Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease (original) (raw)
Abstract
Increasing evidence supports a role for oxidative DNA damage in aging and several neurodegenerative diseases including Alzheimer's disease (AD). Attack of DNA by reactive oxygen species (ROS), particularly hydroxyl radicals, can lead to strand breaks, DNA–DNA and DNA–protein cross-linking, and formation of at least 20 modified bases adducts. In addition, α,β-unsaturated aldehydic by-products of lipid peroxidation including 4-hydroxynonenal and acrolein can interact with DNA bases leading to the formation of bulky exocyclic adducts. Modification of DNA bases by direct interaction with ROS or aldehydes can lead to mutations and altered protein synthesis. Several studies of DNA base adducts in late-stage AD (LAD) brain show elevations of 8-hydroxyguanine (8-OHG), 8-hydroxyadenine (8-OHA), 5-hydroxycytosine (5-OHC), and 5-hydroxyuracil, a chemical degradation product of cytosine, in both nuclear and mitochondrial DNA (mtDNA) isolated from vulnerable regions of LAD brain compared to age-matched normal control subjects. Previous studies also show elevations of acrolein/guanine adducts in the hippocampus of LAD subjects compared to age-matched controls. In addition, studies of base excision repair show a decline in repair of 8-OHG in vulnerable regions of LAD brain. Our recent studies show elevated 8-OHG, 8-OHA, and 5,6-diamino-5-formamidopyrimidine in both nuclear and mtDNA isolated from vulnerable brain regions in amnestic mild cognitive impairment, the earliest clinical manifestation of AD, suggesting that oxidative DNA damage is an early event in AD and is not merely a secondary phenomenon.
ALZHEIMER'S DISEASE—CLINICAL FEATURES
Alzheimer's disease (AD), the most common form of dementia in adults over age 65, is the fourth leading cause of death in the United States and currently affects 4.5 million Americans (1). Estimates indicate that ∼3% of Americans between ages 65 and 74, 19% between ages 75 and 84, and ∼47% over age 85 are victims of the disease (2) and that ∼60% of nursing home patients over age 65 suffer from AD. As the baby boom generation ages and without preventive strategies, it is estimated that there may be 14 million Americans with AD by 2040 (1). Clinically, AD is characterized by a gradual decline of cognitive function from a previous higher level that is manifest as impairment of social and occupational functions. Clinical features of AD include impairment of recent memory, language disturbances, and alterations of abstract reasoning, concentration and thought sequencing (executive function) (3). Based on criteria from the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA), the diagnosis of probable AD is made when patients demonstrate (i) dementia established by clinical examination and documented by mental status tests, (ii) deficits in two or more areas of cognition, (iii) progressive worsening, (iv) no disturbance in consciousness, (v) onset between age 40 and 90, and (vi) no systemic or other brain diseases that could account for the progressive deficits (4). The mean length of life following diagnosis is 8.5 years with a range of 1–25 years (5).
Initially, AD subjects often present clinically with amnestic mild cognitive impairment (MCI), which is thought to be a transition between normal aging and early dementia and at present likely represents the best opportunity for pharmacologic interventions. The clinical criteria for the diagnosis of amnestic MCI are those described by Petersen et al. (6) and include: (i) memory complaints, (ii) objective memory impairment for age and education, (iii) intact general cognitive function, (iv) intact activities of daily living (ADLs), and (v) the subject is not demented. Several other forms of MCI have been described including non-amnestic single domain and multiple domain forms (7). Current data suggest that conversion from MCI to dementia occurs at a rate of 10–15% per year (7) with ∼80% conversion by the sixth year of follow-up; although ∼5% of MCI subjects remain stable or convert back to normal (8,9). Progression from MCI leads to early AD (EAD) that is clinically characterized by (i) a decline in cognitive function from a previous higher level, (ii) decline in one or more areas of cognition in addition to memory, (iii) a clinical dementia rating scale score of 0.5–1, (iv) impaired ADLs, and (v) a clinical evaluation that excludes other causes of dementia. Further progression of the disease leads to late-stage AD (LAD) characterized by severe dementia with profound global cognitive deficits and immobility.
NEUROPATHOLOGIC MANIFESTATIONS OF AD
Pathologically, LAD is characterized by an abundance of neurofibrillary tangles (NFT), senile plaques (SP) or beta amyloid (Aβ) plaques, neuropil thread formation, neuron and synapse loss, and proliferation of reactive astrocytes in the entorhinal cortex, hippocampus, amygdala and association areas of frontal, temporal, parietal and occipital cortex. NFT consist of intracellular deposits of paired helical filaments composed of hyperphosphorylated tau.
SP are present in two forms: (i) diffuse plaques composed of amorphous extracellular deposits of Aβ that lack neurites and (ii) neuritic plaques (NP) composed of extracellular deposits of insoluble Aβ surrounded by dystrophic neurites, reactive astrocytes, and activated microglia. In addition to Aβ present in SP, recent studies suggest soluble Aβ oligomers are present in the AD brain that may represent the main toxic form of Aβ, thus implicating them in the disease process (10–12).
These hallmark pathological lesions are employed for the neuropathological diagnosis of AD using the National Institute on Aging-Reagan Institute (NIA-RI) criteria. The NIA-RI criteria use the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) NP scores in conjunction with Braak staging to provide low, intermediate and high likelihood classifications for the diagnosis of AD. The CERAD criteria use NP densities in three neocortical regions (frontal, temporal, and parietal) to provide an age-related NP score that is used in conjunction with the clinical history to reach a diagnosis of possible, probable, or definite AD. Braak and Braak (13) demonstrated that the pathological process in AD progresses in a topographically predictable manner from the transentorhinal (stages I and II) to entorhinal, hippocampus, amygdala and adjacent temporal cortex (limbic stages III and IV) and then to the isocortex (stages V and VI).
