Substance P is expressed in hippocampal principal neurons during status epilepticus and plays a critical role in the maintenance of status epilepticus (original) (raw)
Abstract
Substance P (SP), a member of the tachykinin family, is widely distributed in the central nervous system and is involved in a variety of physiological processes including cardiovascular function, inflammatory responses, and nociception. We show here that intrahippocampal administration of SP triggers self-sustaining status epilepticus (SSSE) in response to stimulation of the perforant path for periods too brief to have any effect in control rats, and this SSSE generates a pattern of acute hippocampal damage resembling that known to occur in human epilepsy. The SP receptor (SPR) antagonists, spantide II and RP-67,580, block both the initiation of SSSE and SSSE-induced hippocampal damage and terminate established anticonvulsant-resistant SSSE. SSSE results in a rapid and dramatic increase in the expression of preprotachykinin A (a precursor of SP) mRNA and SP in principal neurons in CA3, CA1, and the dentate gyrus as well as in hippocampal mossy fibers. SP also increases glutamate release from hippocampal slices. Enhanced expression of SP during SSSE may modulate hippocampal excitability and contribute to the maintenance of SSSE. Thus, SPR antagonists may constitute a novel category of drugs in antiepileptic therapy.
Feed-forward excitation travels along a hippocampal trisynaptic circuit, in which dentate gyrus (DG) granule cells make excitatory synaptic connections via mossy fibers onto CA3 pyramidal neurons that, in turn, form excitatory synaptic connections onto CA1 pyramidal neurons (1, 2). This hippocampal circuit displays considerable neurochemical plasticity in response to lesions and change in afferent activity (3–6). Substance P (SP), a member of peptides belonging to the tachykinin family, is present in low concentration in the rat hippocampus (7, 8). However, its expression, like that of other neuropeptides, shows considerable plasticity and can be regulated by drugs (9–11), neuronal activity (12, 13), and convulsant kainic acid (14, 15). SP is well established as a neurotransmitter/neuromodulator (16) and is released by various stimuli (17, 18). There is evidence to suggest that SP modulates neuronal membrane excitability by virtue of slow synaptic currents (19), raises intracellular Ca2+ concentrations (20), and enhances responses to excitatory amino acids (21). Using a new animal model (22), we now present evidence that SP plays an important role in the severe seizures seen in self-sustaining status epilepticus (SSSE) and in modulating hippocampal excitability.
MATERIALS AND METHODS
Induction and Analysis of Status Epilepticus (SE).
Male Wistar rats (240–280 g) were implanted under anesthesia (ketamine 60 mg kg−1/xylazine 15 mg kg−1) with a bipolar stimulating electrode into the angular bundle of the perforant path and a bipolar recording electrode into the granule cell layer of the ipsilateral DG. To standardize the position of the electrode, only animals in which population spike amplitude was ≥2 mV were used. Seven days after surgery, animals were stimulated in the awake state for 30 min with 10-s 20-Hz trains (1 ms square wave, 20 V) delivered every minute, together with 2 Hz continuous stimulation as described previously (22). Electroencephalographic (EEG) activity from the DG was monitored and recorded for 24 hr after the end of perforant path stimulation (PPS) by means of a monitor 81 program (Stellate Systems). The software was configured for automatic detection and saving of spikes and seizures. EEG was analyzed offline and the cumulated duration of seizures was calculated. The severity of behavioral seizures was scored according to Racine (23). Control animals underwent surgery and received sham PPS during which they were connected to the stimulator, but no current was passed. All animal experiments were reviewed and approved by the Animal Care Committee of Sepulveda Veteran Administration Medical Center.
Administration of SP Receptor (SPR) Ligands.
We used the protocol previously described (24). Wistar rats were implanted with stimulating electrodes in the angular bundle of the perforant path and recording electrodes, combined with guide cannulas (internal diameter 0.6 mm) in the granule cell layer of the ipsilateral DG. SP (Sigma) that was dissolved in 0.5 μl saline was injected through the guide cannulas by means of a Hamilton microsyringe into the DG of awake rats 5 min before 7-min PPS. SPR antagonists, spantide II (America Peptide, Sunnyvale, CA) and RP-67,580 were injected into the DG of awake rats 5 min before the beginning or 10 min after the end of 30-min PPS.
Immunohistochemistry.
