Fatal Leukemia in Interleukin 15 Transgenic Mice Follows Early Expansions in Natural Killer and Memory Phenotype Cd8+ T Cells (original) (raw)
Abstract
Inflammation likely has a role in the early genesis of certain malignancies. Interleukin (IL)-15, a proinflammatory cytokine and growth factor, is required for lymphocyte homeostasis. Intriguingly, the expression of IL-15 protein is tightly controlled by multiple posttranscriptional mechanisms. Here, we engineered a transgenic mouse to overexpress IL-15 by eliminating these posttranscriptional checkpoints. IL-15 transgenic mice have early expansions in natural killer (NK) and CD8+ T lymphocytes. Later, these mice develop fatal lymphocytic leukemia with a T-NK phenotype. These data provide novel evidence that leukemia, like certain other cancers, can arise as the result of chronic stimulation by a proinflammatory cytokine.
Keywords: interleukin 15, leukemia, transgenic mice, lymphocytes, inflammation
Introduction
IL-15 is a pleiotropic cytokine that is important for both innate and adaptive immune cell homeostasis, as well as peripheral immune function 1. IL-15 shares the common γ chain (γc) and IL-2/15Rβ with IL-2 for signaling, but also utilizes a private IL-15Rα subunit for high affinity binding 2 3 4. Numerous in vitro and in vivo studies have documented a critical role for IL-15 in the development, survival, and function of the NK cell lineage 5 6 7 8 9 10 11 12. Further, IL-15 is required for the normal expansion and/or survival of nonclassical T cells and memory phenotype TCR-α/β CD8+ T cells, while not being essential for their development 11 12 13. Additional studies have documented a role for IL-15 in peripheral immune functions such as T lymphocyte trafficking 14, innate immune IFN-γ production 15, and host defense against infectious pathogens 1. These studies are consistent with the broad expression of IL-15 and IL-15Rα by multiple cell types and tissues, suggesting that this ligand/receptor may mediate a wide range of functions in vivo 2 4. Indeed, the phenotypes of mice deficient in IL-15/IL-15Rα 11 12, compared with mice deficient in IL-2/IL-2Rα 16 17 18, definitively demonstrate a large variety of unique in vivo functions mediated by IL-15 19.
Despite an abundance of transcript in multiple tissues and cell types, IL-15 is poorly translated and secreted. Three primary posttranscriptional checkpoints are responsible for this observation: multiple AUGs in the 5′ UTR 2 20, inefficient long signal peptides (LSPs) and short signal peptides (SSPs; references 21 and 22), and a negative regulator near the COOH terminus of the precursor proteins 22. Through the systematic elimination of these three checkpoints, the synthesis of bioactive human IL-15 protein increased 250-fold in vitro 23. Such tight posttranscriptional control of the IL-15 gene product is unusual for most cytokines thus far characterized, suggesting that constitutively abundant IL-15 protein may somehow be deleterious to the host.
Recently, a connection between chronic inflammatory processes and the genesis of cancer has been appreciated 24. Inflammation resulting from persistent infections has been linked to malignancies, including Helicobacter pylori and gastric carcinoma, schistosomiasis, and bladder cancer, as well as hepatitis C virus and hepatocellular carcinoma 24. The increased production of macrophage migration inhibitory factor (MIF) during H. pylori infection, a molecule that downregulates the p53 tumor suppressor gene during inflammation, provided one direct mechanism whereby the proinflammatory state may result in susceptibility to transforming genetic mutation 25. Further, individuals with polymorphisms at the IL-1β gene, resulting in increased expression of proinflammatory IL-1β during H. pylori infection, have a higher risk of developing gastric cancers 26.
Leukemia is a complex, heterogeneous disorder with multiple molecular etiologies 27. However, direct evidence that mediators of inflammation can, via alterations in proliferation or survival, contribute to leukemogenesis is lacking. We hypothesized that deregulation of IL-15 gene expression, resulting in alterations in lymphocyte homeostasis, could promote malignant transformation in lymphocytes. In the current report, we tested these hypotheses in vivo by engineering transgenic mice that lack posttranscriptional control of IL-15 gene expression, thereby efficiently translating and secreting murine IL-15 protein. These IL-15 transgenic (IL-15tg) mice have early expansions of peripheral blood lymphocytes, specifically NK cells and memory phenotype CD8+ T cells. Later, IL-15tg mice develop a striking leukemic expansion, some composed primarily of clonal CD3+TCR-α/β+ T cells, along with progressive alopecia, multiorgan lymphocytic infiltrates, and premature death, not unlike the leukemia of large granular lymphocytes that occurs in patients 28.
Materials and Methods
Reagents.
The following mAbs reactive with murine cells were purchased from BD PharMingen: CD2 (RM2-5), CD3 (145-2C11), CD4 (RM4-5), CD5 (53-7.3), CD8a (53-6.7), CD8b2 (53-5.8), CD19 (1D3), CD25 (7D4), CD44 (IM7), CD69 (H1.2F3), CD122 (TM-β1), CD62L (MEL-14), Ly6C (AL-21), DX5 (DX5), NK1.1 (PK136), Ly49D (4E5), B220 (RA3-6B2), Mac-1 (M1/70), TCR-β (H57-597), TCR-γ/δ (GL3), Vβ2 (B20.6), Vβ11 (RR3-15), and IFN-γ (XMG1.2), and used as direct conjugates to PE, FITC, or peridinin chlorophyll protein (PerCP). The following mAb were purchased from BD PharMingen and used as isotype controls: hamster IgG (G235-2356), mouse IgG2b/k (49.2), rat IgG2a/k (R35-95), rat IgG2b/k (A95-1), and rat IgM/k (R4-22), and used as direct conjugates to PE, FITC, or PerCP. For immunohistochemical analysis, CD3 (145-2C11) mAb and anti–hamster IgG horseradish peroxidase–conjugated secondary reagents were used (BD PharMingen). For Western analysis the anti–FLAG M2 and M5 (Sigma-Aldrich) and probe 8 (Santa Cruz Biotechnology, Inc.) Abs were used. The following cytokines were provided: recombinant murine (rm)IL-15 (Immunex), recombinant human (rh)IL-2 (Hoffman LaRoche), and rmIL-12 (Genetics Institute).
IL-15 Transgene Design and Construction.
The IL-15 transgene was designed to eliminate posttranscriptional checkpoints thereby optimizing for the overexpression of an efficiently translated and secreted murine IL-15 mature protein (see Fig. 1). The transgene was cloned using standard DNA cloning techniques as follows. The murine mIL-2 signal peptide coding sequence (nucleotides 49–108; sequence data are available from GenBank/EMBL/DDBJ under accession no. K02292) was amplified from the pmut-1 plasmid (American Type Culture Collection) with forward primer (5′-GGCATGTACAGCATGCAGCTCG-3′) and a reverse primer engineered with a NarI restriction site (underlined) (5′-ATCGGCGCCTGCGCTGTTGACAAGGAGCAC-3′). The murine IL-15 cDNA encoding the mature protein (nucleotides 610–951; sequence data are available from GenBank/EMBL/DDBJ under accession no. U14332) was PCR amplified from a full-length mIL-15 cDNA with a forward primer engineered with a NarI restriction site (5′-GATGGCGCCAACTGGATAGATGTAAGATATG-3′) and a reverse primer (5′-GATCGGATCCCTA_TTTGTCATCGTCGTCCTTGTA-GTC_GGACGTGTTGATGAA-3′) engineered with a BamHI restriction site (underlined) and FLAG epitope tag (italics). Both PCR products were TA cloned into the PCR2.1 vector (Invitrogen) and accuracy confirmed by sequencing (ABI 377XL sequencer). The mIL-2SP coding sequence was subcloned into pBluescript II SK (Stratagene) using an EcoRI site. Next, the NarI/BamHI fragment of mIL-15 mature protein sequence was ligated into this construct. The NarI restriction sites used to ligate the mIL-2SP and mIL-15 mature protein ensured maintenance of the proper open reading frame. We next ligated out of frame the BamHI/NotI fragment of the human growth hormone (hGH) gene 29 into the IL-2SP/IL-15 construct, downstream of the IL-15 cDNA. Then the XhoI/HindIII fragment of mouse MHC class I promoter (Dd; references 30 and 31) from pGEM4z-2.7 was ligated into the multiple cloning site of pGEM7zf (Promega). Finally, the resultant XhoI/SalI fragment of Dd promoter was ligated into the construct, upstream of the IL-2 signal peptide, resulting in the final transgenic construct. A sequential digest of the construct with XhoI and NotI, which releases the linearized 5.2-kb transgene from pBluescript, was used for microinjections.
