Peritoneal macrophages express both P-selectin and PSGL-1 (original) (raw)
Abstract
Macrophages, phagocytic cells involved in an early phase of host defense, are known to express the P-selectin ligand, PSGL-1. Heretofore, P-selectin has only been found on platelets and endothelial cells. Here, we demonstrate that peritoneal macrophages isolated by peritoneal lavage of unchallenged mice express P-selectin on the plasma membrane. The peritoneal macrophages synthesize P-selectin, as indicated by metabolic labeling experiments. P-Selectin is constitutively expressed on the extracellular surface of macrophages but is only partially colocalized with PSGL-1. P-Selectin is rapidly translocated from the macrophage plasma membrane to intracellular vesicles and to lysosomes. Peritoneal macrophages assemble into cell strings under flow conditions based upon macrophage–macrophage interactions mediated by P-selectin and PSGL-1. This is the first description of a leukocyte shown to express both P-selectin and PSGL-1.
Keywords: secondary interactions; endocytosis; trafficking; lymphatics
Introduction
Macrophages, derived from monocytes, are an important component of the host defense system. In response to an inflammatory stimulus circulating monocytes migrate out of the blood stream into sites of injury where they differentiate into inflammatory macrophages. Monocytes also continuously emigrate into peripheral tissues, even in the absence of any inflammatory stimulus (van Furth and Cohn, 1968). Recent experiments suggest that these monocytes can also become tissue macrophages (Randolph et al., 1998, 1999). Upon activation by immunologic stimuli, these resident macrophages exit the tissue and transmigrate to the lymphatic system for antigen presentation. Resident alveolar macrophages are able to translocate particulate antigens to the paracortical T cell area of draining lymph nodes (Thepen et al., 1993). During the antigen sensitization phase of contact hypersensitivity, resident dermal macrophages migrate to regional lymph nodes and contribute to the efficiency of sensitization (Sato et al., 1998).
Peritoneal macrophages are a population of resident mononuclear phagocytes of unknown function. In the first 2 h after induction of inflammation, the number of resident macrophages in the peritoneal cavity significantly decreases, suggesting their emigration out of the peritoneal cavity (Haskill and Becker, 1985; Barth et al., 1995). Activated resident peritoneal macrophages migrate into parathymic lymph nodes and to the gut-associated lymphoid tissues (Rosen and Gordon, 1990; Sminia et al., 1995; van Vugt et al., 1995; Bellingan et al., 1996). Adhesion molecules including L-selectin, VLA-4, Mac-1, LFA-1, and PSGL-1 that potentially mediate the interaction of monocytes under flow have been extensively studied (Luscinskas et al., 1994, 1996; Gerszten et al., 1998; Lim et al., 1998) in contrast to the interactions of macrophages with adhesive molecules under flow. Here, we demonstrate that mouse peritoneal macrophages adhere to VCAM-1 under conditions of laminar flow. We also demonstrate that homotypic interactions among macrophages occur in laminar flow and that these interactions are mediated by PSGL-1 and P-selectin on the surface of the peritoneal macrophages. P-Selectin expression has heretofore been thought to be restricted to platelets and endothelial cells. These studies are the first demonstration that a specific leukocyte subtype, resident peritoneal macrophages, can express functional P-selectin.
Results
Peritoneal macrophages bind to VCAM-1 during laminar flow
To determine if peritoneal macrophages bind to an adhesive surface under conditions of laminar flow, isolated mouse peritoneal leukocytes were perfused at 0.5 dynes/cm2 over a VCAM-1–coated surface in a parallel flow chamber. The binding was calcium dependent and was completely inhibited by the presence of 5 mM EDTA. Interaction between adherent and flowing cells results in linear arrays of peritoneal leukocytes parallel to the direction of flow (Fig. 1, A and B, WT). Leukocytes roll over an adherent cell and bind to it on the downstream side. The peritoneal leukocytes forming strings consist entirely of adherent macrophages. All the cells in the string-like clusters stain with F4/80 antibodies that recognize a macrophage-specific antigen (Fig. 1 A, inset, WT). Similar string-like clusters have been observed when monocytes are exposed to laminar flow over an adhesive surface (Luscinskas et al., 1994; Alon et al., 1996; Walcheck et al., 1996; Lim et al., 1998).
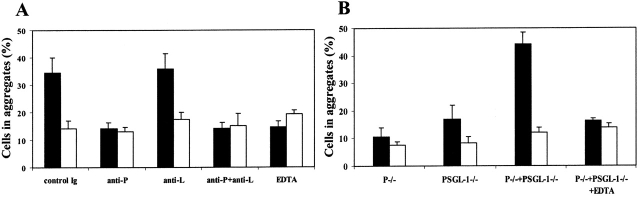
Figure 1.

Secondary interactions between peritoneal macrophages under flow are mediated by PSGL-1 and P-selectin. Leukocytes isolated by peritoneal lavage from mice in the absence of an inflammatory stimulus were perfused into a flow chamber containing a VCAM-1–coated surface for 5 min at 0.5 dyn/cm2 shear stress. Formation of cell strings was observed (A) and quantitated (B). Peritoneal macrophages from wild-type mice, WT; L-selectin null mice, L−/−; P-selectin null mice, P−/−; PSGl-1 null mice, PSGL-1−/− or a mixture of peritoneal macrophages from both P-selectin null mice and PSGL-1 null mice, P−/− + PSGL-1−/−, were introduced into the flow chamber. (A) WT inset, detection of cell strings with Texas red–conjugated F4/80 antibodies; P−/− + PSGL-1−/− inset, peritoneal cells from P-selectin null mice were detected as in A. Peritoneal cells from PSGL-1 null mice were detected with FITC-conjugated F4/80 antibody and the two cell populations mixed. (B) The percentage of macrophages in a microscope field that form strings is indicated. Values are means ± SEM. *, indicates P < 0.001.
The secondary interactions that lead to string-like formations among monocytes rolling on and adhering to selectins, VCAM-1, or inflamed endothelium are blocked with anti–PSGL-1 or anti–L-selectin antibodies (Alon et al., 1996; Lim et al., 1998). To explore whether L-selectin and PSGL-1 mediate this phenomenon in macrophages, we used peritoneal leukocytes from PSGL-1 null or L-selectin null mice to evaluate the role of these adhesion molecules in secondary interactions among peritoneal macrophages adhering to a VCAM-1–coated surface. String formation was unaffected by the absence of L-selectin (Fig. 1, A and B, L−/−). In contrast string formation by peritoneal macrophages was essentially eliminated in the absence of PSGL-1 (Fig. 1, A and B, PSGL1−/−). Because P-selectin is the predominant PSGL-1 ligand in a variety of leukocyte cell adhesion processes in vivo (Yang et al., 1999; Hirata et al., 2000), we examined the properties of peritoneal leukocytes from P-selectin null mice during perfusion over a VCAM-1–coated surface. P-Selectin null leukocytes bind to the VCAM-1 surface but nucleation by adherent macrophages to form cell strings was decreased ∼80–85% (Fig. 1, A and B, P−/−). These results indicate that string formation is independent of L-selectin at a shear stress of 0.5 dyn/cm2, but requires P-selectin and/or PSGL-1.
To demonstrate that nucleation is mediated by interaction of P-selectin and PSGL-1, we perfused a mixture of PSGL-1 null and P-selectin null leukocytes over a VCAM-1-coated surface. Combining the PSGL-1 null and P-selectin null macrophages restored cell string formation (Fig. 1, A and B, P−/− + PSGL1−/−). To observe the distribution of P-selectin–deficient cells and PSGL-1–deficient cells in strings, we labeled cells with two different fluorescent dyes. Strings were formed from cells of the two genotypes, P-selectin null macrophages (green) and PSGL-1 null macrophages (red) (Fig. 1 A, inset, P−/− + PSGL1−/−). Thus, PSGL-1 and P-selectin appear to be the major ligand–receptor pair mediating secondary interactions between resident peritoneal macrophages under these conditions.
