLE1 repress TCRα and TCRβ activity in Jurkat cells. Jurkat cells (2.5 × 106) were transfected by SuperFect for 7–8 hr with wild-type or mutated (marked by × at the top panel) TCRβ, 2 μg (A), or TCRα, 1 μg (B) reporters, with or without 1 μg of TLE1 expression vector. Data presented are the average of five (TCRβ) and six (TCRα) independent transfections carried out in duplicate. Control values of reporter lacking TCR enhancer (5–8%) were substracted. (C) A scheme summarizing the various interactions of AML/CBFα with the adjacently bound factors Ets, Myb, and C/EBP, as well as with the coactivators (arrows) p300/CBP and ALY, and with the negative regulators (bar) Ear 2 and TLE.
Protein–Protein Interactions.
Interaction between immobilized Ni-NTA-purified fused AML proteins and _in vitro_-translated35S-labeled TLE1 protein was performed as described (17). For pull-down experiments, fused proteins (20 μg) immobilized on Ni-NTA beads were incubated with Jurkat postnuclear extract (100 μg) (34) for 2 hr at 4°C in binding buffer A (20 mM Hepes, pH 7.9/50 mM KCl/2.5 mM MgCl2/10% glycerol/1 mM DTT/0.2% BSA/50 mM NaF/1 mM phenylmethyl sulfonyl fluoride (PMSF)/5 μg/ml Aprotinin/20 μg/ml pepstatin A/20 μg/ml leupeptin). The Ni-NTA beads were washed with PBS, associated proteins were eluted, and the bound TLE proteins were detected (24, 34). For in vitro association studies between TLE1 and LEF-1 or β-catenin, each of the latter two was transfected (20 μg DNA) into 293 cells (2.5 × 106). Cells were harvested 48 hr later in immunoprecipitation buffer (20 mM Tris (pH8), 1% Triton X-100, 140 mM NaCl, 10% glycerol, 1 mM EGTA, 1.5 mM MgCl2, 1 mM DTT, 50 mM NaF, 1 mM PMSF, 5 μg/ml Aprotinin, 20 μg/ml pepstatinA and 20 μg/ml leupeptin). Extracts (≈500 μg) containing similar amounts of LEF-1 or β-catenin, were incubated with anti-hemagglutinin (clone 12 CA5, Boehringer Mannheim) bound to Affi-Gel 10 for 2 hrs at 4°C. Bound protein was eluted in sample buffer containing 50 mM citrate and analyzed. Glutathione _S_-transferase (GST) pull-down assays were performed essentially as described (21, 30).
Cell Culture, Transient Transfection, and Reporter Gene Assays.
The human embryonic kidney cell line 293, was cultured at 37°C in DMEM supplemented with 10% fetal calf serum. Jurkat cells were grown in RPMI medium 1640 supplemented with 10% fetal calf serum. 293 cells were transfected by CaPO4 and Jurkat cells by SuperFect (Qiagen, Germany). Jurkat-transfected cells were harvested after 7–8 hr, and 293 cells were harvested after 24 hr. The amount of DNA in the transfections was kept constant by additions of the relevant backbone vector. Luciferase activity was measured by the Luciferase Assay System (Promega).
RESULTS
The VWRPY Motif and Additional C-Terminal Sequences in AML1 and AML2 Are Required for Interactions with Gro/TLE.
To determine whether AML and TLE/Gro can interact with each other, a combination of yeast two-hybrid interaction studies and biochemical analyses of_in vitro_ association and pull-down binding assays were performed.
The various AML-derived constructs used in the interaction trap assays are shown in Fig. 1. AML1b and AML2 both include the RD and the entire TAD, and both end with the VWRPY motif (6, 14). AML1b and AML1a are identical except that the latter contains a different C terminus, lacking the VWRPY (6, 13). Various AML regions, fused to an activation domain (AD-AML), were introduced and expressed in yeast cells that contained, in addition to a lacZ reporter gene, one of the following bait constructs: LexA-Gro, LexA-TLE1, or LexA-t-Gro. The latter is a truncated version of Gro that spans amino acids 251–719, and maintains specific interaction with Hairy (21). Control LexA-β contains Cbfβ, which interacts with all tested RD-containing proteins. Gro interacted strongly with VWRPY-containing AML1b and AML2 but only weakly with AML1a (Fig. 1). t-Gro, which lacks the Q, GP, and CcN domains of Gro (24), interacted with AML1b more stongly than did full-length Gro. This indicated that the SP and WD domains of Gro contain the AML-binding function. TLE1, the human homolog of Gro**,** shares a high degree of sequence similarity with Gro (24). In this assay, AML1b and AML2 interacted significantly with TLE1 yet to a lesser extent than they did with Gro (Fig. 1). The strong AML**:Gro interaction was also observed independently by Aronson et al. (23), who showed that in yeast, Gro interacts weakly with Drosophila Runt, whereas PEBP2αB1, the mouse homolog of Runt, interacts more strongly with Gro. Removal of the RD, in t-AML1b and t-AML1a,** improved the interaction with TLE1, indicating that the N-terminal part of AML was not required for this interaction (Fig. 1). Consistent with this, RD by itself failed completely to interact with either Gro or TLE1. Of note, t-AML1a–TLE1 and AML1b–TLE1 interactions are of similar magnitude, indicating that in addition to the VWRPY motif, other C-terminal sequences may contribute to these interactions.
