Eb1 Proteins Regulate Microtubule Dynamics, Cell Polarity, and Chromosome Stability (original) (raw)
The EB1 family represents a highly conserved group of proteins, present in yeast through humans, that localize to spindle and cytoplasmic microtubules, especially at their distal tips. The budding yeast homologue of EB1, Bim1p, regulates microtubule stability and is important for positioning the mitotic spindle, anchoring it to the bud through astral microtubule attachment to the cortical protein Kar9p. Bim1p interacts functionally with dynactin in a late mitotic cell cycle checkpoint. EB1 proteins in human cells interact physically with the adenomatous polyposis coli (APC) tumor suppressor protein, targeting APC to microtubule plus ends, and with members of the dynactin complex. Here, we review recent studies in yeast and human cells that suggest the involvement of EB1 in promoting microtubule search and capture and in maintaining chromosome stability.
The EB1 Family
Human EB1 is a 35-kD, mildly acidic, leucine zipper protein cloned as an interacting partner with the APC COOH terminus in a yeast two-hybrid screen (see Fig. 1 A; Su et al. 1995). Because loss of heterozygosity at the APC tumor suppressor locus is an early event in most colon cancers, and germline APC mutation leads to the familial adenomatous polyposis (FAP) colon cancer syndrome (Kinzler and Vogelstein 1996), this interaction raises questions about the role of EB1 in facilitating APC functions. However, EB1 proteins are also found in unicellular organisms that lack APC, suggesting a more primitive role that predates the evolution of APC. In this review, we first focus on the functions of EB1 proteins in yeasts, and then we speculate on how these functions may have been adapted in multicellular eukaryotes.
Figure 1.
(A) Domain structure of the 2,843 amino acid APC protein. Only the β catenin, glycogen synthase kinase 3 β (GSK3β), microtubule, discs large, and EB1 binding regions are shown. The sites of most truncation mutations in human colon cancers are marked with arrows (adapted from Polakis 1997). (B) Sequence homology relationships among selected EB1 family members. Percentage identity at the protein level is indicated.
The full diversity of EB1 family members remains unknown at this time. To date, EB1 has been found in every organism and nearly every cell type examined, including neuronal, lymphocytic, and epithelial cells. Genome sequencing projects have revealed that budding yeast possess a single EB1 sequence homologue, called BIM1 (binding to microtubules 1), because the protein interacted with α-tubulin in a two-hybrid screen (Schwartz et al. 1997). The fission yeast homologue _mal3_+ was isolated in a screen for mutations that caused chromosome loss (Beinhauer et al. 1997). Caenorhabditis elegans has two EB1 related genes (GenBank accession numbers VW02B12L.3 and Y59A8B.P; these data were produced by the C. elegans Sequencing Group at the Sanger Centre and can be obtained from http://www.sanger.ac.uk/Projects/C\_elegans/), and Drosophila melanogaster has at least three EB1 family members (GenBank accession numbers [Benson et al. 2000] AAD27859, AAF46575, and AAF57623). The number of EB1 proteins in humans is unknown, but to date EB1, EB2, EB3, and EBF3 have been reported, along with the highly related RP1, RP2, and RP3 proteins (Su et al. 1995; Renner et al. 1997; Su, L.K., and Qi, Y., Association of APC with EB1 family proteins. 1998. 4193 (Abstr.); Juwana et al., 1999; Nakagawa et al., 2000). Recently, EB3 was shown to be neuronally expressed and to interact with a neuron-specific homologue of APC, APCL (Nakagawa et al. 2000). RP1 was identified by its induction upon T lymphocyte activation, and it shares APC binding and subcellular localization with EB1 (Renner et al. 1997; Juwana et al. 1999). The sequence relationships of selected EB1 proteins are shown in Fig. 1 B.
EB1 Proteins Localize to Microtubule Tips
In living yeast cells, overexpressed GFP-Bim1p localizes to the entire microtubule cytoskeleton, including the mitotic spindle, the spindle pole body (the budding yeast centrosome), and cytoplasmic microtubules (Schwartz et al. 1997). However, when native levels of expression are driven from the BIM1 promoter, fluorescence on cytoplasmic microtubules is limited to the microtubule distal tips (presumed to be plus ends) and the spindle pole body (Tirnauer et al. 1999). Together, these studies suggest that Bim1p is capable of binding along the length of microtubules, but that at endogenous levels, it binds preferentially to microtubule ends. Fluorescence at the spindle pole body may represent the physical association of Bim1p with centrosomal proteins, Bim1p binding to the microtubule proximal (minus) ends, or Bim1p binding to the distal (plus) ends of extremely short microtubules. Bim1p may bind to more than one of these structures, as it was present in a partially purified yeast spindle pole body preparation (Wigge et al. 1998).