The rats were anesthetized with pentobarbital and perfused intracardially with 0.1 M phosphate-buffered 4% formaldehyde (for light microscopy) or 2% formaldehyde and 1% glutaraldehyde (for electron microscopy). Serial coronal sections (40 μm thick) through the full septal–temporal axis of the hippocampus were cut on a freezing microtome and incubated with a rabbit anti-SP antiserum (Peninsula Laboratories). For light microscopy, we localized the antiserum with the ABC method, with 3-3′-diaminobenzidine and nickel enhancement. The specificity of the anti-SP antiserum was confirmed by the absence of staining of hippocampus tissue from mice lacking the preprotachykinin A (PPT-A) gene (25). Also, when the primary antiserum was omitted, there was no labeling. For quantification of SP immunoreactivity (SP-ir), we used the stereological method (26) to estimate the numbers of SP-ir neurons and mossy fiber terminals in the DG granule cell layer, CA3 and CA1 pyramidal cell layers. Briefly, four pairs of sections per hippocampus were counted as numbers per unit volume (Nv). The volume of the hippocampus (_V_ref) was estimated by measuring areas of the hippocampus in eight approximately equally spaced sections. The mean hippocampus area multiplied by the number of sections and their thickness is _V_ref. The neuron number (N) = Nv × _V_ref. For electron microscopy, we used a preembedding immunogold protocol in which the primary antiserum was localized with a 1.0 nM colloidal gold-labeled secondary antibody (Amersham) followed by silver intensification (18).
In Situ Hybridization.
The coronal cryostat sections (10 μm thick) from the hippocampus were hybridized with a [35S]dATP-labeled 30-mer oligodeoxynucleotide probe to PPT-A. In situ hybridization and washes were done essentially as described previously (14). Sections were exposed to x-ray film (Amersham) for 3 to 4 days and to Ilford-5D nuclear emulsion (Polysciences) for 3 wk at 4°C and counterstained with cresyl violet. Autoradiograms were digitized with a Nikon MT1-CCD video camera and were analyzed by using the NIH image program for Macintosh computers. Images were quantified by densitometric analysis, and values were corrected to grain/area.
Competition ELISA.
The rats were decapitated and hippocampi were dissected on ice. Tissue extraction was carried out by using 2 M acetic acid as described by Folkesson (27). SP levels were measured by a competition ELISA. The optical density was determined at 492 nm on an automated microtiter plate reader (Molecular Devices). The SP values in samples were calculated by interpolation from the reference dilution curve by using the softmax program (Molecular Devices).
Glutamate Release.
Male Wistar rats (280–300 g) were anesthetized with methoxyflurane and the hippocampi were immediately dissected out and sectioned with a tissue chopper on an ice-cooled plate to yield transverse slices (475 μm thick). Ten slices were placed in a net cage and pre-incubated at 34°C for 3 hr in artificial cerebrospinal fluid containing (in mM) 126.0 NaCl, 4.0 KCl, 1.4 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26.0 NaHCO3, 4.0 glucose equilibrated with 95% 02/5% CO2. One μM of SP (Sigma) was applied into the slice perfusate. Samples of perfusate (0.5 ml) were collected at regular 5-min intervals before, during, and after application of SP. Concentrations of the glutamate in the sample were determined by HPLC with fluorometric detection.
Histologic Analysis.
The rats from different group were perfused intracardially with 0.1 M phosphate-buffered 4% formaldehyde. Serial coronal sections (30 μm thick) were cut on freezing microtome and were stained with cresyl violet to determine neuronal loss. Neuronal quantification was carried out in areas CA1 and CA3 by stereological method (26).
RESULTS
Thirty minutes of PPS induced behavioral stage 4–5 seizures and EEG seizure activity (Fig. 1A) in all animals tested. As reported previously (23, 24), this pattern of behavior and ictal EEG continued past the end of 30-min PPS and lasted from 6 to 24hr in different animals (Fig. 1E).
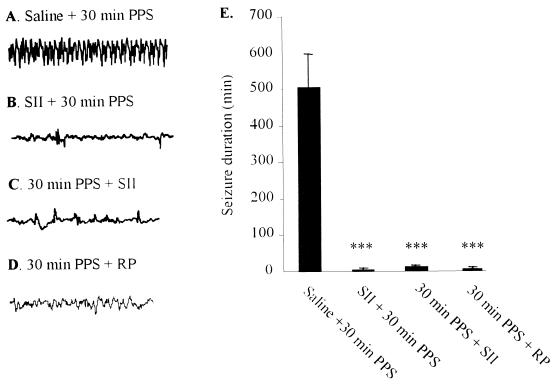
Figure 1.
(A–D) Representative EEGs recorded from hippocampal DG 30 min after the end of 30-min PPS. (E) Effects of SPR antagonists on the duration of seizures induced by 30-min PPS. EEG activity from the DG was recorded for 24 hr after the end of 30-min PPS. Thirty-minute PPS (n = 18) induced EEG seizures lasting on the average 506 ± 100 min. Mean time spent in seizures was reduced to 5 min in rats pretreated with 1 nmol SII. In rats injected with 50 nmol SII and 25 nmol RP-67,580 10 min after 30-min PPS (n = 4 per group), mean postinjection seizure duration was reduced to 8 min and 6 min, respectively. ***P < 0.001, compared with 30-min PPS. Data are mean ± SEM and were analyzed by the Kruskall–Wallis test with the Mann–Whitney test.