We next confirmed the ability of AML to directly interact with TLE by protein-affinity blot analysis (“Far Western assay”), by using purified recombinant AML fusion proteins (Fig.2 A and B).35S-labeled TLE1 bound strongly to immobilized N-terminal truncated AML1b and AML2 (lanes 3 and 4). Binding of TLE1 to immobilized AML1a also was detected (lane 5), whereas nonrelevant control proteins did not show any interaction with TLE1 (lanes 1 and 2). These results further suggest that both the C-terminal region of AML and the VWRPY motif play a role in AML–TLE1 interactions.
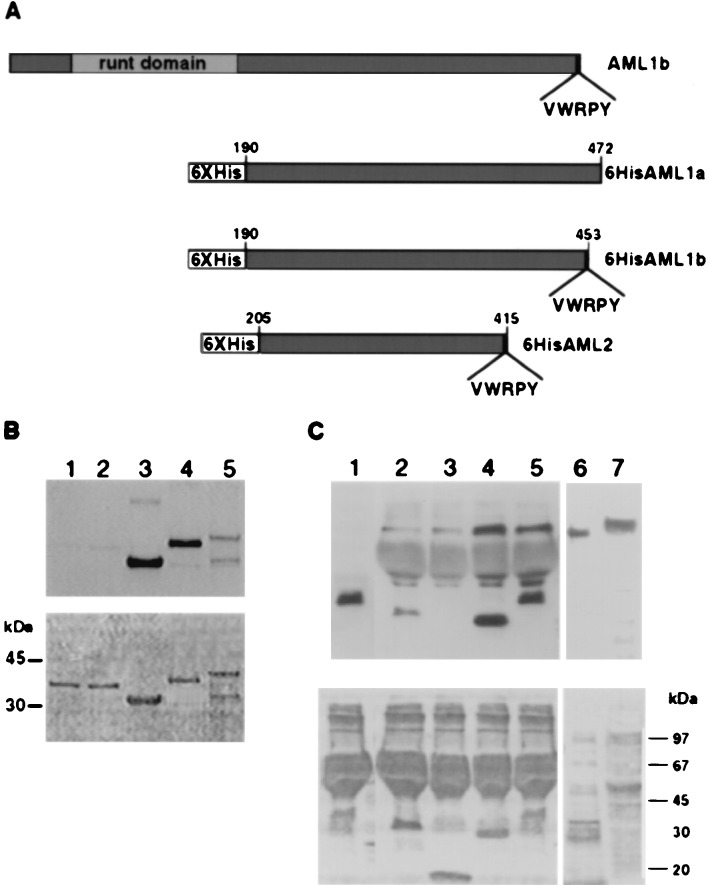
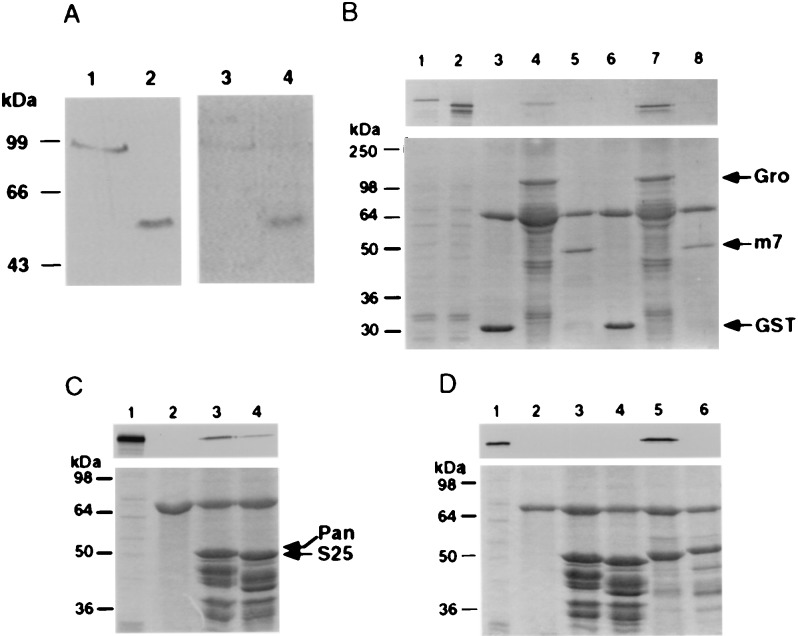
Figure 2.
