Molecular Cloning and Biological Characterization of a Novel Murine Lymphoid Growth Factor (original) (raw)
Abstract
Using a bioassay consisting of the proliferation of a murine B cell line, a cDNA of a gene whose product supports the growth of that cell line was isolated from a thymic stromal cell line. This factor, termed thymic stromal lymphopoietin (TSLP), is a protein of 140 amino acids. The gene encoding TSLP was mapped to murine chromosome 18. Purified recombinant TSLP supported the growth of pre-B cell colonies in vitro, but had no myelopoietic activity. TSLP had comitogenic activity for fetal thymocytes, but was not as potent as interleukin 7 in lobe submersion cultures. Injection of TSLP into neonatal mice induced the expansion of B220+BP-1+ pre-B cells.
Keywords: cytokine, B lymphocyte, growth factor, lymphocyte differentiation, DNA sequence
Introduction
The development of functional B and T lymphocytes from their immature precursor cells has been extensively studied in the last several years. The process of expansion and maturation is regulated by soluble and membrane-bound ligands that have distinct but overlapping roles 1 2. Despite a long list of cytokines that influence the proliferation and differentiation of B and T cell precursors, in vitro systems have been unable to faithfully recapitulate all of the developmental steps observed in vivo, which supports the notion that additional growth factors may exist.
One approach to identification of cytokines that stimulate the process of hematolymphoid development has been to derive stromal cell lines from various tissues and assess their ability to sustain active lymphopoiesis in vitro 3 4 5. We reported previously on the derivation and characterization of a phenotypically unique thymic stromal cell clone, Z210R.1, that when cocultured with lymphoid precursors from murine fetal liver resulted in vigorous B cell development 6. A nonadherent B lymphoid cell line (NAG8/7) was derived from this culture system and this cell line was shown to be dependent on stromal cell–conditioned medium for survival and expansion 6. Using the proliferative response of the NAG8/7 cell line as a bioassay, we have isolated a novel cDNA from a Z210R.1 expression library by direct expression screening. The expressed protein, termed thymic stromal lymphopoietin (TSLP), shares an overlapping but distinct biological profile with IL-7, and utilizes components of the IL-7 receptor system (see Park et al. in this issue [7]).
Materials and Methods
TSLP Assay.
TSLP bioactivity was assessed in the NAG8/7 proliferation assay as described 6. In brief, NAG8/7 cells were seeded into 96-well flat-bottomed tissue culture wells (Corning) at a density of 2 × 104 cells/well in RPMI 1640 (GIBCO BRL), 10% fetal bovine serum (Hyclone), 100 U/ml penicillin, 100 mg/ml streptomycin, 50 μM 2-ME, and 2 mM l-glutamine (GIBCO BRL). Cultures were incubated for 48 h at 37°C in a humidified atmosphere containing 5% CO2. Proliferation was assessed by 3H-TdR incorporation (1 μCi/well was added for the last 16 h of culture).
cDNA Cloning.
RNA was isolated from Z210R.1 cells, oligo-dT–primed cDNA was synthesized and cloned into the pDC406 vector, pools of clones were generated, and transfections into CV-1/EBNA cells were performed, all as described 8. Supernatants from the CV-1/EBNA cells were harvested 3 d after transfection and assayed directly for NAG8/7 cell proliferative activity as described above. After finding a positive pool, a single clone was subsequently isolated from it as described 8. The insert from this clone, clone 17, was then used to screen an oligo-dT–primed Z210R.1 cDNA library made in λgt10, from which two other clones (2-1 and 1A) were isolated. The extent of each clone with reference to Fig. 1 is: clone 17, nucleotides 7–750; clone 2-1, nucleotides 1–750; clone 1A, nucleotides 27–1125.
Figure 1.
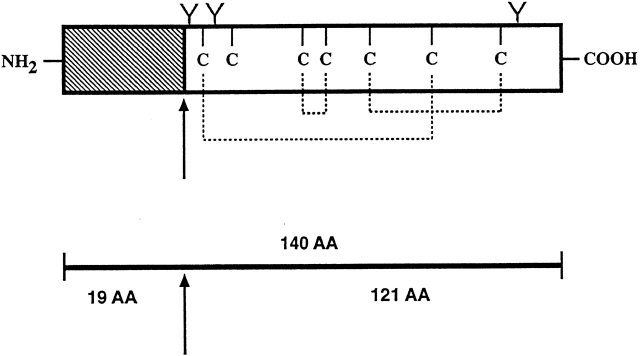
The cDNA and predicted amino acid sequence of murine TSLP. (A) The sequence given is a composite of three clones (see Materials and Methods). The signal peptide is in italics; amino acid numbering begins with the NH2 terminus of the mature protein (Tyr). Potential sites of N-linked carbohydrate addition are underlined. Cysteines are double underlined. Although there is no stretch of polyA at the 3′ end of our clones, it is likely that the AATAAA sequence at nucleotides 1111–1116 represents a polyadenylation signal, since the clone containing it was isolated from an oligo-dT–primed cDNA library. TSLP sequence data are available from EMBL/GenBank/DDBJ under accession no. AF232937. (B) Schematic representation of cysteine residues and potential glycosylation sites.
RNA Hybridization.
PolyA+ RNA (5 μg) was run on agarose gels, transferred to a nylon membrane, and hybridized to a 32P-labeled antisense riboprobe derived from the entire coding region and ∼300 bases of 3′ untranslated region as described 8. The final wash of the filters was for 30 min at 63°C in 0.2× SSC.
Reverse Transcription and PCR Analysis.
RNA was extracted from freshly isolated mouse tissue according to the method of Cathala et al. 9. Contaminating genomic DNA was removed from RNA samples by treatment with DNAase I (Boehringer) as per the manufacturer's instructions. Samples were ethanol precipitated and resuspended in water before reverse transcription (RT) with SuperScript II (GIBCO BRL) and oligo-dT primers as per the manufacturer's instructions. Mock-transcribed samples lacking reverse transcriptase were included in each sample. Reaction products were normalized for cDNA content with the hypoxanthine phosphoribosyltransferase (HPRT) competitor of the polycompetitor construct PQRS provided by R.M. Locksley (University of California at San Francisco, San Francisco, CA; reference 10). Titrated amounts of polycompetitor vector were added to 1 ml aliquots of RT reaction products and PCR amplified with HPRT primers as described previously 10. Densitometry of ethidium bromide–stained PCR products was performed to calculate the equivalence of PQRS vector for each cDNA preparation. Volumes of tissue cDNA containing equivalent amounts of HPRT activity were then amplified to detect TSLP mRNA.
Primers used for TSLP PCR were: (a) 5′-TGCAAGTACTAGTACGGATGGGGC-3′ from the 5′ end of the coding region of the TSLP gene; and (b) 5′-GGACTTCTTGTGCCATTTCCTGAG-3′ from the 3′ end of the coding region of the TSLP gene. This primer pair yields the expected 323-bp product. 0.5–2.0-pg equivalents of HPRT were amplified for 35 or 40 cycles (Omnigene thermocycler) in a 50-μl reaction containing 1.5 mM MgCl2, 400 nM sense and antisense primers, 200 μM dNTP, and 0.25 μl of Taq polymerase (GIBCO BRL). Cycling conditions for all TSLP amplifications were 30 s at 94°C, followed by 30 s at 62°C and a final extension of 10 min at 72°C.
