Vascular Endothelial Growth Factor and Angiopoietin-1 Stimulate Postnatal Hematopoiesis by Recruitment of Vasculogenic and Hematopoietic Stem Cells (original) (raw)
Abstract
Tyrosine kinase receptors for angiogenic factors vascular endothelial growth factor (VEGF) and angiopoietin-1 (Ang-1) are expressed not only by endothelial cells but also by subsets of hematopoietic stem cells (HSCs). To further define their role in the regulation of postnatal hematopoiesis and vasculogenesis, VEGF and Ang-1 plasma levels were elevated by injecting recombinant protein or adenoviral vectors expressing soluble VEGF165, matrix-bound VEGF189, or Ang-1 into mice. VEGF165, but not VEGF189, induced a rapid mobilization of HSCs and VEGF receptor (VEGFR)2+ circulating endothelial precursor cells (CEPs). In contrast, Ang-1 induced delayed mobilization of CEPs and HSCs. Combined sustained elevation of Ang-1 and VEGF165 was associated with an induction of hematopoiesis and increased marrow cellularity followed by proliferation of capillaries and expansion of sinusoidal space. Concomitant to this vascular remodeling, there was a transient depletion of hematopoietic activity in the marrow, which was compensated by an increase in mobilization and recruitment of HSCs and CEPs to the spleen resulting in splenomegaly. Neutralizing monoclonal antibody to VEGFR2 completely inhibited VEGF165, but not Ang-1–induced mobilization and splenomegaly. These data suggest that temporal and regional activation of VEGF/VEGFR2 and Ang-1/Tie-2 signaling pathways are critical for mobilization and recruitment of HSCs and CEPs and may play a role in the physiology of postnatal angiogenesis and hematopoiesis.
Keywords: vascular endothelial growth factor, angiopoietin-1, angiogenesis, hematopoiesis, tyrosine kinase receptors
Introduction
In the adult, the majority of hematopoietic stem cells (HSCs) 1 and circulating endothelial precursor cells (CEPs; references 2 3 4) reside in the bone marrow (BM). However, upon stimulation with various cytokines, HSCs and CEPs can be mobilized to the peripheral circulation and colonize other hematopoietic organs, such as the spleen. In addition, BM failure states secondary to BM fibrosis (myelofibrosis) or certain metastatic neoplastic diseases are associated with relocalization of HSCs from BM to extramedullary hematopoietic macroenvironments, including the spleen resulting in splenomegaly 5. However, the exact identities of the chemocytokines responsible for the mobilization and the mechanism of extramedullary recruitment of BM-derived HSCs and CEPs are not well established.
Given the common prenatal origin of CEPs and HSCs from hemangioblasts, both hematopoietic and vasculogenic cells share several survival and chemotactic signaling pathways. The tyrosine kinase receptors, VEGFR2 (Flk-1, KDR; reference 6) and Tie-2 7, whose expression was originally shown to be restricted to vascular cells, are also shared by subsets of HSCs. Angiopoietin-1 (Ang-1), the natural ligand for Tie-2 8, in conjunction with VEGF, modulates various aspects of angiogenesis such as remodeling 9 10 11 12 and vascular permeability 13. Furthermore, Ang-1 and VEGF exert a synergistic effect in promoting the survival of hematopoietic progenitor cells in the developing aorta-gonad-mesonephros 14 15. In addition to conveying signals that regulate cell proliferation, VEGF and Ang-1 also promote chemotaxis and chemokinesis of endothelial cells 11 12. Since VEGF plasma levels are elevated in the circulation during tumor growth 16 and BM failure states 17, we hypothesized that angiogenic factors may play a role in the pathogenesis of postnatal hematopoietic dysfunction and splenomegaly. In this regard, we speculated that acute or chronic regional overexpression of angiogenic factors, such as VEGF and Ang-1, may regulate hematopoiesis by promoting the extramedullary mobilization and recruitment of HSCs and CEPs.
Adenoviral (Ad) vectors are ideal vectors for the high level expression of angiogenic factors with chemokinetic potential because they allow for regional expression of a given chemokine for durations long enough to exert their physiological effect. The intravenous injection of Ad vectors into SCID mice results primarily in the localization of the Ad vectors to the extramedullary organs, including liver and, to a smaller degree, spleen. Therefore, to mimic physiological conditions of sustained plasma release of VEGF and Ang-1, we delivered Ad vectors expressing Ang-1 and isoforms of VEGF (including soluble VEGF165 and matrix-bound VEGF189) to extramedullary organs of immunocompromised SCID mice. In this regard, Ad vectors are ideal vectors for the expression of factors with chemokinetic potential because they allow for regional expression at high titers long enough to exert their physiological effect.
We demonstrate that plasma elevation of VEGF and/or Ang-1 results in the mobilization of HSCs and CEPs. Combined elevation of VEGF and Ang-1 resulted in remodeling of the BM vasculature with concomitant splenomegaly. These studies suggest that temporal and regional activation of VEGF/VEGFR2 and Ang-1/Tie-2 signaling pathways are critical for mobilization and recruitment of HSCs and CEPs and may play a role in the pathogenesis of hematopoietic dysfunction and physiology of postnatal angiogenesis and hematopoiesis.
Materials and Methods
Animals.
Immunodeficient age- (8 wk), weight- (>20 g), and sex-matched SCID mice (on BALB/c background) were purchased from the The Jackson Laboratory and maintained in filtered air Thorensen units. All mice received the Ad vector expressing VEGF165 (AdVEGF165; 1.5 × 108 PFU) and/or the AdAng-1 (109 PFU), the AdNull vector (no transgene; 109 PFU), or the AdVEGF189 vector (2 × 108 PFU) in a volume of 100 μl, by single intravenous administration on day 0. Each study involved three to four animals. Age-matched female BALB/c and C57BL/6 mice were used in CFU-S assay/allogeneic peripheral blood cell transplantation as recipients.
Ad Vectors.
AdVEGF165 and AdVEGF189 are Ad5-derived E1a-, E3-deficient (E1a-E3-E4+) vectors with an expression cassette in the E1a region containing the murine VEGF165 cDNA/human VEGF189 cDNA driven by the CMV major immediate/early promoter/enhancer. The control vector, AdNull, is similar in design except that it contains no transgene in the expression cassette. AdAng-1 is constructed using the Adeno-Quest™ system from Quantum Biotechnology, Inc. Full-length cDNAs encoding an engineered version of human Ang-1 called Ang-1* were cloned into shuttle vector pQBI-AdCMV5 green fluorescent protein. Linearized plasmid and viral long-arm DNA were cotransfected into 293 cells as described previously 18.
Immunoassays of Cytokines.
Murine VEGF plasma levels were evaluated using commercial ELISA (R&D Systems) following the manufacturer's instructions. Human plasma Ang-1 levels were also measured using a sensitive ELISA (Regeneron).
Peripheral Blood Analysis.
Initially every 2–3 d and later on a weekly basis, retroorbital blood was collected with capillary pipettes (Unopette; Fisher Scientific). Platelets, total white blood cells (WBCs), and granulocytes (polymorphonuclear leukocytes) were counted using a Neubauer hematocytometer (Fisher Scientific). Differential leukocyte counts were obtained by examination of blood smears from each mouse stained with Wright-Giemsa stain (200 cells counted/smear). The plasma samples were collected, stored at −80°C, and assessed later by immunoassay for murine VEGF and human Ang-1.
Flow Cytometry.
A total of 104 to 105 cells were incubated for 30 min at 4°C with the following FITC- or PE-conjugated mAbs: H-2Kb–FITC (AF6-88.5; BD PharMingen); CD11b-FITC (M1/70; BD PharMingen); and H-2Kd–FITC (SF-1-1.1; BD PharMingen). DC101 (VEGFR2)–FITC (ImClone Systems) was developed and characterized as described previously 19. Propidium iodide (Becton Dickinson) was dissolved at 3 mM in PBS and used at 30 μM. The cells were analyzed by two-color flow cytometry using an Coulter Elite flow cytometer (Beckman Coulter).
