Defining therapeutic targets by using adenovirus: Blocking NF-κB inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares anti-inflammatory mediators (original) (raw)
Abstract
The role of the transcription factor NF-κB in the pathogenesis of rheumatoid arthritis has long been a subject of controversy. We used an adenoviral technique of blocking NF-κB through overexpression of the inhibitory subunit IκBα, which has the advantage that it can be used in the diseased tissue itself, with >90% of the synovial macrophages, fibroblasts, and T cells infected. We found that the spontaneous production of tumor necrosis factor α and other pro-inflammatory cytokines is NF-κB-dependent in rheumatoid synovial tissue, in contrast to the main anti-inflammatory mediators, like IL-10 and -11, and the IL-1 receptor antagonist. Of even more interest, IκBα overexpression inhibited the production of matrix metalloproteinases 1 and 3 while not affecting their tissue inhibitor. Blocking NF-κB in the rheumatoid joint thus has a very beneficial profile, reducing both the inflammatory response and the tissue destruction. The adenoviral technique described here has widespread applicability, allowing rapid testing of the effects of blocking a potential therapeutic target in either cultures of normal cells or in the diseased tissue itself.
Despite the success of recent clinical trials of anti-tumor necrosis factor α (TNFα) therapy in rheumatoid arthritis (RA), based on the prior finding that TNFα was of pivotal importance (1–6), there are a number of unresolved questions concerning the pathogenesis of RA. One is whether the mechanism of joint inflammation (synovitis) and destruction are the same or whether these processes are regulated differently. Contrasting opinions concerning this have been argued forcefully: For example, one school of thought suggests that, although TNFα is the cytokine controlling synovitis, IL-1 is the dominant cytokine implicated in cartilage destruction (7). Another variant suggests that the fibroblast-like cells in the joint, its major source of destructive enzymes, have acquired an autonomous “tumor-like” invasive phenotype and are not regulated in the same manner as the synovitis (8). Transplantation experiments of synovial fibroblasts and cartilage into severe combined immunodeficient mice are used to argue this point (9). The prediction from these experiments is that anti-TNF therapy will, as observed in trials, control signs and symptoms of inflammation but may not prevent structural damage and consequential deterioration of physical function in the long term. The anti-TNF trials reported so far have been too brief to resolve this controversy. A deeper understanding of the pathogenesis of RA would help determine how to improve on antirheumatic therapy.
Although it is possible to use increasingly sophisticated molecular methods to construct and analyze animal models of disease by using transgenic and/or gene targeting technology, the study of animal models of complex multigenic disease can only reveal clues as to the actual mechanisms operating in authentic human disease. We have begun to use an approach, applicable in either normal cells or pathological tissues from diseased sites, which permits the inhibition of selected intracellular molecular pathways. Blockade is achieved through our effective method of adenoviral gene transfer into normal human macrophages (10, 11). This method has now enabled us to analyze in detail the effects of NF-κB blockade in rheumatoid synovium, which demonstrates that major destructive enzymes, chiefly produced by fibroblasts, are just as dependent on NF-κB as the pro-inflammatory cytokines produced mainly by macrophages. These results suggest that there are major similarities in the key intracellular pathways in the cells most closely involved with synovitis, the macrophages, and tissue destruction, the fibroblasts.
MATERIALS AND METHODS
Cells.
Synovium from patients with RA undergoing joint surgery was dissociated by cutting into small pieces and was digested with collagenase and DNase (12). The total cell mixture was cultured at 37°C in RPMI medium 1640 with 25 mM Hepes and 2 mM l-glutamine, supplemented with 5% heat-inactivated fetal bovine serum, on 24- or 48-well plates.
Adenoviral Vectors.
Recombinant, replication-deficient adenoviral vectors encoding Escherichia coli β-galactosidase (Advβgal) or having no insert (Adv0) were generously provided by A. Byrnes and M. Wood (Oxford University). An adenovirus encoding porcine IκBα with a cytomegalovirus promoter and a nuclear localization sequence (AdvIκBα) was generously provided by R. de Martin (University of Vienna) (13). Porcine IκBα has >95% homology with the human molecule. Viruses were propagated in the 293 human embryonic kidney cell line and were purified by ultracentrifugation through two cesium chloride gradients (14). The titers of viral stocks were determined through a plaque assay on 293 cells, as described (14). All viruses used were plaque-purified from a master stock to prevent contamination with wild-type adenovirus.
Infection Techniques.
For experiments concerning cytokine or matrix metalloproteinase synthesis, freshly prepared rheumatoid synovial cells were resuspended in 1 ml of serum-free RPMI medium 1640 on a 12-well plate at 2.5–4 million cells per well. After incubation for 1 h, they either were left uninfected or were infected with AdvIκBα or Adv0 at a multiplicity of infection of 40:1. After 2 h, the supernatants were removed and replaced with 0.5 ml of RPMI medium 1640 supplemented with 5% heat-inactivated fetal bovine serum. The nonadherent cells were carefully spun down and were reintroduced to the rheumatoid cell coculture in 0.5 ml of RPMI medium 1640 supplemented with 5% heat-inactivated fetal bovine serum (total volume, thus, of 1 ml).