Chromosomal Mapping.
Interspecific backcross progeny were generated by mating (C57BL/6J × Mus. spretus) F1 females and C57BL/6J males as described 11. A total of 205 N2 mice were used to map the Tslp locus. DNA isolation, restriction enzyme digestion, agarose gel electrophoresis, Southern blot transfer, and hybridization were performed as described 12. All blots were prepared with Hybond-N1 nylon membrane (Amersham Pharmacia Biotech). The probe, an ∼790-bp fragment of mouse cDNA, was labeled with [α-32P]dCTP using a random primed labeling kit (Stratagene); washing was done to a final stringency of 1.0× SSCP, 0.1% SDS, 65°C. A fragment of 8.2 kb was detected in KpnI-digested C57BL6/J DNA and a fragment of 14.5 kb in KpnI-digested M. spretus DNA. A description of the probes and RFLPs for the loci linked to the gene for TSLP (Tslp), including desmoglein, adenomatosis polyposis coli (Apc), and fibroblast growth factor 1, has been reported previously 13 14. Recombination distances were calculated as described using the computer program SPRETUS MADNESS 15. Gene order was determined by minimizing the number of recombination events required to explain the allele distribution patterns.
Purification of CV-1/EBNA–expressed TSLP.
Approximately 1 liter of CV-1/EBNA cell supernatant containing TSLP was diluted 1:4 with 20 mM Tris, pH 7.5 (buffer A), and applied to a 190 ml Q-Sepharose Fast Flow column (Amersham Pharmacia Biotech) equilibrated with buffer A. The column was washed with 500 ml buffer A and then eluted with a 1,000 ml linear gradient of 0–300 mM NaCl in buffer A. TSLP-containing fractions were identified by NAG8/7 bioassay. Active fractions from two Q-Sepharose columns were concentrated to ∼60 ml, adjusted to pH 3.5 with 1 M citric acid, and applied to a 42 ml S-Sepharose Fast Flow column (Amersham Pharmacia Biotech) equilibrated with 20 mM citric acid, 100 mM sodium chloride, pH 3.5 (buffer B). The column was washed with 60 ml buffer B and then eluted with a 500 ml linear gradient of buffer B to 50 mM trisodium citrate, 50 mM sodium chloride, pH 6.5. Active fractions were identified by NAG8/7 bioassay, concentrated to 4 ml using a YM10 ultrafiltration membrane (Amicon) and chromatographed on a 2.6 × 90 cm Sephacryl S-100 HR column (Amersham Pharmacia Biotech) equilibrated with 20 mM Tris, 150 mM, pH 7.5. Active fractions from two Sephacryl S-100 HR columns were pooled, adjusted to pH 2 with 10% TFA in water, and purified by reversed-phase (RP)-HPLC on a 0.46 × 22 cm RP300 column (Applied Biosystems) using a gradient of acetonitrile in 0.1% TFA. The gradient was 0–25% acetonitrile in 10 min followed by 25–50% acetonitrile in 50 min TSLP eluted at ∼39% acetonitrile.
Yeast Expression of TSLP.
A cDNA fragment encoding the gene for TSLP was generated using the PCR and subcloned into the Kluyveromyces lactis expression vector, YIpK. This vector is composed of the yeast integrating vector, YIp5 16, with added sequences that comprise the LAC4 promoter of K. lactis 17 18 and the secretion leader from the α-factor gene of Saccharomyces cerevisiae 19 included between the EcoRI and BamHI sites of Yip5.
The oligonucleotide sequences of the primers used to generate the cDNA fragment encoding TSLP are as follows: 5′ primer, 5′-ATATGGTACCTTTGGATAAAAGATACAACTTTTCTAACTGCAACTTG-3′, and 3′ primer, 5′-ATATCCATGGTTATTCTGGAGATTGCATGAAGGAATA-3′. The fragment allows the fusion of the TSLP cDNA in frame to the α-factor leader sequence at an Asp718 site.
The vector was integrated into the genome of K. lactis strain MW98 by homologous recombination into the LAC4 promoter region. For expression of the recombinant TSLP, yeast cells were grown for 48 h in a medium consisting of 1% yeast extract (Difco), 2% peptone (Difco), and 2% galactose (Sigma-Aldrich). Cells were then removed by centrifugation and the medium was filtered through a 0.45-μ cellulose acetate filter. The recombinant protein was recovered from the supernatant; alternatively, the sterile yeast supernatant was assayed directly for TSLP biological activity.
Purification of Yeast-expressed TSLP.
6.5 L of yeast broth containing TSLP was diluted 1:2 with 20 mM citric acid, 50 mM sodium chloride, pH 3.5, adjusted to pH 3.5 with 1 M citric acid, and applied to a 470-ml S-Sepharose column (Amersham Pharmacia Biotech) equilibrated with the dilution buffer. The column was washed with buffer until the absorbance at 280 nm returned to baseline, and TSLP was then eluted with 50 mM Hepes, 100 mM NaCl, pH 7. Fractions containing TSLP were identified by SDS-PAGE, pooled, diluted 1:2 in buffer A, adjusted to pH 7.5 with 1 M Tris base, and applied to a 182-ml Q-Sepharose Fast Flow column (Amersham Pharmacia Biotech) equilibrated with buffer A. The column was washed with buffer A until the absorbance at 280 nm returned to baseline and then eluted with a 1,200-ml linear gradient of 0–300 mM NaCl in buffer A. TSLP-containing fractions were identified by NAG8/7 bioassay and SDS-PAGE, concentrated to 4.5 ml using Centriprep-10 concentrators (Amicon), and chromatographed on a HiLoad 26/60 Superdex 75 column (Amersham Pharmacia Biotech) equilibrated with PBS. TSLP-containing fractions were identified by NAG8/7 bioassay and SDS-PAGE. SDS-PAGE analysis showed 24- and 30-kD major forms of TSLP that were partially resolved by the last gel filtration chromatography step. A 21-kD minor species of TSLP as well as hyperglycosylated forms of TSLP were also detected.
Peptide Mapping and Disulfide Group Determination.
TSLP (6 mg) purified from CV-1/EBNA cells was digested at 37°C for 16 h with 0.4 μg modified trypsin (Promega) in 45 μl of 0.1 M Tris, pH 8. The digest was diluted 1:2 with 0.1% TFA, adjusted to pH 2 with 10% TFA, and peptides were separated by RP-HPLC on a 0.21 × 15 cm Vydac C18 column (Separations Group) with a linear gradient of acetonitrile (0–60% in 90 min) in 0.1% TFA. The elution was monitored at 214 nm, and peptides were collected manually and sequenced on an HP G1000A protein sequencer (Hewlett Packard). An equivalent digest was also analyzed by RP-HPLC/mass spectrometry 20.
Colony Assays.
