Debcl, a Proapoptotic Bcl-2 Homologue, Is a Component of the Drosophila melanogaster Cell Death Machinery (original) (raw)
Abstract
Bcl-2 family of proteins are key regulators of apoptosis. Both proapoptotic and antiapoptotic members of this family are found in mammalian cells, but no such proteins have been described in insects. Here, we report the identification and characterization of Debcl, the first Bcl-2 homologue in Drosophila melanogaster. Structurally, Debcl is similar to Bax-like proapoptotic Bcl-2 family members. Ectopic expression of Debcl in cultured cells and in transgenic flies causes apoptosis, which is inhibited by coexpression of the baculovirus caspase inhibitor P35, indicating that Debcl is a proapoptotic protein that functions in a caspase-dependent manner. debcl expression correlates with developmental cell death in specific Drosophila tissues. We also show that debcl genetically interacts with diap1 and dark, and that _debcl_-mediated apoptosis is not affected by gene dosage of rpr, hid, and grim. Biochemically, Debcl can interact with several mammalian and viral prosurvival Bcl-2 family members, but not with the proapoptotic members, suggesting that it may regulate apoptosis by antagonizing prosurvival Bcl-2 proteins. RNA interference studies indicate that Debcl is required for developmental apoptosis in Drosophila embryos. These results suggest that the main components of the mammalian apoptosis machinery are conserved in insects.
Keywords: Bcl-2 family, BH domains, baculovirus P35, Dark, caspase
Introduction
Programed cell death by apoptosis is essential to remove unwanted and superfluous cells during animal development and metamorphosis to maintain tissue homeostasis (reviewed in Jacobson et al. 1997; Vaux and Korsmeyer 1999). Genetic studies in Caenorhabditis elegans have identified at least four genes, egl-1, ced-3, ced-4, and ced-9, that are essential for the regulation of all developmentally programed death of somatic cells (reviewed in Metzstein et al. 1998). EGL-1, CED-3, and CED-4 are required for cell death to occur, whereas CED-9 is essential for cell survival (Yuan and Horvitz 1992; Yuan et al. 1993; Hengartner and Horvitz 1994; Conradt and Horvitz 1998). In this developmental cell death pathway, EGL-1 functions upstream of CED-9, while CED-9 interacts with and regulates CED-4, which is required for CED-3 activation (Metzstein et al. 1998). The main apoptotic machinery has been conserved during evolution, and homologues of these C. elegans proteins are found in mammals. As expected, the pathways of cell death are considerably more complex in mammals, where EGL-1, CED-3, and CED-9 are represented by multiple family members. Although there is only one mammalian counterpart of CED-4, named Apaf-1, currently known (Zou et al. 1997), adaptor molecules that act to recruit caspases to death complexes and mediate their activation can be seen as functional homologues of CED-4 (reviewed in Kumar 1999; Kumar and Colussi 1999).
CED-3, a cysteine protease of the caspase family, is the main downstream effector of apoptosis in C. elegans (Yuan et al. 1993). There are at least 14 mammalian homologues of CED-3, some of which play key roles in apoptosis (reviewed in Cryns and Yuan 1998; Nicholson 1999). The adaptor proteins, CED-4 in C. elegans, and Apaf-1 in mammals, are essential for the activation of CED-3 and caspase-9, respectively (Li et al. 1997; Yang et al. 1998). CED-9 and its mammalian homologues, including Bcl-2, Bcl-xL, and Bcl-w, act as inhibitors of caspase activation and function upstream of CED-4/Apaf-1 (reviewed in Adams and Cory 1998; Gross et al. 1999). EGL-1 and its mammalian homologues share a small region of homology (BH3 domain) with CED-9/Bcl-2 proteins and act as proapoptotic proteins upstream of CED-9/Bcl-2 (Conradt and Horvitz 1998; Gross et al. 1999). In addition to these proteins that are distantly related to Bcl-2, mammalian cells also express a number of proapoptotic members of the Bcl-2 family, such as Bax, Bak, and Bok. These proteins, termed the Bax subclass of proteins, contain three Bcl-2 homology (BH) domains, BH1, BH2, and BH3, but lack the NH2-terminal BH4 domain present in some (e.g., CED-9, Bcl-2, Bcl-xL, and Bcl-w) prosurvival members of the Bcl-2 family (Adams and Cory 1998; Gross et al. 1999). Interestingly, no homologues of the Bax subclass of proteins have been found in C. elegans.
In Drosophila melanogaster, six caspases have been discovered so far (Fraser and Evan 1997; Inohara et al. 1997; Song et al. 1997; Chen et al. 1998; Dorstyn et al. 1999a,Dorstyn et al. 1999b). In addition, a CED-4/Apaf-1 homologue, termed Dark/Dapaf-1/HAC-1, has recently been described (Kanuka et al. 1999; Rodriguez et al. 1999; Zou et al. 1999). Although the caspase(s) regulated by Dark is currently unknown, Dark can interact with two known Drosophila caspases, Dredd and Dronc (Kanuka et al. 1999; Rodriguez et al. 1999). So far, no CED-9/Bcl-2–like protein has been reported in the fly. Given the conservation of the cell death machinery, it is anticipated that Drosophila also has Bcl-2–like proteins. In this paper, we described the identification of two Bcl-2 homologues in Drosophila, one of which, named Debcl, was characterized in detail. Debcl is a proapoptotic member of the Bcl-2 family that contains BH1, BH2, and BH3 domains. We show that Debcl is structurally related to the mammalian proapoptotic Bcl-2 family of proteins and functions in the execution of physiological cell death in Drosophila.
Materials and Methods
Cloning of Debcl and 48A-E cDNAs
Debcl and the 48A-E Bcl-2–like proteins were identified as genomic regions encoding putative Bcl-2 family members by TBLASTN searches using Bcl-2 protein sequence (accession numbers of the genomic sequence entries are indicated below). Full-length debcl cDNA sequence of 1,535 bp was obtained from BDGP clones GH01265 and LD12719, purchased from Research Genetics. A 950-bp partial cDNA clone for the 48A-E homologue was isolated from a mixed stage Drosophila embryo cDNA library in λgt11 using a 450-bp probe derived from Drosophila genomic DNA by PCR. Sequencing of this clone confirmed that it also encoded a Bcl-2 family member (Fig. 1 C). However, since the predicted reading frame in the sequence is open at the 5′ end, it is likely that the cDNA clone is not full length.
Figure 1.
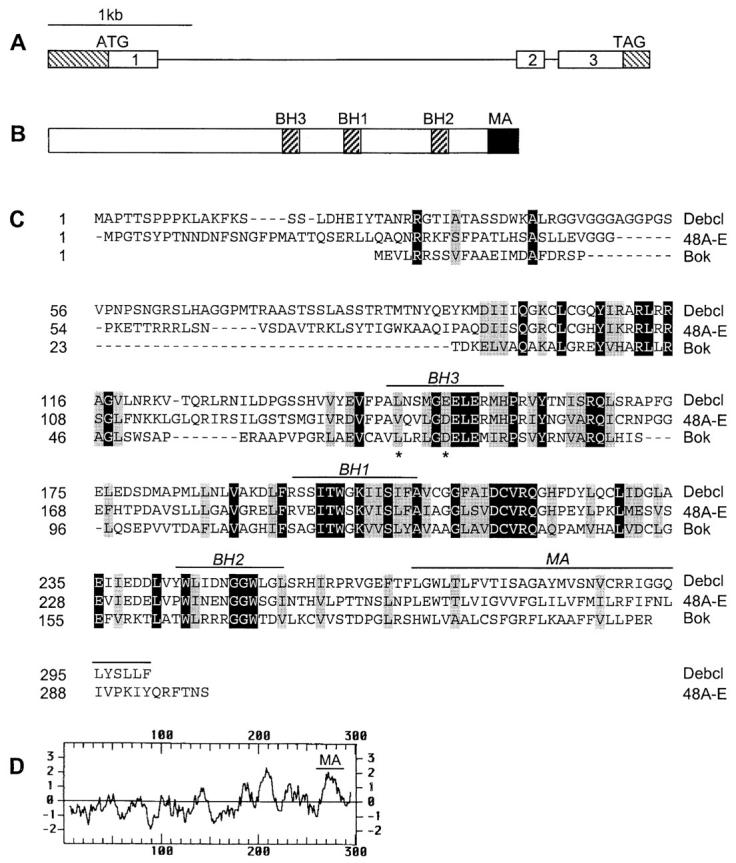
Debcl is a Bcl-2–like protein. A, Genomic structure of the debcl gene at 42E-43A. The noncoding regions of the exons are shown as hatched boxes. B, Debcl protein structure. The relative positions of the three BH domains (BH1, BH2, and BH3) and a membrane anchor (MA) are shown. C, An alignment of the Debcl sequence with Bok and 48A-E Drosophila Bcl-2 homologue. The sequence of the 48A-E homologue was obtained from a partial cDNA sequence isolated by us and the genomic sequence in the data base. The protein sequence of this clone is likely to be incomplete at the NH2 terminus. Residues identical in all three proteins are shown in black boxes and those similar shown in gray boxes. The positions of the two residues in the BH3 domain of Debcl, which were mutated in functional studies in Fig. 5 A, are indicated by an asterisk. D, A Kyte-Doolittle plot of the Debcl protein showing the putative MA region.