Spleen and BM Analysis.
BM was obtained by flushing both femoral bones with 3 ml of cold (4°C) IMDM containing 20% FCS. Manual leukocyte differentials were performed on Wright-Giemsa–stained cytospin preparations of BM cells and splenocytes. The number of megakaryocytes in randomly chosen femoral BM and spleen samples was determined using 400× magnification field light microscopy.
Progenitor Assay.
PBMCs were collected from orbital plexus and isolated after centrifugation over a discontinuous gradient using lympholyte-M. PBMCs (105 cells) were plated in triplicate in 1 ml of 0.8% methylcellulose containing 30% FCS, 1% l-glutamine, 2.5% hemin, 0.05 mM 5-ME, IL-3 (50 ng/ml; R&D Systems), c-kit ligand (20 ng/ml; Immunex), and erythropoietin (2 U/ml; Sandoz) in 35-mm suspension culture dishes. Scoring was performed with an inverted microscope (40×) on day 7. Cells from PBMCs obtained from AdVEGF165- and/or AdAng-1–treated mice were seeded in the colony assays and four CFU types were scored: CFU-GM, BFU-E, CFU-M, and CFU-Mix, 3, 5, 14, 28 d after the onset of treatment. CEPs present in the mobilized PBMCs were quantified as described previously 4 20. Freshly isolated PBMCs obtained from mice treated with AdVEGF and/or AdAng-1 on days 3 and 14 were cultured in endothelial growth medium (M199; GIBCO BRL) supplemented with 20% fetal bovine serum (HyClone), endothelial cell growth factor (30 μg/ml), fibroblast growth factor (FGF) (5 ng/ml, human recombinant basic FGF; Sigma-Aldrich), heparin (5 U/ml), penicillin (100 U/mL), streptomycin (100 μg/ml), and fungizone (0.25 μg/ml). These cells were placed on 12-well gelatin-coated plates. After 2–3 wk, the endothelial colonies were identified by metabolic uptake of DiI-acetylated-LDL (DiI-Ac-LDL; PerImmune, Inc.) by incubating the wells with 1 μg/ml of human DiI-Ac-LDL for 5 h and visualized by fluorescence microscopy. For von Willebrand factor (vWF) staining, cells were rinsed with HBSS and fixed immediately with ethanol for 10 min. DiI-Ac-LDL+vWF+ colonies formed in the first 3 d after vector administration were scored as early outgrowth endothelial colonies, whereas endothelial colonies formed after 14 d of culture were considered as late-outgrowth colonies (CFU-ECs).
CFU-S Assay.
Mobilized PBMCs were obtained from days 3, 5, 7, 14, 21, and 28 after the onset of treatment with various Ad vectors. For each data point, three recipient mice were irradiated with 9 Gy from a 137Cs γ-ray source at a dose rate of _∼_0.90 Gy/min to prevent the production of endogenous spleen colonies. Irradiated mice were injected intravenously via the tail vein with 105 PBMCs within several hours after the completion of irradiation. The mice were killed 12 d later, and their spleens were removed and fixed in Bouin's solution. The number of macroscopic spleen colonies was then scored.
Repopulating Assay.
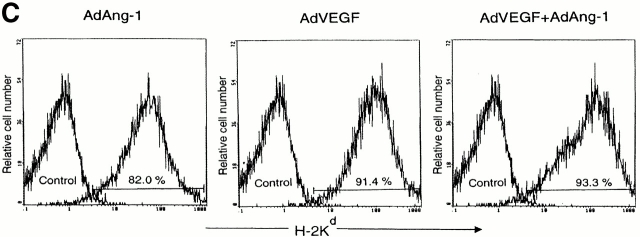
After vector administration, the peripheral blood from SCID mice was collected on day 7. PBMCs (106 cells) were transplanted into lethally irradiated (9.5 Gy) recipient C57BL/6 mice. As a control, PBMCs (106 cells) from AdNull-treated SCID mice were transplanted into irradiated C57BL/6 mice by intravenous injection at day 0. Chimerism was determined by FACS® analysis (donor SCID mice, H-2Kd; recipient C57BL/6 mice, H-2Kb). The chimeric mice were killed at 90, 120, and 150 d after transplantation and BM mononuclear cells (BMMCs) were stained for flow cytometry analysis with H-2Kd–FITC (donor type). The representative percentages of positive populations in BMMCs are shown at day 150 (see Fig. 2 C). The data from age-matched normal C57BL/6 mice are also represented as control. A total of 104 cells were incubated for 30 min at 4°C with FITC- or PE-conjugated mAbs, H-2Kb–FITC (AF6-88.5; BD PharMingen) and H-2Kd–FITC (SF-1-1.1; BD PharMingen). The percentage of positive cells was analyzed by flow cytometry using an Elite flow cytometer (Beckman Coulter). Survival was monitored every day until 150 d after vector administration. As control, BMMCs (106) from SCID mice were transplanted into irradiated C57BL/6 mice by intravenous injection at day 0.
Figure 2.
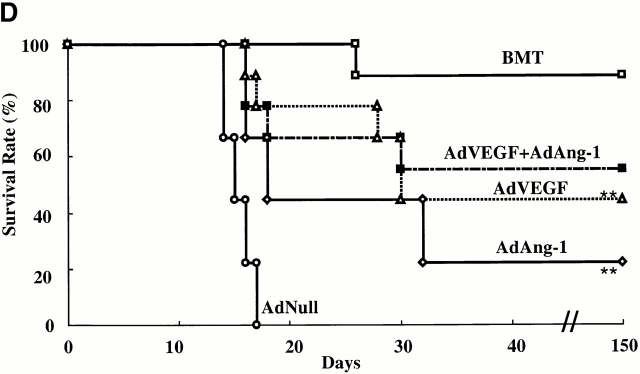
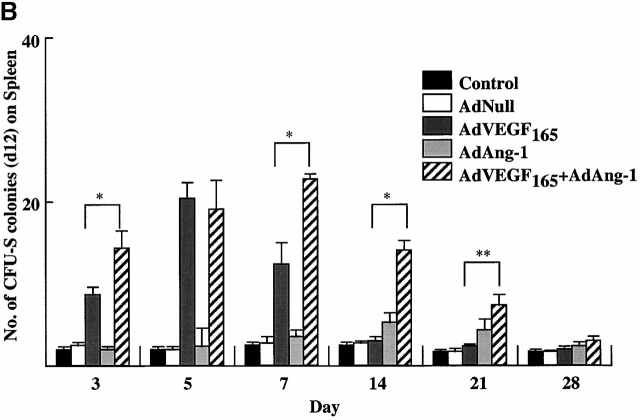
AdVEGF165 and/or AdAng-1 induce peripheral mobilization of hematopoietic progenitor cells and stem cells. (A) The number of mobilized progenitors was determined using standard CFU assays. Large numbers of progenitors were mobilized by VEGF (day 5, predominantly CFU-GM) and Ang-1 (day 14, predominantly CFU-M) (n = 4). The pluripotency of the mobilized cells was determined by CFU-S assay (B) and BM repopulating assay (C and D). Compared with AdVEGF, the combination of AdVEGF plus AdAng-1 induced significant long-term mobilization of CFU-S up to 21 d. Days 3, 7, and 14, *P < 0.01; day 21, **P < 0.05. Ang-1, VEGF, or combined VEGF and Ang-1 promoted mobilization of BM repopulating cells (n = 9). PBMCs (106 cells) from SCID mice (H-2Kd) treated with AdNull, AdVEGF165, AdAng-1, or a combination (AdVEGF165 and AdAng-1) were transplanted into irradiated C57BL/6 (H-2Kb) mice by intravenous injection on day 0. (C) The number of engrafted H-2Kd cells was determined by flow cytometry. Compared with the AdNull group, AdAng-1– and AdVEGF-treated mice showed significant mobilization of cells capable of reconstituting hematopoiesis in lethally irradiated mice. **P < 0.05. In contrast, all the mice transplanted with PBMCs from the peripheral blood of AdNull-treated mice failed to engraft. (D) As a control group, 90% of the mice transplanted with untreated BM (BMT group) were engrafted and survived the effects of lethal irradiation.
