In Situ Tolerance within the Central Nervous System as a Mechanism for Preventing Autoimmunity (original) (raw)
Abstract
Multiple sclerosis (MS) is believed to be an autoimmune disease in which autoreactive T cells infiltrate the central nervous system (CNS). Animal models of MS have shown that CNS-specific T cells are present in the peripheral T cell repertoire of healthy mice and cause autoimmune disease only when they are activated by immunization. T cell entry into the CNS is thought to require some form of peripheral activation because the blood–brain barrier prohibits trafficking of this tissue by naive cells. We report here that naive T cells can traffic to the CNS without prior activation. Comparable numbers of T cells are found in the CNS of both healthy recombinase activating gene (Rag)−/− T cell receptor (TCR) transgenic mice and nontransgenic mice even when the transgenic TCR is specific for a CNS antigen. Transgenic T cells isolated from the CNS that are specific for non-CNS antigens are phenotypically naive and proliferate robustly to antigenic stimulation in vitro. Strikingly, transgenic T cells isolated from the CNS that are specific for myelin basic protein (MBP) are also primarily phenotypically naive but are unresponsive to antigenic stimulation in vitro. Mononuclear cells from the CNS of MBP TCR transgenic but not nontransgenic mice can suppress the response of peripheral MBP-specific T cells in vitro. These results indicate that naive MBP-specific T cells can traffic to the CNS but do not trigger autoimmunity because they undergo tolerance induction in situ.
Keywords: trafficking, T cell, experimental allergic encephalomyelitis, multiple sclerosis, transgenic
Introduction
Activation of self-reactive T cells is one of the first steps in the development of autoimmune disease. The pool of autoreactive T cells available for activation is limited to a large extent by mechanisms of tolerance induction that eliminate self-reactive T cells 1. Animal models of autoimmune disease, however, demonstrate that some self-reactive T cells remain in the periphery. These T cells are described as “ignorant” because they do not cause autoimmune disease when encountering self-antigen in vivo unless the self-antigen is presented in an immunostimulatory context 2 3. Experimental allergic encephalomyelitis (EAE), an animal model of multiple sclerosis (MS), illustrates this phenomenon. EAE is induced by immunization with central nervous system (CNS) antigens such as myelin basic protein (MBP) in CFA 4. Recognition of MBP in this context activates naive T cells circulating in the periphery, which then enter the CNS and initiate destruction of myelin 5. In the absence of experimental intervention, the MBP-specific T cells are not activated and do not induce EAE.
CNS immune privilege is believed to be one of the main components in maintaining ignorance in MBP-specific T cells. Immune privilege is maintained within the CNS by the blood–brain barrier that limits CNS trafficking by leukocytes 6 7 8. Previous studies have suggested that only activated T cells are able to cross the blood–brain barrier and perform immune surveillance of the CNS 7 9 10.
Our studies use TCR transgenic mice specific for MBP1-11 11 12, the predominant epitope targeted in EAE in B10.PL mice 13. MBP1-11–specific T cells in the periphery of TCR transgenic mice are primarily naive and proliferate readily to MBP in vitro 11 14. EAE can be induced in these mice by administration of pertussis toxin or can occur spontaneously in an environment with increased microbial exposure 14. In the studies reported here, we analyzed the ability of the MBP1-11–specific T cells to traffic to the CNS in the absence of any exogenous stimulation. Surprisingly, comparable numbers of T cells were isolated from the CNS of both MBP-specific TCR transgenic mice and wild-type mice. In contrast to wild-type mice, however, most of the MBP-specific T cells found in the CNS of young mice exhibited a naive phenotype rather than an activated or memory phenotype. Similar observations were made in TCR transgenic models specific for nonself antigens. TCR transgenic models differ significantly from wild-type mice in that TCR transgenic mice have few activated/memory peripheral T cells. These results indicate that, in the absence of activated T cells in the periphery, naive T cells can traffic to the CNS. Despite the trafficking of naive MBP-specific T cells through the CNS, the majority of MBP TCR transgenic mice do not develop autoimmunity. We show that the lack of spontaneous EAE is due in part to suppression of the responses of naive MBP-specific T cells within the target organ. Furthermore, CNS cells from MBP TCR transgenic mice are able to suppress the response of nontolerant peripheral MBP-specific T cells in vitro. These data demonstrate that CNS autoimmune disease is regulated in part by the induction of tolerance to CNS antigens in situ.
Materials and Methods
Mice.
MBP TCR1 transgenic mice (B10.PL-H2u-H2-T18a [73NS]/Sn-TgN [TCRα]/BlJg and B10.PL-H2u-H2-T18a [73NS]/Sn-TgN [TCRβ]/C14Jg [11]) and MBP TCR2 transgenic mice 12 were backcrossed onto B10.PL (73 NS)/Sn for at least 11 generations. B10.PL (73 NS)/Sn, C57Bl/6J, and lymphocytic choriomeningitis virus (LCMV) TCR transgenic mice (B6, D2-TgN (TCR LCMV) 327Sdz 15) were obtained from The Jackson Laboratory. Ovalbumin (Ova) TCR transgenic mice (C57BL/6-TgN [TCR Ova] 1100Mjb 16) and Eα52-68:I-Ab (TEa) TCR transgenic mice 17 were provided by Dr. Michael Bevan (University of Washington, Seattle, WA) and Dr. Alexander Rudensky (University of Washington, Seattle, WA), respectively. DO11.10 TCR transgenic mice were provided by Dr. Anne O'Garra (DNAX Research Institute, Palo Alto, CA). Mice were maintained in a specific pathogen-free facility. The Animal Care Committee of the University of Washington approved all procedures used in this report.
Antibodies.
The following monoclonal antibodies were used for flow cytometry: PE-conjugated anti-TCRβ (clone H57-597), hamster anti-IgG (clone Ha4/8), rat anti-IgG (clone R35-95), anti-CD3 (clone 17A2), anti-Vβ8.1,8.2 (clone MR5-2), and anti-Vα2 (clone B20.1); FITC-conjugated anti-CD44 (clone IM7), anti-IgG (clone R35-95), anti-CD3 (clone 500-A2), anti-CD4 (clone GK1.5), anti-CD8 (clone 53-6.7), anti-TCRβ (clone H57.597), and anti-CD45RB (clone 16A); biotin-conjugated anti-TCRβ (clone H57.597), anti-CD49d (clone R1-2), anti-IgG (clone R35-95), anti-Vβ8 (clone MR 5-2), anti-CD45R (clone RA3-6B2), anti-CD8 (clone 53-6.7), anti-Vα2 (clone B20.1), anti-CD4 (clone RM 4-5), anti-CD45RB (clone 16A), and anti-CD62L (clone MEL-14); and purified anti-CD16/CD32 (Fcγ III/II receptor, clone 2.4 G2). All monoclonal antibodies were purchased from BD PharMingen except anti-CD3, which was purchased from Caltag. Biotin-conjugated primary monoclonal antibodies were detected by streptavidin-conjugated Tricolor from Caltag.
Flow Cytometry.
Single-cell suspensions (106) of splenocytes, LN cells, or CNS cells (105) were resuspended in 10 μl of whole mouse serum and anti-CD16/CD32 (0.05 μg/ml) for 15 min at room temperature (RT). Samples were then incubated for 30 min at RT in the dark with primary antibodies diluted in 50 μl of FACS® staining buffer (FSB [0.05% sodium azide and 5% FCS; Atlanta Biologicals] in PBS). Cells were washed, resuspended in 50 μl of secondary antibody diluted in FSB, and incubated for 20 min at RT. Cells were washed and resuspended in either 300 μl of FSB or fixed in FSB with 0.37% formaldehyde. Cells (at least 3 × 105 per sample) were analyzed with a FACScan™ flow cytometer (Becton Dickinson). Fluorescence is plotted on a log scale.