Plasmid Vectors for Expression in Cultured Cells
The 900-bp coding region of debcl was PCR amplified by Pfu polymerase (Stratagene) with an in-frame NH2-terminal HA tag and cloned into mammalian expression vector pcDNA3 (Invitrogen) and inducible Drosophila expression vector, pRmHa.3 (Bunch et al. 1988), to generate pcDNA3-debcl and pMT-debcl, respectively. In pMT-debcl, debcl expression is under control of a metallothionein (MT) promoter (Bunch et al. 1988). A green fluorescent protein (GFP) reporter construct was generated by placing GFP coding region downstream of an actin promoter in insect vector, pPAC-5C. To generate a P35 insect expression construct, the coding region of the baculovirus p35 was PCR-amplified and cloned into pPAC-5C. Debcl BH3 domain mutants L146G and E151G were generated by Quickchange™ method (Stratagene) using pMT-debcl as a template. Vectors for the expression of FLAG-tagged Bcl-2 family members have been described previously (Huang et al. 1997; Moriishi et al. 1999).
mRNA Expression Analysis
Total RNA from various developmental stages of Drosophila or adult flies was prepared using RNAzol B according to the manufacturer's (Tel-Test Inc.) protocol. Poly A+-enriched RNA was prepared using oligo dT magnetic beads (Dynal). Northern blots were prepared and hybridized with a 900-bp debcl open reading frame (ORF) probe as described (Dorstyn et al. 1999a,Dorstyn et al. 1999b). For reverse transcriptase (RT)-PCR, 1 μg total RNA was reverse transcribed using a first strand cDNA synthesis kit (Amersham Pharmacia Biotech). Aliquots of cDNA were subjected to 30 cycle PCR using primers from debcl ORF that generate a PCR product of ∼450 bp. For in situ RNA analysis, antisense and sense digoxygenin-labeled riboprobes were prepared using T7 and SP6 RNA polymerases from linearized pcDNA3-debcl as a template. Digoxygenin labeling was performed according to the manufacturer's instructions (Roche Biochemicals). In situ hybridization to Drosophila embryos and larval tissues was essentially as described (Dorstyn et al. 1999a,Dorstyn et al. 1999b), except that hybridization signals were further amplified using Tyramide Signal Amplification (TSA™) Indirect system according to the protocol supplied by the manufacturer (New England Nuclear Life Science Products).
Cell Death Assays
Schneider L2 (SL2) cells were maintained and transfected using Cellfectin (Life Technology) as described (Chen et al. 1996). For death assays, 1.5 × 106 SL2 cells were cotransfected with 1.6 μg vector or pMT-debcl (wild-type or mutants) and 0.4 μg pPAC-GFP reporter. 24 h later, cells were split into two halves, one of which was treated with 0.7 mM CuSO4 for 8 h (for immunoblots) or 16 h (for death assays). Where indicated, 50 μM zVAD-fmk (Enzyme Systems Inc.) was added to cultures at the time of addition of CuSO4. After fixation with 4% paraformaldehyde, GFP positive cells were counted by fluorescence microscopy. Cell survival was calculated as the percentage of GFP positive cells in CuSO4-treated cells, relative to the percent of GFP positive cells in untreated cells. The results, shown as average percentages ± SEM, were derived from three independent experiments. To check copper-induced Debcl expression, after an 8-h CuSO4 treatment, cells were lysed in SDS-PAGE buffer and lysates subjected to immunoblotting using an αHA antibody (Roche Biochemicals). NIH 3T3 cells were transfected using Fugene6 (Roche Biochemicals) with 1.0 μg of pcDNA3-debcl, and 0.2 μg of a β-galactosidase expression plasmid (pEF-βgal; Kumar et al. 1994). Where indicated, cells were cotransfected with P35, Bcl-2, Bcl-xL, MIHA, and Bcl-xLexpression constructs (described in Uren et al. 1996; Dorstyn and Kumar 1997; Huang et al. 1997). In these experiments, we used 3 μg of the specific inhibitor expression construct mixed with 1 μg of pcDNA3-debcl and 0.2 μg of pEF-βgal. Cells were fixed and stained with X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) 48 h after transfection, and β-galactosidase positive cells were scored for apoptotic morphology (Kumar et al. 1994; Dorstyn and Kumar 1997). Data, presented as percent apoptotic cells as a fraction of total β-galactosidase positive cells ± SEM, were derived from three independent experiments.
Analysis of Apoptotic Cells in Embryos
For TUNEL, embryos were devitellinized and prepared as described (Chen et al. 1996). TUNEL was performed using a kit (Roche Biochemicals) and embryos were mounted in 70% glycerol. Acridine orange staining was according to a published procol (Abrams et al. 1993).
Transgenic Flies and Genetic Interaction Studies
The PCR-amplified 900-bp debcl coding region tagged with HA (see above) was cloned into the pUAST plasmid (Brand and Perrimon 1993). Transgenic flies were generated and maintained essentially as described (Richardson et al. 1995). A homozygous line on the 2nd chromosome was used for the analysis of cell death in larval tissues. For genetic interaction studies, a line on the 3rd chromosome, UAS-debcl#26, was used to generate the strain GMR-GAL4/CyO; UAS-debcl#26/TM6B, which gave rise to adults with severely ablated eyes. This strain was crossed to strains containing GMR-p35; a deficiency of rpr, hid, and grim (Df(3L)H99); a deficiency of diap1 (Df(3L)brm11 or Df(3L)stf-13); a deficiency of diap2 (Df(2R)Jp1); or a P allele mutation of dark, dark CD8 (Rodriguez et al. 1999), and the eye phenotypes of the progeny were compared with GMR-GAL4; UAS-debcl#26 adult eyes using light microscopy or scanning EM as described previously (Richardson et al. 1995). dark CD8 flies were kindly provided by J. Abrams (University of Texas, Southwestern Medical Center, Dallas, TX). All other fly stocks were obtained from the Bloomington stock center.
Debcl/Bcl-2 Interaction Studies
293T cells were cotransfected with HA-tagged Debcl in pcDNA3 and FLAG-tagged control vector or various Bcl-2 family proteins in the presence of a baculoviral P35 expression construct by lipofection as described (Huang et al. 1997). 36 h after transfection, cells were labeled with 100–200 μCi/ml 35S-methionine (New England Nuclear) and cell lysates prepared in lysis buffer (20 mM Tris-HCl, pH 7.4, 135 mM NaCl, 1% Triton X-100, 10% glycerol, supplemented with 0.5 μg/ml Pefabloc, 100 μg/ml soybean trypsin inhibitor, and 1 μg/ml each of leupeptin, aprotinin, and pepstatin). In some experiments, unlabeled cell extracts from transfected cells were used for immunoprecipitation/immunoblotting analyses. Equivalent trichloro-acetic acid precipitable counts (5 × 108 cpm) or 1 mg of cell lysates were used for each immunoprecipitation with 5 μg of a control isotype-matched αEE (Berkeley Antibody Co.), αFLAG M2 (Sigma Chemical Co.) or αHA 11 (Berkeley Antibody Co. or Roche Biochemicals) antibodies. Immunoprecipitations were performed according to a previous protocol (Moriishi et al. 1999) and the immunoprecipitated material fractionated by SDS-PAGE. For 35S-methionine–labeled proteins, the signals were detected by fluorography after signal enhancement using AMPLIFY (Amersham Pharmacia Biotech). For unlabeled proteins, signals were detected by immunoblotting using the ECL detection system (Amersham Pharmacia Biotech).
RNA Interference (RNAi) Methods
RNAi methods were essentially as described (Bhat et al. 1999; Misquitta and Paterson 1999). debcl sense and antisense RNA transcripts were synthesized using the Ambion Megascript kit using linearized pcDNA3-debcl as a template. After purification and annealing, double-stranded RNA was dissolved in injection buffer (5 mM KCl in 0.1 mM phosphate buffer, pH 7.8) at 0.75 mg/ml. Precellularized embryos were injected at 50% egg length as described (Bhat et al. 1999; Misquitta and Paterson 1999). Embryos were aged at 18°C to stage 16 before TUNEL staining.
