GATA-1 as a Regulator of Mast Cell Differentiation Revealed by the Phenotype of the GATA-1low Mouse Mutant (original) (raw)
Abstract
Here it is shown that the phenotype of adult mice lacking the first enhancer (DNA hypersensitive site I) and the distal promoter of the GATA-1 gene (neoΔHS or GATA-1low mutants) reveals defects in mast cell development. These include the presence of morphologically abnormal alcian blue+ mast cells and apoptotic metachromatic− mast cell precursors in connective tissues and peritoneal lavage and numerous (60–70% of all the progenitors) “unique” trilineage cells committed to erythroid, megakaryocytic, and mast pathways in the bone marrow and spleen. These abnormalities, which were mirrored by impaired mast differentiation in vitro, were reversed by retroviral-mediated expression of GATA-1 cDNA. These data indicate an essential role for GATA-1 in mast cell differentiation.
Keywords: mast cells, GATA-1, differentiation, commitment, progenitor cells
Introduction
Considerable progress has been made in recent years in our understanding of mast cell differentiation (1, 2). Mast cells derive from c-kitlow CD34low Sca-1+ progenitor cells, present in the marrow and spleen (3, 4). These give rise in vivo to c-kithigh CD34high Sca-1+/− precursors characterized by extensive proliferation capacity, the presence of small cytoplasmic granules, and absent or low expression of the receptor that binds the Fc portion of the IgE antibody with high affinity (FcɛRI−; references 4 and 5). In the mouse, mast cell precursors circulate in the fetal blood (5) to populate the connective and mucosal tissues where they reside throughout adult life and mature into the respective classes of definitive connective and mucosal mast cells (1, 2), characterized by specific protease expression profiles (6). At least two classes of mast cell progenitors have been identified in the mouse on the basis of the colonies that they give rise to in culture. In fact, a minority of the mixed (erythroid [E],* megakaryocytic [Mk], and granulocytic-monocytic [GM]) colonies detected after 14 d of culture contain mast cells (7), indicating that the progenitor cells from which they derive had mast cell differentiation potential. On the other hand, colonies composed of ≅500 mast cells each, which presumably derive from unilineage progenitor cells, have also been identified after 21 d of semisolid culture stimulated with IL-3 and stem cell factor (SCF; reference 8).
The molecular mechanisms underlying mast cell differentiation are better detailed in vitro in liquid cultures stimulated with IL-3 supplemented with either a fibroblast feeder layer (9) or SCF (10, 11). Under such conditions, mononuclear cells from the marrow (and spleen) give rise to bone marrow–derived mast cells (BMMC), which phenotypically and functionally resemble mast cell precursors purified from the connective tissues of the adult animals. In fact, BMMC are c-kithigh FcɛRI− cells with small alcian blue− cytoplasmic granules (12) that reconstitute connective and mucosal mast cell function when transplanted into mast cell–deficient animals (13) and mature in 21 d into berberine sulfate+ FcɛRI+ mast cells capable of taking up and releasing serotonin after IgE/anti-IgE stimulation (10, 12).
Several genes have been implicated in the control of mast cell differentiation. For some of these, specific roles have been assigned through loss of function studies in the mouse. For example, defective mast cell differentiation results from mutations in either SCF or its receptor, c-kit (14), mutation of the signal transducers Jak3 (15) and PI3K (16), mutation of the transcription factor MITF (17), encoded by the microphtalmia locus (18), and deletion of gp49B1 (19), a member of the immunoglobulin gene superfamily.
GATA-1 (for review see reference 20) is a member of a highly conserved family of zinc finger protein-encoding genes that are expressed in progenitor cells as well as in E cells, megakaryocytes, and mast cells (21–25). Genetic approaches in the mouse have been instrumental in establishing a key role for GATA-1 expression in the regulation of E, Mk, and most recently, eosinophil (26) differentiation but have not as yet demonstrated a requirement in mast cell differentiation. This is due to the fact that GATA-1 loss of function mutants are embryonically lethal whereas mast cell differentiation is better studied in adult animals. Chimeric mice derived from a mixture of normal and GATA-1null embryonic stem cells contain apparently normal numbers of mast cells deriving from the GATA-1null clone (27, 28). In contrast, it has been recently noted that the connective tissue of the skin from heterozygote females carrying a GATA-1 expression mutation (and that survive until adulthood) contains mast cells whose granules react with alcian blue but not with berberine sulfate, suggesting defective mast cell maturation (29). However, both studies fail to identify the precise contribution of the mutant stem cell clone to mast cells differentiated in vivo, leaving the interpretation of the results uncertain. On the other hand, the deletion of GATA-2 has been reported to ablate mast cell differentiation of embryonic stem cells (30) and GATA-2, but not GATA-1, is found expressed in connective mast cells by in situ hybridization (31) and western and RNA blotting techniques (29). Indirect evidence for a functional role for GATA-1 in mast cell differentiation is provided by the demonstration that both mature connective mast cells and BMMC activate GATA-1 expression shortly after IL-3 stimulation (29) and by the presence of GATA consensus sequences in the promoter region of mast cell–specific genes such as carboxypeptidase A (MC-CPA; reference 32) and the α chain of the human IgE receptor (33). Furthermore, ectopic GATA-1 expression activates the expression of the endogenous MC-CPA gene and of the IL n-1 receptor–related T1 gene from the mast cell–specific promoters in immortalized (32) or primary mast cells (34), respectively. However, because the consensus sequences for all of the GATA proteins are similar, the functional role of GATA-1 expression in mast cell differentiation, if any, remains uncertain.
