Melanocyte Destruction after Antigen-Specific Immunotherapy of Melanoma: Direct Evidence of T Cell–Mediated Vitiligo (original) (raw)
Abstract
Current strategies for the immunotherapy of melanoma include augmentation of the immune response to tumor antigens represented by melanosomal proteins such as tyrosinase, gp100, and MART-1. The possibility that intentional targeting of tumor antigens representing normal proteins can result in autoimmune toxicity has been postulated but never demonstrated previously in humans. In this study, we describe a patient with metastatic melanoma who developed inflammatory lesions circumscribing pigmented areas of skin after an infusion of MART-1–specific CD8+ T cell clones. Analysis of the infiltrating lymphocytes in skin and tumor biopsies using T cell–specific peptide–major histocompatibility complex tetramers demonstrated a localized predominance of MART-1–specific CD8+ T cells (>28% of all CD8 T cells) that was identical to the infused clones (as confirmed by sequencing of the complementarity-determining region 3). In contrast to skin biopsies obtained from the patient before T cell infusion, postinfusion biopsies demonstrated loss of MART-1 expression, evidence of melanocyte damage, and the complete absence of melanocytes in affected regions of the skin. This study provides, for the first time, direct evidence in humans that antigen-specific immunotherapy can target not only antigen-positive tumor cells in vivo but also normal tissues expressing the shared tumor antigen.
Keywords: immunotherapy, melanoma, autoimmunity, vitiligo, T lymphocytes
Introduction
The appearance of vitiligo in melanoma patients is thought to be the result of immune-mediated destruction of melanocytes through recognition of shared target antigens and has been associated with clinical response 1 2. However, there has been no direct evidence that either a cell-mediated or humoral event is responsible for melanocyte destruction, and no demonstration that targeting a specific antigen in melanoma patients results in vitiligo.
Current trials of immunotherapy in melanoma have been directed at melanocytic differentiation antigens such as MART-1/MelanA, gp100, and tyrosinase 3 4 5 6. To evaluate the efficacy and potential toxicity of targeting the MART-1 antigen 7, a clinical trial using adoptively transferred CD8+ CTL clones was initiated. In this report, we describe a patient who developed unusual inflammatory skin lesions after an infusion of MART-1–specific CTLs and provide direct evidence in humans that antigen-specific immunotherapy can target not only antigen-positive tumor cells in vivo but also normal melanocytes expressing a tumor-associated self-protein.
Materials and Methods
Generation of MART-1–specific CTL Clones from Peripheral Blood.
Studies using human subjects received prior approval by the Institutional Review Board at the Fred Hutchinson Cancer Research Center and the University of Washington. PBMCs were obtained from donors by leukapheresis. MART-1–specific CTL clones were generated as described previously 8 9. In brief, autologous PBMCs were stimulated using antigen-presenting cells pulsed with the A2-restricted peptide epitope of MART-1 (M27:AAGIGILTV 10). After three cycles of stimulation, T cells were cloned by limiting dilution and expanded for in vitro testing. CTL clones demonstrating specific lysis of M27 peptide–pulsed and MART-1–positive tumor targets in a chromium release assay were selected for infusion. Clones used for infusions 1 and 2 were transduced with the inducible suicide gene, hygromycin-thymidine kinase (HyTK; a gift of Targeted Genetics Corporation, Seattle, WA, and the National Gene Vector Lab, Indianapolis, IN; reference 11). CTLs transduced with HyTK express the resistance marker, hygromycin phosphotransferase, to permit in vitro selection, and the HSV thymidine kinase gene to permit in vivo ablation with low dose ganciclovir should toxicity due to the infused T cells occur. The transduction protocol for CD8+ CTL clones and characterization of HyTK-transduced T cells have been described previously 12.
Construction of Peptide–MHC Tetramers.
Tetramers were made according to the protocol of Altman et al. 13. In brief, human β2-microglobulin and the soluble domain of the HLA-A2 heavy chain (residues 1–276) linked at its COOH terminus to a BirA substrate peptide (BSP) were expressed in Escherichia coli and isolated as an insoluble aggregate. The expressed HLA-A2–BSP and β2-microglobulin subunits were solubilized and refolded together in vitro in the presence of peptide. Folded material was biotinylated by BirA enzyme. HLA-A2–peptide complexes were purified on gel filtration and ion exchange columns. Tetrameric complexes of biotinylated HLA-A2–peptide were produced by mixing purified, biotinylated heterodimer with NeutrAvidin–PE (Molecular Probes) at a molar ratio of 4:1. Tetramers presenting immunogenic epitopes of MART-1 (M27:AAGIGILTV), tyrosinase (T368:YMDGTMSQV), and gp100 (G154:KTWGQYWQV) were constructed.