In some experiments, rheumatoid synovial cells were infected as above, but at a density of 0.4 million cells per well in a final volume of 0.2 ml, on a 96-well plate. Four wells of cells from each category—uninfected, Adv0-infected, and AdvIκBα-infected—were plated. After incubation with adenovirus for 2 h, one well from each category was untreated, one was treated with 20 μg/ml of human recombinant IL-1 receptor antagonist (Cambridge Bioscience, Cambridge, U.K.), one was treated with 20 μg/ml of the A2 anti-TNFα antibody (Centocor), and one was treated with both of these cytokine inhibitors.
For infectibility experiments, cells were resuspended in 0.6 ml of serum-free RPMI medium1640 on a 24-well plate at 1 million cells per well. After incubation for 1 h, they either were left uninfected or were infected with Advβgal or Adv0 at a multiplicity of infection of 40:1. After 2 h, the supernatants were removed and replaced with 0.4 ml of RPMI medium 1640 supplemented with 5% heat-inactivated fetal bovine serum. The nonadherent cells were carefully spun down and were reintroduced to the rheumatoid cell coculture in 0.2 ml of RPMI medium 1640 supplemented with 5% heat-inactivated fetal bovine serum (total volume of 0.6 ml).
Analysis of Infectibility.
Cell were scraped off the plates in the culture medium 48 h after infection and were spun down and washed in fluorescence-activated cell sorter staining solution as described (15). Each batch of uninfected, Adv0-infected, or Advβgal-infected cells then was resuspended in 25 μl of staining solution and was incubated with 125 ng of anti-CD3 PerCP and 500 ng of anti-CD14 PE (both from Becton Dickinson), in a total volume of 45 μl for 45 min at 4°C. They then were incubated at 37°C for 10 min, before 45 μl of a 2 mM solution of Fluorescein di-(β-d)-galactopyranoside (Sigma) was added for 1 min. Addition of excess (10×) ice-cold staining solution was used to stop the reaction. Cell fluorescence was analyzed by fluorescence-activated cell sorter as described (16).
Analysis of Cytokines and Metalloproteases.
In the assays for cytokine production, supernatants were taken off, and nonadherent cells were removed 48 h after infection. They were analyzed for TNFα (17), IL-1β, IL-6, and IL-8 (18), IL-10 (19, 20), IL-11 (21), and interleukin 1 receptor antagonist (IL-1ra) and the p75 soluble TNF receptor (22) by ELISA. The production of matrix metalloproteinase type 1 (MMP 1) (Collagenase), MMP 3 (Stromelysin), and the tissue inhibitor of metalloproteases (TIMP-1) was analyzed by ELISA kits purchased from Amersham Pharmacia. In some experiments, aliquots of 50 μl were taken off after 12, 24, 48, 72, and 110 h. After the nonadherent cells had been removed and reintroduced to the cells in the same amount of medium, these aliquots were analyzed for cytokines and metalloproteases as described above.
Western Blotting.
In this experiment, three batches of 10 × 106 cells each either were left uninfected or were infected with Adv0 or AdvIκBα. Two days after infection, cytosolic and nuclear extracts were prepared as described (23), and proteins were separated by SDS/PAGE on a 10% (wt/vol) polyacrylamide gel, followed by electrotransfer onto nitrocellulose membranes. IκBα and the p42/44 mitogen-activated protein kinases were detected by using antibodies purchased from Santa Cruz Biotechnology.
Statistical Methods.
All statistical testing was performed by using a one-sided, paired-comparisons Student’s t test.
RESULTS
Infectibility of Rheumatoid Synovial Cells with Adenovirus.
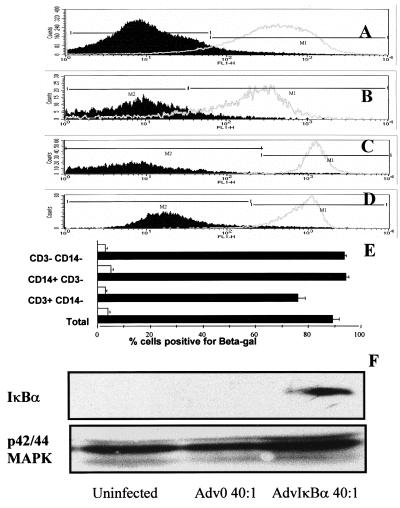
Infectibility of cells derived from rheumatoid synovial tissue was investigated by using the AdvβGal adenovirus. Because 40:1 of AdvβGal infected 95–100% of human macrophages (10) and 30:1 of AdvβGal infected nearly 100% of human fibroblasts (J.B., F.B., B.F., and M.F., unpublished work), we decided to use 10:1 and 40:1 of AdvβGal in initial experiments. It was found that 40:1 of AdvβGal infected nearly 100% of the entire rheumatoid synovial cell population (Fig. 1A) but that 10:1 infected only 60–70% (data not shown). All subsequent experiments thus used a multiplicity of infection of 40:1 of AdvβGal and other adenoviruses.
Figure 1.