Histopathology.
Tissues were fixed in 10% buffered formalin and paraffin embedded. Sections were stained with hematoxylin and eosin and examined under microscopy.
Delivery of Neutralizing VEGFR2 mAb to Mice.
A group of SCID mice were treated with 1.5 × 108 PFU of AdVEGF165, 1.5 × 108 PFU of AdVEGF165 and 109 PFU of AdAng-1, or 109 PFU of AdNull intravenously on day 0. Some AdVEGF165-, AdVEGF165- and AdAng-1–, or AdNull-treated SCID mice received 800 μg of anti-VEGFR2 (clone DC101) mAb intraperitoneally at 2-d intervals from either day 0 or 2.
Recombinant VEGF Induces Splenomegaly in Mice.
A group of BALB/c mice were treated with 100 ng/mice recombinant VEGF or PBS intraperitoneally daily. Recombinant VEGF was purchased from Immunotech.
Statistical Evaluation.
The results are expressed as mean ± SEM. Statistical analyses were performed using the unpaired two-tailed Student's t test. Survival rates were compared between the two groups by the log rank test.
Results
AdAng-1 and AdVEGF165 but Not AdVEGF189 Promote Mobilization of Hematopoietic Cell_s._
Intravenous administration of Ad vectors expressing VEGF165 (AdVEGF165, 1.5 × 108 PFU) resulted in a peak VEGF plasma level (1,640 ± 233 pg/ml) 24 h after injection and a return to pretreatment level on day 35. Doses >1.5 × 108 PFU of AdVEGF165 were significantly toxic and resulted in the death of the treated mice (data not shown). There were no signs of toxicity in mice treated with AdNull vectors at doses up to 109 PFU. In AdAng-1–treated mice (109 PFU), plasma levels of Ang-1 peaked on day 3 after injection (74.1 ± 0.7 ng/ml), returning to the pretreatment level after 8 wk. Plasma VEGF and Ang-1 were not detectable in AdNull-treated mice.
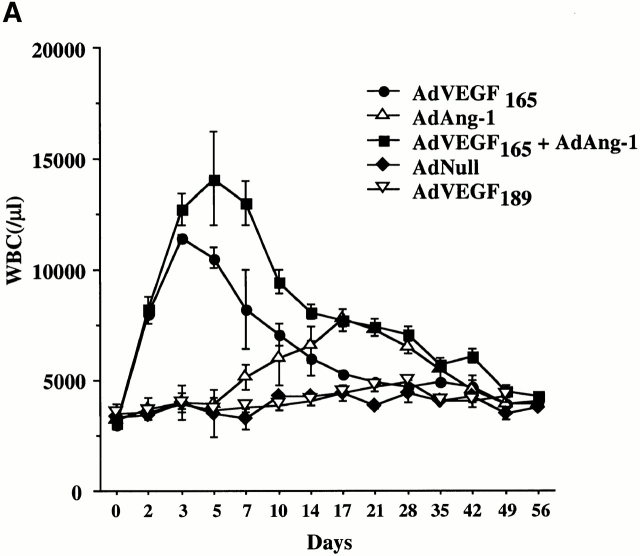
To examine the effects of acute and chronic elevation of circulating VEGF165 or Ang-1 levels in the mobilization of CEPs and HSCs, SCID mice were injected intravenously with AdVEGF165 (1.5 × 108 PFU) and/or AdAng-1 (109 PFU), or AdVEGF189 (2 × 108 PFU). Subsequently, the number of leukocytes, hematopoietic progenitor cells, and HSCs were determined in the peripheral blood. Control mice were injected with 109 PFU of AdNull vector. Compared with AdNull-treated mice, AdVEGF165-treated mice had a fourfold increase in WBCs on day 3, returning to the level of AdNull-treated mice by 5 wk after injection (Fig. 1 A). In contrast, administration of AdAng-1 into SCID mice resulted in only a small increase in WBCs, peaking at day 17 and returning to control levels by 7 wk. However, the combination of AdVEGF165 and AdAng-1 resulted in a synergistic sevenfold increase in WBCs 5 d after vector administration. As expected, injection of AdVEGF189, which results in production of matrix-bound VEGF189, did not result in leukocyte mobilization.
Figure 1.
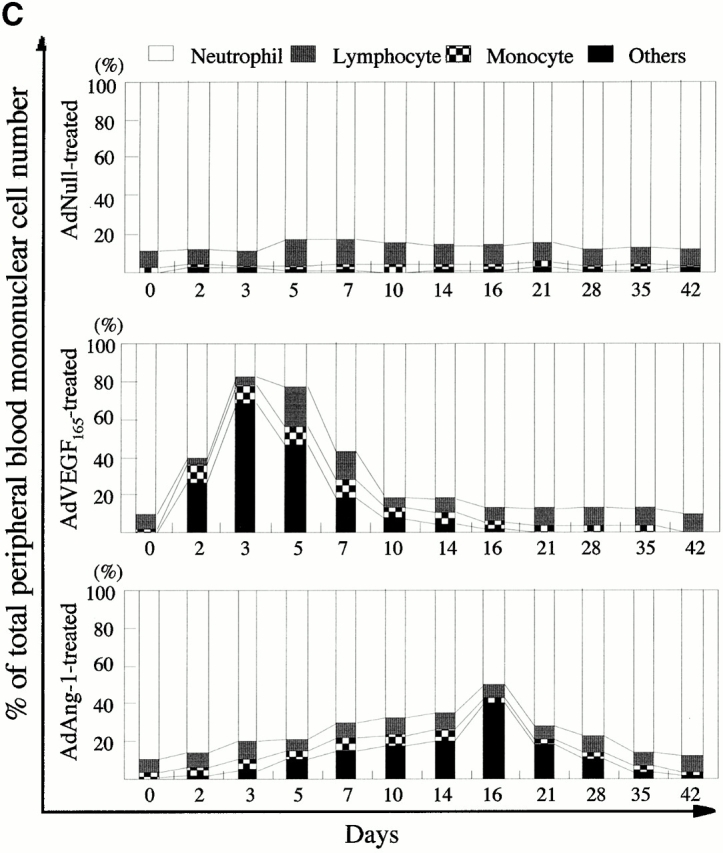
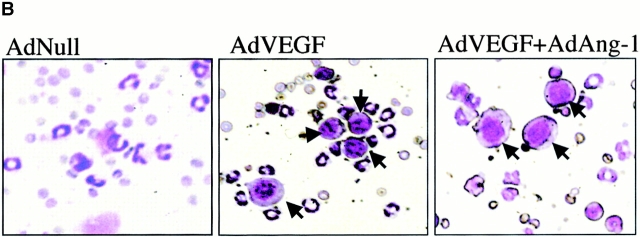
AdVEGF165 and AdAng-1 promote mobilization of mature and immature hematopoietic cells. Immunodeficient SCID mice received AdVEGF165 (1.5 × 108 PFU) and/or AdAng-1 (109 PFU), AdNull vector (109 PFU), or the AdVEGF189 vector (2 × 108 PFU) by a single intravenous administration on day 0. (A) Total WBCs were counted using a Neubauer hematocytometer and stained by crystal violet. n = 6. (B) Morphological characterization of PBMCs was determined by Wright-Giemsa staining. Original magnification: ×200. (C) Differential leukocyte counts were obtained by examining the blood smears from each mouse (200 cells counted/smear). n = 4.