VWRPY-containing AML proteins associate with Gro and TLE. (A) A scheme of 6× His AML proteins used in Far Western and pull-down analyses. Numbers on the 6× His derivatives indicate the regions that are included in the constructs. (B) In vitro association assay between AML and TLE proteins. Three micrograms of 6× His proteins: AML2, 33 kDa, lane 3; AML1b, 37 kDa, lane 4; AML1a, 39 kDa, lane 5; and control nonrelevant fused proteins, Bovine α1 and α2 PAF-AH (1b) subunits of 35 kDa, lanes 1 and 2, were analyzed by Western blotting and reacted with 35S in vitro -translated TLE1. (C) Interaction in Jurkat cell extracts between immobilized AML and TLE. 6× His-AML1-b lane 5, AML2 lane 4 and control nonrelevant proteins: 6× His leptin lane 3 (18 kDa) and the α2 PAF-AH (1b) subunit lane 2 (35 kDa). Bound proteins were analyzed with pan-TLE monoclonal antibodies. Lane 1 AML1-b without Jurkat extract, served as control for nonspecific binding of anti-TLE antibodies to 6× His proteins. The size difference between in vitro_-translated TLE (lane 6) and Jurkat extract TLE (lane 7) may result from expression of four different TLE genes in Jurkat cells. Ponceau staining are shown in the bottom panels of_B and C.
To evaluate the biological significance of the _in vitro_interactions between AML and TLE, we asked whether AML proteins interact with TLE in vivo (Fig. 2C). AML1 and AML2 are highly expressed in the human T cell line Jurkat (6, 14), as are the TLE proteins (34). Indeed, anti-TLE antibodies that reacted with _in vitro_-translated TLE1 (lane 6) readily detected TLE in Jurkat cell extracts (lane 7). Western blot analysis readily detected TLE proteins that were pulled-down from Jurkat cell extracts by immobilized AML1 or AML2 (lanes 4 and 5), but not by two other nonrelated control proteins (lanes 2 and 3). Taken together, the results of the interaction trap, Far Western assay, and pull-down studies clearly demonstrate that Gro and TLE specifically interact with AML1 and AML2 and that, in addition to the VWRPY motif, other C-terminal sequences participate in these interactions.
TLE1 Represses TCRα and TCRβ Activity in Jurkat Cells.
The ability of AML1 and AML2 to pull down TLE from Jurkat extracts suggested that these proteins are interacting in vivo. These associations are puzzling, as transfection experiments have previously shown that AML1 is a transcriptional activator (4, 17, 18, 33, 35), whereas Gro was shown to act as a transcriptional corepressor (21, 22). To examine the possibility that AML1 negatively regulates transcription in conjunction with TLE1, we assessed the ability of TLE1 to repress the activity of TCRα and TCRβ in their native milieu, i.e., T cell Jurkat. Reporter constructs in which luciferase (luc) activity was regulated by either the TCRα or TCRβ enhancers were used; AML–enhancer interactions are necessary for transcriptional activation by these control regions (4, 17, 18, 35, 36). The minimal enhancer of TCRα contains well characterized functional binding sites for several transcription factors, including LEF-1, ATF/CREB, AML, and Ets (refs.17, 18, 31, 35, 37, and 38; Fig.3). The TCRβ minimal enhancer used here contained two well defined AML binding sites and two Ets binding sites but no LEF-1 binding site (Fig. 3). Enhancer activity in Jurkat cells was assayed in TLE1- transfected versus nontransfected cells. Transfection of TCR reporters alone recorded the endogenous level of transcriptional activators. Cotransfection with TLE1 reduced TCRβ activity by two-thirds (Fig.3A), in keeping with the repressive role of TLE1 and given the specific interactions between Gro/TLE1 and AML1 documented above. Significantly, site-directed mutagenesis of AML binding sequences in the TCRβ enhancer markedly reduced both the endogenous activity of TCRβ and, importantly, TLE1-mediated inhibition (Fig.3A). This result indicated that TLE1-mediated transcriptional repression depended largely on the binding of AML to the TCRβ enhancer. Similar results were obtained by transfections of the TCRβ constructs into the early myeloid cell line K562 (not shown).