Accession Numbers
debcl sequence has been deposited in GenBank/EMBL/DDBJ under accession number AF178430. The EST clones (BDGP clones GH01265 and LD12719) from which debcl sequence was derived have the accession numbers AI513093 and AI062455, respectively. debcl genomic sequence is contained in AC007624. Coding region for 48A-E Bcl-2–like protein is contained in AC007473.
Results
Two Bcl-2 Homologues in Drosophila
To identify Bcl-2–like proteins, we searched the Drosophila sequence database using the TBLASTN program and identified two putative Bcl-2 homologues in the genomic regions 42E-43A and 48A-E. The 42E-43A region was represented in two EST clones, which were sequenced in full. The 1,535-bp full-length cDNA for the putative Bcl-2 homologue on 42E-43A contains a 410-bp 5′ untranslated region, a 900-bp coding sequence, and a 225-bp 3′ untranslated region. A comparison of the cDNA and genomic sequences identified three exons in this gene (Fig. 1 A). The cDNA encoded a Bcl-2–like protein consisting of 300 amino acids with an estimated molecular mass of ∼33 kD. This was confirmed by in vitro translation of transcribed RNA (data not shown). We have named this protein Debcl (pronounced debacle) for death executioner Bcl-2 homologue. Debcl contains three of the BH domains, BH1, BH2, and BH3 (Fig. 1B and Fig. C), but lacks the NH2-terminal BH4 domain found in some antiapoptotic members of the Bcl-2 family. The COOH terminus of Debcl contains a putative hydrophobic membrane anchor, similar to that found in many Bcl-2–like proteins (Fig. 1C and Fig. D).
Debcl is most similar to the other putative Drosophila Bcl-2–like protein on region 48A-E, sharing 42% identity and 62% similarity in a 169 amino acid stretch (Fig. 1 C). Among the published mammalian Bcl-2 family members, the homology is mostly limited to the regions that comprise the three BH domains. In this region, Debcl shares the highest degree of homology (35% identity, 52% similarity) with Bok, a proapoptotic Bax subfamily member (Adams and Cory 1998; Gross et al. 1999). Debcl shares 20–30% identity with various prosurvival members of the Bcl-2 family, including A1 (30% identity, 49% similarity), Bcl-2 (25% identity, 41% similarity), Bcl-xL (25% identity, 46% similarity), Mcl-1 (26% identity, 46% similarity), and Bcl-w (21% identity, 41% similarity). The overall structure of Debcl is similar to Bax, Bak, and Bok, all of which contain BH1, BH2, and BH3 domains, a membrane anchor region, and a relatively long NH2-terminal region (Fig. 1, B–D).
debcl Expression Correlates with Cell Death during Drosophila Development
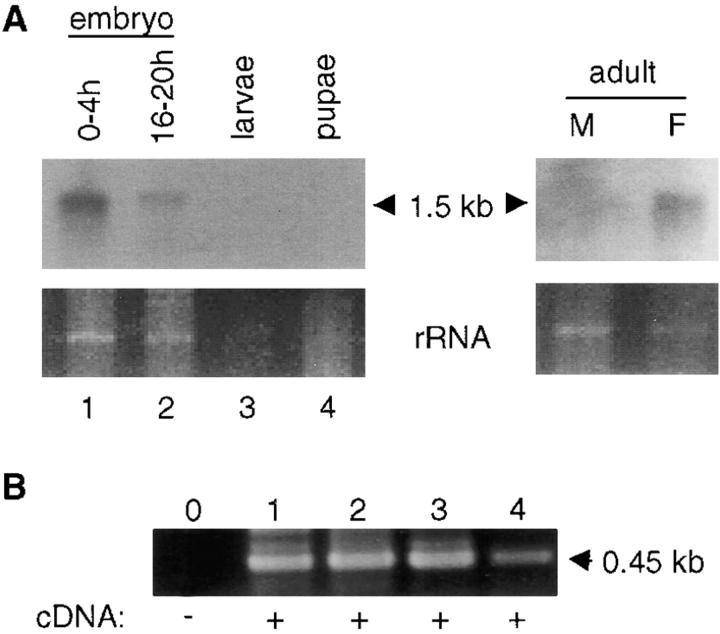
debcl expression was determined by RNA blotting, RT-PCR, and in situ hybridization to Drosophila embryos and tissues. In most cases low levels of debcl mRNA expression was detected. In RNA blots, a 1.5-kb transcript was evident in most developmental stages, but expression was relatively high in 0–4-h embryos and adult female flies (Fig. 2 A). In late embryos, larvae and pupae debcl expression was somewhat reduced and barely detectable by Northern blotting. However, RT-PCR analysis indicated that debcl mRNA was present in all developmental stages examined (Fig. 2 B).
Figure 2.
debcl mRNA expression in Drosophila. A, Northern blot of poly A+-enriched RNA isolated from various developmental stages and adult flies. debcl transcript is detected as a single, ∼1.5-kb band in most samples examined. The lower panels depict portions of the ethidium bromide-stained gels corresponding to the residual ribosomal RNA bands before transfer to membrane. B, RT-PCR analysis of debcl expression. After reverse transcription of RNA from various stages of Drosophila development, PCR was carried out for 30 cycles using _debcl_-specific primers that generate a 450-bp product. Lanes 1–4 in B correspond to lanes 1–4 in A. Note that all samples express debcl transcript.
Because of low expression of debcl, we used tyramide amplification after hybridization to detect debcl mRNA expression in situ (Fig. 3). In early embryos, debcl mRNA was present uniformly, but became more concentrated in the tissues of the gut later in embryogenesis (Fig. 3, A–D). The relatively high levels of debcl RNA in early embryos (Fig. 3 A; data not shown for precellularized embryos) are likely to be derived maternally, as zygotic transcription does not begin until stage 5. From stage 14 embryos, debcl mRNA was detected in regions in the head that correspond to the pharynx and clypeolabrum, where many TUNEL positive cells are detected (Fig. 3 G). Expression could also be detected in a segmentally reiterated pattern in stage 14 embryos (Fig. 3 C) that may correlate with the TUNEL positive cells that are detected in the nervous system at this stage (Fig. 3 G and 4 C). During 3rd instar larval development, debcl expression was detected in the brain lobes in the outer proliferative center (Fig. 3 J), in the posterior part of the eye imaginal disc (Fig. 3 K), and in the gut (Fig. 3 N) where TUNEL positive cells were clearly seen (Fig. 3 O and 4, E and G). debcl expression was also clearly evident in the salivary glands, particularly in the ducts (Fig. 3 L). Because of background staining problems in salivary glands, acridine orange staining instead of TUNEL was used to detect apoptotic cells in this tissue. Using this technique, no apoptotic cells were detected in 3rd instar salivary glands (Fig. 4 K), suggesting that debcl expression may precede cell death in this tissue. High levels of debcl expression was detected in the nurse cell compartment of stage 10a ovaries (Fig. 3 P), which undergo apoptosis at stage 10b (Foley and Cooley 1998). Thus, debcl expression late in embryogenesis, during larval development, and during oogenesis significantly correlates with tissues undergoing apoptosis.
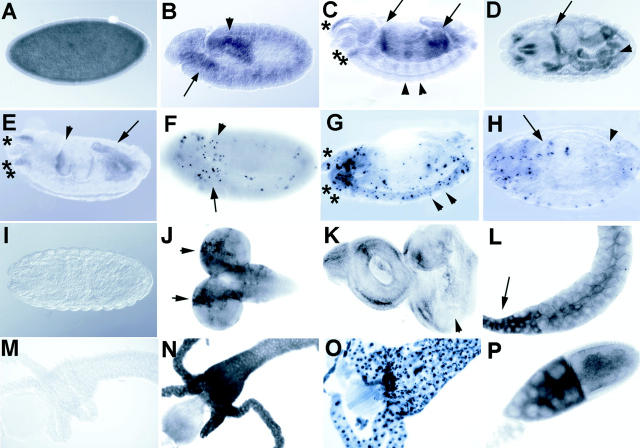
Figure 3.
