B Cell Antigen Receptor Engagement Inhibits Stromal Cell–derived Factor (SDF)-1α Chemotaxis and Promotes Protein Kinase C (PKC)-induced Internalization of CXCR4 (original) (raw)
Abstract
The entry of B lymphocytes into secondary lymphoid organs is a critical step in the development of an immune response, providing a site for repertoire shaping, antigen-induced activation and selection. These events are controlled by signals generated through the B cell antigen receptor (BCR) and are associated with changes in the migration properties of B cells in response to chemokine gradients. The chemokine stromal cell–derived factor (SDF)-1α is thought to be one of the driving forces during those processes, as it is produced inside secondary lymphoid organs and induces B lymphocyte migration that arrests upon BCR engagement. The signaling pathway that mediates this arrest was genetically dissected using B cells deficient in specific BCR-coupled signaling components. BCR-induced inhibition of SDF-1α chemotaxis was dependent on Syk, BLNK, Btk, and phospholipase C (Plc)γ2 but independent of Ca2+ mobilization, suggesting that the target of BCR stimulation was a protein kinase C (PKC)-dependent substrate. This target was identified as the SDF-1α receptor, CXCR4, which undergoes PKC- dependent internalization upon BCR stimulation. Mutation of the internalization motif SSXXIL in the COOH terminus of CXCR4 resulted in B cells that constitutively expressed this receptor upon BCR engagement. These studies suggest that one pathway by which BCR stimulation results in inhibition of SDF-1α migration is through PKC-dependent downregulation of CXCR4.
Keywords: chemokine, lymphocyte, migration, signaling, phospholipase C
The ability to successfully mount an immune response depends on the interaction of multiple cell types, insuring that autoreactivity is avoided, diversity and specificity are achieved, and a memory of the encounter is established. The anatomic compartmentalization of APCs, T and B lymphocytes, and effector cells in secondary lymphoid organs like the spleen or lymph node maximizes the probability of interaction of the cellular and humoral factors necessary to achieve these responses. Zones containing specific cellular populations allow ordered and sequential interactions to take place, thereby insuring that only a small subset of B lymphocytes are stimulated and produce antibody or undergo further somatic hypermutation within germinal centers (GCs)1 (1, 2). Although important during an immune response, changes in the migration and position of B cells inside lymphoid organs are also intimately associated with their stage of maturation. An antigen receptor (AgR)-dependent positive selection process allows some of the newly generated bone marrow B cells to progress to the mature B cell stage. This selection event is associated with follicular relocalization. In those sites, in contact with follicular dendritic cells, B lymphocytes might receive survival signals and become recruited to the long-lived B cell repertoire (3–5).
Above a threshold of AgR engagement, B cells migrate toward or arrest within the periarteriolar lymphoid sheath, where the probability of encountering Ag-specific T cells is maximal. In the absence of cognate interactions with T cells, activated B cells will die, and as a consequence, strong autoreactivity will be purged from the repertoire (6, 7). Rare activated B cells that receive cognate T cell help will proliferate, and some of them will join follicles to form GCs. The GC reaction generates B cells with new migration properties. Thus, plasmocytes can relocate into the red pulp of the spleen, join the peripheral circulation, and enter the bone marrow, where they will produce antibody for an extended period of time (8). Memory B cells will either recirculate or reside in the marginal zone of the spleen, the privileged area for reencounter with blood-borne antigen (1).
Four chemokines with the ability to direct the migration of B lymphocytes are known to be expressed within secondary lymphoid organs. These are B lymphocyte chemoattractant (BLC) (or B cell–attracting chemokine [BCA]-1), which binds to the Burkitt's lymphoma receptor 1 (BLR1) (or CXCR5) (9, 10); secondary lymphoid tissue chemokine (SLC, or 6C-kine, exodus-2) and Epstein-Barr virus– induced molecule 1 ligand chemokine (ELC, or MIP3β), both binding to the chemokine receptor CCR7 (11, 12); and SDF-1α (or pre-B cell growth–stimulating factor [PBSF]), which stimulates cells through CXCR4 (13, 14). The importance of these chemokines in the migration and selection processes is suggested either by the differential expression of their receptors during B cell maturation or by the fact that AgR engagement can modulate the associated chemotactic responses. BLR1, the receptor for BLC, is only expressed when cells mature from newly generated to follicular B cells; this expression is likely to account, at least in part, for the tropism of those cells for follicles. Thus, inactivation of BLR1 by targeted gene disruption is associated with deficits in spleen and Peyer's patch follicles (15, 16). BCR activation has a direct impact on B cell chemotaxis to ELC and SDF-1α, resulting in either enhancement or arrest, respectively (12, 14). Given that AgR signaling controls B cell maturation and is associated with cell relocalization, the direct regulation of chemokine responsiveness by BCR engagement is likely to play a major role in driving the selection and organization of B lymphocytes within lymphoid organs.