Analysis of Antigen-specific T Cell Frequency in the Peripheral Blood and Tissue Samples Using Peptide–MHC Tetramers.
To evaluate in vivo persistence of T cells in the peripheral blood, PBMCs were prepared from samples drawn on day 0 (preinfusion), 1, 7, and 14 after each infusion and cryopreserved so all samples could be analyzed simultaneously. At the completion of the study, these samples were thawed, stained with PE-conjugated M27 peptide–MHC tetramer (M27-tetramer PE; 50 μg/ml), anti-CD8–FITC (1:60), and a cocktail of Cy5-PE–conjugated antibodies (anti-CD4, -CD16, and -CD19) for 40 min at 22°C. Analysis was carried out on the CD8+ Cy5-PE–conjugated lymphocyte subset to eliminate nonspecific tetramer staining. The frequency of MART-1–specific CTLs is presented as a fraction of tetramer-positive, CD8+ lymphocytes over the total number of CD8+ cells. This method detects with high specificity a frequency of antigen-specific T cells population as low as 1/10,000 CD8+ cells or 1/100,000 PBMCs 14 15 16.
Analysis of T cell infiltrates from tissue samples (skin and tumor) using peptide–MHC tetramers was performed as described above except that single cell suspensions were prepared from fresh biopsy samples by mechanical disruption followed by Ficoll gradient separation before staining.
Immunohistochemical Analysis of Antigen Expression in Skin and Tumor Biopsies.
Staining for gp100 (1:100; Dako), MART-1 (1:5; Novocastra Laboratories, Ltd.), and tyrosinase (1:30; Novocastra Laboratories, Ltd.) was performed by a peroxidase-labeled streptavidin–biotin method on an automated stainer (Ventana Medical Systems). Paraffin tissue sections were mounted on aminoalkylsilane-treated glass slides and heat treated to optimize antigen retrieval. Endogenous peroxidase activity was blocked by incubation in hydrogen peroxide/methanol. Immunohistochemical staining involved the sequential application of diluted primary antibody, biotinylated goat secondary antibodies, and then peroxidase-labeled streptavidin. The antigens were visualized by incubation with aminoethylcarbazole substrate in the presence of hydrogen peroxide. Nonimmune mouse IgG in place of specific antibody was used for negative control sections.
Results
Clinical Course and Appearance of Inflammatory Skin Lesions after Adoptive Transfer of MART-1–specific CTLs.
The patient described in this study was a 46-yr-old woman diagnosed with metastatic melanoma of unknown primary who presented with supraclavicular and axillary lymphadenopathy. There was no prior history of vitiligo or ocular symptoms. She was initially treated with a chemoimmunotherapy regimen of carmustine (BCNU), dacarbazine (DTIC), cisplatin, IFN-α, and high dose IL-2 17. She received four cycles of chemoimmunotherapy with a partial response but, 3 mo after her last cycle, developed progressive chest wall and nodal disease. Treatment with adoptively transferred MART-1–specific CTL clones was initiated at that time. A total of five T cell infusions consisting of autologous MART-1–specific CD8+ CTL clones were administered over a 4-mo period. The initial two infusions were carried out using HyTK-transduced CTL clones at cell doses of 1.0 and 3.3 × 109 cells/m2 1 wk apart, followed by a 3-wk period of observation for toxicity. No toxicity was observed and the patient received infusions 3, 4, and 5 consisting of unmodified CTL clones at cell doses of 3.3 × 109 cells/m2 3 wk apart, each accompanied by a 14-d course of subcutaneous IL-2 (500,000, 1,000,000, and 2,000,000 U/m2 daily, respectively).