Mice generated with a targeted deletion of upstream enhancer and promoter sequences of the GATA-1 gene express a reduced level of GATA-1 (35). Such animals, designated GATA-1low mice, are born thrombocytopenic (36, 37) and anemic (38). The few animals that survive to adulthood recover from their anemia (39) and have a normal life span (40). The mutation affects terminal maturation of megakaryocytes by preventing their fragmentation into pro-platelets (36, 37) and of erythroblasts by increasing their apoptotic rate (39). As a consequence of these defects, the hemopoietic tissues of the GATA-1low mice contain high levels of E/Mk progenitor cells (36–39). Here it is shown that mast cell differentiation is also impaired in GATA-1low mice. The defect is similar to that in E and Mk lineages (i.e., amplification of the progenitor compartment, increased apoptotic rate at the precursor level, and defective differentiation of the mature cells) and is reversed in vitro by forced GATA-1 expression. These data provide evidence for a direct role of GATA-1 in mast cell differentiation and suggest possible similarities between the regulatory mechanism(s) of the E/Mk and mast cell differentiation pathways.
Materials and Methods
Mice.
The GATA-1low colony was bred at the animal facilities of the Istituto Superiore di Sanità. Littermates were genotyped by PCR at birth as previously described (39, 40). WBB6FA W/Wv female mice were purchased from The Jackson Laboratory. All the experiments were performed with sex- and age (4–6 mo)-matched mice under protocols approved by the institutional animal care committee.
Tissue Sampling.
Blood was harvested with a capillary tube from the retro-orbital plexus and cells from the peritoneum (peritoneal lavage) were collected by gently washing the peritoneal cavity with 1 ml PBS (GIBCO BRL).
Histological Analysis.
Ears and dorsal skin samples were paraffin embedded according to standard procedures. Slides of consecutive sections were dewaxed, rehydrated, and stained with regular (1% in H2O) and acidified (0.02% in 0.25% glacial acetic acid) toluidine blue (Multilab) and safranin-counterstained alcian blue (kit for acid mucosubstances, pH 2.5; Bio Optica; reference 41). Cells obtained from peritoneal lavages were cytospun onto glass slides (Cytospin 3; Shandon) and stained with May-Grunwald/Giemsa (Sigma-Aldrich) or alcian blue. Samples for transmission electron microscopy were fixed in 2.5% glutaraldehyde, postfixed in OsO4, and embedded in SPURR resin (Polyscience). Light microscopy was analyzed with a Leica Light Microscope (Leica) equipped with a Coolsnap video camera for computerized images (RS Photometrics) while transmission electron microscopy was performed with the EM 109 Zeiss (Oberkochen).
Immunohistochemical and Immunofluorescence Analysis of GATA-1 Expression.
For immunohistochemistry, paraffin embedded samples were cut, stained with the anti–GATA-1 monoclonal antibody (Santa Cruz Biotechnology, Inc.), and developed with the Ultraystain polyvalent HRP Immunostaining kit (Ylem) as described by the manufacturers. Immunofluorescence studies were performed on cytocentrifuged cells fixed in 4% paraformaldehyde for 10 min, washed in PBS, and saturated for 30 min with NET gel (150 mM NaCl, 5 mM EDTA, 50 mM Tris-HCl, pH 7.4, 0.05% NP-40, 0.25% carrageenan λ gelatin, 0.02% NaN3). Slides were incubated first with the anti–GATA-1 antibody (10 μg/ml NET gel) for 1 h, and then washed with NET gel and incubated for 45 min with FITC-conjugated anti–rat IgG antibody (1:50 in NET gel; Santa Cruz Biotechnology, Inc.). After several washes, slides were mounted in glycerol-DABCO containing 5 μg/ml 4–6,diamidino-2-phenyldol (Sigma-Aldrich) to counterstain the nuclei. Cells not incubated with the primary antibody served as negative controls.
Terminal Deoxy Transferase Uridine Triphosphate Nick-end Labeling (TUNEL) Staining.
TUNEL reaction was performed on paraffin sections dewaxed, rehydrated, and incubated with the In Situ Cell Death Detection Kit (Boehringer) as described by the manufacturer. At the end, slides were counterstained with propidium iodide (Sigma-Aldrich).
Flow Cytometry Analysis and Mast Cell Purification.
For flow cytometric analysis, cells were suspended in Ca2+ Mg2+–free PBS supplemented with 1% (vol/vol) bovine serum albumin, 2 mM EDTA, 0.1% NaN3, and labeled with PE-conjugated anti–c-Kit (CD117), anti-CD45R/B220, TER-119, and FITC-conjugated anti-CD34, SCA-1 (all from BD Biosciences), and 2D5 (produced in the laboratory) monoclonal antibodies. To analyze the expression of FcɛRI, cells were first incubated with a monoclonal mouse anti–DNP-IgE (10 μg/ml, clone SPE-7; Sigma-Aldrich) and then washed and incubated with FITC-conjugated rat anti–mouse IgE (αIgE; reference 12). All antibodies were incubated for 30 min on ice at a concentration of 1 μg/106 cells. Cell fluorescence was analyzed with the Coulter Epics Elite ESP cell sorter (Beckman Coulter). Appropriate fluorochrome-conjugated isotype controls were used to gate nonspecific fluorescent signals and dead cells were excluded by propidium iodide staining. Mast cells were purified from B cell immuno-depleted peritoneal lavage by sorting. First, peritoneal cells were incubated with FITC-conjugated CD45R (≅1 μg/106 cells, B220; BD Biosciences) for 30 min on ice, washed twice with PBS, and suspended in 90 μl buffer and 20 μl MACS microbeads conjugated with a monoclonal mouse anti–fluorescein antibody (Miltenyi Biotec). This cell suspension was loaded on a MS+/RS+ MiniMacs column placed in a MACS separator. B220− cells were recovered in the effluent fraction and incubated with PE c-kit for 30 min on ice. The c-kithigh fraction was then sorted with the Coulter Epics Elite. The purity of the sorted fractions was evaluated by reanalysis of the fluorescent cells. Negative controls were represented by cell aliquots concurrently incubated with irrelevant isotype-matched antibodies.