The mechanism by which BCR ligation may lead to SDF-1α unresponsiveness has been addressed in this study by using the genetically defined DT40 B cell system. Targeted disruption of many of the signaling components of the BCR-stimulated pathway have been generated in these cells and have demonstrated great utility in defining the mechanisms by which antigen stimulation of B cells results in cellular activation. DT40 B cells migrate efficiently to SDF-1α and are arrested in their migration by BCR cross-linking. Through the analysis of a series of signaling mutants of DT40 cells, we have established that BCR stimulation results in a calcium-independent, protein kinase C (PKC)-dependent downregulation of the SDF-1α receptor CXCR4. These studies suggest mechanisms by which diverse signals may influence this pathway and thereby modulate redirected migration of B cells inside lymphoid tissues.
Materials and Methods
Reagents.
Human SDF-1α, 12G5 anti-CXCR4, and anti– SDF-1α antibodies were from R & D Systems, Inc.; human serum albumin (HSA) and BSA, FITC-conjugated F(ab′)2 fragment of goat anti–mouse IgG, and PMA were from Sigma Chemical Co. The M4 mAb anti–chicken IgM has previously been described (17).
DT40 Cell Culture and Transfections.
Wild type (wt) and Btk- (18), Syk- (19), phospholipase C (Plc)γ2- (20), BLNK- (21), or IP3R (22)-deficient chicken DT40 cells were maintained in RPMI 1640 supplemented with 10% FBS, 1% chicken serum, 50 mM 2-ME, 2 mM l-glutamine, and antibiotics. The constructions containing wt and SSLKIL→ AALKAA (4A) mutants of human CXCR4 have been described previously (23). Cells were transfected by electroporation at 250 V and 960 μF in PBS (107 cells/0.5 ml). 20 μg expression constructs were cotransfected with 2 μg pBabe-puror vector (24). Transfectants were selected in 0.5 μg/ml puromycin 24 h after electroporation. The presence of CXCR4 surface expression was determined by FACS® analysis with 12G5 mAb and FITC-conjugated secondary antibody. In each condition: DT40-wt + CXCR4 (wt or 4A), Plcg2−\\− + CXCR4 (wt or 4A). Two clones were analyzed for the experiments; they had comparable and homogenous levels of expression ranging from 120 to 200 arbitrary units (data not shown).
Chemotaxis Assay.
DT40 cells (106 cells per condition) were washed and resuspended in 100 ml RPMI 1640 and 0.25% HSA and incubated for 1 h at 39°C in the presence of different concentrations of anti-BCR antibodies. Cells were then added to the top chamber of a 6.5-mm diameter, 5-μm pore polycarbonate transwell culture insert (Costar Corp.); the lower chamber contained RPMI 0.25% HSA alone or supplemented with 100 nM SDF-1α. Migration proceeded for 3 h at 39°C. Transmigrated cells were then vigorously suspended and counted with a FACScan™ (Becton Dickinson) for 20 s at 60 μl/min, with gating on forward and side scatter to exclude debris. 100% migration was obtained by counting cells added directly to the lower chamber.
CXCR4 Surface Expression Analysis.
