DcpS can act in the 5′–3′ mRNA decay pathway in addition to the 3′–5′ pathway (original) (raw)
Abstract
Eukaryotic mRNA degradation proceeds through two main pathways, both involving mRNA cap breakdown. In the 3′–5′ mRNA decay pathway, mRNA body degradation generates free m7GpppN that is hydrolyzed by DcpS generating m7GMP. In the 5′–3′ pathway, the recently identified human Dcp2 decapping enzyme cleaves the cap of deadenylated mRNAs to produce m7GDP and 5′-phosphorylated mRNA. We investigated mRNA decay in human cell extracts by using a new assay for decapping. We observed that 5′-phosphorylated intermediates resulting from decapping appear after incubation of a substrate RNA in human cell extracts, indicating the presence of an active 5′–3′ mRNA decay pathway. Surprisingly, however, the cognate m7GDP product was not detected, whereas abundant amounts of m7GMP were generated. Additional experiments revealed that m7GDP is, unexpectedly, efficiently converted to m7GMP in extracts from various organisms. The factor necessary and sufficient for this reaction was identified as DcpS in both yeast and human. m7GMP is thus a general, pathway-independent, by-product of eukaryotic mRNA decay. m7GDP breakdown should prevent misincorporation of methylated nucleotides in nucleic acids and could generate a unique indicator allowing the cell to monitor mRNA decay.
Amajor step of gene regulation occurs at the level of mRNA turnover (1–3), a process that has been extensively studied in the yeast Saccharomyces cerevisiae. In this organism, degradation of most wild-type mRNAs starts with the removal of the poly(A) tail (deadenylation) (1). A major pathway comprises subsequent hydrolysis of the cap structure by Dcp1/Dcp2, followed by rapid 5′–3′ exonucleolytic degradation by Xrn1 (5′–3′ mRNA decay pathway) (4). In an alternative, minor, pathway, deadenylation is followed by exosome-dependent 3′–5′ degradation of the mRNA body (3′–5′ pathway) (5). In addition, two specialized surveillance pathways exist. On the one hand, mRNAs containing a premature stop codon are degraded by deadenylation-independent decapping and 5′–3′ decay (nonsense-mediated mRNA decay, NMD) (6). On the other hand, mRNAs lacking a stop codon are degraded by exosome-dependent 3′–5′ exonucleolytic trimming (nonstop decay, NSD) (7, 8). Much less is known about mRNA turnover in other organisms. However, the decay pathways identified in yeast are likely to be conserved, because decay intermediates corresponding to these pathways have been identified in both plant and animal cells (9–12). In addition, homologues to the yeast mRNA decay factors are also found in mammals (13–17).
Eukaryotic mRNAs contain at their 5′ end a cap structure containing a guanosine methylated at position 7, attached in an inverted manner (5′–5′) to the first mRNA nucleotide by a triphosphate linkage (18). This specific feature of eukaryotic mRNAs protects them from 5′–3′ exonucleases and serves as a recognition site for factors involved in pre-mRNA splicing, RNA transport, and translation initiation (19). Degradation of eukaryotic mRNAs entails the specific removal of the mRNA cap (20). This process differs in the various decay pathways. A scavenger decapping enzyme, DcpS was shown to hydrolyze the residual m7GpppN cap structure after the complete 3′–5′ degradation of the mRNA by the exosome (21). DcpS releases m7GMP and is unable to cleave cap structures attached to a long RNA chain. Cleavage of mRNA cap linked to the mRNA body occurs in the 5′–3′ and NMD pathways, removing the mRNA from the translatable pool and creating an entry point for 5′–3′ exonucleases. The yeast Dcp1 protein was long thought to mediate this process, on its activation by Dcp2 (22, 23). However, human Dcp2 (hDcp2) was recently shown by us and others to catalyze this reaction (24–26). We have also shown that yeast Dcp2 is catalytically active, demonstrating that this feature is evolutionarily conserved (24); a finding that has been confirmed by others (27). Our studies demonstrate that hDcp2 cleaves mRNA to generate m7GDP and a 5′-phosphorylated RNA (24). hDcp2 thus clearly differs from DcpS, both by the nature of its products as well as by its substrate requirements; hDcp2 does not act on RNAs shorter than 9 nt (24). Thus, human and other eukaryotic cells contain two different activities responsible for cap breakdown: DcpS, producing m7GMP in the 3′–5′ decay and NSD pathways, and hDcp2, producing m7GDP in the 5′–3′ and NMD pathways.
Assessing the contribution of the various pathways to mRNA degradation in vivo is essential to understand the regulation of this process. Analysis of mutants in various decay factors and identification of in vivo mRNA degradation intermediates indicated that the 5′–3′ pathway predominates in yeast (5, 20, 23). Analysis of mRNA decay in human cell extracts in vitro revealed high-level formation of m7GMP; m7GDP being conspicuously absent (21). Taken together with studies on the effect of various mRNA structural modifications on mRNA decay in vitro (21, 28) and immunodepletion experiments (29), these data have led to the suggestion that the exosome-dependent 3′–5′ decay pathway is more important in mammalian cells. It is noteworthy, however, that specific intermediates of the 5′–3′ pathways were detected in human cells (9–12). Thus, the relative contribution of each pathway to in vivo mRNA degradation in human cells remains to be established.