Progenitor Cell Counts.
The frequency of progenitor cells was determined by plating light density (P < 1.080) marrow and spleen cells (0.25–1.0 × 105 cells/plate) or 10 μl blood in standard methylcellulose culture (0.9% wt/vol) containing FBS (30% vol/vol; Sigma-Aldrich) and a combination of recombinant growth factors including 100 ng/ml SCF, 10 ng/ml IL-3 (Sigma-Aldrich), 2 U/ml erythropoietin (EPO; Boehringer), and 50 ng/ml thrombopoietin (TPO; reference 39). Cultures were incubated at 37°C in a humidified incubator containing 5% CO2 in air and scored after 7 d for burst-forming unit (BFU)-E–, CFU-GM–, and mixed (E, Mk, and GM) CFU–derived colonies according to standard morphological criteria. These scoring criteria were confirmed by harvesting each colony with a glass microcapillary and individually transferring the colonies on glass slides for May-Grunwald/Giemsa staining. Alternatively, cells harvested from colonies with similar morphology were pooled and immunophenotyped as described above. The frequency of progenitor cells was also established in collagen-based semisolid cultures (Mega-Cult™-C; StemCell Technologies Inc.) as described by the manufacturer. In brief, 105 light density spleen cells or unfractionated bone marrow cells were added to the basal medium supplemented with SCF, IL-3, EPO, and TPO (at the same concentrations described above) and gel formation was induced by adding cold collagen solution. The mixture (0.75 ml) was dispensed in duplicate wells of the Mega-Cult™-C chamber slide and incubated for 7 d. Gels were then dehydrated, acetone fixed, and stained with toluidine blue.
Culture of BMMC.
Marrow and spleen light density cells (1–2 × 105 cells/ml) were cultured for up to 21 d in Iscove's modified Dulbecco's medium supplemented with FBS (10% vol/vol), 4 nmol/L glutamine, 50 U/ml penicillin, and 50 U/ml streptomycin sulfate and then stimulated with 100 ng/ml SCF and 10 ng/ml IL-3 as previously described (10, 11).
Infection with Phosphoglicerate Kinase Gene (PGK)-GATA-1 Retrovirus.
BMMC were transduced with the retroviral vector containing the mouse GATA-1 gene under the control of the human PGK promoter as previously described (42). In brief, BMMC (105 cells) were cocultivated either with the GP plus E86 producer cell line, which was derived from NIH 3T3 molecularly engineered to express viral gal/pol and ecotropic env genes (43), or with NIH 3T3 (2 × 106 cells each), as control, in Iscove's modified Dulbecco's medium supplemented with FBS (10% vol/vol; Hyclone), 4 nmol/L glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin sulfate, 6 μg/ml polybrene (Sigma-Aldrich), 10 ng/ml SCF, 10 ng/ml IL-3, 1 U/ml EPO, and 50 ng/ml TPO. 48 h later, the nonadherent cells were recovered from the flasks and transferred in the same culture media freshly made without polybrene and additionally analyzed 10 and 17 d later. Because the packaging cell line produces ≅106 viral particle per ml of culture (42), >90% of the GATA-1low cells incubated for 48 h under these infection conditions expressed high levels of GATA-1 by immunofluorescence.
Serotonin Release Assay.
Cells (106/ml) were incubated for 6 h at 37°C with 84.0 Ci/mmol [3H]serotonin (5-hydroxy [3H]tryptamine trifluoroacetate, 2 μCi/ml; Amersham Biosciences), washed twice, and incubated again (20 × 106 cells/ml) for 1 h on ice either with the monoclonal mouse anti–DNP-IgE (10 μg/ml) or with medium. After incubation, cells were washed again, divided into aliquots (0.4 × 106cells/50 μL), and stimulated for 15 min at 37°C with either medium or 2 μg/ml rat monoclonal αIgE (R35-72; BD Biosciences), 1 μg/ml DNP-human serum albumin (Sigma-Aldrich), or 1 μg/ml ionomycin (Sigma-Aldrich). Reactions were terminated with 50 μl cold Hank's balanced salt solution. The cells were removed by centrifugation and the level of [3H]serotonin in the supernatants was measured with the Packard 1600TR liquid scintillator counter (PerkinElmer). The total amount of [3H]serotonin incorporated by the cells was determined by lysing nonstimulated cells with 1% Triton X-100. The levels of serotonin released upon stimulation were expressed as absolute counts per minute and as percent of the total amount of [3H]serotonin incorporated (cpm in supernatants ÷ cpm in cell lysate × 100; reference 5).
RNA Isolation and Semiquantitative RT-PCR Analysis.