Lymphocyte Isolation.
Mice were anesthetized with a mixture of xylazine (7 mg/kg), ketamine (100 mg/kg), and pentobarbital (50 mg/kg) diluted in saline and administered by intraperitoneal injection. Deeply anesthetized mice were perfused with 50 ml of heparinized PBS via the left ventricle of the heart. The spinal cords were removed via insufflation and the brain was dissected. Both tissues were minced, dissociated with a 5-ml syringe plunger, and strained through wire mesh (Small Parts). After centrifugation, cells were resuspended in 37% Percoll (Amersham Pharmacia Biotech), centrifuged at 2,118 g for 15 min, washed, and counted. To prepare cells for proliferation experiments, pooled CNS samples were washed twice with 37% Percoll, resuspended in 30% Percoll, and layered over 70% Percoll. Cells harvested from the gradient interface were washed and resuspended in complete media (DMEM; GIBCO BRL) supplemented with essential amino acids (Irving Scientific), 9.5% FCS, 1.9 mM l-glutamine, 0.95 mM sodium pyruvate, 43 μM β-mercaptoethanol, 95 U/ml penicillin G, 95 μg/ml streptomycin sulfate, and 0.1 mM nonessential amino acids. The amount of contamination of the CNS mononuclear cell preparation with peripheral cells was assessed using proflavin staining. Proflavin is a nuclear dye that crosses the endothelium of most blood vessels easily but does not cross the blood–brain barrier 18 19. In two separate staining experiments in which proflavin hydrochloride (20 μg/ml) was administered intravenously, >80% of the CNS mononuclear cells did not stain positively for proflavin (data not shown).
Splenocytes were purified over a lympholyte M gradient (Cedarlane) and RBCs were lysed before staining. LNs (cervical, inguinal, brachial, and axillary) were teased with tweezers and filtered through wire mesh (Small Parts) to create a single-cell suspension. In some experiments, cervical and inguinal LNs were harvested separately.
T Cell Stimulation Assay.
Single-cell suspensions of LN or CNS mononuclear cells were plated at 1.5 × 104 Vα2+ T cells/well (MBP TCR1, TEa TCR) or 2 × 104 TCR+ T cells/well (MBP TCR2, D011.10, and nontransgenic) in 96-well plates in complete media. APCs (106/well) consisted of splenocytes from the appropriate wild-type mouse depleted of TCR+ cells by panning with anti-Thy 1.2 (clone 30-H12; BD PharMingen)–coated plates 20, or by magnetic bead depletion using biotinylated anti-TCR and streptavidin-coated Dynabeads (Dynal). MBP TCR1 and MBP TCR2 T cells were stimulated in the presence or absence of 30 μM MBP Ac1-11 peptide (Research Genetics) and T cell–depleted APCs. TEa T cells were stimulated in the presence or absence of 5 μg/ml Eα52–68 peptide (a gift from Dr. Alexander Rudensky) and T cell–depleted APCs. D011.10 T cells were stimulated in the presence or absence of 1 μM Ova 323–339 peptide (a gift from Dr. Craig Beeson, University of Washington, Seattle, WA) and T cell–depleted APCs. 5-Bromo-2′-deoxyuridine (BrdU; 25 μg/ml; Sigma-Aldrich) was added after 48 h of culture and cells were harvested 12–16 h later. Proliferation was assessed by staining the cultured cells with PE-labeled anti-Vα2 (for MBP TCR1 and TEa transgenic mice) or anti-TCR monoclonal antibody (for MBP TCR2 and DO11.10 TCR transgenic mice), cell permeabilization, and staining with FITC-labeled anti-BrdU monoclonal antibody (clone 3D4; BD PharMingen) as described previously 21 22. For assessment of anergy in CNS T cells, stimulations were performed as described above except in the presence or absence of 50 U IL-2/ml 23.
Statistics.
The Student's t test was used for a comparison of the means between sample groups. Error bars represent one SD.
Results
MBP-specific T Cells Are Present in the CNS of Healthy Animals.
The high incidence of spontaneous EAE in recombinase activating gene (Rag)−/− MBP TCR transgenic mice suggests that T cells may be activated by encountering MBP epitopes in vivo. Activation of MBP-specific T cells within the CNS is only possible if some naive T cells gain access to this tissue. To investigate this possibility, we isolated lymphocytes from the brain and spinal cord of clinically healthy, 4–7-wk-old, well perfused, MBP TCR1 transgenic mice on the Rag+/+ background. Lymphocytes from individual animals were analyzed to avoid mixing cells from any mice that had preclinical spontaneous EAE with cells from healthy mice. Surprisingly, the number of T cells per gram of CNS tissue isolated from young, healthy MBP TCR1 transgenic mice was comparable to the number isolated from nontransgenic control mice (Fig. 1). This result was unexpected because entry into the CNS is believed to be limited to activated T cells 7 9 10, and TCR transgenic mice typically have few activated T cells in the periphery 14 24.
Figure 1.
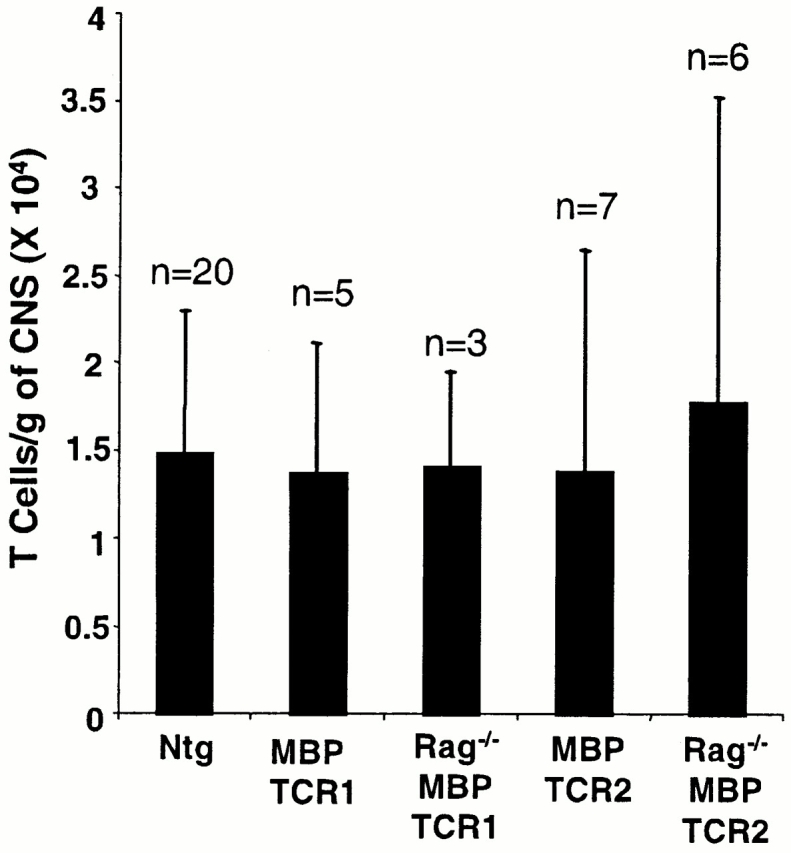
Similar numbers of T cells are isolated from the CNS of MBP TCR transgenic mice and nontransgenic (Ntg) mice on both the Rag−/− and Rag+/+ background. CNS lymphocytes were collected as described in Materials and Methods and stained with anti-TCR monoclonal antibodies. Error bars represent one SD.