Pathologically, subjects with MCI show a significant increase in NP in neocortical regions and a significant increase in NFT in entorhinal cortex, hippocampus and amygdale compared to normal control subjects (14). Braak staging scores of MCI subjects are typically in the range of III–IV. EAD subjects generally meet NIA-RI high likelihood criteria for the histopathologic diagnosis of AD with Braak staging scores of V but have less severe overall NFT and NP formation than LAD.
OXIDATIVE DAMAGE IN MCI AND AD
Although multiple free species, atoms or molecules with an unpaired electron in the outer shell are present in the body, the most common radicals are derived from the reduction of molecular oxygen to water during oxidative phosphorylation and as a group are termed reactive oxygen species (ROS). In normally functioning tissues, a balance is maintained between ROS generation and antioxidant protection mediated through antioxidant enzymes including copper/zinc superoxide dismutase, manganese superoxide dismutase, glutathione peroxidase and catalase among others and small antioxidant molecules such as glutathione, vitamin E, and vitamin C. When the balance between free radical generation and antioxidant capacities shifts toward free radical generation, oxidative stress occurs leading to oxidative damage to lipids, proteins, RNA, and DNA. Oxidative damage to these biomolecules can contribute to loss of function leading to exacerbation of damage. The brain is particularly susceptible to oxidative damage due to its high oxygen consumption rate (∼1/5th consumed oxygen), its high-energy demands, rich abundance of polyunsaturated fatty acids and lipids, and the relatively limited antioxidant capacity relative to other organs (15). In general, ∼2% of oxygen consumed by cells during oxidative phosphorylation is converted to ROS (16), although this may well be higher in subjects with impaired oxidative phosphorylation such as AD. Superoxide generated through free radical leakage is quickly converted to hydrogen peroxide that can diffuse to the cytoplasm and interact with redox active metals including iron or copper to further generate hydroxyl radical through Fenton or Haber–Weiss reactions. Hydroxyl radicals generated through these pathways can further propagate damage to surrounding biomolecules. Several previous studies (17,18) show significant elevation of copper and iron in vulnerable regions of LAD brain suggesting high potential for redox-metal-mediated ROS generation.
The concept of ROS attack of biomolecules leading to cellular damage and death was hypothesized to be the basis of cellular aging by Harman in 1956 (19) and was termed the free radical theory of aging. Subsequently, numerous studies have provided evidence that free radical-mediated damage to cellular function contributes to aging, cancer and multiple age-related neurological diseases including AD (20). Studies of oxidative damage in LAD show significantly increased lipid peroxidation, protein oxidation, and RNA oxidation in multiple neocortical brain regions (15). More recently, studies from our laboratories and others show RNA and protein oxidation and lipid peroxidation are also significantly elevated in vulnerable regions of the MCI brain (15), suggesting oxidative damage may be an early event in the pathogenesis of AD. Unfortunately, studies of the temporal profile of biomolecule oxidative modification have not been carried out and it is currently not clear whether DNA oxidation precedes modification of other cellular molecules.
Although ROS can attack a variety of biomolecules, DNA, particularly mitochondrial DNA (mtDNA), may be the primary target of the free radical damage that contributes to cellular degeneration and aging (21). Indeed, multiple studies show oxidative damage to DNA may be important in cancer (22,23) and, because of its high oxygen consumption rate, may also be important in neuronal damage associated with aging and neurodegenerative diseases.
DNA OXIDATION
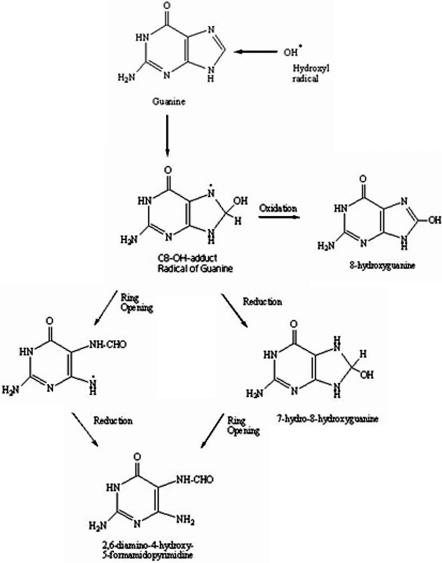
Oxidative attack of DNA by ROS, particularly hydroxyl radicals, can lead to strand breaks, DNA–DNA and DNA–protein cross-linking, and in nuclear DNA (nDNA) of replicating cells sister chromatid exchange and translocation (24,25). As reviewed by Dizdaroglu et al. (26), ROS attack of DNA can lead to the generation of more than 20 oxidized base adducts, the most prominent being 8-hydroxyguanine (8-OHG). Because of its low oxidation potential related to the other three DNA bases, guanine is the most readily oxidized. Attack of C8 of guanine leads to the formation of a guanine C8-OH adduct radical that can undergo three separate pathways (Figure 1): (i) ring opening and subsequent reduction leading to formation of 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyGua); (ii) initial reduction of the guanine C8-OH radical adduct to form 7-hydro-8-hydroxyguanine that, following ring