CD8 T Cells Are Required for the Formation of Ectopic Germinal Centers in Rheumatoid Synovitis (original) (raw)
Abstract
The assembly of inflammatory lesions in rheumatoid arthritis is highly regulated and typically leads to the formation of lymphoid follicles with germinal center (GC) reactions. We used microdissection of such extranodal follicles to analyze the colonizing T cells. Although the repertoire of follicular T cells was diverse, a subset of T cell receptor (TCR) sequences was detected in multiple independent follicles and not in interfollicular zones, suggesting recognition of a common antigen. Unexpectedly, the majority of shared TCR sequences were from CD8 T cells that were highly enriched in the synovium and present in low numbers in the periphery. To examine their role in extranodal GC reactions, CD8 T cells were depleted in human synovium-SCID mouse chimeras. Depletion of synovial CD8 T cells caused disintegration of the GC-containing follicles. In the absence of CD8 T cells, follicular dendritic cells disappeared, production of lymphotoxin-α1β2 markedly decreased, and immunoglobulin (Ig) secretion ceased. Immunohistochemical studies demonstrated that these CD8 T cells accumulated at the edge of the mantle zone. Besides their unique localization, they were characterized by the production of interferon (IFN)-γ, lack of the pore-forming enzyme perforin, and expression of CD40 ligand. Perifollicular IFN-γ+ CD8 T cells were rare in secondary lymphoid tissues but accounted for the majority of IFN-γ+ cells in synovial infiltrates. We propose that CD8+ T cells regulate the structural integrity and functional activity of GCs in ectopic lymphoid follicles.
Keywords: lymphoid follicle, rheumatoid arthritis, lymphoid neogenesis, pathogenesis, CD40
Introduction
Rheumatoid arthritis (RA)*is a chronic inflammatory disease that causes disability due to immune-mediated damage of joints and shortens life expectancy through vascular injury. Multiple genes contribute to disease risk, the strongest known risk factor has been associated with a sequence polymorphism of the HLA-DRB1 gene (1). HLA class II genes are considered to be predictors of disease susceptibility but have also been implicated as markers of disease severity (2). Only recently have HLA-C polymorphisms and the KIR2DS2 gene encoding for an HLA-C–recognizing receptor been identified as risk factors for vasculitic complications in patients with RA (3).
The strong association of disease risk and disease severity with HLA class II molecules has driven the paradigm that CD4 T cells have a critical role in the disease process. Consistent with this model, CD4 T cells are the dominant cell population in rheumatoid lesions and they participate in the formation of ectopic lymphoid microstructures. Germinal centers (GCs) in the inflamed synovial membrane are a characteristic finding in RA (4). Affinity maturation and receptor revisions have been described in such structures (5, 6). Overall, similar molecular pathways seem to be important in the formation of GCs in the rheumatoid joint and in secondary lymphoid tissues (7–9). A comparative study of synovial tissues containing different types of lymphoid microstructures has shown that the production of lymphotoxin (LT)-α1β2 and B lymphocyte chemoattractant (CXCL13), are crucial factors in the emergence of synovial GCs (7). CXCL13 protein was found in endothelial cells and synovial fibroblasts. LT-α1β2 was detected on B cells in the mantle zone, a subset of B cells in the GCs, and on T cells contributing to the lymphoid follicles. An important difference between the ectopic lymphoid follicles and their counterparts in lymph nodes lies in the complete absence of primary follicles in the synovial membrane. Rheumatoid lesions exclusively contain secondary follicles, and follicular dendritic cell networks are only established in the presence of active ongoing GC reactions. GC reactions are typically associated with Ag-reactive responses and are dependent on CD4 T cells to provide help in B cell differentiation (10–12). Therefore, the isolation of such CD4 T cells from rheumatoid lesions should lead to the identification of T cells critically involved in the disease process. This project set out to isolate and characterize CD4 T cells participating in the ectopic lymphoid follicles in RA tissue lesions. Unexpectedly, the majority of TCR sequences isolated from follicular T cells derived from CD8 T cells. Depletion of CD8+ T cells led to the architectural and functional disruption of active GCs. Follicular CD8 T cells were a unique subset of cells expressing CD40 ligand (CD40L) and IFN-γ and lacking perforin. These data have important implications for the understanding of the synovial inflammation in RA. They establish CD8 T cells as being critical in ectopic GC formation. They also suggest that searches for the driving Ag in RA should not exclusively focus on soluble Ags presented in the context of MHC class II molecules but should include endogenous Ags complexing with HLA class I molecules.
Materials and Methods
Study Population.
Synovial tissue specimens were obtained from 81 patients with RA with active synovitis who were undergoing synovial biopsy, synovectomy, or total joint replacement surgery. All patients had unequivocal destructive RA, were seropositive for RF, and fulfilled the American College of Rheumatology (Atlanta, GA) criteria for the diagnosis of RA. PBMCs were obtained at the time of surgery. The protocol was approved by the Mayo Clinic Institutional Review Board and all patients provided written informed consent.
Microdissection of Synovial Lymphoid Follicles and TCR β-Chain Sequencing.
From five patients, lymphoid aggregates with unequivocal GC formation were picked from synovial tissue using 8-μm cryosections and a 50-μm microcapillary under an inverted microscope. In parallel, T cell clusters were picked from interfollicular regions as controls. cDNA from each pick was synthesized using a TCR-BC antisense oligonucleotide (5′-CTGTGCACCTCCTTCCCATTC-3′) and then amplified by PCR with TCR BV- and nested BC-specific primers (13, 14). PCR products were cloned with the TA cloning kit (Invitrogen Life Technologies) and sequenced. To select BV elements used by follicular T cells, immunohistochemical staining with BV-specific mAb was performed on serial tissue sections directly adjacent to the sections used for microdissection. The panel of anti-BV mAb included BV3S1, BV5S1, BV5S2/S3, BV6S7, and BV8 (T Cell Diagnostics and Endogen). From each patient, material from three independent follicular picks was subjected to sequence analysis. Subsequently, 5–9 follicular picks and, in three patients, 3–7 interfollicular areas were screened for specific TCR sequences. In these experiments, cDNA was amplified with the appropriate primers, labeled with digoxigenin, and hybridized with biotinylated oligonucleotides representing the N-D-N region of TCR sequences that were present in the follicles. Hybridized products were detected by PCR-ELISA on streptavidin-coated microtiter plates using horseradish peroxidase–conjugated antidigoxigenin Ab and a colorimetric reaction (Roche Molecular Diagnostics and Boehringer Mannheim). The specificity of the hybridization assay was validated for 15 probes with cDNA from PBMCs. Wells yielding a positive hybridization signal were eluted, the material was reamplified with the appropriate primers, and was sequenced. A cross-reactive TCR, in addition to the expected sequence, was identified for two probes, suggesting that the specificity of the assay was ∼90%.
Limiting Dilution Analysis.
Multiple aliquots (8–10 per dilution) from serially diluted CD4+ and CD8+ T cells (2 × 106, 106, 5 × 105, and 105 cells) were prepared. cDNA from these serial dilutions was amplified with the appropriate TCR-BV and -BC primers, hybridized with the appropriate N-D-N probe derived from the follicular picks, and analyzed by PCR-ELISA (15). In the limiting dilution analysis, a signal ≥10% of the positive control was scored positive to optimize sensitivity while maintaining specificity. Frequencies were estimated by assuming a Poisson distribution.
Flow Cytometry.
Synovial tissue was digested with 3 mg/ml type 1A collagenase, 1 mg/ml hyaluronidase, and 0.1 mg/ml type IV deoxyribonuclease I (16). Synovial tissue mononuclear cells and PBMCs were isolated using a Ficoll gradient, stained, and sorted on a FACSVantage™ flow cytometer (Becton Dickinson). The following Abs were used: FITC- and PE-labeled anti-CD4 and anti-CD8 (Becton Dickinson), and PE-labeled anti-CD40L (Ancell). cDNA from purified subpopulations were obtained and amplified with primers specific for perforin (5′-CTCCTCCTGGGCATCCTTCTC-3′, 5′-ACTGGTCCTGGTGGGTCTTCT-3′) and granzyme A (5′-GAGCCAACAAAACAGATAATG-3′, 5′-AGAAGCCACAGTAAATGAAAG-3′).
Treatment of Human Synovium-SCID Mouse Chimeras with Anti-CD8 mAb.