SP Facilitates SSSE, and SPR Antagonists Block SSSE.
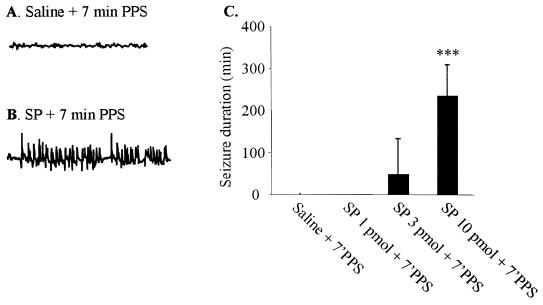
Administration of SP into hippocampus before challenging rats with 7-min PPS [a duration of stimulation which, by itself, was never sufficient to induce SE (22)] produced dose-dependent seizure behaviors (Fig. 2C). Injection of 10 pmol SP before subthreshold PPS induced (in six of six rats) severe SSSE similar in character to that usually induced by 30-min PPS (22, 23). The seizures progressed from facial myoclonus to forelimb clonus and eventually to rearing and falling. These stage 4 and 5 seizures were accompanied by high-frequency high-amplitude electrographic paroxysmal activity in the DG (Fig. 2B) that lasted many hours (Fig. 2C). Control rats given the same volume of vehicle with 7-min PPS did not exhibit seizure activity or develop SE (Fig. 2 A and C), nor did rats injected with 10 pmol of SP mixed with 1 nmol of spantide II, a SPR antagonist (data not shown). In further experiments, we examined the anticonvulsant effects of a SPR antagonist by injecting spantide II into the hippocampus of rats in which SSSE was induced by 30-min PPS. Injection of 1 nmol spantide II had no behavioral effects by itself but injection 5 min before 30-min PPS suppressed EEG spike frequency (Fig. 1B, 2 ± 1/min compared with 30 ± 10/min in 30-min PPS, P < 0.001) and prevented the development of SE in four of four rats (Fig. 1E). Most importantly, administration of spantide II 10 min after the end of 30-min PPS markedly suppressed spike frequency (Fig. 1C, 3 ± 2/min compared with 30 ± 10/min in 30-min PPS, P < 0.001) and reduced seizure duration in a dose-dependent manner. Ten nmol of spantide II reduced the mean duration of postinjection seizures from 506 min to 240 min, while 50 nmol and 250 nmol reduced mean seizure duration to 8 min (Fig. 1E) and 1 min, respectively. Similarly, injection of RP-67,580 (2 and 25 nmol), a selective SPR antagonist, 10 min after the end of 30-min PPS suppressed EEG frequency (Fig. 1D) and reduced the mean duration of postinjection seizures from 506 min to 109 min and 6 min (Fig. 1E), respectively. Treatment with the standard anticonvulsant diazepam (10 mg/kg, i.v.), at the same time, only reduced postinjection seizures to 95 ± 22 min (28).
Figure 2.
(A and B) Representative EEGs were obtained from DG 30 min after injection. (B) Dose response for SP-facilitated EEG seizures in awake rats. ***P < 0.001, compared with saline with 7-min PPS. Data are mean ± SEM and were analyzed by the Kruskall–Wallis test with the Mann–Whitney test.
SP-Facilitated SSSE Causes Hippocampal Damage.
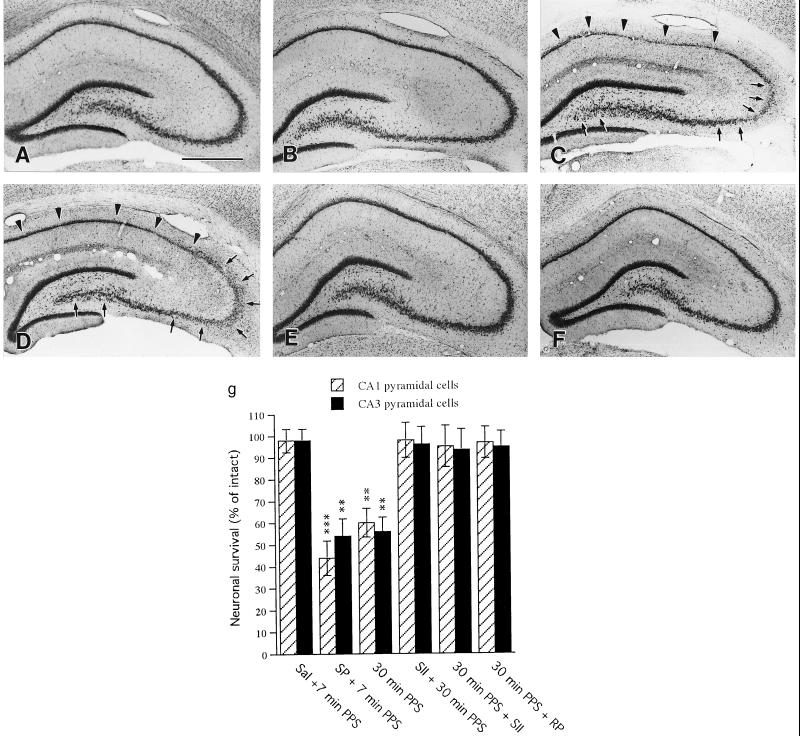
Because SE usually leads to neuronal death, we analyzed the hippocampus histologically. Vehicle injection with 7-min PPS that did not lead to SSSE caused no morphological abnormalities in any subregion of the hippocampus (Fig. 3 B and G) compared with controls (3_A_). In contrast, in all rats (six of six) that developed SSSE as a result of injection of 10 pmol SP combined with 7-min PPS, severe neuronal loss, greater on the side of stimulation, occurred bilaterally throughout the CA1 and CA3 pyramidal cell layers (Fig. 3 C and G), in a distribution resembling that seen in human