In excess of 90% of rheumatoid synovial cells can be infected with adenovirus. β-galactosidase activity in total rheumatoid synovial membrane cell cultures (A), T lymphocytes (B), macrophages (C), or synoviocytes (D) were detected by double gating for size/granularity and cell surface markers, in cultures infected with 40:1 of either Adv0 (broken line) or Advβgal (solid line). Shown is a representative of five experiments. In E, the means of β-galactosidase-positive cells are plotted ± SEM (n = 5). In F, rheumatoid synovial cells were infected with 40:1 of either Adv0 or AdvIκBα, and cytosolic extracts were prepared. IκBα expression was determined by Western blotting, and the expression of the p42–44 mitogen-activated protein kinases was used as a control.
Because the rheumatoid synovium is a complex mixture of cells, it was of interest to study the infectibility of each of the main constituents, macrophages, T lymphocytes, and synoviocytes. This was performed through preincubating the cells with fluorescent antibody markers to CD3 and CD14, which allowed identification of CD3+/CD14− (T lymphocytes), CD14+/CD3− (macrophages), and CD3-/CD14− (chiefly synovial fibroblast) populations. By using human peripheral blood T cells and monocytes and human skin fibroblasts as controls, the forward scatter/side scatter characteristics of these cell populations could be identified. Double gating then was used to ensure that the rheumatoid synovial cell populations chosen to represent T lymphocytes, macrophages, and synoviocytes had the correct size/granularity as well as the surface marker characteristics. As could be expected from earlier results, both macrophages (Fig. 1C) and fibroblasts (Fig. 1D) were easy to infect; surprisingly, >90% of the T lymphocytes in the synovium also were infected by 40:1 of AdvβGal (Fig. 1B).
It was also possible to demonstrate that infection of rheumatoid synovial cells with 40:1 of AdvIκBα, but not Adv0, was able to induce considerable cytosolic overexpression of IκBα (Fig. 1F). The amount of nuclear protein available in these limited amounts of tissue did not permit either Western analysis of nuclear IκBα or electrophoretic mobility shift studies of NF-κB function, which has been reported earlier with this virus by using human macrophages (10, 11).
IκBα Overexpression Inhibits the Production of Pro-Inflammatory Cytokines.
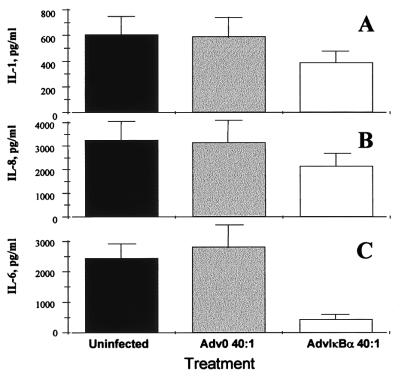
It has been demonstrated that the spontaneous production of TNFα, by rheumatoid synovium as assessed two days after infection, was inhibited by 70% in cells infected with AdvIκBα, as compared with mock-infected cells or cells infected with Adv0 (10). Here, we demonstrate that the spontaneous production of both IL-1β (Fig. 2A) and IL-8 (Fig. 2B) also was inhibited significantly (P < 0.01) by IκBα over-expression, but to a more modest degree (40%, as compared with uninfected or Adv0-infected cells). In contrast, IL-6 was inhibited very potently (P < 0.001) (85%; Fig. 2C). These results agree with previous findings in normal macrophages identifying IL-6 as a pro-inflammatory cytokine strongly dependent on transcriptional regulation by NF-κB (11).
Figure 2.
AdvIκBα infection inhibits the spontaneous production of pro-inflammatory cytokines from rheumatoid synovial cells. Shown is the effect of infection of rheumatoid synovial cells with 40:1 of either Adv0 or AdvIκBα on the spontaneous production of IL-1β (A), IL-8 (B), and IL-6 (C). Error bars indicate SEM (n = 6).
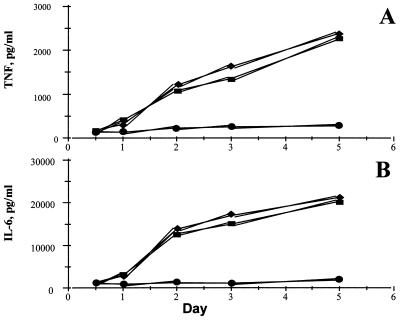
In a separate series of experiments, aliquots of medium from cultured cells from rheumatoid synovial membranes were removed after 12, 24, 48, 72, and 110 h, and cytokine analysis was performed. It was seen (Fig. 3) that, although the production of TNFα and IL-6 from uninfected or Adv0-infected cells gradually increased between day 1 and day 5, the cytokine production from AdvIκBα-infected cells remained almost static, indicating that, once sufficient over-expression of IκBα had been achieved to inhibit NF-κB activation, only very little TNFα and IL-6 is produced.
Figure 3.
IκBα over-expression permanently inhibits TNFα and IL-6 production. Shown is the effect of infection of rheumatoid synovial cells with 40:1 of either Adv0 or AdvIκBα on the spontaneous production of TNFα (A) or IL-6 (B) over time. Symbols denote uninfected (■), Adv0-infected (♦), or AdvIκBα-infected (●) cells. Shown is a representative of three independent experiments.
IκBα Overexpression Does Not Affect the Production of the Major Anti-Inflammatory Cytokines and Mediators.