Resident macrophages isolated from the mouse peritoneum have surface P-selectin
To date P-selectin has been observed only in platelets and endothelial cells (Hsu-Lin et al., 1984; Stenberg et al., 1985; Berman et al., 1986; Bonfanti et al., 1989; McEver et al., 1989). We used flow cytometry to confirm the presence of P-selectin on peritoneal leukocytes. Monoclonal rat anti–P-selectin antibodies bound peritoneal leukocytes (Fig. 2 A) indicating that the mouse peritoneum contains a population of P-selectin–positive leukocytes in the absence of an inflammatory stimulus. In control experiments with peritoneal macrophages from P-selectin–deficient mice, no cell labeling was observed.
Figure 2.
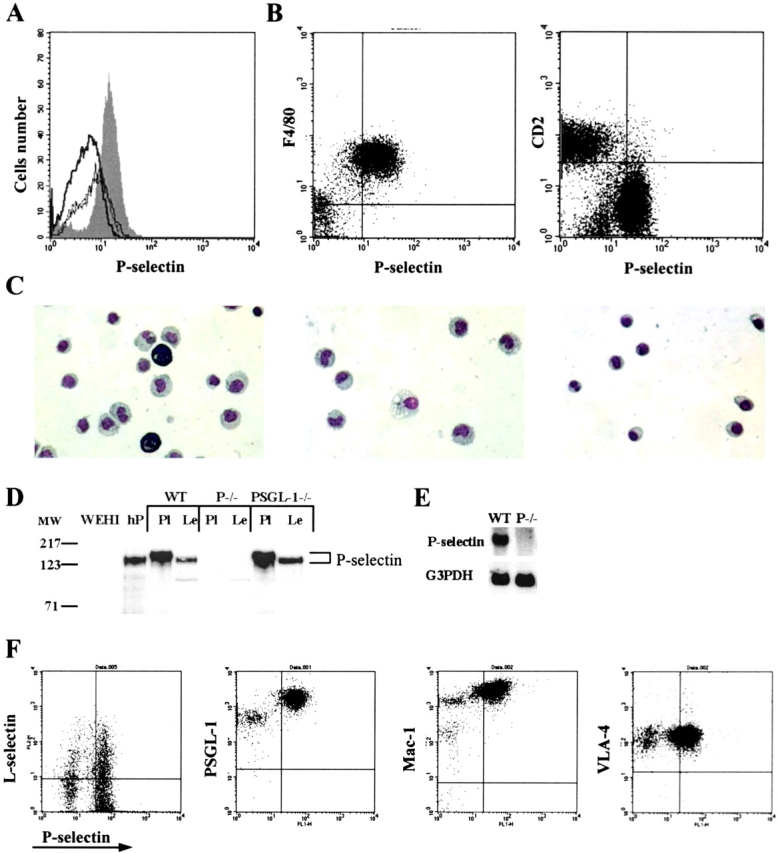
Mouse peritoneal macrophages express P-selectin. Flow cytometry was performed on peritoneal cells freshly isolated from wild-type and P-selectin null mice. (A) Peritoneal leukocytes were labeled with a rat monoclonal anti–P-selectin antibody or with an isotype-matched rat IgG. Cells from wild-type mice labeled with anti–P-selectin antibody (shaded), cells from P-selectin null mice labeled with anti–P-selectin antibody (thin line); cells from wild-type mice labeled with isotype-matched rat IgG (thick line), cells from P-selectin null mice labeled with isotype-matched IgG (dashed line). (B) Peritoneal leukocytes were labeled simultaneously with FITC-conjugated rat monoclonal anti–P-selectin antibodies and with either PE-conjugated rat anti-F4/80 antibodies or PE-conjugated rat anti-CD2 antibodies. (C) Peritoneal cells labeled with FITC-conjugated anti–P-selectin antibodies and separated on a cell sorter according to P-selectin expression were stained with Wright-Giemsa stain and visualized by light microscopy. Left, unfractionated cells; center, P-selectin–positive cells; right, P-selectin–negative cells. (D) Proteins in the lysate from platelets (108) and peritoneal leukocytes (4 × 106) were separated by electrophoresis on 7% SDS-gels under reducing conditions and the proteins transferred to a PVDF membrane. P-Selectin was detected using rabbit antibodies to the cytoplasmic tail of P-selectin and HRP-conjugated goat anti–rabbit IgG. WT, wild-type mice; P−/−, P-selectin null mice; PSGL-1−/−, PSGL-1 null mice; hP, purified human P-selectin (0.1 μg); WEHI, lysate from 4 × 106 WEHI cells; Pl, platelet lysate; Le, peritoneal leukocyte lysate. (E) Total mRNA was isolated from peritoneal leukocytes of wild-type (WT) or P-selectin null (P−/−) mice, the mRNA species separated by gel electrophoresis and transferred to a PVDF membrane. The P-selectin band was detected using radiolabeled probe complementary to exon 3 of mouse P-selectin. (F) Peritoneal leukocytes were labeled simultaneously with FITC-conjugated rat monoclonal anti–P-selectin antibodies and with PE-conjugated rat anti–L-selectin antibodies, PE-conjugated anti–PSGL-1 antibodies, PE-conjugated anti-Mac1 antibodies or PE-conjugated rat anti–VLA-4 antibodies.
To confirm that the peritoneal cells bearing P-selectin are macrophages, we studied the interaction of these cells with antibodies specific for several leukocyte populations. P-Selectin was expressed only by F4/80-positive cells, a population that corresponds to a macrophage subset of leukocytes (Fig. 2 B). In contrast, peritoneal leukocytes that were positive for P-selectin were negative for CD2 that is expressed on peripheral B cells, T cells, and natural killer cells (Fig. 2 B).
To confirm that the P-selectin–positive cells were macrophages, we observed their morphology by light microscopy. Peritoneal cells were isolated and the P-selectin–positive and –negative fractions separated on a FACsorter™ with the aid of P-selectin antibodies. The total cell population (Fig. 2 C, left) and the fractionated cells were stained with Wright-Giemsa stain and analyzed by light microscopy. P-Selectin–positive cells had the morphological features of macrophages (Fig. 2 C, center), whereas P-selectin–negative cells appeared to be lymphocytic or plasmacytic in origin (Fig. 2 C, right). Thus, the P-selectin–positive cells isolated from the peritoneal cavity of mice appear to be macrophages.
We examined lysates of peritoneal leukocytes by Western blot analysis to confirm the presence of P-selectin and to determine its molecular weight. The P-selectin in peritoneal leukocytes was compared with P-selectin in lysates from mouse platelets and P-selectin purified from human platelets. The results of this analysis confirmed the presence of P-selectin on mouse peritoneal macrophages (Fig. 2 D). Anti–P-selectin antibodies stained bands of ∼140,000 molecular weight in platelet lysates and in peritoneal leukocyte lysates from wild-type and PSGL-1–deficient mice. P-Selectin m-RNA was also identified in peritoneal leukocytes from wild-type mice, but not P-selectin null mice, by Northern blot analysis (Fig. 2 E).
L-selectin is expressed on neutrophils, monocytes, and most lymphocytes, whereas PSGL-1 is expressed on essentially all blood leukocytes. We explored whether L-selectin and PSGL-1 were coexpressed with P-selectin on the surface of peritoneal macrophages. A subset of P-selectin bearing peritoneal macrophages also express L-selectin, whereas essentially all of the P-selectin bearing macrophages also express PSGL-1 (Fig. 2 F). P-Selectin bearing peritoneal macrophages express the integrins MAC-1 and VLA-1, important for monocyte trafficking (Fig. 2 F).