epilepsy (29). No detectable cell loss was found in the hippocampi of rats coinjected with spantide II and SP combined with 7-min PPS (data not shown). The hippocampal neuronal loss caused by 30-min PPS (Fig. 3 D and G) was completely prevented in four of four rats by preinjection of the SPR antagonist, spantide II (Fig. 3 E and G) and was virtually abolished in four of four rats by injection of 50 nmol spantide II and 25 nmol RP-67,580 10 min after the end of 30-min PPS (Fig. 3 F and G). These results suggest that SP potentiates neuronal degeneration by facilitating both the initiation and the maintenance of SSSE.
Figure 3.
Photomicrographs of cresyl violet-stained hippocampus 7 days after treatment. Compared with controls (A), there is no detectable cell loss in the hippocampus of rats subjected to 7-min PPS (B). Animals stimulated for the same duration (7-min PPS) after intrahippocampal injection of 10 pmol SP show a narrowing of the pyramidal cell layer throughout the CA1 subfield (arrowheads in C) and a decrease in neuronal density in CA3 (arrows in C). Rats subjected to 30-min PPS display narrow staining throughout CA1 (arrowheads in D) and sparse staining of CA3 (arrows in D). Preinjection with the SPR antagonist, SII, completely prevented the neuronal loss seen after 30-min PPS (E). Injection of SII 10 min after 30-min PPS also reduced neuronal loss to undetectable levels (F). (G) Quantification of neuronal loss in the hippocampus. Data are expressed as mean percent of control ±SEM. Significant differences were calculated by ANOVA with probable least-squares difference Fisher’s test (**P < 0.01, ***P < 0.001). (Bar = 200 μm.)
SSSE Evokes an Increase in SP and in PPT-A mRNA in the Hippocampus.
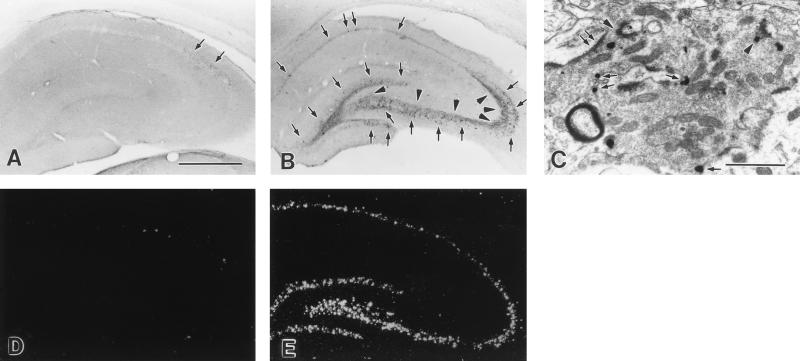
In the intact or electrode-implanted control hippocampus, as reported by previous investigators (7, 8), only a few SP-ir fibers were seen in the subiculum and at the junction of CA2 and CA3; very few SP-ir cells were present in the stratum oriens of CA1 and CA2 and none within the DG granule cell layer (Fig. 4A). SSSE, however, resulted in a dramatic increase in SP-ir in the DG granule cells and in pyramidal cells of CA3 and CA1 both in the stimulated and contralateral hippocampus (Fig. 4B), although SP staining was weaker contralaterally. This change persisted for the 3-day observation period. Twelve hours after 30-min PPS, SP-ir within the granule cell layer was 30-fold higher in SSSE rats than in paired controls. SP-ir within the CA3 and CA1 pyramidal cell layers was 35-fold and 13-fold higher, respectively, than in control rats 24 hr after 30-min PPS (Table 1). Substantial numbers of SP-ir terminals with morphology resembling mossy fibers were also seen in the strata pyramidale and lucidum of CA3 where there normally are no SP-ir mossy fibers (Fig. 4B, Table 1). On electron microscopic examination, abundant SP-ir terminals of mossy fibers that formed asymmetric synapses were seen within the pyramidal cell layer and the stratum lucidum of CA3 (Fig. 4C). Importantly, SP-ir were located both at presynaptic densities and on the extrasynaptic membrane of mossy fiber terminals (Fig. 4C), suggesting that SP may be released both synaptically and extrasynaptically (30, 31).