Figure 1.
Structure of the IL-15 transgene. Three primary posttranscriptional checkpoints were eliminated: 5′ AUGs, the inefficient IL-15 signal peptide, and a COOH terminus retention sequence. Near global overexpression was achieved by the MHC class I promoter, efficient translation and secretion by use of the IL-2 signal peptide, and stabilization of COOH terminus by the addition of the FLAG epitope. The 3′ portion of the hGH gene is fused out of frame for straightforward identification of the transgene by Southern blot and to optimize transgene expression in vivo.
Generation of IL-15tg Mice.
The microinjection fragment was isolated from the vector in a Tris-acetic acid-EDTA (TAE)-buffered 1% agarose gel slice. DNA was purified from the agarose using the Qiaex II purification kit (QIAGEN) and eluted in an injection buffer consisting of 10 mM Tris-HCl pH 7.4 and 0.1 mM EDTA. An aliquot of the 5.2-kb microinjection fragment was used to determine the DNA concentration by direct comparison with the High DNA Mass Ladder (Life Technologies) on an ethidium bromide–stained gel. DNA was injected into a single pronucleus of FVB/N embryos 32 at a concentration of 2 ng/μl. Injected embryos were transferred to the oviducts of pseudopregnant ICR foster mice. Potential transgenic mice were screened by isolating genomic DNA 33 from tail biopsies and testing for transgenic sequences by Southern hybridization (see below).
Southern Analysis.
High molecular weight mouse DNA was isolated from diced tail clips using a proteinase K, phenol/chloroform extraction as described 34. For Southern analysis, 10 μg of genomic DNA was digested to completion with SstI and fractionated on a 1% agarose gel followed by alkaline transfer to a positively charged nylon membrane. DNA blots were hybridized with a 32P-labeled 600-bp probe directed against hGH sequence found in the transgene (see Fig. 1) and analyzed using a Storm 860 PhosphorImager and ImageQuant software (Molecular Dynamics). The expected size of SstI-digested transgene band is 2.6 kb, the unit length fragment obtained from multicopy tandem transgene insertion.
Real Time Quantitative Reverse Transcription PCR.
Mouse tissues were snap-frozen before RNA isolation and reverse transcription (RT) as described 15. IL-15 transgene mRNA transcripts were quantified by the dual-labeled fluorogenic probe method, using a Prism 7700 thermal cycler and sequence detector (PerkinElmer/ABI). Primers used were: IL-15 transgene forward, 5′-CGACGATGACAAATAGGGATCC-3′, reverse, 5′-GACGTCCGGGAGCCTGTA-3′, probe, 5′-FAM AACTCCCCGAACCACTCAGGGTCCT TAMRA-3′; 18S rRNA forward, 5′-CGGCTACCACATCCAAGGAA-3′, reverse, 5′-GCTGGAATTACCGCGGCT-3′, probe, 5′-VIC TGCTGGCACCAGACTTGCCCTC TAMRA-3′. In parallel with experimental samples, standard curves for the IL-15 transgene and 18S rRNA (reference control) of known concentration were quantitated, and absolute copy numbers were calculated. Final quantitation is reported as the absolute copy number of IL-15 transgene transcripts per 106 18S rRNA copies.
Western Blot Analysis.
Splenocyte lysates were loaded directly onto 8–16% gradient gels (Tris-HCL; Bio-Rad Laboratories). Recombinant FLAG protein was used as a positive control (Sigma-Aldrich). Proteins were electrophoresed under denaturing conditions and electroblotted to nitrocellulose membranes at 100 V for 1 h at 4°C. Membranes were blocked overnight with 5% nonfat dry milk in TBS plus 0.5% Tween 20 (TBST) and then incubated for 1.5 h with rabbit anti-FLAG Ab (Santa Cruz Biotechnology, Inc.) diluted 1:400 in TBST plus 2% nonfat dry milk. Membranes were washed with TBST and incubated for 1 h with horseradish peroxidase–conjugated donkey anti–rabbit IgG (Amersham Pharmacia Biotech) secondary Ab diluted 1:3,000 in TBST. Proteins were detected using enhanced chemiluminescence reagents (ECL Plus and ECL hyperfilm; Amersham Pharmacia Biotech).
Murine IL-15 ELISA.
96-well plates were coated with soluble murine IL-15Rα as a primary capture reagent 35. Polyclonal rabbit anti–mouse IL-15 antiserum 15 was used as a secondary detection reagent (Immunex). rmIL-15 (Immunex) was serially diluted for the standard curve, and the assay sensitivity was between 9 and 40 pg/ml.
Tissues and Blood.
For autopsy, mice were anesthetized, killed by cervical dislocation, and weighed. Peritoneal lavage was performed with 10 ml ice cold PBS. Tissues were removed, examined grossly, and processed for RNA or protein isolation (snap frozen in LN2), histology (fixed in 10% neutral buffered formalin), and immunohistochemistry (frozen in OCT medium). Body weight and selected organ weights were determined, and relative body weight ratios were calculated. Spleen, thymus, and lymph nodes were disrupted, RBC lysed, and strained through 70-μm nylon mesh to obtain single cell leukocyte suspensions. Liver leukocytes were isolated after collagenase digestion of liver homogenate and density centrifugation over Lympholyte M (Cedarlane). Bone marrow mononuclear cells were isolated from two femurs by flushing with ice cold PBS. Peripheral blood was harvested from the tail or retro-orbital plexus, and blood smears were prepared. Whole blood was RBC lysed and all leukocyte suspensions were enumerated electronically (Z1 cell counter; Coulter) and manually (hemacytometer) in a blinded fashion.
Histopathology.
Fixed tissues were dehydrated with ethanol, transferred to xylene, and embedded in paraffin using standard histology techniques, and 3-μm sections were cut and stained with hematoxylin and eosin. Peripheral blood smears were fixed in ethanol and Wright/Giemsa stained. All histology samples were reviewed by a pathologist (J. Durbin).
Analysis of NK Cell Function.
Fresh peripheral blood leukocytes were used as effectors, and YAC-1 tumor cells as targets, in a standard 51Cr release assay. For NK cell IFN-γ production, murine leukocytes were costimulated with IL-12 (10 ng/ml) and IL-15 (1 ng/ml) for 48 h, and cell culture supernatants were then harvested and assayed for IFN-γ protein by ELISA 15.
Flow Cytometry.