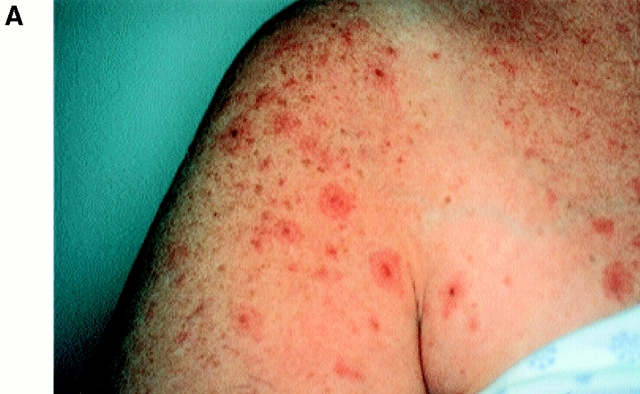
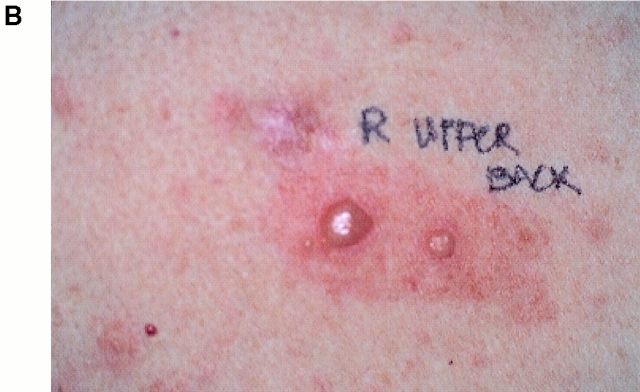
5 d after infusion 3, given with low dose subcutaneous IL-2 (500,000 U/m2), well-demarcated erythematous papules appeared around pigmented areas of skin in a targetoid pattern (Fig. 1). Some lesions developed into bullae and underwent a vitiliginous change, whereas others began to spread and coalesce over the next 3 d to cover most of her back and upper arms (Fig. 2). These lesions were associated with pruritis and a low-grade fever (37.8°C). Because of concern that choroidal melanocytes might be involved, the patient underwent a complete ophthalmologic evaluation. Visual acuity and field of vision were normal. Fundoscopic examination revealed a mild anterior uveitis but no evidence of choroiditis or retinitis. 10 d after the infusion, the infiltrates resolved without intervention. Except for a few depigmented bullae, the patient retained most of her skin pigmentation even in areas of previous inflammation. The IL-2 injections continued to day 14. The patient subsequently received infusions 4 and 5 of MART-1–specific T cells and IL-2 without incident.
Figure 1.
Clinical appearance of inflammatory skin lesions after infusion of autologous MART-1–specific CD8+ CTL clones and 500,000 U/m2/d of IL-2. (A) Close-up of well-circumscribed erythematous papules surrounding pigmented lentiges and nevi on right anterior shoulder. (B) Close-up of vitiliginous bullae appearing in the previous site of raised pigmented nevus on the back.
Figure 2.
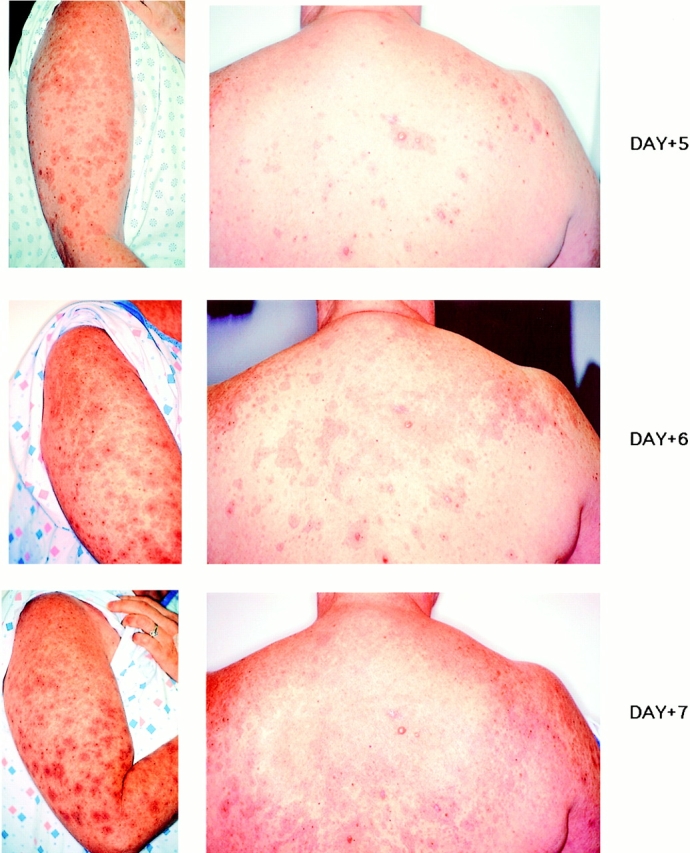
Progression of inflammatory lesions appearing around pigmented areas of skin from days 5–7 after T cell infusion 3.
During the 4-mo period of T cell infusions, clinical and radiographic examination demonstrated stabilization of the tumor masses. 3 mo after the last T cell infusion, the patient developed central nervous system metastases and a rapidly enlarging chest wall mass; she received palliative radiotherapy but expired 6 wk later.
Histological and Immunohistochemical Analysis of Inflammatory Skin Infiltrates after Adoptive Transfer of MART-1–specific T Cells and IL-2 (Infusion 3).
On day 6 after infusion 3, a skin biopsy was obtained over an inflamed site. Histologically, the skin displayed an intense inflammatory infiltrate composed of lymphocytes centered around the dermal–epidermal junction (Fig. 3). Few definite nevus cells could be identified within the degenerating keratinocytes and the changes were consistent with a regressing melanocytic nevus replaced by an intense lymphocytic infiltrate. Further analysis of the lymphocytic infiltrate using horseradish peroxidase–conjugated anti-CD3, -CD4, and -CD8 antibodies demonstrated a predominance of CD3+CD4−CD8+ lymphocytes.
