RNA-Binding Proteins Tia-1 and Tiar Link the Phosphorylation of Eif-2α to the Assembly of Mammalian Stress Granules (original) (raw)
Abstract
In response to environmental stress, the related RNA-binding proteins TIA-1 and TIAR colocalize with poly(A)+ RNA at cytoplasmic foci that resemble the stress granules (SGs) that harbor untranslated mRNAs in heat shocked plant cells (Nover et al. 1989; Nover et al. 1983; Scharf et al. 1998). The accumulation of untranslated mRNA at SGs is reversible in cells that recover from a sublethal stress, but irreversible in cells subjected to a lethal stress. We have found that the assembly of TIA-1/R+ SGs is initiated by the phosphorylation of eIF-2α. A phosphomimetic eIF-2α mutant (S51D) induces the assembly of SGs, whereas a nonphosphorylatable eIF-2α mutant (S51A) prevents the assembly of SGs. The ability of a TIA-1 mutant lacking its RNA-binding domains to function as a transdominant inhibitor of SG formation suggests that this RNA-binding protein acts downstream of the phosphorylation of eIF-2α to promote the sequestration of untranslated mRNAs at SGs. The assembly and disassembly of SGs could regulate the duration of stress- induced translational arrest in cells recovering from environmental stress.
Keywords: RNA-binding proteins, stress, translational control, eIF-2α
The general translational arrest that accompanies environmental stress is initiated by the phosphorylation of eIF-2α, an essential component of the heterotrimeric eIF-2 complex that loads the initiator tRNA (Met-tRNAMet) onto the 40S ribosomal subunit (Berlanga et al. 1998; Gray and Wickens 1998; Srivastava et al. 1998; Harding et al. 1999). The eIF-2 complex binds GTP/GDP, and its activity is regulated by eIF-2B, a guanine nucleotide exchange factor. Phosphorylation of eIF-2α increases the affinity of eIF-2 for eIF-2B, and prevents the exchange of GDP for GTP, thereby inhibiting the formation of preinitiation complexes. Although other initiation factors (e.g., eIF-4E) can also contribute to stress-induced translational arrest, the ability of nonphosphorylatable mutants of eIF-2α to abrogate stress-induced translational arrest (Murtha-Riel et al. 1993) indicates the central importance of eIF-2α in this process.
Stress-induced inhibition of translational initiation allows elongating ribosomes to run off nonheat shock mRNAs, resulting in the disassembly of polyribosomes. In cells allowed to recover from stress in the presence of actinomycin D, synthesis of nonheat shock proteins is rapidly resumed, indicating that preexisting mRNAs are not degraded (Storti et al. 1980; Lindquist 1981; Panniers and Henshaw 1984). Studies of cultured Peruvian tomato cells have shown that untranslated mRNAs accumulate at discrete cytoplasmic foci known as heat stress granules (HSGs) (Nover et al. 1983, Nover et al. 1989; Scharf et al. 1998). In cells that are returned to ambient temperature, these mRNAs rapidly move from HSGs to polyribosomes. If mRNAs sequestered at HSGs are unavailable for translational reinitiation, the assembly and disassembly of these structures might influence the duration of stress-induced translational arrest. Heat shock mRNAs that are selectively translated during stress are excluded from HSGs (Nover et al. 1989), consistent with the proposed role for these structures in translational repression. The further observation that low molecular weight heat shock proteins are prominent components of HSGs (Scharf et al. 1998) suggests that they may be homologous to the mammalian stress granules to which HSP27 (a mammalian low molecular weight heat shock protein) is recruited in heat-stressed chicken embryo fibroblasts (Collier and Schlesinger 1986; Collier et al. 1988) and HeLa cells (Arrigo et al. 1988). Although stress-induced nascent transcripts are excluded from mammalian SGs (Collier et al. 1988) (consistent with the exclusion of HSP mRNAs from tomato HSGs), the association of untranslated mRNAs with mammalian stress granules (SGs) has not been reported.
We have identified three RNA-binding proteins (TIA-1, TIAR, and poly(A)+ binding protein I [PABP-I]) that coaggregate with poly(A)+ RNA at mammalian SGs. TIA-1, TIAR, and PABP-I are members of the RNA recognition motif type family of RNA-binding proteins (Tian et al. 1991; Kawakami et al. 1992, Kawakami et al. 1994). PABP-I resides in the cytoplasm where it promotes the stability and translation of polyadenylated mRNAs (Adam et al. 1986; Query et al. 1989; Burd et al. 1991; Matunis et al. 1993). In contrast, TIA-1 and TIAR are concentrated in the nucleus at steady state but normally shuttle between the nucleus and the cytoplasm (Kedersha, N., and P. Anderson, unpublished observations). Although TIA-1 and TIAR have been implicated in signaling cascades regulating entry into apoptosis (Tian et al. 1991, Tian et al. 1995; Kawakami et al. 1992, Kawakami et al. 1994; Taupin et al. 1995), their precise function has not been determined. Mutant mice lacking TIAR exhibit partial embryonic lethality and defective germ cell maturation, implicating this protein in selective aspects of vertebrate development (Beck et al. 1998). Here, we report that TIA-1 and TIAR act downstream of the stress-induced phosphorylation of eIF-2α to promote the recruitment of untranslated mRNAs to SGs. The ability of phospho-eIF-2α (Srivastava et al. 1998), TIA-1 (Tian et al. 1991), and TIAR (Kawakami et al. 1992) to trigger apoptosis suggests that the assembly and disassembly of SGs might influence the survival of cells subjected to environmental stress.
Materials and Methods
Cell Lines
DU145 and COS-7 cells were obtained from the American Type Culture Collection. Cells were maintained in 10% FBS in DME.
Antibodies and Reagents
Murine mAbs to TIA-1 (ML-29, IgG1) were raised against recombinant protein at ImmunoGen, Inc., screened by ELISA, and shown to recognize TIA-1 but not TIAR, by immunofluorescent staining. Anti-TIAR (murine monoclonal 6E3, IgG2a) and anti–TIA-1/R (murine monoclonal 3E6, IgG2a) have been described previously (Taupin et al. 1995). Antibodies from commercial sources include HSP-27 (StressGen Biotechnologies Corp.), anti-HA (murine mAb clone 16B12, IgG1; Berkeley Antibody Co.), anti–ribosome P antibody (ImmunoVision, Springdale, AR), anti–β-galactosidase mAb (Promega Corp), and anti-T7 (Novagen). Isotype-specific secondary antibodies were obtained from Southern Biotechnology Associates. Hoechst dye (33258), nocodazole, cytochalasin B, cycloheximide, puromycin, anisomycin, and protease inhibitors were obtained from Sigma Chemical Co. Anti–La antibodies were obtained from Dr. Peter Schur (Brigham and Women's hospital, Boston, MA). mAb against eIF-2α was a gift from Dr. Richard Panniers (National Institutes of Health, Bethesda, MD). Anti–PABP-I mAb (10E10) was a gift from Dr. Gideon Dreyfuss (University of Pennsylvania, School of Medicine, Philadelphia, PA).