This protocol was reviewed and approved by the Mayo Clinic Animal Care and Use Committee. NOD.CB17-Prkdc scid/J mice (NOD-SCID) were purchased from The Jackson Laboratory and used at age 6–8 wk. For tissue implantation, the mice were anesthetized with 50 mg/kg pentobarbital intraperitoneal (Abbott Laboratories) and methoxyflurane inhalation (Medical Developments). Pieces of human synovial tissue with inflammatory infiltrates were placed into a subcutaneous pocket on the upper dorsal midline. In this model, complete tissue engraftment occurs within 1 wk (14, 17). Mice were treated with 600 μg/d anti-CD8 mAb (OKT8, CRL-8014; American Type Culture Collection), 600 μg/d mouse IgG2 (R&D Systems), or buffer intraperitoneally for 3 d starting 7–12 d after tissue implantation. Grafts were harvested 1 wk after treatment and embedded in OCT compound (Tissue-Tek; Sakura Finetek) for immunohistochemistry or shock frozen in liquid nitrogen for RNA analysis. To confirm that CD8+ T cells were depleted, CD8 β1-chain transcripts were amplified using reverse transcription-PCR with CD8 β1-chain primers (5′-GGTGCAAACCAACAAGATG-3′, 5′-GGAAATCAACCACACTCAGC-3′).
Immunohistochemistry.
Frozen synovial tissues embedded in OCT compound were cut into 5-μm sections, fixed in acetone for 10 min, and air-dried. The following murine mAbs were used for immunohistochemistry: anti-CD3 (1:100; UCHT1); anti-CD8 (1:100; C8/144B); anti-CD20 (1:50; B-Ly1); anti-CD23 (1:50; MHM6); anti-CD68 (1:300; EBM11; all Dako); antiperforin (δG9; 1:50; Ancell); and anti–IFN-γ (1:50; MAB285; R&D Systems). Sections were incubated with normal goat serum for 10 min at room temperature and then with primary Abs for 1 h. Biotinylated rabbit anti–mouse Ab (1:300; Dako) was used as a secondary Ab. Sections were developed with VectaStain Elite ABC reagents (Vector Laboratories) and 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich). For double labeling, slides were incubated with a second primary Ab and developed using VectorABC substrate for alkaline phosphatase and vector blue (Vector Laboratories). For intracellular cytokine stains, sections were fixed in 4% paraformaldehyde solution, blocked with MOM mouse IgG blocking reagent (Vector Laboratories) for 1 h, and stained with primary Ab after permeabilization with 0.1% saponin in PBS. Sections were counterstained with hematoxylin (Surgipath) or nuclear fast red (Vector Laboratories).
PCR Analysis of Tissue Transcripts.
Total RNA was extracted from synovial tissues with TRIzol (Invitrogen Life Technologies) and first strand cDNA was synthesized using oligo(dT) and AMV reverse transcriptase (Roche Molecular Biochemicals and Boehringer Mannheim; reference 18). cDNA was adjusted to contain equal numbers of β-actin transcripts as determined by PCR-ELISA (17). Adjusted cDNA was amplified under nonsaturating conditions. Human Ig gene transcripts were amplified using the following family-specific primers: VH1 (5′-CAGGTGCAGCTGGTGCAG-3′); VH3 (5′-GAGGTGCAGCTGGTGGAG-3′); VH4 (5′-CAGGTGCAGCTGCAGGAG-3′); Vκ1 (5′-GACATCCAGATGACCCAGTC-3′); Vκ2 (5′-GATATTGTGATGACCCAGACTCC-3′); and Vκ3 (5′-GAAATTGTGTTGACGCAGTCT-3′). The reverse primers were designed to be complimentary to the 5′ end of the γ chain (5′-GTGTTGCTGGGCTTGTGATT-3′) and κ chain constant regions (5′-CTCGTAGTCTGCTTTGCTCA-3′). One constant region primer and three variable region primers were used under optimal multiplex PCR conditions. In addition, the following cytokine- and cell surface molecule–specific primers were used: LT-β (5′-GCAAGGACTGGGGTTTCAGA-3′, 5′-GTAGCCGACGAGACAGTAGA-3′); long isoform of CD21 ([CD21L]; 5′-GTGGATTTACTTTGAAGGGCA-3′, 5′-GGCATGTTTCTTCACACCG-3′); IFN-γ (5′-ACCTTAAGAAATATTTTAATGC-3′, 5′-ACCGAATAATTAGTCAGCTT-3′); IL-1β (5′-GACACATGGGATAACGAGGC-3′, 5′-GGGATCTACACTCTCCAGCTG-3′); and TNF-α (5′-TAGCCCATGTTGTAGCAAACCC3′, 5′-TCGGCAAAGTCGAGATAGTC-3′). If indicated, amplified products were labeled with digoxigenin-11-dUTP (Roche Molecular Biochemicals and Boehringer Mannheim), hybridized with biotin-labeled probes (IFN-γ [5′-ATTTGGCTCTGCATTATTTTTCTGT-3′]; IL-1β [5′-AGCTTTTTTGCTGTGAGTCCCGGAG-3′]; and TNF-α [5′-AAT-GGCGTGGAGCTGAGAGATAAC-3′]), and quantified by PCR-ELISA. The number of cytokine-specific sequences was determined by interpolation on a standard curve and expressed as the number of cytokine sequences per 2 × 106 β-actin sequences. All reactions were done in triplicates, results are expressed as mean ± SD.
ELISA.
Total human IgG in the serum of human synovium-SCID mouse chimeras was measured by solid-phase capture sandwich ELISA (AlerChek).
Results
T Cells with Identical TCR β-Chains Colonize Different Follicles but not Interfollicular Areas.
Synovial tissue samples were collected from a series of 81 patients undergoing synovectomy or arthroplasty. In a subset of 21 patients (Table I), lymphoid aggregates with follicular dendritic cells and characteristic cellular compartmentalization were identified as ectopic GCs (Fig. 1 A); five of these patients were selected for microdissection analysis. All of these patients had multiple GCs in the synovial tissue, while T cell–B cell aggregates without follicular dendritic cells were not detected. In each of these tissues, T cell–B cell clusters were isolated and cDNA was amplified with TCR-BV– and -BC–specific primers. Amplified products from three such clusters from each patient were cloned and sequenced. In all patients and in all dissected follicles, the TCR repertoire in the follicle was diverse. A typical example of the diversity of TCR sequences isolated from three distinct follicles of patient 624 is given in Table II. For each BV family in each of the follicles, 9–15 TCR β-chains were sequenced. The vast majority of the sequences were unique; only few of the TCR sequences were seen repeatedly.
Table I.
Characteristics of Patients with RA who Have Ectopic GCs in the Synovium
| Sex (M/F) | 6/15 |
|---|---|
| Age (y) | |
| Median | 53.8 |
| Range | 17.3–73.8 |
| RF-positive (%) | 100 |
| HLA-DRB1*04 (%) | 90 |
| Disease duration (y) | |
| Median | 14.9 |
| Range | 0.8–53.2 |
Figure 1.
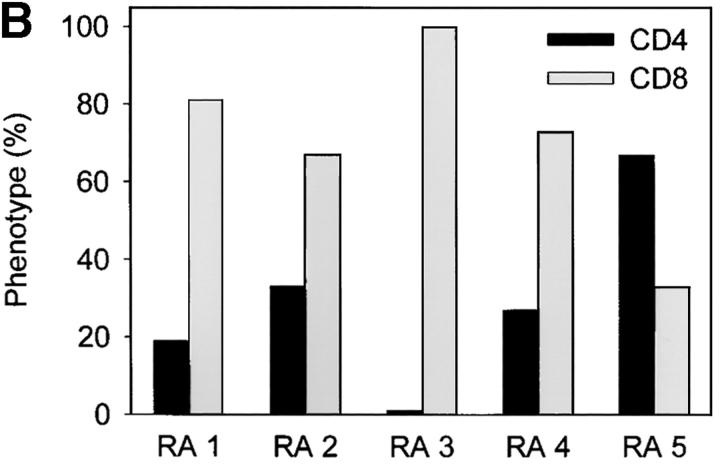
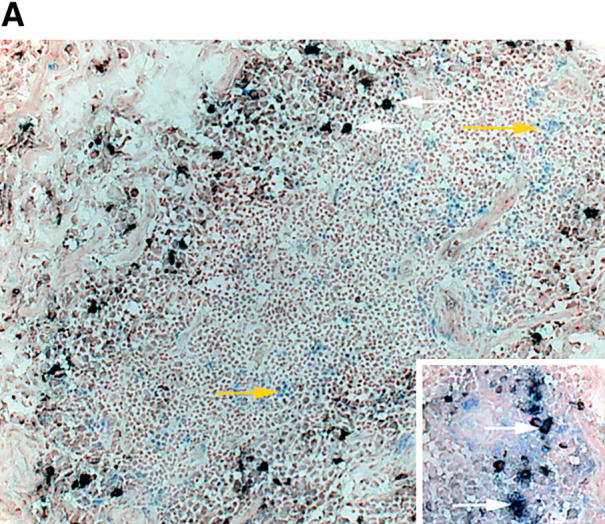
Follicular TCR β-chain sequences preferentially derive from CD8 T cells. Synovial tissue was selected from five patients with RA who had multiple ectopic GCs in the synovial membrane. (A) The distribution of CD4 T cells (left), CD20 B cells (middle), and IgD+ cells (right) is shown in a representative example. The mantle zone is indicated by an asterisk (right). Follicles were microdissected and TCR β-chain sequences were obtained (Tables II and III). In parallel, synovial tissue and peripheral CD4 and CD8 cells were purified by FACS®. cDNA was tested for the presence of these TCR β-chain sequences by PCR-ELISA using N-D-N–specific oligonucleotides as probes. (B) In four of the five patients, the majority of the TCR β-chain sequences were detected in the CD8, and not the CD4, population. (C) To define the localization of the CD8 cells in synovial follicles, tissue sections were stained with anti-CD8 (brown) and anti-CD23 (red, expressed on follicular dendritic cells) mAb. CD8 T cells were found in the perifollicular zone, sometimes within the mantle zone, and only occasionally in the GC. Original magnifications: (left) × 100 and (right) × 400.