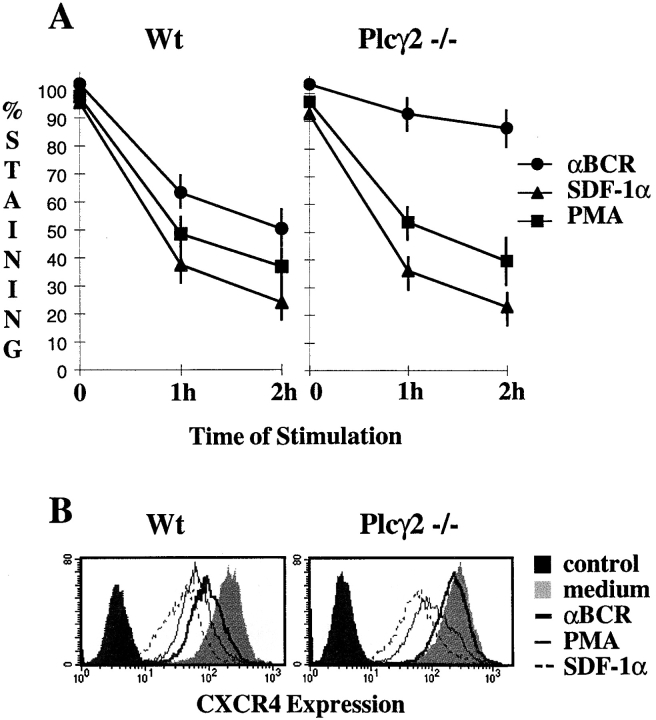
Cells expressing wt or the 4A mutant of human CXCR4 were resuspended in RPMI 1640 and 0.25% HSA at 107 cells/ml. They were then diluted twice with the same buffer or with medium supplemented with 200 nM SDF-1α, 200 nM PMA, or 20 μg/ml anti-BCR mAb and kept either at 4°C (for T = 0) or incubated at 39°C for 1 or 2 h. All subsequent steps were carried out at 4°C. Cells were washed once in staining buffer (PBS, 0.5% BSA, 0.05% azide, and 5% normal goat serum) and incubated in the presence of 12G5 anti-CXCR4 antibodies for 1 h. After two washes, primary antibodies were detected using a FITC-conjugated F(ab′)2 fragment of goat anti–mouse IgG. Signals were acquired on a FACScan™. Results are given as percentage of controls, 100% corresponding to cells incubated in medium alone. No inhibition of 12G5 binding was found when cells were preincubated with SDF-1α, PMA, or anti-BCR at 4°C, showing that modulation of 12G5 binding was the consequence of an active process. Further controls included absence of staining of nontransfected cells by 12G5 mAb (data not shown) or of CXCR4-transfected cells by an isotype control primary antibody (Fig. 3 B).
Figure 3.
BCR engagement induces a Plcγ2-dependent internalization of CXCR4. (A)Human CXCR4 expressing wt or Plcγ2-deficient DT40 cells were incubated with medium alone or medium supplemented with either 100 nM SDF-1α, 100 nM PMA, or 10 μg/ml anti-BCR mAb. Cells were either transferred immediately on ice (T = 0) or after incubation for 1 or 2 h at 39°C (T = 1, 2 h). They were then processed for staining with 12G5 anti-CXCR4 mAb. Values represent the percentage of staining, 100% corresponding to unstimulated cells processed in parallel. They are the mean value of four experiments realized on two different clones for each condition. Error bars, SD. (B)Representative anti-CXCR4 staining of human CXCR4 expressing wt or Plcγ2-deficient DT40 cells after 2 h of incubation in medium alone or medium supplemented with anti-BCR mAb, PMA, or SDF-1α. Control represents staining of untreated cells using an isotype-matched, irrelevant mAb.
Results
DT40 Cells Migrate in Response to SDF-1α and Arrest upon BCR Ligation.
To determine if the chicken B cell line DT40 was an accurate model for SDF-1α–dependent migration and BCR-induced arrest, we characterized the ability of these cells to migrate in response to this chemokine. DT40 cells were placed on the upper side of a transwell apparatus, and human SDF-1α was placed on the opposite side. DT40 cells migrated efficiently (Fig. 1) to this chemokine. The migration was specifically inhibited by anti– SDF-1α antibody but not by an irrelevant antibody (not shown), demonstrating the specificity of this migration effect. Cross-linking of the BCR on DT40 cells with the murine mAb M4 for 1 h resulted in a dose-dependent inhibition of SDF-1α–mediated migration, resulting in full inhibition at 5 μg/ml. The ability of this chicken cell line to respond to the human chemokine further confirms that SDF-1α and its receptor are highly conserved among diverse species. Although the chicken version of SDF-1α and its receptor have not been characterized, it is likely that a high degree of sequence conservation will be found for this species as well.
