Dynamic Tuning of T Cell Reactivity by Self-Peptide–Major Histocompatibility Complex Ligands (original) (raw)
Abstract
Intrathymic self-peptide–major histocompatibility complex class II (MHC) molecules shape the T cell repertoire through positive and negative selection of immature CD4+CD8+ thymocytes. By analyzing the development of MHC class II–restricted T cell receptor (TCR) transgenic T cells under conditions in which the endogenous peptide repertoire is altered, we show that self-peptide–MHC complexes are also involved in setting T cell activation thresholds. This occurs through changes in the expression level of molecules on thymocytes that influence the sensitivity of TCR signaling. Our results suggest that the endogenous peptide repertoire modulates T cell responsiveness in the thymus in order to enforce tolerance to self-antigens.
Keywords: T cell antigen receptors, thymus, tolerance, transgenic mice, CD5
Introduction
The formation of a useful T cell repertoire that can respond to foreign antigens while remaining nonresponsive to self begins in the thymus where immature T cell precursors expressing unique antigen receptors are screened for their ability to interact with self-MHC ligands. Thymocytes that express a TCR capable of engaging self-MHC molecules with low avidity are positively selected to form the peripheral T cell compartment, while those with TCRs that bind to self-MHC ligands too strongly are deleted to prevent the maturation of autoreactive clones 1. The composition of self-peptides bound to intrathymic MHC molecules heavily influences the selection process 2. Fetal thymic organ culture systems using MHC class I–restricted TCR transgenic thymocytes have shown that positive selection of CD8+ T cells is facilitated by specific class I–binding peptides 3 4. Moreover, various mutant and transgenic strains of mice engineered to express a severely limited array of self-peptides on MHC class II exhibit defects in producing a diverse CD4+ T cell repertoire 5 6 7. These experiments indicate that the signals that drive positive selection of a given thymocyte are delivered by specific self-peptides bound to thymic MHC molecules.
Once these signals are received, immature CD4+CD8+ double positive (DP) thymocytes undergo a variety of phenotypic and functional changes. These include upregulation of the surface expression of the TCR and activation markers such as CD69 and CD5 8 9 10 11. Other maturational changes induced in positively selected thymocytes include downregulation of the recombination activating gene (RAG) products involved in TCR gene rearrangement 12 13 14 15 16 17 and commitment to either the CD4+ or CD8+ single positive (SP) lineage. Furthermore, changes in the signaling properties of the TCR occur such that mature T cells become less sensitive to TCR stimulation 18 19.
Studies of the changes that occur during thymocyte maturation have primarily focused, to date, on the events surrounding positive selection by specific intrathymic self-peptides, with the presumption that nonselecting peptides are ignored and play no role in development. Because a wide range of affinities theoretically exists for TCR–ligand interactions in the thymus, we reasoned that for a given TCR there should exist self-peptides that fall just below the threshold for positive selection that may still deliver signals that can induce alterations in thymocyte phenotype or function. Since thymocytes may be more sensitive to TCR stimulation during the early stages of positive selection, they may more readily perceive weak signals from such peptides. Moreover, recent studies indicate that T cell maturation may involve multiple TCR engagements with intrathymic ligands that each induce incremental changes in thymocyte phenotype 20 21 22 23 24. We hypothesized that at least some of these signals may come from TCR interaction with a broad range of intrathymic peptides that include those outside the window of avidity for positive selection.
In this study, we tested this hypothesis by analyzing the phenotype and function of TCR transgenic thymocytes developing in the presence of altered repertoires of MHC class II–bound peptides. We present evidence for signals distinct from those that induce complete positive selection that can be perceived through the TCR of immature thymocytes. These signals are not sufficient for promoting full development to the mature SP stage but are able to alter the expression of molecules such as CD5 and CD2 that are normally associated with positive selection, and increase the signaling threshold through the TCR. The expression levels of these molecules are directly influenced by the endogenous peptide diversity. Our results suggest that immature αβ TCR+ thymocytes perceive a broader range of thymic self-peptide–MHC complexes than previously thought and adjust the signaling properties of the TCR in response to the self-peptide repertoire. This may be important for enforcing the peptide specificity of T cell responses and maintaining tolerance to the self-peptide repertoire.
Materials and Methods
Animals.
6–8-wk-old female C57BL/6J (B6, H-2b) and invariant chain–deficient B6 mice were purchased from The Jackson Laboratory and maintained at the animal facility at the University of Washington. RAG-deficient mice (H-2b) were purchased from Taconic Farms. H-2M–deficient mice (H-2b) were provided by Luc Van Kaer (Vanderbilt University, Nashville, TN). TCli TCR transgenic mice were generated as described previously 25. Triple peptide transgenic mice were generated using constructs described elsewhere 7 26. In brief, purified human invariant chain (Ii)-peptide DNA constructs were coinjected into (B6 × DBA/2) F1 × Ii−/− embryos. Germline transmission of constructs was verified by PCR amplification of tail DNA from the progeny of transgenic founders using primers specific for each transgene. Triple peptide mice were further bred to homozygous Ii−/−, Ii−/− H-2M−/−, or RAG−/− backgrounds. TCliRAG−/− Ii−/− 3p+ mice were generated by crossing TCliRAG−/− Ii−/− mice to triple peptide mice on a RAG−/− Ii−/− background. Mutant and transgenic mice were bred and maintained under specific pathogen-free conditions at the animal facility at the University of Washington.
Radiation Bone Marrow Chimeras.
Bone marrow cells collected from the femurs and tibias of TCR transgenic mice were washed several times in serum-free RPMI 1640 and 5 × 106 cells were injected into the tail vein of each lethally irradiated (950 rads) recipient. Chimeras were given antibiotic-containing water and analyzed 6–10 wk after bone marrow transfer.
Abs and Peptides.