In the cells of higher organisms, EB1 is found in a similar distribution, albeit on a more spatially extensive microtubule array. By indirect immunofluorescence in tissue culture cells, EB1 has been localized to the centrosome, the mitotic spindle, and the distal tips of cytoplasmic microtubules (Berrueta et al. 1998; Morrison et al. 1998). EB1 and RP1 staining also have been visualized at what appear to be microtubule ends within the mitotic spindle, possibly at or near kinetochores (Juwana et al. 1999). Thus, EB1 proteins are found on microtubule plus ends throughout the cell cycle in diverse cell types, a location ideally suited for linking the microtubule cytoskeleton to other structures within the cell. Although EB1 was named before its end-binding properties were known, end binding 1 aptly labels the protein.
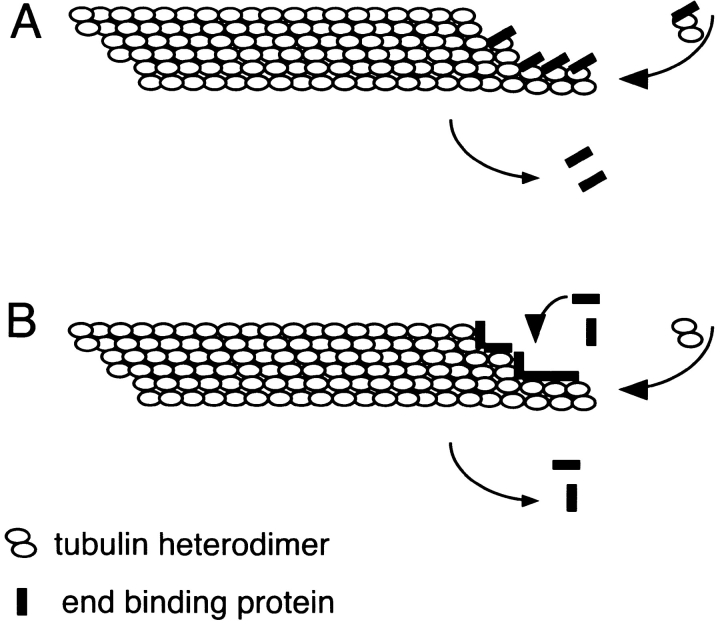
While numerous proteins localize along the lengths of microtubules, specific localization to microtubule plus ends is limited to relatively few. These include the KIN I kinesins, which bind to both plus and minus ends of microtubules in vitro and induce catastrophes (see ; Desai et al. 1999); members of the dynactin complex, a 20S multiprotein complex important for activating cytoplasmic dynein (Pfarr et al. 1990; Steuer et al. 1990; Vaisberg et al. 1993; Echeverri et al. 1996; Busson et al. 1998; Skop and White 1998; Vaughan et al. 1999); and the cytoplasmic microtubule–vesicle linker protein CLIP170. GFP-CLIP170 has been imaged in living tissue culture cells and shown to treadmill specifically along the plus ends of growing (but not shrinking) microtubules (Perez et al. 1999). While the mechanism remains unknown, proteins that bind to microtubule ends rather than along their lengths could do so by using dynamic or structural cues; for example, as diagrammed in Fig. 2, proteins that specifically recognize growing plus ends could copolymerize with tubulin (as proposed for CLIP170; Diamantopoulos et al. 1999), or they could recognize a specific conformation (such as the GTP cap or the unrolled sheet) at the growing microtubule end. It will be interesting to see whether EB1 binds microtubule plus ends directly or via another protein, and whether the microtubule tip localization shared by these proteins translates into physical and functional interactions among them.
Figure 2.
Models for microtubule tip localization. A protein specifically localized to the ends of growing microtubules could bind to tubulin heterodimers and copolymerize into the microtubule (A), or recognize a specific conformation at the growing microtubule end, such as the GTP cap or the unrolled sheet (B). Dissociation from the microtubule could result from exclusion as the protofilament seam closes or from the tension caused by seam closure.