Populations of blast-like cells that are usually localized to the BM were detected at high levels in the peripheral circulation of the AdVEGF165- and/or AdAng-1–treated mice. These cells displayed scant cytoplasm and large nuclei, reminiscent of BM-derived immature hematopoietic progenitor and precursor cells (Fig. 1 B). Compared with AdNull-treated mice, AdVEGF165- and AdVEGF165 plus AdAng-1–treated mice had a dramatic increase in the WBC percentage of blast-like cells (Others; Fig. 1 B) and monocytes during days 2–14, returning almost to baseline 3 wk after the start of treatment (Fig. 1 C). The intravenous administration of AdAng-1 to SCID mice also resulted in increased circulation of blast-like cells, peaking at day 16 and returning to baseline on day 49 (Fig. 1 C). There were no significant changes in the leukocyte levels of AdNull-treated mice.
AdVEGF165 and AdAng-1 Induced Mobilization of Hematopoietic Progenitor Cells with Stem Cell Potential.
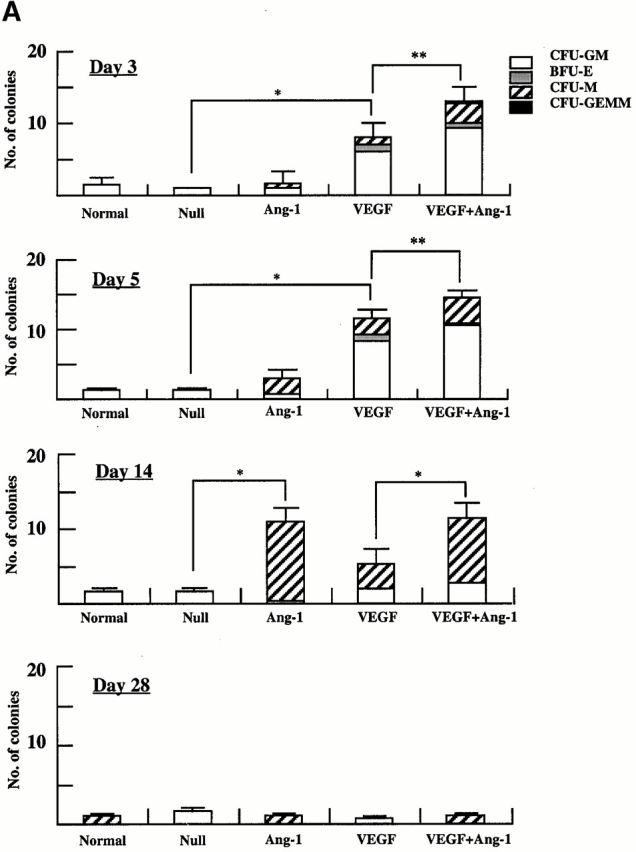
The administration of AdVEGF165 induced mobilization of hematopoietic progenitors to the peripheral circulation. These progenitors comprised colony-forming units (CFU-Cs) such as CFU-M, CFU-GM, BFU-E, and CFU-Mix (CFU-GEMM). Compared with AdNull-treated mice, at days 3 and 5 the majority of the mobilized CFU-Cs consisted of CFU-GM (*P < 0.005). CFU-GMs peaked at day 5 and returned to baseline levels by day 28 (Fig. 2 A). The remaining leukocytes mobilized to the peripheral blood consisted of mature cells including monocytes.
Administration of AdAng-1 promoted a delayed but significant mobilization of CFU-Cs to the peripheral blood. Compared with AdNull or AdVEGF165, at day 14 after injection, AdAng-1–mobilized CFU-Cs comprised predominantly CFU-M (*P < 0.005). The numbers of CFU-Cs returned to control levels on day 28. The combination of both AdVEGF165 and AdAng-1 enhanced the mobilization of CFU-Cs (**P < 0.05).
The number of mobilized pluripotent hematopoietic cells with stem cell potential, capable of forming spleen colonies (CFU-S), was measured by injecting AdVEGF165 and/or AdAng-1 or, as control, AdNull-mobilized PBMCs into irradiated syngeneic mice (Fig. 2 B). Administration of AdVEGF165 induced a 20-fold increase in peripheral blood CFU-S by day 5 of treatment (Fig. 2 B). On the other hand, administration of AdAng-1 induced a 2.5-fold increase in peripheral blood CFU-S 14 d after injection. Coinjection of both AdVEGF165 and AdAng-1 had a synergistic effect on the number of mobilized CFU-S. Administration of AdNull did not result in the mobilization of CFU-S.
The number of mobilized pluripotent hematopoietic cells with BM-repopulating capacity was also determined (Fig. 2 C) by allogeneic transplantation of VEGF165- and/or Ang-1–mobilized PBMCs into lethally irradiated mice. Injection of either AdVEGF165 and/or AdAng-1, but not AdNull, resulted in mobilization of HSCs that were able to engraft and rescue lethally irradiated mice. All mice transplanted with 106 PBMCs from AdNull-treated mice died within 18 d (Fig. 2 D). However, 56% of the mice transplanted with PBMCs from AdVEGF and AdAng-1, 45% of AdVEGF, and 22% of AdAng-1–treated mice survived beyond 150 d. These data suggest that overexpression of VEGF165 and Ang-1 results in mobilization of pluripotent hematopoietic cells with stem cell potential from BM to the peripheral circulation.
AdVEGF165 and AdAng-1 Induce the Mobilization of CEPs.
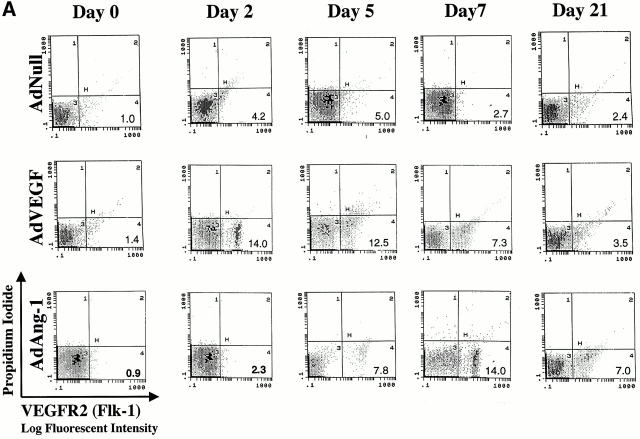
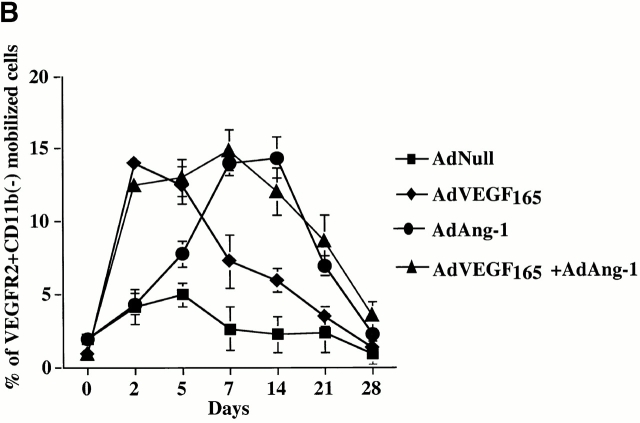
We have shown that a subset of mobilized circulating VEGFR2+ PBMCs contain a unique population of marrow-derived endothelial cells, namely CEPs 2 4. AdVEGF165 caused mobilization of CEPs to the peripheral blood on days 2, 5, and 7, with a return to control levels by day 14 (Fig. 3a and Fig. b). Administration of AdVEGF165 and AdAng-1 into SCID mice resulted in increased levels of circulating VEGFR2+CD11b− cells, 7 d (15.3 ± 2.5%) and 14 d (11.1 ± 4.1%) after the start of treatment (Fig. 3 B). Mobilized CEPs can be distinguished from mature endothelial cells by their capacity to form late-outgrowth colonies (CFU-ECs) 20. In contrast to mature endothelial cells, which form early outgrowth colonies, CEPs form rapidly proliferating endothelial colonies 3–4 wk after initiation of culture. Incubation of mobilized VEGFR2+ PBMCs with VEGF and FGF-2 (basic FGF) on collagen-coated plastic dishes resulted in the generation of early and late outgrowth DiI-Ac-LDL+ endothelial cells (CFU-ECs; Fig. 3C and Fig. D). The majority of the colonies formed (Fig. 3E, Fig. F, and Fig. G) were composed of late outgrowth endothelial cells, suggesting that VEGF and Ang-1 promote the mobilization of BM-derived CEPs.