Figure 3.
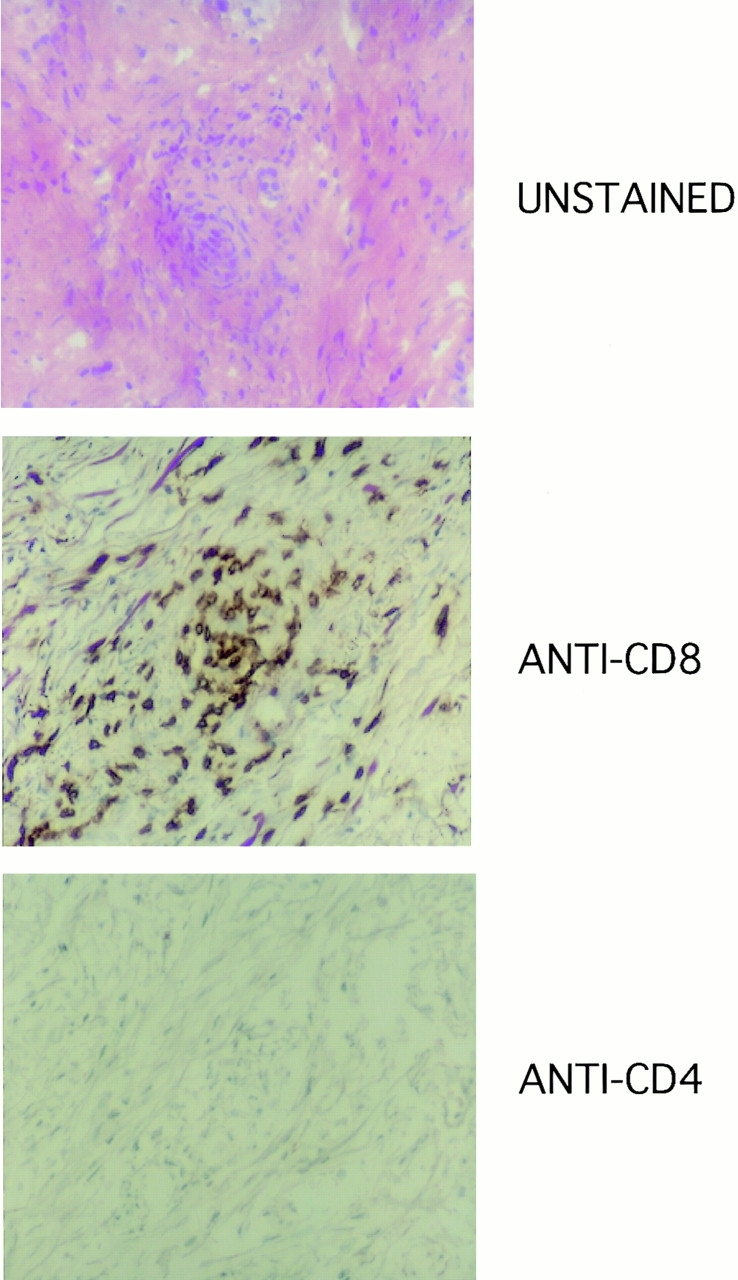
Immunohistochemical staining of inflammatory lesion from skin biopsy obtained on day 6. Hematoxylin and eosin stain demonstrates intense lymphocytic infiltrate in the dermal–epidermal junction in the area of melanocytic nevus. Immunohistochemical staining of the same section demonstrates a predominant population of CD4−CD8+ lymphocytes in infiltrate.
Characterization of Lymphocytes Recovered from the Inflammatory Skin Lesion.
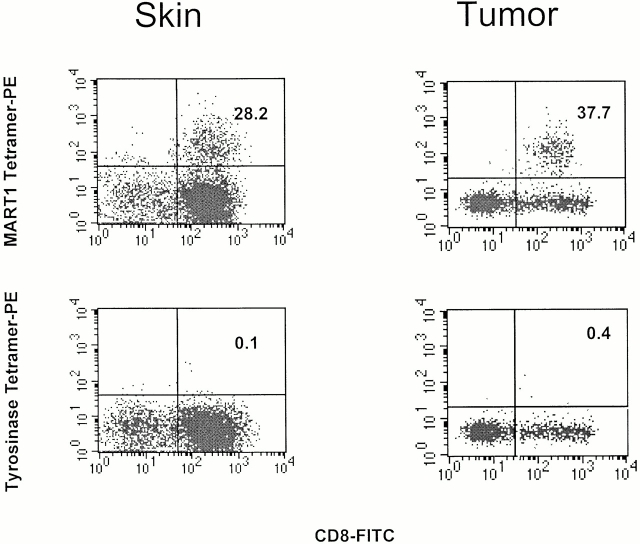
To further characterize the CD8+ infiltrate seen in the skin biopsy at day 6 after infusion 3, a portion of the biopsy specimen was processed into a single cell suspension. Staining of these cells using peptide–MHC tetramers revealed a substantial proportion (28.2%) of CD8+ T cells binding specifically to the M27 peptide–MHC tetramer (Fig. 4). Staining of CD8+ T cells with control tetramers (the tyrosinase T368 and gp100 G154 peptide tetramers) was negative. Simultaneous analysis of the peripheral blood (see below) yielded a frequency of MART-1–specific CTLs (1.2% of all CD8+ cells) that was higher than a preinfusion sample (<0.01%) but significantly lower than that of the skin biopsy sample (28.2%), demonstrating in vivo persistence of infused MART-1–specific CTLs in the peripheral blood and preferential localization to areas of inflammation.