Staining of leukocyte suspensions with fluorochrome-conjugated mAb reacting with cell surface antigens and intracellular IFN-γ was performed as described 36. Forward scatter, side scatter, and fluorescence data were collected on a Coulter XL flow cytometer (Beckman Coulter) and analyzed with the WinMDI software program (Joseph Trotter, Scripps Research Institute, La Jolla, CA). Nonreactive isotype control staining of identical cells was used to set quadrant gates with ≥99% of cells located in the negative quadrant. In flow cytometric histograms, fluorescence data is shown with tic marks in log10 increments.
DNA PCR Assessment of TCR-β Clonality.
Dβ to Jβ rearrangements were analyzed in high molecular weight genomic DNA isolated from fresh peripheral mouse leukocytes using a proteinase K, phenol/chloroform extraction as described 34. PCR reactions (50 μl) contained 100 ng genomic DNA template, 3 pmol of each primer, 0.2 mM of each dNTP, 2 mM MgCl2, and 1 U Taq DNA polymerase in 1X PCR buffer as supplied (PerkinElmer). Reactions were run on a 9700 thermocycler (PerkinElmer) under the following conditions: 3 min at 94°C; 32 cycles of 45 s at 94°C, 90 s at 65°C, and 150 s at 72°C; and 10 min at 72°C. PCR primers spanning the Dβ-Jβ region were used as published 37. Populations that appeared to have clonal or oligoclonal Dβ-Jβ rearrangement were further analyzed by the above method for Vβ usage. Primers located within each of the Vβ regions and the reverse Jβ primers were used as published 37. Ethidium bromide–stained PCR products were run on a 1% agarose gel and visualized under ultraviolet light. To verify that single Vβ-Jβ bands were indeed indicative of a single TCR, PCR products were cloned and at least four different clones from each product sequenced. In all cases, identical sequences were obtained from such Vβ-Jβ products.
Statistical Analysis.
Experimental groups were compared by the Student's t test with P < 0.05 considered significant.
Results
Generation of Transgenic Mice that Overexpress an Efficiently Translated and Secreted IL-15 Mature Protein.
Previous reports have identified major posttranscriptional regulatory mechanisms that control human IL-15 translation and secretion 5 22 23. We constructed a modified murine IL-15 cDNA that lacked the three primary posttranscriptional checkpoints controlling wild-type IL-15, thereby optimizing for IL-15 overexpression (Fig. 1). Modifications to the cDNA included removing upstream AUGs that impeded translation, replacing the inefficiently translated and secreted endogenous IL-15 signal peptides with the IL-2 signal peptide, and stabilizing the COOH terminus of the mature protein with a FLAG epitope tag. Near global overexpression of this modified IL-15 cDNA was driven by the MHC class I Dd promoter 30, and the 3′ portion of the hGH gene was spliced downstream and out of frame to maximize transcription, translation, and processing of the transgene in vivo 29. Transfection of COS-7 cells with an expression plasmid containing the modified IL-15 cDNA resulted in secretion of bioactive murine IL-15 protein (data not shown), confirming that these modifications resulted in efficient IL-15 protein translation and secretion.
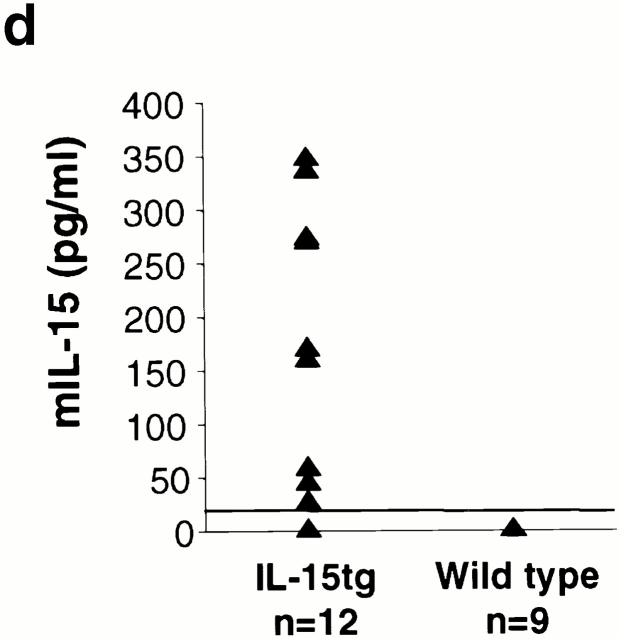
Transgenic mice were created by microinjection of the IL-15 transgene into pronuclear stage FVB/N embryos. Three IL-15tg lines were identified by Southern blot analysis of genomic DNA with a hGH cDNA probe (Fig. 2 a). Although all three IL-15tg lines demonstrate a similar phenotype, the severity varied based upon transgene expression at the transcript level. Here, we report the phenotype of one representative IL-15tg line (3304 in Fig. 2 a). IL-15tg mice developed normally as neonates, but grossly manifested progressive alopecia beginning at 5–6 wk of age (see below). The transgene transcript, now efficiently translated and secreted, was quantified and found to be abundantly expressed in multiple tissues from IL-15tg mice (Fig. 2 b), in a similar pattern as the endogenous IL-15 transcript 2 that is poorly translated and secreted. The transgenic protein was detected by immunoblot analysis of the FLAG epitope and was present in multiple tissues (Fig. 2 c). In addition, IL-15tg mice had measurable serum levels of murine IL-15 (Fig. 2 d). IL-15 protein was detected (mean ± SEM, 186.7 ± 41.8 pg/ml) in the serum of 9 of 12 IL-15tg mice tested. In all (n = 9) wild-type, nontransgenic mice, IL-15 protein was undetectable using this ELISA. Thus, IL-15tg mice express measurable serum levels of IL-15 protein at 6–20 wk of age, whereas wild-type mice do not.
Figure 2.

Detection and expression of the IL-15 transgene. (a) Three FVB/N IL-15tg lines positive by Southern blot analysis with hGH gene probe (3284, 3285, 3304), while negative line (3286) is shown for comparison. Triangle denotes the expected 2.6-kb size of the SstI digested transgene. (b) Real time RT-PCR of tissues from a representative IL-15tg mouse. Results show the mean ± SEM of triplicate measurements of IL-15 transgene expression from total cellular RNA, isolated from IL-15tg tissues. Sm. Int., small intestine; Lg. Int., large intestine; Sk. Musc., skeletal muscle; BM, bone marrow. (c) Immunoblot analysis of splenocyte lysates for transgenic protein with the FLAG epitope tag. Equal amounts of total cellular protein were loaded as follows: lane 1, spleen cells from nontransgenic wild-type FVB mouse; and lanes 2–4, spleen cells from three different IL-15tg mice. (d) IL-15 protein levels in IL-15tg mice. Serum from IL-15tg mice and nontransgenic wild-type controls were analyzed using a specific murine IL-15 ELISA (see Materials and Methods).
Early Lymphocytosis in IL-15tg Mice.
Previous studies have documented a role for IL-15 in the homeostasis of several lymphocyte subsets 1. We therefore examined the peripheral blood of IL-15tg (n = 71) and nontransgenic littermates controls (n = 51) at 6 wk of age. The IL-15tg mice exhibited a significant increase in leukocyte number (31,694 ± 3,267/μl blood), compared with controls (7,983 ± 503/μl blood, P < 10−7; Fig. 3 a). The lymphocyte counts in IL-15tg mice (21,712 ± 2,718/μl blood) were significantly higher compared with controls (3,648 ± 375/μl blood, P < 10−8), demonstrating that the lymphocytosis in the IL-15tg mice was responsible for the elevated leukocyte numbers (Fig. 3 b). Examination of peripheral blood smears from IL-15tg mice confirmed the expansion of small and large granular lymphocytes (Fig. 3c and Fig. d). This lymphocytosis was observed in IL-15tg mice as early as 3 wk of age.