Figure 3.
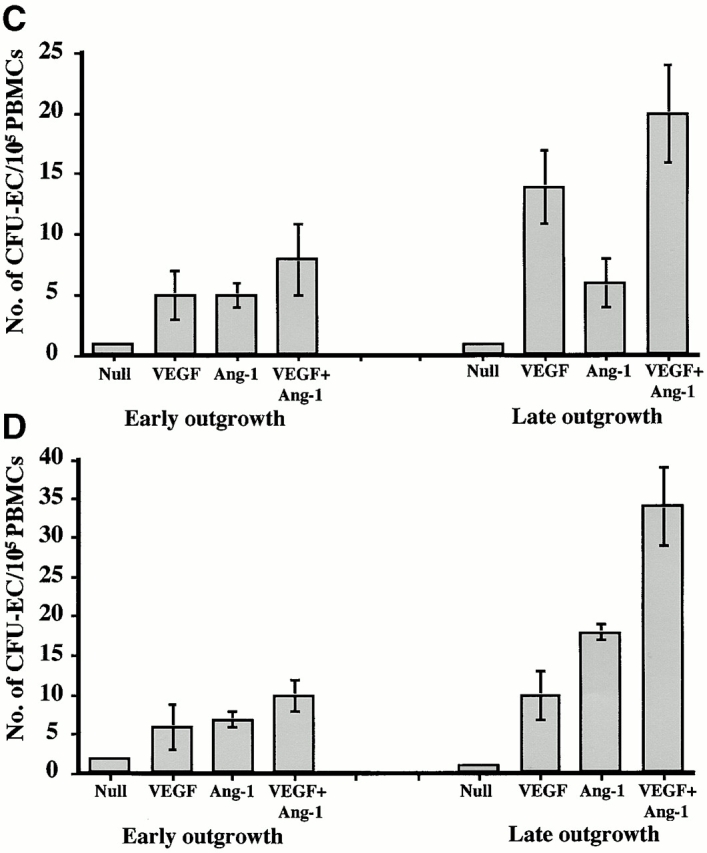
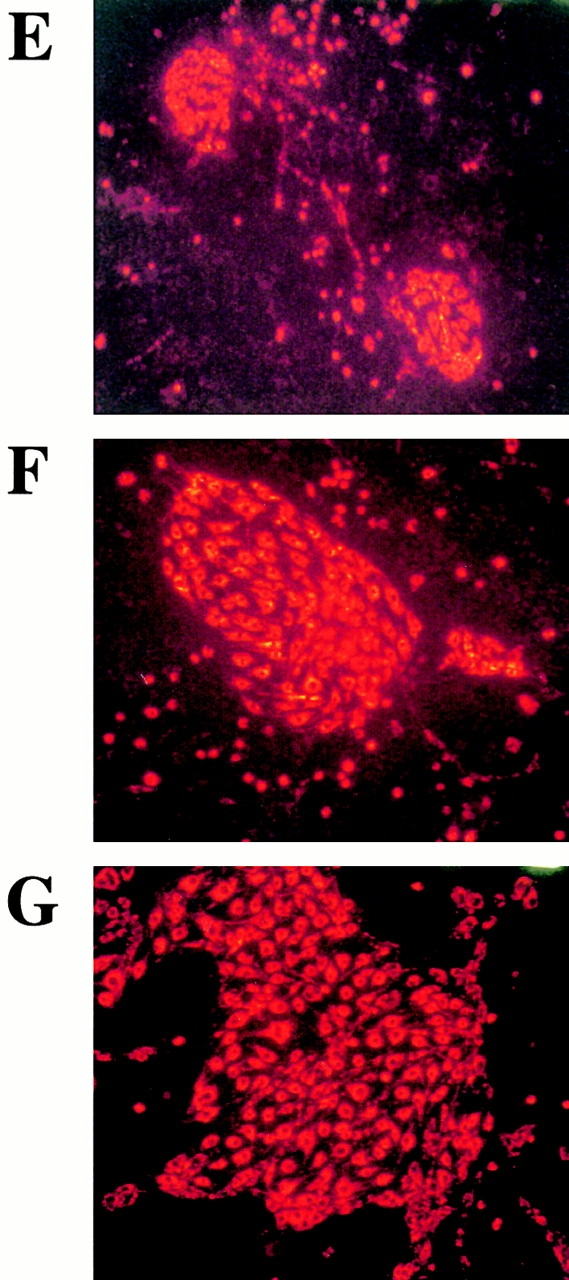
AdVEGF165 and Ang-1 promote mobilization of CEPs with late outgrowth potential. (A) Mobilized PBMCs were isolated from AdVEGF165- and/or AdAng-1– or AdNull-treated SCID mice on days 0–28 and stained with FITC-conjugated anti-VEGFR2 (clone DC101) mAb and propidium iodide. (A) 104 cells were analyzed on a Beckman Coulter Elite flow cytometer, and representative percentages of positive populations in PBMCs are shown. (B) The percentages of mobilized circulating VEGFR2+CD11b− cells from AdVEGF165- and/or AdAng-1– or AdNull-treated SCID mice (n = 3) are shown from days 0 –28. For quantification of CEPs with late outgrowth potential, mobilized PBMCs were obtained from AdVEGF165 and/or AdAng-1 or AdNull-treated SCID mice on days 0–21 and plated in the presence of endothelial growth medium (reference 13) on collagen/fibronectin-coated plastic dishes. Endothelial colonies (CFU-ECs) were identified by vWF immunostaining and metabolic labeling with DiI-Ac-LDL. (C) CFU-ECs proliferated 2 wk after the start of culture (mean ± SEM). Colonies that formed within the first 3 d (early outgrowth) and colonies formed 14 d (late outgrowth) after vector administration were quantified by vWF and DiI-Ac-LDL labeling. (D) The majority of CFU-EC proliferated 3 wk after the start of culture, forming focal DiI-Ac-LDL+ endothe-lial monolayers as seen at 14 (E), 20 (F), and 25 d (G).
Prolonged Plasma Elevation of VEGF165 and Ang-1 Results in Cellular and Vascular Remodeling of BM and Spleen.
The sustained chronic elevation of VEGF165 and Ang-1 within 1 wk resulted in the initial stimulation of hematopoiesis with increased BM cellularity and proliferation of erythroid, myeloid, and megakaryocytic lineages compared with BM of AdNull-treated SCID mice (Fig. 4A and Fig. B). However, by 2–3 wk there was a gradual decrease in BM cellularity and remodeling of the BM architecture with concomitant profound splenomegaly of AdVEGF- and AdAng-1–treated SCID mice. Histological examination of the BM at day 14 demonstrated progressive increase in sinusoidal space and proliferation of capillaries (Fig. 4 C). By day 60, at a time when the plasma levels of VEGF and Ang-1 returned to baseline, the overall BM cellularity and vascularity returned to baseline levels (Fig. 4 D). These BM architectural changes were much less pronounced when either VEGF or Ang-1 was overexpressed.