Role(s) of Budding Yeast EB1 in Spindle Positioning
Once bound, what do EB1 proteins do to microtubules? The first studies of EB1 function have come from yeast mutants. Yeast lacking the BIM1 gene are viable, but their cytoplasmic microtubules are shorter than those in wild-type cells and they show abnormalities in parameters of dynamic instability (see ). In _bim1_Δ cells, microtubules were found to depolymerize more slowly and to undergo fewer transitions and longer pauses, at the expense of growing, than in wild-type cells (Tirnauer et al. 1999). Thus, even though Bim1p increases the depolymerization rate, it promotes net polymerization by increasing both the time spent growing and the rescue frequency, resulting in microtubules that are longer as well as more dynamic. In wild-type budding yeast, microtubules are most dynamic during the G1 phase of the cell cycle, which is the phase when the spindle pole body moves toward the presumptive bud site in preparation for mitosis (Carminati and Stearns 1997). Interestingly, microtubules in the _bim1_Δ mutant are most abnormal during G1, and _bim1_Δ cells are defective in mitotic spindle positioning (Tirnauer et al. 1999). While spindle alignment across the bud neck is unique to budding yeast, the mechanisms used to achieve it are relevant to more universal processes, i.e., the capture of kinetochores by microtubules within the spindle.
In this issue, Adames and Cooper use time-lapse sequences from living yeast cells to dissect the spindle positioning process into two sequential steps (Adames and Cooper 2000). The first step consists of spindle movement to the bud neck, resulting from cytoplasmic microtubule capture and end-on depolymerization at the bud tip (or at the presumptive bud site, earlier in the cell cycle). Consistent with its localization to the microtubule tip, Bim1p is a central component of this first step. Recent studies by Lee et al. 2000 and Korinek et al. 2000 have revealed that Bim1p acts at this stage not just by increasing microtubule dynamicity, but in the capture event itself. By interacting with Kar9p, which is a cortical protein that appears as a dot at the bud tip (Miller and Rose 1998), Bim1p forms a physical link between the microtubule end and the bud, as depicted in Fig. 3. Bim1p and Kar9p coimmunoprecipitate from yeast cells and cofractionate by sucrose gradient sedimentation; in cells lacking Bim1p, microtubules fail to contact the Kar9p dot (Lee et al. 2000), demonstrating the functional importance of this interaction. Proteins with homology to KAR9 have not been found in multicellular eukaryotes, but mechanistically similar interactions are likely to occur.
Figure 3.
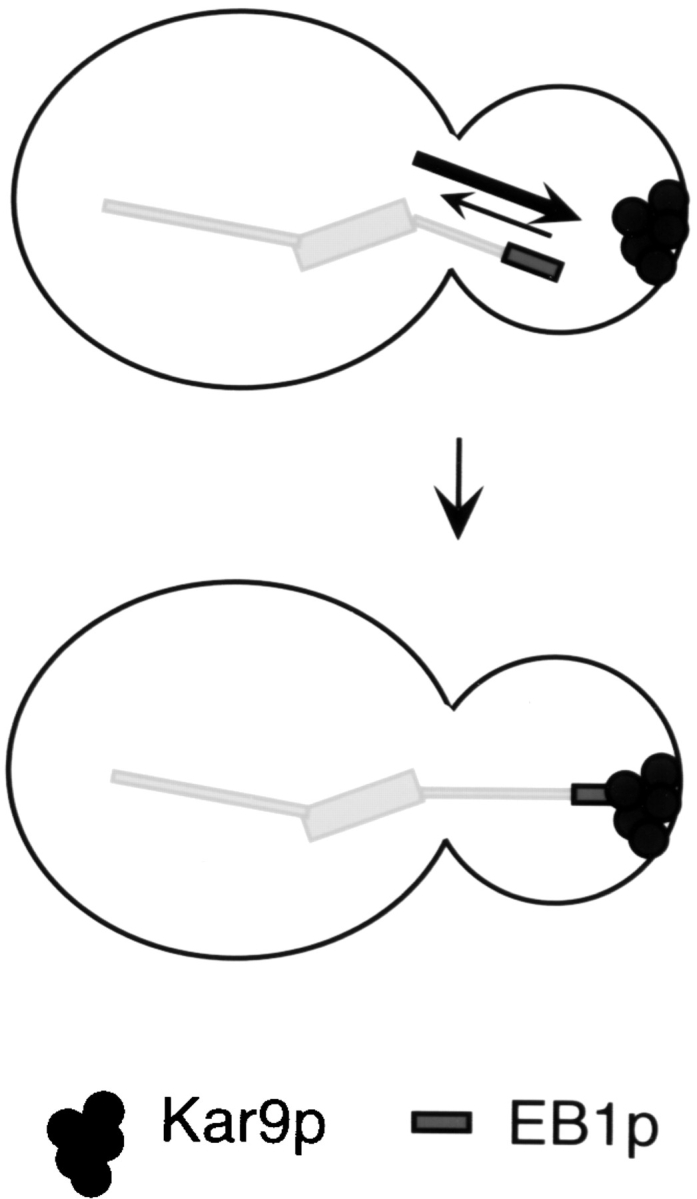
The first step of the spindle orientation process in budding yeast. Microtubule binding proteins and cortical proteins are required. In the example shown, at the microtubule tip, Bim1p both increases microtubule dynamicity (arrows) and binds Kar9p at the cortex, followed by depolymerization (Lee et al. 2000; Korinek et al. 2000). In the subsequent step, the spindle is pulled (and pushed) into the bud neck through dynein-dependent forces.