P-Selectin is synthesized in the peritoneal macrophage
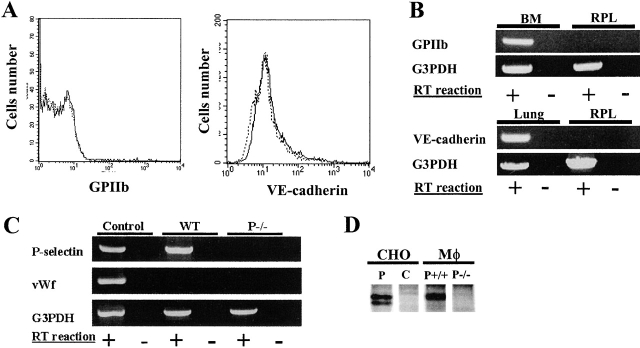
P-Selectin is present on the surface of activated platelets and activated endothelial cells (Hsu-Lin et al., 1984; Stenberg et al., 1985; Sims et al., 1988; Bonfanti et al., 1989; McEver et al., 1989). We explored whether peritoneal macrophage P-selectin might arise from contamination with activated platelets or platelet-derived or endothelial cell–derived microparticles bound to the PSGL-1 bearing leukocytes. Neither a platelet-specific marker, GPIIb, nor an endothelial marker, VE-cadherin, is observed on the surface of peritoneal leukocytes by flow cytometry (Fig. 3 A). Similarly, mRNA for GPIIb or VE-cadherin was not detectable in peritoneal leukocytes by RT-PCR analysis of peritoneal cells (Fig. 3 B). However, using RT-PCR we were able to amplify a portion of P-selectin cDNA including the coding sequence from the lectin domain to the transmembrane domain, indicating that macrophage-associated P-selectin is the membrane bound and not the soluble form of the protein (Fig. 3 C; Johnston et al., 1990). We did not detect any mRNA for von Willebrand factor, which resides within the same intracellular compartments as P-selectin in endothelial cells and platelets (Fig. 3 C; Bonfanti et al., 1989).
Figure 3.
P-selectin is synthesized in peritoneal macrophages. (A) Flow cytometry was performed on peritoneal leukocytes freshly isolated from wild-type mice. Cells were probed with FITC-conjugated anti–glycoprotein IIb, FITC-conjugated rat anti–VE-cadherin or FITC-conjugated, isotype matched control IgG. (Left) Antiglycoprotein IIb, solid line, isotype matched IgG, dotted line. (Right) Anti–VE-cadherin, solid line; isotype matched IgG, dotted line. (B) Reverse transcription was used to prepare cDNA from resident peritoneal leukoctyes (RPL), bone marrow (BM), or lung. Gene-specific primers were used to amplify mRNA fragments for GPIIb, VE-cadherin, and G3PDH. C. Reverse transcription was used to prepare cDNA from total peritoneal leukocytes (WT) and total peritoneal leukocytes from P-selectin null mice (P−/−). Gene-specific primers were used to amplify mRNA fragments for P-selectin, glycerol phosphate dehydrogenase (G3PDH), and von Willebrand factor (vWf). RT reaction, reverse transcriptase. (D) Autoradiograph of metabolically labeled P-selectin immunoprecipitated with rabbit anti–P-selectin antibodies. CHO cells, Chinese hamster ovary cells; P, transfected with P-selectin; C, control CHO cells; MΦ, macrophages; P+/+, P-selectin expressing cells; P−/−, P-selectin null cells.
To directly demonstrate P-selectin synthesis by peritoneal macrophages, plastic-adherent macrophages from wild-type and P-selectin null mice, as well as CHO cells expressing P-selectin and untransfected CHO cells, were grown in media containing [35S]methionine and [35S]cysteine. A band of radiolabeled protein of the proper molecular weight was isolated from wild-type peritoneal macrophages and CHO cells expressing P-selectin using rabbit antibodies against the cytoplasmic tail of P-selectin (Fig. 3 D). Using this antibody no radiolabeled protein was isolated from peritoneal macrophages of P-selectin null mice or from untransfected CHO cells. Together, these results support the conclusion that peritoneal P-selectin is derived from peritoneal leukocytes and is not of platelet or endothelial cell origin.
P-Selectin undergoes internalization in macrophages
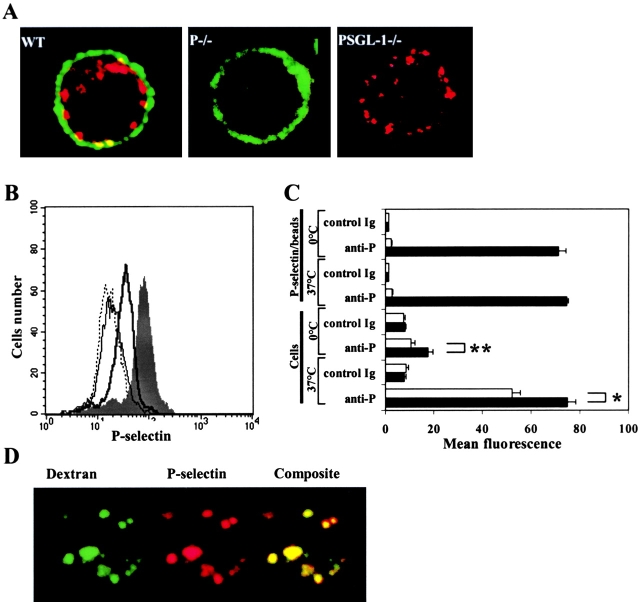
To address more precisely where P-selectin and PSGL-1 localize within peritoneal macrophages, we stained unfixed cells with anti–P-selectin antibodies labeled with Texas red and anti–PSGL-1 antibodies labeled with Alexa 488. Confocal microscopic analyses of stained macrophages show only small areas of colocalization of P-selectin and PSGL-1 (Fig. 4 A). Staining for PSGL-1 appears primarily on the periphery of the cell. P-Selectin is localized primarily in granule-like structures that appear to be beneath the cell surface although some P-selectin was observed on the cell surface. The granular appearance of the staining for P-selectin suggested that much of the P-selectin might be internalized.
Figure 4.
P-Selectin is present on the plasma membrane of peritoneal macrophages and is rapidly internalized. (A) Peritoneal cells from wild-type mice, and P-selectin null and PSGL-1 null mice were fixed and labeled with Texas red–conjugated rat anti–P-selectin antibodies (red) and Alexa 488–conjugated rat anti–PSGL-1 antibodies (green); merge (yellow). Wild-type mice, WT; P-selectin null mice, P−/−; PSGL-1 null mice, PSGL-1−/−. (B) Peritoneal leukocytes were incubated with FITC-conjugated rat anti–P-selectin antibody or with FITC-conjugated isotype matched antibody either at 0° ice or at 37°C and the cells analyzed by flow cytometry. Incubation with anti–P-selectin antibody at 0°, thick line; incubation with anti–P-selectin antibody at 37°C, shaded; incubation with isotype matched antibody at 0°, thin line; incubation with isotype matched antibody at 37°C, dotted line. (C) P-selectin–coated polystyrene microspheres or peritoneal leukocytes from wild-type mice were incubated with FITC-conjugated rat anti–P-selectin antibody or FITC-conjugated isotype matched antibody either at 0° or at 37°C. Microspheres and cells were divided and treated with buffer at pH 7.0 or pH 2.3 and subsequently analyzed for bound fluorescent antibody by flow cytometry. Closed bars, treatment with buffer at pH 7, open bars, treatment with buffer at pH 2.3. *, indicates P < 0.01; **, indicates P < 0.001. The values represent mean ± SEM. (D) Confocal micrographs of permeabilized peritoneal leukocytes from wild-type mice incubated with FITC-conjugated dextran (green) and Texas red–conjugated rat anti–P-selectin antibody (red).
Macrophages were stained with P-selectin either at 0°C or at 37°C. Labeling of peritoneal macrophages with rat anti–P-selectin antibody was strongly dependent on temperature (Fig. 4 B). Cells incubated at 37°C are more strongly labeled with anti–P-selectin than cells labeled at 0°C, suggesting accumulation of P-selectin–specific antibody within the cell. There is no temperature-dependent difference in staining of peritoneal macrophages with the isotype matched IgG. To distinguish surface-bound anti–P-selectin antibodies from those that have been internalized, cells labeled with anti–P-selectin antibodies were exposed to buffer at pH 2.3 or to buffer at pH 7.0. When cells stained with anti–P-selectin antibodies on ice were exposed to buffer at pH 2.3, staining due to anti–P-selectin was reduced almost to the background level observed with isotype matched IgG (Fig. 4 C). In contrast, peritoneal macrophages labeled with anti–P-selectin antibodies at 37°C retained considerable anti–P-selectin antibody when exposed to buffer at pH 2.3 (Fig. 4 C). These results suggest that P-selectin is actively internalized at 37°C and that bound anti–P-selectin antibody is internalized with its target antigen.