Figure 4.
Characterization of lymphocytes harvested from skin biopsy obtained day 6 after CTL infusion 3 (left) and from tumor biopsy (right). Same skin sample as in Fig. 3. Flow cytometry analysis (left) using PE-conjugated M27 tetramer or an irrelevant (T369) tetramer and anti-CD8–FITC antibody demonstrates a population of MART-1–specific CTL clones representing >28% of the CD8+ lymphocyte population recovered from the skin biopsy. Flow cytometry analysis (right) of tumor biopsy demonstrates MART-1–specific CTL staining comprising >37% of CD8+ lymphocytes in tumor sample.
Characterization of Lymphocytes Recovered from Tumor Site.
The patient presented with a supraclavicular mass extending to her right axilla and breast measuring 12 × 17 cm. For a period of 24–48 h after each T cell infusion, the patient noted tenderness and erythema localized to the chest wall tumor. To evaluate if transferred T cells infiltrated the tumor site, core biopsies of the tumor mass were obtained and divided for histologic analysis and for processing into a single cell suspension. Histologically, lymphocytic infiltration of the tumor mass was present with evidence of extensive necrosis. Approximately 38% of CD8+ lymphocytes from this specimen stained with the MART-1 (M27) peptide–MHC tetramer but not with control tetramers (tyrosinase and gp100), demonstrating that infused MART-1–specific T cells localized not only to normal melanocytes in the skin but also to tumor sites (Fig. 4).
In Vivo Persistence of Infused MART-1–specific CTLs.
The preinfusion (day 0) frequency of MART-1–specific T cells in the peripheral blood of patient 1017-3 was <1/10,000. After infusion 1, MART-1–specific T cells appeared in the peripheral blood at a frequency of 1/99 (1.0%) of CD8+ T cells on day 1, but declined rapidly, dropping by day 14 to a frequency one log lower (1/1,232). After T cell infusion 3 and IL-2 (500,000 U/m2/d for 14 d), MART-1–specific T cells maintained a high frequency in vivo (day 1, 1/125; day 7, 1/84; day 14, 1/118) which included the period when the inflammatory skin reaction was observed (days 6–10).
Expression of MART-1 in Tumor and Skin.
To determine if T cell localization to tumor sites and skin was associated with expression of MART-1, immunohistochemistry was performed on tumor and skin samples.
Analysis of a core biopsy of the chest wall tumor obtained preinfusion demonstrated abundant expression of MART-1 by >70% of melanoma cells (data not shown).
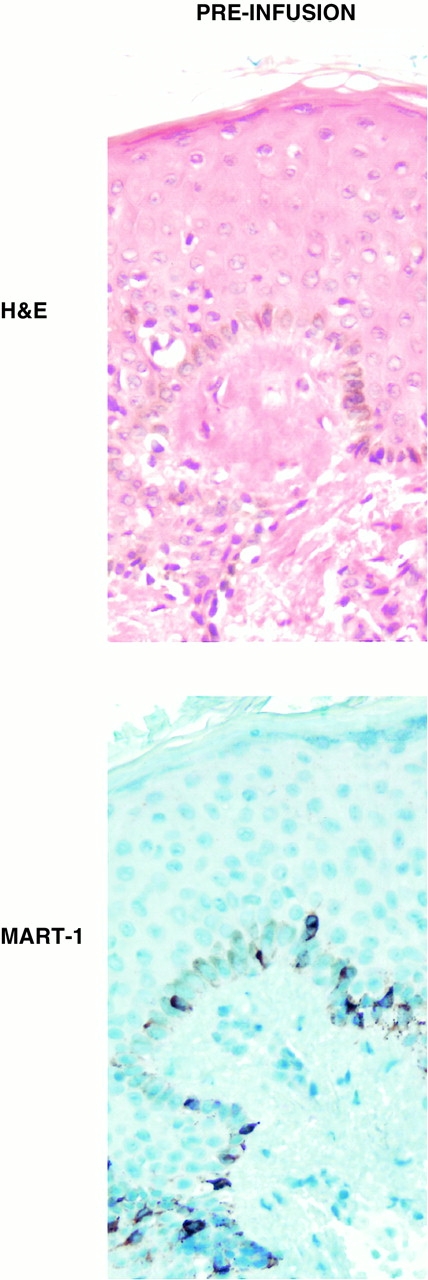
Analysis of a preinfusion skin biopsy demonstrated a layer of melanized keratinocytes underlying pigmented skin and abundant MART-1 expression among melanocytes lining the dermal–epidermal junction (Fig. 5; preinfusion).