In the second step in spindle positioning, the cytoplasmic microtubule binds laterally and slides along the concavity of the bud, pulling the nucleus into the narrow bud neck (Adames and Cooper 2000). This step requires the minus end–directed motor cytoplasmic dynein and the associated dynactin complex. Interestingly, as discussed below, EB1 from human cells coimmunoprecipitates with dynactin components and dynein intermediate chain (Berrueta et al. 1999). In yeast, no physical interaction has been shown, but a functional connection suggests yet another role for Bim1p. It was previously shown that dynein and dynactin mutants delay cytokinesis until spindle position is corrected (Yeh et al. 1995; Muhua et al. 1998), leading to the concept of a cytokinesis checkpoint. BIM1 was one of two genes isolated in a screen for bypass of this checkpoint; cells mutated in the dynactin component ARP1 and lacking BIM1 failed to delay the cell cycle and underwent cytokinesis before spindle position was corrected, resulting in a lethal multinucleate phenotype, and raising the possibility that EB1 proteins play a role in sensing or communicating the spindle position to the cell cycle machinery (Muhua et al. 1998). The role for dynein in spindle movement may be conserved in multicellular organisms (Skop and White 1998), where stereotyped spindle rotations occur during early embryonic and neuronal development (for review see Lu et al. 1998).
Role of Fission Yeast EB1 in Chromosome Segregation
Proteins involved in aligning the spindle to the cortex are expected to overlap with those used within the spindle, during the comparable process of kinetochore capture. Both processes make use of connections between microtubules and a specialized capture site, and in both cases, successful capture is likely to be enhanced by increased microtubule dynamicity as well as by stabilized interactions between the microtubule end and the capture site. The critical events in chromosome segregation are monitored by the highly regulated spindle assembly checkpoint machinery. In fission yeast, the EB1 homologue mal3 + was cloned in a screen for novel mutants that failed to accurately segregate a nonessential minichromosome (Beinhauer et al. 1997). In addition to chromosome loss, _mal3_Δ cells also showed similar changes to _bim1_Δ cells in microtubule length and nuclear position, as well as abnormalities in cell shape and septum placement (Beinhauer et al. 1997). Whether chromosome loss in the _mal3_Δ mutant is due to defective kinetochore capture or to these other abnormalities is currently unknown, but the localization of EB1 proteins to spindle microtubules in diverse organisms suggests that they may act to regulate dynamic instability or microtubule end-on attachments in the spindle. Interestingly, human EB1 substituted functionally for Schizosaccharomyces pombe mal3 + in this study, highlighting the remarkable degree of conservation within the EB1 family.
Interaction of EB1 with APC
Chromosomal instability is a major defect in the cancer of the colon. Could the EB1–APC interaction play a role in regulating chromosome segregation? A large body of work has shown that by regulating β-catenin levels, APC affects the transcription of several oncogenic proteins including c-myc, cyclin D1, and PPARδ (He et al. 1998, He et al. 1999; Tetsu and McCormick 1999; for reviews see Polakis 1997; Barker et al. 2000; Nathke 2000). These transcriptional effects alone may account for the role of APC in preventing cancer development, but the question remains whether the interaction of APC with the cytoskeleton contributes to its tumor suppressor role. Transgenic mice, bearing an APC truncation distal to the β-catenin binding region (designed to address the function of the COOH-terminal, EB1-binding portion of APC; Fig. 1 A), showed no evidence of colon cancer (Smits et al. 1999), and a study of human colon tumors failed to detect somatic mutations in the EB1 coding sequence by reverse transcriptase single strand conformational polymorphism (Jais et al. 1998). However, the possibility remains that disruption of EB1–APC binding may play a role in later, accelerating events once tumorigenesis has been initiated, or it may be permissive for tumorigenesis only in the presence of other abnormalities. Understanding the role of EB1 in colon cancer, cell cycle progression, and chromosome stability is a significant objective for future studies.