The following mAbs directed to mouse cell surface antigens were purchased from BD PharMingen: anti-CD4–PE (RM4-5); anti-CD4–peridinin chlorophyll protein (PerCP) (RM4-5); anti-CD8a–FITC (53-6.7); anti-CD8a–allophycocyanin (APC) (53-6.7); anti-TCR-β–PE (H57-597); anti-TCR-β–PE (H57-597); anti-Vβ6–PE (RR4-7); anti-Vα2–biotin (B20.1); anti-CD69–FITC (H1.2F3); anti-CD5–FITC (53-7.3); anti-CD2–biotin (RM2-5); anti-CD28–biotin (37.51); anti-CD44–biotin (IM7); anti-L-selectin–biotin (MEL-14); anti-LFA-1–FITC (2D7); anti-CD45RB–FITC (16A); and anti-Thy-1.1–FITC (HIS51). Anti-CD3 (145-2C11) mAb was provided by Dr. M. Bevan (University of Washington, Seattle, WA). The following peptide–MHC class II complex–specific mAbs were used: YAe (Eα52–68:I-Ab [27, 28]); Y3P (I-Ab, HB183; American Type Culture Collection); 15G4 (murine class II–associated Ii peptide [CLIP]:I-Ab [29; unpublished observations]); A8 (CD22:I-Ab); and H10 (Rab5:I-Ab; unpublished observations). All peptides were synthesized with a Synergy 432 (Applied Biosystems) automated peptide synthesizer using Fmoc chemistry and analyzed by reverse-phase HPLC. The purity of peptides used was >90%.
Flow Cytometry.
For four-color analyses, ∼5 × 105 cells were incubated on ice for 30 min with PE–, PerCP–, APC–, and biotin–conjugated Abs, washed in PBS containing 1% FCS (Life Technologies) and 0.1% sodium azide, and incubated with fluorescein-avidin D (Vector Laboratories) for an additional 30 min on ice. Labeled cells were washed and analyzed on a FACScan™ or FACScalibur™ flow cytometer (Becton Dickinson) using CELLQuest™ and ReproMac software.
T Cell Assays.
To detect expression of specific peptides on splenocytes from various mouse strains, we used T cell hybridomas specific for I-Eα52–68, IgM377–392, β2-microgobulin (β2m)48–58, CD2225–39, and Rab586–101 peptides presented on I-Ab 30. Titrated numbers of splenocytes were incubated with 105 T cell hybrids for 18–20 h. IL-2 production was measured with the IL-2–dependent HT-2 cell line in a colorimetric Alamar blue assay.
Calcium Flux.
Freshly isolated thymocytes were resuspended at 107 cells per milliliter in media (DMEM/3% FCS) containing 5 μg of Indo-1-AM (Sigma-Aldrich) and incubated for 1 h at 37°C. Light exposure was minimized to prevent photobleaching. Cells were then washed and surface-labeled for flow cytometry with anti-CD4–PE and anti-CD8–FITC mAbs. Labeled thymocytes were washed and resuspended in warm media at 2 × 106 cells/ml. Antigen-presenting cells were B6 splenocytes incubated with various concentrations of peptide at 37°C for 3 h, washed, and resuspended in warm media at 2 × 106 cells/ml. For TCR stimulation, 106 labeled thymocytes were gently mixed with 106 APC and the sample was applied to a FACS Vantage™ (Becton Dickinson) for 45 s to measure the basal intracellular concentration. The sample was then centrifuged for 8 s at 16,000 g to maximize cell contacts, reapplied to the machine, and read for a further 7 min. Changes in intracellular calcium were assessed over time as the ratio of calcium-bound (FL5) to calcium-unbound (FL4) Indo-1 fluorescence. Calcium flux data for gated CD4+ CD8+ thymocytes was analyzed using FlowJo (Tree Star) software.
Thymocyte Deletion Assay.
Total thymocytes from the indicated strains of TCli mice were incubated overnight at 37°C in the presence of B6 LPS blasts cultured with varying doses of human CLIP (hCLIP) peptide. After 18 h, cells were washed and stained with anti-CD4–PerCP, anti-CD8–APC, anti-Vβ6–PE, and anti-CD69–FITC for four-color flow cytometric analysis of thymocyte coreceptor dulling as described above. After drawing a tight-live gate, viable DP thymocytes remaining were calculated as a percent of the starting proportion of CD4hiCD8hi cells in the indicated DP gate.
Results
Generation of Mice Expressing Limited Self-Peptide Repertoires.
First we wished to determine whether immature thymocytes are capable of perceiving signals from nonselecting intrathymic self-peptide–MHC complexes by analyzing hCLIP/I-Ab–specific TCli TCR transgenic thymocytes developing in the presence of a wild-type versus altered repertoire of self-peptides that precluded positive selection. We showed previously that TCli CD4+ T cells are selected relatively poorly, apparently due to competition among TCli thymocytes for selecting peptide–MHC class II complexes that are available in limited abundance in the thymus 25. Nevertheless, we noticed that most DP thymocytes in TCli mice have uniformly upregulated the CD69 and CD5 markers associated with positive selection (data not shown), a phenomenon which has been observed in other TCR transgenic mice 11. The upregulated expression of these molecules on TCli DP thymocytes occurs only in the presence of diverse MHC class II–bound peptides on a wild-type selecting background, but not on the nonselecting Ii-null (Ii−/−) background. However, these markers do not reflect commitment of DP thymocytes to the CD4 SP lineage as assessed by termination of CD8 protein synthesis and downregulation of RAG expression (data not shown). Therefore, it is possible that upregulated CD69 and CD5 expression on TCli DP thymocytes arises from TCR engagement with self-peptides that do not promote positive selection.