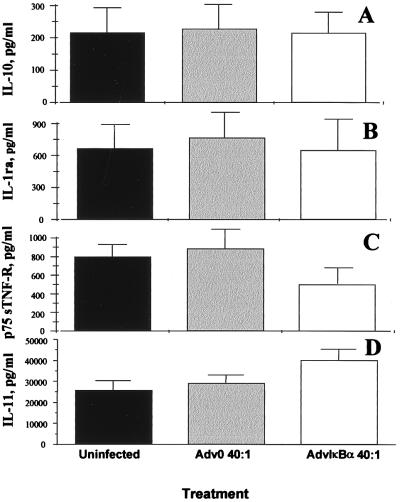
Previous results from human macrophages, the major producers of IL-10 in the rheumatoid joint (24), have indicated that both IL-10 and the IL-1 receptor antagonist are NF-κB-independent in these cells. However, the profound diminution of TNFα and IL-1β induced by NF-κB down-regulation in macrophages causes a modest secondary decrease in IL-10 and IL-1ra (11). In rheumatoid synovial cell cultures, the spontaneous production of both IL-10 and the IL-1 receptor antagonist are unaffected by over-expression of IκBα (Fig. 4 A and B). The production of the p75 soluble TNF receptor was inhibited significantly (P < 0.01), however (Fig. 4C). IL-11, another important immunoregulatory cytokine produced mainly by the fibroblast-like cells in the rheumatoid synovium (21), was up-regulated significantly (P < 0.05) by NF-κB down-regulation (Fig. 4D). It is noteworthy that the classical pro-inflammatory cytokines (TNFα, IL-1, IL-6) are NF-κB-dependent whereas the anti-inflammatory ones (IL-10, IL-11) are not.
Figure 4.
Inhibition of NF-κB through AdvIκBα infection has only marginal effects on the spontaneous production of anti-inflammatory mediators. Shown is the effect of infection of rheumatoid synovial cells with 40:1 of either Adv0 or AdvIκBα on the spontaneous production of IL-10 (A), the IL-1 receptor antagonist (B), the p75 soluble TNF receptor (C), and IL-11 (D). Error bars indicate SEM (n = 6).
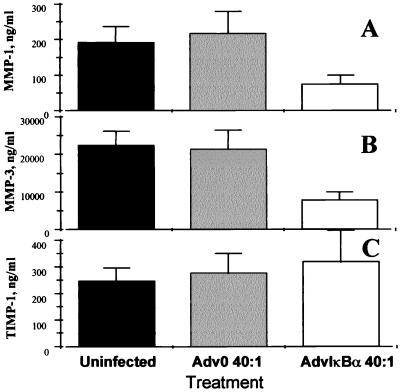
IκBα Overexpression Inhibits the Production of Matrix Metalloproteases but Not Their Inhibitor.
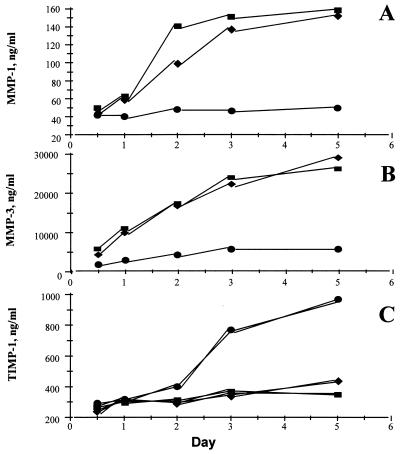
It was observed (Fig. 5 A and B) that IκBα overexpression potently inhibited (P < 0.001) the spontaneous production of both MMP 1 and MMP 3 in rheumatoid synovial cells. These MMPs were assayed because there is evidence that they are produced in RA joints and are important mediators in joint destruction (25–28); quantitative ELISA assays are available to measure their biosynthesis. In contrast, the spontaneous production of their inducible inhibitor, TIMP-1, was not significantly affected after 2 days (Fig. 5C). Furthermore, while studying a time-course of MMP production (Fig. 6A and B), it appears that, although uninfected and Adv0-infected rheumatoid synovial cell cocultures continue producing these enzymes throughout the incubation period, the AdvIκBα-infected cells produce very little of either MMP 1 or MMP 3 after significant IκBα overexpression has been achieved. Again, there is a remarkable contrast to the strong potentiation of TIMP-1 production seen after 3 or 5 days (Fig. 6C).
Figure 5.
IκBα over-expression potently inhibits MMP 1 and MMP 3 production but slightly potentiates the production of TIMP-1. Shown is the effect of infection of rheumatoid synovial cells with 40:1 of either Adv0 or AdvIκBα on the spontaneous production of MMP 1 (A), MMP 3 (B), and TIMP-1 (C). Error bars indicate SEM (n = 6).
Figure 6.
Effect of AdvIκBα infection on the balance between MMP production versus the production of TIMP-1 over time. Shown is the effect of infection of rheumatoid synovial cells with 40:1 of either Adv0 or AdvIκBα on the spontaneous production MMP 1 (A), MMP 3 (B), and TIMP-1 (C) over time. Symbols denote uninfected (■), Adv0-infected (♦), or AdvIκBα-infected (●) cells. Shown is a representative of three independent experiments.