Stress Treatments and Immunofluorescence
Cells were plated on 11-mm glass coverslips in either 24-well or 4-well plates, grown for 2–5 d until cells were fully spread, and the desired degree of confluency was obtained. Cells were subjected to heat shock by floating the plate in a 44°C pan of water in a CO2 incubator for times indicated in the figure legends, and immediately fixed. UV treatment was administered by removing the media from the cells, placing the plate in a Stratolinker, exposing the cells to 100 mJoules, and then immediately adding back media to the wells. Control cells were mock-treated (e.g., media removed without irradiation) and found to be unaffected. For immunofluorescence, a wide range of fixation conditions were tested to determine the optimal conditions for each antibody used. Cells were briefly rinsed in PBS and incubated for 10 min in 2% paraformaldehyde in PBS. This was removed and the cells were immediately immersed in −20°C methanol for 10 min, rinsed in PBS, and incubated in blocking buffer (5% normal goat serum in PBS) for 1 h before the addition of primary antibodies. Primary antibodies were diluted (purified IgG from murine mAbs 3E6 and ML-29 were used at 2.5 μg/ml, anti-HA ascites fluid was used at 1/2,000, anti-T7 ascites fluid was used at 1/5,000, anti–ribosome P Ig was used at 1/5,000, anti-La autoimmune serum was used at 1/1,000, and anti-PABP (10E10) was used at 1/1,000), and cells were incubated in the diluted antibody for 1–12 h, washed several times in PBS, and incubated for 1 h in diluted isotype-specific secondary antibodies (1/200 for FITC-labeled secondary antibodies; 1/2,000 for Texas red–labeled secondary antibodies) in blocking solution supplemented with Hoechst dye 33258 at 50 ng/ml. Cells were washed three times for 10 min in PBS, mounted in vinol mounting media (Fukui et al. 1987), and viewed through a Nikon Eclipse 800 microscope equipped with epifluorescence optics and appropriate filters for detection of FITC, Texas red, or Hoechst dye. Controls were routinely performed to insure the isotype specificity of each secondary antibody used, and to monitor that possible spillover between channels did not occur. Specimens were photographed using either T-max 400 film (Kodak) or Fujichrome 400 film, scanned into Adobe Photoshop, and compiled.
In situ hybridization to detect poly(A)+ RNA was performed essentially as described previously (Spector et al. 1998), with a slight modification to allow double staining for TIAR/TIA-1. In brief, cells were fixed in 2% paraformaldehyde in PBS for 10 min, permeabilized with −20°C methanol for 10 min, and washed twice in 2× SSC. Hybridization was performed in a humid chamber at 43°C, using a biotinylated oligo-dT probe (50-mer) for 4–16 h. Cells were washed three times with 2× SSC and incubated in a 1:2,000 dilution of Alexa 488-labeled streptavidin (Molecular Probes) in 4× SSC containing 0.1% Triton X-100 for 45 min at room temperature. Cells were washed in 4× SSC, and then probed with anti–TIAR/TIA-1 antibody (mAb 3E6, which recognizes both TIAR and TIA-1) in 2× SSC containing 0.1% TX-100 and also containing biotinylated goat anti-streptavidin (1/2,000 dilution; Vector Labs) to amplify the biotinylated dT signal. After a 1-h incubation, cells were again washed in 4× SSC and subjected to a final incubation with a solution containing 1/2,000 dilution of goat anti–mouse IgG Texas red (Southern Biotechnology Associates), 1/2,000 streptavidin Alexa 488, and 0.5 μg/ml Hoechst dye in 2× SSC/0.1% Triton X-100. After 1 h, cells were sequentially washed in 4× SSC and 2× SSC, and mounted. Similar results for poly(A)+RNA were obtained when cells were treated for in situ without counterstaining with anti–TIA-1/R. Controls included cells mock-hybridized in the absence of probe or cells pretreated with NaOH to hydrolyze RNA.
Plasmid Constructions and Transfections
Full-length TIA-1 subcloned into pMT2 was described previously (Tian et al. 1991). TIA-1ΔRRM was constructed using a PCR strategy. The COOH-terminal region of TIA-1 spanning met219 through the last amino acid (glutamine 386) was amplified from pMT2-TIA-1 for 25 cycles (94°C for 1 min, 50°C for 1 min, and 74°C for 1 min) using Tli polymerase (Promega Corp.) and primers with EcoRI and XbaI cloning sites (GGGAATTCATGCGTCAGACTTTTTCACCA and GCTCTAGATTCACTGGGTTTCATACCCTGC), respectively. The insert was cut with EcoRI and XbaI and cloned in-frame with a hemagglutinin (HA) tag in pMT2 that was similarly cut. The final clone was verified by sequencing.
Full-length pMT2-HA-TIA-1, pMT2-HA-TIA-1ΔRRM, and T7-tagged hnRNPA1 cloned into the pCGT7-A1 vector (Caceres et al. 1994) (a gift from Dr. Adrian Krainer, Cold Spring Harbor Laboratory) were transiently transfected into COS cells using SuperFect according to the manufacturer's recommendations. After 24–48 h, cells were cultured in the absence or presence of arsenite (0.5 mM, 30 min) before processing for immunofluorescence microscopy using mAbs reactive with either TIA-1 (mAb ML29) or the T7 tag. Plasmids pETF-eIF-2α, pETF-eIF-2-αS51A, pETF-eIF-2-αS51D, and pETF vector were provided by Dr. Randal Kaufman (University of Michigan, Ann Arbor, MI).
Transient Transfection Assay for SG Formation
The effects of wild-type and mutant eIF-2α on spontaneous and arsenite-induced SG formation were determined by cotransfecting COS cells with a reporter plasmid (pCDNA3-β-galactosidase) and the pETF vector encoding wild-type, mutant eIF-2α, or no insert. COS cells were transfected in 6-well plates using SuperFect according to the manufacturer's directions. Immediately after transfection, the cells were trypsinized and divided among several wells of 24-well plates to insure constant transfection efficiency of parallel samples. Cells were allowed to grow for 24–48 h, processed for fluorescence microscopy, and SDS-PAGE. SG formation was assessed using two-color staining for β-galactosidase and TIA-1 using mAb ML-29. Cells expressing β-galactosidase were counted and scored for SG formation. At least 100 transfected cells were scored per well, and the results of three independent experiments were averaged to produce the data shown in Fig. 7. Samples processed for SDS-PAGE were harvested by trypsin and lysed in SDS-PAGE buffer. Equal amounts of total protein were resolved on SDS-PAGE, blotted onto nitrocellulose, and probed with anti–β-galactosidase (1/2,000) and anti–eIF-2α (1/1,000).
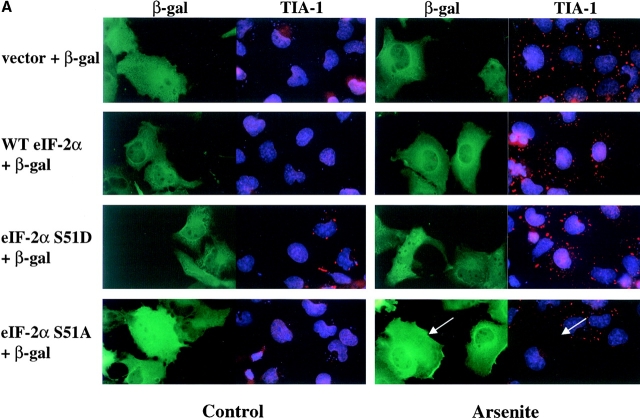
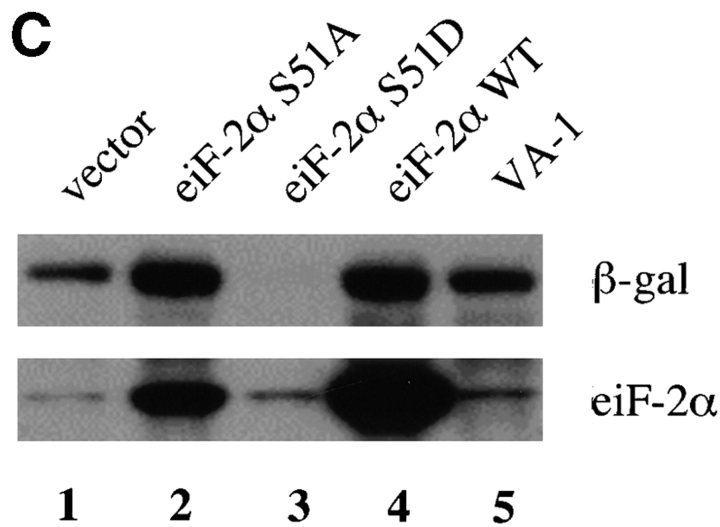
Figure 7.