A second MBP TCR transgenic model (MBP TCR2) also demonstrated normal numbers of T cells within the CNS compared with age-matched nontransgenic mice (Fig. 1). Of eight MBP TCR2 transgenic mice analyzed, only one mouse exhibited an abnormally high number of T cells (1.5 × 106 T cells/g, 100-fold higher than the average T cell count). Data from this mouse were not included in Fig. 1 because of the possibility that this animal was developing spontaneous disease.
The number of T cells in the CNS of Rag−/− MBP TCR transgenic mice was also analyzed to determine if the expression of endogenous TCRs influenced the ability of transgenic T cells to enter the CNS. The number of T cells isolated from the CNS of Rag−/ − MBP TCR transgenic mice were comparable to the number isolated from nontransgenic mice and Rag+/+ TCR transgenic mice (Fig. 1).
T Cells in the CNS of Young TCR Transgenic Mice Are Predominantly Naive.
Previous studies in nontransgenic mice indicated that only activated T cells cross the blood–brain barrier 7 9 10. To investigate whether the T cells found in the CNS of TCR transgenic mice also display an activated phenotype, T cell populations in 4–7-wk-old nontransgenic (controls) and Rag−/− MBP TCR transgenic mice were analyzed using antibodies specific for cell-surface activation/memory markers. In nontransgenic mice, the majority of CNS T cells expressed high levels of CD44 as well as low levels of CD45RB (Fig. 2) and high levels of CD49d (data not shown), consistent with an activated/memory phenotype. Peripheral T cells from nontransgenic mice expressed significantly lower levels of these activation/memory markers compared with CNS T cells (P = 0.002). These observations are consistent with earlier studies showing that the CNS is preferentially trafficked by activated T cells.
Figure 2.
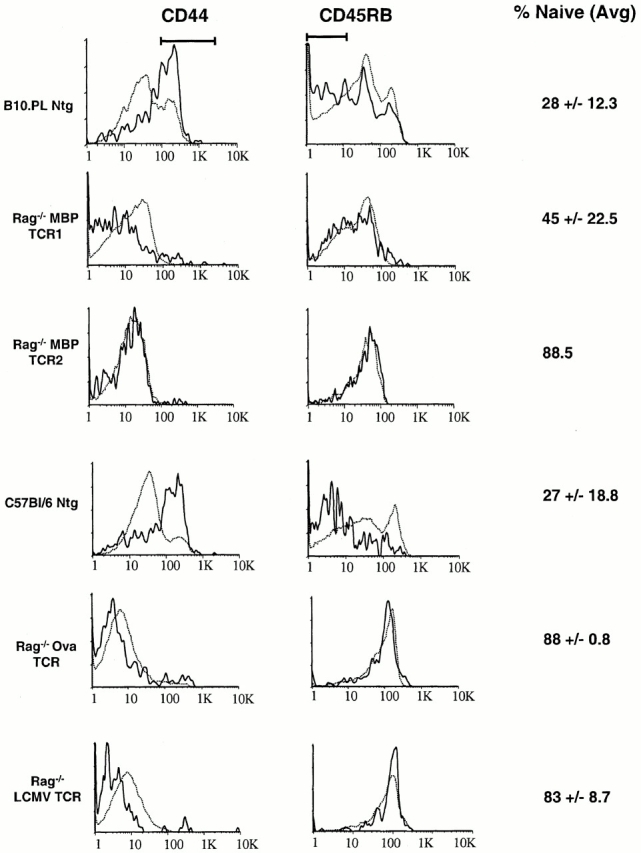
T cells in the CNS of TCR transgenic mice have a predominately naive phenotype. T cells were harvested from the CNS (solid lines) and spleen (dotted lines) as described in Materials and Methods, and stained with monoclonal antibodies specific for TCR, CD44, and CD45RB. The indicated percentage of naive CNS T cells represents the average percentage of TCR+ cells ± one SD that are neither CD44hi nor CD45RBlo (as indicated by the bars).
Our analysis also revealed that the ratio of CD8+/CD4+ T cells is more than twofold higher in the CNS than in the spleen in nontransgenic mice (data not shown). The basis for this CD8+ enrichment in the CNS is not clear; however, it may reflect the fact that a higher percentage of CD44high T cells are found in the CD8+ peripheral T cell subset compared with the CD4+ peripheral T cell subset (28 ± 3.5% versus 19 ± 3.9%, respectively, n = 4) in wild-type mice.
The phenotype of T cells in both the periphery and CNS of Rag−/− MBP TCR transgenic mice was strikingly different from the phenotype seen in nontransgenic mice. The highly restricted TCR repertoire in Rag−/− TCR transgenic mice limits the ability of these T cells to be activated by environmental antigens. Therefore, the percentage of CD44high splenocytes in both Rag−/− MBP TCR1 and Rag−/− MBP TCR2 transgenic mice was 10-fold lower than the percentage in nontransgenic mice (Fig. 2). Unexpectedly, the majority of T cells isolated from the CNS of Rag−/− MBP TCR1 and MBP TCR2 transgenic mice also exhibited a naive phenotype characterized by low levels of CD44 (Fig. 2) and CD49d (data not shown), and primarily intermediate to high levels of CD45RB (Fig. 2).
The data described above suggest that reducing the number of activated T cells in the periphery does not diminish trafficking to the CNS, but instead allows more naive T cells to gain entry. This hypothesis predicts that the CNS of other Rag−/− TCR transgenic mice should also contain similar numbers of T cells as the CNS of wild-type mice, and that the CNS T cells in the TCR transgenic mice should display a naive phenotype. To test these predictions, CNS T cells were isolated from two other Rag−/− TCR transgenic models that were not specific for CNS antigens. In Rag−/− LCMV and Rag−/− Ova TCR transgenic mice, the number of T cells isolated per gram of CNS tissue was again comparable to the number of T cells isolated from nontransgenic control mice (data not shown). T cells isolated from the CNS of both of these transgenic models also express low levels of CD44 and CD49d, and intermediate to high levels of CD45RB (Fig. 2 and data not shown).
In each Rag−/− TCR transgenic model except Rag−/− MBP TCR1 transgenic mice, the percentage of naive T cells (CD45RBhigh, CD44low) found in the CNS is >80% compared with <30% in nontransgenic mice (Fig. 2). Rag−/− MBP TCR1 transgenic mice are unique in that they appear to contain a higher percentage of CD45RBlow T cells in the CNS than the other transgenic models, and consequently a lower percentage of naive T cells. Interestingly, MBP TCR1 transgenic mice on a Rag+/+ background also differ significantly from MBP TCR2 transgenic mice in their incidence of spontaneous EAE. In a conventional animal facility, the incidence of spontaneous EAE is 45% in MBP TCR1 mice but only 11% in MBP TCR2 transgenic mice 25.