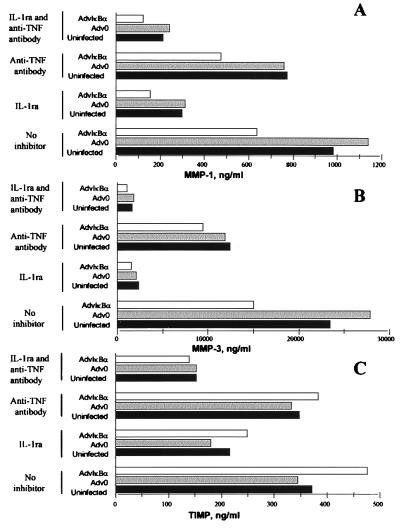
The results in Figs. 5–6 indicate direct or indirect dependence of NF-κB for the production of both MMP 1 and MMP 3, but not for TIMP-1. Both IL-1 and TNFα are known inducers of MMPs, however, and to examine the influence of the down-regulation of these two cytokines on MMP production, a series of experiments was performed with addition of IL-1ra and/or a TNFα neutralizing antibody just after infection of cells. It was seen (Fig. 7A) that, although IL-1 turned out to be a major inducer of MMP 1, the relative inhibition of MMP 1 by IκBα over-expression was largely unaffected by neutralization of either IL-1 or TNFα. Although inhibition of IL-1 through IκBα overexpression (Fig. 2A) may contribute, it appears that MMP 1 is ≈50% under direct NF-κB control (see Fig. 7A; in the presence of IL-1ra, there is still 50% inhibition in AdvIκBα-infected cells), although it cannot be definitely ruled out that indirect effects on some other soluble mediator might be involved. MMP 3 was even more potently driven by IL-1 (Fig. 7B). Neutralization of IL-1 removed the inhibition of MMP 3 seen in AdvIκBα-infected cells, indicating that the majority of the MMP 3 down-regulation by IκBα overexpression is caused by inhibition of IL-1. Although TIMP-1 appeared to be partly IL-1 driven (Fig. 7C), AdvIκBα had little effect on TIMP-1 production under either of the conditions studied.
Figure 7.
Effect of AdvIκBα infection on MMP and TIMP production in rheumatoid synovial cell cultures in which IL-1 and/or TNFα were neutralized. Rheumatoid synovial cells were either left uninfected or infected with 40:1 of Adv0 or AdvIκBα. After the virus was taken off and culture medium was added, the cells in each of the three categories either were left untreated or were treated with 20 μg/ml of the IL-1 receptor antagonist and/or 20 μg/ml of the A2 anti-TNFα antibody. The spontaneous production of MMP 1 (A), MMP 3 (B), and TIMP-1 (C) was assayed after 2 days. Shown is a representative of three complete experiments.
DISCUSSION
The genetic approach to studying cell signaling pathways has many advantages and has led to marked progress and deeper understanding of signal transduction (29, 30). However, this only applies to cells that are easy to transfect efficiently, for example, cell lines such as CV-1 origin SV-40 (COS), Chinese hamster ovary, 293, or Jurkat cells. It is assumed, or hoped, that other cell types will conform to the principles determined in cell lines. There are a number of reasons why this may not be the case, which prompted us to develop a method of using adenovirus to introduce genes into effectively all (>95%) normal human macrophages, thus permitting us to study the effects of blocking signaling pathways in these cells, which are of major importance in chronic inflammation (10). Here, we have extended the use of adenoviral gene transfer and have demonstrate that all major cell types in a pathological site, the rheumatoid synovium, are infected at a very high frequency. This means that adenovirus is a useful tool for studying not only signaling in the macrophages, but also the T lymphocytes and cells lacking markers of T cells or macrophages, which would include mainly fibroblasts with some dendritic cells and endothelium (Fig. 1). The marked infectibility of T cells from the rheumatoid joint was surprising in view of reports in the literature of low infectibility of in vitro activated T lymphocytes (31). However, other authors (32, 33) have reported greater infectibility, provided that a targeting antibody was used; it is possible that the exact state of activation of the T lymphocyte, and which integrins and other receptors are expressed, is of importance for increasing infectability.
The degree of reduction in synthesis of pro-inflammatory cytokines in RA synovium treated with AdvIκBα varied with the cytokine assayed. At 48 h after infection, IL-1β and IL-8 production was reduced by 40% whereas TNFα was reduced by 75% and IL-6 was reduced by 85%. Kinetic studies for the latter two show that the exact degree of inhibition varies with time and sample of diseased tissue. However, the differential degree of inhibition of pro-inflammatory cytokines induced by NF-κB blockade suggests that there are both NF-κB-dependent and NF-κB-independent pathways involved in regulating the pro-inflammatory cytokines produced in the rheumatoid joint. It will be of interest to analyze what these other important transcription factors are. Both activator protein 1 (AP-1) and NF–IL-6 are likely candidates. That there are indeed both NF-κB-dependent and -independent pathways of inducing TNFα and other pro-inflammatory cytokines in macrophages has been clearly demonstrated by using AdvIκBα as an inhibitor: Although lipopolysaccharide-induced TNFα was NF-κB-dependent in human macrophages, zymosan-induced TNFα was not (11), and there are NF-κB-independent pathways of inducing TNFα also in T lymphocytes (34).