To examine the role of hDcp2 in mRNA degradation, we tested whether hDcp2-mediated decapping activity could be detected in human cell extracts. We used a novel assay to detect the specific downstream product of hDcp2 activity. Surprisingly, however, the second product of decapping, m7GDP, was not observed. We solved this dilemma by demonstrating that m7GDP is efficiently converted to m7GMP in yeast and human cell extracts. Furthermore, we identified DcpS as the enzyme mediating this reaction. Thus, DcpS can act in all known mRNA decay pathways and m7GMP is a general, pathway-independent, by-product of mRNA degradation. This observation indicates that both the 5′–3′ and 3′–5′ pathways are active in human cell extracts and has important consequences for understanding the role and regulation of the various mRNA decay pathways in mRNA turnover.
Materials and Methods
Construction of Plasmids. pBS2312 was used for expression of GST-hDcp2-His-6 (24). The pBS2497 GST-DcpS-His-6 expression plasmid contains the DcpS coding sequence PCR-amplified from an image cDNA clone of human DcpS cDNA (30). The PCR product, obtained by using primers OBS590 (5′-CGGCACCATGGC GGACGCAGCT-3′) containing a NcoI site, and primer OBS591 (5′-AAATATCTCGAGTTATTAGTGATGGTGGTGATGATGGCT T TGCTGAGCCTCCTGCAAGA-3′) containing a His-6 tag sequence and a _Xho_I site, was digested with _Nco_I and _Xho_I and cloned in _Nco_I/_Xho_I-digested pBS2312, thus replacing the hDcp2 ORF. Absence of mutations was verified by sequencing.
To express GST-Dcs1-His-6, the Dcs1 ORF was PCR-amplified from yeast genomic DNA (strain FY1679) by using primer OBS466 (5′-AGGAACATGTCTC AACTGCCAACAGA-3′) containing a _Pci_I site, and primer OBS467 (5′-T TATACTCGAGT TAT TAT T TA A A ACCGT TCACA ATCT-3′) containing a _Xho_I site. The PCR product was inserted in _Nco_I/_Xho_I-digested pBS2010 (pET24-His-6-GST-ABD) as a _Pci_I/_Xho_I fragment. In the resulting pBS2425, the Dcs1 ORF is downstream of the His-6-GST sequence. A _Bam_HI/_Dra_I fragment of pBS2425 DNA (starting in the GST sequence and ending just upstream of the Dcs1 stop codon) was cloned in frame with a double-stranded oligonucleotides (OBS567/568; 5′-AAACATCATCACCACCATCACTAATAAC-3′/5′-TCGAGTTATTAGTGATGG TGGTGATGATGTTT-3′) containing a His-6 tag sequence, a stop codon, and _Dra_I/_Xho_I cohesive ends, in _Bam_HI/_Xho_I-digested pBS2312, generating plasmid pBS2499.
In Vitro Decapping Assays. Cap-labeled RNA synthesis, preparation of recombinant proteins, and in vitro decapping reactions were done as described (24). Reaction products were separated by PEI cellulose TLC. As a developing solution, 0.3 M LiCl/1 M formic acid was used. To obtain radiolabeled m7GMP, m7GDP, and m7GpppG, cap-labeled RNA was incubated with human cell extract, recombinant hDcp2, and nuclease P1, respectively. The reaction products were separated by TLC and visualized with a PhosporImager. m7GMP, m7GDP, or m7cap spots were scraped off the TLC plate and eluted in decapping buffer (45 mM Tris·HCl, pH 8/27 mM (NH4)2SO4/9 mM MgAc) with constant shaking for 40 min at room temperature. Insoluble TLC material was removed by centrifugation. Product yield was determined by counting. A total of 800 cpm of m7GMP, m7GDP or m7cap were incubated with 50–150 ng of recombinant protein or 7 μg of cellular extract for 20–60 min at 37°C in a 10-μl reaction volume. Reactions were stopped by phenol-chloroform extraction. Five microliters of such supernatants were analyzed by TLC.
Extract Preparation. Extracts from human HEK293 embryonal kidney cells were prepared by homogenization in DA (Decapping Activity) buffer (20 mM Hepes·KOH, pH 7.6/0.01% Nonidet P-40/20 mM KCl/1 mM MgCl2/10% glycerol/0.1 mM EDTA/1 mM DTT), followed by centrifugation. Protein concentration in the supernatant was determined by Bradford assay (Bio-Rad). For heat inactivation, extracts were incubated for 5 min at 95°C.
The yeast strain BY4147 and its deletion derivatives Δ_dcs2_ and Δ_dcs1_ were purchased from EUROSCARF (Frankfurt, Germany). For yeast extracts, 25-ml cultures were grown to OD600 = 1, centrifuged at 4,500 × g, and washed with 15 ml of ice-cold water. Pellets were resuspended in 1 ml of DA buffer, centrifuged again, and resuspended in 250 μl of DA buffer. Cells were disrupted by vortexing four times for 30 sec with 180-μl glass beads at 4°C. After centrifugation (5 min, 4,500 × g, 4°C), the supernatant was taken and recentrifuged (5 min, 20,000 × g, 4°C). Protein concentrations of cleared lysates were measured by Bradford assays.