In situ analysis of debcl expression during development. A debcl antisense RNA probe labeled with digoxygenin was used to detect debcl expression in situ. A, Uniform staining is evident in a stage 5 cellularized embryo. B, In germ band extended embryo (stage 11), staining is evident in the anterior (arrow) proctodeum and posterior midgut (arrowhead), which are regions that show higher levels of TUNEL positivity (F). C, A lateral view of a germ band retracted embryo (stage 14) showing staining in the gut, particularly in the anterior and posterior midgut (arrows) and staining in the head corresponding to tissues of the clypeolabrum (*) and of the pharynx (**). Staining is also observed in a segmental reiterated pattern (examples indicated by arrowheads), that may correspond to cells in the central and peripheral nervous system, which show positive TUNEL at this stage (G). D, A dorsal view of a stage 16 embryo showing staining in regions in the head and gut (arrow indicates a strong stripe of staining that occurs in the foregut–midgut junction). E, A lateral view of an embryo at stage 16, showing staining in regions of the gut (arrowhead indicates the foregut–midgut junction and arrow indicates the hindgut), and in tissues of the clypeolabrum (*) and pharynx (**). F, TUNEL of an embryo at stage 11, showing a higher level of TUNEL positive cells in the region of the anterior midgut (arrow) and the proctodeum, posterior midgut (arrowhead). G, TUNEL of a stage 14 embryo, showing TUNEL positive cells in a segmentally reiterated pattern in cells of the nervous system (examples indicated by arrowheads) and in the region of the clypeolabrum (*) and pharynx (**). H, TUNEL on a stage 16 embryo showing higher numbers of TUNEL positive cells in the gut (midgut indicated by the arrow, and hindgut indicated by the arrowhead), and in head. I, A stage 16 embryo hybridized with a control sense probe. J, Antisense probe on third instar larval brain lobes showing stronger staining in rows of cells in the region of the outer proliferative center of the brain hemispheres (indicated by arrows), a region that also labels with TUNEL (see Fig. 4 E). K, Antisense probe on a third instar larval eye-antennal disc showing weak staining. The arrowhead indicates the morphogenetic furrow, after which higher levels of staining are observed in some cells corresponding with the region where TUNEL positive cells are observed (see Fig. 4 G). L, Antisense probe on third instar larval salivary glands showing positivity in the duct (arrow). M, Sense control probe on third instar larval gut showing no staining. Also, sense controls on other larval tissues and adult ovaries showed no staining (data not shown). N, Antisense probe on late third instar larval gut showing high levels of staining. O, TUNEL of a late third instar larval gut showing most cells are positive at this stage. P, Antisense probe on ovaries showing high levels of debcl expression in the nurse cells (on the left) and in the oocyte (right) of stage 10a egg chambers. Lower levels of staining are observed subsequent to stage 10 (not shown).
Figure 4.
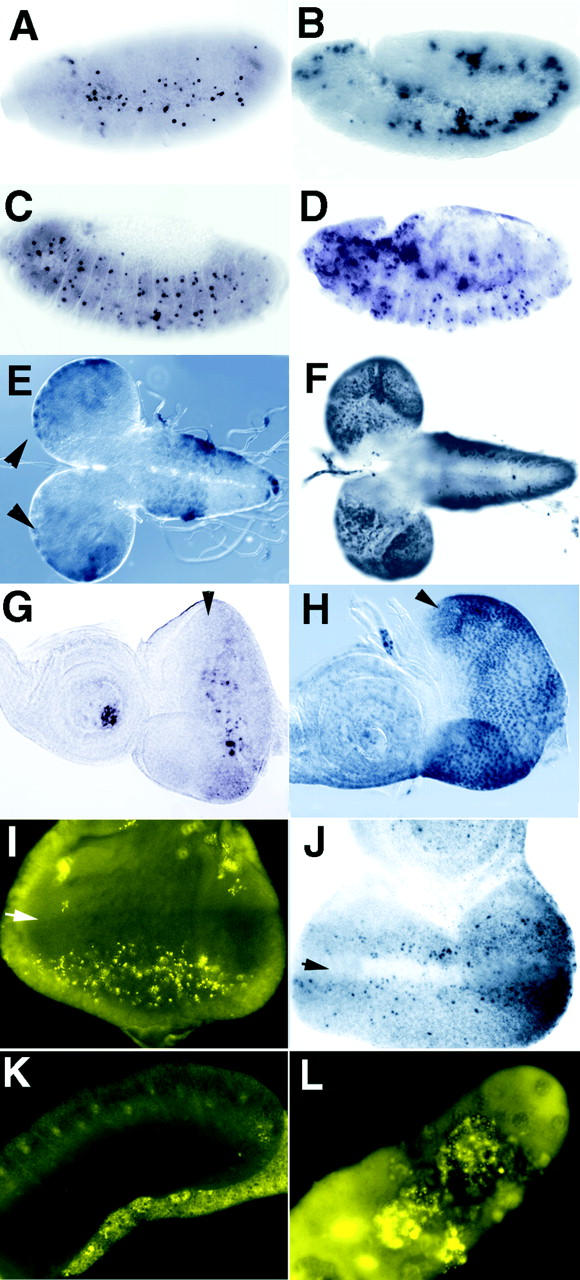
Debcl induces cell death in vivo. Homozygous flies containing debcl under control of the UAS-GAL4 promoter were crossed to various GAL4 drivers and the effect on cell death examined by TUNEL or acridine orange staining. B, D, F, and H represent samples from hsp70-GAL4 × UAS-debcl after heat shock induction. Samples were heat-shocked for 1 h and allowed to recover for 1 h (A and B) or for 3 h (C–H) before fixation and staining. A, A wild-type stage 11 embryo after heat shock, showing a normal pattern of TUNEL. B, A _hsp70_-GAL4 × _UAS_-debcl stage 11 embryo after heat shock-induced expression, showing an increase in TUNEL positive cells relative to A. C, A wild-type stage 13 embryo after heat shock showing a normal pattern of TUNEL. D, A hsp70-GAL4 × _UAS_-debcl stage 13 embryo after heat shock showing an increase in TUNEL positive cells relative to C. Strong TUNEL positive cells are observed in the gut (out of the plane of focus). E, A wild-type 3rd instar larval brain lobe (dorsal view) after heat shock showing low levels of TUNEL staining cells in the brain hemispheres (arrowheads) and in the ventral ganglion. F, A hsp70-GAL4 × UAS-debcl 3rd instar larval brain lobe (dorsal view) after heat shock-induced expression showing an increase in TUNEL positivity relative to E. Note that most of the TUNEL positive cells in the ventral ganglion are out of the plane of focus but extend all the way to the posterior end. G, A wild-type 3rd instar larval eye-antennal disc after heat shock showing only a few TUNEL positive cells. The arrowhead indicates the morphogenetic furrow (also in H, I, and J) after which there are a cluster of TUNEL positive cells. H, A hsp70-GAL4 × _UAS_-debcl 3rd instar larval eye-antennal disc after heat shock expression showing a large increase in TUNEL positive cells relative to G. I, Acridine orange staining of an eye disc from GMR-GAL4 × _UAS_-debcl flies, which results in expression in the posterior region of the eye disc, showing an increase in apoptotic cells in the posterior region. Acridine orange staining of control discs was similar to TUNEL labeling (not shown). J, TUNEL staining of an eye disc from eyeless-GAL4 × _UAS_-debcl flies, which results in expression throughout the eye disc during 2nd instar larval development, and is strong in the anterior region in 3rd instar larvae showing an increase in TUNEL positive cells anterior to the morphogenetic furrow (arrow). K, Acridine orange staining of a 3rd instar larval salivary gland showing essentially no staining, even after long exposure. L, Acridine orange staining of a 3rd instar larval salivary gland from a 109-88-GAL4 × _UAS_-debcl, which results in strong expression in the embryonic and larval salivary glands (not shown), showing that there is strong staining of the large polyploid nuclei.
Debcl Is a Proapoptotic Protein
To investigate whether Debcl is a pro- or antiapoptotic protein in vivo, we generated transgenic flies with the debcl cDNA under control of the yeast UAS-GAL4 promoter. Ectopic expression was then achieved by crossing these flies to various GAL4 drivers. To express debcl in all tissues at various developmental stages, _UAS_-debcl flies were crossed to hsp70-GAL4 flies and embryos or larvae were heat shocked. Heat shock-induced expression of debcl resulted in enhanced levels of TUNEL positive cells in the embryo (Fig. 4B and Fig. D) and in larval tissues (Fig. 4F and Fig. H; data not shown).
Tissue specific drivers were then used to express debcl during larval development. Ectopic expression of debcl in the posterior region of the eye imaginal disc using the GMR-GAL4 driver (Ellis et al. 1993) resulted in increased acridine orange staining cells in the posterior region of the eye (Fig. 4 I). Similarly, expression throughout the eye imaginal disc of 2nd instar larvae using the eyeless-GAL4 driver (Hauck et al. 1999) resulted in increased TUNEL positive cells in the anterior and posterior regions of the eye (Fig. 4 J). We predicted that expression of debcl from eye specific drivers would result in adults with ablated eyes, as does expression of rpr, hid, and grim from the GMR enhancer (White et al. 1994; Grether et al. 1995; Chen et al. 1996). Surprisingly, despite the increase in apoptotic cells seen in the imaginal discs, the adult flies from these crosses exhibited only a mild rough eye phenotype (data not shown), possibly because of the excess number of cells that are normally generated during eye development. However, other UAS-debcl lines, which presumably have a much higher level of expression, resulted in adults with severely ablated eyes when crossed to GMR-GAL4 (see below). We also expressed debcl in the larval salivary gland using a salivary gland specific driver, 109-88-GAL4, which resulted in a massive increase in acridine orange staining cells (Fig. 4 L) and a reduction in the size of the salivary glands (not shown). Thus, debcl induces cell death when ectopically expressed in a number of different tissue types during Drosophila development, indicating that Debcl is a proapoptotic protein of the Bcl-2 family.