Bone marrow colony assays to detect pre-B colony-forming cells (CFU–pre-B) were initiated by a modification of the method as described 21 in which agarose (SeaPlaque; FMC Corp.) was substituted for agar. Granulocyte–macrophage CFU were cultured as described 22. All growth factors used to stimulate colony growth (IL-7, TSLP, GM-CSF, IL-3, CSF-1, and G-CSF) were produced at Immunex Corporation as described previously 23 and used at a plateau concentration of 20 ng/ml. In some experiments, cultures were initiated in methylcellulose cultures (StemCell Technologies Inc.) to allow isolation of individual colonies for phenotypic analysis. Cultures were incubated for 7 d at 37°C in a fully humidified atmosphere of 5% O2, 5% CO2, and 90% N2, and aggregates of >40 cells were scored as a colony using an inverted microscope.
Individual colonies in methylcellulose cultures were removed with a finely drawn Pasteur pipet. Colonies were pooled, washed in PBS, and stained with fluorochrome-labeled Abs recognizing Thy-1, B220, MAC-1, or IgM (BD PharMingen). Samples were analyzed on a FACStarPLUS™ flow cytometer (Becton Dickinson) with a minimum of 5,000 events collected per group.
Thymocyte Assays.
CD4−CD8− thymocytes were purified from adult thymocytes from 10–12-wk-old female C57BL/6J mice (The Jackson Laboratory) by incubation with anti-CD4 and anti-CD8 mAbs and rabbit complement as described 24. Adult CD4−CD8− thymocytes were cultured in 96-well flat-bottomed plates (Costar) at a density of 5 × 104 cells per well in a volume of 0.2 ml of DMEM with 5% fetal bovine serum and 5 × 10−5 M 2-ME. Cultures were incubated for 3 d and were pulsed with 1 mCi of 3H-TdR per well for the last 6 h of incubation. Alternatively, thymic lobe submersion cultures were established by placing day 14 fetal thymic lobes in a 24-well plate (Costar) in 1 ml of medium as described 24. IL-7 was added at a concentration of 50 ng/ml, stem cell factor (SCF) at a concentration of 100 ng/ml, and purified TSLP at 50 ng/ml. Cultures were incubated for 7 d, then cells were harvested and counted.
In Vivo Treatment of Mice with TSLP.
Litters of newborn C57BL/6 mice were derived by timed matings. Beginning at 7 d of age, neonatal mice were injected once daily with 200 ng of purified yeast-derived TSLP, 200 ng of human IL-7, or diluent (10 mg/ml mouse serum albumin) in 0.05 ml intraperitoneally. Injections continued for 10 d with the dose of cytokine being increased twofold after 5 d. Mice were killed within 24 h of the last injection, and spleen and bone marrow were harvested for analysis.
mAbs and Flow Cytometric Analysis.
The following mAbs were used for detection of B cell subpopulations. Fluorochrome- or biotin-conjugated anti-B220, anti–heat-stable antigen (HSA), and anti-CD43 were purchased from BD PharMingen. Biotin-conjugated anti–BP-1 was provided by Dr. Max Cooper (University of Alabama, Birmingham, AL). Fluorescein- or Texas red–conjugated goat anti-IgM (m chain specific) was purchased from Southern Biotechnology Associates, Inc. FITC-conjugated polyclonal goat anti-IgD (δ chain specific) was provided by Dr. Fred Finkelman (University of Cincinnati Medical Center, Cincinnati, OH). Cyanin-conjugated anti-B220 was provided by Dr. Tom Waldschmidt (University of Iowa, Iowa City, IA). For four-color analysis of pre-B cell populations, bone marrow was incubated initially with anti-FcγRII mAb (2.4G2) and normal rat sera, followed by simultaneous addition of Abs specific for pre-B cell markers or surface μ or surface δ as described previously 25. Stained cells were analyzed on a FACStarPLUS™ (Becton Dickinson). Data were analyzed using Repromac (TrueFacts Software).
Results
Isolation of a cDNA Clone Encoding TSLP.
To isolate cDNA clones encoding the B lineage growth and differentiation activity found in supernatants of the Z210R.1 cell line, we chose to utilize an approach involving direct expression in mammalian cells 7 26. A cDNA library was generated in a mammalian expression vector, using mRNA from the Z210R.1 cell line. Pools of cDNA clones were transfected into CV-1/EBNA monkey kidney cells, and 3 d later the supernatants of the transfected cells were assayed for TSLP biological activity using the NAG8/7 responder cell line. After screening 82,000 cDNA clones, a positive pool was found. This pool was then broken down into successively smaller subpools until a single cDNA clone encoding the proliferative activity was isolated. The insert from this clone was then used as a probe to isolate further TSLP clones from a λgt10 cDNA library made from the Z210R.1 cells, in order to confirm the representative nature of the original clone.
The TSLP clones isolated had a single open reading frame of 140 amino acids, including a predicted 19–amino acid signal peptide (Fig. 1 A). The mature 121–amino acid protein contains 3 potential sites for N-linked carbohydrate addition and 7 cysteine residues. This is shown schematically in Fig. 1 B. There is a formal possibility that the natural TSLP protein is larger, since no stop codons are present in the short 5′ untranslated regions contained in our cDNA clones (although if that is the case the additional NH2-terminal amino acids would seem to be unnecessary for proper secretion and biological activity of the molecule). The initiating methionine codon contained in the existing clones does lie within an acceptable version of the Kozak consensus sequence. The 3′ untranslated region is ∼700 nucleotides long.
Expression of TSLP mRNA in Murine Tissues and Cell Lines.
Northern blots revealed a single hybridizing species of mRNA that migrated somewhat faster than 18S rRNA (Fig. 2 A), consistent with the mRNA size of ∼1,150 nucleotides (not counting polyA) estimated from the cDNA clones. In addition to the Z210R.1 cells, several other stromal cell lines expressed TSLP mRNA, as did the EL4 T cell lymphoma, lymphoid organs (thymus, spleen, and bone marrow), and kidney. The pre-B cell lymphoma 70Z/3 was negative, as were brain and liver.
Figure 2.
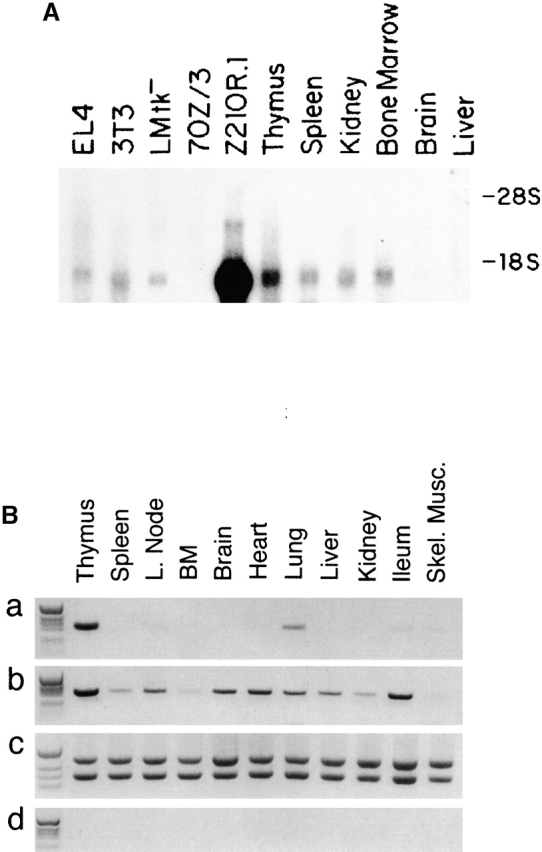
Analysis of TSLP mRNA expression in various tissues. (A) Northern analysis of indicated tissue or cell line polyA+ mRNA with an antisense TSLP probe. (B) RT-PCR of TSLP mRNA expression in various tissues: (a and b) TSLP RT-PCR reaction products from indicated samples of cDNA amplified for 35 or 40 cycles, respectively; (c) normalization of cDNA sample amount using HPRT PCR primers and an HPRT competitor; (d) PCR of mock-transcribed samples. BM, bone marrow; L. node, lymph node; Skel., skeletal.