It has been reported that, in active RA synovium, both pro-inflammatory cytokines and anti-inflammatory mediators such as TGFβ1, IL-10, IL-1ra, and the soluble TNF-receptors are up-regulated and spontaneously produced (35). Because there is persistent disease despite the production of these anti-inflammatory molecules, the effects of pro-inflammatory cytokines predominate; it is thus of great interest that the effect of NF-κB blockade was markedly different on pro-inflammatory mediators than on anti-inflammatory. There was no effect on IL-10 or IL-1ra production, some modest up-regulation of IL-11, and only a moderate reduction of the p75 soluble TNF-R, assayed two days after AdvIκBα infection. Because the p75 soluble TNF-R is markedly up-regulated by pro-inflammatory mediators (lipopolysaccharide and 4β-phorbol 12-myristate 13-acetate (PMA) as well as IL-1 and TNFα) (36), it is possible that much of the inhibition seen in p75 is secondary to the reduced inflammatory effects after AdvIκBα infection.
Many symptoms in patients with RA—pain, stiffness, and joint swelling—depend on pro-inflammatory cytokines, as judged by the rapid onset of benefit after anti-TNFα therapy (1–6). Just as important, if not more so, is the process of joint destruction. In mice with collagen induced arthritis or with a disregulated TNFα transgene, there is joint destruction that can be abolished by blocking TNFα and other pro-inflammatory cytokines (37–39). However, whether this is the case in RA is not yet clear. The identity of the enzymes involved in the breakdown of human articular cartilage, composed primarily of collagen type II and proteoglycans, in RA has been the focus of multiple studies during the last decades and is still a subject of debate (25). There is evidence that MMPs, in particular MMP 1, MMP 13, and the membrane-bound MMPs, are capable of cleaving collagen type II (40, 41). MMP 3 lacks this capacity but does have activity against other components of the extracellular matrix, including proteoglycans, fibronectin, and laminin (25). The involvement of MMP 3 or other MMP enzymes in proteoglycan cleavage is still a matter of debate.
AP-1 has been proposed as the dominant transcription factor for the regulation of MMP 1 and MMP 3 because there are AP-1 sites on both promoters and deletion of AP-1 site(s) inhibited MMP gene activation (42). Also, certain agents that block AP-1 also inhibit the synthesis of MMP 1 and MMP 3 (42, 43). Other authors (44–46) have demonstrated AP-1-independent mechanisms of MMP 1 and MMP 3 induction, however, and some recent studies (47–49) have discussed whether NF-κB might play a role in MMP 1 induction. We investigated whether NF-κB blockade modulated the synthesis of MMP enzymes or TIMP-1, the inducible tissue inhibitor of MMPs, which binds covalently and irreversibly inactivates the enzymatic site of MMPs. AdvIκBα markedly inhibited the degradative enzymes MMP 1 and MMP 3 but had no effect on TIMP-1. Hence, the destructive potential of the synovium is markedly reduced with NF-κB blockade. It is interesting to note, in this context, that the promoter element of MMP 1 has several NF-κB sites (47, 50, 51) and that our own data indicate that MMP 1 is NF-κB-dependent in fibroblasts and chondrosarcoma cells (J.B., F.B., B.F., and M.F., unpublished work), which do not produce IL-1 and TNFα. The MMP 3 promoter has just one NF-κB site (GenBank accession no. U43511), and it is not clear whether the inhibition of MMP 3 seen after AdvIκBα infection is direct or indirect, just reflecting the reductions in IL-1 and TNFα (Fig. 7).
The results presented here indicate that blocking NF-κB in rheumatoid joints would have an uniquely beneficial profile—within 2 days, there is marked reduction in the pro-inflammatory cytokines, without a concomitant reduction in the anti-inflammatory mediators. This should have a markedly beneficial effect on the synovitis especially in the light of the considerable “downstream” effects of blocking the single cytokine TNFα, as discussed (5, 35). Just as importantly, the destructive metalloproteinase enzymes MMP 1 and MMP 3 are reduced, without a concomitant reduction in their inducible inhibitor, TIMP-1. Hence, drugs that block NF-κB in the diseased synovial tissue should inhibit both synovitis and joint destruction. Although none are yet known to be both safe and effective, it is likely that some will be found because of intense screening efforts in the pharmaceutical industry. Based on the above data and finding of nuclear NF-κB in active RA joints (52, 53), NF-κB activation seems to be central to the pathogenesis of RA and perhaps other chronic inflammatory diseases.
There is much excitement in the pharmaceutical industry about the power of “genomics” approaches to define candidate genes as potential therapeutic targets. However, there is a dearth of techniques to rapidly assess which, if any, of the potential candidates are worth pursuing further. By bridging genetic and functional approaches, adenovirus as used here provides a way of accelerating therapeutic development. Because it is possible to generate a wide spectrum of inhibitors by molecular approaches, such as antisense cDNA, dominant negative kinases, or single chain Fv antibodies, this adenoviral approach has the power to ascertain what a specific and potent drug blocking a certain pathway would achieve at the actual disease site.