Table II.
Diversity of TCR β-Chain Sequences Isolated from Individual Synovial Follicles
| Sequence frequency (n) | |||||||
|---|---|---|---|---|---|---|---|
| Follicle | TCR BVsegment | Sequences(n) | 1 | 2 | 3 | 4 | 5 |
| A | 3 | 15 | 11 | 0 | 0 | 1 | 0 |
| 5S2 | 9 | 9 | 0 | 0 | 0 | 0 | |
| B | 3 | 14 | 7 | 2 | 1 | 0 | 0 |
| 5S2 | 9 | 5 | 2 | 0 | 0 | 0 | |
| C | 3 | 15 | 11 | 2 | 0 | 0 | 0 |
| 5S2 | 11 | 11 | 0 | 0 | 0 | 0 |
These results suggested an enormous diversity in each lymphoid follicle, greater than would be expected in an immune response to a single Ag in a GC. To address this question in more detail, we analyzed whether identical TCR sequences could be found in independent follicles, which would indicate the recognition of a limited spectrum of Ags. For each patient, 3–11 TCR sequences were randomly chosen for further analysis (Table III). To optimize sensitivity for detecting shared TCR sequences in different follicles, we used hybridization with TCR N-D-N–specific oligonucleotides. A representative result of these experiments is shown in Table IV. 11 N-D-N–specific oligonucleotides, corresponding to the TCR sequences of one of the index follicles, were used to probe amplified cDNA from nine distinct follicles and seven interfollicular zones. Nine of the 11 TCR β-chain sequences were present in multiple follicles. Only two of the probes were exclusively found in the follicles from which they were derived, and, thus, had a restricted distribution pattern. Six of the 11 oligonucleotides hybridized to amplified material from >50% of all follicles, and, thus, had a broad distribution pattern. Moreover, most of the TCR sequences were only detected in follicles and were not represented among interfollicular T cells. The restriction of follicle-derived TCR sequences to follicular structures, and the sharing of TCR β-chains between independent follicles was seen in all patients and for all TCR BV families analyzed in the microdissected materials. As expected, there was no sharing between different patients.
Table III.
TCR β-Chain Sequences of Follicular T Cells
| TCR segment | ||||
|---|---|---|---|---|
| Patient | Probe | BV | N-D-N sequence | BJ |
| 3544 | 1 | 5S2 | AGC AGC TTG TCT CAG CTA GCG GTT TAC ACC | 2S2 |
| 2 | 5S2 | AGC AGC TTA ATG AGG AAC ACT | 2S4 | |
| 3 | 2 | GCT AGA GAA CAT GGG GGC GCG GGT ACC GGG | 2S2 | |
| 4 | 5S2 | AGC AGC CCA GGC CCA GCG GGA GGG GTG GAT | 2S3 | |
| 5 | 5S2 | ATT CAG CTT GGG GGG GGC ACT GAA | 1S1 | |
| 624 | 1 | 3 | AGC AGC TTA CTA GGG GGC CCA CTC TAC GAG | 2S7 |
| 2 | 3 | AGC AGT TTG GGG ACT AGC GGG GAG CAG | 2S1 | |
| 3 | 3 | AGC AGT TAC GGG GGA GGC TTA GGC GAG CAG | 2S7 | |
| 4 | 3 | AGC AGT TTA GCC AAC CGC TCC TAC | 2S7 | |
| 5 | 3 | ACC GGA CAG GGT TTT AGG GAC GGC GAT GGC | 1S2 | |
| 6 | 3 | AGC AGT ACA TCC CAC TCC CGG GAC AGA GGG | 2S3 | |
| 7 | 3 | AGC AGG AGG TCT GGG AGC CAA | 2S7 | |
| 8 | 5S2 | AGC AGC CCT GGG ACT AGC GGA GGG AAT ACG | 2S3 | |
| 9 | 5S2 | AGC AGC TTT GGC CCA TCC TAC | 2S7 | |
| 10 | 5S2 | AGC AGC TTT ACT AGC GGG AGA CCA GAT | 2S3 | |
| 11 | 5S2 | AGC AGC TTG GGA CAC GGA ACT TAC GAG | 2S7 | |
| 3511 | 1 | 5S2 | AGC AGC TAC TTG GAC CGC CCA CCT GGG GCC | 2S6 |
| 2 | 2 | GCT AGA GGA CAG GGC GAC ACT GAA | 1S1 | |
| 3 | 2 | AGT GCT CCC CTA TCG GAC GGA CCT TAT GGC | 1S2 | |
| 1134 | 1 | 6S4 | AGC AGC TCC TCA GGG GGA ACT ACC TAC | 2S1 |
| 2 | 6S4 | AGC AGC TTA GAC CCG GGA GGA GAT | 2S3 | |
| 3 | 6S6 | AGC GCT GTG TCG GAT ACG | 2S3 | |
| 4 | 6S6 | AGC AGC ACG TAC GGG GTC GAG CAG | 2S1 | |
| 5 | 6S6 | AGC AAC ATA GCG GGG GGG ATG GAT ACG | 2S3 | |
| 6 | 8 | AGC AGT CTC GGG CAG GGC GAT ACC GGG | 2S2 | |
| 7 | 8 | AGC AGT CCC GGG TTC CAA GAG | 2S5 | |
| 8 | 8 | AGC AGT TTC CGA TCG CAC AAT | 2S1 | |
| 9 | 8 | AGC AGT GCC GGG ACT AGC GGG GGA GAG | 2S5 | |
| 10 | 8 | AGC AGC CGA AGG GTT GAG ACC | 2S5 | |
| 11 | 6 | AGC AGC CCC CGG GGC GGG CAG TAC GAG | 2S7 | |
| 2064 | 1 | 2 | GCT AGA ACT AGC GGG GGG TCC TAC ACC GGG | 2S2 |
| 2 | 5S2 | AGC AGC TGG GAC AGG GCT GGG GCC | 2S6 | |
| 3 | 5 | AGC AGA AGG ACA GGG CTT TTG AAC ACT | 1S1 | |
| 4 | 8 | AGC AGT CCC TGG CCG TGG CCC CAG | 1S5 | |
| 5 | 5 | AGC AGC CCG GGA CTA GCG GGG GAC TAC | 2S7 | |
| 6 | 6 | AGC AGC GCG GAC AGG GGT ACA GAT | 2S3 |
Table IV.
Sharing of TCR β-Chain Sequences between Independent Synovial Follicles
| Follicle | Transitional zone | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Probe | A | B | C | D | E | F | G | H | I | A | B | C | D | E | F | G |
| 1 | + | + | + | + | − | + | − | + | + | − | − | − | − | − | − | − |
| 2 | + | + | + | + | + | + | + | + | + | − | − | + | − | + | − | − |
| 3 | + | + | + | − | + | − | + | + | − | − | − | − | − | − | − | − |
| 4 | + | − | + | − | + | − | − | + | + | − | − | − | − | − | − | − |
| 5 | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 6 | + | + | + | − | + | − | − | + | + | − | − | − | − | − | − | − |
| 7 | − | + | + | − | − | + | − | + | + | + | − | + | + | + | + | − |
| 8 | − | − | + | − | − | − | + | − | + | − | − | − | − | − | − | − |
| 9 | − | − | + | + | − | − | − | + | + | − | − | − | − | − | − | − |
| 10 | + | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − |
| 11 | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
In summary, the repertoire of T cells recruited to synovial follicles is broad but nonrandom. Most T cells colonizing one follicle are likely to be also seeded into other distally located follicles.
T Cells Shared Among Independent Synovial Follicles Preferentially Express CD8.
To examine the distribution of TCR sequences that were shared between different synovial follicles, CD4 and CD8 T cells from PBMCs and synovial tissue from the same patient were isolated. cDNA was amplified with the appropriate TCR-BV and -BC primers and hybridized with TCR N-D-N–specific oligonucleotides. Surprisingly, these hybridization experiments demonstrated that 70% of all TCR β-chain sequences were from CD8 and not CD4 T cells. Results for all five patients are given in Fig. 1 B. In four of the five tissues analyzed >65% of all TCRs isolated from follicular T cells were expressed on CD8 T cells. In only one of the patients could the majority of follicular TCR sequences be assigned to CD4 T cells. Fig. 1 C shows immunohistochemical localization of CD8 T cells in follicular synovitis. As a rule, CD8 T cells were found in the perifollicular zones. Occasionally, they migrated into the GCs. Overall, they represented only a small subset of the T cells accumulated in the T cell–B cell clusters. The predominance of TCR mRNA from CD8 T cells in follicular picks may, therefore, indicate that these cells are preferentially activated and have upregulated TCR transcription.