Effect of wild-type and mutant recombinant eIF-2α on the assembly of TIA-1/R+ SGs. (A) COS cells were transiently cotransfected with plasmids encoding β-galactosidase and either vector alone, wild-type eIF-2α, eIF-2α (S51D), or eIF-2α (S51A) as indicated at the left of the figure. After 48 h, cells were cultured in the absence (Control, left paired panels) or presence (Arsenite, right paired panels) of arsenite (1 mM, 1 h) before fixation and processing for two-color immunofluorescence microscopy using mAbs specific for β-galactosidase (green, anti–β-galactosidase) or TIA-1 (red, TIA-1–specific antibody ML-29). In each case, paired views of the same field are presented to allow the identification of transfected and untransfected cells. Arrows in the bottom right panel point out transfected cells in which arsenite-induced assembly of SGs is prevented by the expression of eIF-2α (S51A). (B) Phosphorylation of eIF-2α is required for SG formation. Transiently transfected COS cells were treated and stained as described and were scored (at least 100 transfected cells per treatment) for the presence or absense of TIAR/ 1+ SGs as detected with mAb 3E6, and expressed as a percentage of the total cells scored per treatment. The results of 3 independent experiments were averaged. (C) Effects of mutant eIF-2α on expression of β-galactosidase. COS cells were transiently transfected with the indicated plasmids and total cell lysates were analyzed by Western blot. Upper panel, β-galactosidase; lower panel, eIF-2α.
Results
TIA-1 and TIAR Colocalize at Stress Granules in Response to Stress
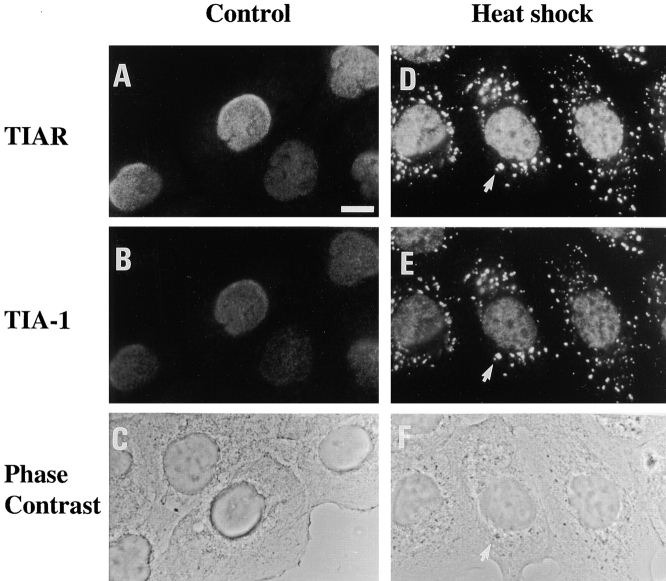
Although TIA-1 and TIAR are concentrated in the nuclei of the DU145 human prostate cell line (Fig. 1A and Fig. B), both proteins rapidly accumulate in the cytoplasm in response to mild heat shock (20 min, 44°C; Fig. 1D and Fig. F). In the cytoplasm, TIA-1 and TIAR colocalize at discrete phase dense particles (Fig. 1, D–F, arrows), which are similar to the SGs that form in heat shocked plant and animal cells (Nover et al. 1983; Collier and Schlesinger 1986; Arrigo et al. 1988; Collier et al. 1988; Scharf et al. 1998; Nover et al. 1989). In addition, TIA-1/R+ SGs are also formed in cells treated with osmotic shock (1 M sorbitol for 60 min, followed by a 30-min recovery in isotonic media), UV irradiation (data not shown) and sodium arsenite (a potent inducer of oxidative stress). Stimuli that do not induce the formation of SGs in DU145 or HeLa cells include: serum starvation, inhibitors of RNA polymerases (actinomycin D, α-amanitin, or DRB), microtubule or actin depolymerizing agents (nocodazole or cytochalasin B), and inflammatory cytokines (TNF-α) or antibody-mediated Fas ligation (data not shown).
Figure 1.
TIA-1 and TIAR coaggregate at SGs. DU 145 cells were untreated (A–C, same field) or exposed to mild heat shock (44°C for 20 min, D–F, same field). Cells were immediately fixed and processed for two-color immunofluorescence using the TIAR-specific antibody 6E3 (A and D) and the TIA-1–specific antibody ML29 (B and E), and photographed separately. TIA-1 and TIAR colocalize at SGs (arrows), which are also visible by phase-contrast microscopy (C and F). Bar, 10 μm.
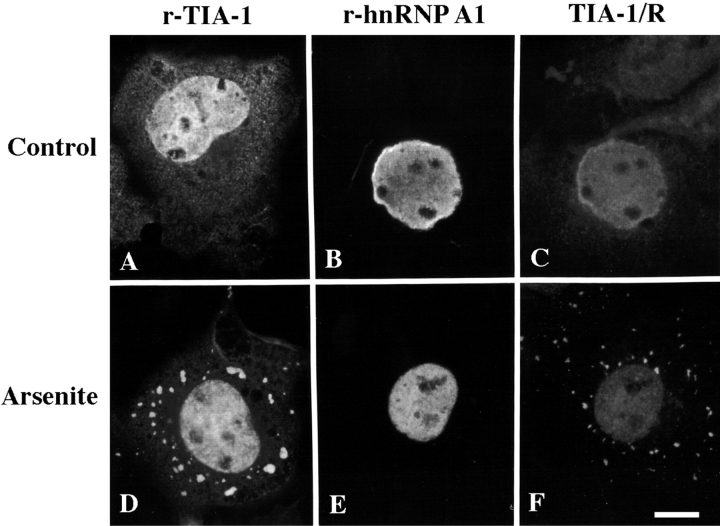
The cytoplasmic accumulation of TIA-1 and TIAR in response to certain stress stimuli could result from inactivation of the cytoplasm-to-nucleus arm of the shuttling machinery. As such, SGs might be sites at which many shuttling proteins accumulate in a general response to stress. We tested this possibility by comparing the subcellular localization of hnRNP A1, an RNA-binding protein that shuttles between the nucleus and the cytoplasm (Pinol-Roma and Dreyfuss 1992), before and during the application of stress. We used arsenite rather than heat shock in these and subsequent experiments (see Fig. 4 and Fig. 5) to allow the delivery of graded doses of stress (Brostrom and Brostrom 1998). Recombinant TIA-1 and T7-tagged recombinant hnRNP A1 were transiently transfected into COS cells using SuperFect. After 24 h, cells were cultured in the absence (Fig. 2, A–C) or presence (Fig. 2, D–F) of arsenite (0.5 mM, 30 min) before processing for immunofluorescence microscopy. Whereas arsenite-induced oxidative stress results in the accumulation of recombinant TIA-1 at SGs (Fig. 2 D), it does not result in the accumulation of recombinant hnRNP A1 at SGs (Fig. 2 E). The stress-induced aggregation of endogenous TIA-1/R (Fig. 2 F, showing the same field as in E) in the hnRNP A1–transfected cells confirms that SGs formed under these conditions specifically exclude hnRNP A1. These results indicate that accumulation at SGs is not a general property of RNA-binding proteins that shuttle between the nucleus and cytoplasm. Additional proteins tested and found not to accumulate at SGs during heat shock include the following: the RNA-binding proteins, La (Meerovitch et al. 1993) and nmt-55/p54nrb (Traish et al. 1997), the major vault protein, ubiquitin-conjugating enzyme UBC9, focal adhesion kinase, the adaptor protein shc, actin, tubulin, vimentin, keratin, and protein phosphatase PP2A (data not shown). In addition, markers for various membrane-bound compartments including the ER (BIP, calreticulin, and calnexin), the Golgi apparatus (lectin vinca villosa), and the mitochondria (MitoTracker dye) showed no consistent association with SGs, suggesting that SGs are free, self-assembling structures rather than arising from a preexisting membranous or cytoskeletal structure.
Figure 4.