Figure 3.



Early lymphocytosis in IL-15tg mice. (a and b) Total white blood cell (WBC) and lymphocyte counts in IL-15tg (n = 71) and nontransgenic littermate control (n = 51) at 6 wk of age. Graphs show the mean ± SEM, with a significant increase in both the absolute white blood cell (*, P < 10−7) and lymphocyte counts (**, P < 10−8) in IL-15tg mice. (c and d) Representative photomicrographs at low (40×) and high (100×) magnification of peripheral blood smears from a 6-wk-old IL-15tg mouse (IL-15tg) and a nontransgenic littermate control (WT Control). Note expansion of large granular lymphocytes in IL-15tg mouse smear.
Early Expansion in NK Cell Number and Function in IL-15tg Mice.
IL-15, acting through the IL-15R complex, has a requisite role in NK cell lineage development 11 12, and provision of exogenous IL-15 expands murine NK cells in vivo 12 38. Immunophenotyping the peripheral blood of IL-15tg mice revealed that the major population of expanded lymphocytes was DX5+CD3− NK cells (Fig. 4 a). In IL-15tg mice, both the percentage (46 ± 1.5% vs. 4 ± 0.2%, P < 10−12) and absolute number (10,161 ± 1,220/μl blood vs. 142 ± 15/μl blood, P < 10-11) of NK cells were consistently and significantly increased, compared with nontransgenic littermate controls (Fig. 4 b). Further, those DX5+CD3− cells coexpressed the Ly49D NK receptor at a frequency comparable to wild-type NK cells (Fig. 4 a).
Figure 4.

Early expansions in NK cell number and function within IL-15tg mice. (a) Flow cytometric analysis of peripheral blood lymphocytes from representative IL-15tg and nontransgenic littermate wild-type controls (WT). NK cells are DX5+CD3−Ly49+/−, whereas T cells are CD3+DX5−Ly49D−. (b) IL-15tg mice (n = 71) have a significant increase in the absolute NK cell number, compared with nontransgenic littermate controls (n = 51; *, P < 10−11). Data shown are the mean ± SEM. (c) Cytotoxicity of fresh leukocytes isolated from IL-15tg or nontransgenic wild-type littermate controls (WT) against YAC-1 tumor targets, without any additional in vitro or in vivo activation. Data shown are the mean ± SEM of triplicate wells from three representative IL-15tg and two wild-type control mice.
To confirm the expanded DX5+CD3− lymphocytes were functionally NK cells, we examined the natural cytotoxicity of fresh peripheral blood leukocytes from IL-15tg mice and non-transgenic controls for their ability to lyse YAC-1 target cells. Leukocytes from IL-15tg mice efficiently lysed YAC-1 targets at relatively low effector/target cell ratios (Fig. 4 c), consistent with the increased percentage of NK cells in IL-15tg mice. Of note, fresh leukocytes from IL-15tg mice were able to efficiently lyse YAC-1 tumor targets without additional in vivo or in vitro activation. As a further indicator of NK cell function, leukocytes from IL-15tg mice were tested for their ability to secrete IFN-γ after monokine costimulation. Abundant IFN-γ protein was measured after 48 h incubation of IL-15tg lymphocytes in IL-12 plus IL-15 (data not shown), similar to that reported for mature NK cells 15. Thus, at 6 wk of age, the peripheral blood of IL-15tg mice contains a major population of functional DX5+CD3−Ly49+/− NK cells.
Early Expansion of Memory Phenotype CD8+ T Cells in IL-15tg Mice.
IL-15 has also been shown to be critical for memory phenotype CD8+ T cell expansion and/or survival in mice 11 12 13 39. Analysis of the T cell compartment revealed that the percentage of TCR-β+CD3+ cells present in the peripheral blood of IL-15tg mice was reduced, due to the NK cell expansion (Fig. 5 a). However, the normal ratio of CD4/CD8 T cells (3.26 ± 0.1) in nontransgenic controls was dramatically inverted within IL-15tg mice (0.59 ± 0.04, P < 10−32). This inversion was due to an expansion of CD8+ T cells (10-fold, P < 10−4), as the absolute number of CD4+ T cells was identical between IL-15tg and nontransgenic littermate controls (Fig. 5b and Fig. c). Further phenotypic analysis of these CD8+ T cells showed that they are CD44hiCD62LloCD69−Ly6Chi, consistent with a memory phenotype (Fig. 6).
Figure 5.
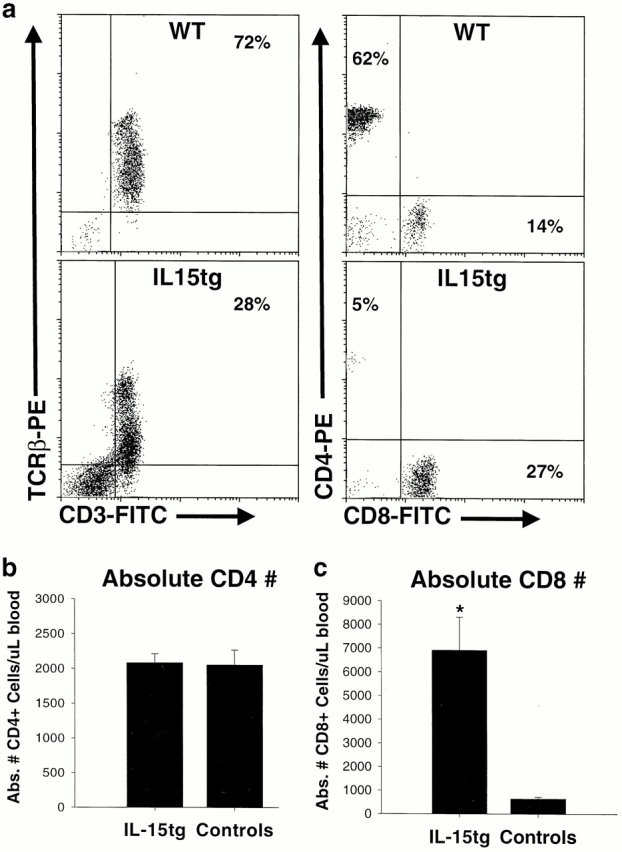
Early expansion of CD8+ T cells within IL-15tg mice. (a) Flow cytometric analysis of peripheral blood lymphocytes from representative IL-15tg and nontransgenic littermate wild-type controls (WT). Most lymphocytes in wild-type mice are CD3+TCR-β+ T cells, and the percentage of this population is reduced in IL-15tg mice due to dilution by the expanded NK cells. The CD4/CD8 ratio is significantly inverted in IL-15tg mice. (b) The absolute number of CD4+ T cells is identical in IL-15tg and control mice. (c) The absolute number of CD8+ T cells is significantly increased in IL-15tg (P < 10−4), compared with control mice. This increase in CD8+ T cells is responsible for the CD4/CD8 ratio. For b and c, data represent the mean CD4 or CD8 counts ± SEM of IL-15tg (n ± 71) and control (n = 51) mice.
Figure 6.
Memory phenotype of expanded CD8+ T cells. Flow cytometric analysis of peripheral blood lymphocytes from a representative IL-15tg mouse at 6 wk of age, demonstrating the CD44hiLy6C+CD69−CD62Llo phenotype, with a nontransgenic littermate control shown for comparison. Similar results were obtained in all IL-15tg mice examined (n = 10). WT, wild-type.