Analysis of other tissues from normal mice by RT-PCR again demonstrated TSLP expression in thymus, spleen, bone marrow, and lung (Fig. 2 B, a). Over several experiments, there was variable expression of TSLP detected in lymph nodes by RT-PCR. Peyer's patches, brain, liver, heart, ileum, and skeletal muscle were negative for TSLP expression by this method. Increasing the number of cycles of the PCR reaction from 35 to 40 allowed detection of a band of appropriate size in many of the tissues tested (Fig. 2 B, b).
Chromosomal Localization of the TSLP Gene.
Tslp was mapped to mouse chromosome 18 by analysis of DNA from interspecific backcrosses between C57BL/6J and M. spretus. The Tslp gene lies between desmoglein 1 (Dsg1) on the centromere-proximal side and Apc on the telomeric side (Fig. 3). Given the close linkage between Tslp and Apc in the mouse, the human homologue of Tslp most likely maps to human chromosome 5q21–q22. Mouse mutations mapping to this general vicinity (chromosome 18) include spm (a sphingomyelinase deficiency) and ataxia (ax).
Figure 3.
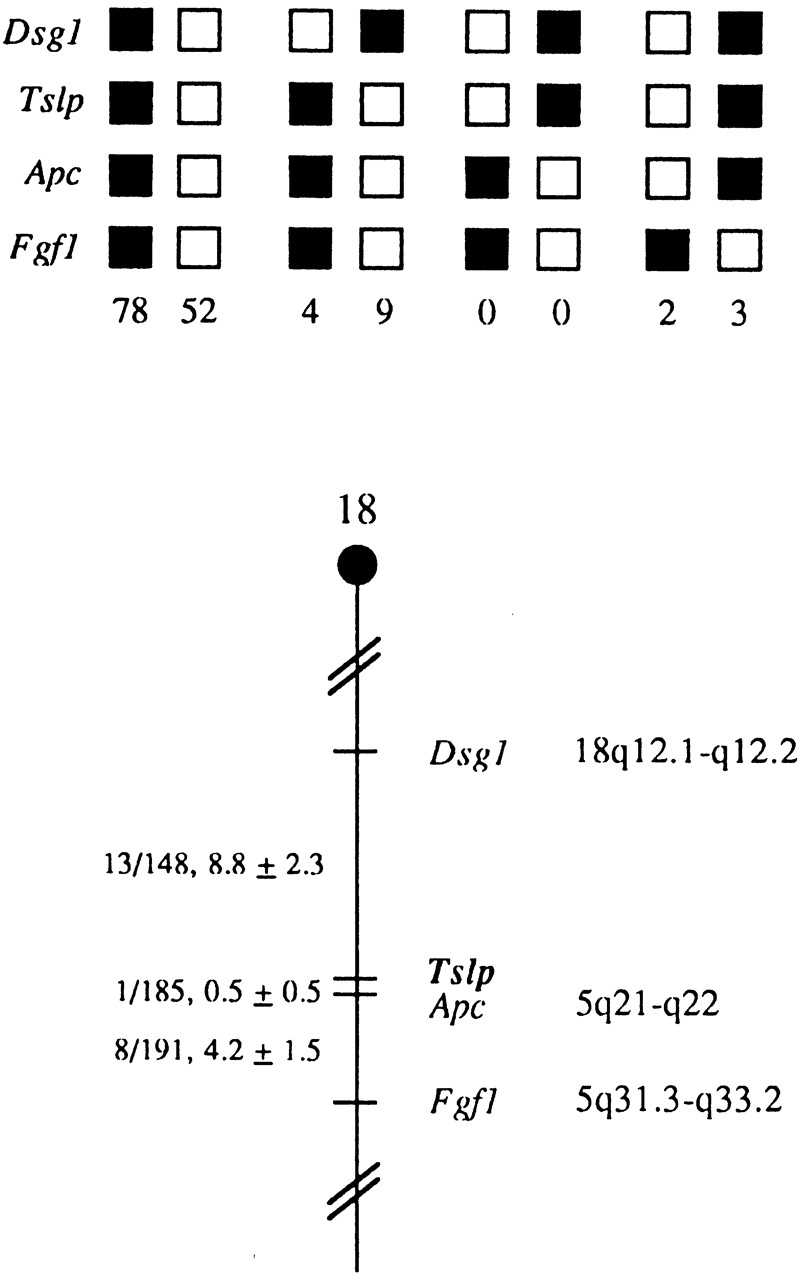
Tslp maps in the proximal region of mouse chromosome 18. Tslp was placed on mouse chromosome 18 by interspecific backcross analysis. The segregation patterns of Tslp and flanking genes in 148 backcross animals that were typed for all loci shown at the top of the figure. For individual pairs of loci, >148 animals were typed. Each column represents the chromosome identified in the backcross progeny that was inherited from the (C57BL/6J × M. spretus)F1 parent. The black boxes represent the presence of a C57BL/6J allele and the white boxes represent the presence of the M. spretus allele. The number of offspring inheriting each type of chromosome is listed at the bottom of each column. A partial chromosome 18 linkage map showing the location of Tslp in relation to linked genes is shown at the bottom of the figure. The number of recombinant animals is presented over the total number of animals typed to the left of the chromosome map between each pair of loci. Recombination frequencies expressed as genetic distance in cM (±1 SE) The positions of loci in human chromosomes, where known, are shown to the right. References for the human map positions of loci cited in this study can be obtained from Genome Data Base (www.gdb.org), a computerized database of human linkage information maintained by The William H. Welch Medical Library of The Johns Hopkins University (Baltimore, MD).
Protein Characterization.