Figure 1.
Migration of DT40 cells in response to SDF-1α and inhibition by BCR engagement. DT40 cells (106 cells per condition) were kept unstimulated or stimulated with increasing concentrations of M4 anti-BCR antibodies for 1 h. They were then assayed for migration in the presence or absence of SDF-1α and blocking anti–SDF-1α mAb. Results are expressed as the mean percentage of input cells that migrated into the lower chamber. Error bars represent the SD of two independent experiments.
BCR-induced Inhibition of SDF-1α Migration Is Plcγ-dependent and Ca2+-independent.
A series of homozygous cell lines deficient in specific BCR signaling components has been generated in DT40 cells (18–22). These mutant lines were tested for their ability to migrate in response to SDF-1α and to arrest upon BCR cross-linking. As seen in Fig. 2, all of the mutants tested, whether deficient in either early (syk) or late (Btk, BLNK, Plcγ2, IP3R) components of BCR-induced signaling, migrated in response to SDF-1α. In contrast, the BCR-induced arrest of SDF-1α–directed migration was observed in some, but not all, of the mutants tested. Although cells deficient for molecules involved in Plcγ2 activation such as syk, BLNK, Btk, and Plcγ2 were unable to mediate BCR-induced arrest of SDF-1 migration, IP3R-deficient cells (generated by deletion of the three IP3 receptor genes) retained their ability to migrate in response to SDF-1α and arrest upon BCR cross-linking. DT40 cells have three IP3 receptors that mediate the efflux of calcium from intracellular stores in response to IP3 (22). A triple knockout of these receptors is unable to trigger intracellular calcium release in response to BCR-induced IP3 stimulation yet maintains its ability to arrest SDF-1α–mediated migration. IP3 and diacylglycerol are both produced in response to Plcγ2 activation. As BCR-induced arrest is Plcγ2 dependent but IP3R independent, it implies that the pathway triggered by Plcγ2, which is affected in BCR- induced arrest of SDF-1α migration, is diacylglycerol dependent, which, in turn, activates PKC. Thus, the dependence on Plcγ2 in the absence of IP3-stimulated release of calcium implies that the mechanism by which BCR cross-linking results in migration arrest to SDF-1α may be dependent upon PKC activation through Plcγ2.
Figure 2.
BCR-induced inhibition of migration is Plcγ2 dependent and calcium independent. Wt DT40 cells or subclones deficient for Btk, Syk, BLNK, Plcγ2, or for the three IP3 receptors (IP3R−/−/−) were assayed for migration with or without preincubation with 10 μg/ml anti-BCR antibodies and in the presence (SDF-1α) or absence (med) of 100 nM SDF-1α in the lower chamber. Results are depicted as in Fig. 1 and represent the mean value of at least four independent experiments. Error bars, SD.
CXCR4, the Receptor for SDF-1α, Is Downregulated by BCR Cross-linking and PKC Activation.