Incubation of peritoneal leukocytes with FITC-conjugated dextran results in colocalization of P-selectin with FITC-dextran particles, a fluid phase marker. Colocalization is likely within endocytic vesicles and lysosomes (Fig. 4 D).
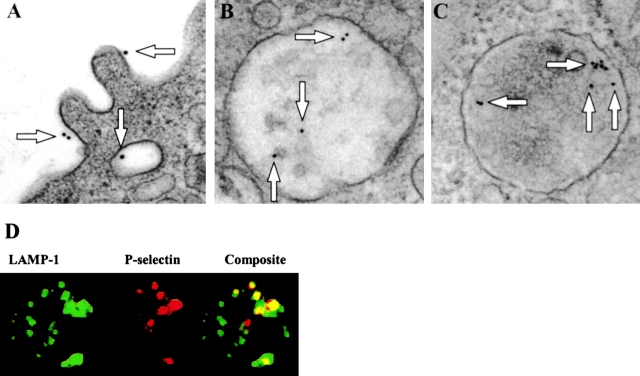
In electron micrographs of cell sections from macrophages incubated at 0°C before fixation, P-selectin detected with nanogold-conjugated antibodies is localized to the external face of the plasma membrane and to the internal face of the membrane of intracellular vesicles (Fig. 5 A). Gold particles were not observed on the peritoneal macrophages of P-selectin–deficient mice. In cell sections from macrophages incubated at 37°C, P-selectin is also detected in intracellular compartments (Fig. 5, B and C). To evaluate the nature of these compartments, cells stained with anti–P-selectin and anti–LAMP-1 antibodies were examined by confocal microscopy. Confocal images show that P-selectin is partially colocalized with the lysosomal marker, LAMP-1, in macrophages (Fig. 5 D).
Figure 5.
P-Selectin is both surface bound and internalized. Electron micrographs of peritoneal leukocytes from wild-type mice incubated at 0°C or at 37°C with rat anti–P-selectin antibody and 10- or 18-nm colloidal gold-conjugated goat anti–rat IgG. Arrows indicate colloidal gold particles. (A) Image from cell at 0°C. (B) Image from cell at 37°C. (C) Image from cell at 37°C. (D) Confocal micrographs of peritoneal leukocytes from wild-type mice incubated at 37°C with FITC-conjugated anti–LAMP-1 antibodies (green) and Texas red–conjugated rat anti–P-selectin antibody (red); merge (yellow). LAMP-1, lysosome-associated protein-1.
Expression of P-selectin by stimulated macrophages
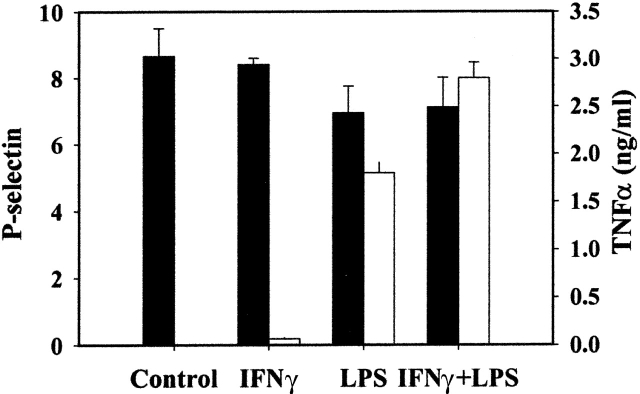
Surface expression of P-selectin in endothelial cells is regulated by two different mechanisms (Weller et al., 1992). Activation of endothelial cells with a secretagogue leads to translocation of P-selectin from the Weibel-Palade body membrane to the cell surface. Activation of endothelial cells with a cytokine leads to de novo P-selectin synthesis followed by P-selectin surface expression. Therefore, we investigated whether activation of resident peritoneal macrophages by cytokines alters P-selectin surface expression.
Macrophages are activated through a two-stage mechanism, a priming stage and a triggering stage (Adams and Hamilton, 1987). We determined the level of P-selectin surface expression on peritoneal macrophages after 24 h of stimulation with IFNγ, LPS or IFNγ, and LPS. The extent of macrophage stimulation was determined by measuring secretion of TNFα into the cell supernatant. Activation of resident peritoneal macrophages with either agent or the combination of agents did not alter the level of P-selectin on the macrophage plasma membrane (Fig. 6). In contrast, the secretion of TNFα was significantly stimulated after activation of macrophages with LPS with or without IFNγ.
Figure 6.
Expression of P-selectin by activated macrophages. Resident peritoneal macrophages were stimulated with 20 ng/ml of IFNγ and/or 100 ng of LPS for 24 h. The level of P-selectin expression on the surface of resting or stimulated macrophages was determine by FACS®. Mean fluorescence of P-selectin signal was determined as the difference between signal obtained after staining of macrophages with FITC-labeled anti–P-selectin antibodies and FITC-labeled control IgG (black bars). The concentration of TNFα in the culture supernatants was measured by ELISA (white bars). Values represent mean ± SD.
Aggregation of peritoneal macrophages
Expression of both an adhesion receptor and its physiological ligand by the same cell can potentially promote formation of cell aggregates. Therefore, we analyzed the number of cell aggregates in peritoneal lavage harvested from P-selectin null, PSGL-1 null, and wild-type mice. When determined from cells maintained at 37°C, the percentage of cells in aggregates in isolated peritoneal lavage from PSGL-1 (8%) and P-selectin (7%) null mice were equivalent to the percentage observed in wild-type mice (11%). Because we demonstrated temperature-dependent internalization of macrophage surface-bound P-selectin, we compared the number of cell aggregates in peritoneal lavage after incubation of cells at 0°C. Incubation of wild-type peritoneal cells at 0°C significantly increased calcium-dependent cell aggregation (Fig. 7 A). This cell aggregation is P-selectin mediated and was not affected by the blocking of the function of another PSGL-1 ligand, L-selectin (Fig. 7 A).
Figure 7.
P-Selectin mediated aggregation of peritoneal leukocytes. Peritoneal leukocytes were incubated for 1 h under the conditions indicated. The percentage of cells in aggregates (>2 cells/aggregate) was determined by phase-contrast microscopy. (A) Wild-type peritoneal leukocytes incubated at 0°C (black bars) or at 37°C (white bars) in the presence or absence of blocking antibodies and in the presence of 2 mM CaCl2 or 5 mM EDTA. (B) Peritoneal leukocytes from P-selectin null (P−/−), PSGL-1 null (PSGL-1−/−), P-selectin null plus PSGL-1 null (P−/− + PSGL-1−/−), and P-selectin null plus PSGL-1 null in the presence of EDTA (P−/− + PSGL-1−/− + EDTA) were incubated in the presence of 2 mM CaCl2 at 0°C (black bars) or at 37°C (white bars). Values are mean ± SD.
To demonstrate that PSGL-1 is the primary P-selectin ligand in macrophage aggregation, we examined the temperature dependent aggregation of a mixture of P-selectin null and PSGL-1 null cells. Significant temperature and calcium ion–dependent macrophage aggregation was observed in the mixture of P-selectin null and PSGL-1 null cells (Fig. 7 B) indicating the role of PSGL-1 as the P-selectin ligand in this process.
Discussion
P-Selectin is localized in the membranes of the α granules of platelets and the Weibel-Palade bodies of endothelial cells. Upon cell activation P-selectin is translocated to the plasma membrane of these cells (Stenberg et al., 1985; Berman et al., 1986; Bonfanti et al., 1989; McEver et al., 1989). P-Selectin on the surface of activated platelets and endothelial cells is available to interact with its ligand on myeloid cells (Larsen et al., 1989; Hamburger and McEver, 1990). The P-selectin ligand on the surface of myeloid cells has been identified as PSGL-1 (Moore et al., 1992; Sako et al., 1993). PSGL-1 has also been demonstrated on subsets of T lymphocytes (Moore and Thompson, 1992). These adhesion molecules mediate tethering of leukocytes to P-selectin expressed by stimulated endothelial cells and platelets.