Recombinant TSLP expressed in mammalian cells (CV-1/EBNA) was purified to near homogeneity using a four-step procedure. The final two steps, gel filtration chromatography and RP-HPLC, partially resolved TSLP into a 23-kD major species and an 18-kD minor species as shown by SDS-PAGE. These estimations are in good agreement with those of natural TSLP expressed by Z210R.1 cells (24 and 20 kD), as determined by radioiodination of partially purified natural TSLP, binding to NAG8/7 cells, and elution followed by SDS-PAGE (data not shown). The molecular weight difference for the two forms is probably due to differences in N-linked glycosylation, as _N_-glycanase treatment reduced their molecular weights to ∼14,000 (data not shown), which is close to the calculated molecular weight from the cDNA sequence 13. Purified TSLP was analyzed by 30 cycles of NH2-terminal sequence analysis. The sequence corresponded to that shown in Fig. 1 A beginning at residue 20, tyrosine. This is in agreement with the cleavage site predicted from the cDNA sequence 27. Amino acids were not identified on cycles 6 and 16, which are predicted to be cysteine residues, and cycle 7, which is predicted to be an asparagine and an N-linked glycosylation site. Asparagine on cycle 2, which is also predicted to be an N-linked glycosylation site, was detected, suggesting that it is not glycosylated. Analysis by SDS-PAGE under reducing and nonreducing conditions produced similar results, suggesting that TSLP is not dimerized by an intermolecular disulfide bond (data not shown).
TSLP contains seven cysteine residues, which could potentially form as many as three disulfide bonds. To identify the disulfide bonding pattern, purified TSLP from CV-1/EBNA cells was digested under native conditions with trypsin and the resulting peptides were identified by protein sequencing. A single sequence was obtained for residues 14–28 indicating that cys-16 was not disulfide linked. This result was confirmed by RP-HPLC/mass spectrometry of a TSLP tryptic digest which showed a mass of 1,765.96 compared with the expected mass of 1,766.07. A single sequence was also obtained for residues 32–47, which contains two cysteine residues. RP-HPLC/mass spectrometry showed a mass of 1,851.76, which compares favorably with the expected mass of 1,852.14 for this peptide with an internal disulfide bond between cys-38 and cys-44. Sequences corresponding to residues 1–13 and 73–87 coeluted, suggesting that cys-6 and cys-79 are disulfide linked. Similarly, sequences corresponding to residues 48–65 and 88–111 coeluted, suggesting that cys-59 and cys-102 are disulfide linked. Therefore, cysteine residues 6 and 79, 38 and 44, and 59 and 102 form three disulfide bonds, whereas cysteine residue 16 is not disulfide linked.
TSLP Effects on Lymphoid Progenitors and Myeloid Colony-forming Cells.
Recombinant TSLP expressed transiently in mammalian cells (CV-1/EBNA) or expressed in yeast (K. lactis), and purified as described was tested for biological activity in the NAG8/7 bioassay. A dose-dependent stimulation of thymidine incorporation was observed with both preparations of TSLP having comparable specific activity of ∼20,000 U/μg protein (data not shown).
Pre-B cells have previously been shown to grow clonally in semisolid medium when stimulated with IL-7 21 23. We tested the ability of TSLP to support the clonal growth of normal murine bone marrow cells under conditions that are permissive for pre-B colony growth in agarose cultures. Parallel cultures of normal murine bone marrow cells were initiated in the presence of either IL-7 or TSLP at various concentrations. Growth of colonies was observed in the presence of either cytokine (data not shown). Colonies were composed of 40 or more small mononuclear cells that had the morphological appearance of resting lymphocytes. Clone size was noticeably larger in the presence of IL-7 than TSLP, and the number of colonies at plateau concentrations of IL-7 was approximately twofold greater than the number of colonies developing in the highest effective concentration of TSLP (data not shown). Colony growth in TSLP was strictly dose dependent, with half-maximal colony formation stimulated by ∼2 ng/ml of purified K. lactis or mammalian expressed TSLP (data not shown). Treatment of murine bone marrow with anti-B220 plus complement eliminated the growth of colonies in response to IL-7 or TSLP, but had no effect on granulocyte and/or macrophage colonies in response to GM-CSF, IL-3, CSF-1, or G-CSF (data not shown). The addition of 20 ng/ml of purified TSLP to cultures of whole bone marrow or bone marrow depleted of B220+ cells (by either complement lysis or magnetic bead separation) and stimulated with plateau concentrations of GM-CSF, IL-3, CSF-1, or G-CSF had no effect on the number of myeloid colonies (data not shown). Thus, TSLP stimulates B cell precursor growth but has no demonstrable effect on myeloid committed precursor cells in murine bone marrow.
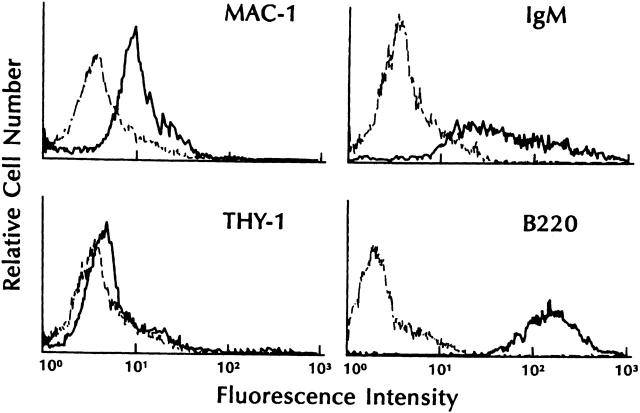
Previous studies 6 had shown that cocultures of Z210R.1 stromal cells with fetal liver cells resulted in the development of surface μ+ cells. To ascertain that this activity was indeed due to TSLP, cultures of B220+surface Ig (sIg)M− bone marrow cells were established in methylcellulose in the presence of TSLP. After 7 d of culture, cells were isolated from harvested colonies, stained, and analyzed by flow cytometry. These cells expressed B220, surface IgM, and low levels of Mac-1, but were negative for expression of Thy-1 (Fig. 4).
Figure 4.
Cell surface phenotype of bone marrow colonies grown in TSLP. Whole bone marrow cells were cultured for 7 d in methylcellulose plus TSLP (20 ng/ml). Colonies were plucked from the semisolid media, pooled, and disrupted. Cells were stained with the appropriate reagents as indicated and analyzed via flow cytometry.
Since TSLP was cloned from a stromal cell line derived from murine thymus, its ability to support thymocyte growth was evaluated. TSLP was tested for growth-stimulating activity on cultures of whole thymocytes or purified adult CD4−CD8− thymocytes both alone and with other cytokines. TSLP failed to stimulate a proliferative response either alone or in the presence of other cytokines in cultures of whole adult thymocytes (data not shown). In cultures of CD4−CD8− thymocytes, TSLP had minimal proliferative activity alone, but significant synergy was seen when TSLP was combined with IL-1 (Table ). There was no stimulatory activity of TSLP with other cytokines (IL-2, IL-3, IL-4, or IL-7) in this type of assay (data not shown). Previously, it has been shown that IL-7 stimulates significant growth of thymocytes in submersion cultures of intact day 14 fetal thymic lobes and that SCF (c-kit ligand) synergizes with IL-7 in this culture system 24. The activity of TSLP was assessed in this culture system, and it can be seen in Table that in contrast to IL-7, TSLP had minimal growth-stimulating activity on its own and did not synergize with SCF.
Table 1.
Synergistic Activity of TSLP on IL-1–induced CD4−CD8− Thymocyte Proliferation
| IL-1β | ||
|---|---|---|
| TSLP | None | 20 ng/ml |
| cpm | ||
| None | 84 | 6,063 |
| 1/10 | 112 | 21,968 |
| 1/50 | 114 | 16,677 |
| 1/250 | 95 | 9,113 |
| Control SN | ||
| 1/10 | 120 | 7,037 |
| 1/50 | 105 | 7,443 |
| 1/250 | 61 | 6,981 |
Table 2.