The activity of the TCRα enhancer was also reduced by cotransfection with TLE1 (Fig. 3B). However, TLE1 caused a greater reduction in TCRβ activity than it did in TCRα activity. As expected, mutation in the LEF-1 binding site decreased the activity of TCRα; however, surprisingly, it also reduced the inhibition by TLE1 (Fig. 3B). This result raised the possibility that in the context of the TCRα enhancer, TLE1 also interacted with LEF-1. Therefore, we further examined the interactions between LEF-1 and TLE1 (below).
TLE1 Inhibits LEF-1:β-Catenin-Mediated Transcriptional Stimulation.
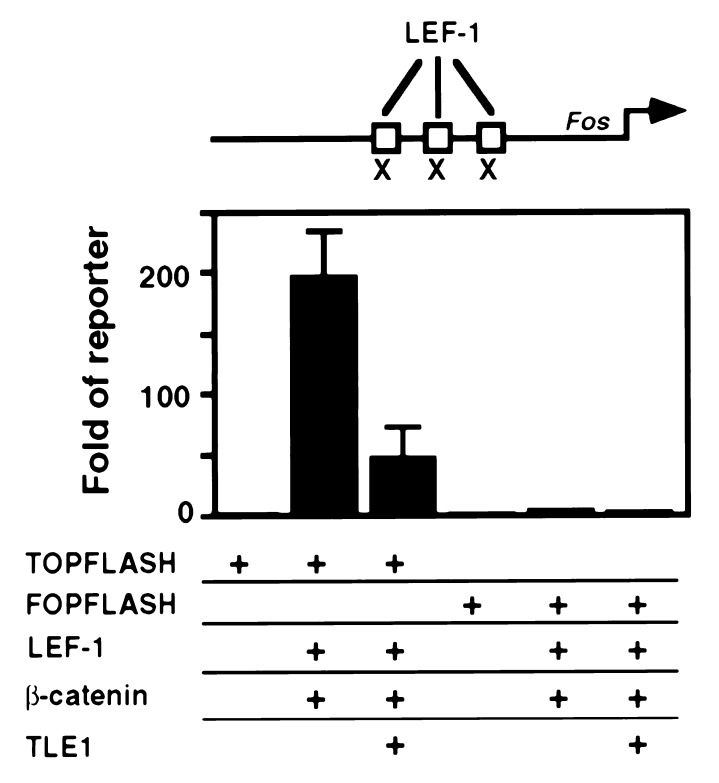
It has recently become evident that LEF-1 and its_Drosophila_ homolog Pangolin (Pan) are involved in the Wnt/Wg signaling pathway through their interactions with β-catenin and its highly conserved Drosophila homolog Armadillo (Arm) (29, 39, 40). β-Catenin/Arm is a co-activator of LEF-1/Pan in stimulating transcription from multimerized LEF-1 binding sites (39). To investigate the possibility that TLE1 counteracts β-catenin-dependent LEF-1 activity, we employed two luc reporter constructs which contained either multimeric LEF-1 binding sites (TOPFLASH) or mutated LEF-1 binding sites (FOPFLASH) (39). Cotransfection of TOPFLASH with LEF-1 and β-catenin resulted in more than 180-fold increase of luc activity relative to TOPFLASH alone (Fig.4). As expected, cotransfection with the mutated construct FOPFLASH resulted in no activity. Addition of TLE1 to the cotransfection assay markedly reduced the stimulation effect of LEF-1 and β-catenin on the TOPFLASH-luc activity. These data show that TLE1 is capable of repressing LEF-1/β-catenin-mediated transcriptional stimulation in vivo.
Figure 4.
TLE1 abrogates LEF- and β-catenin-dependent activity of TOPFLASH. 293 cells (0.5 × 106) were transfected by CaPO4 for 24 hr with 0.5 μg of reporter plasmids containing three LEF-1 binding sites (TOPFLASH) or three mutated LEF-1 binding sites (marked by × at the top panel, FOPFLASH), together with 2 μg of expression plasmid of LEF-1 and β-catenin, in the presence or absence of 2 μg of TLE1 expression vector.