Total RNA was prepared with a commercial guanidine thiocyanate/phenol method (Trizol; GIBCO BRL) using 20 μg glycogen (Hoffmann LaRoche Ltd.) as a carrier. 1 μg total RNA was reverse transcribed at 42°C for 30 min in 20 μl of 10 mM Tris-HCl, pH 8.3, containing 5 mM MgCl2, 1 U RNase inhibitor, 2.5 U Moloney Murine Leukemia Virus reverse-transcriptase, and 2.5 μM random hexamers (all from PerkinElmer). Gene expression was analyzed by amplifying 2.5 μL reverse-transcribed cDNA in 100 μl of 10 mM Tris-HCl, pH 8.3, containing 2 mM MgCl2, dNTP (200 μM each), 0.1 μCi [α32P]dCTP (specific activity 3,000 Ci/mmol; Amersham Biosciences), 2 U AmpliTaq DNA polymerase, and sense and 100 nM each antisense primers specific for β2 microglobulin (39), GATA-1 (39), GATA-2 (39), MITF (44), MC-CPA (45), MMCP-6 (45), and MMCP-7 (46). PCR conditions were as follows: 60 s at 95°C, 60 s at 55°C, and 60 s at 72°C for β2 microglobulin, GATA-1, and MMCP-6, and 60 s at 95°C, 60 s at 60°C, and 60 s at 72°C for GATA-2, MITF, and MMCP-7. All of the reactions were performed using a GeneAmp 2400 thermocycler (PerkinElmer) and analyzed in the linear range of amplification (20–35 cycles in all of the cases). Positive (RNA from adult marrow and 32D cells) and negative (mock cDNA) controls were included in each experiment. 20 μL aliquots were removed from the PCR mixture after selected cycles of amplification, and the amplified bands were separated by electrophoresis on 4% polyacrylamide gel for semiquantitative amplification analysis (47). Gels were dried using a Biorad apparatus (Hercules) and exposed to Hyperfilm-MP (Amersham Biosciences) for 2 h at −70°C. All procedures were performed according to standard protocols (48).
Statistical Analysis.
Statistical analysis was performed by analysis of variance (Anova test) using Origin 3.5 software for Windows (Microcal Software Inc.).
Results
GATA-1low Mice Contain Many Mast Cell Precursors but Normal Numbers of Morphologically Abnormal Mast Cells in Connective Tissues and Peritoneal Lavage.
Histological sections of the ears from the GATA-1low mice and their normal littermates are presented in Figs. 1 and 2 . No difference was observed between the number of mast cells revealed by toluidine blue and alcian blue staining in the wild-type animals (Fig. 1). In contrast, in the GATA-1low animals, numerous metachromatic− granule-containing cells but normal numbers of mature mast cells were detected by toluidine blue and alcian blue/safranin staining, respectively, in the connective tissue just above the middle cartilage (the trabeculated structure recognizable between two skin layers at the lower level of magnification in Fig. 1 B) of the ear (Figs. 1 and 2; reference 49). The frequency per mm2 of total granule-containing cells in the connective region of the ear and skin from the GATA-1low mice and from littermates was 991 ± 226 versus 259 ± 193 (ear) and 475 ± 14 versus 112 ± 18 (skin), respectively (P < 0.01 in both cases), whereas the frequency of mature mast cells in mutants versus controls was 363 ± 17 versus 193 ± 70 (P < 0.05) in the connective tissues of the ear and 157 ± 37 versus 104 ± 15 (P < 0.08) in the skin (Table I).
Figure 1.
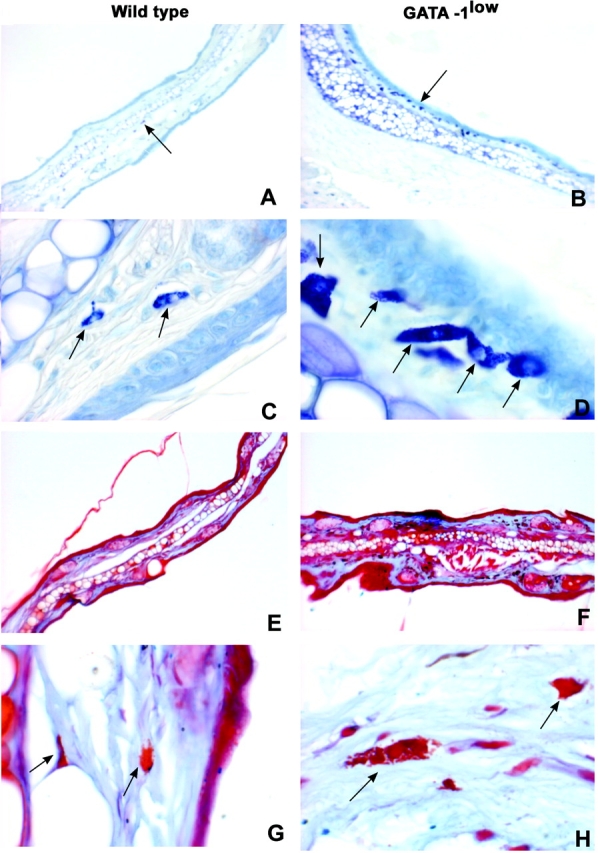
Connective tissue of the ear of the GATA-1low mice contains high numbers of mast cell precursors (metachromatic− after toluidine blue) but normal numbers of more mature (alcian blue+ safranin+) mast cells. Toluidine blue (A–D) and alcian blue/safranin (E–H) staining of consecutive paraffin-embedded sections of the ear from one representative GATA-1low mouse (right panels) and a wild-type (left panels) littermate, as indicated. The presence of mast cells and their precursors is indicated by arrows. The results are representative of replicative analysis from at least four mutants and four normal mice. ×10 in A, B, E, and F and ×100 in C, D, G, and H.