Acknowledgments
We thank Professor R. N. Maini for his encouragement and helpful suggestions. The AdvIκBα adenovirus was kindly provided by Dr. Rainer de Martin (Department of Vascular Biology and Thrombosis Research, University of Vienna, Austria). This work was funded by the Arthritis Research Campaign (U.K.), the Wellcome Trust, the European Community Training and Mobility of Researchers Fellowships, and the Swedish Institute.
ABBREVIATIONS
Adv0
adenovirus with no insert
Advβgal
adenovirus encoding β-galactosidase
AdvIκBα
adenovirus encoding porcine IκBα
IL-1ra
interleukin 1 receptor antagonist
TNF
tumor necrosis factor
RA
rheumatoid arthritis
MMP 1
matrix metalloproteinase type 1
TIMP
tissue inhibitor of metalloproteases
AP-1
activator protein 1
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Elliott M J, Maini R N, Feldmann M, Long-Fox A, Charles P, Katsikis P, Brennan F M, Walker J, Bijl H, Ghrayeb J, et al. Arthritis Rheum. 1993;36:1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- 2.Elliott M J, Maini R N, Feldmann M, Kalden J R, Antoni C, Smolen J S, Leeb B, Breedveld F C, Macfarlane J D, Bijl H, et al. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 3.Rankin E C C, Choy E H S, Kassimos D, Kingsley G H, Sopwith S M, Isenberg D A, Panayi G S. Br J Rheumatol. 1995;34:334–342. doi: 10.1093/rheumatology/34.4.334. [DOI] [PubMed] [Google Scholar]
- 4.Moreland L W, Baumgartner S W, Schiff M H, Tindall E A, Fleischmann R M, Weaver A L, Ettlinger R E, Cohen S, Koopman W J, Mohler K, et al. N Engl J Med. 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann M, Elliott M J, Woody J N, Maini R N. Adv Immunol. 1997;64:283–350. doi: 10.1016/s0065-2776(08)60891-3. [DOI] [PubMed] [Google Scholar]
- 6.Maini R N, Breedveld F C, Kalden J R, Smolen J S, Davis D, Macfarlane J D, Antoni C, Leeb B, Elliott M J, Woody J N, et al. Arthritis Rheum. 1998;41:1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Van Lent P L, Van De Loo F A, Holthuysen A E, Van Den Bersselaar L A, Vermeer H, Van Den Berg W B. J Rheumatol. 1995;22:2250–2258. [PubMed] [Google Scholar]
- 8.Zvaifler N J, Firestein G S. Arthritis Rheum. 1994;37:783–789. doi: 10.1002/art.1780370601. [DOI] [PubMed] [Google Scholar]
- 9.Muller-Ladner U, Kriegsmann J, Franklin B N, Matsumoto S, Geiler T, Gay R E, Gay S. Am J Pathol. 1996;149:1607–1615. [PMC free article] [PubMed] [Google Scholar]
- 10.Foxwell B M J, Browne K, Bondeson J, Clarke C, de Martin R, Brennan F M, Feldmann M. Proc Natl Acad Sci USA. 1998;95:8211–8215. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bondeson J, Brown K A, Brennan F M, Foxwell B M J, Feldmann M. J Immunol. 1999;162:2939–2945. [PubMed] [Google Scholar]
- 12.Buchan G, Barrett K, Turner M, Chantry D, Maini R N, Feldmann M. Clin Exp Immunol. 1988;73:449–455. [PMC free article] [PubMed] [Google Scholar]
- 13.Wrighton C J, Hofer-Warbinek R, Moll T, Eytner R, Bach F H, de Martin R. J Exp Med. 1996;183:1013–1022. doi: 10.1084/jem.183.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham F L, Prevec L. Mol Biotechnol. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- 15.Clarke C J P, Taylor-Fishwick D A, Hales A, Chernajovsky Y, Sugamura K, Feldmann M, Foxwell B M J. Eur J Immunol. 1995;25:2961–2966. doi: 10.1002/eji.1830251037. [DOI] [PubMed] [Google Scholar]
- 16.Nolan G P, Fiering S, Nicolas J F, Herzenberg L. Proc Natl Acad Sci USA. 1988;85:2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelberts I, Moller A, Schoen G J M, van der Linden C J, Buurman W A. Lymphokine Cytokine Res. 1991;10:60–76. [PubMed] [Google Scholar]
- 18.Butler D M, Maini R N, Feldmann M, Brennan F M. Eur Cytokine Network. 1995;6:225–230. [PubMed] [Google Scholar]
- 19.Abrams J S, Ronocarlo M G, Yssel H, Andersson U, Gleich G I, Silver J E. Immunol Rev. 1993;125:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 20.Foey A D, Parry S L, Williams L M, Feldmann M, Foxwell B M J, Brennan F M. J Immunol. 1998;160:920–928. [PubMed] [Google Scholar]
- 21.Hermann J A, Hall M A, Maini R N, Feldmann M, Brennan F M. Arthritis Rheum. 1998;41:1388–1397. doi: 10.1002/1529-0131(199808)41:8<1388::AID-ART7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Bauerle P, Henkel T. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 23.Whiteside S T, Visvanathan K V, Goodbourn S. Nucleic Acids Res. 1992;20:1531–1538. doi: 10.1093/nar/20.7.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsikis P, Chu C Q, Brennan F M, Maini R N, Feldmann M. J Exp Med. 1994;179:1517–1527. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matrisian L M. Trends Genet. 1990;6:121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- 26.Clark I H, Powell L K, Ramsey S, Hazleman B L, Cawston T E. Arthritis Rheum. 1993;36:372–379. doi: 10.1002/art.1780360313. [DOI] [PubMed] [Google Scholar]