One important aspect of the EB1–APC interaction may be its role in directing protein localization. Like EB1, APC localizes to the microtubule cytoskeleton, as well as to the leading edges of migrating epithelial cells (Nathke et al. 1996), and EB1 and RP1 colocalize with APC in the membrane protrusions of fibrosarcoma cells (Juwana et al. 1999). Recently, GFP–APC protein has been imaged in living cells and shown, like CLIP170, to associate specifically with the plus ends of growing microtubules, but, unlike CLIP170, to drop off the ends of shrinking microtubules as a preserved granular structure (Mimori-Kiyosue et al. 2000). In colon cancer cell lines that exclusively contain truncated APC (unable to bind EB1), endogenous EB1 localization is unchanged, suggesting that EB1 binds to microtubule tips independent of APC (Berrueta et al. 1998; Morrison et al. 1998). Transfection of these cells with GFP-APC constructs containing or lacking the EB1 binding region demonstrated that the EB1 binding region directed APC localization to microtubule tips; GFP-APC constructs lacking the EB1 binding region (but still able to bind microtubules) localized nonspecifically to microtubules, whereas APC constructs containing the EB1 binding region localized to the tips (Askham et al. 2000). This result is consistent with the earlier observation that APC possesses a microtubule binding domain and is capable of bundling microtubules in vitro (Munemitsu et al. 1994). Taken together, these findings suggest that while EB1 is not required for the binding of APC to microtubules, it may target APC to the ends, facilitating delivery or interaction of APC with specific sites at the cell membrane. In colon polyps, abnormalities in APC targeting could synergize with rapid cell proliferation to disrupt cell migration and/or accelerate aneuploidy.
Could the EB1–APC interaction have a role outside of the colon? FAP patients exhibit a variety of extracolonic manifestations, including skin and central nervous system tumors, suggesting a role for APC in other polarized tissues. Moreover, the finding of additional APC isoforms in Drosophila and the neuronal specific interaction between EB3 and APCL leaves open the possibility that other members of the EB1 or APC families may play an as-yet-undiscovered role in normal development and possibly in other disorders (Hayashi et al. 1997; Hamada et al. 1999; McCartney et al. 1999; Yu et al. 1999; Nakagawa et al. 2000). Binding between EB1 and APC is downregulated during mitosis, possibly because of mitotic phosphorylation of APC (Trzepacz et al. 1997; Askham et al. 2000). How cell cycle changes affect EB1, and how EB1 interacts with APC in other cell types are also open questions.
Interaction of EB1 with Dynactin Components
In higher eukaryotes, EB1 interacts with components of the dynactin complex, the activator for cytoplasmic dynein. Cytoplasmic dynein is a minus end–directed microtubule-based motor that, with the dynactin complex, participates in Golgi dynamics, vesicle transport, and focusing the poles of the mitotic spindle (for review see Karki and Holzbaur 1999). EB1 has been shown to coimmunoprecipitate the dynactin components p150glued, p50/dynamitin, and the intermediate chain of dynein, from lymphocytes and epithelial cells (Berrueta et al. 1999). This interaction occurred independently of microtubules, because it was preserved in cells treated with the microtubule-depolymerizing agent nocodazole, and independently of APC, as it occurred in cell lines lacking the COOH terminus of APC. Like EB1, dynactin has been localized to the plus ends of cytoplasmic microtubules at cortical sites in epithelial cells (Busson et al. 1998). As with APC, EB1 might regulate dynactin localization, or, reciprocally, dynactin may help to load EB1 onto the microtubules. A role for dynactin analogous to that in yeast is suggested by a study of the early spindle rotations of the developing C. elegans embryo (Skop and White 1998). Dynactin was shown to localize to the cortical sites and to be required for these spindle rotations. It will be interesting to see whether the C. elegans and D. melanogaster EB1 family members interact with dynactin and whether EB1 proteins play a role in spindle rotations in these organisms.
Effect of Paclitaxel on EB1 Localization
EB1 is a microtubule end–binding protein with several potential roles in normal cellular processes. Is it possible that anticancer therapies might affect EB1 function? Paclitaxel is a chemotherapeutic agent that reduces microtubule dynamicity without depolymerizing the microtubule cytoskeleton, and it is thought to cause malignant cells to arrest in mitosis because of spindle damage and, subsequently, to undergo apoptotic cell death. In tissue culture cells, paclitaxel treatment disrupted the localization of EB1 to the microtubules (Morrison et al. 1998). The mechanisms of this effect could include a change in the structure of the microtubule polymer (along its length or at its tip), or a requirement of dynamic microtubules to confer EB1 binding. The idea that the antitumor effects of paclitaxel could be mediated through EB1 must remain speculative, as this drug has shown little activity against colon cancer in clinical trials. Alternatively, the lack of clinical efficacy of paclitaxel in colon cancer may result from the absence of the EB1–APC interaction specifically in this setting. Both situations highlight the important possibility that natural products that affect the microtubule cytoskeleton might interact with or perturb endogenous microtubule end–binding proteins.