IL-15tg Mice Develop Fatal Lymphocytic Leukemia with Age.
Between 12 and 30 wk of age, most IL-15tg mice develop a cluster of signs including decreased activity, weight loss, and difficulty breathing. These signs progress for 3–7 d when the mice become acutely moribund and are killed (average 19.6 ± 1.1 wk of age). In evaluable IL-15tg mice, a massive elevation in peripheral blood leukocyte number, consisting mainly of lymphocytes, was observed (mean 186,582 ± 32,433/ul blood, range 47,000–606,000/ul blood, n = 22). Immunophenotyping revealed that although DX5+CD3− NK cells remained elevated, the major lymphocyte population was consistently CD3+ TCR-β+DX5hi/lo/neg T cells. Representative flow cytometric analyses of the blood from several IL-15tg mice that developed such massive lymphocyte outgrowth are shown in Fig. 7 a. The expanded CD3+ T cells and NK cells were observed in multiple lymphoid tissues, including blood, spleen, and bone marrow (Fig. 7 b). Flow cytometric analyses of expanded lymphocyte populations isolated from 22 different IL-15tg mice revealed a consistent phenotype of CD3+TCR-β+CD2+CD5+CD44+CD8+/− DX5hi/lo/neg. In two additional cases, CD3−TCR-β−CD8−CD4−DX5+ lymphocyte populations were expanded, likely representing a murine NK cell leukemia (data not shown), and in no case was CD4 expression observed. Concurrently, these mice demonstrated massive splenomegaly (Fig. 7 c) with grossly enlarged livers. Analysis of peripheral blood smears confirmed the dramatic lymphocyte expansion, some of which had striking blast-like morphological features and prominent nucleoli (Fig. 7 d).
Figure 7.
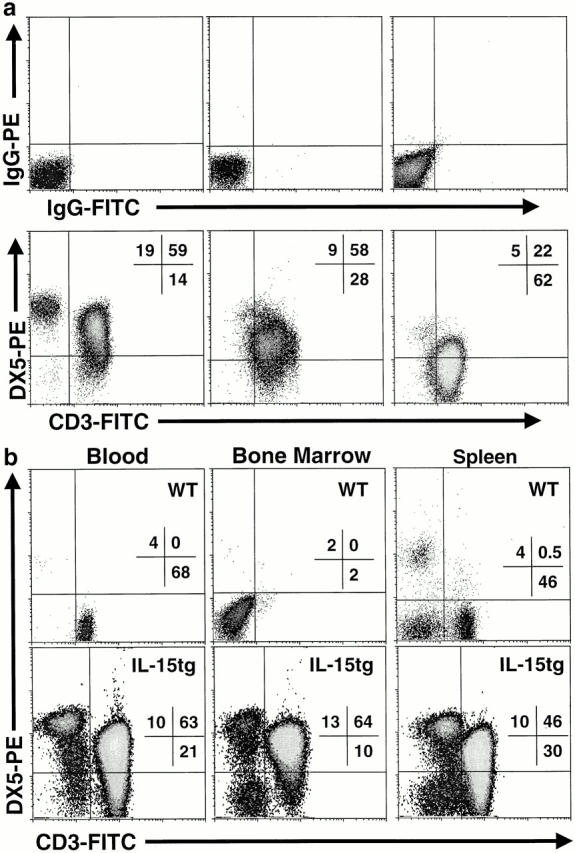
IL-15tg mice develop fatal lymphocytic leukemia with age. (a) Flow cytometric analyses of peripheral blood lymphocytes isolated from representative IL-15tg mice immediately before death. Although the DX5+CD3− NK cell expansion persisted, the major lymphocyte population is CD3+TCR-β+DX5hi/lo/neg T cells. Results shown are from three different IL-15tg mice with isotype control staining shown above the DX5 and CD3 stained cells for each IL-15tg leukemia. (b) Similar lymphocyte expansions are evident in multiple lymphoid tissues from leukemic IL-15tg mice (blood, spleen, bone marrow), as illustrated by a representative IL-15tg mouse. Tissues from a nontransgenic littermate control are shown for comparison. WT, wild-type. (c) Leukemic IL-15tg mice have gross splenomegaly, as evidenced by increased spleen to body weight ratio. Graph shows the mean spleen/body weight ratio ± SEM of 22 leukemic IL-15tg mice. (d) Photomicrographs (100×) illustrating the morphology of the leukemic lymphocytes from peripheral blood smears of four representative IL-15tg mice.
We next examined expanded populations of CD3+ TCR-β+ lymphocytes from IL-15tg mice for clonality of the TCR-β locus. The analyses were performed by DNA PCR with primers that amplify all possible rearrangements of the Jβ1 and Jβ2 loci followed by another DNA PCR assay that amplifies all possible Vβ-Jβ rearrangements 37. An example of a clonal TCR-β+ T cell population isolated from a mouse that developed fatal lymphocytic leukemia is shown in Fig. 8 a. Amplification of the Jβ2 region demonstrated that a single J segment (Jβ2.7) was used by these lymphocytes (Fig. 8 b). After identifying the Jβ clonal population, PCR amplification of all possible V-Jβ2 rearrangements identified the rearranged Vβ present (Vβ16) in the genomic DNA (Fig. 8 c). Through such experiments, expanded lymphocytes from 9 of 19 (48%) IL-15tg mice were determined to be clonal, consistent with leukemia. Importantly, mice from two independent IL-15tg lines developed clonal lymphocytic leukemia, suggesting that this phenotype was not due to the location of the transgene insertion. As further evidence of TCR-β clonality, the amplified Vβ-D-Jβ products from a subset of the leukemias (n = 4), were cloned and sequenced. Each of these leukemias had a distinct TCR Vβ usage and 100% of clones contained identical TCR-β rearrangements at the DNA level (n = 4/4 leukemias, data not shown). Flow cytometric studies on TCR Vβ expression also provided evidence that the lymphocyte expansions were clonal (Fig. 8 d).
Figure 8.

Clonal TCR-β T cell expansion in IL-15tg mice. (a) Schematic of an example rearranged TCR-β gene that was observed in an IL-15tg mouse that died of fatal lymphocytic leukemia. (b) DNA PCR gel showing a monoclonal Jβ2.7 usage in the expanded lymphocytes from an IL-15tg mouse (tg), compared with polyclonal control (C). (c) DNA PCR gel showing a monoclonal Vβ16-Dβ2-Jβ2.7 usage in the expanded lymphocytes from an IL-15tg mouse. A faint band is visible with the prominently used Vβ4, and is present on most gels examining the FVB/N TCR-β. (d) Example of a clonal TCR Vβ6+ T cell population in a different IL-15tg mouse with fatal leukemia by flow cytometry. Blood cells were gated on CD3+ lymphocytes and assessed for expression of the Vβ6 TCR.
Progressive Alopecia and Multiorgan Lymphocytic Infiltrate of IL-15tg Mice.
Animals with dramatically elevated lymphocyte counts were killed for pathologic analysis. All IL-15tg mice were remarkable for whole body alopecia (Fig. 9), splenomegaly, and lymphadenopathy. Microscopic examination of tissues harvested from these animals showed diffuse lymphocytic infiltrates, most prominent in the peritoneum and surrounding intraabdominal organs. In addition, all showed marked skin involvement and expansion of splenic white pulp. Effacement of normal architecture by this infiltrate was seen in enlarged lymph nodes. Prominent lung and liver infiltrates were seen in most animals with highly elevated lymphocyte counts.
Figure 9.