TLE1/Gro Interacts with LEF-1/Pan but Not with β-Catenin.
The above results raised the question as to whether TLE1 abrogated LEF-1/β-catenin activity by physically interacting with LEF-1 or by tethering β-catenin, thus preventing it from cofunctioning with LEF-1. To address this issue, we have examined the interactions between these proteins by _in vitro_association assay. Far Western blots performed with35S-labeled TLE1 and immobilized LEF-1 or β-catenin showed that TLE1 interacted with LEF-1 but not with β-catenin (Fig. 5A, lanes 3 and 4). In addition, immobilized GST-Gro retained35S-labeled Pan in a pull-down assay (Fig.5B, lane 4).
Figure 5.
TLE1/Gro interactions with LEF-1/Pan. (A). TLE1 interacts with LEF-1 and not with β-catenin.In vitro association of 35S-labeled TLE1 with LEF-1 or β-catenin that were immunoprecipitated (IP) from overexpressing 293 cells. Lanes 1 and 2: IP LEF-1 and β-catenin reacted with anti-HA antibody. Lanes 3 and 4: interaction of IP LEF-1 and β-catenin with 35S-labeled TLE1. (B). Gro interacts with Pan. In vitro association between immobilized GST-Gro and _in vitro_-translated35S-labeled Pan or ΔPan lacking the first 50 aa. GST and GST-E(spl)m7 served as negative controls. Lanes 1 and 2: 10% of labeled Pan or ΔPan input, respectively. GST, GST-Gro and GST-E(spl)m7 incubated with labeled Pan were run in lanes 3,4, and 5, respectively; GST, GST-Gro, and GST-E(spl)m7 incubated with labeled ΔPan were run in lanes 6,7, and 8, respectively. (C) Pan (1–130) is adequate for interaction with Arm. _In vitro_association between immobilized GST-Pan (1–130) and35S-Arm. Lanes: 1, 10% of 35S-Arm input; 2, GST; 3,GST-Pan (1–130); 4, GST-PanS25 (1–130). (D) Pan (1–130) is not adequate for the interaction with Gro. In vitro association between immobilized GST-Pan (1–130) and35S Gro. Lanes: 1, 10% of 35S Gro input; 2, GST; 3, GST-Pan (1–130); 4, GST-PanS25 (1–130); 5, GST-E(spl)m7 as a positive control; 6, GST-E(spl)m7ΔWRPW-lacks the C-terminal tetrapeptide motif necessary for mediating m7-Gro interaction (21). Below B, C and D, are Coomassie blue-stained gels.
Pan Interacts with Arm and Gro Through Different Domains.
As noted before, Drosophila Pan is a target of the Wnt/Wg signaling pathway and activates transcription through its interaction with Arm (29, 39). It was reported that the N-terminal 130 aa of Pan are sufficient for in vitro interactions with β-catenin (29). Additionally, the Pan variants PanS 25 and PanS 28, carrying point mutations in amino acids 31 and 43, respectively, have a mutant phenotype and show reduced binding to β-catenin in vitro, suggesting that N-terminal amino acids 1–50 are required for functional interactions between Pan and Arm/β-catenin (29). Because TLE1 and Gro interacted with LEF-1 and Pan, respectively (Fig.5 A and B), we tested whether Gro and Arm bind coincident domains on Pan and thus compete for interaction with Pan. As previously shown by others for β-catenin (29), immobilized GST-Pan (1–130) specifically retained 35S-labeled Arm (Fig. 5C, lane 3). In contrast, it failed to retain35S-labeled Gro (Fig. 5D, lane 3). However, _in vitro_-synthesized Pan lacking the first 50 aa readily interacted with GST-Gro (Fig. 5B, lane 7). These results indicate that structural requirements for Pan–Arm interaction differ than those for Pan–Gro, although they do not rule out in any way the possibility of indirect competition by Gro and Arm over the binding to Pan.
DISCUSSION
Here we have described the identification and characterization of protein–protein interactions between the corepressors TLE/Gro and the transcriptional regulators AML and LEF-1/Pan. In AML, the terminal VWRPY motif was required for the association with Gro/TLE, consistent with previous findings by Aronson et al. (23), along with additional C-terminal sequences. In Pan, we have shown that the interaction interface required for contacting Gro/TLE is distinct from that used for Pan–β-catenin interactions. Transactivation assays demonstrated that TLE1 repressed transcription mediated by the TCRα and TCRβ enhancers. Provocatively, these data imply a putative function for AML and LEF-1 in gene repression, in addition to their previously well characterized activity as transcriptional activators (see below), and suggest that TLE may inhibit Wnt signaling by repressing β-catenin-dependent transcriptional activation mediated by LEF-1.