Figure 4.
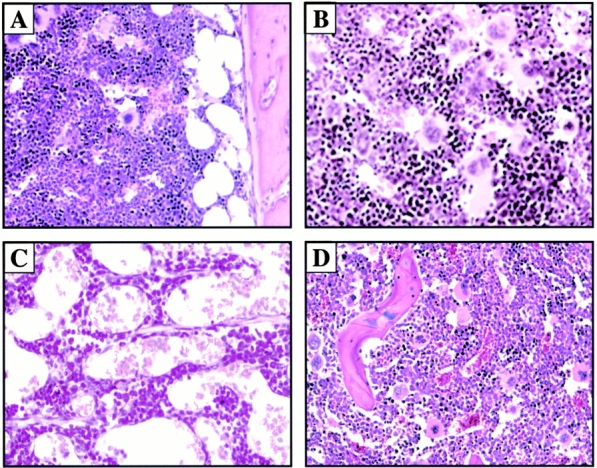
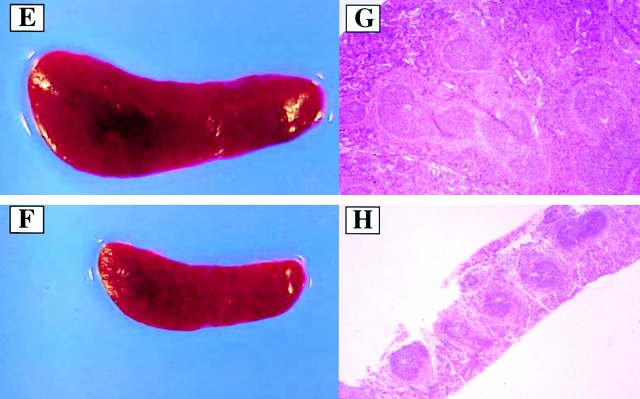
Chronic elevation of AdVEGF and AdAng-1 induces vascular remodeling of BM with concomitant splenomegaly. Administration of AdVEGF165 and/or AdAng-1 or AdNull was performed as described in the legend to Fig. 1. On days 7, 14, and 60 after inoculation three mice in each group were killed, and their organs were collected and processed for histological analysis. Paraffin sections of the spleen and BM were stained by hematoxylin and eosin. Representative BM sections from day 7 after AdNull (A) and day 7 after AdVEGF165 plus AdAng-1 (B) are shown. BM cellularity was increased in AdVEGF165 plus AdAng-1–treated SCID mice at day 7 compared with AdNull-treated mice. (C) On day 14, sinusoidal space and proliferation of capillaries were progressively increased in the BM of AdVEGF165 plus AdAng-1–treated SCID mice. (D) BM cellularity and vascularity returned to baseline levels on day 60. Macroscopic and microscopic observations of spleens from BALB/c mice treated with recombinant VEGF (E and G) or PBS as a control (F and H) on day 10 are shown. These mice were injected subcutaneously with 100 ng of recombinant VEGF or only vehicle, on a daily basis. There was a remarkable increase in the size of the spleen and follicular hyperplasia 10 d after VEGF treatment compared with control. Original magnifications: (A, C, D, G, and H) ×200; (B) ×400; (E and F) ×20.
Given that SCID mice have an abnormal splenic structure, evaluation of the splenic cellular architecture after injection with VEGF or Ang-1 is not feasible. Therefore, immunocompetent BALB/c mice were injected subcutaneously with 100 ng of recombinant VEGF on a daily basis for 2 wk. After 2 wk, at the time when there was a relative decrease in BM cellularity (1.9 ± 0.3 × 107/femur from VEGF-treated mice compared with 2.5 ± 0.1 × 107/femur for normal BALB/c mice, P < 0.01), there was a remarkable increase in spleen size (Fig. 4E and Fig. F). Compared with the control group (Fig. 4 H), there was increased cellularity and follicular hyperplasia in the spleen of VEGF-treated group (Fig. 4 G; 0.3 ± 0.2 × 108 compared with 2.4 ± 0.3 × 108 for normal BALB/c mice, P < 0.005). Splenomegaly and follicular hyperplasia were reversed after cessation of treatment.
Mobilization of Leukocytes by AdVEGF165 Is Mediated Primarily through VEGFR2 Signaling.
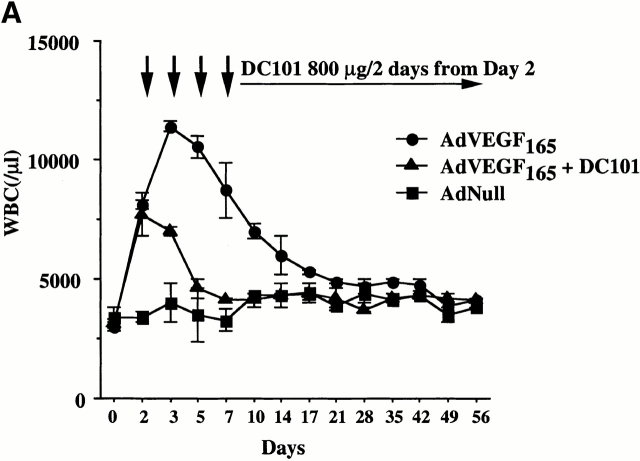
Murine VEGFR2 (Flk-1), which is expressed on subsets of HSCs and CEPs, mediates signals that may not only promote proliferation but also stimulate chemotaxis of endothelial cells. Therefore, we postulated that the primary mechanism whereby VEGF induces mobilization of HSCs and CEPs is through activation of VEGFR2. Injection of a neutralizing mAb to murine VEGFR2 (clone DC101) 2 d after AdVEGF165 injection significantly blocked VEGF165-induced mobilization of leukocytes (Fig. 5a and Fig. b) and CFU-Cs (Fig. 5 C) to the peripheral circulation. However, DC101 failed to inhibit Ang-1–induced migration of HSCs, suggesting that Ang-1–delayed mobilization of HSCs and CEPs requires Tie-2 signaling and is therefore independent of the VEGFR2/VEGF signaling pathway.
Figure 5.