Debcl Function Requires the BH3 Domain and Is Caspase-dependent
To characterize further the biological activity of Debcl, we expressed debcl in Drosophila SL2 cells under the control of an inducible insect promoter. Within 16 h of transfection, Debcl induced apoptosis in a majority of the transfected SL2 cells (Fig. 5 A). By 48 h, all debcl transfected cells had been lost (not shown). This cell death was partially inhibited by the cell permeable peptide caspase inhibitor zVAD-fmk and much more effectively by baculovirus caspase inhibitor P35, indicating that Debcl-induced apoptosis is, at least in part, mediated by caspases. While zVAD-fmk is an efficient inhibitor of many mammalian caspases, it is not known whether it can inhibit Drosophila caspases as effectively. Therefore, the partial inhibition of Debcl-induced cell death by zVAD-fmk may reflect its inability to efficiently inhibit all Drosophila caspases. To confirm that Debcl's cell killing function is dependent on caspase activity, we crossed debcl transgenic flies with _GMR_-p35 flies. As discussed below and shown in Fig. 6, in the resulting flies the effect of Debcl in eye ablation was significantly reduced.
Figure 5.
Debcl function requires BH3 domain and caspases. A, Cell death assays in Drosophila SL2 cells. SL2 cells, cotransfected with pMT-debcl (wild-type or BH3 mutants) and pPAC-GFP, were treated with CuSO4 for 16 h to induce Debcl expression. Cell loss due to apoptosis was monitored by counting residual GFP positive cells after CuSO4 treatment. In the upper panel, expression of HA-Debcl after an 8-h treatment with CuSO4 (i.e., before the onset of apoptosis) is shown. Debcl-induced cell death was significantly inhibited by the caspase inhibitors, baculovirus P35 and zVAD-fmk, or mutations in conserved residues of the Debcl BH3 domain (L146G and E151G mutants). Although not shown here, in transfected cells, both Debcl BH3 domain mutants were expressed as efficiently as the wild-type Debcl protein. B, Debcl induces apoptosis in mammalian cells. NIH 3T3 cells were cotransfected with the Debcl expression construct alone or mixed with the Bcl-2, Bcl-xL, P35, MIHA, or IAP expression constructs, and a β-galactosidase expression vector. β-galactosidase positive cells were scored for apoptotic morphology 48 h later. Note that both the prosurvival Bcl-2 proteins and the caspase inhibitors significantly inhibit Debcl-induced apoptosis. Debcl-induced cell death was concentration-dependent and use of higher amount of expression vector resulted in death of >95% of the cells (not shown). In both A and B, data (mean ± SEM) were derived from three independent experiments.
Figure 6.
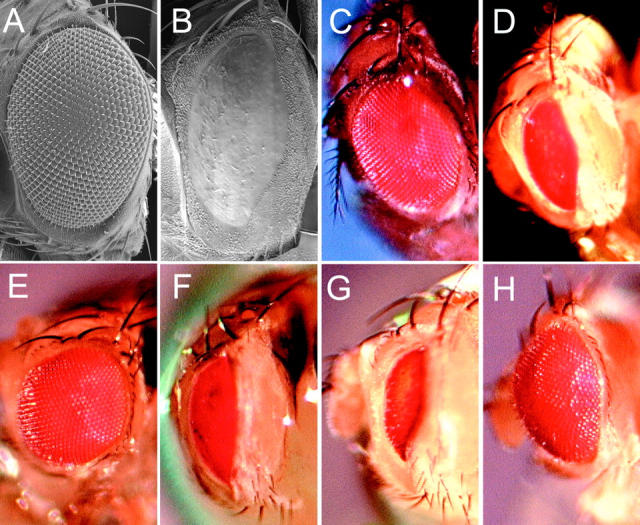
Genetic interactions of GMR-p35, the Df(3L)H99 genes, diap1 and dark with GMR-GAL4; UAS-debcl#26. The effects of GMR-p35 and reducing the dosage of the Df(3L)H99 genes, diap1 and dark genes on the eye phenotype of heterozygous GMR-GAL4; UAS-debcl#26 flies, were examined after crossing GMR-GAL4/CyO; UAS-debcl#26/TM6B flies to the relevant stocks. A, Scanning electron micrograph of a wild-type adult eye (Canton S). B, Scanning electron micrograph of GMR-GAL4; UAS-debcl#26 adult eye, showing severe ablation of the eye. C, Photograph of a wild-type eye (Canton S). D, Photograph of GMR-GAL4; UAS-debcl#26 adult eye, showing severe ablation of the eye and patches of reduced pigmentation. E, Photograph of GMR-GAL4; UAS-debcl#26/GMR-p35 adult eye, showing strong suppression of the ablated eye phenotype. F, Photograph of GMR-GAL4; UAS-debcl#26/Df(3L)H99 (removing rpr, hid, and grim) adult eye, showing little effect on the ablated eye phenotype. G, Photograph of GMR-GAL4; UAS-debcl#26/Df(3L)brm11 (removing diap1) adult eye, showing enhancement of the ablated eye phenotype. Similar results were obtained using another diap1 deficiency, (Df(3L)stf-13). H, Photograph of GMR-GAL4/darkCD8 (hypomorphic allele); UAS-debcl#26 showing suppression.
In several proapoptotic Bcl-2 members, the BH3 domain is essential for their killing function (Adams and Cory 1998; Gross et al. 1999). To determine whether the BH3 domain in Debcl was required for its proapoptotic function, we generated two substitution mutants (L146G and E151G) of the Debcl BH3 domain and analyzed their killing activity in SL2 cells. The 146L residue is conserved in the BH3 domains of most proapoptotic Bcl-2 members, whereas 151E corresponds to an acidic residue in most BH3 domains. Whereas the L146G mutation partially inhibited apoptosis induction by Debcl, E151G mutation completely abrogated Debcl-mediated cell killing (Fig. 5 A). Drosophila proteins Grim, Reaper, and Hid are able to induce apoptosis in mammalian cells (McCarthy and Dixit 1998; Claveria et al. 1998; Haining et al. 1999), despite the fact that mammalian homologues of these proteins have not been found. To determine whether Debcl can also induce apoptosis in mammalian cells, we cloned debcl cDNA in a mammalian expression vector and transfected it into NIH 3T3 cells. Most of the _debcl_-transfected cells underwent apoptosis (Fig. 5 B). When Debcl was cotransfected with expression vectors carrying caspase inhibitors P35, MIHA, or IAP (reviewed in Ekert et al. 1999), a substantial decrease in apoptosis was evident. These results indicate that Debcl-induced killing is dependent upon its BH3 domain and requires caspase function. In addition to caspase inhibitors, coexpression of prosurvival Bcl-2 and Bcl-xL proteins also significantly inhibited Debcl-induced apoptosis (Fig. 5 B).
Genetic Interactions of debcl with p35, H99, diap1, and dark
After screening a number of UAS-debcl lines, two lines (UAS-debcl#26 on chromosome III and UAS-debcl#18 on chromosome II) were found that, when crossed to GMR-GAL4, gave rise to adults with severely ablated eyes (Fig. 6B and Fig. D). To use this phenotype to examine genetic interactions, a stock was generated containing GMR-GAL4 (2nd chromosome) and UAS-debcl#26. To examine whether the rough eye phenotype was due to the activity of caspases, we crossed GMR-p35 to these flies and examined the eye phenotype of the progeny. As shown in Fig. 6 E, GMR-p35 significantly improved the severe rough eye phenotype of GMR-GAL4; UAS-debcl#26 eyes (Fig. 6 D). These results confirm that Debcl functions in a caspase-dependent fashion upstream of caspase activation.
To determine the involvement of rpr, hid, or grim in the GMR-GAL4; UAS-debcl#26 eye phenotype, we crossed these flies to a deficiency that removes all three genes (Df(3L)H99). If rpr, hid, or grim are rate limiting for Debcl function, then suppression of the GMR-GAL4; UAS-debcl#26 eye phenotype would be expected. However, no significant suppression of this phenotype was observed (Fig. 6 F), suggesting that the GMR-GAL4; UAS-debcl#26 eye phenotype is not dependent on the gene dosage of rpr, hid, or grim.