opening, leads to FapyGua, and (iii) oxidation of the guanine C8-OH radical adduct to form 8-OHG [adapted from (26,27)]. Recent studies suggest that the end products of ROS attack of guanine may depend on oxygen tension with increased 8-OHG formation occurring under elevated oxygen tension, whereas FapyGua formation is more likely under reduced oxygen tension (28). Because of the ease of oxidation of guanine, 8-OHG has been shown to be the predominant marker of DNA oxidation using a variety of analytical techniques including immunohistochemistry, 32P or fluorescent post-labeling of nucleosides enzymatically digested from DNA, comet assays, capillary electrophoresis with electrochemical detection (ECD), UV-Visible or laser induced fluorescence detection, high pressure liquid chromatography (HPLC) coupled with ECD or mass spectrometry (MS) or gas chromatography (GC) coupled with MS (26). Of these analytical approaches, immunoassays have primarily been used in the analysis of urine samples or other dilute biological specimens (29), whereas comet assays have been used in the analysis of DNA damage following exposure of cell cultures (lymphocytes) to toxic agents to evaluate repair of strand breaks (29,30). For the analysis of oxidation of DNA isolated from tissue specimens, most studies have used HPLC/ECD, HPLC/MS or GC/MS. Although HPLC/ECD provides detection limits of ∼20 fmol, it suffers from the potential for interference from co-eluting compounds, a problem shared with capillary electrophoresis with ECD or optical methods of detection. Additionally, the small sample volumes used for capillary electrophoresis lead to poorer sensitivities and detection limits for 8-OHG compared to other techniques (29). Because of the difficulties associated with these techniques, most recent studies of DNA oxidation have used HPLC or GC coupled with MS for unequivocal identification of the oxidized base adducts. Additionally, MS allows identification of multiple-base adducts in a single run providing a more complete picture of DNA oxidation. For analysis by HPLC/MS, DNA samples are enzymatically digested to nucleosides [8-hydroxyguanosine (8OhdG)] followed by HPLC, whereas samples for GC are hydrolyzed in formic acid to individual bases (8-OHG) before derivatization to increase volatility. For consistency, 8-OHG will be used in this review to represent oxidized guanine regardless of the method of DNA digestion. Both HPLC/MS and GC/MS use stable-labeled internal standards for identification and quantification of DNA base adducts.
Figure 1.
Reaction pathway for hydroxyl radical attack of guanine to form 8-hydroxyguanine and 2,6-diamino-4-hydroxy-5-formamidopyrimidine [adapted from Ref. (26)].
Although sample processing of DNA for GC/MS is more extensive, recent comparisons of LC/MS and GC/MS in the analysis of 8-OHG from calf thymus DNA showed similar results for the two techniques with comparable detection limits for HPLC (35 fmol/ µg DNA) and GC (30 fmol/ µg DNA) (31), suggesting additional processing necessary for GC/MS does not lead to artifactual elevation of 8-OHG levels.
DNA oxidation in MCI and LAD
The study of DNA damage and its possible contribution to neurodegeneration in AD began in 1990 when Mullaart et al. (32) showed a 2-fold increase in DNA strand breaks in the brain in AD. The increase in strand breaks was hypothesized to activate poly (ADP-ribose) polymerase (PARP), a zinc finger DNA-binding protein (33) that could cause depletion of intracellular NAD+ and depletion of energy stores resulting in cell death (34). Additionally, Su et al. (35), using terminal deoxynucleotidyl transferase labeling, showed increased strand breaks in non-NFT bearing neurons in AD that was associated with nitrotyrosine labeling, suggesting DNA in neurons in AD may be subject to oxidative damage mediated by peroxynitrite prior to NFT formation.
The study of specific oxidized DNA bases in the brain in aging and AD was initiated in 1993 by Mecocci et al. (36), who used HPLC/ECD to show an age-dependent increase in levels of 8-OHG in nDNA and mDNA specimens from cerebral cortex of 10 normal control subjects aged 42–97. In a follow-up study, the same group analyzed nDNA and mtDNA from frontal, temporal, and parietal lobes and cerebellum of 13 AD and 15 age-matched normal control subjects and showed a significant 3-fold increase in 8-OHG in mtDNA isolated from parietal lobe of AD (37). This study did not show significant differences in levels of 8-OHG in nDNA from AD subjects. In subsequent studies, Lyras et al. (38) used GC/MS and showed increased 8-OHG, 8-hydroxyadenine (8-OHA) and 5-hydroxycytosine (5-OHC) in total DNA from AD parietal lobe compared to age and gender matched control subjects. This study also showed increased thymine glycol, 4,6-diamino-5-formamido-pyrimidine (FapyAde), FapyGua, and 5-hydroxyuracil (5-OHU), a degradation product of cytosine in various brain regions in AD.