The large variety of protein isoforms encoded by AML1 (13,14) provide ample potential possibilities for protein–protein interactions, with the VWRPY motif being only one of several interacting sequences. Notably, AML1 interacts with Ets, Myb, and C/EBP when bound to nearby binding sites (4) as well as with the nonDNA-binding proteins PEBP2β/CBFβ, ALY, Ear2, and p300/CBP (refs. 2, 18, 41, and 42; Fig. 3C).
Gro and TLE are widely expressed and seem to act as dedicated co-repressors for a specific subset of DNA-binding transcriptional regulators. In Drosophila, these include the Hairy-related basic helix–loop–helix protein repressors, Engrailed (En) and Dorsal (21, 30, 43, 44). Unlike the interactions between Gro/TLE and Runt/AML, which rely on a common VWRPY motif (or on Hairy’s WRPW), Gro’s interactions with En and Dorsal are mediated by the eh1 and rel homology domains, respectively (30, 43, 44). LEF-1 also lacks a VWRPY pentapeptide, but it does contain a somewhat similar pentapeptide, FRQPY, at position 223–227 within a highly conserved Pan/LEF-1 region. Further work will clarify the potential significance of this sequence.
AML and LEF-1/TCF are both members of extended gene families, highly conserved between Drosophila and vertebrates. Contrary to other transcriptional activators, AML1 and LEF-1/TCF are unable to activate transcription through multimerized binding sites in vitro (4, 31, 38, 45), suggesting that their mode of regulation may be context-dependent. LEF-1 overcomes this deficiency by association with β-catenin, a component of the Wnt signaling pathway (39, 46), whereas the AML1 copartner has yet to be identified. In mammals, AML1 and LEF-1/TCF are initially expressed in several tissues during development (47, 48) and are found subsequently in adult lymphocytes. Coexpression of both AML1 and LEF-1 in T lymphocytes correlates well with their cobinding to the TCRα enhancer, their interaction with the coactivator ALY (18), and as shown here, with their ability to interact with Gro/TLE. Thus, AML1 and LEF-1 may share various partner proteins, including ALY and TLE.
To date, AML1 and LEF-1/TCF have been shown to promote gene expression, so their interactions with the Gro/TLE co-repressors are intriguing. In flies, Gro has been implicated in negative transcriptional regulation and, in its absence, all of its associated partner proteins fail to silence their respective target genes (21, 23,43, 44). Indeed, recruitment of Gro was even shown to alter an activator to a repressor (30). Similarly, alliance with Gro/TLE may convert the AML1 and LEF-1/TCF to being transcriptional repressors. However, a second possibility exists, that Gro/TLE act to ensure that AML and LEF-1/TCF target genes are silenced until the conditions are ripe for activation. In this scenario, AML is not acting as a transcriptional repressor, but rather under appropriate conditions Gro/TLE is displaced by a co-activator, thereby enabling transactivation to occur. Further experiments will allow us to distinguish between these two possibilities.
Acknowledgments
We thank Rolf Kemler, Marc van de Wetering, Hans Clevers, Konrad Basler, Avri Ben-Zeev, Al Courey, Amos Oppenheim, and Lucas Walzer for plasmids, cells, and reagents, and Yigal Burstein, Avri Ben-Zeev and Beny Geiger for discussions and advice. This work was supported by grants from the Cooperation Program in Cancer Research of the Deutsches Krebsforschungszentrum (DKFZ) and Israel’s Ministry of Science (MOS); the Commission of the European Community’s Biomedicine and Health research program BIOMED II; and the Shapell Family Biomedical Research Foundation at the Weizmann Institute. Z.P. is supported by the Israel Cancer Research Fund (RCDA), by the Israel Science Foundation, and by the Fund for the Advancement of Medicine in memory of Eliyahu and Tatiana Leszczynski. S.S. is a Scholar of the Fonds de la Recherche en Sante du Quebec and a Killam Scholar of the Montreal Neurological Institute.