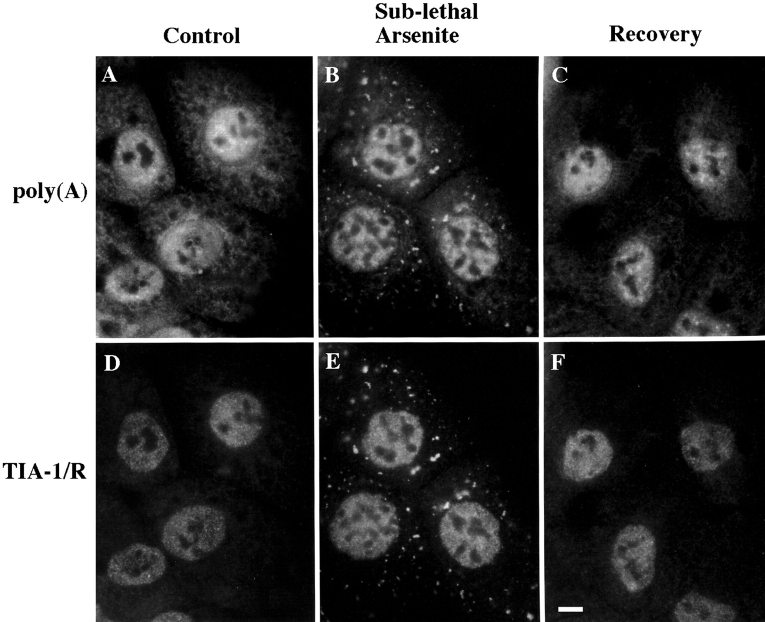
TIA-1/R and poly(A)+ RNA coaggregate at SGs. Untreated (A and D), arsenite-treated and immediately fixed (0.5 mM for 1 h, B and E), or arsenite-treated as in B and E and allowed to recover in arsenite-free media for 3 h (C and F) DU145s were processed for in situ immunofluorescence to detect poly(A) (A–C), and counterstained for TIA-1/R (D–F, anti-3E6). Paired fields (A and D, B and F, C and F) are shown. Bar, 10 μm.
Figure 5.
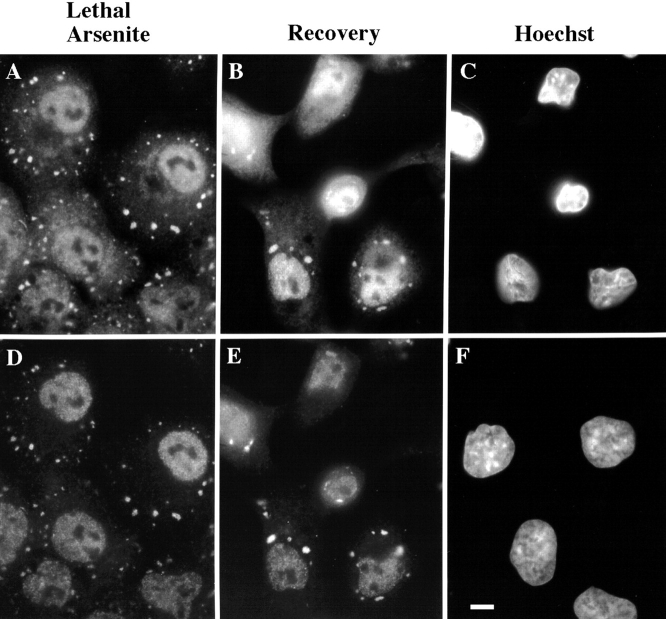
Lethal stress induces the irreversible assembly of SGs. DU145 cells were cultured in the absence (F) or presence (A–E) of arsenite (2 mM, 1 h), immediately fixed (A and D) or allowed to recover for 3 h in the absence of arsenite (B, C, and E) before processing for visualization of poly(A)+ RNA (A and B), TIA-1/R (D and E), or DNA (C and F, Hoechst dye). Bar, 10 μm.
Figure 2.
Recombinant TIA-1, but not hnRNP A1, accumulates at SGs in arsenite-treated COS transfectants. COS cells were transfected with plasmid vectors encoding recombinant TIA-1 (A and D) or T7-tagged recombinant hnRNP A1 (B, E, C, and F) using Superfect. After 24 h, cells were cultured in the absence (A–C) or presence (D–F) of arsenite (0.5 mM, 30 min) before processing for visualization of recombinant proteins using mAbs reactive with TIA-1 (anti-3E6, the level of expression of recombinant TIA-1 greatly exceeds that of endogenous TIA-1/R, allowing the identification of transfected cells; A and D), T7 (B and E) or endogenous TIA-1/R (C and F, anti-3E6). Paired fields (B and C and E and F) are shown. Bar, 10 μm.
HSP27, Poly(A)+ RNA, and PABP-I Are Components of SGs
Low molecular weight heat shock proteins are prominent components of SGs in both plant and animal cells (Arrigo et al. 1988; Collier et al. 1988; Scharf et al. 1998). To clarify the relationship between TIA-1/R+, tomato, and mammalian SGs, we used immunofluorescence microscopy to compare the subcellular localization of TIA-1/R and HSP27 in DU145 cells before and after heat shock. Whereas TIA-1/R are concentrated in the nucleus in the absence of stress (Fig. 3 A), heat shock induces the formation of TIA-1/R+ SGs (Fig. 3 B). As previously reported (Arrigo et al. 1988; Loktionova et al. 1996), heat shock induces the translocation of HSP27 from the cytoplasm to the nucleus (Fig. 3, compare C with D). A subpopulation of the HSP27 that remains in the cytoplasm coaggregates with TIA-1/R at SGs (Fig. 3 D). Whereas virtually all cytoplasmic TIA-1/R associates with SGs formed in response to many different stresses, little or no HSP27 associates with SGs formed in response to arsenite or UV irradiation (data not shown). These results suggest that HSP27+ SGs constitute a subset of TIA-1/R+ SGs, and argue against a direct role for HSP27 in nucleating SG formation.
Figure 3.
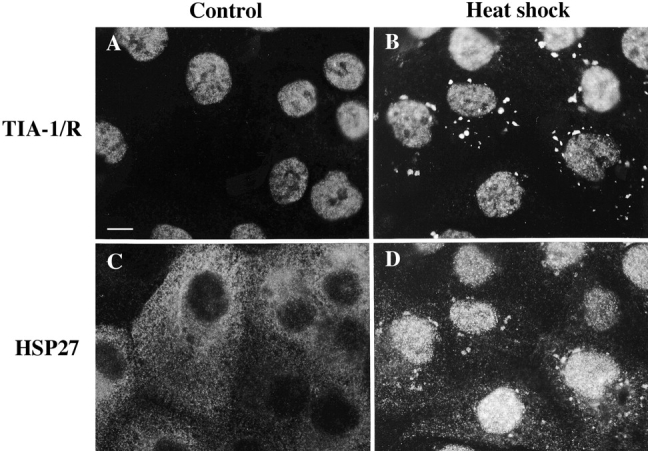
Recruitment of HSP27 to SGs. DU145 cells were untreated (A and C) or exposed to heat shock (45°C for 20 min, B and D) and stained for both TIA-1/R (A and B, anti-3E6) and HSP27 (C and D). Bar, 10 μm.
In cultured tomato cells, SGs are sites at which untranslated mRNA accumulates in response to stress (Nover et al. 1989). Therefore, we used in situ hybridization to compare the subcellular localization of poly(A)+ RNA and TIA-1/R in DU145 cells before and after arsenite-induced oxidative stress. In untreated cells, both poly(A)+ RNA (Fig. 4 A) and TIA-1/R (Fig. 4 D) are distributed between the nucleus and the cytoplasm. After exposure to a sublethal concentration of arsenite (0.5 mM) for 1 h (Fig. 4 B), poly(A)+ RNA accumulates at discrete cytoplasmic aggregates that are coincident with TIA-1/R+ SGs (Fig. 4 E). When arsenite-treated cells are allowed to recover for 3 h in arsenite-free media, aggregates of poly(A)+ RNA (Fig. 4 C) and TIA-1/R (Fig. 4 F) coincidentally disappear. No signal was observed in cells processed for in situ hybridization in the absence of oligo-dT or in cells stained with an isotype-matched control mAb (data not shown). In situ hybridization revealed that poly(A)+ RNA also accumulates at SGs formed in response to sublethal heat shock (data not shown).