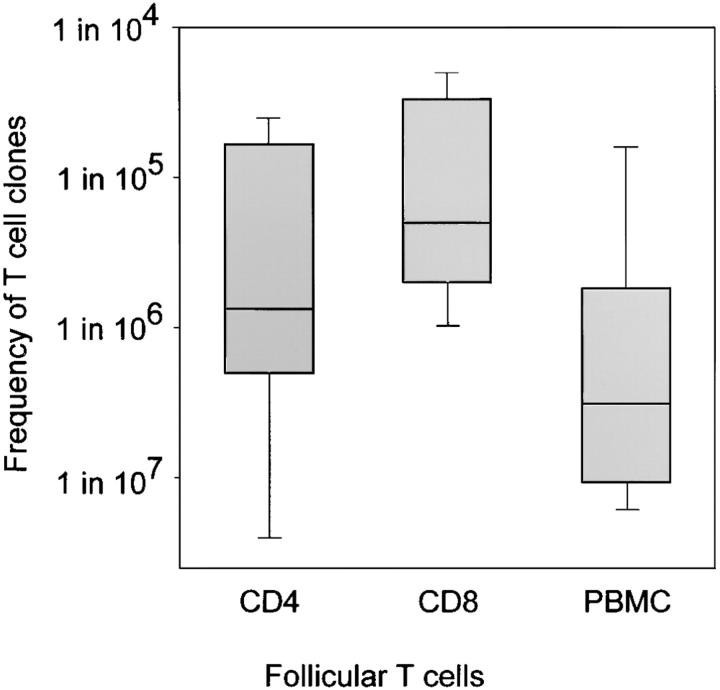
Previous studies have shown that CD8 T cells undergo clonal expansion and attain large clonal size throughout all lymphoid compartments, particularly in patients with RA. To examine whether CD8 T cells homing to the perifollicular zones were part of such clonally expanded T cells, their frequency in the peripheral blood was determined by a limiting dilution assay and PCR amplification/oligonucleotide hybridization to detect specific TCR sequences. All TCR N-D-N probes generated from microdissected T cells gave positive hybridization results on peripheral T cells. TCR β-chains expressed by synovial tissue CD4 T cells were found at an average frequency of 1-in-750,000 peripheral blood CD4 T cells (Fig. 2) . TCR probes derived from synovial CD8 T cells were represented at an average of 1-in-200,000 circulating CD8 T cells. These findings indicated that T cells colonizing the synovial follicles were not part of large clonal expansions. Randomly selected TCR β-chains appear at a frequency of ∼1-in-5 × 106 T cells in patients with RA (15). Thus, T cells participating in synovial follicles were slightly enriched, consistent with them being memory cells; however, they clearly did not reach the frequencies expected for globally expanded T cells. Their presence in the tissue indicated a highly selective accumulation, as can be expected from Ag-specific T cells driving GC reactions.
Figure 2.
Follicular T cells are relatively enriched in the synovial tissue. Frequencies of TCR β-chain sequences isolated from synovial follicles were determined in peripheral blood by limiting dilution. TCR amplification and hybridization with N-D-N–specific oligonucleotides was used to detect the presence of specific sequences. Results are shown as box plots displaying medians, 25th–75th percentile as the box and the 10th and 90th percentiles as whiskers. T cells expressing TCR β-chains derived from synovial tissue follicles were infrequent in peripheral blood. Sequences used by follicular CD8 T cells were slightly more frequent than those isolated from follicular CD4 T cells. Arbitrarily chosen TCR sequences from PBMCs of patients with RA have a median frequency of 1-in-5 × 106 T cells. The enrichment of T cells with shared TCR β-chains in the synovial follicles is consistent with them being memory cells.
CD8 T Cells in Synovial Follicles Express CD40L and Produce IFN-γ, but Lack Perforin.
T cell involvement in GC formation has been related to CD40–CD40L interaction between B cells and CD4+ T cells (19, 20). We have recently described that tissues from patients with extranodal GC formation selectively contain a specialized subset of CD8 T cells, CD8+CD40L+ T cells (16). Therefore, we explored whether CD8 T cells contributing to follicles in the synovial tissue expressed CD40L. In the patients studied, CD40L was found on 10–12% of synovial CD4 and CD8 T cells (data not shown). Mononuclear cells from synovial tissue specimens of three of the five patients were sorted into CD40L+ and CD40L− subsets. These T cell subsets were then analyzed for the presence of TCR sequences amplified from the T cells colonizing the follicles. Seven of 11 TCR sequences were detected in the CD40L+ subset (Table V). The majority of follicular CD8 TCR sequences were represented in CD40L+ T cells and were at least tenfold enriched in comparison with synovial CD40L− T cells.
Table V.
Representative Follicular TCR Sequences in Synovial Tissue CD40L+ and CD40L–T Cell Subsets
| Presence in | |||||
|---|---|---|---|---|---|
| CD4 | CD8 | Patient | TCRprobe | 10 × 103 CD40L−cells | 103 CD40L+cells |
| + | 2064 | 3 | − | − | |
| + | 4 | − | − | ||
| + | 5 | − | − | ||
| + | 6 | − | + | ||
| + | 3544 | 1 | − | + | |
| + | 2 | − | − | ||
| + | 3 | − | + | ||
| + | 4 | − | + | ||
| + | 5 | + | + | ||
| + | 3511 | 1 | − | + | |
| + | 2 | − | + |
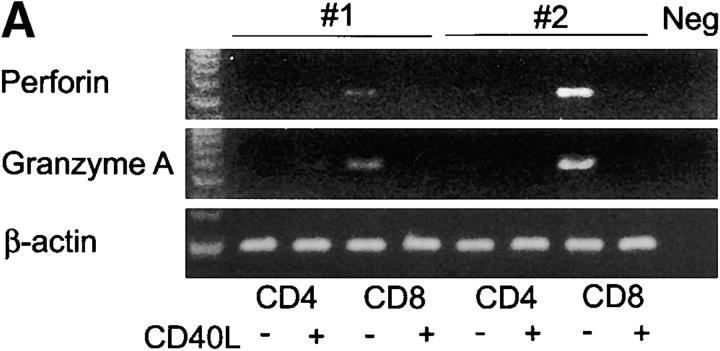
To determine whether CD8+CD40L+ were functionally distinct, all four T cell subpopulations (CD4+ and CD8+ T cells fractionated into CD40L– and CD40L+ subsets by FACS®) were examined for the transcription of perforin and granzyme A. As shown in Fig. 3 A, transcripts for perforin and granzyme A were exclusively detected in CD8+ T cells lacking CD40L. CD8 cells positive for CD40L did not have the capability to synthesize the cytolytic molecules, perforin and granzyme A. This finding was confirmed by immunohistochemistry (Fig. 3 B). Synovial lymphoid follicles contained only very few perforin-positive cells, most of which did not express the CD8 marker. The majority of CD8 T cells in the follicle were perforin negative.
Figure 3.