One mechanism by which BCR activation could lead to arrest of SDF-1α– mediated migration might result from BCR-induced downregulation of the SDF-1α receptor from the cell surface by a PKC-dependent internalization of the SDF-1α receptor. Previous studies have demonstrated that the SDF-1 receptor, CXCR4, is rapidly internalized upon activation of PKC by phorbol esters that, in turn, can be blocked by inhibitors of PKC (25, 26). To determine if a BCR-induced, PKC- dependent internalization of the CXCR4 pathway is present in DT40 cells, the human SDF-1α receptor, CXCR4, was stably transfected into wt and Plcγ2-mutant DT40 cells. Cell surface expression of human CXCR4 on DT40 cells is downregulated in response to BCR cross-linking, phorbol ester treatment, or SDF-1α exposure (Fig. 3). However, BCR-induced downregulation of CXCR4 is blocked in the Plcγ2-deficient DT40 background, which correlates with the inability of this mutant to display BCR-mediated arrest of SDF-1α migration. To determine if Plcγ2 is upstream, downstream, or pleiotropic in relation to CXCR4, this mutant was tested for its ability to respond to phorbol esters or SDF-1α. CXCR4 surface expression is downregulated normally in Plcγ2-deficient cells in response to phorbol esters or SDF-1α (Fig. 3), indicating that Plcγ2 lies upstream of CXCR4 in the BCR-induced internalization pathway and that SDF-1α–induced internalization is independent of Plcγ2 activation. These results thus suggest that the BCR-induced arrest of SDF-1α–directed migration may be due in part to CXCR4 internalization triggered by BCR-mediated stimulation of Plcγ2 and PKC activation. In addition, they show that SDF-1α and BCR activation lead to CXCR4 surface downregulation through different pathways in DT40 B cells.
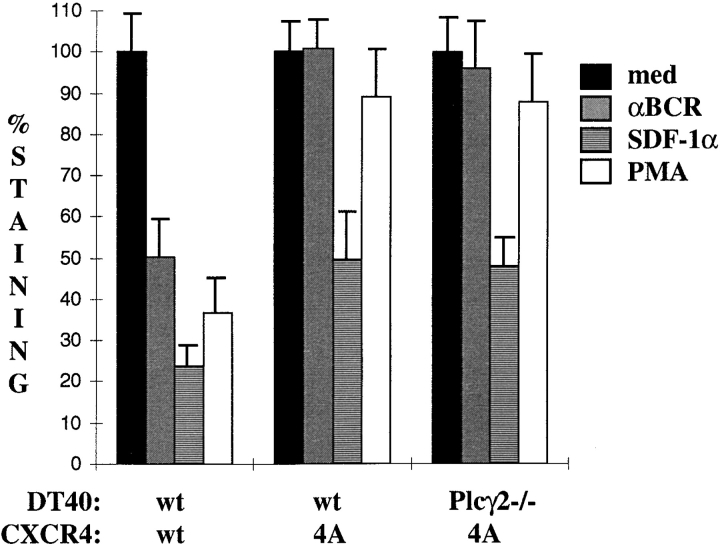
The SSXXIL Motif in the COOH Terminus of CXCR4 Is Required for BCR-induced Receptor Internalization.
Signoret et al. (23) have demonstrated that a SSXXIL motif, similar to that required for endocytosis of CD4 and the TCR complex, is required for phorbol ester–induced, but not ligand-induced, internalization of CXCR4. To determine the contribution of this motif to the BCR-induced internalization of CXCR4 in DT40 cells, we generated stable transfectants of DT40 wt or Plcγ2-mutant cells expressing a CXCR4 mutant in which the SSLKIL sequence was replaced by AALKAA. As seen in Figs. 3 and 4, wt DT40 cells expressing the wt human CXCR4 receptor internalize this receptor in response to BCR cross-linking, SDF-1α treatment, and PMA stimulation. In contrast, the SSLKIL→ AALKAA mutant CXCR4 receptor (designated 4A in Fig. 4), whether expressed in wt or Plcγ2-mutant DT40 cells, was incapable of BCR- or PMA-induced internalization (Fig. 4) but retained significant receptor downmodulation in response to SDF-1α. The 4A mutant CXCR4 was unable to migrate in response to SDF-1α, either in the presence or absence of BCR cross-linking (data not shown). The basis for this migration defect has not been determined. BCR- and Plcγ2-dependent internalization of CXCR4 thus appears to utilize the same pathway as PMA, a PKC-dependent downmodulation of this receptor.
Figure 4.
An SSXXIL internalization motif present in CXCR4 is required for its surface downregulation after BCR engagement. Wt or Plcγ2-deficient DT40 cells transfected with wt or 4A mutants of human CXCR4 were kept unstimulated or incubated with either 100 nM SDF-1α, 100 nM PMA, or 10 μg/ml anti-BCR mAb for 2 h at 39°C. They were then stained for CXCR4 surface expression. Results represent the percentage of staining, 100% corresponding to cells incubated directly at 4°C. Results represent the mean value of four experiments realized on two different clones for each condition. Error bars, SD.