Figure 5.
Preinfusion and postinfusion skin biopsies stained with hematoxylin and eosin (H&E) (top) or anti–MART-1 horseradish peroxidase antibody (bottom). In the preinfusion sample, melanized keratinocytes and melanocytes are present in the dermal–epidermal layer. Abundant expression of MART-1 antigen is seen in basal melanocytes. In the postinfusion sample, melanocytes are absent, but residual melanin is observed coating melanophages (black arrowheads) and within melanophages (white arrowheads). No MART-1 staining is seen.
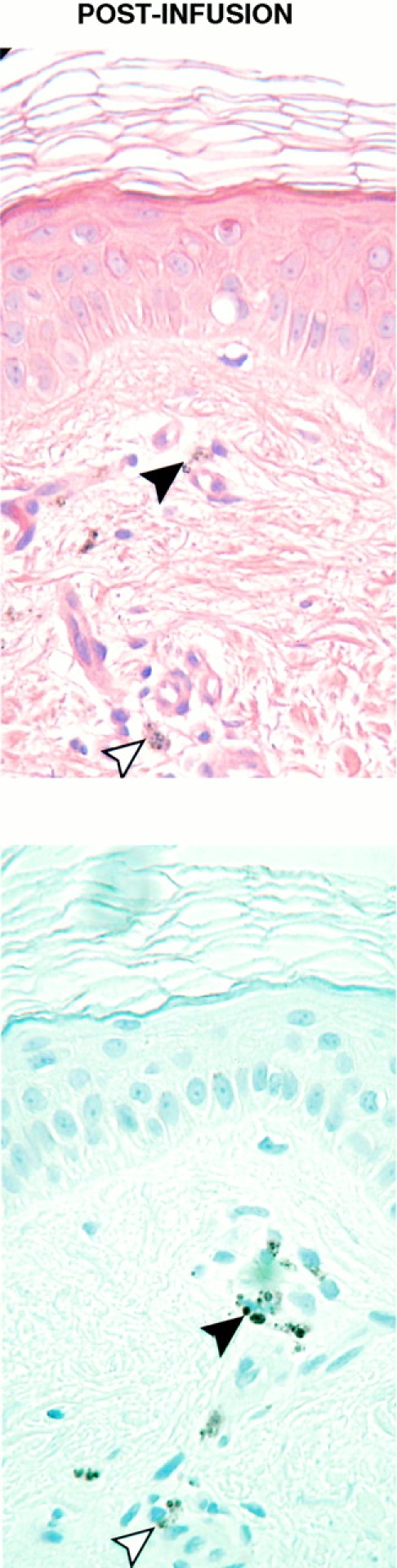
Examination of a skin biopsy obtained from a previously inflamed and pigmented site obtained 3 wk after infusion 3 demonstrated no T cell infiltrate and histologic changes consistent with a regressed nevus: presence of melanophages, residual melanin, dermal fibrosis, a lentiginous pattern of keratinocytes, and no melanocytes confirmed by the complete absence of MART-1 staining (Fig. 5; postinfusion). This finding was confirmed in multiple skin biopsies.
Discussion
In this study, we describe a patient who developed unique inflammatory lesions circumscribing pigmented areas of skin after an infusion of MART-1–specific CD8+ T cell clones. These lesions resulted in loss of cutaneous melanocytes in involved regions and provide direct evidence that vitiligo after immunotherapy of melanoma can be attributed to melanocyte-specific CD8+ CTLs. CTL-mediated destruction of melanocytic nevi as a precedent to the development of vitiligo is consistent with animal models in which depigmentation occurs after immunization to murine homologues of human melanoma antigens, tyrosinase-related protein (TRP)-1 and gp100 18 19. However, the mechanism of vitiligo in humans after immunotherapy has never been clearly defined. Although antibody- or T cell–mediated events have been postulated in patients who develop nonmelanoma-associated vitiligo 20 21, neither T cells nor antibodies have been identified or recovered from the site of lesions. This study suggests that one mechanism by which patients receiving immunotherapy may develop vitiligo is the induction of CTLs reactive to melanocytic differentiation antigens such as MART-1. After cognate antigen recognition, such CTLs may mediate melanocyte destruction by direct cytotoxicity or initiation of a localized inflammatory response.