Here, we demonstrate that peritoneal macrophages isolated from normal mice synthesize and express a functional form of P-selectin. This is the first example of a leukocyte that produces P-selectin. In contrast to platelets and endothelial cells, peritoneal macrophage P-selectin is constitutively expressed on the plasma membrane. We have demonstrated the presence of P-selectin on the cell surface by flow cytometry, by fluorescence and electron microscopy. However, distribution of P-selectin and PSGL-1 on the cell surface, as analyzed by fluorescence microscopy, reveals that, although there is some colocalization of the two proteins, PSGL-1 appears on the cell surface membrane, whereas most of the P-selectin is intracellular.
In cells with regulated secretory granules sequences within the cytoplasmic tail of P-selectin target this protein to the storage granules (Disdier et al., 1992; Koedam et al., 1992). Sequences within the C1 and C2 domains of the P-selectin cytoplasmic tail have been implicated in delivery of P-selectin to secretory granules during expression in heterologous cells (Modderman et al., 1998; Blagoveshchenskaya et al., 1999). After the stimulation of endothelial cells, P-selectin is redistributed to the plasma membrane from storage granules and then rapidly internalized (Subramanian et al., 1993; Steiadi et al., 1995; Hattori et al., 1989). When P-selectin is expressed in heterologous cells lacking regulated secretory granules, it is transported to the cell surface and then rapidly endocytosed for degradation (Green et al., 1994; Blagoveshchenskaya et al., 1998b). The cytoplasmic tail of P-selectin contains a lysosomal targeting signal in the C1 domain and lysosomal avoidance signals in the C2 domain (Blagoveshchenskaya et al., 1998a,b). Together these signals regulate P-selectin transport among early and late endosomes and lysosomes (Blagoveshchenskaya et al., 1998a). These same sequences within the P-selectin cytoplasmic tail likely also regulate distribution of the protein in peritoneal macrophages. In the absence of secretory granules, macrophages direct P-selectin to the plasma membrane. However, P-selectin is found primarily in vesicular compartments within peritoneal macrophages. Colocalization with dextran particles places P-selectin in endosomes, whereas colocalization with LAMP-1 places P-selectin in lysosomes. These results reflect the pathways of P-selectin expression and transport established in model systems of cells lacking regulated secretory organelles. Although de novo P-selectin synthesis is observed in endothelial cells after cytokine stimulation, macrophage activation does not appear to alter P-selectin surface expression.
Secondary leukocyte–leukocyte interactions under flow conditions have been demonstrated previously for monocytes flowing over surfaces coated with P-selectin, E-selectin, or TNF-α–treated endothelial cells (Lim et al., 1998) and for neutrophils flowing over surfaces coated with E-selectin, P-selectin, L-selectin, PNAd, VCAM-1, cytokine-stimulated endothelial cells, and PSGL-1 or neutrophil monolayers (Alon et al., 1996; Walcheck et al., 1996). L-selectin has been demonstrated to mediate the secondary interactions resulting in string-like cell formations for both monocytes and neutrophils under hydrodynamic shear stress of 1.5–3.0 dynes/cm2 (Alon et al., 1996; Walcheck et al., 1996; Lim et al., 1998). PSGL-1 has been demonstrated to be the counter ligand for this interaction for both neutrophils and monocytes (Walcheck et al., 1996; Lim et al., 1998). However, macrophages are not found in blood but in the afferent lymph. The shear stress in the lymphatic system is much less than that in blood. Shear stress above a threshold level is critical for the optimal interaction of L-selectin with its ligands (Finger et al., 1996). At 0.7 dynes/cm2 wall shear stress, neutrophils flowing over a P-selectin–coated surface do not form string-like formations; secondary accumulation of neutrophils does not occur at this wall shear stress as this is an L-selectin–mediated interaction (Alon et al., 1996). Failure to observe a contribution of L-selectin to peritoneal macrophage string formation in our system may be a result of insufficient wall shear stress to induce L-selectin–PSGL-1 binding. In contrast, P-selectin does not require shear above a critical threshold to promote and maintain interactions with its counterreceptor (Finger et al., 1996).
The functional significance of our observation that peritoneal macrophages express both the receptor and the counterreceptor, P-selectin and PSGL-1, remains unknown. We have demonstrated that at low temperature, when P-selectin internalization is slowed, homotypic peritoneal macrophage aggregates form (Fig. 7). It is possible that under certain physiologic conditions, P-selectin expression on the macrophage surface is stabilized. The presence of P-selectin and PSGL-1 on the same cell would potentially yield macrophage aggregates within the peritoneum. These peritoneal macrophages would not only adhere to each other but also to neutrophils, monocytes, and subsets of T lymphocytes. These cell aggregates may be part of a host defense system that clears the peritoneum of bacteria or other microbial particles. These aggregates may nucleate the formation of granulomas or possibly participate in the formation of giant cell granulomas.
Alternatively, P-selectin on the macrophage surface may be important in the immune response. Specifically, P-selectin and PSGL-1 may represent a ligand pair that contributes to the binding energy between macrophages and T lymphocytes. The interaction of antigen presenting cells with the lymphocyte T cell receptor complex is of low affinity, and several other ligand pairs are known to support both cell–cell interaction as well as cell signaling in the immunologic synapse (Lee et al., 1998; Grakoui et al., 1999). It remains plausible that in the case of peritoneal macrophage–T lymphocyte interaction, P-selectin on the macrophage participates in recognition and binding of T cell PSGL-1. LPS-induced up-regulation of P-selectin mRNA has been observed in both human dendritic cells and in Kupffer cells (Essani et al., 1995; Baltathakis et al., 2001) suggesting the potential for similar mechanisms in these cells.
In summary, these results suggest a functional role for the dual appearance of P-selectin and PSGL-1 on the peritoneal macrophage. These adhesion molecules may be important for microbial defense or for antigen presentation and immune response. In vivo experiments using genetically modified mice will offer an approach to testing these hypotheses.
Materials and methods
Mice
L-selectin–deficient mice were a gift from T. Tedder (Duke University, Durham, NC). P-Selectin–deficient mice and C57BL/6J mice were obtained from Jackson Laboratory. PSGL-1–deficient mice were prepared as described previously (Yang et al., 1999) and backcrossed five generations with C57BL/6J. All mice were 8–12 wk old when used for the described experiments. All experimental procedures on animals were approved by the Animal Care and Use Committee of Beth Israel Deaconess Medical Center.
Reagents
Rat antibodies specific to murine PSGL-1 (2PH1), P-selectin (RB40.34), L-selectin (MEL-14), CD41 (MWReg30), CD11b (M1/70), CD49D (MFR4.B), CD2 (RM2–5), and polyclonal rabbit anti–P-selectin antibodies were obtained from BD Biosciences. F4/80 antibodies were obtained from Serotec Ltd. Goat anti–VE-cadherin antibodies (C19) were obtained from Santa Cruz Biotechnology, Inc. Colloidal gold-conjugated goat anti–rat antibodies (10 or 18 nm particles) were obtained from Jackson ImmunoResearch Laboratories. Rabbit anti–P-selectin cytoplasmic tail antibodies were prepared as described previously (Chong et al., 1994). The soluble form of mouse VCAM-1 was from R&D Systems. The soluble form of recombinant human PSGL-1, a gift from Genetics Institute (Andover, MA), was characterized in our laboratory (Croce et al., 1998). P-Selectin was purified from human platelets (Larsen et al., 1992). Texas red was obtained from Molecular Probes. Wright-Giemsa solution and low endotoxin BSA were obtained from Sigma-Aldrich.
Isolation of resident leukocytes from mouse peritoneum
Mice were killed and their peritoneal contents collected by flushing with 10 ml of ice-cold PBS containing 2 mM EDTA and 10 U/ml of heparin. Total leukocyte counts were determined using a counter (model T890; Beckman Coulter).