Cell Yield from Fetal Thymus Lobe Submersion Cultures
| Culture conditions | Cell yield/well |
|---|---|
| ×10−5 | |
| No cytokine | 0.26 |
| TSLP | 0.51 |
| IL-7 | 5.9 |
| SCF | 1.1 |
| TSLP + SCF | 2.6 |
| IL-7 + SCF | 11.5 |
| TSLP + IL-7 | 3.7 |
| TSLP + IL-7+ SCF | 11.8 |
Effects of TSLP or IL-7 Administration on B Lymphopoiesis In Vivo.
Four distinct stages of adult murine B cell maturation were defined by Hardy et al. through the use of multiparameter flow cytometry 28. In that study, one of the earliest committed B cell precursors, the pre–pro-B cell was defined by the expression of CD45R and CD43 (leukosialin) on sIgM− bone marrow cells. This cell is thought to give rise to the pro-B cell that is defined by decreased expression of CD43 and increased expression of HSA. The transition from pro-B cell to pre-B cell is determined by the induction of expression of BP-1. To ascertain the effects of TSLP administration in vivo, 7-d-old neonatal mice were injected daily intraperitoneally with either TSLP or IL-7 for 10 d. The next day, spleen and bone marrow were harvested for assessment of pre-B cell subpopulations by flow cytometry as described 28. The dosage and schedule of cytokine administration were based on previous studies done with IL-7 (Morrissey, P.J., unpublished observations). The phenotypic composition of bone marrow pre-B cell populations was determined by four-color flow cytometry. Gates were set on the sIgM−sIgD− bone marrow cells, and this population was analyzed for expression of B220 and BP-1. As can be seen in Fig. 5 A, mice treated with either TSLP or IL-7 had a significantly greater percentage of B220+BP-1+ pre-B cells. This observation is consistent with previous work that has shown that IL-7 induces BP-1 expression on pre-B cells and increases this population when administered to mice 29 30. Further phenotypic analysis was performed to determine if other changes could be observed in earlier populations of B cell precursors. Thus, CD43 and HSA expression were analyzed in the sIgM−B220+ cell population. As can be seen in Fig. 5 B, there was no appreciable difference in the expression of CD43 or HSA in the B220+sIgM− pre-B cells between control, TSLP-, and IL-7–treated mice. In these cytokine-treated mice, there was significant splenomegaly (twofold over controls), which was due predominantly to an accumulation of B220+sIgM+ B cells (data not shown). Again, there was no significant difference in numbers or phenotype of the splenic B cell population between IL-7– and TSLP-treated mice. These results indicate that TSLP administration enhances B cell production and increases B cell numbers, and that there is no appreciable difference between TSLP and IL-7 in the parameters examined in this study.
Figure 5.
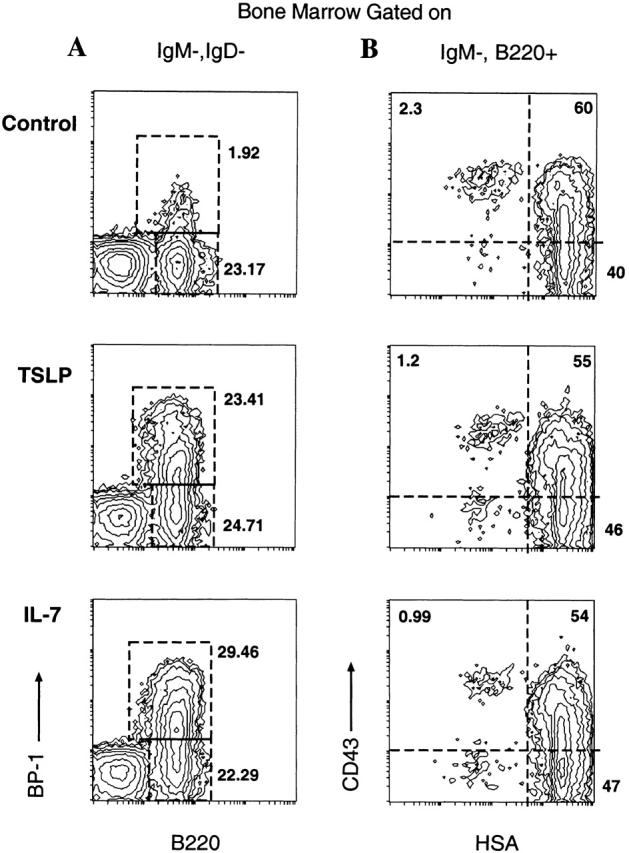
Analysis of bone marrow pre-B cells from mice treated with TSLP or IL-7. Neonatal C57BL/6 mice were injected with MSA, TSLP, or IL-7 for 10 d as described above (see Materials and Methods). 1 d after cessation of treatment, bone marrow was harvested, stained, and analyzed. (A) BP-1 and B220 expression on sIgM−sIgD− bone marrow cells. (B) CD43 (S7) and HSA (M1/69) expression of IgM−B220+ bone marrow cells.
Discussion
The cloning of a novel murine gene that encodes a protein with lymphopoietic activity is described. The activity was initially identified in a coculture system of an adherent thymic stromal cell line with a medullary phenotype, Z210R.1, and day 15 fetal liver 6. Interestingly, the nonadherent lymphoid-like cells that grew in these cocultures phenotypically resembled B cells. A clonal lymphoid line (NAG8/7) was derived from long term cocultures, and this line expresses B220, 6C3 (BP-1), and is surface μ+, but κ chain negative. Importantly, NAG8/7 cells proliferated in response to conditioned medium from the Z210R.1 cell line and thus became the basis of a bioassay that allowed for the preliminary characterization of the growth factor and the eventual cloning of the cDNA encoding this activity.
The cDNA encoding the NAG8/7 growth activity was cloned from a library made from the Z210R.1 cell line. Based on the nucleotide sequence, the mature TSLP protein consists of 121 amino acids with 3 potential N-glycosylation sites and 7 cysteine residues. Interestingly, IL-7 is a 129–amino acid protein with 2 N-linked glycosylation sites and 7 cysteine residues. Comparison of both the nucleotide and amino acid sequences, however, reveals no significant degree of homology between TSLP and IL-7. Additionally, TSLP maps to mouse chromosome 18, whereas IL-7 maps to mouse chromosome 3.
Northern and RT-PCR analysis of mRNA prepared from various tissues and cell lines revealed detectable expression of TSLP in spleen, thymus, kidney, lung, and bone marrow of normal mice. Brain, liver, heart, ileum, and skeletal muscle were negative. Variable expression of TSLP was detected in lymph nodes by RT-PCR. IL-7 has been shown to be expressed in spleen, thymus, and kidney, but not liver, by Northern analysis. In addition, there is evidence to suggest that IL-7 gene expression may be complicated by the use of differential splicing and/or polyadenylation 3. No such evidence has been found to date from the studies of gene expression of TSLP.