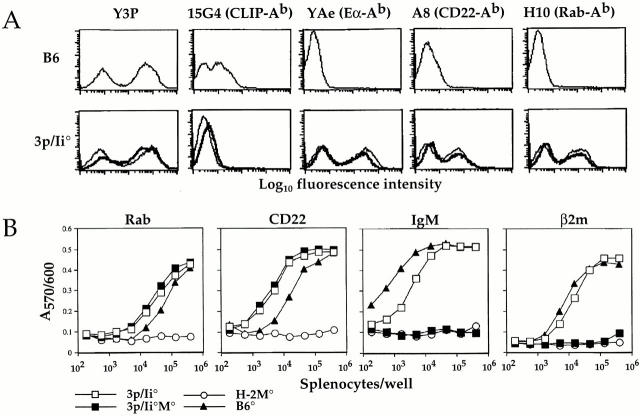
To test this hypothesis, we analyzed the development of TCli thymocytes in thymic environments that displayed skewed repertoires of self-peptides yet maintained normal levels of class II expression. Previously, we reported a strategy for generating mice with different arrays of self-peptides on class II molecules that are expressed at a wild-type level 7. This involved reconstitution of murine Ii-deficient (Ii−/−) mice and mice doubly deficient for both Ii and H-2M (Ii−/−M−/−) with a human genomic Ii transgene construct containing an embedded high affinity I-Ab–binding peptide in place of the CLIP region that associates with class II. We have generated three such constructs, each containing a different, naturally occurring I-Ab peptide (Eα52–68, CD2225–39, and Rab586–101; unpublished observations). For this study, we created mice that overexpress all three peptides by coinjection of the three constructs into fertilized zygotes. The triple peptide mice were bred to the Ii−/− (3p/Ii−/−) or Ii−/−M−/− (3p/Ii−/−M−/−) background. Both 3p/Ii−/− and 3p/Ii−/−M−/− mice exhibit wild-type levels of class II expression and similarly high expression of all three transgene-encoded peptides as detected by complex-specific Abs and T cell assays using endogenous peptide-specific T cell hybrids (Fig. 1). A small fraction of the class II molecules in 3p/Ii−/− mice do not display the transgene-encoded peptides but present a diverse set of endogenous peptides such as those derived from IgM and β2m (Fig. 1 B). However, when the triple peptide transgenes are expressed on the Ii−/− M−/− background, the diversity of such “background” peptides appears to be reduced, as reflected by our inability to detect the IgM and β2m peptides by specific T cell hybrids (Fig. 1 B). Therefore, these mice provide thymic environments with peptide repertoires that are limited to predominant expression of three peptides combined with low levels of a presumably more or less diverse set of other endogenous peptides.
Figure 1.
Splenocytes from 3p/Ii−/− and 3p/Ii−/−M−/− triple peptide transgenic mice present a limited repertoire of endogenous peptides. (A) High surface expression of I-Ab–bound Eα, CD22, and Rab5-derived peptides as indicated by flow cytometric analysis of splenocytes using peptide:I-Ab complex–specific Abs. Splenocytes from wild-type B6 (top), 3p/Ii−/− (bottom, thin lines), and 3p/Ii−/−M−/− (bottom, thick lines) mice were stained with Y3P (I-Ab–specific), 15G4 (mouse CLIP:I-Ab–specific), YAe (Eα:I-Ab–specific), A8 (CD22:I-Ab–specific), or H10 (Rab5:I-Ab–specific) Ab to assess surface class II/peptide expression. (B) 3p/Ii−/−M−/− splenocytes exhibit a further reduced endogenous peptide repertoire relative to 3p/Ii−/− splenocytes. Titrated numbers of splenocytes from B6 (▴), H-2M−/− (○), 3p/Ii−/− (□), and 3p/Ii−/−M−/− (▪) mice were used to stimulate T cell hybridomas specific for Rab5, CD22, IgM, or β2m peptides. IL-2 production by T cells was assessed by screening assay supernatants with the Alamar Blue colorimetric assay using the IL-2–dependent HT-2 cell line and measuring the OD570/600 absorbance.
Intrathymic Self-Peptides Induce Altered Expression Levels of Accessory Molecules on DP Thymocytes Independent of Positive Selection.
First we constructed bone marrow chimeras to determine whether upregulation of CD69 and CD5 on TCR transgenic DP cells and maturation into CD4 SP thymocytes could be mediated by either the major or minor peptides expressed in 3p/Ii−/− and 3p/Ii−/−M−/− mice. Bone marrow from RAG-deficient TCli mice (TCliRAG−/−) was transferred into lethally irradiated wild-type B6, 3p/Ii−/−, and 3p/Ii−/−M−/− hosts. As predicted, we found that TCli→B6 chimeras exhibit normal positive selection of TCli CD4+ T cells and upregulated expression of CD69 and CD5 on DP thymocytes similar to that seen in unmanipulated TCli mice (Fig. 2 A, and data not shown). In contrast, positive selection of TCli T cells is completely blocked at the DP stage of development in both 3p/Ii−/− and 3p/Ii−/−M−/− hosts. However, interestingly, the DP thymocytes in these mice present a range of CD69 and CD5 expression (Fig. 2 B). The level of upregulation of these markers on the TCli thymocytes appears to parallel the endogenous peptide diversity expected in the different hosts. TCli DP thymocytes developing in wild-type B6 mice express the highest levels of CD69 and CD5 and exhibit progressively lower expression of these markers in 3p/Ii−/− and 3p/Ii−/−M−/− mice. DP thymocytes from TCli→3p/Ii−/−M−/− chimeras exhibit the lowest levels of these markers comparable to those seen on DP cells of wild-type mice and Ii-deficient TCli mice (data not shown). These results demonstrate that increased expression of CD69 and CD5 on DP thymocytes can occur in the absence of positive selection and the relative levels of these markers appear to be influenced by the diversity of endogenous thymic peptide–MHC ligands. Since a wide array of different self-peptides appears to be necessary for maximal expression of these molecules, distinct peptides may induce different levels of CD69/CD5 upregulation depending on the avidity of the TCR interaction with these ligands.
Figure 2.
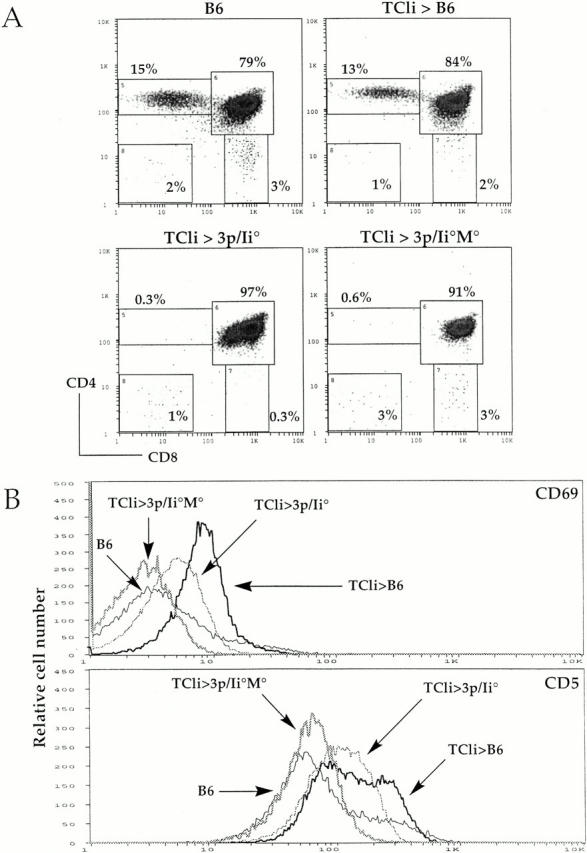
Upregulation of CD69 and CD5 in TCli DP thymocytes can occur in the absence of positive selection. (A) CD4/CD8 flow cytometry density plots for B6 mice, TCliRAG−/−→B6, TCliRAG−/−→3p/Ii−/−, and TCliRAG−/−→3p/Ii−/−M−/− bone marrow chimeras, with percentages of cells falling into each gate indicated. (B) Flow cytometry histograms of CD69 and CD5 expression on gated DP thymocytes from the indicated mice. Three chimeras of each type were analyzed yielding similar FACS® profiles.