Summary
Functional studies of yeasts deficient in the microtubule end–binding EB1 proteins demonstrate their roles in several aspects of microtubule search and capture, cell polarization, and chromosome stability. In cells that require a cell cycle delay to correct spindle position abnormalities, Bim1p may participate in the checkpoint machinery as well. The EB1–APC interaction in higher eukaryotes could have arisen evolutionarily to take advantage of specific targeting to the microtubule tip. In multicellular organisms, the interaction between EB1 and dynactin components may have been adapted to exert force on microtubule structures in complex cellular behaviors important for development and cell migration. Many questions about EB1 function remain: how does EB1 influence chromosome segregation? Are EB1 proteins in multicellular organisms important for both spindle and cytoplasmic microtubule behaviors? What role does the loss of the EB1–APC interaction play in the pathogenesis of colon cancer? Are EB1 proteins important for dynactin or dynein function? And might drugs that specifically target EB1 play a role in cancer therapy? Future studies to address these questions are eagerly awaited.
Acknowledgments
The authors thank Ewan Morrison for the communication of results before publication, and Tim Mitchison and David Pellman for critical and helpful review of the manuscript.
J.S. Tirnauer is supported by a grant (K08 DK 02578) from the National Institutes of Health.
Dynamic instability, usually measured at the microtubule plus (distal) end, is defined by four parameters: the rates of growth, corresponding to polymerization; and shrinkage, corresponding to depolymerization; and the frequencies of transitions between growth and shrinkage (catastrophes) and between shrinkage and growth (rescues) (reviewed in Desai and Mitchison 1997). Two other terms used in reference to microtubule dynamics are pauses, periods during which length appears constant, reflecting either a non dynamic state or a state such as treadmilling in which polymerization at the plus end and depolymerization at the minus end are coupled; and dynamicity (Toso et al. 1993), a composite measurement of the total tubulin dimers gained or lost per unit time. Dynamicity is increased by faster growth or shrinkage or more frequent transitions. Microtubule length is likewise affected by all four parameters. Classically, greater dynamicity correlates with shorter length, and EB1 is unusual but not unique in increasing both dynamicity and microtubule length.
Measurements of dynamic instability in living cells can be affected by multiple factors; in addition to biological differences, methodological variables include cell temperature, choice of fluorescent protein marker, and imaging and software configurations. As an example, two studies of microtubule dynamics in the _bim1_Δ mutant (Tirnauer et al. 1999; Adames and Cooper 2000) both showed as the major effect reduced dynamicity and increased pausing, but changes in individual parameters differed somewhat between the studies.
References
- Adames N.R., Cooper J.A. Microtubule interactions with the cell cortex causing nuclear movements in Saccharomyces cerevisiae . J. Cell Biol. 2000;149:863–874. doi: 10.1083/jcb.149.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askham J.M., Moncur P., Markham A.F., Morrison E.E. Regulation and function of the interaction between the APC tumour suppressor protein and EB1. Oncogene. 2000;19:1950–1958. doi: 10.1038/sj.onc.1203498. [DOI] [PubMed] [Google Scholar]
- Barker N., Morin P.J., Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv. Cancer Res. 2000;77:1–24. doi: 10.1016/s0065-230x(08)60783-6. [DOI] [PubMed] [Google Scholar]
- Beinhauer J.D., Hagan I.M., Hegemann J.H., Fleig U. Mal3, the fission yeast homolog of the human APC-interacting protein EB-1, is required for microtubule integrity and the maintenance of cell form. J. Cell Biol. 1997;139:717–728. doi: 10.1083/jcb.139.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D.A., Karsch-Mizrachi I., Lipman D.J., Ostell J., Rapp B.A., Wheeler D.L. GenBank. Nucl. Acids Res. 2000;28:15–18. doi: 10.1093/nar/28.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L., Kraeft S.-K., Tirnauer J.S., Schuyler S., Chen L.B., Hill D.E., Pellman D., Bierer B.E. The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules. Proc. Natl. Acad. Sci. USA. 1998;95:10596–10601. doi: 10.1073/pnas.95.18.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L., Tirnauer J.S., Schuyler S.C., Pellman D., Bierer B.E. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr. Biol. 1999;9:425–428. doi: 10.1016/s0960-9822(99)80190-0. [DOI] [PubMed] [Google Scholar]