Discussion
AgR signaling determines B cell maturation, selection, and orientation within lymphoid organs. Progression from newly generated B cells into MZ and follicular B cells is driven by BCR signaling and is associated with specific anatomic localization inside the spleen. Supra-threshold AgR engagement redirects B cells from follicles, MZ, or blood circulation toward the periarteriolar lymphoid sheath. Depending on their ability to direct cognate interaction with T cells, a humoral response will emerge or B cells will die in a few days (2, 5, 6, 27).
It is now clearly established that chemokines play an important role in these relocalization processes. Thus, the expression of BLR1 is associated with follicular B cell maturation and is required for their tropism in the spleen and Peyer's patch, whereas SDF-1α and SLE responses are rapidly regulated upon BCR engagement (12, 14, 16). The BCR-induced downregulation of CXCR4 demonstrated here offers a first example in which differential AgR engagement might promote differential responsiveness to a chemokine and allow repertoire-based interclonal competition for migration toward a restricted, chemokine-secreting environment (28). However, as seen in Fig. 3, BCR cross-linking results in a twofold reduction in CXCR4 expression. Although this change in expression may account for some of the migration inhibition seen, it suggests that other pathways may be involved as well. The generation of CXCR4 mutants that are deficient in BCR-induced downregulation yet retain chemotactic response to SDF-1α will allow further dissection of the contribution of this pathway to the antigen-driven compartmentalization of lymphocytes. In addition, the definition of SDF-1α secretion sites will provide important clues toward the understanding of B cell migration and selection.
In vitro studies have shown that pro-B cells are dependent on contact with stromal cells and cytokines for survival, whereas cells expressing the pre-B cell receptor are only dependent on soluble factors (29, 30). Bone marrow stromal cells produce SDF-1α, and pro-B cells respond to this chemokine (13, 31). It is tempting to transpose our data from the early steps of pro-B to pre-B cell transition. Thus, like BCR, pre-BCR signaling might induce the downregulation of CXCR4 and block SDF-1α–dependent migration of pre-B cells toward stromal cells. Therefore, CXCR4 downregulation might allow B cells to lose stromal cell tropism upon successful rearrangement of their IgH gene and signaling through the pre-B cell receptor. This mechanism could guarantee the restriction of rare niches to pro-B cells. In agreement with the importance of SDF-1 during early B cell differentiation, SDF-1 and CXCR4 knockout mice show a profound defect in pro-B cell production (32–34). The present definition of a pathway from BCR to the CXCR4 receptor and of a motif responsible for this coupling may allow the construction of mutants to directly test the role of this pathway in vivo. Such analysis might provide insights that will define how Ag-dependent competitive migration participates in B cell maturation and response to Ag.
Acknowledgments
This work was supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH); the National Institute of Allergy and Infectious Diseases, NIH; the Irvington Institute for Immunological Research; and the Association pour la Recherche contre le Cancer.