Our studies used GC/MS with selective ion monitoring (GC/MS-SIM) and specific-stable labeled internal standards to quantify 8-OHG, FapyGua, 8-OHA, FapyAde and 5-OHC in nDNA isolated from frontal, temporal and parietal lobes and cerebellum of LAD and age-matched normal control subjects. All specimens used in our studies were from short postmortem interval (PMI) autopsies (2–4 h) of subjects followed longitudinally in the University of Kentucky Alzheimer's Disease Center. Results of the analyses showed statistically significant elevations of 8-OHG, 8-OHA, and 5-OHU in temporal and parietal lobes in AD compared to age-matched control subjects (39). We did not observe significant differences in FapyGua or FapyAde in this initial study. More recently, in a study comparing levels of nDNA oxidation to mtDNA oxidation in specimens isolated from LAD subjects and age-matched normal control subjects, we found statistically significant elevations of 8-OHG, 8-OHA, 5-OHC and FapyAde in mtDNA from parietal and temporal lobes of LAD patients (40). We also observed significantly increased 5-OHC in mtDNA from LAD frontal lobe. Analysis of nDNA showed significantly increased 8-OHG in DNA from temporal and parietal lobes, 8-OHA in all three neocortical areas, 5-OHC in frontal and temporal lobes, 5-OHU in temporal lobe and FapyAde in temporal lobe and cerebellum in LAD. Comparison of levels of oxidation in mtDNA relative to levels of oxidation in nDNA showed a 10-fold increase in mtDNA oxidation, particularly 8-OHG, compared to nDNA consistent with mtDNAs proximity to ROS generation in the mitochondria and the relatively limited DNA repair capacity in mitochondria (39,40). Although there have been multiple studies that show DNA damage is increased in isolated DNA, there have been relatively few studies of cellular distribution of DNA damage. Previous studies using antibodies against 8-OHG in RNA and 8-OhdG in DNA showed immunostaining for both adducts was more pronounced in LAD subjects compared to age-matched normal control subjects (41). Characterization of immunostaining showed the presence of oxidized nucleotides was not restricted to NFT-bearing neurons or to cells closely localized to SP.
In studies of peripheral DNA damage in AD, Mecocci et al. (42,43) showed significant elevations of 8-OHG in lymphocytes isolated from 40 AD subjects compared with samples isolated from 39 age-matched control subjects that negatively correlated with plasma antioxidant levels. In additional studies of DNA damage in AD lymphocytes, Kadioglu et al. (30) used comet assays and lesion specific DNA repair endonucleases (endonuclease III for oxidized pyrimidines and formamido pyrimidine glycosylase for oxidized purines) to show significantly increased levels of oxidized purines and pyrimidines in lymphocytes isolated from 24 AD subjects compared to 21 age-matched normal controls.
Although there have been several studies of DNA oxidation in LAD, there have been few studies of DNA oxidation in MCI. In our most recent studies, we analyzed DNA base adducts in nDNA and mtDNA from temporal, parietal and frontal lobes (neocortex) and cerebellum of eight longitudinally followed amnestic MCI patients compared to six longitudinally followed normal control subjects, all with short PMI autopsies (44). These analyses showed statistically significant elevations of 8-OHG in nDNA from MCI frontal, and temporal lobes and mtDNA from temporal lobes of MCI patients compared to normal control subjects. We also observed significantly elevated 8-OHA and FapyAde in nDNA and FapyAde in mtDNA from all three neocortical lobes of MCI patients compared to normal controls. It is interesting that levels of base adducts in MCI were not significantly different from those observed in LAD subjects, suggesting oxidative damage to DNA in addition to other biomolecules is an early event in the pathogenesis of neuron degeneration in AD and may play a meaningful role in progression of the disease.
In addition to direct oxidation by ROS, DNA can also be modified by aldehydic by-products of lipid peroxidation. Attack of polyunsaturated fatty acids by ROS leads to the production of several aldehydic by-products including straight chain (C3–C10) aldehydes and α,β-unsaturated aldehydes including _trans_-4-hydroxy-2-nonenal (HNE) and acrolein. Previous studies from our laboratory and others (15) showed significant elevations of HNE and acrolein in LAD brain and CSF and in vulnerable regions of MCI and EAD brain. Because of the reactivity of α,β-unsaturated aldehydes, they can react with deoxyguanosine through an initial Michael addition of the exocyclic amino group followed by ring closure of _N_−1 onto the aldehydic group to generate a bulky exocyclic 1−_N_2-propanodeoxyguanosine adduct (45). These adducts are biologically relevant in that they may promote DNA–DNA and DNA–protein cross-linking (46). In addition, the presence of these bulky exocyclic adducts may alter transcription factor binding and limit transcription of essential proteins. Although several studies show elevated levels of lipid peroxidation products in LAD brain, there have been few studies of aldehydic DNA adducts. In a recent study using isotope dilution capillary LC/MS/MS, we showed a statistically significant 2-fold increase in levels of acrolein/guanosine adducts in nDNA isolated from the hippocampus of eight LAD subjects compared to age-matched control subjects (47). In contrast, levels of the HNE/guanosine adduct in nDNA from parietal lobe and hippocampus of LAD subjects were not significantly altered compared to normal control subjects (45). These findings were consistent with earlier studies of Gotz et al. (48), who showed no significant differences in HNE/guanosine adducts using 32P post-labeling of deoxyguanosine adducts from DNA isolated from hippocampus, parietal cortex and cerebellum of LAD subjects compared to controls.
Although multiple studies show an accumulation of oxidatively modified DNA adducts in the progression of AD, the potential consequences of accumulated DNA oxidative modifications in post-mitotic cells such as neurons are unclear. The most likely impact is on binding of transcription factors. A number of redox-sensitive genes contain multiple guanines in critical transcription factor binding sites that are particularly vulnerable to oxidative modification. Previous studies of the role of oxidized guanine in the p50-binding subunits of nuclear factor kappa beta (NF-κβ), which contains four guanines in a row, showed the substitution of 8-OHdG for guanine 1 led to enhanced p50-binding affinity, whereas oxidative modification of guanine 3 reduced p50 binding (49). The presence of oxidized guanine at guanines 3 and 4 had no effect on p50 binding. Additionally, the presence of a single 8-OHdG led to inhibition of transcription factor binding to AP-1 and Sp1 (50) that could lead to diminished transcription of critical antioxidant enzymes.