- Busson S., Dujardin D., Moreau A., Dompierre J., De May J.R. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 1998;9:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- Carminati J.L., Stearns T. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J. Cell Biol. 1997;138:629–641. doi: 10.1083/jcb.138.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A., Mitchison T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- Desai A., Verma S., Mitchison T.J., Walczak C. Kin I kinesins are microtubule destabilizing enzymes. Cell. 1999;96:69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Diamantopoulos G.S., Perez F., Goodson H.V., Batelier G., Melki R., Kreis T.E., Rickard J.E. Dynamic localization of CLIP-170 to microtubule plus ends is coupled to microtubule assembly. J. Cell Biol. 1999;144:99–112. doi: 10.1083/jcb.144.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri C.J., Paschal B.M., Vaughan K.T., Vallee R.B. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada F., Murata Y., Nishida A., Fujita F., Tomoyasu Y., Nakamura M., Toyoshima K., Tabata T., Ueno N., Akiyama T. Identification and characterization of E-APC, a novel Drosophila homologue of the tumour suppressor APC. Genes Cells. 1999;4:465–474. doi: 10.1046/j.1365-2443.1999.00272.x. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Rubinfeld B., Souza B., Polakis P., Wieschaus E., Levine A.J. A Drosophila homolog of the tumor suppressor gene adenomatous polyposis coli down-regulates beta-catenin but its zygotic expression is not essential for the regulation of Armadillo. Proc. Natl. Acad. Sci. USA. 1997;94:242–247. doi: 10.1073/pnas.94.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T.C., Chan T.A., Vogelstein B., Kinzler K.W. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T.C., Sparks A.B., Rago C., Hermeking H., Zawel L., da Costa L.T., Morin P.J., Vogelstein B., Kinzler K.W. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Jais P., Sabourin J.C., Bombled J., Rougier P., Lasser P., Duvillard P., Benard J., Bressac-de Paillerets B. Absence of somatic alterations of the EB1 gene adenomatous polyposis coli-associated protein in human sporadic colorectal cancers. Br. J. Cancer. 1998;78:1356–1360. doi: 10.1038/bjc.1998.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juwana J.P., Henderikx P., Mischo A., Wadle A., Fadle N., Gerlach K., Arends J.W., Hoogenboom H., Pfreundschuh M., Renner C. EB/RP gene family encodes tubulin binding proteins. Intl. J. Cancer. 1999;81:275–284. doi: 10.1002/(sici)1097-0215(19990412)81:2<275::aid-ijc18>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Karki S., Holzbaur E.L. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 1999;1:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- Kinzler K.W., Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Korinek W.S., Copeland M.J., Chaudhuri A., Chant J. Molecular linkage underlying microtubule orientation toward cortical sites in yeast. Science. 2000;287:2257–2259. doi: 10.1126/science.287.5461.2257. [DOI] [PubMed] [Google Scholar]
- Lee L., Tirnauer J.S., Li J., Schuyler S.C., Liu J.Y., Pellman D. Positioning of the mitotic spindle by a cortical-microtubule capture mechanism. Science. 2000;287:2260–2262. doi: 10.1126/science.287.5461.2260. [DOI] [PubMed] [Google Scholar]
- Lu B., Jan L.Y., Jan Y.N. Asymmetric cell divisionlessons from flies and worms. Curr. Opin. Genet. Dev. 1998;8:392–399. doi: 10.1016/s0959-437x(98)80108-1. [DOI] [PubMed] [Google Scholar]
- McCartney B.M., Dierick H.A., Kirkpatrick C., Moline M.M., Baas A., Peifer M., Bejsovec A. Drosophila APC2 is a cytoskeletally associated protein that regulates wingless signaling in the embryonic epidermis. J. Cell Biol. 1999;146:1303–1318. doi: 10.1083/jcb.146.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.K., Rose M.D. Kar9p is a novel cortical protein required for cytoplasmic microtubule orientation in yeast. J. Cell Biol. 1998;140:377–390. doi: 10.1083/jcb.140.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y., Shiina N., Tsukita S. Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J. Cell Biol. 2000;148:505–517. doi: 10.1083/jcb.148.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison E.E., Wardleworth B.N., Askham J.M., Markham A.F., Meredith D.M. EB1, a protein which interacts with the APC tumour suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene. 1998;17:3471–3477. doi: 10.1038/sj.onc.1202247. [DOI] [PubMed] [Google Scholar]