Direct analysis of the T cell population present in inflammatory skin lesions and tumor nodule biopsies was performed using peptide–MHC tetramers 22. The inflammatory lesions appeared after T cell infusion 3 at a time when the precursor frequency of MART-1–specific CTLs in the peripheral blood was ∼1% of all CD8+ cells. However, the infiltrating MART-1–specific T cell population in the inflamed skin biopsy specimen and a tumor nodule harvested at this time represented >28% of CD8+ T cells in the specimen, demonstrating that transferred CTLs preferentially migrated and localized to antigen-positive tissue.
Histologically, melanocytes in the inflamed tissue were replaced by a T cell infiltrate. Lesions of high antigen density, e.g., a raised, pigmented mole (Fig. 3), rapidly blistered and developed vitiligo. However, other involved areas did not undergo depigmentation and subsequent infusions of an equivalent cell dose of MART-1–specific CTLs with IL-2 (infusions 4 and 5) failed to elicit an inflammatory response, even in areas of pigmentation. To explain this discordance, postinfusion skin biopsies of previously inflamed sites were taken and analyzed for the presence of melanocytes and MART-1 expression. Histology revealed no residual melanocytes, but pigmented keratinocytes and melanophages were seen. Residual melanin can often appear in these latter cell types after melanocytic destruction and account for the observed pigmentation. Immunohistochemical staining for melanosomal proteins (MART-1, gp100, and tyrosinase) was negative, in contrast to normal skin where localized staining is observed within melanocytes. Thus, one explanation is that, during the initial inflammatory process (after infusion 3), MART-1–specific CTLs eradicated skin melanocytes but pigmentation persisted due to residual melanin in keratinocytes and melanophages. In the absence of melanocytes and the MART-1 antigen, no skin toxicity occurred with subsequent infusions of MART-1–specific CTLs and IL-2.
IL-2 therapy alone (without T cells) had been administered previously to the patient and was insufficient to induce an autoimmune reaction, suggesting that the coadministration of antigen-specific T cells was essential to the observed response. The absence of observable toxicity after infusions of MART-1–specific T cells alone (infusions 1 and 2) suggests that coadministered IL-2 (first given with infusion 3) may be equally important to the observed T cell–mediated toxicity. IL-2 may facilitate a breach in tolerance by maintaining or expanding the population of infused MART-1–specific T cells. Analysis of T cell persistence demonstrated sustained levels of transferred MART-1–specific CTLs during IL-2 administration of >1/100, significantly higher than that observed after T cell transfer in the absence of IL-2. Another effect of the administered IL-2 may be increased vascular permeability 23. Although the dose of IL-2 was only 500,000 U/m2, or at least 20 times lower than doses typically associated with clinical signs of vascular leakage, it is possible that even at this low dose, a subclinical effect on vascular permeability was sufficient to permit transmigration of circulating MART-1–specific T cells to the dermal junction where sampling of antigen-positive melanocytes could occur.
Of note, in our patient and in other patients described previously as developing vitiligo after melanoma therapy, the skin toxicity was self-limited and without chronic sequelae. Our patient experienced a mild anterior uveitis but this resolved uneventfully without specific therapy and there was no evidence of posterior uveitis involving choroidal melanocytes or the pigmented retina. This discordance in toxicity may be due to the locally immunosuppressive intraocular microenvironment 24.
Toxicity in this patient was self-limited and restricted to cutaneous melanocytes. Should this prove to be the only significant adverse effect in subsequent trials, further studies using larger T cell doses and increased numbers of infusions in patients with lower tumor burden are warranted.
Acknowledgments
We wish to acknowledge the clinical assistance of Dr. Daniel Markowitz.
This work was supported by National Institutes of Health grant R01 CA71849, a Burroughs Wellcome Fund Career Award, and the Cancer Research Institute (C. Yee).