ABBREVIATIONS
RD
runt domain
TCR
T cell receptor
TAD
transactivation domain
GST
glutathione _S_-transferase
Note
While this paper was under review, Thirunavukkarasu et al. (49) have reported that TLE2 inhibits the transactivation function of another member of the RD family; the osteoblast-specific transcription factor Osf2 (originally cloned as Cbfa1/AML3), and that the VWRPY motif is required for this inhibition.
References
- Kagoshima H, Shigesada K, Satake M, Ito Y, Miyoshi H, Ohki M, Pepling M, Gergen P. Trends Genet. 1993;9:338–341. doi: 10.1016/0168-9525(93)90026-e. [DOI] [PubMed] [Google Scholar]
- 2.Speck N A, Stacy T. Crit Rev Eukaryotic Gene Expression. 1995;5:337–364. doi: 10.1615/critreveukargeneexpr.v5.i3-4.60. [DOI] [PubMed] [Google Scholar]
- 3.Meyers S, Hiebert S W. Crit Rev Eukaryoic Gene Expression. 1995;5:365–383. doi: 10.1615/critreveukargeneexpr.v5.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 4.Ito Y, Bae S-C. In: Oncogenes as Transcriptional Regulators. Yaniv M, Ghysdael J, editors. Vol. 2. Basel, Switzerland: Birkhauser; 1997. pp. 107–132. [Google Scholar]
- 5.Miyoshi H, Shimizu K, Kozu T, Maseki N, Kaneko Y, Ohki M. Proc Natl Acad Sci USA. 1991;88:10431–10434. doi: 10.1073/pnas.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levanon D, Negreanu V, Bernstein Y, Bar-Am I, Avivi L, Groner Y. Genomics. 1994;23:425–432. doi: 10.1006/geno.1994.1519. [DOI] [PubMed] [Google Scholar]
- 7.Okuda T, Deursen J V, Hiebert S W, Grosveld G, Downing J R. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Stacy T, Binder M, Mari’n-Padilla M, Sharpe A H, Speck N A. Proc Natl Acad Sci USA. 1996;93:344–349. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto F, Thornell T, Crompton T, Denzel A, Gilmour K C, Rosewell I R, Stamp G W H, Beddington R S P, Mundlos S, Olsen B R, Selby P B, Owen M J. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 10.Komori T H, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson R T, Gao Y H, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 11.Look A T. Science. 1997;278:1059–1064. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 12.Ghozi M C, Bernstein Y, Negreanu V, Levanon D, Groner Y. Proc Natl Acad Sci USA. 1996;93:1935–1940. doi: 10.1073/pnas.93.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyoshi H, Ohira M, Shimizu K, Mitani K, Hirai H, Imai T, Yokoyama K, Soeda E, Ohki M. Nucleic Acids Res. 1995;23:2762–2769. doi: 10.1093/nar/23.14.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levanon D, Bernstein Y, Negreanu V, Ghozi M C, Bar-Am I, Aloya R, Goldenberg D, Lotem J, Groner Y. DNA and Cell Biol. 1996;15:175–185. doi: 10.1089/dna.1996.15.175. [DOI] [PubMed] [Google Scholar]
- 15.Zeng C, van Wijnen A J, Stein J L, Meyers S, Sun W, Shopland L, Lawrence J B, Penman S, Lian J B, Stein G S, Hiebert S W. Proc Natl Acad Sci USA. 1997;94:6746–6751. doi: 10.1073/pnas.94.13.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben Aziz, R., Levanon, D., Karn, H., Kidron, D., Goldenberg, D., Lotem, J., Polak-Chaklon, S. & Groner, Y. (1998) Cell Death Diff. in press. [DOI] [PubMed]
- 17.Giese K, Kingsley C, Kirshner J R, Grosschedl R. Genes Dev. 1995;9:995–1008. doi: 10.1101/gad.9.8.995. [DOI] [PubMed] [Google Scholar]
- 18.Bruhn L, Munnerlyn A, Grosschedl R. Genes Dev. 1997;11:640–653. doi: 10.1101/gad.11.5.640. [DOI] [PubMed] [Google Scholar]
- 19.Bienz M. Curr Opin Cell Biol. 1998;10:366–372. doi: 10.1016/s0955-0674(98)80013-6. [DOI] [PubMed] [Google Scholar]