Figure 2.
Connective mast cells from the GATA-1low mice exhibit abnormal morphology and express lower levels of GATA-1 than the cells of normals. Acidified toluidine blue staining (A and B) and GATA-1–specific immunohistochemistry (C and D) of consecutive paraffin-embedded sections from the skin at the base of the ear from a GATA-1low mouse and its normal littermate (right and left panels) and transmission electron microscopy (E and F) of the same specimens. Negative controls for the GATA-1 staining were represented by slides incubated with an irrelevant isotype-matched antibody (not depicted). The presence of mast cells and their precursors is indicated by arrows. Similar results were obtained in at least four independent analysis. ×10 in A and B, ×100 in C and D and the inserts, and ×4,400 in E and F.
Table I.
Frequency of Mast Cell Precursors and Mature Mast Cells in Ear and Skin Sections and in Peritoneal Lavages of Normal and GATA-1low Mice
| Ear(cells/mm2) | Skin(cells/mm2) | Peritoneal lavages(percent of total nucleated cells) | ||||
|---|---|---|---|---|---|---|
| Toluidine blue+ | Alcian blue+ | Toluidine blue+ | Alcian blue+ | May-Grunwald | Alcian blue+ | |
| Normals | 300 | 260 | 106 | 100 | 2.6 | 3.6 |
| 173 | 120 | 97 | 91 | 2.98 | 2.4 | |
| 306 | 200 | 132 | 120 | 3.9 | 3.3 | |
| Mean (±SD) | 259 ± 75 | 193 ± 70 | 112 ± 18 | 104 ± 15 | 3.3 ± 0.7 | 3.1 ± 0.6 |
| GATA-1low | 1,160 | 330 | 492 | 140 | 11.8a | 4.6 |
| 733 | 306 | 466 | 200 | 12.2 | 4.6 | |
| 1,080 | 363 | 470 | 132 | 9.6 | 3.9 | |
| Mean (±SD) | 991 ± 226 | 333 ± 17 | 475 ± 14 | 157 ± 37 | 11.2 ± 1.4 | 4.4 ± 0.4 |
| P | <0.01 | <0.05 | <10−5 | <0.08 | <10−3 | <0.05 |
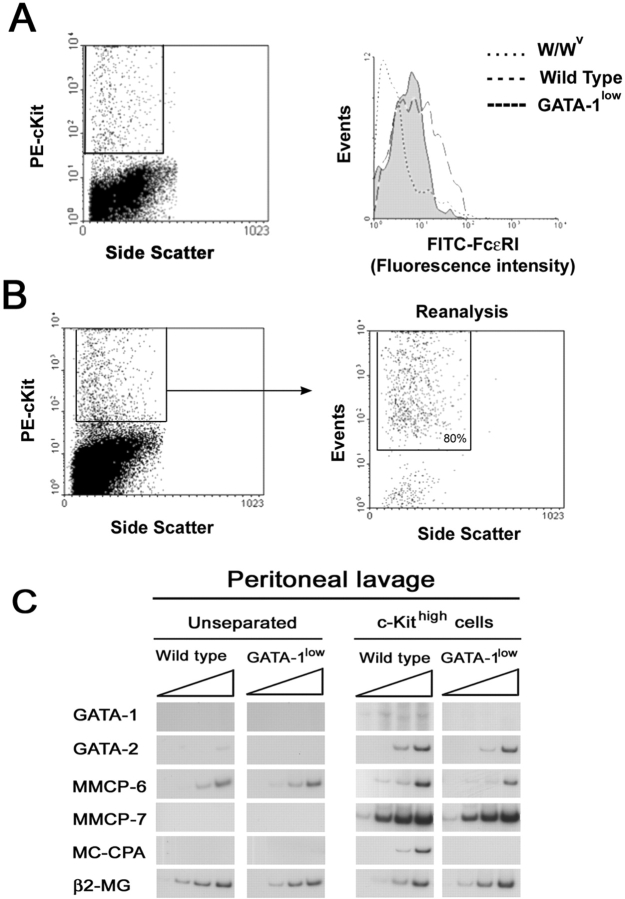
Similar to findings in connective tissues, peritoneal lavage from the mutant mice contained more metachromatic− granule-containing cells than lavage from controls, whereas they contained similar numbers of alcian blue+ cells (Table I). The frequencies of the two cell populations in peritoneal lavage of the GATA-1low mice and normal littermates was 11.2 ± 1.4 versus 3.3 ± 0.7 (P < 0.001) and 4.4 ± 0.4 versus 3.1 ± 0.6 (P < 0.05), respectively. Of note, although dividing cells were never detected in lavages from normal mice, dividing cells represented 0.3% of all the granule-containing cells present in lavages of the GATA-1low mice. The higher incidence of immature mast cells in peritoneal lavages of the GATA-1low mice was confirmed by FACS® determination of the frequency of cells expressing c-kit and FcɛRI (Fig. 3 A). In lavages of both mutant and normal mice, the majority (>75%) of the cells were B220+ (B cells; not depicted). Lavages from the mutant mice contained more c-kit+ cells than those from normal mice (4–6% vs. 1–2%, respectively), although the frequency (and the levels of expression) of FcɛRI+ cells was markedly reduced in the GATA-1low samples (30 vs. 50% of the c-kithigh cells, respectively; Fig. 3 A).
Figure 3.