References
- Bystryn J.C., Rigel D., Friedman R.J., Kopf A. Prognostic significance of hypopigmentation in malignant melanoma. Arch. Dermatol. 1987;123:1053–1055. [PubMed] [Google Scholar]
- Rosenberg S.A., White D.E. Vitiligo in patients with melanomanormal tissue antigens can be targets for cancer immunotherapy. J. Immunother. Emphasis Tumor Immunol. 1996;19:81–84. [PubMed] [Google Scholar]
- Tjandrawan T., Martin D.M., Maeurer M.J., Castelli C., Lotze M.T., Storkus W.J. Autologous human dendriphages pulsed with synthetic or natural tumor peptides elicit tumor-specific CTLs in vitro. J. Immunother. 1998;21:149–157. doi: 10.1097/00002371-199803000-00009. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A., Zhai Y.F., Yang J.C., Schwartzentruber D.J., Hwu P., Marincola F., Topalian S.L., Restifo N.P., Seipp C.A., Einhorn J.H. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J. Natl. Cancer Inst. 1998;90:1894–1900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A., Yang J.C., Schwartzentruber D.J., Hwu P., Marincola F.M., Topalian S.L., Restifo N.P., Dudley M.E., Schwarz S.L., Spiess P.J. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle F.O., Alijagic S., Gilliet M., Sun Y., Grabbe S., Dummer R., Burg G., Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C.H., Robbins P.F., Rivoltini L., Topalian S.L., Miki T., Rosenberg S.A. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl. Acad. Sci. USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C., Savage P.A., Lee P.P., Davis M.M., Greenberg P.D. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- Riddell S.R., Greenberg P.D. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J. Immunol. Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Sakaguchi K., Robbins P.F., Rivoltini L., Yannelli J.R., Appella E., Rosenberg S.A. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2–restricted tumor infiltrating lymphocytes. J. Exp. Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupton S.D., Brunton L.L., Kalberg V.A., Overell R.W. Dominant positive and negative selection using a hygromycin phosphotransferase-thymidine kinase fusion gene. Mol. Cell. Biol. 1991;11:3374–3378. doi: 10.1128/mcb.11.6.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell S.R., Greenberg P.D., Overell R.W., Loughran T.P., Gilbert M.J., Lupton S.D., Agosti J., Scheeler S., Coombs R.W., Corey L. Phase I study of cellular adoptive immunotherapy using genetically modified CD8+ HIV-specific T cells for HIV seropositive patients undergoing allogeneic bone marrow transplant. The Fred Hutchinson Cancer Research Center and the University of Washington School of Medicine, Department of Medicine, Division of Oncology. Hum. Gene Ther. 1992;3:319–338. doi: 10.1089/hum.1992.3.3-319. [DOI] [PubMed] [Google Scholar]
- Altman J.D., Moss P.A.H., Goulder P.J.R., Barouch D.H., McHeyzer-Williams M.G., Bell J.I., McMichael A.J., Davis M.M. Phenotypic analysis of antigen-specific T lymphocytes Science 274 1996. 94 96[published erratum at 280:1821] [DOI] [PubMed] [Google Scholar]
- Ogg G.S., McMichael A.J. Quantitation of antigen-specific CD8+ T-cell responses. Immunol. Lett. 1999;66:77–80. doi: 10.1016/s0165-2478(98)00161-8. [DOI] [PubMed] [Google Scholar]
- Dunbar P.R., Ogg G.S., Chen J., Rust N., van der Bruggen P., Cerundolo V. Direct isolation, phenotyping and cloning of low-frequency antigen-specific cytotoxic T lymphocytes from peripheral blood. Curr. Biol. 1998;8:413–416. doi: 10.1016/s0960-9822(98)70161-7. [DOI] [PubMed] [Google Scholar]
- Lee P.P., Yee C., Savage P.A., Fong L., Brockstedt D., Weber J.S., Johnson D., Swetter S., Thompson J., Greenberg P.D. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- Thompson J.A., Gold P.J., Fefer A. Outpatient chemoimmunotherapy for the treatment of metastatic melanoma Semin. Oncol. 24Suppl. 41997. S44 S48 [PubMed] [Google Scholar]
- Bloom M.B., Perry-Lalley D., Robbins P.F., Li Y., el-Gamil M., Rosenberg S.A., Yang J.C. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J. Exp. Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk W.W., Lee D.S., Surman D.R., Irvine K.R., Touloukian C.E., Chan C.C., Carroll M.W., Moss B., Rosenberg S.A., Restifo N.P. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in micerequirement for CD4(+) T lymphocytes. Proc. Natl. Acad. Sci. USA. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg G.S., Dunbar P.R., Romero P., Chen J.L., Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J. Exp. Med. 1998;188:1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Bystryn J.C. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch. Dermatol. 1995;131:314–318. [PubMed] [Google Scholar]
- McMichael A.J., O'Callaghan C.A. A new look at T cells. J. Exp. Med. 1998;187:1367–1371. doi: 10.1084/jem.187.9.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestingi T., Thompson J.A. Management of IL-2 toxicity. In: Vogelzang N.J., Scardino P.T., Shipley W.U., Coffey D.S., editors. Comprehensive Textbook of Genito-Urinary Oncology. Williams & Wilkins; Baltimore: 1996. pp. 255–261. [Google Scholar]
- Streilein J.W., Wilbanks G.A., Taylor A., Cousins S. Eye-derived cytokines and the immunosuppressive intraocular microenvironmenta review Curr. Eye Res. 11Suppl.1992. 41 47 [DOI] [PubMed] [Google Scholar]