- Muhua L., Adames N.R., Murphy M.D., Shields C.R., Cooper J.A. A cytokinesis checkpoint requiring the yeast homolog of an APC-binding protein. Nature. 1998;393:487–491. doi: 10.1038/31014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S., Souza B., Muller O., Albert I., Bubinfeld B., Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54:3676–3681. [PubMed] [Google Scholar]
- Nakagawa H., Koyama K., Murata Y., Morito M., Akiyama T., Nakamura Y. EB3, a novel member of the EB1 family preferentially expressed in the central nervous system, binds to a CNS-specific APC homologue. Oncogene. 2000;19:210–216. doi: 10.1038/sj.onc.1203308. [DOI] [PubMed] [Google Scholar]
- Nathke I.S., Adams C.L., Polakis P., Sellin J.H., Nelson W.J. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathke I.S. The adenomatous polyposis coli protein. Mol. Pathol. 2000;52:169–173. doi: 10.1136/mp.52.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F., Diamantopoulos G.S., Stalder R., Kreis T.E. CLIP-170 highlights growing microtubule ends in vivo. Cell. 1999;96:517–527. doi: 10.1016/s0092-8674(00)80656-x. [DOI] [PubMed] [Google Scholar]
- Pfarr C.M., Coue M., Grissom P.M., Hays T.S., Porter M.E., McIntosh J.R. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature. 1990;345:263–265. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim. Biophys. Act. 1997;1332:F127–F147. doi: 10.1016/s0304-419x(97)00008-5. [DOI] [PubMed] [Google Scholar]
- Renner C., Pfitzenmeier J.P., Gerlach K., Held G., Ohnesorge S., Sahin U., Bauer S., pfreundschuh M. RP1, a new member of the adenomatous polyposis coli-binding EB1-like gene family, is differentially expressed in activated T cells. J. Immunol. 1997;159:1276–1283. [PubMed] [Google Scholar]
- Schwartz K., Richards K., Botstein D. Bim1 encodes a microtubule-binding protein in yeast. Mol. Biol. Cell. 1997;8:2677–2691. doi: 10.1091/mbc.8.12.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skop A.R., White J.G. The dynactin complex is required for cleavage plane specification in early Caenorhabditis elegans embryos. Curr. Biol. 1998;8:1110–1116. doi: 10.1016/s0960-9822(98)70465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits R., Kielman M.F., Breukel C., Zurcher C., Neufeld K., Jagmohan-Changur S., Hofland N., van Dijk J., White R., Edelmann W., Kucherlapati R., Khan P.M., Fodde R. Apc1638Ta mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev. 1999;13:1309–1321. doi: 10.1101/gad.13.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer E.R., Wordeman L., Schroer T.A., Sheetz M.P. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature. 1990;345:266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- Su L.-K., Burrell M., Hill D.E., Gyuris J., Brent R., Wiltshire R., Trent J., Vogelstein B., Kinzler K.W. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- Tetsu O., McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tirnauer J.S., O'Toole E., Berrueta L., Bierer B., Pellman D. Yeast Bim1p promotes the G1-specific dynamics of microtubules. J. Cell Biol. 1999;145:993–1007. doi: 10.1083/jcb.145.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso R.J., Jordon M.A., Farrell K.W., Matsumoto B., Wilson L. Kinetic stabilization of microtubule dynamic instability in vitro by vinblastine. Biochemistry. 1993;32:1285–1293. doi: 10.1021/bi00056a013. [DOI] [PubMed] [Google Scholar]
- Trzepacz C., Lowy A.M., Kordich J.J., Groden J. Phosphorylation of the tumor suppressor adenomatous polyposis coli (APC) by the cyclin-dependent kinase p34. J. Biol. Chem. 1997;272:21681–21684. doi: 10.1074/jbc.272.35.21681. [DOI] [PubMed] [Google Scholar]
- Vaisberg E.A., Koonce M.P., McIntosh J.R. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J. Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan K.T., Tynan S.H., Faulkner N.E., Echeverri C.J., Vallee R.B. Colocalization of cytoplasmic dynein with dynactin and CLIP 170 at microtubule distal ends. J. Cell Sci. 1999;112:1437–1447. doi: 10.1242/jcs.112.10.1437. [DOI] [PubMed] [Google Scholar]
- Wigge P.A., Jensen O.N., Homes S., Soues S., Mann M., Kilmartin J.V. Analysis of the Saccharomyces spindle pole by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. J. Cell Biol. 1998;141:967–977. doi: 10.1083/jcb.141.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E., Skibbens R.V., Cheng J.W., Salmon E.D., Bloom K. Spindle dynamics and cell cycle regulation of dynein in the budding yeast, Saccharomyces cerevisiae . J. Cell Biol. 1995;130:687–700. doi: 10.1083/jcb.130.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Waltzer L., Bienz M. A new Drosophila APC homologue associated with adhesive zones of epithelial cells. Nat. Cell Biol. 1999;1:144–151. doi: 10.1038/11064. [DOI] [PubMed] [Google Scholar]