Mast cells in peritoneal lavage from GATA-1low mice express lower levels of FcɛRI and of the proteases MMCP-6 and MC-CPA than the corresponding cells from normal littermates. (A) The FACS® analysis of cells from peritoneal lavage from normal and GATA-1low mice stained with PE c-kit and FITC-FcɛRI is presented. Cells from the peritoneal lavage of the mast cells deficient W/Wv mice (reference 51) were analyzed in parallel as negative control. The gating used for the FcɛRI analysis is shown in a representative dot plot of PE c-kit versus side scatter analysis. Very similar c-kit/side scatter dot plots were observed in all the cases. The isotype control for the FcɛRI analysis is not shown because it is superimposable to the histogram obtained with cells from the W/Wv mouse. (B) The gating used to purify c-kithigh cells from the peritoneal lavage and a representative reanalysis after sorting of the purified cells is presented. In all of the cases, >80% of the sorted cells were c-kithigh upon reanalysis. The semiquantitative RT-PCR analysis for the expression of β2 microglobulin (β2-MG), GATA-1, GATA-2, MMCP-6, MMCP-7, and MC-CPA in unseparated or c-kithigh cells of peritoneal lavage from GATA-1low and normal littermates is presented in C. Each product was amplified for increasing number of cycles (20, 25, 30, and 35), as indicated by the triangle on the top of the panels. Similar results were obtained in two additional experiments.
Mast cells of the GATA-1low animals presented an abnormal morphology characterized by a larger size (Figs. 1 and 2). Morphological differences were confirmed by observations under transmission electron microscopy of thin sections from the ears (Fig. 2, E and F). This analysis not only confirmed the larger size of the mutant cells, but revealed further abnormalities that included more disperse chromatin structure and heterogeneity in size and electron density of cytoplasmic granules (50).
To detail the molecular defects of the GATA-1low mast cells, the levels of expression of the mast cell–specific proteases MMCP-6, MMCP-7, and MC-CPA were analyzed by semiquantitative RT-PCR in unfractionated or purified c-kithigh cells from peritoneal lavages of GATA-1low and normal littermates (Fig. 3 C). In agreement with the low frequency of mast cells in the peritoneal lavages, most of the genes investigated were not amplified from cDNA of unfractionated cell preparations. On the other hand, all of the genes considered were amplified from cDNA from c-kithigh cells purified from normal peritoneal lavage (Fig. 3 C). In contrast, when cDNA from c-kithigh cells purified from GATA-1low animals was used as template, fragments specific for MC-CPA were never amplified and amplification of MMCP-6 was markedly reduced. Interestingly, GATA-2 was amplified at the same levels from c-kithigh cells purified from both mouse strains whereas GATA-1 was amplified with cDNA from wild-type but not GATA-1low littermates.
To confirm that the GATA-1low mutation impaired GATA-1 expression in mast cells, the presence of GATA-1 protein was compared in ear and peritoneal lavage cells by immunohistochemical (Fig. 2) and immunofluorescence (unpublished data) microscopy, respectively. GATA-1 protein was readily detectable in mast cells of the normal mice with both techniques, but was barely detectable in GATA-1low cells (Fig. 2, C and D).
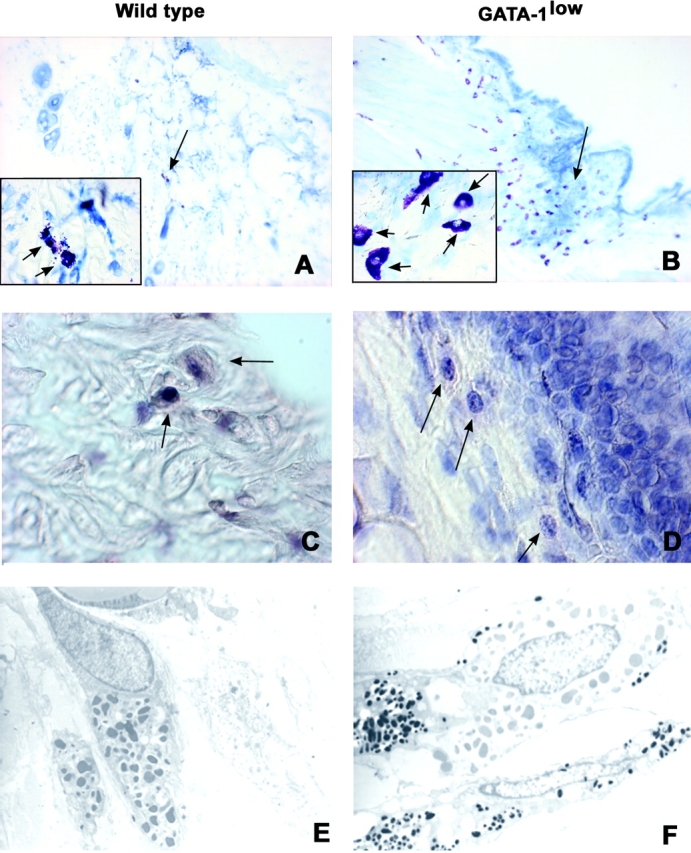
To clarify why the connective tissues of GATA-1low mice contained normal numbers of mature cells in spite of their high content of mast cell precursors, the frequency of apoptotic cells in the connective tissues was evaluated by TUNEL staining of ear sections (Fig. 4) . Very few (<19 nuclei/mm2) fluorescent nuclei were identified in ear sections of normal mice (Fig. 4, A and C). In contrast, high numbers of fluorescent cells (134 ± 2/mm2) were observed in ear sections of GATA-1low mice in which high numbers of mast cells had been identified (Fig. 4, B and D). The cells with apoptotic nuclei were positively identified as mast cell precursors by toluidine blue staining (not depicted).