In contrast to the reversible recruitment of poly(A)+ RNA to TIA-1/R+ SGs in cells treated with a sublethal dose of arsenite, cells exposed to a lethal dose of arsenite exhibit SGs that persist until the apoptotic death of the cell. Fig. 5 shows the colocalization of poly(A)+ RNA (Fig. 5 A) and TIA-1/R (Fig. 5 D) at SGs induced by arsenite (2 mM, 1 h). After 3 h in the absence of arsenite, poly(A)+ RNA (Fig. 5 B) and TIA-1/R (Fig. 5 E) remain at SGs. At this time, Hoechst staining reveals nuclear DNA condensation characteristic of apoptosis (Fig. 5 C), whereas the nuclei of untreated cells appear normal (Fig. 5 F). Thus, the persistence of SGs correlates with the level of stress. We used antibodies reactive with ribosomal P antigen to confirm that the large ribosomal subunit is not a component of SGs, consistent with the idea that these particles are sites at which untranslated mRNAs accumulate in stressed cells (data not shown).
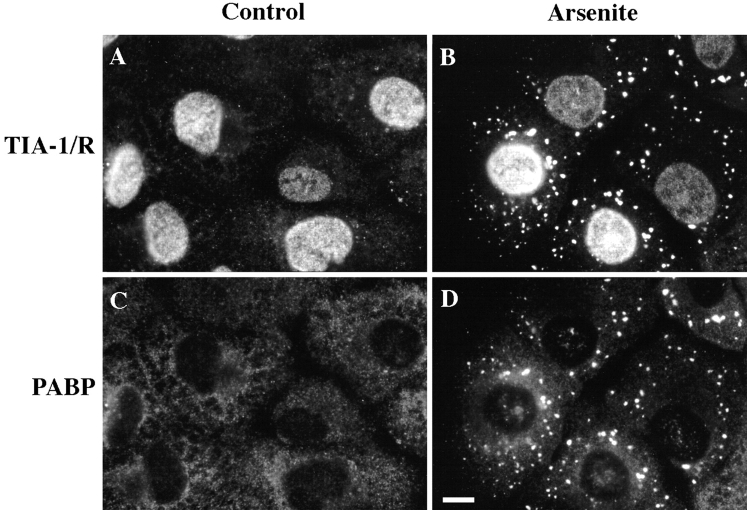
The identification of poly(A)+ RNA as a component of SGs suggested that proteins known to associate with poly(A)+ RNA might also be components of SGs. Therefore, we compared the subcellular localization of PABP-I in DU145 cells in the absence or presence of arsenite. As expected, TIA-1/R resides in the nucleus (Fig. 6 A), whereas PABP-I resides in the cytoplasm (Fig. 6 C, same field as A) in unstressed cells. In response to arsenite-induced oxidative stress, both TIA-1/R (Fig. 6 B) and PABP-I (Fig. 6 D, same field as B) coaggregate at SGs. Whereas the cytoplasmic aggregation of TIA-1/R is nearly quantitative, a significant amount of PABP-I is excluded from these cytoplasmic aggregates, consistent with its association with mRNAs that continue to be translated during stress (i.e., heat shock mRNAs). Sucrose gradient fractionation (data not shown) was performed on both stressed and unstressed cells, and confirmed that PABP-I exhibited a shift from large polysomes in unstressed cells to monosomes and small polysomes in stressed cells, while both TIAR and TIA-1 were found in the light mRNP fractions smaller than monosomes in both stressed and unstressed cells.
Figure 6.
PABP-I is a component of SGs. Control (A and C) or arsenite-treated (0.5 mM for 40 min, B and D) DU145 cells were fixed, permeabilized, and processed for two-color immunofluorescence using antibodies to TIA-1/R (A and B, anti-3E6) or PABP-I (C and D). Both TIA-1/R and PABP-I are clearly recruited to the SGs upon stress (B and D, paired views of same field). Bar, 10 μm.
Phosphorylation of eIF-2α Is Necessary and Sufficient for the Assembly of TIA-1/R+ SGs
The rapid assembly and disassembly of poly(A)+ RNA–containing SGs in DU145 cells subjected to arsenite treatment or heat shock suggests that SGs are sites at which untranslated mRNAs accumulate in response to stress. The primary determinant of stress-induced translational arrest is the phosphorylation of eIF-2α that prevents the assembly of the 43S preinitiation complex. Therefore, we monitored the assembly of SGs in COS transfectants expressing wild-type or mutant eIF-2α. As shown in Fig. 7 A, COS cells were cotransfected with cDNAs encoding β-galactosidase (as a marker of transfected cells) and either vector alone, wild-type eIF-2α, or mutant eIF-2α, and then processed for two-color immunofluorescence microscopy to visualize β-galactosidase (green) and TIA-1 (red). In the absence of arsenite, cells transfected with the S51D phosphomimetic eIF-2α mutant formed TIA-1/TIAR+ SGs, whereas most cells transfected with vector alone, wild-type eIF-2α or the S51A nonphosphorylatable eIF-2α mutant did not (Fig. 7 A, left). In situ hybridization confirmed that the SGs assembled in S51D-transfected cells include poly(A)+ RNA (data not shown). The induction of SGs by S51D correlates with the ability of the S51D mutant to inhibit protein translation of cotransfected β-galactosidase as seen by immunofluorescence (β-galactosidase staining in the S51D cells was appreciably dimmer) and confirmed by Western blot (see below). When transfected cells were treated with arsenite, S51A nonphosphorylatable mutant eIF-2α prevented the formation of TIA-1/TIAR+ SGs (arrows, right), whereas wild-type and S51D mutants did not (Fig. 7 A, right).
The percentage of transfected cells containing TIA-1/R+ SGs (averaged over three independent experiments) is presented in Fig. 7 B. Cells transfected with the reporter β-galactosidase plasmid and empty pETF vector displayed SGs in 25% of the transfected COS cells, even without exposing the cells to arsenite (Fig. 7 B). In cells transfected with wild-type eIF2-α or the nonphosphorylatable eIF2-α mutant S51A, the percentage of spontaneous SGs dropped to <13% and 5%, respectively. We reasoned that the presence of spontaneous SGs in the cells transfected with vector alone could be due to phosphorylation of endogenous eIF-2α by PKR, which phosphorylates eIF-2α in response to dsRNA and is activated by transfection with many plasmids (Davies et al. 1989). To test this hypothesis, we cotransfected a plasmid encoding VA-1 (designated pAdvantage), an adenoviral pol III RNA that inhibits PKR and is used to enhance the translational efficiency of transfected plasmids (Davies et al. 1989). Cotransfection with VA-1 reduced the transfection-induced assembly of SGs to 8% from 25% (Fig. 7 B, column 5), but did not prevent SG formation when the cells were subjected to arsenite treatment, suggesting that kinases other than PKR are likely to phosphorylate eIF-2-α in response to arsenite treatment.
In addition to serving as a marker of transfected cells, the expression of β-galactosidase was quantified by Western blot analysis (Fig. 7 C, top) to assess the degree of translational enhancement or repression mediated by the cotransfected wild-type and mutant forms of eIF-2α and VA-1 RNA. The amount of β-galactosidase obtained was significantly increased when either mutant S51A (Fig. 7 C, lane 2) or wild-type (Fig. 7 C, lane 4) eIF-2α was cotransfected relative to the amount obtained with cotransfected empty vector (Fig. 7 C, lane 1). The cotransfection of the PKR inhibitor VA-1 RNA (Fig. 7 C, lane 5) modestly increased β-galactosidase expression relative to the vector control. In contrast, cotransfection of S51D mutant eIF-2α reduced the expression of β-galactosidase to undetectable levels by Western blot (Fig. 7 C, top, lane 3), although protein was detectable in individual cells by immunofluorescence. In addition to repressing the expression of β-galactosidase, S51D mutant eIF-2α dramatically reduced its own expression (Fig. 7 C, bottom, lane 3), although it was weakly detectable in individual cells by immunofluorescence (data not shown). Taken together, the data indicate that the phosphorylation of eIF-2α induces SGs and reduces expression of transfected proteins.