In our analyses of T cell phenotype, we also examined the expression of CD62L, an LN homing receptor typically expressed on naive T cells 26 27. In the periphery of both nontransgenic and TCR transgenic mice, CD62L was predominantly expressed on naive T cells (defined by expression of CD44, CD49d, or CD45RB) as expected. Surprisingly, in nontransgenic and TCR transgenic models, CD62L expression was observed on CNS T cells that exhibited activation/memory markers as well as on naive T cells, and did not correlate with either phenotype. CNS lymphocytes were enriched for CD62Llow cells compared with splenocytes in nontransgenic animals (CNS, 47 ± 7%; spleen, 10 ± 7%; n = 3), Rag−/− MBP TCR2 transgenic mice (CNS, 46 ± 11%; spleen, 7 ± 2%; n = 3), Rag−/− Ova transgenic mice (CNS, 64 ± 10%; spleen, 10 ± 1%; n = 4), and Rag−/− LCMV transgenic mice (CNS, 52 ± 17%; spleen, 9 ± 4%; n = 3). This general enrichment of CD62Llow cells in the CNS and lack of correlation with expression of activation markers suggest that CD62L is not strictly a naive T cell marker, and its downregulation in some cases may occur independently of T cell activation.
MBP-specific T Cells Convert to Memory Cells with Age.
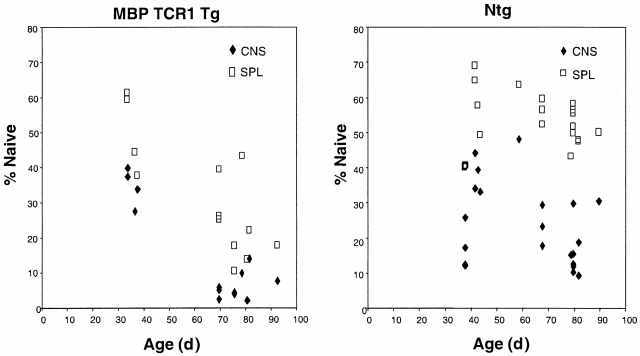
The observation that young Rag−/− MBP TCR1 transgenic mice have a similar percentage of CD45RBlow T cells in the CNS and spleen as nontransgenic mice suggested that interactions with the MBP-specific TCR on these cells promotes the accumulation of T cells with a memory phenotype. Antigenic stimulation could be provided by MBP itself, by a cross-reactive self-antigen, or environmental antigen. In wild-type mice, the percentage of memory T cells increases with age in the peripheral lymphoid compartments, presumably due to an increased number of encounters with environmental antigens 28. We hypothesized that a similar increase in the number of memory T cells should be observed in MBP TCR1 transgenic mice if T cells in these animals are continuously interacting with antigen. Analyzing T cell populations in the spleen and CNS of MBP TCR1 transgenic mice and nontransgenic mice as a function of age tested this prediction. Rag+/+ instead of Rag−/− MBP TCR1 transgenic mice were analyzed because older Rag−/− MBP TCR transgenic mice exhibit a 100% incidence of spontaneous EAE 12. In contrast, Rag+/+ MBP TCR1 transgenic mice exhibit EAE primarily between 5 to 10 wk of age, but very few cases of EAE are observed in mice >69 d of age 14. Older MBP TCR1 transgenic mice (>69 d) exhibited a significant increase in the percentage of activated/memory cells (CD45RBlow, CD44high) in both the CNS and the periphery compared with young MBP TCR1 transgenic mice (P = 0.0006; Fig. 3 and Table ). Furthermore, the age-dependent decrease in naive T cells was significantly greater in MBP TCR1 transgenic mice than in nontransgenic mice (P = 0.0003). A similar age-dependent decrease in naive T cells is seen in MBP TCR2 transgenic mice (data not shown).
Figure 3.
The number of CNS T cells displaying a naive phenotype significantly decreases as MBP TCR1 transgenic mice age. CNS (♦) and splenic (□) T cells were harvested from 21 nontransgenic and 13 MBP TCR1 transgenic mice of different ages and stained with anti-TCR, anti-CD44, and anti-CD45RB monoclonal antibodies. Percent naive represents the percentage of TCR+ cells that are neither CD44hi nor CD45RBlo, as indicated in Fig. 2.
Table 1.
Activated/Memory Phenotypes of CNS and Splenic T Cells from Older (>10 wk) TCR Transgenic and Nontransgenic Mice
| Percentage ofnaive | Percentage ofCD49d high | Percentage ofCD44 high | Percentage ofCD45RB low | |||||
|---|---|---|---|---|---|---|---|---|
| CNS | Spleen | CNS | Spleen | CNS | Spleen | CNS | Spleen | |
| B10.PL | 17.7 ± 7.9 | 51.7 ± 4.9 | 37.7 | 12.2 | 57.9 ± 11.1 | 26.8 ± 7.4 | 63.9 ± 5.8 | 32.9 ± 2.8 |
| NTG | n = 9 | n = 9 | n = 1 | n = 1 | n = 10 | n = 10 | n = 11 | n = 11 |
| MBP | 6.8 ± 3.8 | 24.4 ± 11.1 | ND | ND | 69.3 ± 15.2 | 31.7 ± 9.3 | 79 ± 6.5 | 63.6 ± 11.4 |
| TCR1 | n = 9 | n = 9 | n = 9 | n = 9 | n = 10 | n = 10 | ||
| MBP | 12.1 ± 0.7 | 38.8 ± 0.1 | ND | ND | 39.5 ± 7 | 17.2 ±11 | 78.8 ± 8.6 | 49.9 ± 2.4 |
| TCR2 | n = 2 | n = 2 | n = 5 | n = 5 | n = 2 | n = 2 | ||
| C57Bl/6 | 18.7 | 41.2 | ND | ND | 62.7 | 36.4 | 65.3 | 34.4 |
| Ntg | n = 1 | n = 1 | n = 1 | n = 1 | n = 1 | n = 1 | ||
| Rag−/− | 84.4 | 84.6 | 8.3 ± 2.1 | 1 ± 1 | 19.3 ± 11.8 | 8.5 ± 1.7 | 7.7 | 9.8 |
| Ova | n = 1 | n = 1 | n = 3 | n = 3 | n = 4 | n = 4 | n = 1 | n = 1 |
Several observations support the idea that the increase in memory T cells in Rag+/+ MBP TCR transgenic mice results from interactions via the MBP-specific TCR rather than interactions with environmental antigens via endogenously rearranged TCRs. First, the absolute number of CNS T cells per gram of tissue in older MBP TCR transgenic mice is significantly greater than the number found in older nontransgenic mice (1.4 × 104 CNS T cells in nontransgenic mice, n = 11; 6.0 × 104 CNS T cells in MBP TCR1 mice, n = 10, P = 0.0007; 7.1 × 104 CNS T cells in MBP TCR2 mice, n = 3, P = 0.007). This observation suggests that expression of the MBP TCR results in more antigenic stimulation than the diverse repertoire of TCRs expressed in nontransgenic mice. Second, the absolute number of both total MBP-specific (Vα2+) T cells and CD45RBlow Vα2+ T cells increases with age within the CNS in MBP TCR1 transgenic mice, suggesting that the increase in memory T cells does not simply reflect an increase in T cells with endogenously rearranged TCR chains (data not shown, P = 0.00004). These data are in contrast to results from MHC class II–restricted TCR transgenic mice specific for a nonself antigen, pigeon cytochrome C 24. In this model, there was an increase in peripheral memory T cells as the animals aged; however, this increase was completely in the transgene-negative T cells rather than the transgene-positive T cells. Our data demonstrate that as MBP TCR transgenic mice age, an increasing number of T cells expressing MBP-specific TCRs are activated and converted to a memory phenotype in the absence of spontaneous EAE.
Tolerance Is Induced in MBP-specific Naive T Cells That Enter the CNS.