Next, we tested whether the inhibitor of apoptosis (IAP) homologue, diap1, genetically interacted with debcl, by examining the GMR-GAL4; UAS-debcl#26 eye phenotype when the dosage of diap1 was halved. Halving the dosage of diap1, using two different deficiencies, resulted in a strong enhancement of the GMR-GAL4; UAS-debcl#26 eye phenotype (Fig. 6 G). Furthermore, there was a significant reduction in the number of flies expected containing either of the diap1 deficiencies and GMR-GAL4; UAS-debcl#26. This was possibly due to leaky expression of the GMR-GAL4; UAS-debcl#26 construct in other tissues during development and the enhancement of this effect by reducing the dose of diap1. Thus, diap1 genetically interacts with debcl. We did not observe any genetic interaction between debcl and diap2 when a diap2 deficiency was crossed with GMR-GAL4; UAS-debcl#26 (data not shown).
Recently, a mutation in the Drosophila Apaf1/ced4 homologue, dark, has been described (Rodriguez et al. 1999). To assess the effect of reducing the dosage of dark on the GMR-GAL4; UAS-debcl#26 eye phenotype, a hypomorphic allele of dark (darkCD8) was crossed to GMR-GAL4/CyO; UAS-debcl#26/TM6B flies. As shown in Fig. 6 H, reducing the dosage of dark suppressed the rough eye phenotype of GMR-GAL4; UAS-debcl#26 flies. Therefore, dark genetically interacts with debcl.
Debcl Interacts with Bcl-2 and its Prosurvival Homologues
Since Debcl induces cell death, which is partly inhibited by the overexpression of Bcl-2 and Bcl-xL, an attractive hypothesis is that Debcl binds to and neutralizes prosurvival Bcl-2 homologues. Because no prosurvival Bcl-2–like proteins have been identified so far in Drosophila, we tested if Debcl can bind to any of the known mammalian or viral prosurvival homologues of Bcl-2. In transient overexpression experiments in mammalian cells, Debcl associated with Bcl-2 and most of its functional homologues, although the binding to Bcl-xL, Mcl-1, and adenovirus E1B19K protein was relatively weaker (Fig. 7). For these interaction studies, we used a method involving radiolabeled cell extracts that allows the simultaneous detection of two interacting proteins in the same sample (Fig. 7 A). We also used conventional immunoblotting of the immunoprecipitated proteins and obtained similar results (Fig. 7 B). These data clearly show that Debcl can interact with most of the known prosurvival Bcl-2 proteins and is likely to induce cell death by the same molecular mechanisms as other proapoptotic Bcl-2–related proteins. These results further provide evidence for the functional conservation of Bax-like proteins in mammals and flies. We also tested whether Debcl interacts with the proapoptotic members of Bcl-2 family. In coimmunoprecipitation experiments, Debcl did not associate with any of the BH3-only proteins (Bik, Bid, Bad, and Bim) or the BH1-, BH2-, and BH3- containing proteins (Bax and Bak; data not shown).
Figure 7.
Debcl interacts with multiple prosurvival Bcl-2 family members. HA-tagged Debcl protein was coexpressed with the control vector or FLAG-tagged Bcl-2 family protein and a P35 expression vector. A, 35S-labeled cell lysates were immunoprecipitated (IP) with an isotype-matched control antibody (top), an αHA antibody (middle), or an αFLAG antibody (3rd panel). In the lower panel, extracts from untransfected 293T cells were immunoprecipitated with the control, αHA, and αFLAG antibodies to determine nonspecific interactions. B, Further immunoblot analyses of immunoprecipitates were carried out to confirm the identity of various tagged proteins in transfected cells. In these experiments, unlabeled lysates prepared from transfected cells were used for immunoblotting (WB) and IPs. The top two panels depict the same blot probed sequentially with the rat αHA and the mouse αFLAG antibodies, respectively. In the αFLAG panel, the faint band around 35 kD is residual Debcl signal in the stripped blot. In the bottom two panels, mouse αFLAG immunoprecipitated proteins were immunoblotted with the rat αHA and the mouse αFLAG antibodies, respectively. The Ig light (L) and heavy (H) chain bands are indicated. Note that while αFLAG antibody can pull down both FLAG-tagged proteins (indicated by *) and the associated Debcl protein, αHA antibody mostly immunoprecipitates HA-tagged Debcl, not the associated proteins (not shown). This result suggests that the binding of αHA antibody to HA-Debcl precludes interaction between Debcl and Bcl-2 family members.
Debcl Is Required for Embryonic Cell Death
Currently, no specific debcl mutants are available. Therefore, to examine the in vivo function of Debcl, we carried out RNAi studies to inhibit debcl gene function. RNAi is a powerful technique to disrupt the function of specific genes (Hunter 1999). Additionally, RNAi has the advantage of ablating maternally contributed mRNA that is difficult to achieve genetically. It was originally used in C. elegans, but recently has been successfully applied to Drosophila (Bhat et al. 1999; Misquitta and Paterson 1999). Precellularized embryos were injected with debcl double-stranded RNA, aged until stage 16, and analyzed by TUNEL staining. As shown in Fig. 8B–E, debcl RNAi resulted in a large reduction in TUNEL positive cells in the embryos. We analyzed at least 200 embryos injected with the debcl double-stranded RNA and, in all cases, a 50–90% reduction in TUNEL positive cells was evident. In control experiments, embryos injected with the injection buffer did not show any inhibition of apoptosis (as in Fig. 8 F). In fact, most of the buffer-injected embryos showed slightly higher numbers of TUNEL positive cells, as compared with uninjected embryos (compare Fig. 8A and Fig. F). These RNAi results indicate that debcl gene function is required for programed cell death in the embryos. Additionally, these data suggest that maternally deposited debcl mRNA may be required for normal cell death in fly embryos.
Figure 8.
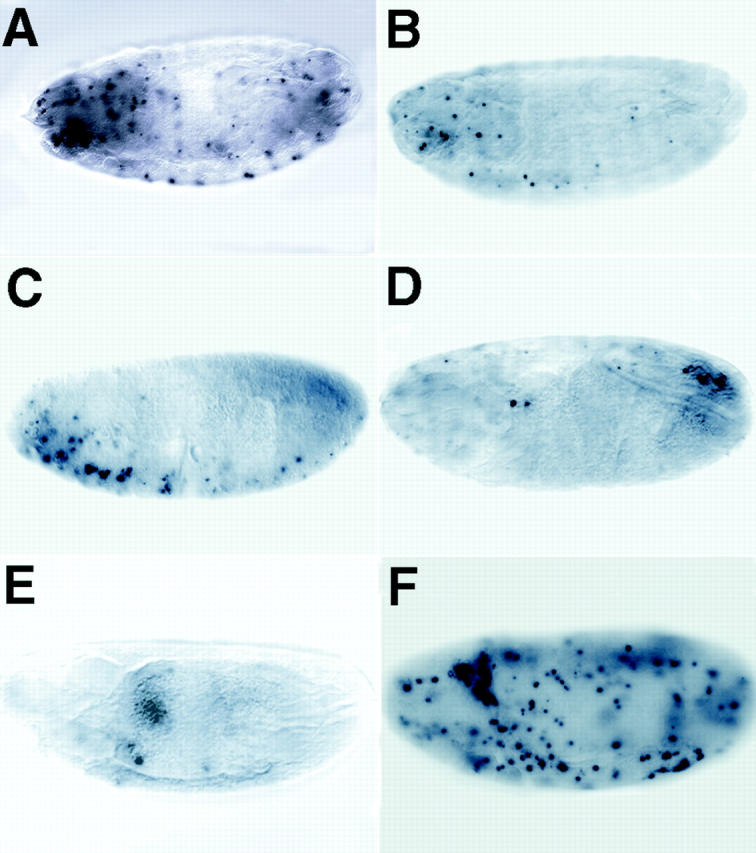
debcl is required for developmental cell death in embryos. RNAi was used to ablate debcl function in embryos. Precellularized embryos were injected with double-stranded debcl RNA and aged to stage 16 before fixation and TUNEL labeling. A, An uninjected control embryo showing the normal pattern of TUNEL labeling. B–E, Typical examples of injected embryos from the debcl RNAi experiment showing that the number of TUNEL positive cells is dramatically reduced compared with the control (A; see Fig. 3 H). F, A buffer-injected control shows that the injection procedure does not inhibit apoptosis, but instead an increase in TUNEL positive cells is observed (compare A and F).