Figure 4.
Connective region of the ear from the GATA-1low mice contains high numbers of apoptotic cells. TUNEL staining of representative paraffin-embedded sections of the ear from wild-type (A and C) and GATA-1low (B and D) mice, as indicated. Negative controls were represented by tissue sections treated with a fluorescent deoxyuridine triphosphate-free TUNEL reaction mixture and are not presented for convenience. Fluorescent nuclei were not detected in negative controls (not depicted), were rare (<5/mm2) in sections from the wild-type ear (A and C), and were numerous (134 ± 2/mm2) in the connective region just below the epithelium of the sections from the GATA-1low ear (B and D). ×10 and ×40 in the top and bottom panels, respectively.
Hematopoietic Tissues from the GATA-1low Mice Contain High Numbers of a “Unique” Trilineage (E-Mk-Mast Cell) Progenitor Cell.
It has been reported that hematopoietic tissues from the GATA-1low mice contain high numbers of E and Mk progenitor cells whereas the number of myeloid progenitors is normal (36–39). It has also been demonstrated that tissues of mutant mice contain a unique progenitor cell, bipotent for the E and Mk lineage, which gives rise to colonies with a typical morphology and whose cells, once transferred into fresh medium, proliferate for at least a couple of passages (36). Similar colonies (the morphology of one of them is presented in Fig. 5 A) were also detected in this study. However, when single colonies were harvested from the dish and individually transferred onto glass slides for morphological analysis, it was recognized that they contained, in addition to E and Mk cells, granule-containing cells that resembled mast cell precursors (Fig. 5 B, Mc). To exclude the possibility that such a mast cell component represented an artifact due to cell contaminants picked up from the methyl-cellulose, marrow and spleen cells from the GATA-1low mice were also cultured in dishes made semisolid with collagen and stimulated with the same mixture of growth factors used for the standard cultures. After 7 d, the collagen gels were dried and stained with toluidine blue and/or with acethyl-colinesterase. Also in this analysis, GATA-1low, but not normal marrow and spleen cells, gave rise in 7 d to colonies that were identified in situ as containing erythroblasts, megakaryocytes (acethyl-colinesterase+), and mast cells (metachromatic− granule-containing cells; unpublished data). This unique trilineage progenitor cell is termed CFU-EMkMc. CFU-EMkMc were below the level of detection in marrow, spleen, and blood cultures of normal littermates (Table II). In contrast, they represented 65–72% of all progenitor cells present in the marrow and spleen of GATA-1low animals (Table II). As many as 315 ± 65 and 800 ± 150 CFU-EMkMc–derived colonies were detected per 105 nucleated cells of the spleen and marrow from GATA-1low mice. These colonies were detectable at a frequency of 12 ± 4 per μL in the blood of the GATA-1low mice (Table II).
Figure 5.
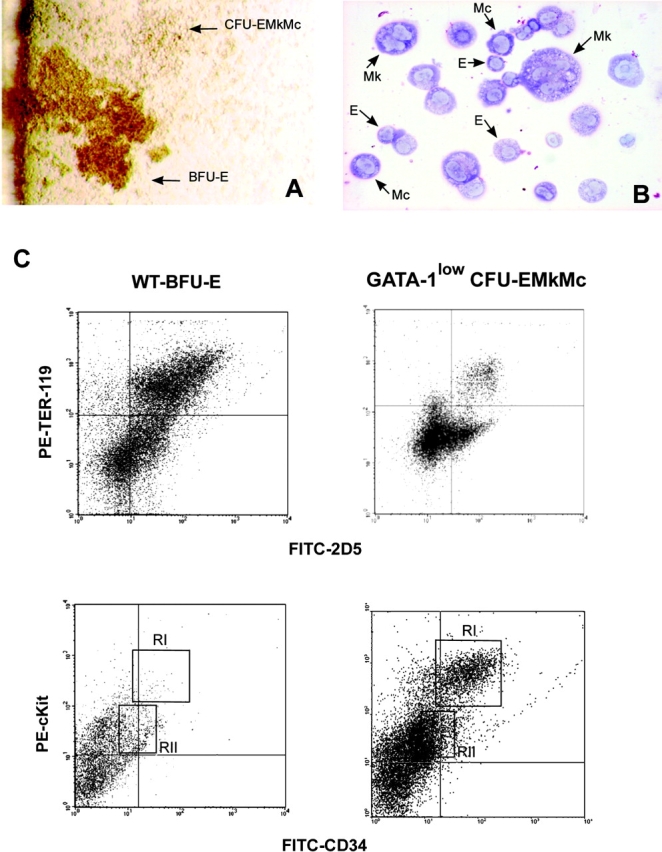
Bone marrow of GATA-1low mice contains trilineage progenitor cells that give rise to colonies composed of erythroblasts, megakaryocytes, and mast cells. (A) The morphology of a trilineage colony, as compared with that of a BFU-E–derived colony growing in its neighborhood is presented. The colony was photographed at day 7 in bone marrow cultures of GATA-1low mice stimulated with SCF, IL-3, EPO, and TPO. (B) May-Grunwald/Giemsa staining of the smear of a representative trilineage colony is presented. Erythroid (E), megakaryocytic (Mk), and mast (Mc) cells are indicated by arrows. Similar results were observed on smears of at least 10 additional individual colonies. ×10 and ×100 in the left and right panels, respectively. (C) FACS® analysis of cells pooled from CFU-EMkMc–derived colonies immunophenotyped by double staining with TER-119/2D5 (E/Mk markers) and c-kit/CD34 (mast cell markers) antibodies is presented. Cells pooled from BFU-E–derived colonies grown in the corresponding cultures from normal mice (WT-BFU-E) were immunophenotyped in parallel with the same antibodies for comparison. RI and RII indicate regions of the dot plots corresponding to the antigenic profile c-kitlow CD34low and c-kithigh CD34high, characteristic of progenitor cells (reference 52) and mast cells, respectively. Negative controls were represented by cells stained with appropriate isotype matched irrelevant antibodies (not depicted). Similar results were obtained in three independent experiments.