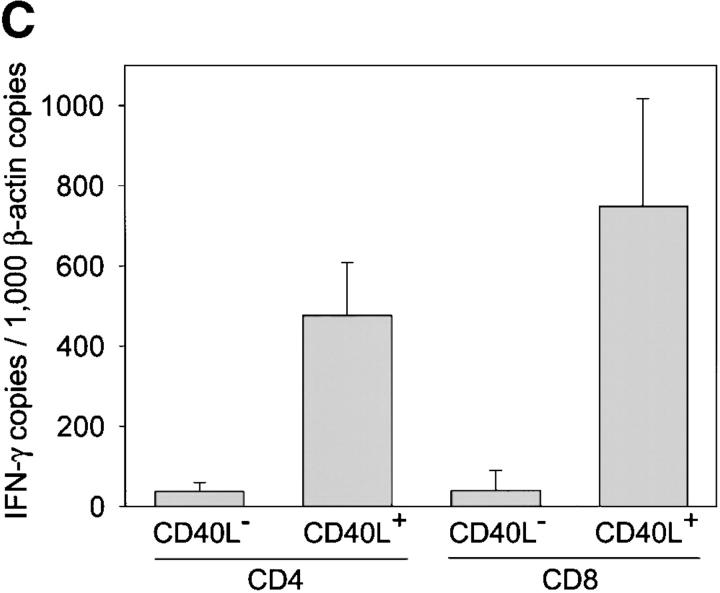
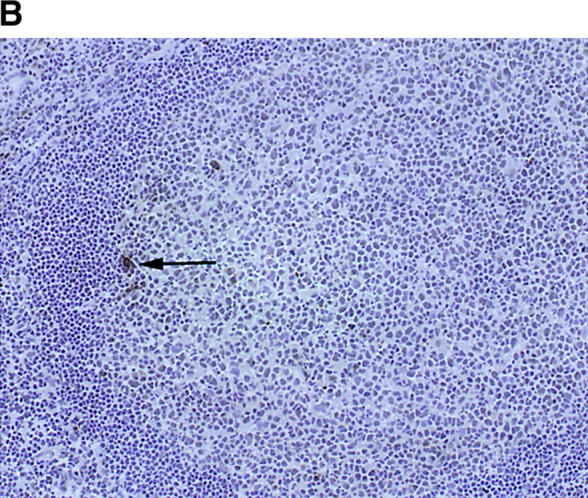
Functional profiles of synovial CD8+ T cell subsets. TCR sequences that were derived from synovial follicles were found in purified synovial CD8+CD40L+ T cells, suggesting that follicular CD8+ T cells may have a distinct functional profile (Table V). Synovial tissue CD8+ T cells were separated into CD40L+ and CD40L− populations by FACS® and analyzed for the transcription of perforin, granzyme A, and IFN-γ. (A) Results for two patients are shown. Expression of perforin and granzyme A was restricted to CD8+CD40L− T cells. (B) Immunohistochemistry confirmed that the majority of CD8 T cells (blue, yellow arrows) in the synovial lymphoid follicles lacked perforin expression. Perforin-positive cells (brown, black arrows) were absent in most follicles (left) and only occasionally seen in others (right). These perforin-positive cells did not coexpress CD8 and were presumably NK or NK T cells. Original magnification: × 200. (C) IFN-γ–specific transcripts were produced at significantly higher levels in CD40L+ than CD40L− T cells. IFN-γ was semiquantified by PCR-ELISA and is shown as mean copy numbers ± SD of triplicate measurements.
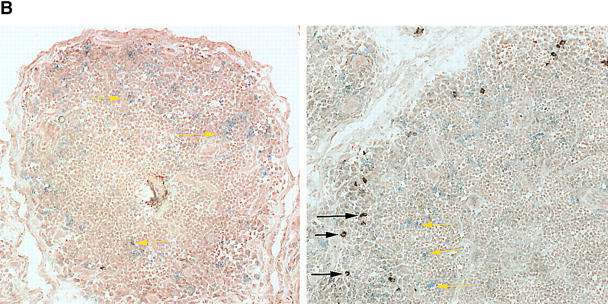
In the absence of cytotoxic function, the major function of CD8+CD40L+ T cells is likely related to cytokine production. To pursue this possibility, CD4+ and CD8+, CD40L+ and CD40L– T cells were analyzed for the expression of IFN-γ transcripts. IFN-γ gene-specific sequences were abundant in the CD4+CD40L+ population but were even more so in CD8+CD40L+ cells (Fig. 3 C). In contrast, IFN-γ mRNA was barely detected in CD40L− T cells. Production of IFN-γ protein in follicular CD8 T cells was confirmed by immunohistochemical studies in tissue sections. Two-color staining identified IFN-γ–producing CD8 T cells in the outer layers of the follicles in close proximity to the follicular mantle zone of small B-lymphocytes immediately surrounding the GC (Fig. 4 A). These experiments also indicated that most of the IFN-γ–releasing cells in the rheumatoid synovium belonged to the CD8 subset. The presence of such IFN-γ–producing CD8 T cells in perifollicular zones is typical for GC formation in the rheumatoid synovium and is not seen in tonsils (Fig. 4 B). Tonsillar lymphoid follicles rarely contained IFN-γ–producing cells and, if present, they were CD8−. The few IFN-γ+ cells found in some follicles did not express a CD8 marker.
Figure 4.
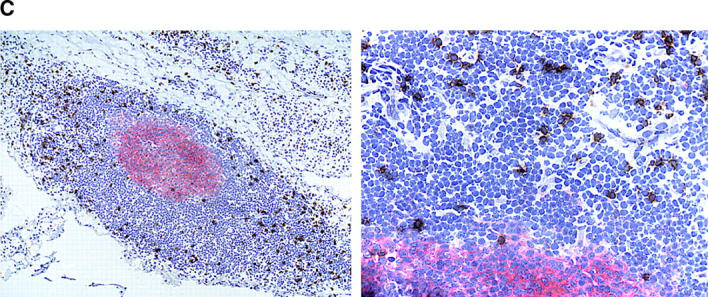
IFN-γ production by follicular CD8 T cells. (A) Synovial tissue sections were stained with anti-CD8 (blue) and anti–IFN-γ (brown) mAb. Most of the IFN-γ–producing cells expressed CD8 (white arrows). CD8+IFN-γ+ cells were located in the perifollicular zone at the outer edge of the mantle zone. (B) Yellow arrows mark CD8+IFN-γ− cells. In contrast, IFN-γ–producing cells were extremely infrequent in tonsillar follicles, where they had a CD8− phenotype (black arrow). Original magnifications: × 200; inset, × 400.
In summary, CD8+ T cells that selectively home to follicular structures in the rheumatoid synovium display a functional profile compatible with their involvement in Ag-specific T cell–B cell interactions and GC reactions.
Depletion of Synovial CD8 T Cells Inhibits the Production of Tissue Cytokines.
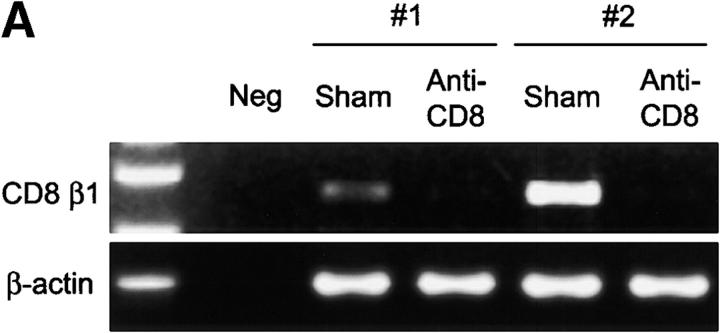
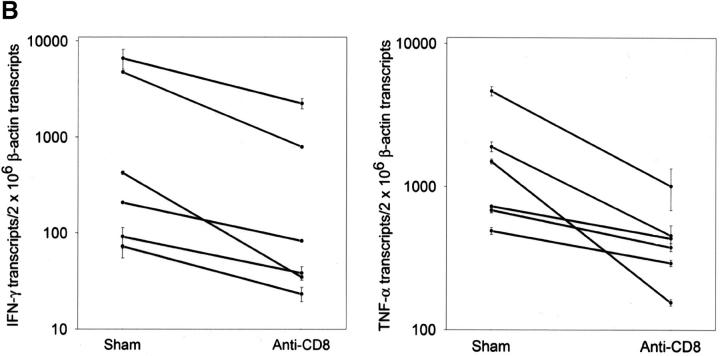
To examine the contribution of CD8 T cells to rheumatoid synovitis, particularly to the formation and maintenance of extranodal GCs, we performed depletion studies in synovium-SCID mouse chimeras. SCID mice were implanted with human synovium collected from six patients with RA with typical GC formation. After tissue engraftment, SCID mouse chimeras carrying tissue from the same donor were assigned to a buffer control group, a control mAb-treated group, or an anti-CD8 mAb treated group. A dose of 600 μg/d anti-CD8 mAb was sufficient to deplete CD8 T cells from the human tissue (Fig. 5 A). In all experiments, depletion of CD8 T cells resulted in a marked reduction of tissue IFN-γ transcription (P = 0.005; Fig. 5 B). CD8-depleted tissues contained <30% of control levels of IFN-γ–specific transcripts. Treatment with control Ig did not have any effect. Elimination of synovial CD8 T cells not only suppressed the production of IFN-γ mRNA, but it also caused a sharp reduction in the transcription of TNF-α (Fig. 5 B). Tissues from the anti-CD8–treated chimeras contained fivefold less TNF-α–specific sequences than the control tissues (P = 0.003).
Figure 5.
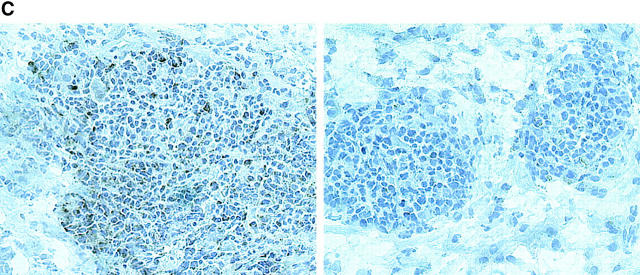
Depletion of synovial CD8 T cells suppresses IFN-γ and TNF-α production. Synovial tissues from patients with RA were engrafted into NOD-SCID mice. Chimeras were treated with anti-CD8 mAb; synovial tissue grafts were explanted after 7 d and analyzed for cytokine transcription. Anti-CD8 mAb treatment effectively depleted CD8 cells from the synovial tissue. (A) Transcripts for the CD8 β-chain were amplified by PCR in tissue extracts prepared from the grafts of sham or anti-CD8 treated chimeras. (B) After depletion of synovial CD8 T cells, in situ transcription of IFN-γ and TNF-α was significantly diminished. Results from six experiments with anti-CD8 mAb– and sham-treated mice are shown. Transcript numbers are adjusted relative to 2 × 106 β-actin transcripts. Data are given as the mean ± SD of triplicate measurements by PCR-ELISA. (C) Immunohistochemical analysis of tissues retrieved from anti–CD8–treated mice (right) demonstrated that the tissues were depleted of IFN-γ+ cells (brown) in contrast to sham-treated mice (left). Ab-mediated depletion of CD8 T cells resulted in the disintegration of synovial follicles and the formation of cell clusters composed of dysmorphic lymphocytes.