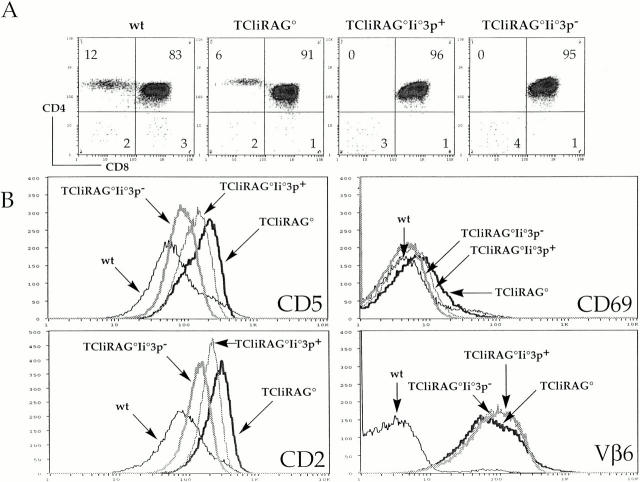
It was possible that the presentation of a wild-type, diverse set of self-peptides on bone marrow–derived antigen-presenting cells might play a role in the upregulation of CD69 and CD5 seen in the DP thymocytes of the TCli→3p/Ii−/− chimeras. Although the graded expression of these markers in the different chimeras makes it unlikely, TCR engagement with the selecting ligand on bone marrow–derived cells rather than on thymic epithelial cells may upregulate CD69 and CD5 without promoting positive selection. To exclude this possibility, we bred 3p/Ii−/− mice to TCliRAG−/− mice to generate TCliRAG−/−Ii−/−3p+ mice and analyzed T cell development in these animals. We also examined TCliRAG2−/−Ii−/−3p− littermates as controls in these experiments. As seen in the TCli→3p/Ii−/− chimeras, positive selection of CD4+ T cells is abrogated in TCliRAG−/−Ii−/−3p+ mice and TCliRAG−/−Ii−/−3p− littermates (Fig. 3 A). Nevertheless, CD69 and CD5 are upregulated in TCliRAG−/−Ii−/−3p+ DP thymocytes to a level that is intermediate between that in TCliRAG−/− and TCliRAG−/−Ii−/−3p− DP thymocytes (Fig. 3 B). Increased expression of these markers is less dramatic than seen in the chimeras, but the overall pattern remains the same. Increased expression of CD2 and LFA-1 was also observed in TCliRAG−/−Ii−/−3p+ DP thymocytes, whereas expression levels of the transgenic TCR (Vβ6) remained constant on selecting and nonselecting backgrounds (Fig. 3 B, and data not shown). Other molecules such as CD28, CD44, and L-selectin were upregulated only in TCli DP thymocytes on a normal selecting background, but not in TCliRAG−/−Ii−/−3p+ or TCliRAG−/−Ii−/−3p− DP thymocytes (data not shown). These results show that, despite the lack of positive selection, DP thymocytes can receive signals from thymic self-peptides that alter the surface expression levels of several T cell accessory molecules. The magnitude of upregulation of these molecules is proportional to the endogenous peptide diversity.
Figure 3.
DP thymocytes in TCliRAG−/−Ii−/−3p+ mice are not positively selected but exhibit increased expression of CD5, CD2, and CD69. (A) CD4/CD8 flow cytometry density plots for wild-type B6, TCliRAG−/−, TCliRAG−/−Ii−/−3p+, and TCliRAG−/− Ii−/−3p− mice, with percentages of cells falling into each quadrant indicated. (B) Flow cytometry histogram overlays of CD5, CD2, CD69, and TCR Vβ6 expression on gated DP thymocytes from the indicated mice. Two mice of each strain were analyzed yielding similar FACS® profiles. Thymic cellularity for all mice analyzed was in the normal range expected (1–3 × 108).
MHC-dependent Changes in Cell Surface Marker Phenotype in Preselection DP Thymocytes from Wild-Type Mice.
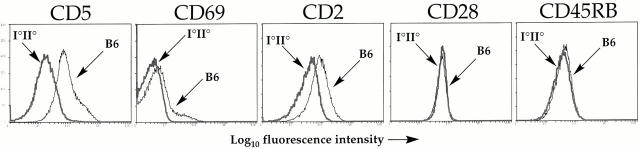
Next we determined whether similar ligand-dependent upregulation of the same molecules could be observed on normal polyclonal DP thymocytes in which the majority of cells are not positively selected. DP thymocytes from B6 mice do indeed exhibit higher expression of CD69, CD5, and CD2 than DP thymocytes in MHC class I and class II double-deficient (I−/−II−/−) mice, whereas expression of other molecules such as CD28 and CD45RB are identical (Fig. 4). This result demonstrates that all immature DP thymocytes from normal mice can perceive signals from MHC ligands that upregulate several accessory molecules before positive selection. Although it is unclear whether this is dependent on specific peptides, the results with the TCli TCR suggest that specific peptides are involved in this upregulation.
Figure 4.
The majority of polyclonal DP thymocytes from normal mice have received MHC-dependent signals that upregulate surface levels of specific accessory molecules independently of positive selection. Shown are histogram overlays of expression of the indicated molecules on DP thymocytes from wild-type B6 versus MHC class I and class II double-deficient (I−/−II−/−) mice.
Surface CD5 and CD2 Expression Levels Correlate with Thymocyte Sensitivity to Antigen.