Macrophage binding studies
25 μl mouse recombinant VCAM-1 in 50 mM sodium bicarbonate containing 100 mM NaCl, pH 8.5, at a final concentration of 5 μg/ml was coated on a polystyrene dish and incubated overnight at 4°C. The dish was washed three times with PBS and blocked with BSA (20 mg/ml in PBS) for 2 h at 37°C. A VCAM-1–coated plate was assembled in a parallel plate laminar flow chamber (Glycotech) and mounted on the stage of an inverted phase-contrast microscope (model Labovert FS; Leitz). To generate the desired shear stress, the outlet of the flow chamber was connected to an automated syringe pump (Harvard Apparatus).
Cells were prepared according to Chen et al. (1999). Resident leukocytes harvested from mouse peritoneum were washed twice with cation-free HBSS containing 2 mg/ml BSA, 2 mM EDTA, and 10 mM Hepes, pH 7.4. Cells were resuspended in the same buffer to a final concentration 107 cells/ml and kept at 4°C until use. For laminar flow assays, peritoneal cells were diluted in a binding buffer (HBSS containing BSA and where indicated, EDTA) to the desired concentration and perfused through the flow chamber at the indicated shear stress. Cell interactions with the surface-bound ligands were visualized with a 10× objective and videotaped using a digital color video camera (model SSC-S320; Sony) and a video recorder (model HS-U790; Mitsubishi).
String formation by peritoneal macrophages mediated by secondary macrophage–macrophage interactions was studied on VCAM-1–coated substrate. Peritoneal cells (106 cells/ml) were perfused at 0.5 dyn/cm2 shear stress over a surface coated with VCAM-1. After 5 min of perfusion, free-floating cells were washed away and several fields with adherent cells were recorded for further analysis. A string was defined as three or more leukocytes aligned in the direction of flow and that were not separated by more than 10 μm. String formation was quantitated as the percentage of leukocytes in strings compared with the total number of adherent cells in the field of view.
Flow cytometric analysis and FACsorting™
Peritoneal cells were isolated, washed, and resuspended in 200 μl of FACS® solution. After incubation for 5 min with 5 μl of anti-CD16/CD32 antibodies to minimize nonspecific binding, cells were stained for 30 min at 4°C with fluorescently labeled antibodies to the indicated cell surface antigens and analyzed by flow cytometry.
Purification of peritoneal leukocytes by FACsorting™ based on P-selectin expression was performed using a FACStar Plus™ cell sorter. Resident peritoneal cells, 107, were incubated for 30 min at 4°C with 20 μg/ml FITC-conjugated rat anti–P-selectin antibodies in FACS® solution.
Fluorescent microscopy
For flow studies, peritoneal cells were labeled with Texas red–conjugated F4/80 antibodies or with calcein. After washing, labeled cells were mixed at a 1:1 ratio to a final concentration of 106 cells/ml. Cells were perfused for 5 min over the VCAM-1–coated surface. Adherent cells were washed with PBS, fixed for 15 min with 4% PFA solution, and examined by fluorescent microscopy. Excitation was at either 488 or 621 nm on a fluorescence microscope (model AX70; Olympus) equipped with a 40× water immersion objective.
For confocal microscopy, live or fixed Leucoperm permeabilized cells were stained with 20 μg/ml of antigen-specific mAbs directly conjugated to Texas red, Alexa 488, or FITC. After staining for 40 min at the indicated temperature, cells were washed twice with PBS at 4°C and examined microscopically using an 100× oil immersion objective.
Northern blot and RT-PCR analysis of gene expression
Peritoneal cells (8 × 106) in RPMI medium were plated on a 60 × 15-mm tissue culture dish. After a 2-h incubation, nonadherent cells were removed by washing with 2 mM EDTA in PBS. Total RNA from adherent cells (>98% macrophages) was isolated using TRIzol reagent (Life Technologies). Northern blots were performed using the NorthernMax-Gly system according to the manufacturer's protocol (Ambion). The P-selectin probe corresponding to exon 3 of P-selectin was radiolabeled by PCR using [32P]dCTP incorporation.
For RT-PCR analysis, RNA from total mouse peritoneal leukocytes, lung, and bone marrow was isolated and the mRNA reverse transcribed using oligo (dT)12–18 primers and SuperScript Rnase H− reverse transcriptase (Life Technologies). The resulting cDNAs were used for amplification of mouse proteins. The gene-specific primers were: vWF, forward (2647–2673), 5′-CTTGGAGCTATTGCAGGCAGAGGAATG-3′ and reverse (4163–4137), 5′-AGGCAGATCTCATACCTGAAAGGGTTC-3′; P-selectin, forward (311–333), 5′-GGACCTGGGTGGGAACAAATAAG-3′ and reverse (2170–2145), 5′-TTGTAGAAGCCACTGCACCACCCAAG-3′; GPIIb forward (720–749), 5′-ATTGAGAACATCATCTCCACGTACCGC-3′ and reverse (998–972), 5′-CGAGTGCCCGAAATATGAAGCCATCTG-3′; VE-cadherin, forward (224–249), 5′-CGCTGCCCCACTATGTGAAAGATCAG-3′ and reverse (452–427), 5′-TTGACAGTGAAGCTGGAAGGTTGTTC-3′.
Western blot analysis
500 μl of mouse blood was diluted twice with HBSS and 2 mM EDTA. Platelet-rich plasma was isolated by centrifugation at 400 g for 7 min. The platelets from platelet-rich plasma were washed twice with PBS and treated with lysis buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 10 mM NaF, 10 mM sodium phosphate, and 10 mM sodium pyrophosphate) containing protease inhibitors (benzamidine, aprotinin, leupeptin, pepstatin A, and PMSF). Mouse peritoneal cells were treated with lysis buffer. The proteins from the cell lysates were separated under the reducing conditions on 7% SDS-PAGE and transferred to PVDF membrane. The membrane was blocked for 2 h with 3% BSA in PBS and rabbit antibodies specific for P-selectin cytoplasmic tail were added to a final concentration of 10 μg/ml. After 2 h of incubation, the membrane was washed and bound antibodies were detected using goat anti–rabbit IgG conjugated to peroxidase.
Internalization assay
Freshly isolated peritoneal cells were first incubated for 40 min at 0°C in FACS® solution to inhibit endocytosis. After incubation, cells were stained with FITC-labeled monoclonal anti–P-selectin antibodies or isotype-matched control IgG for 30 min at the indicated temperature. After washing with PBS at 4°C, half of the sample was incubated for 2 min in PBS, pH 7.0, at 4°C. The other half of the sample was treated with low pH buffer (500 mM NaCl, 0.2 N acetic acid, pH 2.3). The optimal pH for stripping antibody from the cell surface was determined using 10 μm polystyrene beads (Polysciences Inc.) with covalently bound mouse P-selectin. After incubation at low and neutral pH, cells were washed twice with PBS at 4°C and the mean fluorescence was determined by flow cytometry on a FACSCaliber™ after gating on the macrophage population.
EM
Peritoneal leukocytes were washed twice with FACS® solution and incubated for 30 min at 0°C with rat anti–P-selectin antibodies. The cells were incubated for 30 min at either 0°C or 37°C with goat anti-IgG on 10- or 18-nm gold particles. Cells were washed twice with PBS and fixed for 15 min in 2% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4. Fixed cells were pelleted and resuspended in 5% low gelling temperature sea plaque agarose. Agarose blocks were fixed after in 1% osmium tetroxide in cacodylate buffer for 1 h and stained enbloc with 2% uranyl acetate in water overnight. Blocks were washed, dehydrated, infiltrated in Epon 812 propylene oxide, and polymerized for 48 h at 60°C. 80-nm sections were placed on formavir-coated nickel grids, counterstained with uranyl acetate and lead citrate, and viewed on an electron microscope (model JEM 100CX; JEOL).