Figure 4.
Seizure-induced neurochemical changes in the hippocampus. In the control hippocampus (A), some SP-ir fibers are seen at the junction of CA2 and CA3, and a few SP-ir cells are present in the stratum oriens of CA1 and CA2 (arrows). Twenty-four hours after 30-min PPS (B), there is a dramatic increase in SP-ir cell bodies in CA3, CA1, in the granule cell layer (arrows), and in mossy fibers (arrowheads). (C) Electron micrograph of a typical mossy terminal in CA3 was obtained from a rat killed 24 hr after 30-min PPS. The SP-immunoreactive immunogold particles in the mossy fiber terminal are located at a presynaptic density (arrowheads) and in extrasynaptic areas along the internal face (arrows) of the terminal membrane. Asymmetric synapses on the dendrites of CA3 pyramidal neurons (double arrows) are typical features of excitatory connections. (D and E) PPT-A mRNA expression in the hippocampus. In the control hippocampus (D), a few PPT-A mRNA-positive neurons are scattered in the stratum oriens of CA1, CA2, and CA3. Very few PPT-A mRNA-positive cells are present in the pyramids of Ammon’s horn. Three hours after 30-min PPS (E), a dramatic increase in PPT-A mRNA is observed in the dentate granule cell layer and in all pyramidal cell layers. (Bars = 200 μm in A, B, D, and E, 1 μm in C).
Table 1.
Expression of SP-ir and PPT-A mRNA in the hippocampus after the 30-min perforant path stimulation
| Time after PPS | DG granule cells | CA3 pyramidal cells | CA1 pyramidal cells | CA3 mossy fibers | ||||
|---|---|---|---|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | Ipsilateral | Contralateral | Ipsilateral | Contralateral | |
| SP-ir | ||||||||
| 12 hr | 3,017 ± 130* | 1,077 ± 35* | 2,093 ± 144* | 1,373 ± 109* | 784 ± 65* | 717 ± 129* | 613 ± 71* | 430 ± 54* |
| 1 day | 467 ± 95* | 358 ± 46† | 3,533 ± 158* | 2,857 ± 88* | 1,372 ± 91* | 1,134 ± 111* | 652 ± 53* | 457 ± 57* |
| 3 days | 358 ± 30† | 217 ± 33‡ | 351 ± 35‡ | 307 ± 33‡ | 426 ± 87‡ | 82 ± 91‡ | 274 ± 19* | 223 ± 16† |
| 7 days | 101 ± 12 | 102 ± 13 | 114 ± 15 | 74 ± 13 | 101 ± 20 | 96 ± 14 | 101 ± 9 | 97 ± 7 |
| PPT-A mRNA | ||||||||
| 3 hr | 2,867 ± 264* | 2,339 ± 239* | 1,619 ± 141* | 864 ± 31* | 875 ± 45* | 751 ± 42* | ||
| 12 hr | 2,645 ± 239* | 1,863 ± 123* | 2,077 ± 118* | 884 ± 70* | 1,062 ± 65* | 858 ± 66* | ||
| 1 day | 407 ± 65 | 325 ± 69 | 2,393 ± 221* | 2,050 ± 184* | 1,040 ± 81* | 891 ± 74* | ||
| 3 days | 102 ± 12 | 100 ± 10 | 154 ± 20 | 119 ± 15 | 109 ± 12 | 107 ± 14 |
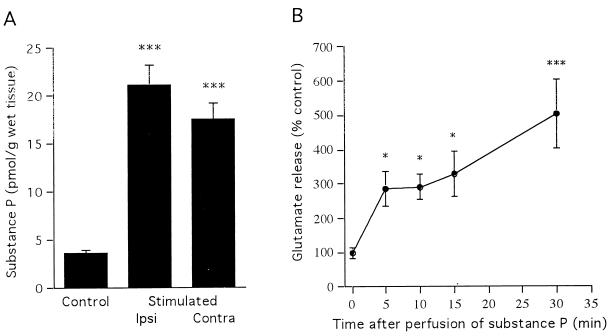
We also studied the expression of PPT-A mRNA in the hippocampus after SSSE by in situ hybridization. PPT-A mRNA distribution in the control hippocampus was in agreement with the immunocytochemical results. A few PPT-A mRNA-positive neurons were scattered in the stratum oriens of CA1 and CA3. Very few PPT-A mRNA-positive cells were present in the pyramidal layer of Ammon’s horn (Fig. 4D). However, 3 hr after 30-min PPS, PPT-A mRNA had dramatically increased in the DG granule cells as well as in the CA3 and CA1 pyramidal cell layers of the hippocampus (Fig. 4E, Table 1). This change persisted for the 3-day observation period. Consistent with the immunohistochemical and in situ hybridization data, a marked elevation in SP content was detected in both hippocampi after SSSE by ELISA (Fig. 5A).