Mutant TIA-1 Prevents the Recruitment of Poly(A)+ RNA to SGs
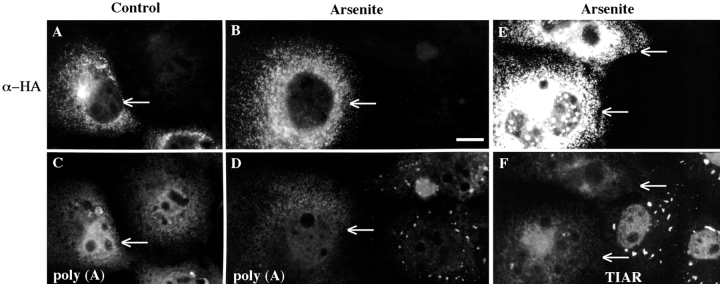
The potential for TIA-1 to directly promote the assembly of SGs was determined by comparing the localization of TIA-1/R and poly(A)+ RNA in COS cells expressing HA-tagged recombinant TIA-1ΔRRM, a truncation mutant that is incapable of binding RNA (Tian et al. 1991), in the absence or presence of arsenite. Recombinant wild-type TIA-1 is distributed between the nucleus and the cytoplasm in a distribution similar to the endogenous protein and accumulates at SGs in cells treated with arsenite (Fig. 2). In contrast, recombinant HA-TIA-1ΔRRM is concentrated in the cytoplasm, where it forms homogeneous microaggregates (Fig. 8 A). In situ hybridization reveals that the distribution of poly(A)+ RNA is similar in transfected and untransfected cells (Fig. 8 C, arrows point to transfected cells). In cells subjected to arsenite-induced stress, the distribution of recombinant HA-TIA-1ΔRRM is not significantly altered (Fig. 8 B). Whereas poly(A)+ RNA accumulates at SGs in untransfected cells (Fig. 8 D), SG formation is prevented in cells transfected with HA-TIA-1ΔRRM (Fig. 8B and Fig. D, paired views of the same field). The aggregation of endogenous TIAR (revealed using mAb 6E3, which recognizes an epitope within the RRM domains deleted in the mutant construct) is also prevented in cells transfected with HA-TIA-1ΔRRM (Fig. 8E and Fig. F, paired views of the same field, arrows point to transfected cells). Even in the absence of stress, the expression of HA-TIA-1ΔRRM coaggregates all detectable full-length endogenous TIAR and TIA-1 into the cytoplasmic microaggregates. These results indicate that TIA-1ΔRRM blocks both the recruitment of endogenous TIAR/1 and of poly(A)+ RNA to SGs in response to arsenite, thereby acting as a transdominant inhibitor of SG formation.
Figure 8.
Effect of HA-TIA-1ΔRRM on arsenite-induced assembly of SGs. COS cells were transiently transfected with recombinant HA-TIA-1ΔRRM, allowed to express protein for 48 h, and either untreated (A and C, paired views of same fields) or treated (B and D–F, paired views of same fields) with 0.5 mM sodium arsenite for 1 h before fixation and double staining for poly(A)+ RNA by in situ hybridization (C and D), and either HA-TIA-1ΔRRM (A, B, E) or endogenous TIAR (F) by immunofluorescence microscopy using mAbs reactive with the HA tag (A, B, and E) or endogenous TIAR (6E3). Arrows point out transfected cells. Bar, 10 μm.
Discussion
Heat stress granules are phase-dense particles that appear in the cytoplasm of plant (Nover et al. 1983, Nover et al. 1989; Scharf et al. 1998) and animal (Collier and Schlesinger 1986; Arrigo et al. 1988; Collier et al. 1988) cells exposed to supra-ambient temperatures. In cultured Peruvian tomato cells, SGs have been shown to harbor untranslated mRNAs that accumulate as a consequence of stress-induced translational arrest (Nover et al. 1989; Scharf et al. 1998). We have used in situ hybridization to show that poly(A)+ RNA is also a component of mammalian SGs formed in response to a variety of environmental stresses (i.e., heat, UV, hyperosmolarity, arsenite). Intact ribosomes are not components of poly(A)+ RNA–containing SGs, which is consistent with the hypothesis that mammalian SGs, like their plant counterparts, harbor untranslated mRNAs. The phase-dense cytoplasmic aggregates formed in heat shocked HeLa cells have been reported to contain HSP27 (Collier and Schlesinger 1986; Arrigo et al. 1988; Collier et al. 1988). We have confirmed that HSP27 is a component of SGs formed in heat shocked DU145 cells. Surprisingly, HSP27 is not a component of SGs formed in arsenite-treated or UV-irradiated DU145 cells, indicating that HSP27 is not a general component of all SGs (operationally defined as the cytoplasmic foci at which untranslated mRNAs accumulate in response to stress). In addition to HSP27, we have identified the RNA-binding proteins TIA-1, TIAR, and PABP-I as components of SGs. TIA-1 and TIAR are invariant components of (poly(A)+RNA–containing)-SGs formed by diverse stimuli in every cell type examined (i.e., DU145, MCF7, COS, NIH3T3, primary fibroblasts, HeLa, C6 glimoma, CaOV3, SW1573, and rat-1 cells; Kedersha, N., and P. Anderson, unpublished results), suggesting that these proteins are core components of SGs that might recruit untranslated mRNAs to these cytoplasmic foci in response to many stimuli other than heat shock.
Our results redefine mammalian SGs as RNP aggregates or cytoplasmic foci at which untranslated mRNAs are actively sequestered by TIA-1/TIAR in response to the stress-induced phosphorylation of eIF-2α. This expanded definition includes the SGs induced by heat shock that include low molecular weight HSPs as well as SGs induced by arsenite and UV irradiation, which do not include low molecular weight HSPs as major components. By this definition, SGs are discrete cytoplasmic foci that arise as a result of altered RNA metabolism. Many nuclear structures such as nuclear speckles, nuclear dots, coiled bodies, GEMS, and nucleoli constitute prominent nuclear substructures whose morphology is a byproduct of RNA metabolism (Spector 1993). The SG appears to be an analogous cytoplasmic structure whose morphology is dependent on events downstream of the stress-induced phosphorylation of eIF-2α. A family of stress-activated eIF-2α kinases appears to monitor different types of cellular stress. Whereas PKR is a double-stranded RNA-dependent kinase that is activated by viral infection and selected environmental stresses (Williams 1997), PERK/PEK is a resident ER protein that is activated by changes in glycoprotein metabolism (Shi et al. 1998; Harding et al. 1999). A vertebrate orthologue of yeast, GCN2 kinase has been proposed to regulate the cellular response to amino acid starvation (Olsen et al. 1998). Finally, a heme-regulated eIF-2α kinase that ensures the balanced synthesis of globin chains and heme during erythrocyte maturation might also monitor the stress response in nonerythroid cells (Berlanga et al. 1998). Whereas VA-1, an adenoviral RNA that specifically inactivates PKR (Mathews and Shenk 1991), inhibits transfection-induced assembly of SGs, it does not inhibit arsenite-induced assembly of SGs. This result suggests that PKR, and at least one other eIF-2α kinase can regulate the assembly of SGs. PERK/PEK is not significantly activated by arsenite (Harding et al. 1999), suggesting that heme-regulated eIF-2α kinase, GCN2, or an unidentified eIF-2α kinase is responsible for the VA-1–resistant assembly of SGs in cells treated with arsenite. It will be important to compare the assembly of SGs in response to environmental stresses that target different eIF-2α kinases.