- 20.Wainwright S M, Ish-Horowicz D. Mol Cell Biol. 1992;12:2475–2483. doi: 10.1128/mcb.12.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paroush Z, Finley R L, Jr, Kidd T, Wainwright S M, Inghan P W, Brent R, Ish-Horowicz D. Cell. 1994;79:805–815. doi: 10.1016/0092-8674(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 22.Fisher A L, Ohsako S, Caudy M. Mol Cell Biol. 1996;16:2670–2677. doi: 10.1128/mcb.16.6.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aronson B D, Fisher A L, Blechman K, Caudy M, Gergen J P. Mol Cell Biol. 1997;17:5581–5587. doi: 10.1128/mcb.17.9.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stifani S, Blaumueller C M, Redhead N J, Hill R E, Artavanis-Tasakonas S. Nat Genet. 1992;2:119–127. doi: 10.1038/ng1092-119. [DOI] [PubMed] [Google Scholar]
- 25.Parkhurst S M. Trends Genet. 1998;14:130–132. doi: 10.1016/s0168-9525(98)01407-3. [DOI] [PubMed] [Google Scholar]
- 26.Gyuris J, Golemis E, Chertkov H, Brent R. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 27.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1987–1997. [Google Scholar]
- 28.Ogawa E, Inuzuka M, Maruyama M, Satake M, Naito-Fujimoto M, Ito Y, Shigesada K. Virology. 1993;194:314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- 29.Brunner E, Peter O, Schweizer L, Basler K. Nature (London) 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- 30.Dubnicoff T, Valentine S A, Chen G, Shi T, Lengyel J A, Paroush Z, Courey A J. Genes Dev. 1997;11:2952–2957. doi: 10.1101/gad.11.22.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Travis A, Amsterdam A, Belanger C, Grosschedl R. Genes Dev. 1991;5:880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- 32.Krimpenfort P, Jong R d, Uematsu Y, Dembic Z, Ryser S, Boehmer H V, Steinmetz M, Berns A. EMBO J. 1988;7:745–750. doi: 10.1002/j.1460-2075.1988.tb02871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogawa E, Maruyama M, Kagoshima H, Inuzuka M, Lu J, Satake M, Shigesada K, Ito Y. Proc Natl Acad Sci USA. 1993;90:6859–6863. doi: 10.1073/pnas.90.14.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Husain J, Lo R, Grbavec D, Stifani S. Biochem J. 1996;317:523–531. doi: 10.1042/bj3170523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayall T P, Sheridan M R, Montminy M R, Jones K A. Genes Dev. 1997;11:887–899. doi: 10.1101/gad.11.7.887. [DOI] [PubMed] [Google Scholar]
- 36.Sun W, Graves B J, Speck N A. J Virol. 1995;69:4941–4949. doi: 10.1128/jvi.69.8.4941-4949.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho L-C, Bhat N K, Gottschalk L R, Lindsten T, Thompson C B, Papas T S, Leiden J M. Science. 1990;250:814–818. doi: 10.1126/science.2237431. [DOI] [PubMed] [Google Scholar]
- 38.Waterman M L, Jones K A. New Biol. 1990;2:621–636. [PubMed] [Google Scholar]
- 39.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 40.Behrens J, von Kries J P, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Nature (London) 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 41.Ahn M-Y, Huang G, Bae S-C, Wee H-J, Kim W-Y, Ito Y. Proc Natl Acad Sci USA. 1998;95:1812–1817. doi: 10.1073/pnas.95.4.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitabayashi I, Yokoyama A, Shimizu K, Ohki M. EMBO J. 1998;17:2994–3004. doi: 10.1093/emboj/17.11.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiménez G, Paroush Z, Ish-Horowicz D. Genes Dev. 1997;11:3072–3082. doi: 10.1101/gad.11.22.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tolkunova E N, Fujioka M, Kobayashi M, Deka D, Jaynes J B. Mol Cell Biol. 1998;18:2804–2814. doi: 10.1128/mcb.18.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaiman A L, Lenz J. J Virol. 1996;70:5618–5629. doi: 10.1128/jvi.70.8.5618-5629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 47.Simeone A, Daga A, Calabi F. Dev Dyn. 1995;203:61–70. doi: 10.1002/aja.1002030107. [DOI] [PubMed] [Google Scholar]
- 48.Oosterwegel M, van de Wetering M, Timmerman J, Kruisbeek A, Destree O, Meijlink F, Clevers H. Development (Cambridge, UK) 1993;118:439–448. doi: 10.1242/dev.118.2.439. [DOI] [PubMed] [Google Scholar]
- 49.Thirunavukkarasu K, Mahajan M, McLarren K W, Stifani S, Karsenty G. Mol Cell Biol. 1998;18:4197–4208. doi: 10.1128/mcb.18.7.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]