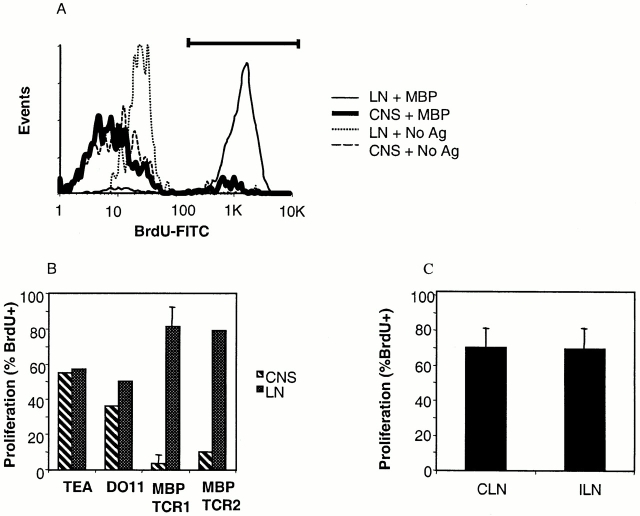
The observation that activated/memory MBP-specific T cells are found in higher numbers in the CNS of healthy, older transgenic mice than in younger mice was inconsistent with the fact that the incidence of spontaneous EAE is significantly higher in younger rather than older mice. Therefore, we investigated the possibility that, in contrast to activated T cells, naive MBP-specific transgenic T cells entering the CNS undergo tolerance in situ and become unresponsive to MBP. CNS T cells isolated from 14–17 healthy MBP TCR1 transgenic mice were pooled and cultured in vitro with MBP Ac1-11. T cell–depleted splenocytes from wild-type mice were added as APCs. Vα2+ T cells isolated from the CNS of MBP TCR1 transgenic mice did not proliferate in response to antigen (Fig. 4A and Fig. B, P = 0.004). In contrast, Vα2+ T cells from LNs of the same animals plated at the same density as the CNS T cells proliferated robustly in response to MBP Ac1-11. CNS T cells isolated from MBP TCR2 transgenic mice also did not proliferate in response to antigen, whereas LN T cells proliferated strongly (Fig. 4 B). To determine whether the induction of nonresponsiveness in T cells within the CNS was unique to T cells specific for MBP, two different MHC class II–restricted TCR transgenic mouse models specific for non-CNS antigens were analyzed using the same experimental protocol. Both CNS and LN T cells harvested from TEa TCR transgenic mice specific for the MHC class II Eα peptide and DO11.10 TCR transgenic mice specific for ovalbumin proliferated equally when stimulated with Eα peptide and Ova peptide, respectively, in vitro (Fig. 4 B). Thus, nonresponsiveness of T cells from the CNS was observed only when the T cells were specific for a CNS antigen.
Figure 4.
CNS T cells from MBP TCR1 and MBP TCR2 transgenic mice do not proliferate in response to antigen while MBP transgenic T cells recovered from CNS-draining cervical LNs (CLN) or nondraining inguinal LNs (ILN) proliferate well. (A) Flow cytometric analysis measuring the proliferation of pooled CNS T cells purified from spinal cords of 17 MBP TCR1 transgenic mice and LN T cells from the same animals. CNS and LN T cells were separately incubated with irradiated T cell–depleted wild-type splenic APCs with and without MBP Ac 1-11. Proliferating T cells are identified as BrdU+ cells as indicated by the bar. (B) Comparison of the proliferation of CNS and LN T cells from different TCR transgenic models in response to antigenic stimulation. Experiments were performed as described in panel A and the data represent an average of three experiments using MBP TCR1 transgenic mice and one experiment each pooling 15–17 MBP TCR2, DO11.10, and TEa TCR transgenic mice. For MBP TCR1 and TEa TCR transgenic mice, proliferation of Vα2+ CNS and LN T cells is shown. For MBP TCR2 and DO11.10 TCR transgenic mice, proliferation of TCR+ rather than Vα2+ T cells is shown. (C) The percentage of proliferating (BrdU+) Vα2+ T cells was determined for T cells harvested from the CLN and ILN (n = 2). Mice used for all of these experiments were 4–12 wk of age.
Previous studies of tolerance to tissue-specific antigens have implicated draining LNs as the site for induction of T cell tolerance. Therefore, we investigated the possibility that nonresponsiveness may be induced in the MBP-specific T cells by presentation of MBP epitopes in the cervical LNs that are believed to drain the CNS 29 30. In the experiments in Fig. 4 B, T cells from multiple peripheral LNs draining different tissues were pooled together such that nonresponsiveness in the cervical LNs might not have been detected. Fig. 4 C compares the proliferative responses to MBP Ac1-11 of Vα2+ T cells isolated from the cervical and inguinal LNs of MBP TCR1 transgenic mice. T cells from both types of LNs proliferated strongly to MBP peptide, supporting the idea that induction of tolerance in MBP-specific T cells occurs within the CNS itself.
Mononuclear Cells within the CNS of MBP TCR Transgenic Mice Actively Suppress the Response of MBP-specific T Cells from the Periphery.
To investigate the mechanism of tolerance induction in the CNS, we first determined if the MBP-specific T cells isolated from the CNS were anergic. CNS T cells from both MBP TCR1 and MBP TCR2 transgenic mice were harvested and stimulated with MBP Ac1-11 and irradiated APCs in the presence and absence of IL-2. CNS T cells isolated from both transgenic models remained nonresponsive to antigen even in the presence of IL-2, whereas LN T cells from the same mice proliferated robustly (data not shown). Therefore, the lack of proliferation observed with MBP-specific T cells from the CNS was not due to an ability to produce IL-2. In all experiments described so far, no more than 21 MBP TCR transgenic mice were pooled per experiment. In one additional experiment, 36 MBP TCR1 TCR transgenic mice were pooled in order to expand the number of wells exposed to IL-2. In this experiment, both CNS and LN T cells proliferated in the presence and absence of IL-2 (data not shown). No proliferation was detected in any of the other experiments using MBP TCR transgenic CNS T cells (n = 5). Therefore, we suspect that the unusually large number of mice pooled in this experiment included a mouse with subclinical EAE. The incidence of spontaneous EAE in the colony from which these mice were obtained is 15%. T cells in the CNS of mice with subclinical EAE will already be activated and would be expected to proliferate in vitro.
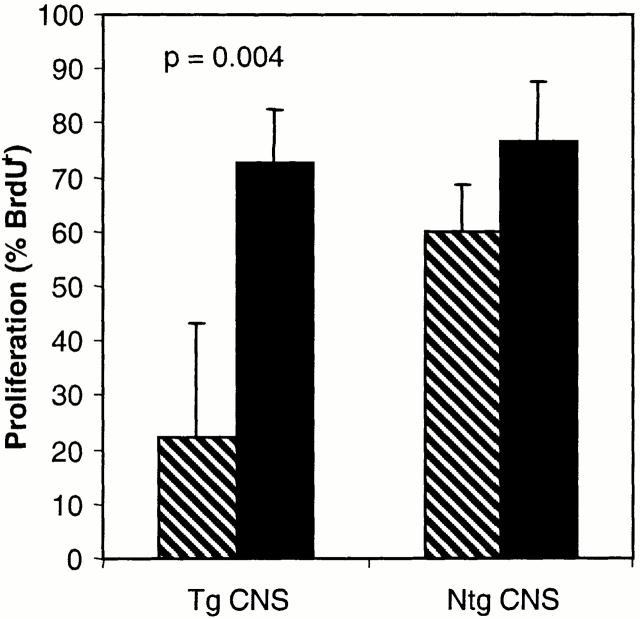
We next investigated whether the mononuclear cells isolated from the CNS of MBP TCR transgenic mice could actively suppress the responses of T cells isolated from the periphery of the same mice that have not undergone tolerance induction. In control experiments, LN T cells (2 × 104) from MBP TCR1 or TCR2 transgenic mice were cultured with and without MBP peptide and either irradiated APCs alone or with irradiated APCs and CNS cells isolated from 15 to 17 nontransgenic mice. The transgenic LN T cells proliferated strongly to MBP Ac1-11 in both the presence and absence of CNS mononuclear cells isolated from nontransgenic mice (Fig. 5). These results confirm our observations made with the TEa and DO11.10 TCR transgenic mice that CNS mononuclear cells do not generally suppress T cell proliferation. Interestingly, CNS cells isolated from MBP TCR transgenic mice had a very different effect in these experiments. Proliferation of MBP-specific LN T cells was significantly inhibited upon incubation with CNS cells isolated from MBP TCR transgenic mice compared with incubation with peptide and irradiated APCs alone (Fig. 5, P = 0.004). These data are the results of four independent experiments using CNS cells from MBP TCR transgenic mice. Thus, mononuclear cells present only in MBP TCR transgenic mice and not nontransgenic mice are capable of mediating bystander suppression of nontolerant MBP-specific peripheral T cells.