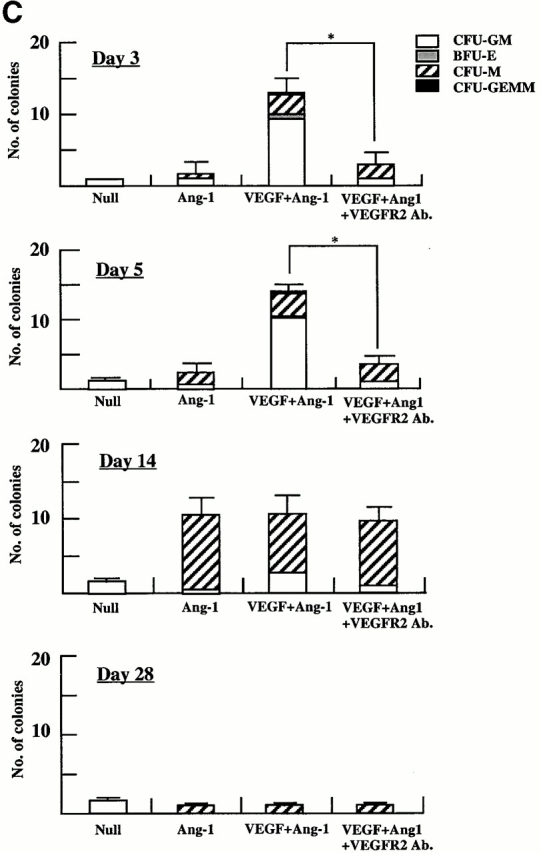
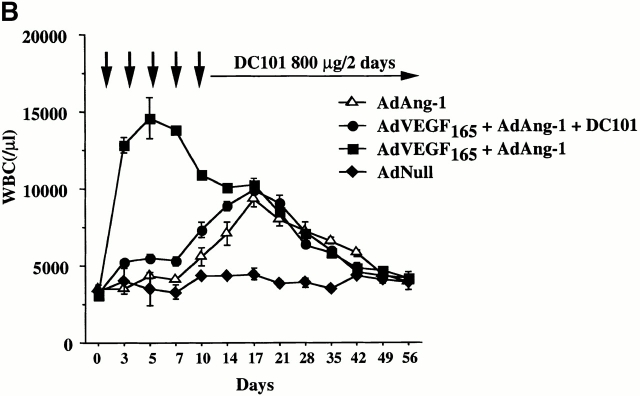
Neutralizing mAb to VEGFR2 inhibits mobilization of leukocytes and hematopoietic progenitor cells induced by AdVEGF165 but not AdAng-1. (A) SCID mice were inoculated with AdVEGF165 (1.5 × 108 PFU) or AdNull (109 PFU) on day 0. Half of the AdVEGF165-treated mice received 800 μg of anti-VEGFR2 (DC101) mAb at 2-d intervals starting from day 2. Total WBCs are expressed as the mean ± SEM (n = 8). •, AdVEGF165; ▴, AdVEGF165 + DC101; ▪, AdNull. (B) SCID mice inoculated with AdVEGF165 (1.5 × 108 PFU) and AdAng-1 (109 PFU) received 800 μg of anti-VEGFR2 (DC101) at 2-d intervals starting from day 0. AdAng-1 (109 PFU) inoculated SCID mice were used as a control. Total WBCs were quantified using a Neubauer hematocytometer. All data are expressed as the mean ± SEM (n = 8). ▵, AdAng-1; •, AdVEGF165 + AdAng-1 + DC101; ▪, AdVEGF165 + AdAng-1; ♦, AdNull. (C) PBMCs from AdAng-1 alone, AdVEGF165 + AdAng-1 (treated or untreated with DC101), or AdNull-treated mice were seeded into the colony assays and four types of CFU (CFU-GM, BFU-E, CFU-M, and CFU-GEMM) were scored at 3, 5, 14, and 28 d after the onset of treatment. All data are expressed as the mean ± SEM (n = 4). Data points achieving statistical significance are shown. *P < 0.005.
Discussion
In this report, we have demonstrated that the elevation of VEGF165 and/or Ang-1 plasma levels by Ad vectors results in robust mobilization of CEPs and hematopoietic progenitors and HSCs (CFU-S, marrow repopulating cells) to the peripheral circulation. Although VEGF165 alone could also induce splenomegaly and increase BM cellularity, the sustained elevation of both VEGF165 and Ang-1 was essential to induce significant remodeling of BM vascular architecture, with concomitant mobilization to the extramedullary organs, resulting in profound splenomegaly. Elevation of Ang-1 alone did not affect the size of the spleen or cellularity of the BM, suggesting that combined signaling through VEGFR2/VEGF and Ang1/Tie-2 signaling pathways plays a key role in the regulation of organ-specific hematopoietic activity.
The overexpression of VEGF165 induces a rapid (24–48 h) mobilization of CEPs and HSCs, suggesting that the effect of VEGF165 is mediated through a chemokinetic process. However, plasma elevation of Ang-1 requires at least 7 d to induce mobilization of CEPs and HSCs. The physiological effect of VEGF is most likely not mediated through BM endothelial cell leakiness since studies have shown that Ang-1 prevents VEGF-induced vascular permeability 13 18. In this regard, Ang-1 should have inhibited the VEGF-induced mobilization of the HSCs and CEPs. In contrast, Ang-1 promoted the mobilization of HSCs and CEPs and synergized with VEGF in augmenting its effect. Collectively, these data suggest that VEGF alone or in combination with Ang-1 induce mobilization by directly interacting with stem cells or promoting remodeling of the BM vasculature. It is conceivable that Ang-1 may alter the adhesion molecule repertoire of BM endothelial cells or HSCs, resulting in enhanced mobilization. Alternatively, Ang-1 may sustain or promote the survival of Tie-2+ HSCs and CEPs, inducing cell cycle shifts that may favor their mobilization to the peripheral circulation.
VEGF has been shown to exert its pleiotropic effects on endothelial cells through its receptors VEGFR1 and VEGFR2. VEGFR2 has been shown to be the principal mitogenic receptor for VEGF 21 22, whereas VEGFR1 conveys signals for vascular remodeling. However, VEGFR1 is expressed by various mature hematopoietic cells, including dendritic and monocytic cells 23 24, whereas VEGFR2 is expressed by HSCs and CEPs 4. Therefore, VEGF165-induced mobilization of mature cells such as monocytes may be mediated though VEGFR1, whereas mobilization of HSCs is most likely mediated through VEGFR2 6. Neutralizing mAb to VEGFR2 inhibited VEGF165-induced mobilization and splenomegaly, suggesting that interaction of VEGF165 with VEGFR2 is essential for mobilization and recruitment of HSCs. Delayed mobilization in response to elevation in circulating Ang-1 is most likely induced by interaction with either Tie-2+ on the stem cells 19 or because of remodeling of the BM microenvironment.
Most in vivo experiments assessing the chemoattractant properties of VEGF165 have been performed with injection of recombinant VEGF165. However, given that the half-life of VEGF165 in the circulation is only several minutes, it has been difficult to evaluate the role of chronic VEGF165 release, as it may occur during tumor growth and metastasis or myelodysplastic syndromes. Likewise, recombinant Ang-1 is unstable and previous studies using Ang-1 protein have failed to demonstrate a functional endpoint 21. It is suggested that in contrast to VEGF165, whereby injection of recombinant protein can induce mobilization of stem cells, Ang-1 protein does not have enough biochemical stability to exert any biological effect. Furthermore, given the delayed mobilization seen with Ang-1 treatment, injection of AdAng-1 into SCID mice as opposed to immunocompetent mice was the only means of sustaining the level of Ang-1 long enough in vivo to observe its effects. Therefore, the use of Ad vectors as regional delivery vehicles for VEGF165 and Ang-1 provides ideal tools for studying the role of these angiogenic factors in mobilization and recruitment of HSCs and CEPs. In addition, given that VEGF189 is matrix bound, Ad vectors producing VEGF189 provide an ideal mechanism to deliver this matrix-bound isoform of VEGF regionally to the extramedullary hematopoietic microenvironments such as the liver.
We show that long-term overexpression of both VEGF165 and Ang-1 is essential to induce an initial increase in BM cellularity followed by remodeling of the BM architecture, with depletion of sinusoidal spaces of hematopoietic cells and a parallel increase in BM vascularization and splenomegaly. These results suggest that regional expression of VEGF165 and Ang-1 in extramedullary organs may act synergistically to modulate postnatal hematopoiesis by inducing extramedullary shifts in hematopoiesis or remodeling of vascular architecture of sinusoidal BM endothelial cells. Recently, it has been shown that plasma VEGF levels are significantly elevated in myelodysplastic syndromes 17, where the BM microenvironment is damaged by fibrotic tissue. This raises the possibility that elevation of VEGF may serve as one of the primary adaptive responses to compensate for BM myelofibrosis, by promoting the relocalization and recruitment of HSCs to other hematopoietic microenvironments such as the spleen, resulting in splenomegaly.
Mobilization of CEPs to the peripheral circulation may play a critical role in regulating postnatal angiogenesis and vasculogenesis. Based on the data presented here, high levels of VEGF produced by tumors may result in the mobilization of BM-derived CEPs to the peripheral circulation and enhance their recruitment into the tumor vasculature. Given that CEPs may also contribute to vascular healing, overexpression of VEGF165 may promote the peripheral blood mobilization and recruitment of CEPs to the injured vascular bed, thereby accelerating wound healing.