The biological activities of TSLP and IL-7 share interesting commonalities. Friend et al. have shown that coculture of day 15 fetal liver with the Z210R.1 cells resulted in the generation of a significant proportion of cells that were surface μ+, although the appearance of significant numbers of μ+ cells was relatively later in the culture period 6. However, similar experiments performed with IL-7 or IXN/2AB stromal cell lines also result in the growth of pre-B cells that are uniquely surface μ−. Thus, these findings suggest that TSLP appears to support the ability of pre-B cells to differentiate to a more mature stage than IL-7. Interestingly, Ray et al. reported that B220+ day 15 fetal liver cells grown in suspension culture for 4 d with TSLP were B220+, but remained largely surface μ−, similar in phenotype to those grown in IL-7 31. However, there was an equivalent proliferative response of these cells when subsequently stimulated with IL-7 and TSLP. Here we demonstrate that the colonies that arose from B220+sIgM− bone marrow cells from adult mice grown in semisolid medium with TSLP for 7 d were sIgM+. The reasons for these somewhat discrepant results are not immediately clear, although the culture systems, starting cell populations, and concentrations of growth factors used are different. Thus, the issue of the comparative activities of TSLP and IL-7 on pre-B cells remains to be elucidated.
Ray et al. also reported that TSLP, similar to IL-7, was not able to support the development of B220+ fetal liver cells to the stage of becoming responsive to maturation to IgM-secreting cells when cultured secondarily with LPS and S17 stromal cells 31. In addition, neither IL-7 nor TSLP can act as a comitogen for mature splenic B cells stimulated with immobilized anti-Ig in vitro (Maliszewski, C., Immunex Corp., personal communication). Thus, we can conclude that IL-7 and TSLP are both able to support B lymphopoiesis from early progenitor cells but that TSLP may support B cell development to a more mature stage than IL-7.
Since TSLP was cloned from a thymic-derived stromal cell line, its effect on T lymphopoiesis was also studied in vitro. TSLP had little effect on the proliferation of adult CD4−CD8− thymocytes in cell suspension culture either alone or with IL-2 or IL-7, but did synergize with IL-1. In lobe submersion culture using day 15 fetal thymus lobes, the addition of IL-7 and IL-7 plus SCF dramatically increased the cell yield per cultured thymic lobe 24. In this system, TSLP had a minimal effect either alone or in combination with SCF. Thus, at least in these culture systems, TSLP does not appear to have potent growth-stimulating abilities for CD4−CD8− thymocytes.
It is interesting to consider the biological activity of TSLP described here with the observations generated from the study of cytokine and cytokine receptor gene knockout mice. Although many cytokine or cytokine receptor genes have been deleted, only mice with targeted disruptions of the IL-7, IL-7R, or γc gene show profound T and B cell lymphopenia 32 33 34. Interestingly, IL-7R−/− mice have a B cell maturational arrest at an earlier stage than IL-7−/− mice, and the lymphopenia in the IL-7R, IL-7, and γc knockout mice is not absolute, suggesting that another ligand that may utilize the IL-7R but not γc would be able to support a low level of lymphopoiesis. Park et al. 7 describe the cloning of a unique receptor for TSLP and propose the use of the IL-7R, but not γc, by TSLP. Mice injected with TSLP have the identical phenotype as mice injected with IL-7, showing enhanced B lymphopoiesis and increased B cell numbers in the spleen 30. However, if TSLP were purely a “functionally redundant” cytokine, it might be predicted that there would be a minimal effect of deleting the IL-7 gene. Since there is severe lymphopenia in these animals, it must be concluded that IL-7 plays a predominant role in lymphopoiesis. Interestingly, a preliminary report of TSLP transgenic mice where the transgene is driven by the proximal lck promoter describes the development of profound pathology and the transgenic line with high copy number surviving only to 3–4 wk of age 35. At lower transgene copy number, extensive pathology consistent with chronic inflammation and autoimmunity develops.
We had invested a substantial amount of effort in attempts to clone a human homologue of TSLP with no success (Sims, J.E., and K. Garka, data not shown). Reduced stringency hybridization of a murine TSLP riboprobe to filters containing human mRNA showed a hybridizing species of a size similar to that of murine TSLP mRNA, yet we were unable to clone a corresponding cDNA from several libraries derived from the same mRNA sources. Genomic Southern blots of human DNA digested with several different restriction enzymes showed a single band that hybridized to the murine TSLP probe. We isolated genomic clones that appeared to correspond to this hybridizing band, but by sequence they clearly did not represent human TSLP. More recently, however, we have identified a human expressed sequence tag that resembles murine TSLP. This impression is confirmed by isolation of full-length cDNA clones corresponding to the same mRNA from which the expressed sequence tag was derived (Sims, J.E., R.F. DuBose, and S.D. Lyman, unpublished data). It remains to be seen whether the biological activities of the mouse and human TSLP are less divergent than their sequences.
Acknowledgments
The authors are grateful for the efforts of John Hix and Melissa Peterson in protein purification and peptide mapping, and Bruce Hess and Kurt Shanebeck for assistance with flow cytometric analysis of bone marrow cells.
These studies were funded by Immunex Corporation and in part by National Institutes of Health grants AI44160 and AI24137.
Footnotes
Abbreviations used in this paper: Apc, adenomatosis polyposis coli; HPRT, hypoxanthine phosphoribosyltransferase; HSA, heat-stable antigen; RP, reversed-phase; RT, reverse transcription; SCF, stem cell factor; sIg, surface Ig; TSLP, thymic stromal lymphopoietin.