For Xenopus laevis oocyte extracts, 40 stage-VI oocytes were homogeneized in 100 μl of J buffer (10 mM Hepes·KOH, pH 7.2/70 mM KCl/1 mM MgCl2/2mMDTT/0.1 mM EDTA/10% glycerol and 0.1 mM PMSF containing 1 μg/ml protease inhibitor mixture; Roche Diagnostics). After centrifugation for 10 min at 15,294 × g at 4°C, the supernatant was used.
RNA Ligation Assay. To detect the downstream product of decapping, 5′ monophosphate RNA, a 106-nt-long capped RNA was synthesized in vitro by T7 transcription in the presence of cap analog (5′-GGGCCAACCGCATCTGCAGCGAGCAACTGAGA AGCCA AGACTGAGCCGGCGGCCGCGGCGCAGCGA ACGAGCAGTGACCGTGCTCCTACCCAGCTCTGCTTCACAG-3′). Transcribed RNAs were phenol-chloroform extracted, ethanol precipitated, and checked for integrity on agarose gels. One nanogram of RNA was combined with 0.5 μg of total RNA from yeast as a carrier and incubated for 1 h at 37°C with 5 units of calf intestinal phosphatase (Biolabs, Northbrook, IL) and 20 units of RNaseOUT (Invitrogen) in a 5-μl reaction to remove background 5′-phosphorylated RNAs. After phenol-chloroform extraction and precipitation, the RNA was incubated with recombinant hDcp2 or human HEK293 cell extract as a source of decapping activity for 30 min at 37°C (see above). The decapped RNA was phenol-chloroform extracted, ethanol precipitated, and subsequently ligated to a 44-nt-long RNA oligonucleotide (5′-CGACUGGAGCACGAGGACACUGACAUGGACUGAAGGAGUAGAAA-3′) for 1 h at 37°C in a 5-μl reaction containing 125 ng of RNA oligonucleotide, 5 units of T4 RNA ligase (Roche Diagnostics), 20 units of RNaseOUT, and the provided reaction buffer. After phenol-chloroform extraction and precipitation, cDNA was generated by reverse transcription using primer OBS388 (5′-CTGTGAAGCAGAGCTGGGTAGGA-3′) complementary to the 3′ end of the substrate RNA. Reverse transcription reactions contained 1 mM dNTPs, 100 units of SuperScript II reverse transcriptase (Invitrogen), provided reaction buffer, 10 mM DTT, and 20 units of RNaseOUT in a total volume of 10 μl. Reactions were incubated at 42°C for 50 min, heat inactivated at 70°C for 15 min, and treated with 1 unit of RNase H (Invitrogen) at 37°C for 20 min. A PCR was then performed with primer OBS461 (5′-CGACTGGAGCACGAGGACACTGA-3′) specific for the RNA oligonucleotide and the nested primer OBS484 (5′-TGGGTAGGAGCACGGTCACTGCT-3′) specific for the substrate RNA (see also Fig. 1_A_). PCR products were analyzed by on 3% agarose gels (Nusieve GTG agarose, BMA).
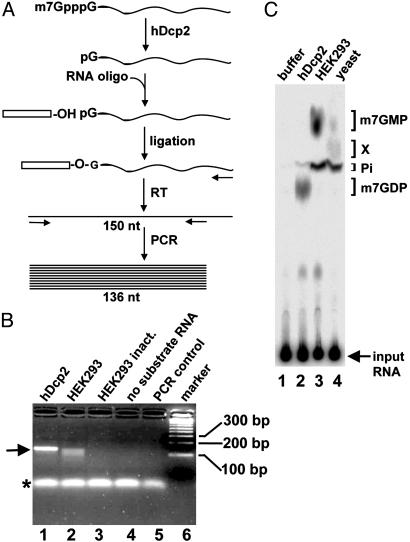
Fig. 1.
Human HEK293 cell extract contains hDcp2-like decapping activity. (A) Outline of the RNA ligation assay used to detect decapped 5′ monophosphate RNA. In vitro transcribed, capped RNA (106 nt) is incubated with a source of decapping activity. The resulting 5′ monophosphate RNA is ligated to an RNA oligonucleotide. The ligation product is then reverse-transcribed (RT), and the resulting cDNA is PCR-amplified by using a primer complementary to the RNA oligonucleotide and a nested downstream primer. (B) Agarose gel analysis of PCR products. Decapping with hDcp2 (lane 1) and HEK293 whole cell extract (lane 2) is shown. Controls include heat-inactivated HEK293 whole cell extract (lane 3), reactions with HEK293 whole cell extract without substrate RNA (lane 4), and PCR without template (lane 5). A DNA size marker is in lane 6 with the fragment indicated on the right. The PCR product corresponding to full-length decapped substrate RNA is indicated by an arrow (left). Note that lane 2 contains faster migrating species in addition to the full-length product. An asterisk indicates nonspecific PCR products formed independently of the template and used as loading control. (C) Analysis of m7GDP formation with hDcp2 and human and yeast cell extract. Cap-labeled RNA was incubated with buffer (lane 1), 50 ng of GST-hDcp2-His-6 (24) (lane 2), 7 μg of human HEK293 whole cell extract (lane 3), or 7 μg of yeast BY4741 wild-type whole cell extract (lane 4). Reaction products were separated by TLC along with unlabeled standards that were revealed by fluorescence; their migration positions are indicated on the right. Pi, inorganic phosphate. An unidentified product X was formed upon incubation with yeast extract. The input RNA, which remained at the origin, is indicated by an arrow.