The ability of TIA-1ΔRRM to function as a transdominant inhibitor of the arsenite-induced recruitment of poly(A)+ RNA to SGs implicates TIA-1 (and possibly TIAR) as an effector of SG assembly. The two functional domains of TIA-1 (and TIAR) confer the requisite properties required for the assembly of SGs: self-aggregation and RNA binding (Dember et al. 1996). The COOH terminus of TIA-1 (i.e., TIA-1ΔRRM) is structurally related to both high molecular weight glutenin (27% amino acid sequence identity over the last 90 amino acids of TIA-1) (Shani et al. 1994), and prion protein (38% amino acid identity over 50 amino acids with the introduction of one gap), both of which are glutamine-rich proteins prone to self-aggregation (Prusiner 1989; Prusiner et al. 1990). Recombinant TIA-1ΔRRM forms cytoplasmic microaggregates when overexpressed in COS cells, indicating that this prion-related domain can self-aggregate in vivo. Moreover, these microaggregates recruit endogenous TIA-1 and TIAR in the absense of stress (data not shown), and prevent the relocalization of TIA-1, TIAR, and poly(A)+ RNA into SGs in response to arsenite-induced oxidative stress. By trapping endogenous TIA-1/R in TIA-1ΔRRM microaggregates, it is likely that the mutant blocks the recruitment of poly(A)+ RNA to SGs by disrupting the normal shuttling of TIA-1/R, thereby preventing its association with nuclear mRNA transcripts.
In addition to promoting the assembly of SGs, other studies suggest that TIA-1 and TIAR contribute to the translational arrest of specific mRNAs. Recently, TIAR has been shown to be a component of a translational repressor complex that binds to an AU-rich element in the 3′ untranslated region of TNF-α transcripts (Gueydan et al. 1999). In resting macrophages, TNF-α transcripts are abundantly expressed, but excluded from polysomes (Crawford et al. 1997; Han et al. 1990). In response to lipopolysaccharide, TNF-α transcripts move to polysomes, allowing the translation and secretion of TNF-α protein (Crawford et al. 1997). It remains to be determined whether posttranscriptional regulation of TNF-α occurs at the level of translational initiation, polyadenylation, or mRNA stability. Other recent results indicate that AU-rich element–binding proteins related to TIA-1 and TIAR can promote the assembly of cytoplasmic aggregates that might be sites of cytoplasmic deadenylation or readenylation (Antic and Keene 1998; Ford et al. 1999). These results suggest that the ability of TIA-1 and TIAR to promote the aggregation, and/or translational repression, of mRNA may be part of a general translational control mechanism. It is likely that the assembly of microscopically visible SGs in response to stress constitutes an extreme example of this regulatory mechanism.
A role for SGs as effectors of stress-induced translational arrest could explain the imperfect correlation between phosphorylation of eIF-2α and translational arrest that exists in some experimental systems. Thus, translational arrest in heat shocked HeLa cells occurs in the absence of detectable phospho-eIF-2α (Duncan and Hershey 1989). More remarkably, heat shock–induced phosphorylation of eIF-2α is transient under conditions in which translational arrest is persistent (DeBenedetti and Baglioni 1986). These results suggest that phospho-eIF-2α is required for the initiation, but not the maintenance, of translation arrest. If dephosphorylation of eIF-2α restores the initiation machinery in the continued presence of stress, sequestration of mRNA at the SG may become a major determinant of translational arrest. Indeed, this mechanism might also explain in part how heat shock transcripts can be translated in stressed cells, inasmuch as heat shock transcripts are selectively excluded from heat SGs in tomato cells (Nover et al. 1989; Scharf et al. 1998). As we have now identified eIF-2α phosphorylation as the molecular event that initiates SG formation, and have more completely defined the composition and nature of the SG, the issue of whether mammalian SGs directly influence the duration and specificity of stress-induced translational arrest can be addressed in future studies.
Acknowledgments
The authors thank Susan Kim for help with graphics, Dr. P.J. Utz for reagents and advice about sucrose gradients, and members of the Anderson lab for suggestions and advice. We also thank Dr. Michel Streuli for advice and valuable discussions. We thank Drs. Randal Kaufman and Bryan Williams for cDNAs encoding eIF-2.
This work was supported, in part, by the National Institutes of Health grants AI33600 and CA67929, and by a grant from the Arthritis Foundation. P. Anderson is a Scholar of the Leukemia Society of America.
Footnotes
Abbreviations used in this paper: HA, hemagglutinin; HSG, heat shock granule; HSP, heat shock protein; PABP-I, poly (A)+ binding protein I; RNP, ribonuclear protein, SG, stress granule.
References
- Adam S., Nakagawa T., Swanson M., Woodruff T., Dreyfuss G. mRNA polyadenylate-binding proteingene isolation and sequencing and identification of a ribonucleoprotein consensus sequence. Mol. Cell. Biol. 1986;6:2932–2943. doi: 10.1128/mcb.6.8.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antic D., Keene J. Messenger RNP complexes containing human ELAV proteinsinteractions with cytoskeleton and translational apparatus. J. Cell Sci. 1998;111:183–197. doi: 10.1242/jcs.111.2.183. [DOI] [PubMed] [Google Scholar]
- Arrigo A.-P., Suhan J., Welch W. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol. Cell. Biol. 1988;8:5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A., Miller I., Anderson P., Streuli M. RNA-binding protein TIAR is essential for primordial germ cell development. Proc. Natl. Acad. Sci. USA. 1998;95:2331–2336. doi: 10.1073/pnas.95.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga J., Herrero S., De Haro C. Characterization of the hemin-sensitive eukaryotic initiation factor 2a kinase from mouse nonerythroid cells. J. Biol. Chem. 1998;273:32340–32346. doi: 10.1074/jbc.273.48.32340. [DOI] [PubMed] [Google Scholar]
- Brostrom C., Brostrom M. Regulation of translational initiation during cellular responses to stress. Prog. Nucleic Acid Res. 1998;58:79–125. doi: 10.1016/s0079-6603(08)60034-3. [DOI] [PubMed] [Google Scholar]
- Burd C., Matunis E., Dreyfuss G. The multiple RNA-binding domains of the mRNA poly(A)-binding protein have different RNA-binding activities. Mol. Cell. Biol. 1991;11:3419–3424. doi: 10.1128/mcb.11.7.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres J., Stamm S., Helfman D., Krainer A. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science. 1994;265:1706–1709. doi: 10.1126/science.8085156. [DOI] [PubMed] [Google Scholar]
- Collier N.C., Schlesinger M.J. The dynamic state of heat shock proteins in chicken embryo fibroblasts. J. Cell Biol. 1986;103:1495–1507. doi: 10.1083/jcb.103.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier N.C., Heuser J., Levy M.A., Schlesinger M.J. Ultrastructural and biochemical analysis of the stress granule in chicken embryo fibroblasts. J. Cell Biol. 1988;106:1131–1139. doi: 10.1083/jcb.106.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford E.K., Ensor J.E., Kalvakolanu I., Hasday J.D. The role of 3′ poly (A) tail metabolism in tumor necrosis factor-alpha regulation. J. Biol. Chem. 1997;272:21120–21127. doi: 10.1074/jbc.272.34.21120. [DOI] [PubMed] [Google Scholar]
- Davies M., Furtando M., Hershey J., Thimmappaya B., Kaufman R. Complementation of adenovirus-associated RNA I gene deletion by expression of a mutant eukaryotic translation initiation factor. Proc. Natl. Acad. Sci. USA. 1989;86:9163–9167. doi: 10.1073/pnas.86.23.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBenedetti A., Baglioni C. Activation of hemin-regulated initiation factor-2 kinase in heat shocked HeLa cells. J. Biol. Chem. 1986;261:338–342. [PubMed] [Google Scholar]