Progressive alopecia in IL-15tg mice. Photograph of a representative 18-wk-old IL-15tg mouse, illustrating the progressive alopecia that involves 50–90% of the skin surface area. The alopecia typically begins at 4–6 wk of age initially involving the head and proximal limbs. A sex- and age-matched wild-type FVB mouse is shown below for comparison.
Fig. 10 illustrates the skin, lung, and liver pathology found in IL-15tg mice. Skin infiltrates were dense within the dermis with individual lymphoid cells present within the epidermis (Fig. 10b and Fig. c). Also remarkable was the presence of mast cells within the dermal infiltrate. Multiple skin ulcerations were evident, and acute inflammatory cells were present at these foci. In immunohistochemical studies on frozen tissue, the invading lymphocytes were CD3 positive (data not shown), whereas no mouse NK cell marker is available for use on tissue sections. The pattern of cutaneous involvement found in these animals is very similar to that seen in patients with NK/T cell lymphomas 40. Other skin changes consistent with long-term inflammation include epidermal hyperplasia, hyperkeratosis, and loss of adnexal structures (e.g., hair follicles).
Figure 10.

Multiorgan lymphocytic infiltration in IL-15tg mice. Histology sections of skin (a–c), lung (d–f), and liver (g–i) stained with hematoxylin and eosin. Low power (10×) micrographs contrast wild-type (a, d, and g) and IL-15tg (b, e, and h) tissues. High power (40×) micrographs demonstrate the lymphocytic morphology of the infiltrating cells in the IL-15tg mice (c, f, and i). See Results for detailed description of the pathology.
A spectrum of lung involvement was observed. In the least affected mice, dense lymphoid infiltrates were identified around blood vessels. In more affected animals, lung involvement was diffuse, involving the walls of alveolar spaces. Fig. 10d and Fig. e, are photomicrographs, taken at 10× and 40× magnification, of a lung section from a transgenic animal. Lymphoid cells are seen surrounding a lymphatic channel, itself containing large lymphocytes as well as marked interstitial involvement. Fig. 10h and Fig. i, demonstrate the common pattern of hepatic disease, with perivascular infiltrates and sinusoidal involvement.
Discussion
IL-15 is a pleiotropic cytokine important in lymphocyte homeostasis and unusual in its tight posttranscriptional control 1. Here, we report the phenotype of transgenic mice engineered to efficiently translate and secrete murine IL-15, which is normally abundant as transcript in multiple tissues but poorly translated and secreted. IL-15tg mice exhibit peripheral blood lymphocytosis, with significant expansions in both the NK cell and memory phenotype CD8+ TCR-α/β T cell compartments early in life. These results demonstrate that IL-15 is a growth factor for these cell types in vivo, and are consistent with gene targeting studies demonstrating that IL-15Rα−/− and IL-15−/− mice lack NK cells and are severely deficient in memory/activated phenotype CD8+ T cells 11 12. Similarly, mice that lack inducible IL-15 gene expression due to disruption of the IFN regulatory factor (IRF)-1 gene 9 10, or are deficient in other IL-15R components 41 42, exhibit NK cell and CD8+ T cell defects. Continual translation and secretion of the proinflammatory cytokine in the IL-15tg mice over several months eventually led to the manifestation of T-NK lymphocytic leukemia in a significant fraction of mice.
The exogenous provision of high doses of rhIL-15 to wild-type or IL-15−/− mice for 1 wk results in transient NK cell increases 12 38. IL-15 has been shown to drive NK cell differentiation from human 7 43 and murine 44 IL-2/15Rβ+ NK cell precursors in the bone marrow, as well as support mature NK cell survival in the absence of serum or other factors 8. This suggests that multiple mechanisms, including increased differentiation and prolonged survival in the periphery, are likely responsible for the accumulation of NK cells observed in IL-15tg mice. It also supports the notion that IL-15 may provide a physiologic set point that normally regulates the total number of NK cells present in vivo. We have shown previously that IL-15 augments NK cell function 6, and consistent with this, fresh NK cells from IL-15tg mice exhibit potent cytolytic activity against YAC-1 tumor target cells.
Similarly, exogenous provision of rhIL-15 to mice for 1 wk increases memory/activated-phenotype CD8+ T cell numbers 12 13. Previous studies have suggested an important role for IL-15 in the antigen-independent maintenance of the memory CD8 T cell pool 12 13 39 45. The consistent increase in CD8+CD44hiLy6C+ T cells within IL-15tg mice supports a role for IL-15 as one homeostatic control for this population. Definitive evidence that IL-15 maintains memory phenotype CD8+ T cells would require adoptive transfer experiments into IL-15−/− and wild-type mice. In IL-15tg mice, housed within specific pathogen-free environments, it is unclear what antigens are driving the CD8+CD44hi T cell expansion. We speculate that these expansions could represent an exaggerated response to normally encountered nonpathogenic antigens in the specific pathogen-free environment. An expansion of benign CD8+ T cell clones has been observed in normal, aged mice and humans 46. These CD8+ T cell clones expand slowly in vivo, do not bear markers of activation, do not cause malignancies, and may potentially arise in response to chronic stimulation with a viral or autoantigen. The early, benign expansion of CD8+CD44hi T cells in IL-15tg mice may somehow be related to such cells. However, the latter are initially polyclonal, whereas the subsequent malignant clonal expansion of lymphocytes in the IL-15tg mice is heterogeneous with regard to CD8 and even CD3 expression, i.e., TCR-α/β+CD8+, TCR-α/β+CD8−, or TCR-α/β−DX5+. Nonetheless, the potential role of IL-15 in the clonal expansion of benign CD8+ T cells seen in elderly mice and humans warrants investigation.
Nishimura et al. recently described transgenic mice that globally overexpress the endogenous long signal peptide form of IL-15, and also observed an increase in functional CD8+CD44hi T cells in lymph nodes and spleen 45, yet there was no NK cell expansion or leukemia reported. A recent report of transgenic mice expressing a chimeric hIL-4R/mIL-15R on T cells suggests that there are unique properties of CD8+CD44hi T cells, in addition to high IL-2/15Rβ expression, that allows them to selectively proliferate in response to IL-15 signals 47. It is interesting that IL-15 appears to control the level of both NK and memory phenotype CD8+ T cells as both of these effectors act coordinately for the clearance of many intracellular pathogens 48 49. Collectively, these data point toward potential clinical utility for time-limited provision of low amounts of IL-15 in the expansion of immune effector cells in immunodeficient patients, or as an adjuvant to boost cellular immune responses after vaccination.
IL-15tg mice develop dramatic and fatal lymphocyte elevations after several months of chronic proinflammatory cytokine stimulation. Clinical findings such as weight loss, reduced activity, and respiratory distress, occur immediately before premature death. In a subset of these mice we have documented that the extraordinary lymphocyte elevation is comprised of a clonal population which, with their clinical course and histopathology, is consistent with the development of leukemia. However, in the remainder of IL-15tg mice that lack clonal lymphocyte expansion yet are otherwise characterized by an identical clinical course, other processes such as autoimmune reactions may contribute to the observed polyclonal lymphocyte expansions and multiorgan infiltration.
The striking leukemic manifestation of this disease suggests that the malignant cell is likely derived from blood or bone marrow, as opposed to a peripheral lymphoid tissues. The chronic proliferation and/or extended survival of lymphocytes in IL-15tg mice likely contribute to the accumulation of additional transforming mutations, as has been postulated for other cancers 24. In support of this, IL-15 has been shown to costimulate stem cell proliferation 7, and extend the survival of normal NK cells 8 and T cells 50 by preventing apoptosis. Studies to sequentially analyze these cells for such secondary genetic alterations are underway, with the hope of providing new insights into the pathogenesis of lymphocytic leukemia.