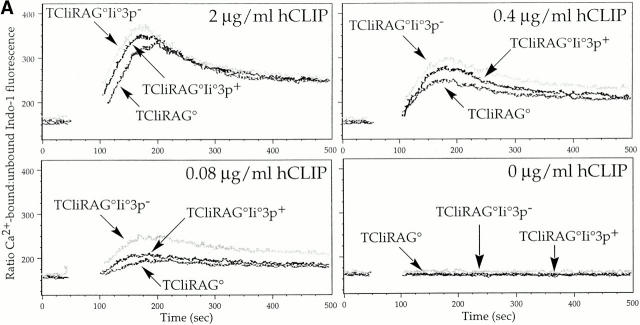
Studies of CD5 function in thymocytes have suggested that it plays a role in dampening signals delivered through the TCR, as thymocytes from CD5−/− mice are hyperresponsive to TCR stimulation and the selection of T cells in such mice is altered in a manner consistent with enhanced TCR signaling 31. CD5 function is believed to be related to its constitutive association with the phosphotyrosine phosphatase src homology 2 domain–bearing protein tyrosine phosphatase 1 (SHP-1) through an immunoreceptor tyrosine-based inhibitory–like motif (ITIM), which may negatively regulate phosphorylation of substrates involved in the TCR signaling pathway 32. Similarly, certain strains of TCR transgenic mice deficient in CD2 exhibit increased positive selection, suggesting that CD2 may also negatively regulate TCR signaling in thymocytes 33. Therefore, we tested whether the increased levels of CD5 and CD2 observed on TCliRAG−/− and TCliRAG−/−Ii−/−3p+ DP thymocytes correlated with decreased sensitivity to TCR stimulation. To characterize the responsiveness of DP thymocytes to TCR stimulation, we assessed the magnitude of calcium flux in thymocytes upon stimulation with peptide-pulsed syngeneic splenocytes. TCliRAG−/−, TCliRAG−/−Ii−/− 3p+, and TCliRAG−/−Ii−/−3p− thymocytes expressing high, intermediate, and low levels of CD5 at the DP stage, respectively, were loaded with the Ca2+-binding fluorescent dye Indo-1, stimulated with B6 splenocytes, pulsed with titrated amounts of the cognate hCLIP peptide, and analyzed by FACS® for the degree of Ca2+ flux expressed as the ratio of Ca2+-bound to unbound Indo-1. It should be noted that despite the difference in CD5 and CD2 expression, TCR levels are similar between DP thymocytes from all three strains of mice (Fig. 3 B). In agreement with our prediction, we found that DP thymocytes from TCliRAG−/−Ii−/− mice show the greatest Ca2+ flux, followed by DP thymocytes from TCliRAG−/−Ii−/−3p+ mice, and finally TCliRAG−/− DP thymocytes (Fig. 5 A). At lower peptide concentrations, the extent of flux is decreased for all DP populations, yet the difference in magnitude between the DP thymocytes remains obvious (Fig. 5 A).
Figure 5.
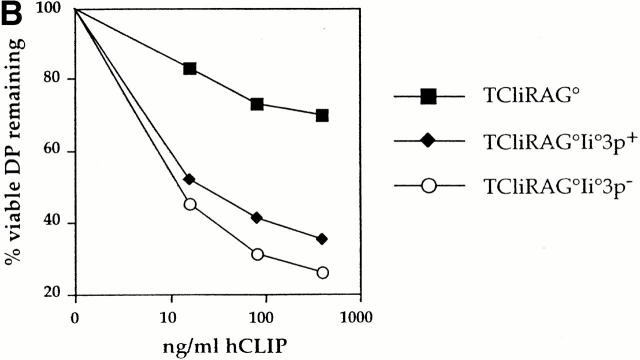
TCR sensitivity to cognate ligand is inversely proportional to CD5/CD2 expression on DP thymocytes. (A) TCli DP thymocytes developing in the presence of reduced self-peptide diversity flux calcium greater than TCli DP thymocytes from a wild-type selecting background. The ratio of calcium-bound to calcium-free Indo-1 is shown as the FL5/FL4 fluorescence ratio after Indo-loaded thymocytes from TCliRAG−/−, TCliRAG−/−Ii−/−3p+, or TCliRAG−/−Ii−/−3p− mice were stimulated with syngeneic wild-type splenocytes pulsed with various concentrations of hCLIP. Breaks in the traces correspond to the time during which thymocytes and antigen-presenting cells were centrifuged together to form conjugates before resumed analysis on the flow cytometer. (B) TCli DP thymocytes developing in the presence of reduced self-peptide diversity are more sensitive to in vitro deletion by cognate peptide. Thymocytes from TCliRAG−/−, TCliRAG−/−Ii−/−3p+, or TCliRAG−/−Ii−/−3p− mice were cultured overnight with B6 LPS blasts presenting varying doses of hCLIP and analyzed for coreceptor dulling by flow cytometry as a measure of deletion. Data are presented as the percentage of starting proportional of viable coreceptorhi DP thymocytes remaining after culture with the indicated doses of peptides.
We also examined the sensitivity of TCli DP thymocytes to deletion by the cognate ligand in vitro. In this assay, thymocytes from TCliRAG−/−, TCliRAG−/−Ii−/−3p+, and TCliRAG−/−Ii−/−3p− mice were cultured overnight with varying doses of hCLIP presented by normal B6 splenic LPS blasts and assessed for the extent of CD4/CD8 coreceptor dulling by flow cytometry as a measure of deletion. The results are shown as the percentage of the starting proportion of viable coreceptorhi DP thymocytes remaining at the different peptide concentrations. In agreement with the calcium flux data, we observed that TCliRAG−/− DP thymocytes, which expressed the highest levels of CD5 and CD2, show the greatest resistance to in vitro deletion (Fig. 5 B). In contrast, TCliRAG−/−Ii−/−3p+ and TCliRAG−/− Ii−/−3p− DP thymocytes, which express lower levels of CD5 and CD2, exhibit significant coreceptor dulling even at the lowest concentration of antigen. Consistent with their higher expression levels of CD5 and CD2, TCliRAG−/−Ii−/−3p+ DP thymocytes are slightly less sensitive to deletion than TCliRAG−/−Ii−/−3p− DP thymocytes. Taken together with the calcium flux data, these results indicate that the sensitivity of immature DP thymocytes to TCR stimulation is modulated by the makeup of the self-peptide repertoire and appears to be inversely proportional to the expression levels of CD5 and CD2.