DNA REPAIR IN MCI AND LAD BRAIN
Although considerable evidence suggests oxidative damage to DNA is associated with AD, beginning in MCI, there have been relatively few studies of DNA repair in the progression of the disease. Initial studies of DNA repair in AD showed increased levels of excision repair-cross-complementing gene products 80 and 89 (51), leading the authors to hypothesize that the AD brain is subject to increased oxidative damage and the elevation of repair gene products is an attempt to increase repair capacities. Studies of repair of alkylation damage of lymphoblasts from AD and control subjects exposed to alkylating agents showed familial AD patients repaired significantly less alkylation damage than cells from normal control subjects (52). Additional studies of subjects with sporadic AD showed some subjects demonstrated similar patterns as observed for familial AD subjects, although further studies are needed to confirm the initial findings (52). Using alkaline filter elution techniques, Boerrigter et al. (53) showed diminished repair of single-strand breaks in lymphocytes from 15 AD patients with two first degree relatives with AD compared to control subjects. Comparison of repair capacity of lymphocytes from 28 AD subjects with no or one first degree relative with AD showed no significant differences compared to controls (53). Studies of repair of DNA double-strand breaks by a non-homologous end joining mechanism that uses the DNA-dependent protein kinase (DNA-PK) complex show DNA-PK protein levels and end joining activities are significantly lower in AD midfrontal cortex compared to normal controls (54). Together, these data suggest repair of strand breaks induced by oxidative damage or other agents may be deficient in AD and may contribute to neurodegeneration.
Studies of repair systems responsible for recognition and repair of specific DNA lesions in the progression of AD have been relatively limited. Using immunohistochemistry, Furuta et al. (55) showed levels of MTH-1, an oxidized purine nucleoside triphosphatase that efficiently hydrolyzes oxidized DNA bases including 8-OHG, were significantly reduced in CA3 of the hippocampus in AD, although they were significantly elevated in entorhinal cortex. The authors suggested that regional differences in MTH-1 expression may have relevance to the regional neuron vulnerability observed in AD (55). More recently Jacobsen et al. (56) showed significantly decreased levels of proteins in the Mre 11 DNA repair complex in neurons of AD neocortex compared to age-matched normal control subjects. The Mre 11 complex consisting of Rad50, Mre11 and Nbs1 participates in DNA damage recognition and repair (57–59) and impairment may contribute to neurodegeneration in AD.
Based on the observation of increased 8-OHG in the progression of AD, it has been postulated that in addition to increased oxidative stress there may be diminished capacity for repair of 8-OHG in AD. To repair 8-OHG, the brain employs 8-oxoguanine glycosylase-1 (OGG1), which functions in the base excision repair pathway by releasing the modified base and creating an abasic site (60). Following removal of 8-OHG, the gap is filled by DNA polymerase and ligated by DNA ligase (61). Mammalian cells code for OGG1, a functional homolog of E. Coli Mut M that recognizes and excises 8-OHG residues paired with C in DNA (62), and is present in mitochondria and nuclei. Nuclear OGG1 (OGG1-α) and the mitochondrial isoform (OGG1-β) are both coded by the same gene and have an identical sequence of 315 amino acids from the N-terminus (63). Differences in the two proteins are in their C-termini where OGG1-α has a nuclear localization sequence coded by exon 7, whereas OGG1-β has a mitochondrial targeting sequence coded by exon 8 (63). Following translocation to mitochondria, the targeting sequence is cleaved by the mitochondrial processing peptidase in the matrix (64,65).
Our initial study of OGG1 excision activity in AD brain showed significantly decreased nuclear OGG1 activity in vulnerable regions (hippocampus and superior and middle temporal gyri) but not cerebellum of LAD subjects compared with cognitively normal control subjects (66). Unfortunately, we did not have sufficient material to measure 8-OHG levels in the same samples to correlate levels of OGG1 activity and levels of 8-OHG. In more recent studies, analysis of DNA from 14 AD patients and 10 normal control subjects identified mutations in the gene that codes for OGG1 in four AD subjects (67). Two of the mutations identified for a common single base (C796) dilution, whereas the other two mutations were nucleotide alterations that lead to single amino acid substitutions. Analysis of OGG1 activity in subjects with mutations showed a significant decrease in activity.
Additionally, recent studies of Iida et al. (68) using immunohistochemistry showed levels of the mitochondrial form of OGG1 (OGG1-β) were significantly decreased in orbitofrontal cortex in LAD compared to normal control subjects, although most staining was associated with NFT-bearing neurons and dystrophic neurites. In studies of OGG1 in aging, incision activity was decreased in peripheral blood lymphocytes (69) and fibroblasts (70,71) as a function of age. In studies of aging mice, OGG1 activity was significantly lower in aged mice in multiple brain regions (61). In contrast, studies of OGG1 in mitochondrial fractions prepared from aged human fibroblasts and liver of aged mice showed significantly higher levels of OGG1-β activity (63). Although these data appear contradictory in light of elevated levels of 8-OHG in aging, Szczesny et al. (63) showed a large fraction of mitochondrial OGG1-β in aged animals is stuck to the mitochondrial membrane in the precursor form and is not translocated to and processed in the matrix that is essential for functional activity. In more recent unpublished studies, we found significantly decreased levels of nuclear OGG1 protein and activity in neocortical lobes of MCI subjects compared to normal controls. We also observe a significant negative correlation between OGG1 activities and levels of 8-OHG. Together, these data suggest that excision of 8-OHG by OGG1 is limited in the progression of AD and may contribute to the accumulation of DNA damage and neurodegeneration in AD.