- 27.Manicourt D H, Fujimoto N, Obata K, Thonar E J. Arthritis Rheum. 1995;38:1031–1039. doi: 10.1002/art.1780380803. [DOI] [PubMed] [Google Scholar]
- 28.Brennan F M, Browne K A, Green P A, Jasper J-M, Maini R N, Feldmann M. Br J Rheumatol. 1997;36:643–650. doi: 10.1093/rheumatology/36.6.643. [DOI] [PubMed] [Google Scholar]
- 29.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. Nature (London) 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 30.Woronicz J D, Gao X, Cao Z, Rothe M, D. V G. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Endo R I, Nemerow G R. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wickham T J, Lee G M, Titus J A, Sconocchia G, Bakacs T, Kovesdi I, Segal D M. J Virol. 1997;71:7663–7669. doi: 10.1128/jvi.71.10.7663-7669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mentel R, Dopping G, Wegner U, Seidel W, Liebermann H, Dohner L. J Med Virol. 1997;51:252–257. [PubMed] [Google Scholar]
- 34.Goldfeld A E, Strominger J L, Doyle C. J Exp Med. 1991;174:73–81. doi: 10.1084/jem.174.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldmann M, Brennan F M, Maini R N. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 36.Pennica D, Lam V T, Mize N K, Weber R F, Lewis M, Fendly B M, Lipari M T, Goeddel D V. J Biol Chem. 1992;267:21172–21178. [PubMed] [Google Scholar]
- 37.Thorbecke G J, Shah R, Leu C H, Kuruvilla A P, Hardison A M, Palladino M A. Proc Natl Acad Sci USA. 1992;89:7375–7379. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams R O, Feldmann M, Maini R N. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kollias G. Res Immunol. 1993;144:342–347. doi: 10.1016/s0923-2494(93)80078-d. [DOI] [PubMed] [Google Scholar]
- 40.Knauper V, Cowell S, Smith B, Lopez-Otin C, O’Shea M, Morris H, Zardi L, Murphy G. J Biol Chem. 1997;272:7608–7616. doi: 10.1074/jbc.272.12.7608. [DOI] [PubMed] [Google Scholar]
- 41.Cowell S, Knauper V, Stewart M L, D’Ortho M P, Stanton H, Hembry R M, Lopez-Otin C, Reynolds J J, Murphy G. Biochem J. 1998;331:453–458. doi: 10.1042/bj3310453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fini M E, Cook J R, Mohan R, Brinckerhoff C E. In: Matrix Metalloproteinases. Parks W C, Mecham R P, editors. San Diego: Academic; 1998. pp. 299–356. [Google Scholar]
- 43.Hui A, Min W X, Tang J, Cruz T F. Arthritis Rheum. 1998;41:869–876. doi: 10.1002/1529-0131(199805)41:5<869::AID-ART15>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 44.Buttice G, Quinones S, Kurkinen M. Nucleic Acids Res. 1991;19:3723–3731. doi: 10.1093/nar/19.13.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quinones S, Buttice G, Kurkinen M. Biochem J. 1994;302:471–477. doi: 10.1042/bj3020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borghaei R C, Rawlings P L, Mochan E. Arthritis Rheum. 1998;41:1398–1406. doi: 10.1002/1529-0131(199808)41:8<1398::AID-ART8>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tewari M, Tuncay O C, Milchman A, Reddy P J, Reddy C D, Cressman D E, Taub R, Newton R C, Tewari D S. Arch Oral Biol. 1996;41:461–468. doi: 10.1016/0003-9969(96)00148-3. [DOI] [PubMed] [Google Scholar]
- 48.Borghaei B H, Borghaei R C, Ni X, Pease E, Thornton R, Mochan E. Inflamm Res. 1997;46:S177–S178. doi: 10.1007/s000110050168. [DOI] [PubMed] [Google Scholar]
- 49.Reddy P J, Tewari M, Hamid Q A, Tuncay O C, Tewari D S. Proc Soc Exp Biol Med. 1998;218:238–243. doi: 10.3181/00379727-218-44293. [DOI] [PubMed] [Google Scholar]
- 50.Angel P, Imagawa M, Chiu R, Stein B, Imbra R J, Herrlich P, Karin M. Cell. 1987;49:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- 51.Vincenti M P, Coon C I, Brinckerhoff C E. Arthritis Rheum. 1998;41:1987–1994. doi: 10.1002/1529-0131(199811)41:11<1987::AID-ART14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 52.Marok R, Winyard P G, Coumbe A, Kus M L, Gaffney K, Blades S, Mapp P I, Morris C J, Blake D R, Kaltschmidt C, et al. Arthritis Rheum. 1996;39:583–591. doi: 10.1002/art.1780390407. [DOI] [PubMed] [Google Scholar]
- 53.Sioud M, Mellbye O, Forre O. Clin Exp Rheumatol. 1998;16:125–134. [PubMed] [Google Scholar]