Discussion
In this paper, we have described the identification of two Bcl-2 homologues in Drosophila. We have shown that one of these, Debcl, is a proapoptotic protein. Given that the existence of a proapoptotic Bcl-2 protein in Drosophila is now established, it can be envisaged that antiapoptotic Bcl-2 proteins are also present in insects. Unlike in C. elegans, which has a single Bcl-2 homologue, CED-9, Drosophila contains at least two such proteins. Interestingly, Debcl-like proteins, which are structurally similar to the mammalian Bax subclass, are not found in C. elegans. Also, considering that Drosophila contains at least six caspases, most of which have been implicated in apoptosis execution, it is likely that the degree of complexity of apoptotic pathways in the fly is much closer to that in mammals than in the worm.
Although debcl expression is low during embryonic and larval development, the expression appears to correlate with cell death in various tissues. With the exception of early embryos, our TUNEL data suggest that debcl may be expressed mainly in the cells that are destined to die. The function of higher debcl mRNA levels in early embryos is currently not known. Transgenic experiments show that ectopic Debcl expression is accompanied by potent apoptosis induction. This may explain why debcl expression is mostly limited to cells that are committed to undergo programed cell death during development. The closest mammalian relative of Debcl, Bok, is mainly expressed in adult reproductive tissues (Hsu et al. 1997). In adult female flies, debcl expression is relatively high and may be mainly contributed by the ovaries. Thus, debcl may function throughout embryonic and larval development, and also in the apoptosis of nurse cells in the adult ovaries. Our in vitro and in vivo data with P35 clearly shows that _debcl_-induced cell death is caspase-dependent and that Debcl lies upstream of caspases in the death pathway. Additionally, an inhibition of debcl eye phenotype in dark mutant flies further substantiates the finding that Debcl lies upstream of the Dark-mediated caspase activation pathway (Fig. 9). Given that debcl RNAi suppresses most of the programed cell death in embryos, debcl is likely to be a critical regulator of cell death during embryogenesis. Since embryos injected with the debcl double-stranded RNA do not progress into larval development, it was not possible to study the function of debcl later in development using RNAi technique.
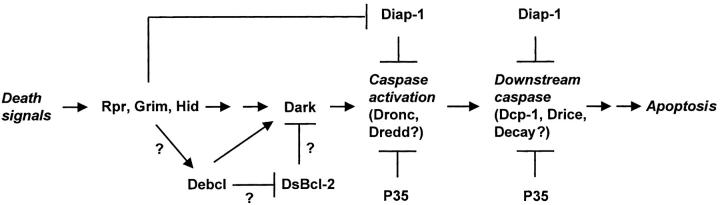
Figure 9.
Possible position of Debcl in the Drosophila cell death pathway leading to caspase activation. Several genetic and biochemical studies have established the possible hierarchy between various components of the Drosophila cell death machinery (reviewed in Abrams 1999). The studies described in this paper place Debcl upstream of the P35-inhibitable, Dark-mediated caspase activation pathway. Debcl may lie downstream of the proteins of the H99 complex (Reaper, Grim, and Hid). However, further experiments are required to firmly place Debcl and H99 in the same genetic pathway. Similar to mammalian death pathways, Debcl may function by antagonizing a yet undiscovered prosurvival Bcl-2–like protein(s) (shown as DsBcl-2) in Drosophila.
Our genetic data show that gene dosage of rpr, hid, and grim have no effect on Debcl-induced apoptosis. This suggests that debcl either lies downstream of the H99 genes or on a separate pathway (summarized in Fig. 9). Our data also show that diap1, but not diap2, genetically interacts with debcl. Since diap1's function as a prosurvival gene is antagonized by the genes of H99 complex (Wang et al. 1999), it is possible that debcl is a component of this pathway (Fig. 9). On the other hand, as diap1 is known to inhibit caspases (Wang et al. 1999), the enhancement of debcl transgenic phenotype when diap1 dosage is halved simply may be due to reduced caspase inhibition in these flies. Future studies involving generation of debcl mutant flies and crossing these flies with rpr, hid, and grim transgenic flies should address whether the H99 genes and debcl indeed lie in the same death pathway.
Debcl binds to many of the mammalian prosurvival Bcl-2 homologues. Thus, it is likely that Debcl functions in a manner analogous to the mammalian or worm proapoptotic Bcl-2–like proteins (Adams and Cory 1998; Gross et al. 1999). These proteins probably act by binding to, and neutralizing the activity of, Bcl-2 or its closest relatives. Bcl-2 and its putative Drosophila homologue(s) probably control the activation of initiator caspases by targeting the adaptor molecules, such as Apaf-1/CED-4/Dark, by regulating the release of apoptogenic factors, such as cytochrome c from the mitochondria (Green and Reed 1998). Bcl-2 or its homologues may perform this function by maintaining organelle integrity, possibly by regulating the mitochondrial membrane pores, such as those containing voltage-dependent anion channel (VDAC; Shimizu et al. 1999), or by controlling a CED-4/CED-3 containing “apoptosome” complex (Hengartner 1997). Most proapoptotic proteins are normally sequestered away from Bcl-2 until receipt of a death stimulus (Adams and Cory 1998; Gross et al. 1999).
An additional level of complexity in mammals is provided by observations that some proapoptotic Bcl-2 relatives, such as Bax and Bak that contain the BH1, BH2, and BH3 regions, may induce caspase-independent death (Xiang et al. 1996; Antonsson et al. 1997), possibly by forming pores in membranes (Antonsson et al. 1997). Although Debcl belongs to this subclass of proteins, as discussed above, our studies indicate that Debcl-induced apoptosis is caspase-dependent. Mutation data indicate that the BH3 domain of Debcl is essential for Debcl-mediated cell killing. It is possible that BH3-mediated interactions with prosurvival members of the Bcl-2 family are necessary for the proapoptotic function of Debcl, in a manner similar to BH3-only proteins (Adams and Cory 1998; Gross et al. 1999).
The prosurvival Bcl-2 proteins have not been reported in Drosophila to date. One possible candidate may be the putative product of the gene at 48A-E locus (Fig. 1). The available cDNA and genomic sequence can encode a putative protein with BH1, BH2, and BH3 domains (Fig. 1 D) similar to Debcl. As we do not currently have information about the NH2-terminal region of this protein, we do not know if the 48A-E protein contains a BH4-like region. Further analysis will determine whether the 48A-E protein is a proapoptotic or a prosurvival Bcl-2 homologue in the fly. Given that Drosophila homologues of CED-3, CED-4, and CED-9 have now been discovered, it is likely that flies also contain BH3-only proteins, such as EGL-1.
Acknowledgments
We thank J. Beaumont for technical assistance, J. Abrams for dark mutant flies, D. Vaux for MIHA cDNA, Y.N. Jan for 109-88-GAL4 fly stocks, K. White for TUNEL protocol, U. Theopold for help with SL2 cells, and W. Sullivan for protocols on RNAi.
This work was supported by the Wellcome Trust and the National Health and Medical Research Council. S. Kumar and H. Richardson are Wellcome Senior Fellows in Medical Science. D.C.S. Huang is a Special Fellow of the Leukemia Society of America.
Footnotes
Paul A. Colussi and Leonie M. Quinn contributed equally to this work.
Abbreviations used in this paper: BH domain, Bcl-2 homology domain; GFP, Aequorea victoria green fluorescent protein; RNAi, RNA interference; RT, reverse transcriptase; SL2 cells, Schneider L2 cells.