Figure 5.
(A) SP content (as measured by ELISA) of the hippocampus 24 hr after the end of 30-min PPS (n = 3). (B) Time course of perfusate concentration of glutamate (n = 4) in response to superfusion of hippocampal slices with 1-μM SP. Basal concentrations are determined as mean values from six consecutive fractions before treatment with SP (0 min), and subsequent samples are expressed as the percent of basal concentration. Data are mean ± SEM and were analyzed by ANOVA with probable least-squares difference Fisher’s test (*P < 0.05, ***P < 0.001).
SP Enhances Release of Glutamate.
Superfusion of hippocampal slices with 1 μM SP for 5 min resulted in a significant 2- to 3-fold increase in the perfusate concentration of glutamate, compared with basal values obtained before SP stimulation by 30 min of perfusion, SP caused a 5-fold increase in glutamate concentration in the hippocampal perfusate (Fig. 5B).
DISCUSSION
The present study demonstrates that intrahippocampal injection of SP triggers SSSE in response to brief subthreshold stimulation, and that this SSSE generates a pattern of acute hippocampal damage resembling that known to occur in human epilepsy. Furthermore, SPR antagonists blocked both the initiation of SSSE and SSSE-induced hippocampal neuronal damage and terminated established anticonvulsant-resistant SSSE, preventing hippocampal damage in the process. Take together, these results suggest that SP contributes to both the initiation phase and the maintenance phase of SSSE.
A critical issue in understanding the mechanism by which SP facilitates SSSE is based on the localization of SP expression. The data presented here indicate that SSSE causes a dramatic increase in SP-ir and in PPT-A mRNA in both types of hippocampal principal neurons, which is consistent with previous studies demonstrating that the expression of SP is increased by various stimuli (9–13, 32). In one report, the distribution of SP expressed in the granule cells and mossy terminals after stimulation of the entorhinal cortex was described as “normal” (33), but many studies, including ours, demonstrated neither SP (7, 8, 34, 35) nor PPT-A mRNA (14, 15) in principal cells in normal rats. Taken together with ELISA results that confirmed the elevation of SP content after SSSE, our data indicate that SSSE results in new expression of SP in the principal cells of the hippocampus, where SP is normally undetectable. The expression after SSSE of SP immunoreactivity within the mossy fiber terminals that form excitatory asymmetric synapses with the CA3 pyramidal cell may suggest the establishment of a new chemical circuit in which SP is now released and thereby further enhances synaptic excitability.
Our studies support a proconvulsant role for SP, since intrahippocampal injection of SP facilitated SSSE while injection of SPR antagonists prevented and stopped it. These results contrast with one report that some effects of SP on entorhinal cortex neurons can be inhibitory (36). These differences may reflect in vitro vs. in vivo effects, regional diversity, or differences in behavior between isolated neurons and those integrated into the complex synaptic circuitry of the brain. Other studies in vivo showed that SP has powerful excitatory effects when applied iontophoretically to cortical neurons (37–39), that systemic administration of a SPR antagonist reduced kainic acid-induced seizure activity (15), and that intracerebroventricular injection of a SPR antagonist suppressed EEG activity (40). Various mechanisms have been suggested for the excitatory effects of SP. It is conceivable that SP, like _N_-methyl-d-aspartate (NMDA), modulates neuronal membrane excitability by virtue of slow synaptic currents (19, 41, 42) and raises intracellular Ca2+ concentration through Ca2+ influx and release of intracellular stores (20, 43). An increase in intracellular Ca2+ might produce a protein kinase C-dependent phosphorylation of the NMDA receptor, partially removing its voltage-dependent Mg2+ blockade (44) and increasing glutamate sensitivity. At the resting membrane potential, glutamate could activate more NMDA receptors, resulting in a larger and more prolonged excitatory postsynaptic potential (21, 45) and could contribute further to the maintenance phase of SSSE.
Further insight into possible mechanisms through which SP influences hippocampal seizure activity has been revealed in our present study in which SP dramatically increased release of glutamate. These results are consistent with evidence in vitro (46, 47) and in vivo (48, 49) that SP and neurokinin A release glutamate and aspartate from the spinal cord. SE and neuronal death often result from excess release of glutamate (50–52). Thus, SP-mediated excitation of hippocampal neurons might be caused, in part, by the facilitation of glutamate release, which may cause a cascade of events leading to SSSE and cell death. In this regard, the success of SPR antagonists in blocking the maintenance phase of SSSE at a time when the standard anticonvulsant diazepam fails to stop seizures raises the possibility of treating anticonvulsant-resistant SSSE with antagonists of SP and other neurokinins.