Results
Human HEK293 Cell Extracts Contain hDcp2-Like Decapping Activity. We recently identified hDcp2 as the human mRNA decapping enzyme that acts on capped mRNA to produce m7GDP and a 5′ phosphorylated RNA in vitro (24). To examine the role of hDcp2 in mRNA degradation, we asked whether hDcp2-mediated decapping could be detected in human cellular extracts. Previous studies failed to detect m7GDP formation in human cell extracts (21, 26). Therefore, we developed an RNA ligation assay to specifically detect the downstream product generated by hDcp2 that we characterized previously (24), namely 5′-phosphorylated RNA (Fig. 1 A). A 106-nt-long capped in vitro transcribed RNA was used as a substrate for the decapping reactions. Decapped RNAs generated during these reactions were ligated to a synthetic RNA oligonucleotide of 44 nt by using RNA ligase, a reaction requiring the presence of a 5′-phosphorylated extremity. cDNAs were synthesized by using a primer complementary to the 3′ end of the substrate RNA and amplified by PCR using a primer specific for the RNA oligonucleotide and a downstream nested primer complementary to the substrate RNA. The PCR products were visualized by ethidium bromide staining after agarose gel electrophoresis (Fig. 1_B_). When recombinant hDcp2 was incubated with the substrate RNA, a product migrating at the expected position for the full-length PCR product (136 nt) was observed (Fig. 1_B_, lane 1). No product was observed when heat-inactivated extract was used or when the substrate RNA was omitted, demonstrating the integrity of the starting RNA and the specificity of the PCR reaction (lanes 3 and 4). When the substrate RNA was incubated with human HEK293 extract, a product comigrating with the product observed on incubation with hDcp2 was formed; in addition, faster migrating products were detected (lane 2). To characterize these different species, DNA fragments were gel-purified and cloned. Sequence analysis (data not shown) confirmed that the slowest migrating species contained DNA fragments with the complete sequence of the substrate RNA fused to the RNA oligonucleotide, demonstrating the presence of a bona fide decapping activity in the extracts. The faster migrating fragments lacked increasing parts of the 5′ end of the substrate RNA. Given the known link between mRNA decapping and 5′–3′ exonucleolytic trimming during mRNA decay (1), this result strongly suggests that these products may represent 5′–3′ degradation intermediates. Although we cannot formally rule out that these shortened products result from endonucleolytic cleavages, we can conclude from the assay that these molecules contain 5′ phosphorylated extremities and, thus, are not generated by nonspecific RNases generating a 5′ OH group (31).
Overall, the results described above demonstrated that the 5′–3′ mRNA degradation pathway is active in human cell extracts. Recent experiments have, however, failed to reveal m7GDP production in cellular extracts (21, 26). Thus, we tested for the formation of m7GDP after incubation of RNA carrying a single radiolabeled phosphate in the cap structure (m7Gp*ppG with p* representing the radioactive phosphate, see Materials and Methods) in our human HEK293 extracts. Reaction products were resolved by TLC using a system allowing the resolution of the major expected products (24). Consistent with previous observations (21, 26), m7GDP was not detected (Fig. 1_C_, lane 3), whereas abundant amounts of m7GMP and inorganic phosphate were formed. This observation revealed an inconsistency between the two assays for hDcp2-mediated cap cleavage. Because the 5′–3′ pathway might be poorly active in human cell extracts, we tested m7GDP formation in extracts derived from yeast cells where the 5′–3′ pathway is known to be the major mRNA decay route. Intriguingly, m7GDP was also not observed in yeast extracts (Fig. 1_C_, lane 4). In this case, low levels of m7GMP were generated together with an unidentified phosphorylated product (thereafter named “X”) and high amounts of free phosphate. These results suggested that the accumulation of m7GDP might not be a reliable indicator of decapping activity. Together, our data indicate that the 5′–3′ decay pathway is active in HEK293 cellular extracts, whereas the absence of m7GDP was possibly caused by its rapid conversion rather than a lack of its formation.
m7GDP Is Unstable in Human, Yeast, and Xenopus Extracts. To test the fate of m7GDP in extract, we produced a radiolabeled species by hDcp2-mediated decapping of cap-labeled RNA. The m7GDP generated was purified from TLC plates (see Materials and Methods). As controls, m7GpppG and m7GMP, formed by incubation with P1 nuclease or HEK293 cell extracts, respectively, were also purified by using the same strategy. As expected, m7GpppG cap was quantitatively converted to m7GMP in human cell extracts (Fig. 2_A_). In yeast extracts (Fig. 2 A, lane 4), product X was generated with only traces of m7GMP. More importantly, m7GDP was rapidly and efficiently converted to m7GMP in human cell extracts and product X in yeast cell extracts (Fig. 2_B_). This demonstrated that m7GDP is unstable in extracts, being rapidly converted to breakdown products. Incubation of m7GMP in HEK293 cell extracts demonstrated that it was essentially stable with only a fraction being converted to free phosphate (Fig. 2_C_). Interestingly, in yeast extracts, m7GMP was converted to species X as well as some free phosphate.