Cell aggregation studies
Leukocytes (2.5 × 105) isolated from the peritoneum of wild-type mice, P-selectin null mice, or PSGL-1 null mice were resuspended in 50 μl of HBSS containing 2 mM CaCl2. Cells were incubated at the indicated temperature in the presence or absence of 10 μg/ml blocking antibodies or 5 mM EDTA for 1 h. Aggregates were observed and quantified by phase-contrast microscopy using a 10× objective. Aggregates were defined as containing two or more cells.
Measurement of P-selectin expression and TNFα production in stimulated macrophages
Resident peritoneal cells were incubated on dishes for 4 h. Nonadherent cells were removed by washing and adherent macrophages were cultured with 20 ng/ml IFNγ and/or 100 ng/ml LPS for 24 h. Adherent cells were harvested by scraping dishes and stained with FITC-labeled rat anti–P-selectin antibodies or FITC-labeled isotype matched control IgG. Stained cells were analyzed on a FACSCalibur™ flow cytometer. Concentrations of TNFα in the culture supernatants were determined by ELISA (R&D Systems).
Metabolic radiolabeling
CHO cells stably transfected with human P-selectin (2 × 107) or 5 × 107 resident peritoneal macrophages from wild-type or P-selectin–deficient mice were grown in 150 μCi/ml [35S]methionine/cysteine containing DME supplemented with 10% FCS for 14 h. Cells were washed three times with cold PBS, and adherent macrophages and CHO cells harvested by scrapping. Cells were sedimented by centrifugation and lysed in buffer containing 1% Triton X-100, 0.1% SDS, 0.5% DOC, 50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.02% NaN3, and protease inhibitors. After a 1-h incubation at 4°C, cell lysates were clarified by centrifugation at 10,000 g for 15 min. Supernatants were precleared with protein G–Sepharose after a 4-h incubation with 10 μg/ml rabbit nonimmune IgG. Cell lysates were incubated overnight at 4°C with rabbit antibodies directed against the cytoplasmic tail of P-selectin (5 μg/ml). Immunoprecipitates were collected by incubation of cell lysates with protein G–Sepharose (Amersham Biosciences) for 4 h at 4°C. Supernatant was removed and the Sepharose beads washed three times with lysis buffer containing 500 mM NaCl. Bound protein was eluted from Sepharose beads by incubation with SDS sample buffer containing mercaptoethanol. Eluted proteins were separated by SDS-PAGE. Autoradiographs were analyzed using an imager (model Typhoon 9400; Amersham Biosciences).
Acknowledgments
This work is dedicated to the memory of Dr. P. Gottlieb.
We thank Drs. Takako Hirata and Paul Gottlieb for helpful discussions about these studies.
Support for this work was provided by grants from the National Heart, Lung, and Blood Institute.
References
- Adams, D.O., and T.A. Hamilton. 1987. Molecular transductional mechanisms by which IFN gamma and other signals regulate macrophage development. Immunol. Rev. 97:5–27. [DOI] [PubMed] [Google Scholar]
- Alon, R., R.C. Fuhlbrigge, E.B. Finger, and T.A. Springer. 1996. Interactions through L-selectin between leukocytes and adherent leukocytes nucleate rolling adhesions on selectins and VCAM-1 in shear flow. J. Cell Biol. 135:849–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltathakis, I., O. Alcantara, and D.H. Boldt. 2001. Expression of different NF-kappaB pathway genes in dendritic cells (DCs) or macrophages assessed by gene expression profiling. J. Cell. Biochem. 83:281–290. [DOI] [PubMed] [Google Scholar]
- Barth, M.W., J.A. Hendrzak, M.J. Melnicoff, and P.S. Morahan. 1995. Review of the macrophage disappearance reaction. J. Leukoc. Biol. 57:361–367. [DOI] [PubMed] [Google Scholar]
- Bellingan, G.H., H. Caldwell, S.E. Howie, I. Dransfield, and C. Haslett. 1996. In vivo fate of inflammatory macrophages during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J. Immunol. 157:2577–2585. [PubMed] [Google Scholar]
- Berman, C.L., E. Yeo, J.D. Wencel-Drake, B.C. Furie, M.H. Ginsberg, and B. Furie. 1986. A platelet alpha granule membrane protein that is incorporated into the plasma membrane during activation. Characterization and subcellular localization of PADGEM protein. J. Clin. Invest. 78:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya, A.D., E.W. Hewitt, and D.F. Cutler. 1998. a. A balance of opposing signals within the cytoplasmic tail controls the lysosomal targeting of P-selectin. J. Biol. Chem. 273:27896–27903. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya, A.D., J.P. Norcott, and D.F. Cutler. 1998. b. Lysosomal targeting of P-selectin is mediated by a novel sequence within its cytoplasmic tail. J. Biol. Chem. 273:2729–2737. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya, A.D., E.W. Hewitt, and D.F. Cutler. 1999. A complex web of signal-dependent trafficking underlies the triorganellar distribution of P-selectin in neuroendocrine PC12 cells. J. Cell Biol. 145:1419–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti, R., B.C. Furie, B. Furie, and D.D. Wagner. 1989. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 73:1109–1112. [PubMed] [Google Scholar]
- Chen, C., J.L. Mobley, O. Dwir, F. Shimron, V. Grabovsky, R.R. Lobb, Y. Shimizu, and R. Alon. 1999. High affinity very late antigen-4 subsets expressed on T cells are mandatory for spontaneous adhesion strengthening but not for rolling on VCAM-1 in shear flow. J. Immunol. 162:1084–1085. [PubMed] [Google Scholar]
- Chong, B.H., B. Murray, M.C. Berndt, L.C. Dunlop, T. Brighton, and C.N. Chesterman. 1994. Plasma P-selectin is increased in thrombotic consumptive platelet disorders. Blood. 83:1535–1541. [PubMed] [Google Scholar]
- Croce, K., S.J. Freedman, B. Furie, and B. Furie. 1998. Interaction between soluble P-selectin and soluble P-selectin glycoprotein ligand 1: equilibrium binding analysis. Biochemistry. 37:16472–16480. [DOI] [PubMed] [Google Scholar]
- Disdier, M., J.H. Morrissey, R.D. Fugate, D.F. Bainton, and R.P. McEver. 1992. Cytoplasmic domain of P-selectin (CD62) contains the signal for sorting into the regulated secretory pathway. Mol. Biol. Cell. 3:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essani, N.A., G.M. McGuire, A.M. Manning, and H. Jaeschke. 1995. Differential induction of mRNA for ICAM-1 and selectins in hepatocytes, Kupffer cells and endothelial cells during endotoxemia. Biochem. Biophys. Res. Commun. 211:74–82. [DOI] [PubMed] [Google Scholar]
- Finger, E.B., K.D. Puri, R. Alon, M.B. Lawrence, U.H. von Andrian, and T.A. Springer. 1996. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 379:266–269. [DOI] [PubMed] [Google Scholar]
- Gerszten, R.E., Y.C. Lim, H.T. Ding, K. Snapp, G. Kansas, D.A. Dichek, C. Cabanas, F. Sanchez-Madrid, M.A. Gimbrone, Jr., A. Rosenzweig, and F.W. Luscinskas. 1998. Adhesion of monocytes to vascular cell adhesion molecule-1-transduced human endothelial cells: implications for atherogenesis. Circ. Res. 82:871–878. [DOI] [PubMed] [Google Scholar]
- Grakoui, A., S.K. Bromley, C. Sumen, M.M. Davis, A.S. Shaw, P.M. Allen, and M.L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science. 285:221–227. [DOI] [PubMed] [Google Scholar]
- Green, S.A., H. Setiadi, R.P. McEver, and R.B. Kelly. 1994. The cytoplasmic domain of P-selectin contains a sorting determinant that mediates rapid degradation in lysosomes. J. Cell Biol. 124:435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger, S.A., and R.P. McEver. 1990. GMP-140 mediates adhesion of stimulated platelets to neutrophils. Blood. 75:550–554. [PubMed] [Google Scholar]