CONCLUSIONS
Multiple studies show increased oxidation of mtDNA and nDNA in MCI and LAD. The levels of 8-OHG, the predominant marker of DNA oxidation, in MCI are comparable to those observed in LAD, suggesting DNA oxidation occurs early in the progression of AD. In addition, considerable evidence suggests there is diminished capacity for repair of a variety of DNA lesions in the progression of AD. The observation of increased mtDNA and nDNA oxidation in MCI, the earliest detectable phase of AD suggests that DNA damage is not a secondary event in the pathogenesis of AD but may contribute in a meaningful way to neurodegeneration observed in AD. Although the studies reviewed here suggest DNA oxidation and diminished repair capacities may play a role in the progression of AD, considerably more work is needed to clarify the mechanisms of DNA oxidation in the disease process.
ACKNOWLEDGEMENTS
Work in the authors’ laboratories was funded by grants from the National Institute on Aging (P30-AG0-28383, 5P01-AG0-5119) and a grant from the Abercrombie Foundation. The authors thank Paula Thomason for editorial assistance and Geri Gerke for assistance with figure preparation. Funding to pay the Open Access publication charges for this article was provided by the Abercrombie Foundation.
Conflict of interest statement. None declared.
REFERENCES
- 1.Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 2.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch. Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4th. Washington, DC: American Psychiatric Publishing; 2000. [Google Scholar]
- 4.National Institute on Aging and Reagan Institute. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol. Aging. 1997;18(Suppl. 4):S1–S2. [PubMed] [Google Scholar]
- 5.Jost BC, Grossberg GT. The natural history of Alzheimer's disease: a brain bank study. J. Am. Geriatr. Soc. 1995;43:1248–1255. doi: 10.1111/j.1532-5415.1995.tb07401.x. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Morris JC. In: Mild Cognitive Impairment. Petersen RC, editor. New York: Oxford University; 2003. pp. 15–39. [Google Scholar]
- 8.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 9.DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003;2:15–21. doi: 10.1016/s1474-4422(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 10.Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol. Aging. 2006;27:570–575. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 12.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 13.Braak H, Braak E. In: Neurodegenerative Diseases. Calne D, editor. Philadelphia, PA: WB Saunders Co.; 1994. pp. 585–613. [Google Scholar]
- 14.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch. Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer's disease. J. Neurosci. Res. 2007 doi: 10.1002/jnr.21346. [Epub ahead of print] doi: 10.1002/jnr.21346. [DOI] [PubMed] [Google Scholar]
- 16.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 17.Cornett CR, Markesbery WR, Ehmann WD. Imbalances of trace elements related to oxidative damage in Alzheimer's disease brain. Neurotoxicology. 1998;19:339–345. [PubMed] [Google Scholar]
- 18.Deibel MA, Ehmann WD, Markesbery WR. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer's disease: possible relation to oxidative stress. J. Neurol. Sci. 1996;143:137–142. doi: 10.1016/s0022-510x(96)00203-1. [DOI] [PubMed] [Google Scholar]
- 19.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 20.Markesbery WR, Montine TJ, Lovell MA. In: The Pathogenesis of Neurodegenerative Disorders. Mattson MP, editor. Totowa, NJ: Humana Press; 2001. pp. 21–57. [Google Scholar]
- 21.Markesbery WR, Lovell MA. DNA oxidation in Alzheimer's disease. Antioxid. Redox Signal. 2006;8:2039–2045. doi: 10.1089/ars.2006.8.2039. [DOI] [PubMed] [Google Scholar]
- 22.Ames BN. Endogenous DNA damage as related to cancer and aging. Mutat. Res. 1989;214:41–46. doi: 10.1016/0027-5107(89)90196-6. [DOI] [PubMed] [Google Scholar]
- 23.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem. J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford DR, Suzuki T, Sesay J, Davies KJ. Analysis of gene expression following oxidative stress. Methods Mol. Biol. 2002;196:155–162. doi: 10.1385/1-59259-274-0:155. [DOI] [PubMed] [Google Scholar]
- 25.Davies KJ. Oxidative stress: the paradox of aerobic life. Biochem. Soc. Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- 26.Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic. Biol. Med. 2002;32:1102–1115. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- 27.Steenken S. Purine bases, nucleosides, and nucleotides: aqueous solution redox chemistry and transformation reaction of their radical cations and e and OH adducts. Chem. Rev. 1989;89:503–520. [Google Scholar]