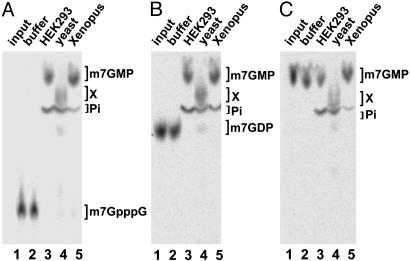
Fig. 2.
m7GDP conversion in human, yeast, and Xenopus cell extracts. (A) Radiolabeled m7GpppG was purified from TLC plates (Materials and Methods) and incubated with buffer (lane 2), HEK293 whole cell extract (lane 3), yeast BY4741 wild-type whole cell extract (lane 4), and whole cell extracts from Xenopus oocytes (lane 5). Lane 1 contains the input of the substrate without incubation. (B) As described for A, with radiolabeled m7GDP as substrate. (C) As described for A, with m7GMP as substrate. In all cases, 7 μg of cellular extract was used. Reaction products were separated by TLC. The migration positions of unlabeled standards are indicated on the sides. The unidentified product X resulting from incubation with yeast extract is also indicated.
Taken together, these results demonstrate that m7GDP is unstable in extract, being efficiently converted to m7GMP in human and product X in yeast. The stability of m7GMP differs between extracts; it is only slowly hydrolyzed to free phosphate in human cell extracts, whereas it is efficiently converted to the uncharacterized species X and some free phosphate in yeast extracts. Because m7GMP is an obligatory intermediate in the formation of product X (see below), these results indicate that m7GDP is also converted in m7GMP in yeast extract before being further rapidly metabolized in product X. Interestingly, conversion of m7GDP into m7GMP was also observed in Xenopus extracts (Fig. 2_B_, lane 5) and Drosophila cell extracts (data not shown), demonstrating, in addition, that this is a conserved feature of eukaryotic cells.
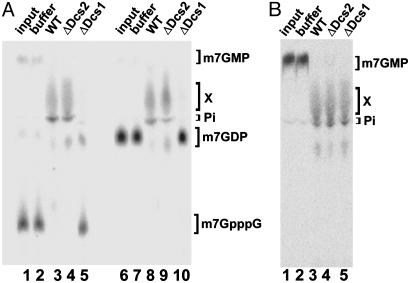
Both Yeast and Human DcpS Catalyze the Conversion of m7GDP into m7GMP. The conversion of m7GDP into m7GMP was reminiscent of DcpS activity, because this enzyme hydrolyzes m7GpppG to m7GMP (21). Thus, we decided to test whether the yeast DcpS or related proteins are required for this reaction in extracts. Interestingly, yeast cells contain, in addition to the DcpS activity harbored by the Dcs1p protein encoded by the DCS1 gene, a highly similar protein, Dcs2p, encoded by the DCS2 gene. However, no DcpS activity has been reported for the product of DCS2 (32). Both DCS genes are not essential. Thus, we were able to test the requirement for the corresponding factors in the conversion of m7GDP into m7GMP by analyzing the fate of radiolabeled m7GDP in extracts from deletion strains and isogenic wild-type controls (Fig. 3_A_). m7GpppG was converted to product X in extracts from wild-type and Δ_dcs2_ cells (Fig. 3_A_, lanes 3 and 4) but remained stable in a Δ_dcs1_ cell extract (lane 5). Interestingly, m7GDP was also stabilized in extracts from Δ_dcs1_ cells (lane 10), indicating that Dcs1p function is required for the conversion of m7GDP in yeast cell extracts. This effect is specific, because conversion of m7GDP to product X occurred efficiently in extracts prepared from isogenic and Δ_dcs2_ cells (lanes 8 and 9). To test whether the Dcs1p and/or the Dcs2p proteins affect the conversion of m7GMP to product X (see Fig. 2_C_), radiolabeled m7GMP was also incubated in the three extracts (Fig. 3_B_). In all cases, m7GMP was quantitatively converted to product X. Thus, we conclude that Dcs1p is required for the conversion of m7GDP and m7GpppG, but that neither Dcs1p nor Dcs2p is absolutely required for the conversion of m7GMP into product X.
Fig. 3.
m7GDP is stable in extracts from Δdcs1 cells. (A) Radiolabeled m7GpppG (lanes 1–5) or m7GDP (lanes 6–10) were incubated with buffer (lanes 2 and 7), BY4741 wild-type (WT) yeast extract (lanes 3 and 8), Δ_dcs2_ yeast extract (lanes 4 and 9), and Δ_dcs1_ yeast extract (lanes 5 and 10). Lanes 1 and 6 contain the input m7GpppG and m7GDP, respectively. Reaction products were separated by TLC; the migration positions of unlabeled standards are indicated on the right. Unidentified product X is also indicated. (B) m7GMP is converted to product X and free phosphate in all yeast extracts. Radiolabeled m7GMP was incubated with buffer (lane 2), BY4741 WT yeast extract (lane 3), Δ_dcs2_ yeast extract (lane 4), and Δ_dcs1_ yeast extract (lane 5). Lane 1 contains the input. Migration positions of m7GMP, product X, and free phosphate (Pi) are indicated.