IL-15 was originally identified as a 4-α helix bundle cytokine with similar in vitro biological properties as IL-2, consistent with their shared receptor components (IL-2/15Rβγc; reference 2). Interestingly, Ishida and et al. generated mice that globally overexpress recombinant IL-2 driven by a MHC class I promoter 51. These IL-2 transgenic mice develop a mild lymphocytic skin infiltrate composed of T cells, but do not demonstrate excessive lymphocyte expansions or malignant transformation such as leukemia. To our knowledge, IL-15tg mice are unique among cytokine transgenics in their induction of leukemia. Collectively, these data strongly support existing data that show little in vivo redundancy when comparing the roles of IL-2 and IL-15 in health and disease.
The chronic lymphocytosis and subsequent leukemia observed in IL-15tg mice share some clinical features and manifestations with the human disease large granular lymphocytic (LGL) leukemia 28. These include extra-lymphoid involvement, a chronic course evolving to an acute expansion, and the prevalence of T cell subtypes. Further, the histopathology of the lymphocytic infiltrates in IL-15tg mice resembles those observed in NK-T lymphomas 40 52. Lymphocytes isolated from patients with LGL leukemia express all three components of the IL-15R complex (αβγ), and increased IL-15 expression was noted in macrophages from these patients 53. In addition, early in vitro propagation of LGL leukemia cell lines can be maintained in the presence of IL-15. We are currently investigating whether increased IL-15 protein expression in the bone marrow or other tissues of LGL leukemia patients may contribute to the initiation or pathophysiology of this disease.
Acknowledgments
We thank Drs. Lewis and Perlmutter for the hGH gene plasmids, Dr. Natarajan Muthusamy for the H-2Dd promoter plasmid, Dr. Said Sif for technical assistance with the immunoblot assays, and Drs. Karl Theil and Amy Gewirtz for help with blood smear photomicrographs. We gratefully acknowledge Drs. Natarajan Muthusamy, Robert Baiocchi, and Matthew Strout for their helpful discussion. We also thank Drs. Marc Dalod and Christine Biron for insightful discussion and technical assistance with the murine IL-15 ELISA.
This work was supported by grants CA-68458, CA-65670, and P30CA-16058 from the National Institutes of Health. T.A. Fehniger is the recipient of Medical Scientist Program (MSP) and Bennett Fellowships from The Ohio State University College of Medicine. J.B. VanDeusen is supported by T-32 CA-09498, and M.A. Cooper by the Howard Hughes Medical Institute Medical Student Research Fellowship.
Footnotes
Abbreviations used in this paper: hGH, human growth hormone; IL-15tg, IL-15 transgenic; LGL, large granular lymphocytic; RT, reverse transcription.
T.A. Fehniger and K. Suzuki contributed equally to this work.
References
- Fehniger T.A., Caligiuri M.A. Interleukin 15biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- Grabstein K.H., Eisenman J., Shanebeck K., Rauch C., Srinivasan S., Fung V., Beers C., Richardson J., Schoenborn M.A., Ahdieh M. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- Giri J.G., Ahdieh M., Eisenman J., Shanebeck K., Grabstein K., Kumaki S., Namen A., Park L.S., Cosman D., Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri J.G., Kumaki S., Ahdieh M., Friend D.J., Loomis A., Shanebeck K., DuBose R., Cosman D., Park L.S., Anderson D.M. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford R.N., Grant A.J., Burton J.D., Peters C., Kurys G., Goldman C.K., Brennan J., Roessler E., Waldmann T.A. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson W.E., Giri J.G., Lindemann M.J., Linett M.L., Ahdieh M., Paxton R., Anderson D., Eisenmann J., Grabstein K., Caligiuri M.A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrozek E., Anderson P., Caligiuri M.A. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–2640. [PubMed] [Google Scholar]
- Carson W.E., Fehniger T.A., Haldar S., Eckhert K., Lindemann M.J., Lai C.F., Croce C.M., Baumann H., Caligiuri M.A. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J. Clin. Invest. 1997;99:937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara K., Hida S., Azimi N., Tagaya Y., Sato T., Yokochi-Fukuda T., Waldmann T.A., Taniguchi T., Taki S. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature. 1998;391:700–703. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- Ohteki T., Yoshida H., Matsuyama T., Duncan G.S., Mak T.W., Ohashi P.S. The transcription factor interferon regulatory factor 1 (IRF-1) is important during the maturation of natural killer 1.1+ T cell receptor- alpha/beta+ (NK1+ T) cells, natural killer cells, and intestinal intraepithelial T cells. J. Exp. Med. 1998;187:967–972. doi: 10.1084/jem.187.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce J.P., Boone D.L., Chai S., Swain R.E., Dassopoulos T., Trettin S., Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Kennedy M.K., Glaccum M., Brown S.N., Butz E.A., Viney J.L., Embers M., Matsuki N., Charrier K., Sedger L., Willis C.R. Reversible defects in natural killer and memory CD8 T cell lineages in IL-15–deficient mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Sun S., Hwang I., Tough D.F., Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson P.C., Liew F.Y. Chemoattraction of human blood T lymphocytes by interleukin-15. J. Exp. Med. 1995;181:1255–1259. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T.A., Yu H., Cooper M.A., Suzuki K., Shah M.H., Caligiuri M.A. IL-15 costimulates the generalized Shwartzman reaction and innate immune IFN-γ production in vivo. J. Immunol. 2000;164:1643–1647. doi: 10.4049/jimmunol.164.4.1643. [DOI] [PubMed] [Google Scholar]
- Schorle H., Holtschke T., Hunig T., Schimpl A., Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- Kundig T.M., Schorle H., Bachmann M.F., Hengartner H., Zinkernagel R.M., Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- Willerford D.M., Chen J., Ferry J.A., Davidson L., Ma A., Alt F.W. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- Ma A., Boone D.L., Lodolce J.P. The pleiotropic functions of interleukin 15not so interleukin 2–like after all. J. Exp. Med. 2000;191:753–756. doi: 10.1084/jem.191.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford R.N., Battiata A.P., Burton J.D., Sharma H., Waldmann T.A. Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region/IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. Proc. Natl. Acad. Sci. USA. 1996;93:2897–2902. doi: 10.1073/pnas.93.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onu A., Pohl T., Krause H., Bulfone-Paus S. Regulation of IL-15 secretion via the leader peptide of two IL-15 isoforms. J. Immunol. 1997;158:255–262. [PubMed] [Google Scholar]
- Tagaya Y., Kurys G., Thies T.A., Losi J.M., Azimi N., Hanover J.A., Bamford R.N., Waldmann T.A. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc. Natl. Acad. Sci. USA. 1997;94:14444–14449. doi: 10.1073/pnas.94.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford R.N., DeFilippis A.P., Azimi N., Kurys G., Waldmann T.A. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J. Immunol. 1998;160:4418–4426. [PubMed] [Google Scholar]