- 28.Breen AP, Murphy JA. Reactions of oxyl radicals with DNA. Free Radic. Biol. Med. 1995;18:1033–1077. doi: 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- 29.Peoples MC, Karnes HT. Recent developments in analytical methodology for 8-hydroxy-2′-deoxyguanosine and related compounds. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;827:5–15. doi: 10.1016/j.jchromb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Kadioglu E, Sardas S, Aslan S, Isik E, Esat Karakaya A. Detection of oxidative DNA damage in lymphocytes of patients with Alzheimer's disease. Biomarkers. 2004;9:203–209. doi: 10.1080/13547500410001728390. [DOI] [PubMed] [Google Scholar]
- 31.Dizdaroglu M, Jaruga P, Rodriguez H. Measurement of 8-hydroxy-2′-deoxyguanosine in DNA by high-performance liquid chromatography-mass spectrometry: comparison with measurement by gas chromatography-mass spectrometry. Nucleic Acids Res. 2001;29:E12. doi: 10.1093/nar/29.3.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullaart E, Boerrigter ME, Ravid R, Swaab DF, Vijg J. Increased levels of DNA breaks in cerebral cortex of Alzheimer's disease patients. Neurobiol. Aging. 1990;11:169–173. doi: 10.1016/0197-4580(90)90542-8. [DOI] [PubMed] [Google Scholar]
- 33.Lautier D, Poirier D, Boudreau A, Alaoui Jamali MA, Castonguay A, Poirier G. Stimulation of poly(ADP-ribose) synthesis by free radicals in C3H10T1/2 cells: relationship with NAD metabolism and DNA breakage. Biochem. Cell Biol. 1990;68:602–608. doi: 10.1139/o90-085. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 35.Su JH, Deng G, Cotman CW. Neuronal DNA damage precedes tangle formation and is associated with up-regulation of nitrotyrosine in Alzheimer's disease brain. Brain Res. 1997;774:193–199. doi: 10.1016/s0006-8993(97)81703-9. [DOI] [PubMed] [Google Scholar]
- 36.Mecocci P, MacGarvey U, Kaufman AE, Koontz D, Shoffner JM, Wallace DC, Beal MF. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann. Neurol. 1993;34:609–616. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- 37.Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Ann. Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 38.Lyras L, Cairns NJ, Jenner A, Jenner P, Halliwell B. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer's disease. J. Neurochem. 1997;68:2061–2069. doi: 10.1046/j.1471-4159.1997.68052061.x. [DOI] [PubMed] [Google Scholar]
- 39.Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer's disease. J. Neurochem. 1998;71:2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer's disease. J. Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 41.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J. Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mecocci P, Polidori MC, Cherubini A, Ingegni T, Mattioli P, Catani M, Rinaldi P, Cecchetti R, Stahl W, et al. Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Arch. Neurol. 2002;59:794–798. doi: 10.1001/archneur.59.5.794. [DOI] [PubMed] [Google Scholar]
- 43.Mecocci P, Polidori MC, Ingegni T, Cherubini A, Chionne F, Cecchetti R, Senin U. Oxidative damage to DNA in lymphocytes from AD patients. Neurology. 1998;51:1014–1017. doi: 10.1212/wnl.51.4.1014. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J. Neurochem. 2006;96:825–832. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Lovell MA, Lynn BC. Detection and quantification of endogenous cyclic DNA adducts derived from trans-4-hydroxy-2-nonenal in human brain tissue by isotope dilution capillary liquid chromatography nanoelectrospray tandem mass spectrometry. Chem. Res. Toxicol. 2006;19:710–718. doi: 10.1021/tx0502903. [DOI] [PubMed] [Google Scholar]
- 46.Kozekov ID, Nechev LV, Moseley MS, Harris CM, Rizzo CJ, Stone MP, Harris TM. DNA interchain cross-links formed by acrolein and crotonaldehyde. J. Am. Chem. Soc. 2003;125:50–61. doi: 10.1021/ja020778f. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Lovell MA, Lynn BC. Development of a method for quantification of acrolein-deoxyguanosine adducts in DNA using isotope dilution-capillary LC/MS/MS and its application to human brain tissue. Anal. Chem. 2005;77:5982–5989. doi: 10.1021/ac050624t. [DOI] [PubMed] [Google Scholar]
- 48.Gotz ME, Wacker M, Luckhaus C, Wanek P, Tatschner T, Jellinger K, Leblhuber F, Ransmayr G, Riederer P, et al. Unaltered brain levels of 1,N2-propanodeoxyguanosine adducts of trans-4-hydroxy-2-nonenal in Alzheimer's disease. Neurosci. Lett. 2002;324:49–52. doi: 10.1016/s0304-3940(02)00163-5. [DOI] [PubMed] [Google Scholar]
- 49.Hailer-Morrison MK, Kotler JM, Martin BD, Sugden KD. Oxidized guanine lesions as modulators of gene transcription. Altered p50 binding affinity and repair shielding by 7,8-dihydro-8-oxo-2′-deoxyguanosine lesions in the NF-kappaB promoter element. Biochemistry. 2003;42:9761–9770. doi: 10.1021/bi034546k. [DOI] [PubMed] [Google Scholar]
- 50.Ghosh R, Mitchell DL. Effect of oxidative DNA damage in promoter elements on transcription factor binding. Nucleic Acids Res. 1999;27:3213–3218. doi: 10.1093/nar/27.15.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hermon M, Cairns N, Egly JM, Fery A, Labudova O, Lubec G. Expression of DNA excision-repair-cross-complementing proteins p80 and p89 in brain of patients with Down Syndrome and Alzheimer's disease. Neurosci. Lett. 1998;251:45–48. doi: 10.1016/s0304-3940(98)00488-1. [DOI] [PubMed] [Google Scholar]