Acknowledgments
We thank Drs. Kimberly Topp (University of California, San Francisco) and Eileen Curran (Amgen) for critically reading the manuscript and Dr. Igor Spigelman for providing RP-67580. This work was supported by National Institutes of Health Grant NS13515 and the Research Service of the Veteran Administration (C.G.W.) and by the Clinical Investigator Development Award NS01792 from National Institutes of Health (R.S.).
ABBREVIATIONS
DG
dentate gyrus
SP
substance P
SPR
SP receptor
EEG
electroencephalographic
PPS
perforant path stimulation
PPT-A
preprotachykinin A
SE
status epilepticus
SP-ir
SP immunoreactivity
SSSE
self-sustaining SE
References
- 1.Andersen P, Bliss T V, Lomo T, Olsen L I, Skrede K K. Acta Physiol Scand. 1969;76:4A–5A. doi: 10.1111/j.1748-1716.1969.tb04499.x. [DOI] [PubMed] [Google Scholar]
- 2.Pare D, deCurtis M, Llinas R. J Neurosci. 1992;12:1867–1881. doi: 10.1523/JNEUROSCI.12-05-01867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gall C, Lauterborn J, Isackson P, White J. Prog Brain Res. 1990;83:371–390. doi: 10.1016/s0079-6123(08)61263-7. [DOI] [PubMed] [Google Scholar]
- Marksteiner J, Saria A, Krause J E. Neuroscience. 1992;51:107–120. doi: 10.1016/0306-4522(92)90475-h. [DOI] [PubMed] [Google Scholar]
- 5.Sperk G, Marksteiner J, Gruber B, Bellmann R, Mahata M, Ortler M. Neuroscience. 1992;50:831–846. doi: 10.1016/0306-4522(92)90207-i. [DOI] [PubMed] [Google Scholar]
- 6.de Lanerolle N C, Brines M L, Kim J H, Williamson A, Philips M F, Spencer D D. Epilepsy Res Suppl. 1992;9:205–219. [PubMed] [Google Scholar]
- 7.Roberts G W, Woodhams P L, Polak J M, Crow T J. Neuroscience. 1984;11:35–77. doi: 10.1016/0306-4522(84)90214-8. [DOI] [PubMed] [Google Scholar]
- 8.Davies S, Kohler C. Anat Embryol. 1985;173:45–52. doi: 10.1007/BF00707303. [DOI] [PubMed] [Google Scholar]
- 9.Bannon M J, Elliott P J, Bunney E B. Brain Res. 1987;427:31–37. doi: 10.1016/0169-328x(87)90041-6. [DOI] [PubMed] [Google Scholar]
- 10.Tempel A, Kessler J A, Zukin R S. J Neurosci. 1990;10:741–747. doi: 10.1523/JNEUROSCI.10-03-00741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerfen C R, McGinty J F, Young W S d. J Neurosci. 1991;11:1016–1031. doi: 10.1523/JNEUROSCI.11-04-01016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendry S H, Jones E G, Burstein N. J Neurosci. 1988;8:1225–1238. doi: 10.1523/JNEUROSCI.08-04-01225.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindefors N, Brodin K, Stiller C O, Persson H, Brodin E. Neuroscience. 1991;45:73–80. doi: 10.1016/0306-4522(91)90104-v. [DOI] [PubMed] [Google Scholar]
- 14.Brene S, Lindefors N, Ballarin M, Persson H. Neuroscience. 1992;50:611–618. doi: 10.1016/0306-4522(92)90450-g. [DOI] [PubMed] [Google Scholar]
- 15.Zachrisson O, Lindefors N, Brene S. Mol Brain Res. 1998;60:291–295. doi: 10.1016/s0169-328x(98)00191-0. [DOI] [PubMed] [Google Scholar]
- 16.Otsuka M, Yoshioka K. Physiol Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- 17.Mantyh P W, DeMaster E, Malhotra A, Ghilardi J R, Rogers S D, Mantyh C R, Liu H, Basbaum A I, Vigna S R, Maggio J E. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Mantyh P W, Basbaum A I. Nature (London) 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- 19.Thompson S W N, King A E, Woolf C J. Eur J Neurosci. 1990;2:638–649. doi: 10.1111/j.1460-9568.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 20.Rusin K I, Bleakman D, Chard P S, Randic M, Miller R J. J Neurochem. 1993;60:952–960. doi: 10.1111/j.1471-4159.1993.tb03242.x. [DOI] [PubMed] [Google Scholar]
- 21.Cumberbatch M J, Chizh B A, Headley P M. Br J Pharmacol. 1995;115:1005–1012. doi: 10.1111/j.1476-5381.1995.tb15911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazarati A M, Wasterlain C G, Sankar R, Shin D. Brain Res. 1998;801:251–253. doi: 10.1016/s0006-8993(98)00606-4. [DOI] [PubMed] [Google Scholar]