Discussion
Thymic self-peptide–MHC complexes play a critical role in positive and negative selection of developing T cells. It has been traditionally viewed that TCR engagement with specific endogenous peptides in the thymus will lead to one or the other of these events. However, what has not been clear is whether thymocyte interactions with self-peptides that are below the avidity threshold for positive and negative selection have any significant biological effects. Since a continuum of TCR–ligand avidities are possible with a diverse self-peptide repertoire, it can be readily envisioned that thymocytes may encounter peptide–MHC complexes that fall just outside the window of positive selection. Also, previous studies have suggested that positive selection may involve multiple TCR engagements with intrathymic ligands, but it has not been clear whether the same ligands or different ones are associated with each “hit.”
In this study, we have demonstrated for the first time that immature DP thymocytes in both normal and TCR transgenic animals can perceive signals delivered by thymic self-peptide–MHC ligands in vivo that appear to fall below the threshold for positive selection. These signals are sufficient to induce alterations in the expression of several accessory molecules that can regulate TCR signaling. We also show that DP thymocytes maturing in thymi harboring distinct self-peptide repertoires exhibit differential TCR sensitivity to antigen in a manner that correlates with the altered expression of negative regulatory molecules such as CD5 and CD2 in the various backgrounds analyzed.
It has been shown that CD5 expression can be regulated by the avidity of the selecting ligand interaction as a means of fine tuning the TCR signaling response in developing T cells 11. Our findings reveal that, in addition to the selecting ligand(s), a broader spectrum of self-peptides than previously thought is able to interact with the TCR of immature thymocytes at the DP stage of T cell development and cause alterations in thymocyte surface phenotype and TCR sensitivity. TCR engagement with these intrathymic peptide–MHC ligands results in a signal that induces cells to upregulate not just CD5, but also CD69, CD2, and perhaps other accessory molecules, but does not promote lineage commitment. Importantly, since greater upregulation of these molecules is seen on more diverse peptide–MHC backgrounds, the avidity with which these peptides engage the TCR likely influences the extent of alteration in surface marker expression levels that occurs. In our model, full positive selection to the SP lineage would require TCR engagement with an even more specific subset of self-peptides that may have higher affinity for the TCR and induce higher levels of CD69, CD5, and CD2 expression. Obviously, peptides with affinities higher than that allowed for positive selection would induce deletion. Thus, distinct sets of peptides on cortical thymic epithelial cells can be sensed by developing thymocytes and induce variable maturational changes in these cells. In this way, TCR sensitivity can be adjusted via a rheostat-like mechanism whereby self-peptides variably tune the levels of TCR signaling modifying molecules such as CD5 and CD2.
It may be argued that quantitative differences in signaling delivered by the positively selecting ligand(s) may induce distinct outcomes (e.g., alteration in cell surface phenotype versus commitment to the SP lineage). For example, the CD69/CD5/CD2 upregulation on DP thymocytes in the absence of positive selection seen in the TCli→3p/Ii−/− chimeras and TCliRAG−/−Ii−/−3p+ mice may simply reflect TCR interaction with decreased amounts of the selecting ligand still present in these mice that would normally induce complete maturation at a higher density. Although this is a valid explanation, it seems unlikely because the uniform upregulation of these markers observed suggests that most transgenic DP thymocytes have received a TCR signal. If the upregulation resulted from TCR interaction with low levels of the selecting ligand, we would instead expect to see a bimodal distribution of expression of these molecules, reflecting competition for low levels of the putative ligand. Our results instead suggest that the ligands responsible for the CD69/CD5/CD2 upregulation are expressed fairly abundantly.
It is also highly unlikely that the increased expression of CD69, CD5, and CD2 on DP thymocytes in the absence of lineage commitment is due to negative selection occurring in TCliRAG−/−Ii−/−3p+ mice. In deletion assays, we consistently observe much higher elevation of CD69 on TCli DP thymocytes upon addition of the cognate peptide than observed on DP thymocytes of TCliRAG−/−Ii−/−3p+ mice or TCli→3p/Ii−/− chimeras (data not shown).
The peptide-specific upregulation of CD5 and CD2 on DP thymocytes appears related to decreased TCR sensitivity, since we find that DP thymocytes expressing higher levels of CD5/CD2 exhibit decreased reactivity to cognate ligand compared with those bearing lower levels of CD5/CD2. Although this evidence is correlative, it agrees with reports suggesting that CD5 and CD2 negatively regulate TCR signaling 31 32 33 34. We speculate that CD5/CD2 upregulation in thymocytes in response to intrathymic peptide ligands serves to modulate TCR responsiveness by raising the activation threshold such that emerging T cells are not overtly reactive to the self-peptide repertoire. Such a mechanism may explain the decreased sensitivity of thymocytes upon maturation observed by several investigators 18 19 35. In those reports, T cell maturation from the DP to SP lineage was shown to be accompanied by reduced sensitivity to weak agonist ligands but not to strong agonists, whereas we observe less responsiveness to the cognate agonist ligand in DP thymocytes that develop in the presence of an increased diversity of self-peptides. However, the comparisons being made are different since those studies do not examine DP thymocytes on nonselecting versus selecting environments. Nevertheless, the dissimilar results may perhaps be due to the different TCRs analyzed and the different assays of TCR sensitivity employed. In those studies, TCR responsiveness was primarily measured by CD69 upregulation, while we assessed reactivity by calcium flux and in vitro deletion assays.
A theoretic model of dynamic tuning of activation thresholds during thymocyte development has been elegantly described previously 36, and a role for CD5 in this process is supported by the finding that CD5 expression levels on mature T cells parallel the signaling intensity or avidity of the positively selecting TCR-peptide–MHC interaction 11. However, it has not been previously reported that TCR interactions with peptides other than those involved in positive or negative selection are capable of modulating expression of CD5 expression. We show that an apparently broad range of peptides may interact with the TCR of developing DP thymocytes and modify the expression of not only CD5, but also CD2, CD69, and likely many other molecules that regulate TCR responsiveness. Because we can show that these molecules are upregulated proportionally to the self-peptide diversity, our results support an analogue process of TCR tuning in which the expression of various accessory molecules is variably regulated, rather than switched on or off. Thus, depending on the strength of the TCR–peptide interactions encountered in the thymus, CD5 and CD2 expression on immature thymocytes may be upregulated to different extents in order to establish a baseline of responsiveness that precludes reactivity to self-ligands. The thymic environment exhibiting greater self-peptide diversity promotes higher CD5 expression since it would presumably include peptide–MHC complexes that can engage the TCR with higher avidity.