- 52.Jones SK, Nee LE, Sweet L, Polinsky RJ, Bartlett JD, Bradley WG, Robison SH. Decreased DNA repair in familial Alzheimer's disease. Mutat. Res. 1989;219:247–255. doi: 10.1016/0921-8734(89)90007-6. [DOI] [PubMed] [Google Scholar]
- 53.Boerrigter ME, van Duijn CM, Mullaart E, Eikelenboom P, van der Togt CM, Knook DL, Hofman A, Vijg J. Decreased DNA repair capacity in familial, but not in sporadic Alzheimer's disease. Neurobiol. Aging. 1991;12:367–370. doi: 10.1016/0197-4580(91)90024-e. [DOI] [PubMed] [Google Scholar]
- 54.Shackelford DA. DNA end joining activity is reduced in Alzheimer's disease. Neurobiol. Aging. 2006;27:596–605. doi: 10.1016/j.neurobiolaging.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Furuta A, Iida T, Nakabeppu Y, Iwaki T. Expression of hMTH1 in the hippocampi of control and Alzheimer's disease. Neuroreport. 2001;12:2895–2899. doi: 10.1097/00001756-200109170-00028. [DOI] [PubMed] [Google Scholar]
- 56.Jacobsen E, Beach T, Shen Y, Li R, Chang Y. Deficiency of the Mre11 DNA repair complex in Alzheimer's disease brains. Brain Res. Mol. Brain Res. 2004;128:1–7. doi: 10.1016/j.molbrainres.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 57.Kastan MB, Lim DS. The many substrates and functions of ATM. Nat. Rev. Mol. Cell Biol. 2000;1:179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- 58.Nelms BE, Maser RS, MacKay JF, Lagally MG, Petrini JH. In situ visualization of DNA double-strand break repair in human fibroblasts. Science. 1998;280:590–592. doi: 10.1126/science.280.5363.590. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi-Iwai Y, Sonoda E, Sasaki MS, Morrison C, Haraguchi T, Hiraoka Y, Yamashita YM, Yagi T, Takata M, et al. Mre11 is essential for the maintenance of chromosomal DNA in vertebrate cells. Embo. J. 1999;18:6619–6629. doi: 10.1093/emboj/18.23.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Souza-Pinto NC, Croteau DL, Hudson EK, Hansford RG, Bohr VA. Age-associated increase in 8-oxo-deoxyguanosine glycosylase/AP lyase activity in rat mitochondria. Nucleic Acids Res. 1999;27:1935–1942. doi: 10.1093/nar/27.8.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imam SZ, Karahalil B, Hogue BA, Souza-Pinto NC, Bohr VA. Mitochondrial and nuclear DNA-repair capacity of various brain regions in mouse is altered in an age-dependent manner. Neurobiol. Aging. 2006;27:1129–1136. doi: 10.1016/j.neurobiolaging.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Tchou J, Kasai H, Shibutani S, Chung MH, Laval J, Grollman AP, Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc. Natl Acad. Sci. USA. 1991;88:4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szczesny B, Hazra TK, Papaconstantinou J, Mitra S, Boldogh I. Age-dependent deficiency in import of mitochondrial DNA glycosylases required for repair of oxidatively damaged bases. Proc. Natl Acad. Sci. USA. 2003;100:10670–10675. doi: 10.1073/pnas.1932854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gakh O, Cavadini P, Isaya G. Mitochondrial processing peptidases. Biochim. Biophys. Acta. 2002;1592:63–77. doi: 10.1016/s0167-4889(02)00265-3. [DOI] [PubMed] [Google Scholar]
- 65.Pfanner N. Protein sorting: recognizing mitochondrial presequences. Curr. Biol. 2000;10:R412–415. doi: 10.1016/s0960-9822(00)00507-8. [DOI] [PubMed] [Google Scholar]
- 66.Lovell MA, Xie C, Markesbery WR. Decreased base excision repair and increased helicase activity in Alzheimer's disease brain. Brain Res. 2000;855:116–123. doi: 10.1016/s0006-8993(99)02335-5. [DOI] [PubMed] [Google Scholar]
- 67.Mao G, Pan X, Zhu BB, Zhang Y, Yuan F, Huang J, Lovell MA, Lee MP, Markesbery WR, et al. Identification and characterization of OGG1 mutations in patients with Alzheimer's disease. Nucleic Acids Res. 2007;35:2759–2766. doi: 10.1093/nar/gkm189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iida T, Furuta A, Nishioka K, Nakabeppu Y, Iwaki T. Expression of 8-oxoguanine DNA glycosylase is reduced and associated with neurofibrillary tangles in Alzheimer's disease brain. Acta. Neuropathol. (Berl.) 2002;103:20–25. doi: 10.1007/s004010100418. [DOI] [PubMed] [Google Scholar]
- 69.Chen SK, Hsieh WA, Tsai MH, Chen CC, Hong AI, Wei YH, Chang WP. Age-associated decrease of oxidative repair enzymes, human 8-oxoguanine DNA glycosylases (hOgg1), in human aging. J. Radiat. Res. (Tokyo) 2003;44:31–35. doi: 10.1269/jrr.44.31. [DOI] [PubMed] [Google Scholar]
- 70.Goukassian D, Gad F, Yaar M, Eller MS, Nehal US, Gilchrest BA. Mechanisms and implications of the age-associated decrease in DNA repair capacity. Faseb. J. 2000;14:1325–1334. doi: 10.1096/fj.14.10.1325. [DOI] [PubMed] [Google Scholar]
- 71.Kaneko T, Tahara S, Taguchi T, Kondo H. Accumulation of oxidative DNA damage, 8-oxo-2′-deoxyguanosine, and change of repair systems during in vitro cellular aging of cultured human skin fibroblasts. Mutat. Res. 2001;487:19–30. doi: 10.1016/s0921-8777(01)00100-8. [DOI] [PubMed] [Google Scholar]