- 23.Racine R G. Electroenceph Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 24.Mazarati A, Liu H, Wasterlain C G. Neuroscience. 1999;89:167–173. doi: 10.1016/s0306-4522(98)00320-0. [DOI] [PubMed] [Google Scholar]
- 25.Cao Y Q, Mantyh P W, Carlson E J, Gillespie A M, Epstein C J, Basbaum A I. Nature (London) 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 26.West M J. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 27.Folkesson R, Neil A, Terenius L. J Neurosci Methods. 1985;14:169–176. doi: 10.1016/0165-0270(85)90032-9. [DOI] [PubMed] [Google Scholar]
- 28.Mazarati A, Wasterlain C G. Neurosci Abstr. 1997;23:1687. [Google Scholar]
- 29.Babb T L, Lieb J P, Brown W J, Pretorius J, Crandall P H. Epilepsia. 1984;25:721–728. doi: 10.1111/j.1528-1157.1984.tb03483.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhu P C, Thureson-Klein A, Klein R L. Neuroscience. 1986;19:43–54. doi: 10.1016/0306-4522(86)90004-7. [DOI] [PubMed] [Google Scholar]
- 31.Pow D V, Golding D W. Neuroscience. 1987;22:1145–1149. doi: 10.1016/0306-4522(87)92989-7. [DOI] [PubMed] [Google Scholar]
- 32.Neumann S, Doubell T P, Leslie T, Woolf C J. Nature (London) 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 33.Borhegyi Z, Leranth C. J Comp Neurol. 1997;384:41–58. doi: 10.1002/(sici)1096-9861(19970721)384:1<41::aid-cne3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 34.Shults C W, Quirion R, Chronwall B, Chase T N, O’Donohue T L. Peptides (Tarrytown, NY) 1984;5:1097–1128. doi: 10.1016/0196-9781(84)90177-3. [DOI] [PubMed] [Google Scholar]
- 35.Shults C W, Johnston P, Gage F H. Brain Res. 1987;426:290–296. doi: 10.1016/0006-8993(87)90882-1. [DOI] [PubMed] [Google Scholar]
- 36.Maubach K A, Cody C, Jones R S G. Neuroscience. 1998;83:1047–1062. doi: 10.1016/s0306-4522(97)00469-7. [DOI] [PubMed] [Google Scholar]
- 37.Phillis J W, Limacher J J. Brain Res. 1974;69:158–163. doi: 10.1016/0006-8993(74)90383-7. [DOI] [PubMed] [Google Scholar]
- 38.Lamour Y, Dutar P, Jobert A. Neuroscience. 1983;10:107–117. doi: 10.1016/0306-4522(83)90085-4. [DOI] [PubMed] [Google Scholar]
- 39.Albus K, Chao H H-A, Hicks T P. Brain Res. 1992;587:353–357. doi: 10.1016/0006-8993(92)91019-b. [DOI] [PubMed] [Google Scholar]
- 40.Grabow T S, Gaumann D M, Yaksh T L. Brain Res. 1989;489:223–230. doi: 10.1016/0006-8993(89)90854-8. [DOI] [PubMed] [Google Scholar]
- 41.Urban L, Randic M. Brain Res. 1984;290:336–341. doi: 10.1016/0006-8993(84)90952-1. [DOI] [PubMed] [Google Scholar]
- 42.Stanfield P R, Nakajima Y, Yamaguchi K. Nature (London) 1985;315:498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- 43.Heath M J, Womack M D, MacDermott A B. J Neurophysiol. 1994;72:1192–1198. doi: 10.1152/jn.1994.72.3.1192. [DOI] [PubMed] [Google Scholar]
- 44.Chen L, Huang L Y. Nature (London) 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- 45.Urban L, Thompson S W, Dray A. Trends Neurosci. 1994;17:432–438. doi: 10.1016/0166-2236(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 46.Kawagoe R, Onodera K, Takeuchi A. Biomed Res. 1986;7:253–259. [Google Scholar]
- 47.Kangrga I, Randic M. J Neurosci. 1990;10:2026–2038. doi: 10.1523/JNEUROSCI.10-06-02026.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skilling S R, Smullin D H, Larson A A. J Neurosci. 1990;10:1309–1318. doi: 10.1523/JNEUROSCI.10-04-01309.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smullin D H, Skilling S R, Larson A A. Pain. 1990;42:93–101. doi: 10.1016/0304-3959(90)91095-Z. [DOI] [PubMed] [Google Scholar]
- 50.Meldrum B S. Epilepsia. 1991;32:S1–S3. doi: 10.1111/j.1528-1157.1991.tb05879.x. [DOI] [PubMed] [Google Scholar]
- 51.Coyle J T, Puttfarcken P. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 52.Lipton S A, Rosenberg P A. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]