Depletion of IFN-γ–producing cells was confirmed by immunohistochemistry. Fig. 5 C shows that synovial tissue sections from anti-CD8 treated chimeras were negative for IFN-γ–producing lymphocytes.
Depletion of CD8 T Cells Disrupts the Function of Synovial Tissue GCs.
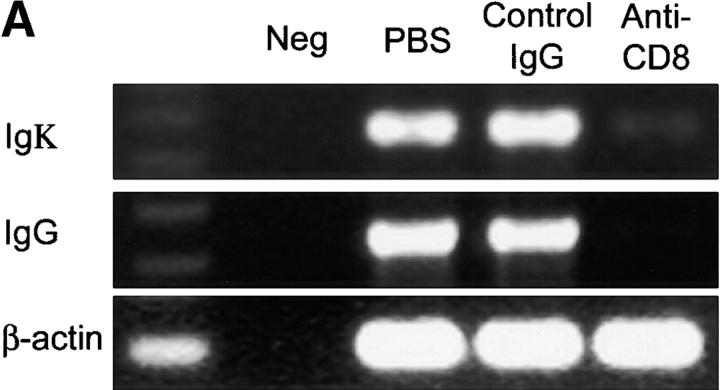
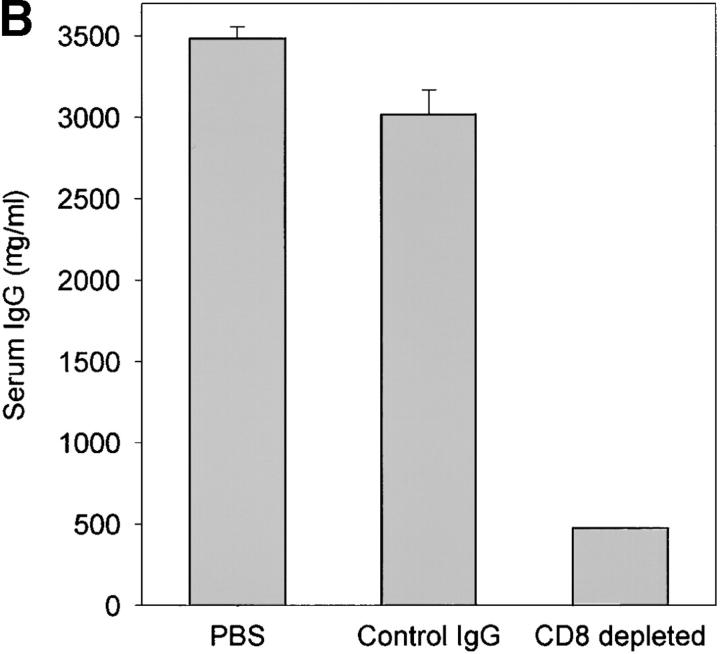
Injection of anti-CD8 not only depleted IFN-γ–producing cells; it also led to a dramatic change in the lymphoid microstructures. In the absence of CD8 T cells, GCs were no longer maintained (Fig. 5 C). T cell–B cell follicles disintegrated, and dysmorphic lymphocytes were assembled in small clusters. To evaluate the effects of these microstructural changes on B cell function, we compared Ig production in tissue samples with and without CD8 T cells. Synovial tissue removed from sham-treated chimeras contained high levels of Igκ- and IgG-specific sequences (Fig. 6 A). Ab-mediated removal of CD8 T cells was associated with a marked reduction of Igκ transcripts and a complete loss of IgG transcripts. To quantify the secretion of Ig, serum from the chimeras was collected just before the explanation of the human tissue grafts, and human Ig was measured. Synovial tissue grafts obviously released large amounts of IgG (Fig. 6 B) but produced only minimal amounts when CD8 T cells were depleted. Again, treatment with isotype-matched control Ig did not have any effect.
Figure 6.
Ig production in synovial GCs is CD8 T cell dependent. Human synovium-SCID mouse chimeras were either sham-treated or treated with anti-CD8 mAb or isotype-matched control IgG as described in Fig. 5. Serum and synovial tissues were collected from the mice 7 d after the treatment.(A) cDNA from the tissues were amplified with Igκ- and IgG-specific primer sets in multiplex PCR. Anti-CD8 mAb treatment but not treatment with control IgG clearly diminished the transcription of Ig H and L chains. (B) Human IgG was quantified in serum from the treated and sham-treated mice by ELISA. Tissue depleted of CD8 T cells secreted minimal amounts of human IgG. Results are shown as mean ± SD of triplicate measurements. Similar results were seen in experiments with tissues from two additional patients.
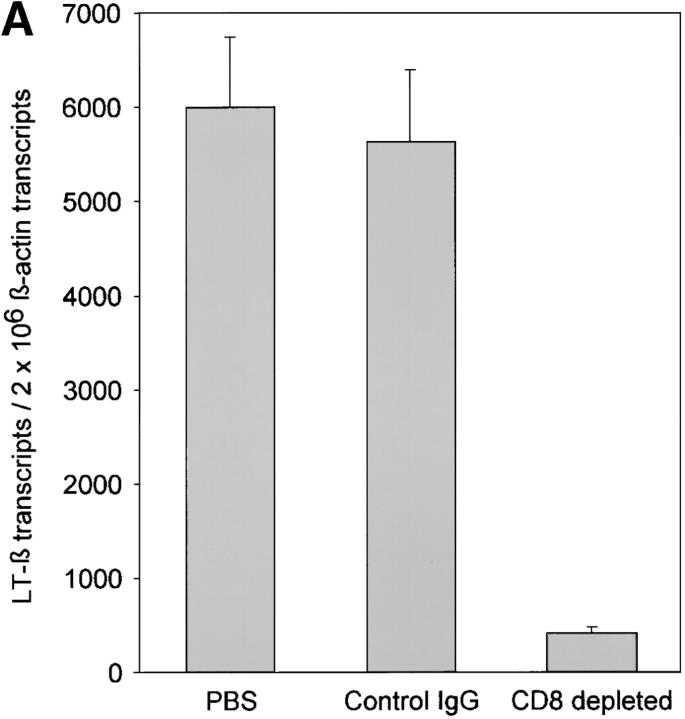
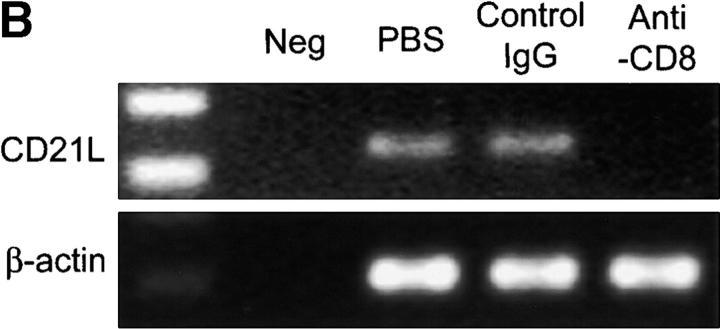
Functional GCs depend on the presence of FDCs and LT-α1β2. Our previous studies have demonstrated that LT-α1β2 also has a central position in extranodal lymphoid neogenesis in the rheumatoid joint (7). Among a series of cytokines and chemokines evaluated in rheumatoid synovium with distinct types of lymphoid microstructures, LT-α1β2 levels had independent predictive power in identifying tissues with GCs. Therefore, levels of LT-β mRNA were semiquantified in control grafts and anti-CD8–treated grafts. Depletion of CD8 T cells correlated with an almost complete loss of LT-β transcription (Fig. 7 A). CD8 T cell depleted follicles were also unable to retain FDCs. Anti-CD8–treated grafts no longer contained transcripts for CD21L, the most sensitive marker for the presence of FDCs in the synovium (Fig. 7 B). These data implicated CD8 T cells as being critical in regulating LT-α1β2 expression and retaining FDCs in the synovium, thereby maintaining functional GCs.
Figure 7.