References
- Abrams J.M. An emerging blueprint for apoptosis in Drosophila . Trends Cell Biol. 1999;9:435–440. doi: 10.1016/s0962-8924(99)01646-3. [DOI] [PubMed] [Google Scholar]
- Abrams J.M., White K., Fessler L.I., Steller H. Programmed cell death during Drosophila embryogenesis. Development. 1993;117:29–43. doi: 10.1242/dev.117.1.29. [DOI] [PubMed] [Google Scholar]
- Adams J.M., Cory S. The Bcl-2 protein familyarbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Antonsson B., Conti F., Ciavatta A., Montessuit S., Lewis S., Martinou I., Bernasconi L., Bernard A., Mermod J.J., Mazzei G. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- Bhat M.A., Izaddoost S., Lu Y., Cho K.O., Choi K.W., Bellen H.J. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–845. doi: 10.1016/s0092-8674(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bunch T.A., Grinblat Y., Goldstein L.S. Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids Res. 1988;16:1043–1061. doi: 10.1093/nar/16.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Nordstrom W., Gish B., Abrams J.M. grim, a novel cell death gene in Drosophila . Genes Dev. 1996;10:1773–1782. doi: 10.1101/gad.10.14.1773. [DOI] [PubMed] [Google Scholar]
- Chen P., Rodriguez A., Erskine R., Thach T., Abrams J.M. Dredd, a novel effector of the apoptosis activators reaper, grim, and hid in Drosophila . Dev. Biol. 1998;201:202–216. doi: 10.1006/dbio.1998.9000. [DOI] [PubMed] [Google Scholar]
- Claveria C., Albar J.P., Serrano A., Buesa J.M., Barbero J.L., Martinez A.C., Torres M. Drosophila grim induces apoptosis in mammalian cells. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:7199–7208. doi: 10.1093/emboj/17.24.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt B., Horvitz H.R. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2–like protein CED-9. Cell. 1998;93:519–529. doi: 10.1016/s0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- Cryns V., Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- Dorstyn L., Kumar S. Differential inhibitory effects of CrmA, P35, IAP and three mammalian IAP homologs on apoptosis in NIH-3T3 cells following various death stimuli. Cell Death Differ. 1997;4:570–579. doi: 10.1038/sj.cdd.4400281. [DOI] [PubMed] [Google Scholar]
- Dorstyn L., Colussi P.A., Quinn L.M., Richardson H., Kumar S. DRONC, an ecdysone-inducible Drosophila caspase Proc. Natl. Acad. Sci. USA. 96 1999. 4307 4312a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorstyn L., Read S.H., Quinn L.M., Richardson H., Kumar S. DECAY, a novel Drosophila caspase related to mammalian caspase-3 and caspase-7 J. Biol. Chem 274 1999. 30778 30783b [DOI] [PubMed] [Google Scholar]
- Ekert P.G., Silke J., Vaux D.L. Caspase inhibitors. Cell Death Differ. 1999;6:1081–1086. doi: 10.1038/sj.cdd.4400594. [DOI] [PubMed] [Google Scholar]
- Ellis M.C., O'Neill E.M., Rubin G.M. Expression of Drosophila glass protein and evidence for negative regulation of its activity in non-neuronal cells by another DNA-binding protein. Development. 1993;119:855–865. doi: 10.1242/dev.119.3.855. [DOI] [PubMed] [Google Scholar]
- Foley K., Cooley L. Apoptosis in late stage Drosophila nurse cells does not require genes within the H99 deficiency. Development. 1998;125:1075–1082. doi: 10.1242/dev.125.6.1075. [DOI] [PubMed] [Google Scholar]
- Fraser A.G., Evan G.I. Identification of a Drosophila melanogaster ICE/CED-3–related protease, drICE. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2805–2813. doi: 10.1093/emboj/16.10.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.R., Reed J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Grether M.E., Abrams J.M., Agapite J., White K., Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- Gross A., McDonnell J.M., Korsmeyer S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Haining W.N., Carboy-Newcomb C., Wei C.L., Steller H. The proapoptotic function of Drosophila Hid is conserved in mammalian cells. Proc. Natl. Acad. Sci. USA. 1999;96:4936–4941. doi: 10.1073/pnas.96.9.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck B., Gehring W.J., Walldorf U. Functional analysis of an eye specific enhancer of the eyeless gene in Drosophila . Proc. Natl. Acad. Sci. USA. 1999;96:564–569. doi: 10.1073/pnas.96.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner M.O. Apoptosis. CED-4 is a stranger no more. Nature. 1997;388:714–715. doi: 10.1038/41873. [DOI] [PubMed] [Google Scholar]
- Hengartner M.O., Horvitz H.R. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994;76:665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- Hsu S.Y., Kaipia A., McGee E., Lomeli M., Hsueh A.J. Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc. Natl. Acad. Sci. USA. 1997;94:12401–12406. doi: 10.1073/pnas.94.23.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D.C., O'Reilly L.A., Strasser A., Cory S. The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:4628–4638. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C.P. Geneticsa touch of elegance with RNAi. Curr. Biol. 1999;9:R440–R442. doi: 10.1016/s0960-9822(99)80276-0. [DOI] [PubMed] [Google Scholar]
- Inohara N., Koseki T., Hu Y., Chen S., Nunez G. CLARP, a death effector domain-containing protein interacts with caspase-8 and regulates apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:10717–10722. doi: 10.1073/pnas.94.20.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M.D., Weil M., Raff M.C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Kanuka H., Sawamoto K., Inohara N., Matsuno K., Okano H., Miura M. Control of the cell death pathway by Dapaf-1, a Drosophila Apaf-1/CED-4–related caspase activator. Mol. Cell. 1999;4:757–769. doi: 10.1016/s1097-2765(00)80386-x. [DOI] [PubMed] [Google Scholar]
- Kumar S. Mechanisms mediating caspase activation in cell death. Cell Death Differ. 1999;6:1060–1066. doi: 10.1038/sj.cdd.4400600. [DOI] [PubMed] [Google Scholar]
- Kumar S., Colussi P.A. Prodomains-adaptors-oligomerizationthe pursuit of caspase activation in apoptosis. Trends Biochem. Sci. 1999;24:1–4. doi: 10.1016/s0968-0004(98)01332-2. [DOI] [PubMed] [Google Scholar]
- Kumar S., Kinoshita M., Noda M., Copeland N.G., Jenkins N.A. Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1 beta-converting enzyme. Genes Dev. 1994;8:1613–1626. doi: 10.1101/gad.8.14.1613. [DOI] [PubMed] [Google Scholar]
- Li P., Nijhawan D., Budihardjo I., Srinivasula S.M., Ahmad M., Alnemri E.S., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- McCarthy J.V., Dixit V.M. Apoptosis induced by Drosophila reaper and grim in a human system. Attenuation by inhibitor of apoptosis proteins (cIAPs) J. Biol. Chem. 1998;273:24009–24015. doi: 10.1074/jbc.273.37.24009. [DOI] [PubMed] [Google Scholar]
- Metzstein M.M., Stanfield G.M., Horvitz H.R. Genetics of programmed cell death in C. eleganspast, present and future. Trends Genet. 1998;14:410–416. doi: 10.1016/s0168-9525(98)01573-x. [DOI] [PubMed] [Google Scholar]
- Misquitta L., Paterson B.M. Targeted disruption of gene function in Drosophila by RNA interference (RNA-i)a role for nautilus in embryonic somatic muscle formation. Proc. Natl. Acad. Sci. USA. 1999;96:1451–1456. doi: 10.1073/pnas.96.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriishi K., Huang D.C., Cory S., Adams J.M. Bcl-2 family members do not inhibit apoptosis by binding the caspase activator Apaf-1. Proc. Natl. Acad. Sci. USA. 1999;96:9683–9688. doi: 10.1073/pnas.96.17.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson D.W. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- Richardson H., O'Keefe L.V., Marty T., Saint R. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development. 1995;121:3371–3379. doi: 10.1242/dev.121.10.3371. [DOI] [PubMed] [Google Scholar]
- Rodriguez A., Oliver H., Zou H., Chen P., Wang X., Abrams J.M. Dark is a Drosophila homolog of Apaf-1/CED-4 and functions in an evolutionarily conserved death pathway. Nat. Cell Biol. 1999;1:272–279. doi: 10.1038/12984. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Narita M., Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Song Z., McCall K., Steller H. DCP-1, a Drosophila cell death protease essential for development. Science. 1997;275:536–540. doi: 10.1126/science.275.5299.536. [DOI] [PubMed] [Google Scholar]
- Uren A.G., Pakusch M., Hawkins C.J., Puls K.L., Vaux D.L. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc. Natl. Acad. Sci. USA. 1996;93:4974–4978. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D.L., Korsmeyer S.J. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Wang S.L., Hawkins C.J., Yoo S.J., Müller H.-A.J., Hay B.A. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell. 1999;98:453–463. doi: 10.1016/s0092-8674(00)81974-1. [DOI] [PubMed] [Google Scholar]
- White K., Grether M.E., Abrams J.M., Young L., Farrell K., Steller H. Genetic control of programmed cell death in Drosophila . Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- Xiang J., Chao D.T., Korsmeyer S.J. BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Chang H.Y., Baltimore D. Essential role of CED-4 oligomerization in CED-3 activation and apoptosis. Science. 1998;281:1355–1357. doi: 10.1126/science.281.5381.1355. [DOI] [PubMed] [Google Scholar]
- Yuan J., Horvitz H.R. The Caenorhabditis elegans cell death gene ced-4 encodes a novel protein and is expressed during the period of extensive programmed cell death. Development. 1992;116:309–320. doi: 10.1242/dev.116.2.309. [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H.M., Horvitz H.R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zou H., Henzel W.J., Liu X., Lutschg A., Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- Zou L., Song Z., Tittel J., Steller H. HAC-1, a Drosophila homolog of Apaf-1 and CED-4 functions in developmental and radiation-induced apoptosis. Mol. Cell. 1999;4:745–755. doi: 10.1016/s1097-2765(00)80385-8. [DOI] [PubMed] [Google Scholar]