Figure 5.
CNS mononuclear cells harvested from MBP TCR transgenic but not nontransgenic mice inhibit proliferation of MBP-specific peripheral LN cells in vitro. MBP TCR transgenic T cells isolated from peripheral LN cells were incubated with MBP Ac1-11 and irradiated APCs as described in the legend to Fig. 4 without (black bars) and with (hatched bars) additional CNS mononuclear cells. CNS mononuclear cells were isolated from 15–17 MBP TCR transgenic or nontransgenic mice. These data represent four experiments using CNS mononuclear cells from MBP TCR2 transgenic mice and three experiments using CNS mononuclear cells from nontransgenic mice. Mice used for these experiments were 4–12 wk of age. Tg, transgenic.
Discussion
Our studies show that multiple mechanisms are responsible for maintaining the immunologically privileged status of the CNS. Previous work indicated that immunological privilege was the result of the specialized structure of the blood–brain barrier that limited T cell trafficking to activated T cells 7 9 10. Our data support the notion that T cells found in the CNS of wild-type mice are predominantly activated/memory T cells. However, recent experiments in sheep demonstrated that naive lymphocytes infused directly into the blood could circulate through the cerebral spinal fluid (CSF 31). Our experiments suggest a basis for reconciling these apparently conflicting findings. We show that the same number of T cells are found in the CNS of nontransgenic, TCR transgenic, and even Rag−/− TCR transgenic mice that have very few activated T cells in the periphery. Thus, a similar number of T cells traffic through the CNS of healthy animals regardless of the number of activated T cells present in the periphery. The CNS of nontransgenic mice is enriched in activated/memory T cells, suggesting that activated T cells have a competitive advantage over naive T cells in crossing the blood–brain barrier. However, as the number of activated T cells in the periphery decreases, more naive T cells are able to enter the CNS. This is most clearly illustrated in Rag−/− TCR transgenic mice in which there are very few activated T cells in the periphery and the vast majority of T cells in the CNS exhibit a naive phenotype.
The increased ability of activated/memory T cells to cross the blood–brain barrier may explain our finding in nontransgenic mice that more CD8+ T cells are found within the CNS than CD4+ T cells. In the periphery of nontransgenic mice, the activated/memory T cell population contains a greater percentage of CD8+ than CD4+ T cells. The CD44high T cell population in the periphery has been shown to have a higher percentage of CD8+ T cells relative to CD4+ T cells in other models as well 32. Alternatively, experiments in a different system suggest that CD8+ T cells may preferentially traffic through the CNS 33. In these studies, dendritic cells and moth–cytochrome C peptide were injected into the CSF of MHC class II–restricted moth–cytochrome C peptide TCR transgenic mice. Preferential recruitment of MHC class I–restricted cells to the CNS was observed even though MHC class II molecules presented the stimulating antigen. These findings and our results are both consistent with enhanced re-circulation or accumulation of CD8+ T cells through the CNS.
An important implication of our results is that circulation of autoreactive naive T cells through the CNS represents a potential trigger of autoimmune disease. Although the degree of trafficking through the CNS by naive T cells is greater in Rag−/− TCR transgenic mice than in nontransgenic mice, some trafficking of the CNS by naive T cells occurs continuously in nontransgenic animals. We demonstrate that initiation of autoimmunity by these naive T cells in the CNS is specifically prevented by the induction of tolerance in situ. Naive MBP-specific T cells isolated from the CNS are unresponsive to antigen while MBP-specific T cells from the periphery of the same animals are fully functional in response to antigen stimulation. Induction of tolerance is unique to MBP-specific T cells present in the CNS, as CNS T cells isolated from TCR transgenic mice specific for non-CNS antigens are not tolerized and proliferate robustly in response to antigen in vitro.
Previous studies of T cell tolerance to tissue-specific antigens indicated that tolerance occurred in LNs draining the relevant tissue. For example, T cells specific for antigens expressed in the pancreas undergo deletion within the LNs draining the pancreas 34 35 36 37 38. In contrast, we demonstrate that induction of tolerance in MBP-specific T cells occurs within the target organ itself rather than within a lymphoid tissue. First, we confirmed that our CNS T cell isolation procedures from MBP TCR transgenic mice produced T cells that were present behind the blood–brain barrier in vivo without significant contamination of peripheral T cells (see Materials and Methods). Second, we examined T cells isolated from cervical LNs for evidence of tolerance because these LNs are believed to drain the CNS 29 30. We found that T cells from these LNs proliferated as robustly as T cells from other LNs and significantly more than T cells from the CNS. Thus, tolerance does not appear to occur in the draining LNs but rather occurs in the CNS itself.
It is not yet clear whether in situ tolerance induction is unique to the CNS and constitutes an aspect of immune privilege of this organ, or whether tissue-specific tolerance occurs in other organs as well. Exposure of naive T cells to antigens within parenchymal tissues has been suggested as a basis for some forms of neonatal tolerance. T cell trafficking through extra-lymphoid tissues is thought to occur more extensively in neonates than in adult mice. However, in at least one example the tolerance induced in neonates by increased trafficking of peripheral tissues was systemic tolerance and did not result in the presence of tolerant T cells exclusively in the relevant tissue 39. In contrast to these findings, we demonstrate that the tolerance to MBP within the CNS is tissue-specific rather than systemic.
Our investigation of the mechanism of tolerance induction in naive MBP-specific T cells entering the CNS showed that tolerance does not occur through downregulation of TCR or CD4 because the expression of these receptors on CNS T cells is comparable to that observed on peripheral T cells (data not shown). Inhibition of proliferation of CNS MBP-specific T cells is not mediated by inhibitory factors found in the CSF or brain during inflammation that appear to modulate T cell activity 40 41. These factors operate within the microenvironment of the inflamed CNS and would not be present in our in vitro cultures. In addition, naive CD4+ T cells specific for non-CNS antigens proliferate equally well when they are isolated from either the CNS or LNs, and it is only T cells from MBP TCR transgenic mice that are nonresponsive. Our data suggest instead that the interaction of naive MBP-specific T cells with antigen in the CNS results in an irreversible state of nonresponsiveness that is maintained even in the absence of soluble factors found in CNS microenvironments during inflammation. The nonresponsiveness induced in MBP-specific T cells in situ was not reversed by addition of exogenous IL-2 in vitro; however, in vivo induction of T cell anergy is not necessarily overcome by addition of exogenous IL-2 42 43 44 45 46. Influenza hemagglutinin–specific TCR transgenic T cells that have been anergized in vivo have been shown to contain high levels of IL-4 and IL-10 mRNA, suggesting that the anergic T cells may function as regulatory T cells 46. This phenomenon could explain our finding that mononuclear cells isolated from the CNS of MBP TCR transgenic mice are capable of suppressing the proliferation of nontolerant peripheral MBP-specific T cells in vitro. Investigation is underway to determine which cell types mediate the suppression and whether this bystander suppression is mediated by soluble factors and/or is cell contact dependent.