Depletion of CD8 T cells disrupts synovial GCs. GCs depend on the production of LT-α1β2 and the presence of FDCs. LT-β transcripts were semiquantified by PCR-ELISA. (A) Treatment with anti-CD8 mAb significantly reduced the relative number of LT-β transcripts in the tissues. Results from one of three experiments are shown as mean ± SD of triplicate measurements. CD8 T cell depletion abrogated CD21L transcription, confirming that FDC networks were no longer present. (B) Synovial tissues retrieved from control and anti-CD8–treated chimeras were assayed for the presence of CD21L transcript, which is exclusively produced by FDCs.
Discussion
This study establishes an unexpected and critical role of CD8 T cells in the formation and maintenance of ectopic GC reactions in rheumatoid synovitis. Depletion of synovial CD8 T cells caused the disintegration of lymphoid follicles and abrogated the production of Ig. CD8 T cells accumulating in the perifollicular region of synovial follicles expressed a unique phenotype and functional profile; they lacked perforin but produced IFN-γ and expressed CD40L. Data presented here suggest that the process of lymphoorganogenesis in the tertiary lymphoid tissue of the rheumatoid joint uses novel cellular pathways and support the model that the presence of CD8+IFN-γ+CD40L+ perforin− T cells is a disease risk factor in RA.
The inflammatory lesion in the synovial membrane of rheumatoid joints is heterogeneous, containing distinct patterns of lymphoid microstructures (21, 22). Most frequently, T cells, B cells, and macrophages are arranged as diffuse infiltrates that lack characteristic topographies. About 50% of patients form follicular aggregates of T and B cells. In a subset of these patients, classic GCs are generated (5, 7). In general, CD8 T cells are a minor population in the synovial lesion. So far, they have been assumed to be restricted to the interfollicular zones. A prior study demonstrated that the frequency of tissue-infiltrating CD8 T cells correlated with the presence of active GC reactions (16). CD8 T cells with a unique phenotype of being CD40L+ can be localized in the outer layer of follicles. Here, we show that these CD8 T cells produce IFN-γ in situ, lack expression of perforin, and hold a critical position in the functional integrity of the GCs.
Comparison of CD8 frequencies in peripheral blood and synovial tissue ruled out that the accumulation of CD8 T cells in synovial follicles was merely a reflection of repertoire abnormalities in patients with RA. CD8 T cells are known to undergo clonal expansion (23–25), and recent studies have proven that the entire T cell repertoire of patients with RA is contracted (15, 26). Perifollicular CD8 TCR sequences collected by micromanipulation were 10–100-fold enriched in the tissue, and frequencies in the periphery were compatible with them being normal memory cells; they were not in the range that is seen with oligoclonal CD8+CD28null T cells. These data suggest that follicular CD8+ T cells are selectively enriched, as one would expect in Ag-specific responses.
A series of studies has identified EBV-derived proteins as the Ag driving clonally expanded synovial CD8 T cells (27). Scotel et al. (28) identified the Ag as the EBV transactivators, BZLF1 and MBLF1. However, EBV gene expression is not only detected in synovial tissues from patients with RA but also in samples from osteoarthritic joints (29). A nonspecific accumulation of EBV-specific CD8 T cells in inflamed tissues has been suggested by HLA tetramer studies (30, 31). In contrast, the CD8+perforin− T cells described here were not reactive to EBV and could not be activated by autologous EBV-transformed lymphoblasts (data not shown). The nature of the Ag recognized by these CD8 cells remains to be identified.
Elimination of CD8 T cells in synovium-SCID mouse chimeras demonstrated that IFN-γ transcription in the tissue was largely dependent on these cells. Immunohistochemical stains confirmed that the overwhelming majority of IFN-γ–producing cells expressed CD8 and were characterized by a unique site of residence. CD8 T cell depletion also inhibited TNF-α production. This could either be a direct effect of CD8 depletion or a result of the loss of IFN-γ, which is critical in the survival of TNF-α–producing macrophages in synovial lesions (18). Unexpected was the profound consequences of CD8 depletion on the structural and functional integrity of synovial GCs. The microarchitecture collapsed with dysmorphic cells clumping together. Consistent with the structural disintegration, CD8-depleted tissues produced only minimal amounts of human Ig, and production of LT-α1β2 was greatly impaired. FDC networks were no longer maintained, as confirmed by the disappearance of CD21L, a robust marker for FDCs in the synovial membrane (17, 32).
As opposed to secondary lymphoid tissues, FDC networks only exist in the synovium if GC reactions are ongoing. Previous studies have shown that tissue concentration of LT-α1β2 is the strongest predictor for the presence of FDCs in the synovial tissue. LT-α1β2 is, at least in part, produced by B cells in the mantle zone of the follicle. Thus T cell–B cell activation, presumably through the recognition of Ag, represents a condition sine qua non for ectopic lympho-organogenesis and FDC recruitment/maturation in the synovium. In this context, the positioning of CD8+IFN-γ+ T cells in the topography of the rheumatoid lesion provides important clues. It has been assumed that T cell–B cell interactions typically occur at the edge of the GC, at the border to the mantle zone. CD8+IFN-γ+ T cells are located at the outer circumference of the mantle zone. In this area, CD83+ interdigitating dendritic cells are found and could be possible partners. More importantly, this positioning would make mantle zone B cells, which have not yet encountered their specific Ag, the partner of this T cell subset.
Provided that CD8+ cells exhibit a helper function in GC formation, the question arises as to the essential elements in CD8 T cell–B cell interaction. We have previously identified two distinct subsets of CD8 T cells in the synovial tissue of RA joints (16). These subsets were distinguished by the presence of perforin and the transcription of IFN-γ, which appeared to be mutually exclusive functional characteristics of two CD8 T cell subpopulations (33). The subset of CD8+IFN-γ+perforin− T cells expressed CD40L and could function as Th cells for B cells. It remains to be determined how IFN-γ production has a place in this process; this cytokine is generally not considered to be crucial in GC reactions.
Collectively, CD8 T cells were found to be critical in establishing/maintaining GC reactions in the rheumatoid joint. Although in the minority, they represent the majority of IFN-γ–producing cells, and the depletion experiments showed that CD4 T cells alone are not sufficient. Nevertheless, CD4 T cells clearly participate in GC reactions. Our microdissection studies painted a very similar picture for CD4 and CD8 T cells. TCRs from both T cell subsets were enriched in the GCs and were shared between different follicles from the same patients, clearly indicating that CD4 and CD8 T cells recognize Ag in the GCs. For CD4 T cells, our results parallel the findings of Zheng et al. (34), who could trace TCR sequences of CD4 T cells from periarteriolar sheets to several follicles. Therefore, our results indicate that CD4 and CD8 T cells act in concert to form GCs in the synovial tissue. Our findings also allow for conclusions on the nature of the Ag handled in the tertiary lymphoid structure. B cells encounter Ag in the blood or in specialized areas of the lymph node on the surface of Ag-transporting cells or follicular dendritic cells. Upon Ag uptake, the B cells process it and present it to their Ag-specific T cell partner to receive help and enter the GCs. Ingestion by endocytosis or phagocytosis should target the Ag toward the endocytic processing pathway to be presented in the context of HLA class II molecules and to be recognized by CD4+ T cells (35–37). Peptides bound to HLA class I molecules derive from a different pool, e.g., the endogenous pool of Ag produced within the cell itself, and access the endoplasmic reticulum. Stimulation of HLA class I–restricted CD8+ T cells to endogenous Ag may, therefore, be a critical event. B cells in the mantle zone of synovial follicles may harbor an Ag that drives the response of CD8+ T cells, eventually giving rise to a GC reaction.
This study has immediate implications for our understanding of RA and the definition of potential therapeutic targets. Treatment aimed at CD8 T cells has not been explored. Because only a minor subset of CD8 T cells has the functional capabilities described here, experimental therapies targeting at CD8+IFN-γ+ T cells may be worthwhile. Also, focusing the search for arthritogenic Ag, with an emphasis on endogenous Ag presented at the outer border of the mantle zone in synovial T cell–B cell follicles, could overcome a central hurdle in RA, namely the identification of the primary Ag recognition event that leads to disease.
Acknowledgments
The authors thank James W. Fulbright for manuscript and figure preparation.
This research was supported by grants from the National Institutes of Health (R01AR42527, R01AR41974, and R01AI44142).
Footnotes
*
Abbreviations used in this paper: CD21L, CD21 long isoform; GC, germinal center; LT, lymphotoxin; RA, rheumatoid arthritis.