- Dember L.M., Kim N.D., Liu K.-Q., Anderson P. Individual RNA recognition motifs of TIA-1 and TIAR have different RNA binding specificities. J. Biol. Chem. 1996;271:2783–2788. doi: 10.1074/jbc.271.5.2783. [DOI] [PubMed] [Google Scholar]
- Duncan R., Hershey J.W.B. Protein synthesis and protein phosphorylation during heat stress, recovery, and adaption. J. Cell Biol. 1989;109:1467–1481. doi: 10.1083/jcb.109.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L.P., Watson J., Keene J.D., Wilusz J. ELAV proteins stabilize deadenylated intermediates in a novel in vitro mRNA deadenylation/degradation system. Genes Dev. 1999;13:188–201. doi: 10.1101/gad.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y.S., Yumura S., Yumur T.K. Agar-overlay immunofluorescencehigh resolution of cytoskeletal components and their changes during chemotaxis. Methods Cell Biol. 1987;28:347–356. doi: 10.1016/s0091-679x(08)61655-6. [DOI] [PubMed] [Google Scholar]
- Gray N.K., Wickens M. Control of translation initiation in animals. Annu. Rev. Cell Dev. Biol. 1998;14:399–458. doi: 10.1146/annurev.cellbio.14.1.399. [DOI] [PubMed] [Google Scholar]
- Gueydan C., Droogmans L., Chalon P., Huez G., Caput D., Kruys V. Identification of TIAR as a protein binding to the translational regulatory AU-rich element of tumor necrosis factor a mRNA. J. Biol. Chem. 1999;274:1–5. doi: 10.1074/jbc.274.4.2322. [DOI] [PubMed] [Google Scholar]
- Han J., Brown T., Beutler B. Endotoxin-responsive sequences control cachectin/tumor necrosis factor biosynthesis at the translational level. J. Exp. Med. 1990;171:465–475. doi: 10.1084/jem.171.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H.P., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Kawakami A., Tian Q., Duan X., Streuli M., Schlossman S.F., Anderson P. Identification and functional characterization of a TIA-1 related nucleolysin. Proc. Natl. Acad. Sci. USA. 1992;89:8681–8685. doi: 10.1073/pnas.89.18.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A., Tian Q., Streuli M., Poe M., Edelhoff S., Disteche C.M., Anderson P. Intron-exon organization and chromosomal localization of the human TIA-1 gene. J. Immunol. 1994;152:4937–4945. [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981;293:311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- Loktionova S., Ilyinskaya O., Gavai V., Kabakov A. Distinct effects of heat shock and ATP depletion on distribution and isoform patterns of human HSP27 in endothelial cells. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1996;392:100–104. doi: 10.1016/0014-5793(96)00792-2. [DOI] [PubMed] [Google Scholar]
- Mathews M., Shenk T. Adenovirus associated RNA and translational control. J. Virol. 1991;65:5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis M.J., Matunis E.L., Dreyfuss G. PUB1a major yeast poly(A)+ RNA-binding protein. Mol. Cell. Biol. 1993;13:6114–6123. doi: 10.1128/mcb.13.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K., Svitkin Y.V., Lee H.S., Lejbkowicz F., Kenan D.J., Chan E.K., Agol V.I., Keene J.D., Sonenberg N. La autoantigen enhances and corrects aberrant translation of polivirus RNA in reticulocyte lysate. J. Virol. 1993;67:3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtha-Riel P., Davies M.V., Scherer B.J., Choi S.-Y., Hershey J.W., Kaufman R.J. Expression of a phosphorylation-resistant eukaryotic initiation factor 2 alpha-subunit mitigates heat shock inhibition of protein synthesis. J. Biol. Chem. 1993;268:12946–12951. [PubMed] [Google Scholar]
- Nover L., Scharf K.D., Neumann D. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol. Cell. Biol. 1983;3:1648–1655. doi: 10.1128/mcb.3.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L., Scharf K.-D., Neumann D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol. Cell. Biol. 1989;9:1298–1308. doi: 10.1128/mcb.9.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen D., Jordan B., Chen D., Wek R., Cavener D. Isolation of the gene encoding the Drosophila melanogaster homolog of the Saccharomyces cerevisiae GCN2 eIF-2alpha kinase. Genetics. 1998;149:1495–1509. doi: 10.1093/genetics/149.3.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panniers R., Henshaw E.C. Mechanism of inhibition of polypeptide chain initiation in heat shocked ehrlich ascites tumor cells. Eur. J. Biochem. 1984;140:209–214. doi: 10.1111/j.1432-1033.1984.tb08088.x. [DOI] [PubMed] [Google Scholar]
- Pinol-Roma S., Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature. 1992;355:730–732. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- Prusiner S.B. Scrapie prions. Annu. Rev. Microbiol. 1989;43:345–374. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- Prusiner S.B., Scott M., Foster D., Pan K.-M., Groth D., Mirenda C., Torchia M., Yang S.-L., Serban D., Carlson G.A. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990;63:673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Query C.C., Bently R.C., Keene J.D. A common RNA recognition motif identified within a defined U1 RNA binding domain fo the 70 K U1 snRNP protein. Cell. 1989;57:89–101. doi: 10.1016/0092-8674(89)90175-x. [DOI] [PubMed] [Google Scholar]
- Scharf K.-D., Heider H., Hohfeld I., Lyck R., Schmidt E., Nover L. The tomato Hsf systemHsfA2 needs interaction with HsfA1 for efficient nuclear import and may be localized in cytoplasmic heat stress granules. Mol. Cell. Biol. 1998;18:2240–2251. doi: 10.1128/mcb.18.4.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani N., Tosenberg N., Kasarda D., Galili G. Mechanisms of assembly of wheat high molecular weight glutenins inferred from expression of wild-type and mutant subunits in transgenic tobacco. J. Biol. Chem. 1994;269:8924–8930. [PubMed] [Google Scholar]
- Shi Y., Vattem K.M., Sood R., An J., Liang J., Stramm L., Wek R.C. Identification and characterization of pancreatic eukaryotic initiation factor 2 a subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. Macromolecular domains within the cell nucleus. Annu. Rev. Cell Biol. 1993;9:265–315. doi: 10.1146/annurev.cb.09.110193.001405. [DOI] [PubMed] [Google Scholar]
- Spector, D., R. Goldman, and L. Leinwand. 1998. In situ hybridization to RNA. In Cells: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 116.110–116.111.
- Srivastava S.P., Kumar K.U., Kaufman R.J. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J. Biol. Chem. 1998;273:2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- Storti R.V., Scott M.P., Rich A., Pardue M.L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980;22:825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Taupin J.L., Tian Q., Kedersha N., Robertson M., Anderson P. The RNA-binding protein TIAR is translocated from the nucleus to the cytoplasm during Fas-mediated apoptotic cell death. Proc. Natl. Acad. Sci. USA. 1995;92:1629–1633. doi: 10.1073/pnas.92.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q., Streuli M., Saito H., Schlossman S.F., Anderson P. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell. 1991;67:629–639. doi: 10.1016/0092-8674(91)90536-8. [DOI] [PubMed] [Google Scholar]
- Tian Q., Taupin J., Elledge S., Robertson M., Anderson P. Fas-activated serine/threonine kinase phosphorylates TIA-1 during Fas-mediated apoptosis. J. Exp. Med. 1995;182:865–874. doi: 10.1084/jem.182.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traish A., Huang Y., Pavao M., Pronovost M., Ashba J., McAneny D., Moreland R. Loss of expression of a 55 kD protein (nmt-55) in estrogen receptor-negative human breast cancer. Diag. Mol. Path. 1997;In press doi: 10.1097/00019606-199708000-00005. [DOI] [PubMed] [Google Scholar]
- Williams B. Role of the double stranded RNA-activated protein kinase (PKR) in cell regulation. Biochem. Soc. Trans. 1997;25:509–513. doi: 10.1042/bst0250509. [DOI] [PubMed] [Google Scholar]