- Haskill, S., and S. Becker. 1985. Disappearance and reappearance of resident macrophages: importance in C. parvum-induced tumoricidial activity. Cell. Immunol. 90:179–189. [DOI] [PubMed] [Google Scholar]
- Hattori, R., K.K. Hamilton, R.D. Fugate, R.P. McEver, and P.J. Sims. 1989. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J. Biol. Chem. 264:7768–7771. [PubMed] [Google Scholar]
- Hirata, T., G. Merrill-Skoloff, M. Aab, J. Yang, B.C. Furie, and B. Furie. 2000. P-Selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J. Exp. Med. 192:1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu-Lin, S.C., C. Berman, B.C. Furie, D. August, and B. Furie. 1984. A platelet membrane protein expressed during activation and secretion: studies using a monoclonal antibody specific for thrombin-activated platelets. J. Biol. Chem. 259:9121–9126. [PubMed] [Google Scholar]
- Johnston, G.I., G.A. Bliss, P.J. Newman, and R. McEver. 1990. Structure of the human gene encoding granule membrane protein-140, a member of the selectin family of adhesion receptors for leukocytes. J. Biol. Chem. 265:21381–21385. [PubMed] [Google Scholar]
- Koedam, J.A., E.M. Cramer, E. Briend, B. Furie, B.C. Furie, and D.D. Wagner. 1992. P-Selectin, a granule membrane protein of platelets and endothelial cells, follows the regulated secretory pathway in AtT-20 cells. J. Cell Biol. 116:617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, E., A. Celi, G. Gilbert, B.C. Furie, J. Erban, R. Bonfanti, D.D. Wagner, and B. Furie. 1989. A receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 59:305–312. [DOI] [PubMed] [Google Scholar]
- Larsen, G.R., D. Sako, T.J. Ahern, M. Shaffer, J. Erban, S.A. Sajer, R.M. Gibson, D.D. Wagner, B.C. Furie, and B. Furie. 1992. P-selectin and E-selectin. Distinct but overlapping leukocyte ligand specificities. J. Biol. Chem. 267:11104–11110. [PubMed] [Google Scholar]
- Lee, K.M., E. Chuang, M. Griffin, R. Khattri, D.K. Hong, W. Zhang, D. Stgraus, L.E. Samuelson, C.B. Thompson, and J.A. Bluestone. 1998. Molecular basis of T cell inactivation by CTLA-4. Science. 282:2263–2266. [DOI] [PubMed] [Google Scholar]
- Lim, Y.C., K. Snapp, G.S. Kansas, R. Camphausen, H. Ding, and F.W. Luscinskas. 1998. Important contributions of P-selectin glycoprotein ligand-1-mediated secondary capture to human monocyte adhesion to P-selectin, E-selectin and TNF-α-activated endothelium under flow in vitro. J. Immunol. 161:2501–2508. [PubMed] [Google Scholar]
- Luscinskas, F.W., G.S. Kansas, H. Ding, P. Pizcueta, B.E. Schleiffenbaum, T.F. Tedder, and M.A. Gimbrone. 1994. Monocyte rolling, arrest and spreading on IL-4 activated vascular endothelium under flow is mediated via sequential action of L-selectin, β1-integrins, and β2-integrins. J. Cell Biol. 125:1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas, F.W., H. Ding, P. Tan, D. Cumming, T.F. Tedder, and M.E. Gerritsen. 1996. L- and P-selectins, but not CD49d (VLA-4) integrins, mediate monocyte initial attachment to TNF-alpha-activated vascular endothelium under flow in vitro. J. Immunol. 157:326–335. [PubMed] [Google Scholar]
- McEver, R.P., J.H. Beckstead, K.L. Moore, L. Marshall-Carlson, and D.F. Bainton. 1989. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J. Clin. Invest. 84:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modderman, P.W., E.A. Beuling, L.A. Govers, J. Calafat, H. Janssen, A.E. Von dem Borne, and A. Sonnenberg. 1998. Determinants in the cytoplasmic domain of P-selectin required for sorting to secretory granules. Biochem. J. 336:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, K.L., and L.F. Thompson. 1992. P-selectin (CD62) binds to subpopulations of human memory T lymphocytes and natural killer cells. Biochem. Biophys. Res. Commun. 186:173–181. [DOI] [PubMed] [Google Scholar]
- Moore, K.L., N.L. Stults, S. Diaz, D.F. Smith, R.D. Cummings, A. Varki, and R.P. McEver. 1992. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J. Cell Biol. 118:445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph, G.J., S. Beaulieu, S. Lebecque, R.M. Steinman, and W. Muller. 1998. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 282:480–483. [DOI] [PubMed] [Google Scholar]
- Randolph, G.J., K. Inaba, D.F. Robbiani, R.M. Steinman, and W.A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 11:753–761. [DOI] [PubMed] [Google Scholar]
- Rosen, H., and S. Gordon. 1990. Adoptive transfer of fluorescence-labeled cells shows that resident peritoneal macrophages are able to migrate into specialized lymphoid organs and inflammatory sites in the mouse. Eur. J. Immunol. 20:1251–1258. [DOI] [PubMed] [Google Scholar]
- Sako, D., X.J. Chang, K.M. Barone, G. Vachino, H.M. White, G. Shaw, T. Veldman, K.M. Bean, T.J. Ahern, B. Furie, et al. 1993. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 75:1179–1186. [DOI] [PubMed] [Google Scholar]
- Sato, K., Y. Imai, and T. Irimura. 1998. Contribution of dermal macrophage trafficking in the sensitization phase of contact hypersensitivity. J. Immunol. 161:6835–6844. [PubMed] [Google Scholar]
- Sims, P.J., E.M. Faioni, T. Wiedmer, and S.J. Shattil. 1988. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in membrane receptor for coagulation factor Va and express prothrombinase activity. J. Biol. Chem. 263:18205–18212. [PubMed] [Google Scholar]
- Sminia, T., M. Soesatyo, M. Ghufron, and T. Thepen. 1995. The migration of peritoneal cells towards the gut. Adv. Exp. Med. Biol. 371A:61–65. [DOI] [PubMed] [Google Scholar]
- Steiadi, H., M. Disdier, S.A. Green, W.M. Canfield, and R.P. McEver. 1995. Residues throughout the cytoplasmic domain affect the internalization efficiency of P-selectin. J. Biol. Chem. 270:26818–26826. [DOI] [PubMed] [Google Scholar]
- Stenberg, P.E., R.P. McEver, M.A. Shuman, Y.V. Jacques, and D.F. Bainton. 1985. A platelet α-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J. Cell Biol. 101:880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, M., J.A. Koedam, and D.D. Wagner. 1993. Divergent fates of P- and E-selectins after their expression on the plasma membrane. Mol. Biol. Cell. 4:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thepen, T., E. Caassen, K. Hoeben, J. Breve, and G. Kraal. 1993. Migration of alveolar macrophages from alveolar space to paracortical T cell area of the draining lymph node. Adv. Exp. Med. Biol. 329:305–310. [DOI] [PubMed] [Google Scholar]
- van Furth, R., and Z.A. Cohn. 1968. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 128:415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt, E., M. van Pelt, R.H. Beelen, and E.W. Kamperidjk. 1995. Migration of rat dendritic cells and macrophages from the peritoneal cavity to the parathymic lymph nodes. Adv. Exp. Med. Biol. 378:163–167. [DOI] [PubMed] [Google Scholar]
- Walcheck, B., K.L. Moore, R.P. McEver, and T.K. Kishimoto. 1996. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. J. Clin. Invest. 98:1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller, A., S. Isenmann, and D. Vestweber. 1992. Cloning of the mouse endothelial selectins. Expression of both E- and P- selectin is inducible by tumor necrosis factor alpha. J. Biol. Chem. 267:15176–15183. [PubMed] [Google Scholar]
- Yang, J., T. Hirata, K. Croce, G. Merrill-Skoloff, B. Tchernychev, E. Williams, R. Flaumenhaft, B.C. Furie, and B. Furie. 1999. Targeted gene disruption demonstrates that PSGL-1 is required for P-selectin mediated but not E-selectin mediated neutrophil rolling and migration. J. Exp. Med. 190:1769–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]