References
- Cumano A., Kee B.L., Ramsden D.A., Marshall A., Paige C.J., Wu G.E. Development of B lymphocytes from lymphoid committed and uncommitted progenitors. Immunol. Rev. 1994;137:5–33. doi: 10.1111/j.1600-065x.1994.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Billips L.G., Petitte D., Dorshkind K., Narayanan R., Chiu C.-P., Landreth K.S. Differential roles of stromal cells, interleukin-7, and kit-ligand in the regulation of B lymphopoiesis. Blood. 1992;79:1185–1192. [PubMed] [Google Scholar]
- Namen A.E., Schmierer A.E., March C.J., Overell R.W., Park L.S., Urdal D.L., Mochizuki D.Y. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J. Exp. Med. 1988;167:988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine J., Takeda K., Tatsumi Y., Ogata M., Miyake K., Hamaoka T., Fujiwara H. Role of a thymic stromal cell clone in inducing the stage-specific differentiation of various subpopulations of double negative thymocytes. J. Immunol. 1991;147:1147–1152. [PubMed] [Google Scholar]
- Williams D.E., Eisenman J., Baird A., Rauch C., Van Ness K., March C.J., Park L.S., Martin U., Mochizuki D.Y., Boswell H.S. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990;63:167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- Friend S.L., Hosier S., Nelson A., Foxworthe D., Williams D.E., Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp. Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- Park L.S., Martin U., Garka K., Gliniak B., Di Santo J.P., Muller W., Largaespada D.A., Copeland N.G., Jenkins N.A., Farr A.G., Ziegler S.F., Morrissey P.J., Paxton R., Sims J.E. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptorformation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 2000;192:659–669. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan C.J., Slack J.L., Mosley B., Cosman D., Lupton S.D., Brunton L.L., Grubin C.E., Wignall J.M., Jenkins N.A., Brannan C.I. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J.F., Mendez B., West B.L., Karin M., Martial J.A., Baxer J.D. A method for isolation of transitionally active ribonucleic acid. DNA. 1983;2:329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Reiner S.L., Zheng S., Corry D.B., Locksley R.M. Constructing polycompetitor cDNAs for quantitative PCR. J. Immunol. Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- Copeland N.G., Jenkins N.A. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet. 1991;7:113–118. doi: 10.1016/0168-9525(91)90455-y. [DOI] [PubMed] [Google Scholar]
- Jenkins N.A., Copeland N.G., Taylor B.A., Lee B.K. Organization, distribution and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus . J. Virol. 1982;43:26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice M.J., Gilbert D.J., Kinzler K.W., Vogelstein B., Buchberg A.M., Ceci J.D., Matsuda Y., Chapman V.M., Patriotis C., Makris A. A molecular genetic linkage map of mouse chromosome 18 reveals extensive linkage conservation with human chromosomes 5 and 18. Genomics. 1992;13:1281–1288. doi: 10.1016/0888-7543(92)90047-v. [DOI] [PubMed] [Google Scholar]
- Buxton R.S., Wheeler G.N., Pidsley S.C., Marsden M.D., Adams M.J., Jenkins N.A., Gilbert D.A., Copeland N.G. Mouse desmocollin (Dsc3) and desmoglein (Dsg1) genes are closely linked in the proximal region of chromosome 18. Genomics. 1994;21:510–516. doi: 10.1006/geno.1994.1309. [DOI] [PubMed] [Google Scholar]
- Green, E.L. 1981. Linkage, recombination and mapping. Genetics and Probability in Animal Breeding Experiments. Oxford University Press, New York. 77–113.
- Struhl K., Stinchcomb D.T., Scherer S., Davis R.W. High-frequency transformation of yeastautonomous replication of hybrid DNA molecules. Proc. Natl. Acad. Sci. USA. 1979;76:1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg J.A., van der Laken K.J., van Ooyen A.J.J., Renniers T.C.H.M., Rietveld K., Schaap A., Brake A.J., Bishop R.J., Schultz K., Moyer D. Kluyveromyces as a host for heterologous gene expressionexpression and secretion of prochymosin. Biotechnology. 1990;8:135–139. doi: 10.1038/nbt0290-135. [DOI] [PubMed] [Google Scholar]
- Ruzzi M., Breunig K.D., Fica A.G., Hollenberg C.P. Positive regulation of the β-galactosidase gene from Kluyveromyces lactis is mediated by an upstream activation site that shows homology to the GAL upstream activation site of Saccharomyces cerevisiae . Mol. Cell. Biol. 1987;7:991–997. doi: 10.1128/mcb.7.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MFα)a putative α-factor precursor contains four tandem copies of mature α-factor. Cell. 1982;30:933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Covey T.R., Huang E.C., Henion J.D. Structural characterization of protein tryptic peptides via liquid chromatography/mass spectrometry and collision-induced dissociation of their doubly charged molecular ions. Anal. Chem. 1991;63:1193–1200. doi: 10.1021/ac00013a003. [DOI] [PubMed] [Google Scholar]
- Lee G., Namen A.E., Gillis S., Ellingsworth L.R., Kincade P.W. Normal B cell precursors responsive to recombinant murine IL-7 and inhibition of IL-7 activity by transforming growth factor-β. J. Immunol. 1989;142:3875–3883. [PubMed] [Google Scholar]
- Williams D.E., Straneva J.E., Shen R.-N., Broxmeyer H.E. Purification of murine bone-marrow-derived granulocyte-macrophage colony-forming cells. Exp. Hematol. 1987;15:243–250. [PubMed] [Google Scholar]
- Williams D.E., Morrissey P.J., Mochizuki D.Y., de Vries P., Anderson D., Cosman D., Boswell H.S., Cooper S., Grabstein K.H., Broxmeyer H.E. T-cell growth factor P40 promotes the proliferation of myeloid cell lines and enhances erythroid burst formation by normal murine bone marrow cells in vitro. Blood. 1990;76:906–911. [PubMed] [Google Scholar]
- Morrissey P.J., McKenna H., Widmer M.B., Braddy S., Voice R., Charrier K., Williams D.E., Watson J.D. Steel factor (c-kit ligand) stimulates the in vitro growth of immature CD3−/CD4−/CD8− thymocytessynergy with IL-7. Cell. Immunol. 1994;157:118–131. doi: 10.1006/cimm.1994.1210. [DOI] [PubMed] [Google Scholar]
- Grabstein K.H., Waldschmidt T.J., Finkelman F.D., Hess B.W., Alpert A.R., Boiani N.R., Namen A.E., Morrissey P.J. Inhibition of murine B and T lymphopoiesis in vivo by an anti–interleukin 7 monoclonal antibody. J. Exp. Med. 1993;178:257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namen A.E., Lupton S., Hjerrild K., Wignall J., Mochizuki D.Y., Schmierer A., Mosley B., March C.J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R.R., Carmack C.E., Shinton S.A., Kemp J.D., Hayakawa K. Resolution and characterization of pro-B and pre–pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch P.A., Burrows P.D., Namen A., Gillis S., Cooper M.D. Bone marrow stromal cells and interleukin-7 induce coordinate expression of the BP-1/6C3 antigen and pre-B cell growth. Int. Immunol. 1990;2:697–705. doi: 10.1093/intimm/2.8.697. [DOI] [PubMed] [Google Scholar]
- Morrissey P.J., Conlon P., Charrier K., Braddy S., Alpert A., Williams D.E., Namen A.E., Mochizuki D. Administration of IL-7 to normal mice stimulates B-lymphopoiesis and peripheral lymphadenopathy. J. Immunol. 1991;147:561–568. [PubMed] [Google Scholar]
- Ray R.J., Furlonger C., Williams D.E., Paige C.J. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro . Eur. J. Immunol. 1996;26:10–16. doi: 10.1002/eji.1830260103. [DOI] [PubMed] [Google Scholar]
- Di Santo J.P., Kühn R., Müller W. Common cytokine receptor γ chain (γc)-dependent cytokinesunderstanding in vivo functions by gene targeting. Immunol. Rev. 1995;148:19–34. doi: 10.1111/j.1600-065x.1995.tb00091.x. [DOI] [PubMed] [Google Scholar]
- Peschon J.J., Morissey P.J., Grabsein K.H., Ramsdell F.J., Maraskovsky E., Gliniak B.C., Park L.S., Ziegler S.F., Williams D.E., Ware C.B. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J. Exp. Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Freeden-Jeffry U., Vieira P., Lucian L.A., McNeil T., Burdach S.E.G., Murray R. Lymphopenia in interleukin 7 gene–deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A., Friend S., Forbush K., Perlmutter R. Lymphoid and myeloid alterations in transgenic mice expressing thymic stromal cell derived lymphopoietin (TSLP) 9th International Congress of Immunology 333Abstr.1995. [Google Scholar]