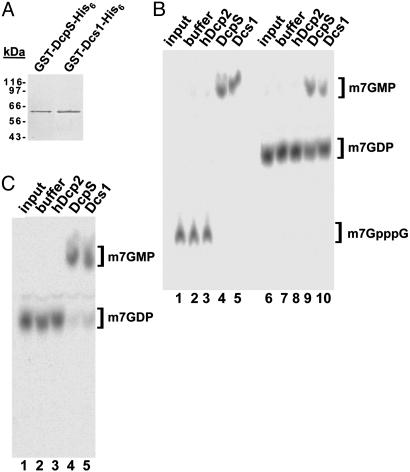
The results reported above suggest that Dcs1p might control the activity responsible for m7GDP conversion or that it may be directly responsible for catalyzing this reaction. To test the latter possibility, the Dcs1p coding sequence was cloned in a bacterial expression plasmid containing an N-terminal GST tag and a C-terminal His-6 tag (see Materials and Methods). In parallel, we cloned the human DcpS coding sequence in the same plasmid. Both proteins were expressed in Escherichia coli and subjected to two sequential purification steps on glutathione agarose and Ni-nitrilotriacetic acid affinity columns. This procedure allowed us to obtain highly pure proteins devoid of detectable contaminants (Fig. 4_A_; the human and yeast recombinant proteins comigrate as their molecular weights differ by only 2 kDa). These recombinant proteins were tested for their ability to hydrolyze m7GpppG cap and m7GDP (Fig. 4_B_). Consistent with previous reports, both proteins efficiently converted m7GpppG to m7GMP, indicating that they were active (lanes 4 and 5). In addition, both proteins catalyzed the conversion of m7GDP into m7GMP, although only a fraction of the substrate was converted under these conditions (lanes 9 and 10). Complete conversion was observed with slightly higher protein amounts (150 ng) and increased reaction time (1 h) (Fig. 4_C_). Purified GST-hDcp2-His-6 was used as a control; as expected, no activity was seen for this protein, ruling out the presence of contaminating activities (24).
Fig. 4.
Both yeast Dcs1p and human DcpS hydrolyze m7GDP to m7GMP. (A) Purified recombinant GST-DcpS-His-6 and GST-Dcs1-His-6 were fractionated by SDS/PAGE and detected by Coomassie staining. A protein size marker was run on the side. The two recombinant proteins have similar molecular mass (64 vs. 65 kDa, including the tags). (B) Radiolabeled m7GpppG (lanes 1–5) and m7GDP (lanes 6–10) were incubated with buffer (lanes 2 and 7), GST-hDcp2-His-6 (lanes 3 and 8), GST-DcpS-His-6 (lanes 4 and 9), and GST-Dcs1-His-6 (lanes 5 and 10). In all cases, 50 ng of protein was used. The inputs of m7GpppG and m7GDP are shown in lanes 1 and 6, respectively. Reaction products were separated by TLC; migration positions of unlabeled standards are indicated on the right. (C) m7GDP was incubated with buffer, GST-hDcp2-His-6, GST-DcpS-His-6, and GST-Dcs1-His-6 as in B; here, 150 ng of protein was used instead of 50 ng, and the incubation time was extended to1h(Materials and Methods).
These results demonstrate that both yeast Dcs1p as well as human DcpS are sufficient to convert m7GDP to m7GMP. In yeast, m7GMP is further converted to product X. Further substantiating this conclusion, we observed that addition of recombinant Dcs1p to a Δ_dcs1_ yeast extract fully restored its ability to convert m7GDP to product X (data not shown).
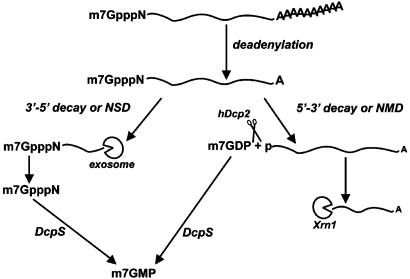
In summary, our results demonstrate that m7GDP, the product of hDcp2-mediated decapping, is rapidly converted to m7GMP by DcpS. Thus, DcpS can act in all known mRNA decay pathways involving either 5′–3′ or 3′–5′ degradation of the mRNA body to produce m7GMP (Fig. 5).
Fig. 5.
Model showing the pathway-independent formation of m7GMP. After deadenylation, a mRNA can be degraded 3′–5′ by the exosome generating free m7GpppN. Alternatively, the mRNA can undergo decapping by hDcp2, generating m7GDP and a 5′-phosphorylated mRNA. RNAs degraded by the NSD and NMD pathways also generate m7GpppN and m7GDP, respectively. Both products are converted to m7GMP by DcpS. Thus, mRNA decay leads to the formation of m7GMP, irrespective of the pathway followed.