As evidenced by the relative plasma levels of Ang-1 and VEGF, higher levels of Ang-1 are necessary to induce mobilization of HSCs and CEPs. Indeed, at doses >1.5 × 108 PFU of AdVEGF165, elevations of plasma VEGF were associated with significant toxicity and resulted in the death of the treated mice. Ang-1 does not exert any major physiological effect at the lower doses (5 × 108 PFU); however, when administered at higher doses (109 PFU) it had a potent synergistic effect with VEGF in mobilizing HSCs and CEPs. These differences in the dose response may be due to the variable receptor densities or differential physiological effects of the VEGF receptors and Tie-2. It is also conceivable that expression of VEGFR2 (Flk-1) on HSCs and CEPs is significantly higher than Tie-2.
These data set forth a novel paradigm for efficient mobilization of HSCs and CEPs to the peripheral circulation. This strategy facilitates recovery of large numbers of pluripotent HSCs and CEPs, which may be used for transplantation or treatment of a wide variety of genetic and malignant disorders. Whether transplantation of mobilized CEPs in conjunction with mobilized HSCs will exert a synergistic effect to enhance engraftment and reconstitution of hematopoiesis is the subject of future studies.
Acknowledgments
We are grateful to Drs. John Rudge, Ella Ioffe, and George D. Yancopoulos (Regeneron Pharmaceutical, Inc.) for providing the Ad vectors and Ang-1 ELISA data and for their helpful discussions during the preparation of this manuscript. We thank Harry G. Satterwhite, Dr. George Lam, Mannix S. Quitriano, Maureen Sullivan, Koji Shido, and Sinae R. Park for their helpful assistance and Margaret Choy for critical reading.
S. Rafii is supported by National Heart, Lung, and Blood Institute (NHLBI) grants R01 HL58707, R01 HL61849, Program Project U01 HL66952 (Project 2), Pilot Project P01 HL59312, the Dorothy Rodbell Foundation for Sarcoma Research, and the Rich Foundation. M.A.S. Moore is supported by NHLBI grant R01 HL61401 and the Gar Reichman Fund of Cancer Research Institute. R.G. Crystal is supported in part by NHLBI grant R01 HL57318, Program Project U01 HL66952-01, the Will Rogers Memorial Fund, and Gen Vec, Inc. (Gaithersburg, MD). K. Hattori is the recipient of a fellowship from the Uehara Memorial Foundation (Tokyo, Japan). B. Heissig is the recipient of a fellowship from the Dr. Mildred Scheel Stiftung für Krebsforschung (Bonn, Germany).
Footnotes
Abbreviations used in this paper: Ad, adenovirus; Ang-1, angiopoietin-1; BM, bone marrow; BMMC, BM mononuclear cell; CEP, circulating endothelial precursor cell; FGF, fibroblast growth factor; HSC, hematopoietic stem cell; VEGF, vascular endothelial growth factor; vWF, von Willebrand factor; WBC, white blood cell.
References
- Moore M.A. Stem cell proliferationex vivo and in vivo observations. Stem Cells. 1997;1:239–248. doi: 10.1002/stem.5530150832. [DOI] [PubMed] [Google Scholar]
- Rafii S. Circulating endothelial precursorsmystery, reality, and promise. J. Clin. Invest. 2000;105:17–19. doi: 10.1172/JCI8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Kalka C., Masuda H., Chen D., Silver M., Kearney M., Magner M., Isner J.M., Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Peichev M., Naiyer A.J., Pereira D., Zhu Z., Lane W.J., Williams M., Oz M.C., Hicklin D.J., Witte L., Moore M.A., Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(1) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- Frey B.M., Rafii S., Teterson M., Eaton D., Crystal R.G., Moore M.A. Adenovector-mediated expression of human thrombopoietin cDNA in immune-compromised miceinsights into the pathophysiology of osteomyelofibrosis. J. Immunol. 1998;160:691–699. [PubMed] [Google Scholar]
- Ziegler B.L., Valtieri M., Porada G.A., De Maria R., Muller R., Masella B., Gabbianelli M., Casella I., Pelosi E., Bock T. KDR receptora key marker defining hematopoietic stem cells. Science. 1999;285:1553–1558. doi: 10.1126/science.285.5433.1553. [DOI] [PubMed] [Google Scholar]
- Hashiyama M., Iwama A., Ohshiro K., Kurozumi K., Yasunaga K., Shimizu Y., Masuho Y., Matsuda I., Yamaguchi N., Suda T. Predominant expression of a receptor tyrosine kinase, TIE, in hematopoietic stem cells and B cells. Blood. 1996;87:93–101. [PubMed] [Google Scholar]
- Davis S., Aldrich T.H., Jones P.F., Acheson A., Compton D.L., Jain V., Ryan T.E., Bruno J., Radziejewski C., Maisonpierre P.C., Yancopoulos G.D. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- Suri C., Jones P.F., Patan S., Bartunkova S., Maisonpierre P.C., Davis S., Sato T.N., Yancopoulos G.D. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Holash J., Maisonpierre D., Compton D., Boland P., Alexander C.R., Zagzag D., Yancopoulos G.D., Wiegland S.J. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- Koblizek T.I., Weiss C., Yancopoulos G.D., Deutsch U., Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr. Biol. 1998;8:529–532. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- Witzenbichler B., Maisonpierre P.C., Jones P., Yancopoulos G.D., Isner J.M. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J. Biol. Chem. 1998;273:18514–18521. doi: 10.1074/jbc.273.29.18514. [DOI] [PubMed] [Google Scholar]
- Thurston G., Suri C., Smith K., McClain J., Sato T.N., Yancopoulos G.D., McDonald D.M. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Hamaguchi I., Huang X.L., Takakura N., Tada J., Yamaguchi Y., Kodama H., Suda T. In vitro hematopoietic and endothelial cell development from cells expressing TEK receptor in murine aorta-gonad-mesonephros region. Blood. 1999;93:1549–1556. [PubMed] [Google Scholar]
- Huang X.L., Takakura N., Suda T. In vitro effects of angiopoietins and VEGF on hematopoietic and endothelial cells. Biochem. Biophys. Res. Commun. 1999;264:133–138. doi: 10.1006/bbrc.1999.1472. [DOI] [PubMed] [Google Scholar]
- Damert A., Machein M., Breier G., Fujita M.Q., Hanahan D., Risau W., Plate K.H. Up-regulation of vascular endothelial growth factor expression in a rat glioma is conferred by two distinct hypoxia-driven mechanisms. Cancer Res. 1997;57:3860–3864. [PubMed] [Google Scholar]
- Aguayo A., Estey E., Kantarjian H., Mansouri T., Gidel C., Keating M., Giles F., Estrov Z., Barlogie B., Albitar M. Cellular vascular endothelial growth factor is a predictor of outcome in patients with acute myeloid leukemia. Blood. 1999;94:3717–3721. [PubMed] [Google Scholar]
- Thurston G., Rudge J.S., Ioffe E., Zhou H., Ross L., Croll S.D., Glazer N., Holash J., McDonald D.M., Yancopoulos G.D. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- Hsu H.C., Ema H., Osawa M., Nakamura Y., Suda T., Nakauchi H. Hematopoietic stem cells express Tie-2 receptor in the murine fetal liver. Blood. 2000;96:3757–3762. [PubMed] [Google Scholar]
- Lin Y., Weisdorf D.J., Solovey A., Hebbel R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F., Rossant J., Yamaguchi T.P., Gertsenstein M., Wu X.F., Breitman M.L., Schuh A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Fong G.H., Rossant J., Gertsenstein M., Breitman M.L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Barleon B., Sozzani S., Zhou D., Weich H.A., Mantovani A., Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- Clauss M., Weich H., Breier G., Knies U., Rockl W., Waltenberger J., Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J. Biol. Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]