Abbreviations used in this paper
BLR1
Burkitt's lymphoma receptor 1
GCs
germinal centers
HSA
human serum albumin
PKC
protein kinase C
Plc
phospholipase C
wt
wild type
References
- 1.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 2.MacLennan IC, Liu YJ, Johnson GD. Maturation and dispersal of B-cell clones during T cell-dependent antibody responses. Immunol Rev. 1992;126:143–161. doi: 10.1111/j.1600-065x.1992.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 3.Gu H, Tarlinton D, Muller W, Rajewsky K, Forster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991;173:1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lortan JE, Roobottom CA, Oldfield S, MacLennan IC. Newly produced virgin B cells migrate to secondary lymphoid organs but their capacity to enter follicles is restricted. Eur J Immunol. 1987;17:1311–1316. doi: 10.1002/eji.1830170914. [DOI] [PubMed] [Google Scholar]
- 5.Goodnow CC, Cyster JG, Hartley SB, Bell SE, Cooke MP, Healy JI, Akkaraju S, Rathmell JC, Pogue SL, Shokat KP. Self-tolerance checkpoints in B lymphocyte development. Adv Immunol. 1995;59:279–368. doi: 10.1016/s0065-2776(08)60633-1. [DOI] [PubMed] [Google Scholar]
- 6.Fulcher DA, Lyons AB, Korn SL, Cook MC, Koleda C, Parish C, Fazekas DS, Basten A. The fate of self-reactive B cells depends primarily on the degree of antigen receptor engagement and availability of T cell help. J Exp Med. 1996;183:2313–2328. doi: 10.1084/jem.183.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 8.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 9.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 10.Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Nature. 1998;391:799–803. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngo VN, Tang HL, Cyster JG. Epstein-Barr virus–induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J Exp Med. 1998;188:181–191. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell–derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bleul CC, Schultze JL, Springer TA. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J Exp Med. 1998;187:753–762. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt KN, Hsu CW, Griffin CT, Goodnow CC, Cyster JG. Spontaneous follicular exclusion of SHP1-deficient B cells is conditional on the presence of competitor wild-type B cells. J Exp Med. 1998;187:929–937. doi: 10.1084/jem.187.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 17.Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch JV. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 18.Takata M, Kurosaki T. A role for Brutons tyrosine kinase in B cell antigen receptor–mediated activation of phospholipase Cγ2. J Exp Med. 1996;184:31–40. doi: 10.1084/jem.184.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurosaki T, Johnson SA, Pao L, Sada K, Yamamura H, Cambier JC. Role of the Syk autophosphorylation site and SH2 domains in B cell antigen receptor signaling. J Exp Med. 1995;182:1815–1823. doi: 10.1084/jem.182.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takata M, Homma Y, Kurosaki T. Requirement of phospholipase Cγ2 activation in surface immunoglobulin M–induced B cell apoptosis. J Exp Med. 1995;182:907–914. doi: 10.1084/jem.182.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishiai M, Kurosaki M, Pappu R, Okawa K, Ronko I, Fu C, Shibata M, Iwamatsu A, Chan AC, Kurosaki T. BLNK required for coupling Syk to PLCγ2 and Rac1-JNK in B cells. Immunity. 1999;10:117–126. doi: 10.1016/s1074-7613(00)80012-6. [DOI] [PubMed] [Google Scholar]
- 22.Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-triphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO (Eur Mol Biol Organ) J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Signoret N, Rosenkilde MM, Klasse PJ, Schwartz TW, Malim MH, Hoxie JA, Marsh M. Differential regulation of CXCR4 and CCR5 endocytosis. J Cell Sci. 1998;111:2819–2830. doi: 10.1242/jcs.111.18.2819. [DOI] [PubMed] [Google Scholar]
- 24.Morgenstern JP, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amara A, Gall SL, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier JL, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1α–dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Signoret N, Oldridge J, Pelchen-Matthews A, Klasse PJ, Tran T, Brass LF, Rosenkilde MM, Schwartz TW, Holmes W, Dallas W, et al. Phorbol esters and SDF-1 induce rapid endocytosis and downmodulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139:651–664. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Martin F, Forbush KA, Perlmutter RM, Kearney JF. Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int Immunol. 1997;9:27–41. doi: 10.1093/intimm/9.1.27. [DOI] [PubMed] [Google Scholar]
- 28.Goodnow CC, Cyster JG. Lymphocyte homing: the scent of a follicle. Curr Biol. 1997;7:R219–R222. doi: 10.1016/s0960-9822(06)00105-9. [DOI] [PubMed] [Google Scholar]
- 29.Melchers F, Strasser A, Bauer SR, Kudo A, Thalmann P, Rolink A. Cellular stages and molecular steps of murine B-cell development. Cold Spring Harb Symp Quant Biol. 1989;1:183–189. doi: 10.1101/sqb.1989.054.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre–pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Apuzzo M, Rolink A, Loetscher M, Hoxie JA, Clark-Lewis I, Melchers F, Baggiolini M, Moser B. The chemokine SDF-1, stromal cell-derived factor 1, attracts early stage B cell precursors via the chemokine receptor CXCR4. Eur J Immunol. 1997;27:1788–1793. doi: 10.1002/eji.1830270729. [DOI] [PubMed] [Google Scholar]
- 32.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 33.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 34.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]