- Cordon-Cardo C., Prives C. At the crossroads of inflammation and tumorigenesis. J. Exp. Med. 1999;190:1367–1370. doi: 10.1084/jem.190.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J.D., Shoaibi M.A., Maestro R., Carnero A., Hannon G.J., Beach D.H. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 1999;190:1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Omar E.M., Carrington M., Chow W.H., McColl K.E., Bream J.H., Young H.A., Herrera J., Lissowska J., Yuan C.C., Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- Caligiuri M.A., Bloomfield C.D. Molecular biology of leukemia. In: Devita V.T., Hellman S., Rosenberg S.A., editors. CancerPrinciples and Practice of Oncology. 6th ed. Lippincott; Philadelphia, PA: 2000. pp. 2389–2404. [Google Scholar]
- Zambello R., Semenzato G. Large granular lymphocytosis. Haematologica. 1998;83:936–942. [PubMed] [Google Scholar]
- Lewis D.B., Yu C.C., Forbush K.A., Carpenter J., Sato T.A., Grossman A., Liggitt D.H., Perlmutter R.M. Interleukin 4 expressed in situ selectively alters thymocyte development. J. Exp. Med. 1991;173:89–100. doi: 10.1084/jem.173.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich C., Scangos G., Tanaka K., Jay G. Regulated expression of a murine class I gene in transgenic mice. Mol. Cell. Biol. 1986;6:1339–1342. doi: 10.1128/mcb.6.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seipelt R.L., Spear B.T., Snow E.C., Peterson M.L. A nonimmunoglobulin transgene and the endogenous immunoglobulin mu gene are coordinately regulated by alternative RNA processing during B-cell maturation. Mol. Cell. Biol. 1998;18:1042–1048. doi: 10.1128/mcb.18.2.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo M., Schroeder A.C., Mobraaten L.E., Gunning K.B., Hanten G., Fox R.R., Roderick T.H., Stewart C.L., Lilly F., Hansen C.T. FVB/Nan inbred mouse strain preferable for transgenic analyses. Proc. Natl. Acad. Sci. USA. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. Manipulating the Mouse EmbryoA Laboratory Manual 1994. Cold Spring Harbor Laboratory Press, ; Plainview, New York: pp. 497 pp [Google Scholar]
- Grunebaum L., Cazenave J.P., Camerino G., Kloepfer C., Mandel J.L., Tolstoshev P., Jaye M., De la Salle H., Lecocq J.P. Carrier detection of Hemophilia B by using a restriction site polymorphism associated with the coagulation Factor IX gene. J. Clin. Invest. 1984;73:1491–1495. doi: 10.1172/JCI111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchatz H., Leung B.P., Wei X.Q., McInnes I.B., Liew F.Y. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritisa role for IL-15 in development of antigen-induced immunopathology. J. Immunol. 1998;160:5654–5660. [PubMed] [Google Scholar]
- Fehniger T.A., Shah M.H., Turner M.J., VanDeusen J.B., Whitman S.P., Cooper M.A., Suzuki K., Wechser M., Goodsaid F., Caligiuri M.A. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12implications for the innate immune response. J. Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- Gartner F., Alt F.W., Monroe R., Chu M., Sleckman B.P., Davidson L., Swat W. Immature thymocytes employ distinct signaling pathways for allelic exclusion versus differentiation and expansion. Immunity. 1999;10:537–546. doi: 10.1016/s1074-7613(00)80053-9. [DOI] [PubMed] [Google Scholar]
- Munger W., DeJoy S.Q., Jeyaseelan R., Sr., Torley L.W., Grabstein K.H., Eisenmann J., Paxton R., Cox T., Wick M.M., Kerwar S.S. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factorcomparison with interleukin-2. Cell. Immunol. 1995;165:289–293. doi: 10.1006/cimm.1995.1216. [DOI] [PubMed] [Google Scholar]
- Ku C.C., Murakami M., Sakamoto A., Kappler J., Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- Natkunam Y., Smoller B.R., Zehnder J.L., Dorfman R.F., Warnke R.A. Aggressive cutaneous NK and NK-like T-cell lymphomasclinicopathologic, immunohistochemical, and molecular analyses of 12 cases. Am. J. Surg. Pathol. 1999;23:571–581. doi: 10.1097/00000478-199905000-00012. [DOI] [PubMed] [Google Scholar]
- DiSanto J.P., Muller W., Guy-Grand D., Fischer A., Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohteki T., Ho S., Suzuki H., Mak T.W., Ohashi P.S. Role for IL-15/IL-15 receptor beta-chain in natural killer 1.1+ T cell receptor-alpha beta+ cell development. J. Immunol. 1997;159:5931–5935. [PubMed] [Google Scholar]
- Yu H., Fehniger T.A., Fuchshuber P., Thiel K.S., Vivier E., Carson W.E., Caligiuri M.A. Flt3 ligand promotes the generation of a distinct CD34+ human natural killer cell progenitor that responds to interleukin-15. Blood. 1998;92:3647–3657. [PubMed] [Google Scholar]
- Williams N.S., Moore T.A., Schatzle J.D., Puzanov I.J., Sivakumar P.V., Zlotnik A., Bennett M., Kumar V. Generation of lytic natural killer 1.1+, Ly-49− cells from multipotential murine bone marrow progenitors in a stroma-free culturedefinition of cytokine requirements and developmental intermediates. J. Exp. Med. 1997;186:1609–1614. doi: 10.1084/jem.186.9.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H., Yajima T., Naiki Y., Tsunobuchi H., Umemura M., Itano K., Matsuguchi T., Suzuki M., Ohashi P.S., Yoshikai Y. Differential roles of interleukin 15 mRNA isoforms generated by alternative splicing in immune responses in vivo. J. Exp. Med. 2000;191:157–170. doi: 10.1084/jem.191.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C.C., Kotzin B., Kappler J., Marrack P. CD8+ T-cell clones in old mice. Immunol. Rev. 1997;160:139–144. doi: 10.1111/j.1600-065x.1997.tb01034.x. [DOI] [PubMed] [Google Scholar]
- Gasser S., Corthesy P., Beerman F., MacDonald H.R., Nabholz M. Constitutive expression of a chimeric receptor that delivers IL-2/IL-15 signals allows antigen-independent proliferation of CD8+CD44high but not other T cells. J. Immunol. 2000;164:5659–5667. doi: 10.4049/jimmunol.164.11.5659. [DOI] [PubMed] [Google Scholar]
- Biron C.A., Nguyen K.B., Pien G.C., Cousens L.P., Salazar-Mather T.P. Natural killer cells in antiviral defensefunction and regulation by innate cytokines. Annu. Rev. Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Scharton-Kersten T.M., Sher A. Role of natural killer cells in innate resistance to protozoan infections. Curr. Opin. Immunol. 1997;9:44–51. doi: 10.1016/s0952-7915(97)80157-4. [DOI] [PubMed] [Google Scholar]
- Bulfone-Paus S., Ungureanu D., Pohl T., Lindner G., Paus R., Ruckert R., Krause H., Kunzendorf U. Interleukin-15 protects from lethal apoptosis in vivo. Nat. Med. 1997;3:1124–1128. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Nishi M., Taguchi O., Inaba K., Minato N., Kawaichi M., Honjo T. Effects of the deregulated expression of human interleukin-2 in transgenic mice. Int. Immunol. 1989;1:113–120. doi: 10.1093/intimm/1.2.113. [DOI] [PubMed] [Google Scholar]
- Natkunam Y., Warnke R.A., Zehnder J.L., Cornbleet P.J. Aggressive natural killer-like T-cell malignancy with leukemic presentation following solid organ transplantation. Am. J. Clin. Pathol. 1999;111:663–671. doi: 10.1093/ajcp/111.5.663. [DOI] [PubMed] [Google Scholar]
- Zambello R., Facco M., Trentin L., Sancetta R., Tassinari C., Perin A., Milani A., Pizzolo G., Rodeghiero F., Agostini C. Interleukin-15 triggers the proliferation and cytotoxicity of granular lymphocytes in patients with lymphoproliferative disease of granular lymphocytes. Blood. 1997;89:201–211. [PubMed] [Google Scholar]