Despite tolerance induction of naive MBP-specific CNS T cells, spontaneous EAE still occurs in a percentage of the MBP TCR transgenic mice, primarily within an age window of 5–12 wk 14. Interestingly, our studies show that at the age when the incidence of spontaneous EAE in MBP TCR1 transgenic mice declines, the number of MBP-specific T cells in the CNS of these mice doubles and their phenotype converts to that of an activated/memory T cell. This increase in activated/memory T cells is due in part to expression of the MBP-specific TCR because the number of memory T cells is greater in the CNS of older TCR transgenic mice than in the CNS of age-matched nontransgenic mice, and much of this increase in MBP TCR transgenic mice can be accounted for by an increase in MBP-specific (Vα2+) memory cells. Thus, although T cells in older MBP TCR transgenic mice appear to have encountered antigen more frequently than T cells in older nontransgenic mice, this encounter is not correlated with initiation of disease. Spontaneous EAE may be prevented in older MBP TCR transgenic mice by the development of nontransgenic CD4+ regulatory T cells that have been shown to be critical in prevention of spontaneous EAE 12 47 48. These regulatory T cells must not function by decreasing exposure of MBP-specific T cells to antigen since memory MBP-specific T cells accumulate in older MBP TCR transgenic mice, but rather the regulatory T cells must change the outcome of this interaction.
In summary, our experiments suggest a model in which naive MBP-specific T cells that escape thymic or peripheral tolerance traffic at a low frequency through the CNS in normal individuals. These T cells are prevented from initiating CNS autoimmune disease by in situ tolerance when they encounter antigen within the CNS. The spontaneous autoimmune disease that occurs in some mice may be caused by a failure of tolerance at several levels. Peripheral events could activate MBP-specific T cells either by immunization, as is the common mechanism for the induction of EAE, or via molecular mimicry mediated by an infectious agent 49 50 51 52 53. Once activated, the MBP-specific T cells are no longer susceptible to tolerance in situ and can initiate autoimmune disease. Alternatively, autoimmunity could develop because tolerance occurring within the CNS is circumvented. Potential mechanisms for overcoming tolerance in the CNS include an infection that may increase costimulation and MHC expression in situ, genetic defects in the tolerance mechanisms, or peripheral infections that may influence antigen presentation within the CNS through the release of soluble factors. These experiments reveal a new form of immunoregulation of CNS-specific T cells that must be considered in the study of the pathogenesis of CNS autoimmunity.
Acknowledgments
The authors thank Eric Huseby, Antoine Perchellet, and Audrey Seamons for critical reading of the manuscript, and Jessica Weber for assistance in animal husbandry.
These investigations were supported by a grant from the National Multiple Sclerosis Society (RG 2559A3/1) to J. Goverman. J. Goverman is supported in part by a Harry Weaver Junior Faculty Award (2080-A-2) from the National Multiple Sclerosis Society. T. Bragg was supported by a Senior Postdoctoral Fellowship from the National Multiple Sclerosis Society (FG 1193-A-1). N. Ordonez was supported in part by a Howard Hughes Undergraduate Research Internship.
Footnotes
P. von Dassow's present address is Scripps Institution of Oceanography, Marine Biology Research Division, University of California San Diego, La Jolla, CA 92093-0202.
Abbreviations used in this paper: CNS, central nervous system; CSF, cerebral spinal fluid; EAE, experimental allergic encephalomyelitis; FSB, FACS staining buffer; LCMV, lymphocytic choriomeningitis virus; MBP, myelin basic protein; MS, multiple sclerosis; Ova, ovalbumin; Rag, recombinase activating gene; RT, room temperature.
References
- Huseby E.S., Goverman J. Tolerating the nervous systema delicate balance. J. Exp. Med. 2000;191:757–760. doi: 10.1084/jem.191.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.F., Heath W.R. Self-ignorance in the peripheral T-cell pool. Immunol. Rev. 1993;133:131–150. doi: 10.1111/j.1600-065x.1993.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Nossal G.J. Tolerance and ways to break it. Ann. NY. Acad. Sci. 1993;690:34–41. doi: 10.1111/j.1749-6632.1993.tb43993.x. [DOI] [PubMed] [Google Scholar]
- Paterson P.Y. Experimental autoimmune (allergic) encephalomyelitisinduction, pathogenesis, and suppression. In: Mescher P.A., Mueller-Eberhard H.S., editors. Textbook of Immunopathology. Grune and Stratton; New York: 1976. pp. 179–213. [Google Scholar]
- Martin R., McFarland H.F. Immunology of multiple sclerosis and experimental allergic encephalomyelitis. In: Raine C.S., McFarland H.F., Tourtellotte W.W., editors. Multiple SclerosisClinical and Pathogenic Basis. Chapmand and Hall; London: 1997. pp. 221–242. [Google Scholar]
- Barker C.F., Billingham R.E. Immunologically privileged sites. Adv. Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- Williams K.C., Hickey W.F. Traffic of hematogenous cells through the central nervous system. Curr. Top. Microbiol. Immunol. 1995;202:221–245. doi: 10.1007/978-3-642-79657-9_15. [DOI] [PubMed] [Google Scholar]
- Weller R.O., Engelhardt B., Phillips M.J. Lymphocyte targeting of the central nervous systema review of afferent and efferent CNS-immune pathways. Brain Pathol. 1996;6:275–288. doi: 10.1111/j.1750-3639.1996.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Linnington H., Lassmann H., Meyermann R. Cellular immune reactivity within the CNS. Trends Neurosci. 1986;9:271–277. [Google Scholar]
- Hickey W.F., Hsu B.L., Kimura H. T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Goverman J., Woods A., Larson L., Weiner L.P., Hood L., Zaller D.M. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- Lafaille J.J., Nagashima K., Katsuki M., Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- Zamvil S., Mitchell D.J., Moore A.C., Kitamura K., Steinman L., Rothbard J.B. T cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- Brabb T., Goldrath A.W., von Dassow P., Paez A., Liggitt H.D., Goverman J. Triggers of autoimmune disease in a murine T-cell receptor transgenic model for multiple sclerosis. J. Immunol. 1997;159:497–507. [PubMed] [Google Scholar]
- Pircher H., Burki K., Lang R., Hengartner H., Zinkernagel R.M. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Hogquist K.A., Jameson S.C., Heath W.R., Howard J.L., Bevan M.J., Carbone F.R. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Grubin C.E., Kovats S., deRoos P., Rudensky A.Y. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Peralta L.A. Hematic and fluid barriers in the optic nerve. J. Comp Neurol. 1966;126:109–121. doi: 10.1002/cne.901260109. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Peralta L.A. Hematic and fluid barriers of the retina and vitreous body. J. Comp. Neurol. 1968;132:109–124. doi: 10.1002/cne.901320106. [DOI] [PubMed] [Google Scholar]
- Kruisbeek, A. 1994. In vitro assays for mouse lymphocyte function. In Current Protocols in Immunology. John Wiley and Sons, Inc., West Sussex, UK. 3.5–3.13. [DOI] [PubMed]