Discussion
We recently identified recombinant hDcp2 as the human mRNA decapping enzyme that cleaves capped mRNAs to produce m7GDP and 5′-phosphorylated downstream RNA products (24). By using a new RNA ligation-based assay, we have now established that decapping occurs in human cell extracts. In addition, we have observed shorter RNA degradation intermediates, consistent with 5′–3′ exonucleolytic digestion of decapped RNA, even though we cannot fully exclude at this stage that they represent endonucleolytically cleaved species. A striking observation was that the second product of the decapping reaction, namely m7GDP, was not detected in human or yeast cell extracts. Our results demonstrate that the absence of m7GDP reflects its rapid conversion into m7GMP rather than a lack of formation. In any case, based on the appearance and downstream processing of both products of this reaction, our results demonstrate unequivocally that the 5′–3′ mRNA decay pathway is active in human cell extracts. Previous studies based exclusively on the release of the nucleotide product generated by cap cleavage have given contradictory results. m7GDP formation was reported to occur exclusively in extracts supplemented with m7GpppG competitor and by using AU-Rich element-containing transcripts (33). However, a later study reported that only m7GMP is formed in extracts, whereas neither the original results nor the effect of cap analog addition could be reproduced (26). In the latter study, formation of a product comigrating with m7GDP was only observed when polysomal extracts or ribosomal salt washes were used. A complicating factor in this issue is the low resolving power of the TLC systems used in most studies. Indeed, the commonly used developing solution 0.45 M ammonium sulfate does not allow for a good separation of m7GDP and m7GMP and does not resolve m7GDP and free phosphate (24), (34). When this system is used, it is thus very difficult to distinguish hDcp2-mediated decapping activity (generating m7GDP) from DcpS-activity (generating m7GMP) and degradation by-products thereof (free phosphate). We recently introduced an improved TLC system (see Materials and Methods; ref. 24) that allows for a much better separation of the different reaction products. Bergman et al. (34) reported an alternative denaturing acrylamide gel-based assay that also allows a faithful resolution of the different reaction products. Clearly, the use of an optimal assay is essential to ensure reliable RNA degradation product identification. However, our results demonstrate that this issue is further complicated by the rapid and quantitative conversion of m7GDP in m7GMP in extracts. As a consequence, these molecules cannot serve as indicators of specific mRNA degradation pathways as previously thought. Indeed, m7GDP is not detectable, although the formation of m7GMP cannot be taken as a distinguishing feature of the 3′–5′ pathway. Therefore, the exclusive presence of m7GMP does not indicate that the 3′–5′ pathway is the only decay pathway active in extracts. In contrast, because we detect the only specific product of mRNA degradation by using a novel ligation assay, namely 5′-phosphorylated RNA, we conclude that the 5′–3′ pathway is active in human cell extracts. The previous detection of decapped RNA isolated from mouse cells (9) also argues for the presence of such a pathway in mammalian cells. However, further work will be required to evaluate the relative contributions of both pathways to general mRNA degradation. Our new assay for decapping will be useful for this purpose.
We also demonstrate that human DcpS and its yeast homologue Dcs1p are responsible for catalyzing the conversion of m7GDP to m7GMP. These enzymes have thus at least two natural substrates, m7GpppG and m7GDP. Although m7GpppG may be a somewhat better substrate, extracts contain sufficient enzymatic activity to convert all of the m7GDP generated by decapping, because no m7GDP was detected. The absence of m7GDP is a particularly striking observation in the case of yeast because the decapping-dependent 5′–3′ mRNA decay pathway predominates in these cells (1). Interestingly, in yeast m7GMP formed by m7GDP hydrolysis is efficiently further transformed into an unidentified compound X. Additional experiments will be necessary to determine the exact nature of this product as well as to identify the factor(s) responsible for this reaction.
The efficient conversion of m7GDP in m7GMP may serve several cellular functions. On one hand, m7GDP may need to be eliminated to prevent its incorporation into nucleic acids. Indeed, we and others (24, 29) have shown previously that m7GDP generated by decapping can be efficiently converted by nucleotide diphosphate kinase to m7GTP that would be a potential substrate for RNA polymerase (and several other enzymes). In theory, m7GDP could also be transformed into m7dGDP and m7dGTP that could be incorporated into the cellular DNA. However, these processes do not seem to have dramatic consequences in vivo because yeast strains lacking Dcs1 are viable (32) even though they are unable to convert m7GDP m7GMP in vitro (Fig. 3_A_). On the other hand, the conversion of m7GDP to m7GMP generates a product common to all known mRNA degradation pathways (Fig. 5). This unique by-product, or derivative(s) thereof, may thus serve as a cellular indicator to coordinate mRNA decay with other cellular processes. Further analysis will reveal whether this is indeed the case.
Acknowledgments
We thank members of the Séraphin laboratory for critical reading of the manuscript, Céline Faux for technical assistance, and Prof. Elmar Wahle for discussion. E.v.D. was supported by fellowships from the Centre National de la Recherche Scientifique (CNRS) and the Fondation pour la Recherche Médicale. This work was funded by La Ligue contre le Cancer (Equipe Labellisée 2001) and the CNRS.
Abbreviations: NMD, nonsense-mediated mRNA decay; NSD, nonstop decay.