Importantly, this mechanism would also progressively limit the number of peptides from which the developing thymocyte may perceive signals, resulting in gradually increasing peptide specificity for the final stages of positive selection. Increasing desensitization of thymocytes as a consequence of multiple TCR engagement with low avidity ligands leading to CD5 upregulation may progressively increase the affinity thresholds that define positive and negative selection. One intriguing possibility that this notion raises is that the selecting peptides involved in the late steps of development may differ for a given TCR depending on its previous history of encounters with intrathymic peptide ligands. The increased TCR signaling thresholds set during thymocyte maturation results in the generation of peptide-specific, rather than peptide-promiscuous, T cells.
Our data suggest that, through modulation of regulatory molecules upon TCR interaction with the continuum of thymic self-peptides, T cells establish cellular activation thresholds during maturation in the thymus to prevent reactivity to self. However, the self-peptide repertoire in the periphery may not mirror that of the thymus, especially in light of data showing that the expression of cathepsins regulating antigen processing are differentially regulated in thymic epithelial cells versus bone marrow–derived antigen-presenting cells 37. Thus, T cells may require further tuning of TCR sensitivity to the self-peptide repertoire in the periphery. We have preliminary data showing that several T cell accessory molecules such as CD2, CD5, and CD28 are altered in splenic CD4+ T cells compared with thymic CD4 SP thymocytes, suggesting that the adjustment of TCR sensitivity may continue in mature T cells in the periphery. We also found that adoptive transfer of TCli CD4+ T cells into hosts expressing altered sets of self-peptides results in changes in CD5 expression. This suggests that TCR tuning through alterations in signaling regulatory molecules may not be fixed in the thymus but may be a continuous process throughout the lifetime of the T cell. Further experiments should determine whether this hypothesis is valid.
In conclusion, we have shown that αβ TCR+ immature thymocytes can sense signals from intrathymic self-peptide–MHC complexes that initiate alterations in surface phenotype and TCR sensitivity independently of positive selection. This is likely to be a critical process by which developing T cells are tuned to ignore self-peptides. This scenario is appealing since it ensures that T cell reactivity is adjusted according to the makeup of the self-peptide repertoire such that all cross-reacting self-peptides that may potentially be activating ligands become inert ligands. The outcome of this process is a T cell pool that is tolerant to the self-peptide repertoire while remaining poised to deal with foreign antigen.
Acknowledgments
The authors would like to thank Cara Plata and Deb Wilson for their excellent animal care; Sue Eastman and Marcela Gomez for their excellent technical assistance; Sally Clarke for help in construction of bone marrow chimeras; Ann Norment, Steve Levin, and Kathy Allen for advice in calcium flux experiments; and Eugene Huang for advice in thymocyte suspension deletion assays.
This work was supported by grants from the Howard Hughes Medical Institute and the National Institutes of Health (A.Y. Rudensky) and by the Howard Hughes Predoctoral Fellowship in Biological Sciences (P. Wong).
Footnotes
Abbreviations used in this paper: APC, allophycocyanin; β2m, β2-microglobulin; CLIP, class II–associated Ii peptide; DP, double positive; Ii, invariant chain; PerCP, peridinin chlorophyll protein; RAG, recombination activating gene; SP, single positive.
References
- Alam S.M., Travers P.J., Wung J.L., Nasholds W., Redpath S., Jameson S.C., Gascoigne N.R. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- Jameson S.C., Bevan M.J. T-cell selection. Curr. Opin. Immunol. 1998;10:214–219. doi: 10.1016/s0952-7915(98)80251-3. [DOI] [PubMed] [Google Scholar]
- Hogquist K.A., Jameson S.C., Heath W.R., Howard J.L., Bevan M.J., Carbone F.R. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Ashton-Rickardt P.G., Bandeira A., Delaney J.R., Van Kaer L., Pircher H.P., Zinkernagel R.M., Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- Sant'Angelo D.B., Waterbury P.G., Cohen B.E., Martin W.D., Van Kaer L., Hayday A.C., Janeway C.A., Jr. The imprint of intrathymic self-peptides on the mature T cell receptor repertoire. Immunity. 1997;7:517–524. doi: 10.1016/s1074-7613(00)80373-8. [DOI] [PubMed] [Google Scholar]
- Surh C.D., Lee D.S., Fung-Leung W.P., Karlsson L., Sprent J. Thymic selection by a single MHC/peptide ligand produces a semidiverse repertoire of CD4+ T cells. Immunity. 1997;7:209–219. doi: 10.1016/s1074-7613(00)80524-5. [DOI] [PubMed] [Google Scholar]
- Barton G.M., Rudensky A.Y. Requirement for diverse, low-abundance peptides in positive selection of T cells. Science. 1999;283:67–70. doi: 10.1126/science.283.5398.67. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Matzinger P., Seder R.A., Paul W.E., Schwartz R.H. Activation events during thymic selection. J. Exp. Med. 1992;175:731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita I., Nagata T., Tada T., Nakayama T. CD69 cell surface expression identifies developing thymocytes which audition for T cell antigen receptor-mediated positive selection. Int. Immunol. 1993;5:1139–1150. doi: 10.1093/intimm/5.9.1139. [DOI] [PubMed] [Google Scholar]
- Swat W., Dessing M., von Boehmer H., Kisielow P. CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur. J. Immunol. 1993;23:739–746. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- Azzam H.S., Grinberg A., Lui K., Shen H., Shores E.W., Love P.E. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turka L.A., Schatz D.G., Oettinger M.A., Chun J.J., Gorka C., Lee K., McCormack W.T., Thompson C.B. Thymocyte expression of RAG-1 and RAG-2termination by T cell receptor cross-linking. Science. 1991;253:778–781. doi: 10.1126/science.1831564. [DOI] [PubMed] [Google Scholar]