- Tough D.F., Sprent J. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon S.R., MacKay V.L., Fink P.J. A functionally compromised intermediate in extrathymic CD8+ T cell deletion. Immunity. 1995;3:321–333. doi: 10.1016/1074-7613(95)90117-5. [DOI] [PubMed] [Google Scholar]
- Harrington C.J., Paez A., Hunkapiller T., Mannikko V., Brabb T., Ahearn M., Beeson C., Goverman J. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity. 1998;8:571–580. doi: 10.1016/s1074-7613(00)80562-2. [DOI] [PubMed] [Google Scholar]
- Linton P.J., Haynes L., Klinman N.R., Swain S.L. Antigen-independent changes in naive CD4 T cells with aging. J. Exp. Med. 1996;184:1891–1900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverman J. Tolerance and autoimmunity in TCR transgenic mice specific for myelin basic protein. Immunol. Rev. 1999;169:147–159. doi: 10.1111/j.1600-065X.1999.tb01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin W.M., Weissman I.L., Butcher E.C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Bradley L.M., Watson S.R., Swain S.L. Entry of naive CD4 T cells into peripheral lymph nodes requires L- selectin. J. Exp. Med. 1994;180:2401–2406. doi: 10.1084/jem.180.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst D.N., Hobbs M.V., Torbett B.E., Glasebrook A.L., Rehse M.A., Bottomly K., Hayakawa K., Hardy R.R., Weigle W.O. Differences in the expression profiles of CD45RB, Pgp-1, and 3G11 membrane antigens and in the patterns of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J. Immunol. 1990;145:1295–1302. [PubMed] [Google Scholar]
- Weller R.O., Engelhardt B., Phillips M.J. Lymphocyte targeting of the central nervous systema review of afferent and efferent CNS-immune pathways. Brain Pathol. 1996;6:275–288. doi: 10.1111/j.1750-3639.1996.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Cserr H.F., Knopf P.M. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the braina new view. Immunol. Today. 1992;13:507–512. doi: 10.1016/0167-5699(92)90027-5. [DOI] [PubMed] [Google Scholar]
- Seabrook T.J., Johnston M., Hay J.B. Cerebral spinal fluid lymphocytes are part of the normal recirculating lymphocyte pool. J. Neuroimmunol. 1998;91:100–107. doi: 10.1016/s0165-5728(98)00164-7. [DOI] [PubMed] [Google Scholar]
- Lerner A., Yamada T., Miller R.A. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalin A. Eur. J. Immunol. 1989;19:977–982. doi: 10.1002/eji.1830190604. [DOI] [PubMed] [Google Scholar]
- Carson M.J., Reilly C.R., Sutcliffe J.G., Lo D. Disproportionate recruitment of CD8+ T cells into the central nervous system by professional antigen-presenting cells. Am. J. Pathol. 1999;154:481–494. doi: 10.1016/S0002-9440(10)65294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster I., Lieberam I. Peripheral tolerance of CD4 T cells following local activation in adolescent mice. Eur. J. Immunol. 1996;26:3194–3202. doi: 10.1002/eji.1830261253. [DOI] [PubMed] [Google Scholar]
- Kurts C., Heath W.R., Carbone F.R., Allison J., Miller J.F., Kosaka H. Constitutive class I–restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Kosaka H., Carbone F.R., Miller J.F., Heath W.R. Class I–restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Heath W.R., Kosaka H., Miller J.F., Carbone F.R. The peripheral deletion of autoreactive CD8+ T cells induced by cross-presentation of self-antigens involves signaling through CD95 (Fas, Apo- 1) J. Exp. Med. 1998;188:415–420. doi: 10.1084/jem.188.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.J., Kurts C., Kreuwel H.T., Holst K.L., Heath W.R., Sherman L.A. Ontogeny of T cell tolerance to peripherally expressed antigens. Proc. Natl. Acad. Sci. USA. 1999;96:3854–3858. doi: 10.1073/pnas.96.7.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alferink J., Tafuri A., Vestweber D., Hallmann R., Hammerling G.J., Arnold B. Control of neonatal tolerance to tissue antigens by peripheral T cell trafficking. Science. 1998;282:1338–1341. doi: 10.1126/science.282.5392.1338. [DOI] [PubMed] [Google Scholar]
- Irani D.N. The susceptibility of mice to immune-mediated neurologic disease correlates with the degree to which their lymphocytes resist the effects of brain-derived gangliosides. J. Immunol. 1998;161:2746–2752. [PubMed] [Google Scholar]
- Gordon L.B., Nolan S.C., Ksander B.R., Knopf P.M., Harling-Berg C.J. Normal cerebrospinal fluid suppresses the in vitro development of cytotoxic T cellsrole of the brain microenvironment in CNS immune regulation. J. Neuroimmunol. 1998;88:77–84. doi: 10.1016/s0165-5728(98)00077-0. [DOI] [PubMed] [Google Scholar]
- Rellahan B.L., Jones L.A., Kruisbeek A.M., Fry A.M., Matis L.A. In vivo induction of anergy in peripheral V beta 8+ T cells by staphylococcal enterotoxin B. J. Exp. Med. 1990;172:1091–1100. doi: 10.1084/jem.172.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandoola A., Cho E.A., Yui K., Saragovi H.U., Greene M.I., Quill H. Reduced CD3-mediated protein tyrosine phosphorylation in anergic CD4+ and CD8+ T cells. J. Immunol. 1993;151:2355–2367. [PubMed] [Google Scholar]
- Moutschen M., Trebak M., Greimers R., Colombi S., Boniver J. Subset-specific analysis of calcium fluxes in murine AIDS. Int. Immunol. 1996;8:1715–1727. doi: 10.1093/intimm/8.11.1715. [DOI] [PubMed] [Google Scholar]
- Tanchot C., Guillaume S., Delon J., Bourgeois C., Franzke A., Sarukhan A., Trautmann A., Rocha B. Modifications of CD8+ T cell function during in vivo memory or tolerance induction. Immunity. 1998;8:581–590. doi: 10.1016/s1074-7613(00)80563-4. [DOI] [PubMed] [Google Scholar]
- Buer J., Lanoue A., Franzke A., Garcia C., Von Boehmer H., Sarukhan A. Interleukin 10 secretion and impaired effector function of major histocompatibility complex class II–restricted T cells anergized in vivo. J. Exp. Med. 1998;187:177–183. doi: 10.1084/jem.187.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Villagomez D., Wang Y., Lafaille J.J. Regulatory CD4+ T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Keere F., Tonegawa S. CD4+ T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti-myelin basic protein T cell receptor transgenic mice. J. Exp. Med. 1998;188:1875–1882. doi: 10.1084/jem.188.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami R.S., Oldstone M.B.A. Amino acid homology between the encephalitogenic site of myelin basic protein and virusmechanism for autoimmunity. Science. 1985;230:1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- Jahnke U., Fisher E.H., Alvord E.C., Jr. Sequence homology between certain viral proteins and proteins related to encephalomyelitis and neuritis. Science. 1985;229:282–287. doi: 10.1126/science.2409602. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K.W., Strominger J.L. Molecular mimicry in T cell-mediated autoimmunityviral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P.J., Paquette J.S., Ciurli C., Antel J.P., Ouellet F. Myelin basic protein and human coronavirus 229E cross-reactive T cells in multiple sclerosis. Ann. Neurol. 1996;39:233–240. doi: 10.1002/ana.410390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M.B. Molecular mimicry and immune-mediated diseases. FASEB (Fed. Am. Soc. Exp. Biol.) J. 1998;12:1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]