References
- 1.Caponigro, G. & Parker, R. (1996) Microbiol. Rev. 60**,** 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilusz, C. J., Wormington, M. & Peltz, S. W. (2001) Nat. Rev. Mol. Cell Biol. 2**,** 237–246. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell, P. & Tollervey, D. (2001) Curr. Opin. Cell Biol. 13**,** 320–325. [DOI] [PubMed] [Google Scholar]
- 4.Hsu, C. L. & Stevens, A. (1993) Mol. Cell. Biol. 13**,** 4826–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs, J. S., Anderson, A. R. & Parker, R. P. (1998) EMBO J. 17**,** 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czaplinski, K., Ruiz-Echevarria, M. J., Gonzalez, C. I. & Peltz, S. W. (1999) BioEssays 21**,** 685–696. [DOI] [PubMed] [Google Scholar]
- 7.van Hoof, A., Frischmeyer, P. A., Dietz, H. C. & Parker, R. (2002) Science 295**,** 2262–2264. [DOI] [PubMed] [Google Scholar]
- 8.Frischmeyer, P. A., van Hoof, A., O'Donnell, K., Guerrerio, A. L., Parker, R. & Dietz, H. C. (2002) Science 295**,** 2258–2261. [DOI] [PubMed] [Google Scholar]
- 9.Couttet, P., Fromont-Racine, M., Steel, D., Pictet, R. & Grange, T. (1997) Proc. Natl. Acad. Sci. USA 94**,** 5628–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gera, J. F. & Baker, E. J. (1998) Mol. Cell. Biol. 18**,** 1498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgs, D. C. & Colbert, J. T. (1994) Plant Cell 6**,** 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim, S. K. & Maquat, L. E. (1992) EMBO J. 11**,** 3271–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daugeron, M. C., Mauxion, F. & Seraphin, B. (2001) Nucleic Acids Res. 29**,** 2448–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salgado-Garrido, J., Bragado-Nilsson, E., Kandels-Lewis, S. & Seraphin, B. (1999) EMBO J. 18**,** 3451–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, S. G., Lee, I., Park, S. H., Kang, C. & Song, K. (1995) Genomics 25**,** 660–666. [DOI] [PubMed] [Google Scholar]
- 16.Bashkirov, V. I., Scherthan, H., Solinger, J. A., Buerstedde, J. M. & Heyer, W. D. (1997) J. Cell Biol. 136**,** 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell, P., Petfalski, E., Shevchenko, A., Mann, M. & Tollervey, D. (1997) Cell 91**,** 457–466. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee, A. K. (1980) Microbiol. Rev. 44**,** 175–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis, J. D., Gunderson, S. I. & Mattaj, I. W. (1995) J. Cell Sci. Suppl. 19**,** 13–19. [DOI] [PubMed] [Google Scholar]
- 20.van Hoof, A. & Parker, R. (2002) Curr. Biol. 12**,** R285–R287. [DOI] [PubMed] [Google Scholar]
- 21.Wang, Z. & Kiledjian, M. (2001) Cell 107**,** 751–762. [DOI] [PubMed] [Google Scholar]
- 22.Beelman, C. A., Stevens, A., Caponigro, G., LaGrandeur, T. E., Hatfield, L., Fortner, D. M. & Parker, R. (1996) Nature 382**,** 642–646. [DOI] [PubMed] [Google Scholar]
- 23.Dunckley, T. & Parker, R. (1999) EMBO J. 18**,** 5411–5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Dijk, E., Cougot, N., Meyer, S., Babajko, S., Wahle, E. & Seraphin, B. (2002) EMBO J. 21**,** 6915–6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lykke-Andersen, J. (2002) Mol. Cell. Biol. 22**,** 8114–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, Z., Jiao, X., Carr-Schmid, A. & Kiledjian, M. (2002) Proc. Natl. Acad. Sci. USA 99**,** 12663–12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steiger, M., Carr-Schmid, A., Schwartz, D. C., Kiledjian, M. & Parker, R. (2003) RNA 9**,** 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee, D., Gao, M., O'Connor, J. P., Raijmakers, R., Pruijn, G., Lutz, C. S. & Wilusz, J. (2002) EMBO J. 21**,** 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen, C. Y., Gherzi, R., Ong, S. E., Chan, E. L., Raijmakers, R., Pruijn, G. J., Stoecklin, G., Moroni, C., Mann, M. & Karin, M. (2001) Cell 107**,** 451–464. [DOI] [PubMed] [Google Scholar]
- 30.Lennon, G., Auffray, C., Polymeropoulos, M. & Soares, M. B. (1996) Genomics 33**,** 151–152. [DOI] [PubMed] [Google Scholar]
- 31.Usher, D. A. (1969) Proc. Natl. Acad. Sci. USA 62**,** 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, H., Rodgers, N. D., Jiao, X. & Kiledjian, M. (2002) EMBO J. 21**,** 4699–4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao, M., Wilusz, C. J., Peltz, S. W. & Wilusz, J. (2001) EMBO J. 20**,** 1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergman, N., Opyrchal, M., Bates, E. J. & Wilusz, J. (2002) RNA 8**,** 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]