References
- 1.Nepom, G.T. 1998. Major histocompatibility complex-directed susceptibility to rheumatoid arthritis. Adv. Immunol. 68:315–332. [DOI] [PubMed] [Google Scholar]
- 2.Weyand, C.M., T.G. McCarthy, and J.J. Goronzy. 1995. Correlation between disease phenotype and genetic heterogeneity in rheumatoid arthritis. J. Clin. Invest. 95:2120–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yen, J.H., B.E. Moore, T. Nakajima, D. Scholl, D.J. Schaid, C.M. Weyand, and J.J. Goronzy. 2001. Major histocompatibility complex class I–recognizing receptors are disease risk genes in rheumatoid arthritis. J. Exp. Med. 193:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young, C.L., T.C. Adamson, J.H. Vaughan, and R.I. Fox. 1984. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthr. Rheum. 27:32–39. [DOI] [PubMed] [Google Scholar]
- 5.Schroder, A.E., A. Greiner, C. Seyfert, and C. Berek. 1996. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 93:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itoh, K., E. Meffre, E. Albesiano, A. Farber, D. Dines, P. Stein, S.E. Asnis, R.A. Furie, R.I. Jain, and N. Chiorazzi. 2000. Immunoglobulin heavy chain variable region gene replacement as a mechanism for receptor revision in rheumatoid arthritis synovial tissue B lymphocytes. J. Exp. Med. 192:1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takemura, S., A. Braun, C. Crowson, P.J. Kurtin, R.H. Cofield, W.M. O'Fallon, J.J. Goronzy, and C.M. Weyand. 2001. Lymphoid neogenesis in rheumatoid synovitis. J. Immunol. 167:1072–1080. [DOI] [PubMed] [Google Scholar]
- 8.Ruddle, N.H. 1999. Lymphoid neo-organogenesis: lymphotoxin's role in inflammation and development. Immunol. Res. 19:119–125. [DOI] [PubMed] [Google Scholar]
- 9.Cyster, J.G. 1999. Chemokines and cell migration in secondary lymphoid organs. Science. 286:2098–2102. [DOI] [PubMed] [Google Scholar]
- 10.Kelsoe, G. 1996. The germinal center: a crucible for lymphocyte selection. Semin. Immunol. 8:179–184. [DOI] [PubMed] [Google Scholar]
- 11.Przylepa, J., C. Himes, and G. Kelsoe. 1998. Lymphocyte development and selection in germinal centers. Curr. Top. Microbiol. Immunol. 229:85–104. [DOI] [PubMed] [Google Scholar]
- 12.McHeyzer-Williams, L.J., D.J. Driver, and M.G. McHeyzer-Williams. 2001. Germinal center reaction. Curr. Opin. Hematol. 8:52–59. [DOI] [PubMed] [Google Scholar]
- 13.Goronzy, J.J., P. Bartz-Bazzanella, W. Hu, M.C. Jendro, D.R. Walser-Kuntz, and C.M. Weyand. 1994. Dominant clonotypes in the repertoire of peripheral CD4+ T cells in rheumatoid arthritis. J. Clin. Invest. 94:2068–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takemura, S., P.A. Klimiuk, A. Braun, J.J. Goronzy, and G.M. Weyand. 2001. T cell activation in rheumatoid synovitis is B cell dependent. J. Immunol. 107:4710–4718. [DOI] [PubMed] [Google Scholar]
- 15.Wagner, U.G., K. Koetz, C.M. Weyand, and J.J. Goronzy. 1998. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 95:14447–14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner, U.G., P.J. Kurtin, A. Wahner, M. Brackertz, D.J. Berry, J.J. Goronzy, and C.M. Weyand. 1998. The role of CD8+ CD40L+ T cells in the formation of germinal centers in rheumatoid synovitis. J. Immunol. 161:6390–6397. [PubMed] [Google Scholar]
- 17.Brack, A., H.L. Rittner, B.R. Younge, C. Kaltschmidt, C.M. Weyand, and J.J. Goronzy. 1997. Glucocorticoid-mediated repression of cytokine gene transcription in human arteritis-SCID chimeras. J. Clin. Invest. 99:2842–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klimiuk, P.A., H. Yang, J.J. Goronzy, and C.M. Weyand. 1999. Production of cytokines and metalloproteinases in rheumatoid synovitis is T cell dependent. Clin. Immunol. 90:65–78. [DOI] [PubMed] [Google Scholar]
- 19.Calderhead, D.M., Y. Kosaka, E.M. Manning, and R.J. Noelle. 2000. CD40-CD154 interactions in B-cell signaling. Curr. Top. Microbiol. Immunol. 245:73–99. [DOI] [PubMed] [Google Scholar]
- 20.Grewal, I.S., and R.A. Flavell. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111–135. [DOI] [PubMed] [Google Scholar]
- 21.Klimiuk, P.A., J.J. Goronzy, J. Björnsson, R.D. Beckenbaugh, and C.M. Weyand. 1997. Tissue cytokine patterns distinguish variants of rheumatoid synovitis. Am. J. Pathol. 151:1311–1319. [PMC free article] [PubMed] [Google Scholar]
- 22.Weyand, C.M., A. Braun, S. Takemura, and J.J. Goronzy. 2001. Lymphoid microstructures in rheumatoid arthritis. In Current Directories Autoimmunity. J.J. Goronzy and C.M. Weyand, editors. Karger, Basel. 168–187. [DOI] [PubMed]
- 23.Posnett, D.N., R. Sinha, S. Kabak, and C. Russo. 1994. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J. Exp. Med. 179:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzgerald, J.E., N.S. Ricalton, A.C. Meyer, S.G. West, H. Kaplan, C. Behrendt, and B.L. Kotzin. 1995. Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J. Immunol. 154:3538–3547. [PubMed] [Google Scholar]
- 25.Hingorani, R., J. Monteiro, R. Furie, E. Chartash, C. Navarrete, R. Pergolizzi, and P.K. Gregersen. 1996. Oligoclonality of Vβ3 TCR chains in the CD8+ T cell population of rheumatoid arthritis patients. J. Immunol. 156:852–858. [PubMed] [Google Scholar]
- 26.Goronzy, J.J., and C.M. Weyand. 2001. Thymic function and peripheral T-cell homeostasis in rheumatoid arthritis. Trends Immunol. 22:251–255. [DOI] [PubMed] [Google Scholar]
- 27.David-Ameline, J., A. Lim, F. Davodeau, M.A. Peyrat, J.M. Berthelot, G. Semana, C. Pannetier, J. Gaschet, H. Vie, J. Even, and M. Bonneville. 1996. Selection of T cells reactive against autologous B lymphoblastoid cells during chronic rheumatoid arthritis. J. Immunol. 157:4697–4706. [PubMed] [Google Scholar]
- 28.Scotet, E., J. David-Ameline, M.A. Peyrat, A. Moreau-Aubry, D. Pinczon, A. Lim, J. Even, G. Semana, J.M. Berthelot, R. Breathnach, et al. 1996. T cell response to Epstein-Barr virus transactivators in chronic rheumatoid arthritis. J. Exp. Med. 184:1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edinger, J.W., M. Bonneville, E. Scotet, E. Houssaint, H.R. Schumacher, and D.N. Posnett. 1999. EBV gene expression not altered in rheumatoid synovia despite the presence of EBV antigen-specific T cell clones. J. Immunol. 162:3694–3701. [PubMed] [Google Scholar]
- 30.Scotet, E., M.A. Peyrat, X. Saulquin, C. Retiere, C. Couedel, F. Davodeau, N. Dulphy, A. Toubert, J.D. Bignon, A. Lim, et al. 1999. Frequent enrichment for CD8 T cells reactive against common herpes viruses in chronic inflammatory lesions: towards a reassessment of the physiopathological significance of T cell clonal expansions found in autoimmune inflammatory processes. Eur. J. Immunol. 29:973–985. [DOI] [PubMed] [Google Scholar]
- 31.Tan, L.C., A.G. Mowat, C. Fazou, T. Rostron, H. Roskell, P.R. Dunbar, C. Tournay, F. Romagne, M.A. Peyrat, E. Houssaint, et al. 2000. Specificity of T cells in synovial fluid: high frequencies of CD8+ T cells that are specific for certain viral epitopes. Arthr. Res. 2:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, Y.J., J. Xu, O. de Bouteiller, C.L. Parham, G. Grouard, O. Djossou, B. de Saint-Vis, S. Lebecque, J. Banchereau, and K.W. Moore. 1997. Follicular dendritic cells specifically express the long CR2/CD21 isoform. J. Exp. Med. 185:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appay, V., D.F. Nixon, S.M. Donahoe, G.M. Gillespie, T. Dong, A. King, G.S. Ogg, H.M. Spiegel, C. Conlon, C.A. Spina, et al. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng, B., S. Han, and G. Kelsoe. 1996. T helper cells in murine germinal centers are antigen-specific emigrants that downregulate Thy-1. J. Exp. Med. 184:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison, L.A., A.E. Lukacher, V.L. Braciale, D.P. Fan, and T.J. Braciale. 1986. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J. Exp. Med. 163:903–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niedermann, G., E. Geier, M. Lucchiari-Hartz, N. Hitziger, A. Ramsperger, and K. Eichmann. 1999. The specificity of proteasomes: impact on MHC class I processing and presentation of antigens. Immunol. Rev. 172:29–48. [DOI] [PubMed] [Google Scholar]
- 37.Chapman, H.A. 1998. Endosomal proteolysis and MHC class II function. Curr. Opin. Immunol. 10:93–102. [DOI] [PubMed] [Google Scholar]