- Borgulya P., Kishi H., Uematsu Y., von Boehmer H. Exclusion and inclusion of α and β T cell receptor alleles. Cell. 1992;69:529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- Brandle D., Muller C., Rulicke T., Hengartner H., Pircher H. Engagement of the T-cell receptor during positive selection in the thymus down-regulates RAG-1 expression. Proc. Natl. Acad. Sci. USA. 1992;89:9529–9533. doi: 10.1073/pnas.89.20.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandle D., Muller S., Muller C., Hengartner H., Pircher H. Regulation of RAG-1 and CD69 expression in the thymus during positive and negative selection. Eur. J. Immunol. 1994;24:145–151. doi: 10.1002/eji.1830240122. [DOI] [PubMed] [Google Scholar]
- Kouskoff V., Vonesch J.L., Benoist C., Mathis D. The influence of positive selection on RAG expression in thymocytes. Eur. J. Immunol. 1995;25:54–58. doi: 10.1002/eji.1830250111. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M., Graf D., Lovatt M., Bommhardt U., Zamoyska R., Fisher A.G. How many thymocytes audition for selection? J. Exp. Med. 1997;186:1149–1158. doi: 10.1084/jem.186.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey G.M., Schober S.L., Endrizzi B.T., Dutcher A.K., Jameson S.C., Hogquist K.A. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J. Exp. Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas B., Stefanova I., Yasutomo K., Dautigny N., Germain R.N. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10:367–376. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- Anderson G., Hare K.J., Jenkinson E.J. Positive selection of thymocytesthe long and winding road. Immunol. Today. 1999;20:463–468. doi: 10.1016/s0167-5699(99)01524-8. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Miazek A. Positive selection of T cellsrescue from programmed cell death and differentiation require continual engagement of the T cell receptor. J. Exp. Med. 1995;181:1975–1984. doi: 10.1084/jem.181.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandoola A., Cibotti R., Punt J.A., Granger L., Adams A.J., Sharrow S.O., Singer A. Positive selection as a developmental progression initiated by αβ TCR signals that fix TCR specificity prior to lineage commitment. Immunity. 1999;10:301–311. doi: 10.1016/s1074-7613(00)80030-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson R.W., Anderson G., Owen J.J., Jenkinson E.J. Positive selection of thymocytes involves sustained interactions with the thymic microenvironment. J. Immunol. 1995;155:5234–5240. [PubMed] [Google Scholar]
- Williams O., Tarazona R., Wack A., Harker N., Roderick K., Kioussis D. Interactions with multiple peptide ligands determine the fate of developing thymocytes. Proc. Natl. Acad. Sci. USA. 1998;95:5706–5711. doi: 10.1073/pnas.95.10.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P., Goldrath A.W., Rudensky A.Y. Competition for specific intrathymic ligands limits positive selection in a TCR transgenic model of CD4+ T cell development. J. Immunol. 2000;164:6252–6259. doi: 10.4049/jimmunol.164.12.6252. [DOI] [PubMed] [Google Scholar]
- Barton G.M., Rudensky A.Y. An altered invariant chain protein with an antigenic peptide in place of CLIP forms SDS-stable complexes with class II αβ dimers and facilitates highly efficient peptide loading. Int. Immunol. 1998;10:1159–1165. doi: 10.1093/intimm/10.8.1159. [DOI] [PubMed] [Google Scholar]
- Murphy D.B., Lo D., Rath S., Brinster R.L., Flavell R.A., Slanetz A., Janeway C.A., Jr. A novel MHC class II epitope expressed in thymic medulla but not cortex. Nature. 1989;338:765–768. doi: 10.1038/338765a0. [DOI] [PubMed] [Google Scholar]
- Murphy D.B., Rath S., Pizzo E., Rudensky A.Y., George A., Larson J.K., Janeway C.A., Jr. Monoclonal antibody detection of a major self peptide. MHC class II complex. J. Immunol. 1992;148:3483–3491. [PubMed] [Google Scholar]
- Liljedahl M., Winqvist O., Surh C.D., Wong P., Ngo K., Teyton L., Peterson P.A., Brunmark A., Rudensky A.Y., Fung Leung W.P., Karlsson L. Altered antigen presentation in mice lacking H2-O. Immunity. 1998;8:233–243. doi: 10.1016/s1074-7613(00)80475-6. [DOI] [PubMed] [Google Scholar]
- Kovats S., Grubin C.E., Eastman S., deRoos P., Dongre A., Van Kaer L., Rudensky A.Y. Invariant chain–independent function of H-2M in the formation of endogenous peptide–major histocompatibility complex class II complexes in vivo. J. Exp. Med. 1998;187:245–251. doi: 10.1084/jem.187.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarakhovsky A., Kanner S.B., Hombach J., Ledbetter J.A., Muller W., Killeen N., Rajewsky K. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- Perez-Villar J.J., Whitney G.S., Bowen M.A., Hewgill D.H., Aruffo A.A., Kanner S.B. CD5 negatively regulates the T-cell antigen receptor signal transduction pathwayinvolvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol. Cell. Biol. 1999;19:2903–2912. doi: 10.1128/mcb.19.4.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh S.J., Killeen N., Tarakhovsky A., Littman D.R., Teh H.S. CD2 regulates the positive selection and function of antigen-specific CD4−CD8+ T cells. Blood. 1997;89:1308–1318. [PubMed] [Google Scholar]
- Pena-Rossi C., Zuckerman L.A., Strong J., Kwan J., Ferris W., Chan S., Tarakhovsky A., Beyers A.D., Killeen N. Negative regulation of CD4 lineage development and responses by CD5. J. Immunol. 1999;163:6494–6501. [PubMed] [Google Scholar]
- Sebzda E., Kundig T.M., Thomson C.T., Aoki K., Mak S.Y., Mayer J.P., Zamborelli T., Nathenson S.G., Ohashi P.S. Mature T cell reactivity altered by peptide agonist that induces positive selection. J. Exp. Med. 1996;183:1093–1104. doi: 10.1084/jem.183.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Z., Singer A. Tuning of activation thresholds explains flexibility in the selection and development of T cells in the thymus. Proc. Natl. Acad. Sci. USA. 1996;93:14747–14752. doi: 10.1073/pnas.93.25.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Roth W., Wong P., Nelson A., Farr A., Deussing J., Villadangos J.A., Ploegh H., Peters C., Rudensky A.Y. Cathepsin Lcritical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280:450–453. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]