Table II.
Frequency of Progenitor Cells in the Marrow and Spleen of Normal and GATA-1low Animals
| Number of progenitor cells | |||
|---|---|---|---|
| Normal mice | GATA-1low mutants | P | |
| Bone marrow(CFU/105 cells) | |||
| BFU-E | 149 ± 33 | 165 ± 33 | <0.01 |
| CFU-GM | 165 ± 25 | 145 ± 16 | >0.05 |
| CFU-EMkMc | 0 | 800 ± 150 | <0.001 |
| Spleen(CFU/105 cells) | |||
| BFU-E | 15 ± 3 | 110 ± 21 | <0.05 |
| CFU-GM | 40 ± 5 | 56 ± 16 | >0.05 |
| CFU-EMkMc | 0 | 315 ± 65 | <0.001 |
| Blood(CFU/10 μL) | |||
| BFU-E | 1 ± 1 | 14 ± 3 | <0.05 |
| CFU-GM | 3 ± 2 | 9 ± 2 | >0.05 |
| CFU-EMkMc | 0 | 12 ± 4 | <0.05 |
The cells present in the CFU-EMkMc–derived colonies were further characterized by FACS® analysis. Cells pooled from normal BFU-E–derived colonies were analyzed in parallel as control (Fig. 5 C). The cells were double stained with either TER-119/2D5 to detect E and Mk cells, or with CD117 that recognizes c-kit and CD34 to detect mast cell precursors. The majority of the cells pooled from the BFU-E–derived colonies cultured from normal mice expressed, as expected, TER-119 and/or 2D5 but very few, if any, expressed high levels of c-kit and/or CD34 (Fig. 5 C). c-kithigh and CD34high cells were also below the level of detection in pools of CFU-GM–derived colonies obtained from the same cultures (unpublished data). In contrast, only some cells from the CFU-EMkMc–derived colonies expressed TER-119 and/or 2D5 whereas 16% of them coexpressed high levels of CD34 and of c-kit (Fig. 5 C). c-kithigh CD34high cells were positively identified as mast cells by sorting and May-Grunwald staining (not depicted).
To further characterize the differentiation potential of mast cells present in the CFU-EMkMc–derived colonies, cells pooled from these colonies (as well as cells pooled from BFU-E– and CFU-GM–derived colonies obtained from normal mice) were cultured in liquid culture stimulated with SCF and IL-3, plus or minus a murine fibroblast feeder layer (Fig. 6 , NIH 3T3). Cells pooled from normal BFU-E– or CFU-GM–derived colonies failed to proliferate and survived for 1–2 wk or less when transferred into liquid culture (Fig. 6 E). In contrast, cells harvested at day 7 from CFU-EMkMc–derived colonies proliferated for up to 50 d under these conditions (Fig. 6 E). The high proliferative capacity was confirmed in limiting dilution experiments in which single cells were deposited in 96-multiwell plates. Cell proliferation occurred in >98% of the wells provided that the cultures had been stimulated with both SCF and IL-3 (unpublished data).
Figure 6.

Defective maturation of mast cell precursors in vitro in CFU-EMkMc–derived colonies. Phase contrast microscopic observation (A and B) and May-Grunwald/Giemsa staining (C and D) of CFU-EMkMc–derived cells grown for 2 wk either in suspension or on fibroblast feeder layer (on the left and right panels, as indicated) in the presence of SCF and IL-3. ×100 in both. (E) The growth curves of CFU-EMkMc–derived cells (straight lines) grown for up to 45 d in cultures stimulated with SCF and IL-3 either in suspension culture (▪) or on a fibroblast underlayer (▿) are presented. The vertical dotted lines indicate weekly demipopulations. The growth curves of cells pooled from BFU-E– (green circles) and CFU-GM (red circles)–derived colonies obtained from cultures of normal mice (dashed lines) and grown in parallel under the same conditions described above are also presented as controls. The results are presented as the mean (±SD) of at least three separate experiments. FACS® analysis for double expression of c-kit/CD34 (dot plots) and FcɛRI (histograms) of CFU-EMkMc–derived cells grown for 2 wk in suspension culture (F) or on a fibroblast underlayer (G) is shown. The cells analyzed for FcɛRI expression had been gated as c-kit+. For convenience, only staining with an irrelevant antibody is shown for the FcɛRI (dotted line) analysis. The c-kit− CD34+ cells present in G were separated by FACS® and identified as contaminating fibroblasts. Similar results were obtained in three additional experiments.
After the second week of liquid culture, the CFU-EMkMc–derived cells acquired a homogeneous morphology of granule-containing cells that coexpressed high levels of c-kit and CD34 (Fig. 6, F and G) but did not react with alcian blue (unpublished data). Some of the c-kit+ cells coexpressed FcɛRI, although the levels of FcɛRI expression remained below those expressed by normal BMMC throughout the time in culture (compare Fig. 6 F with 7 A). Of interest, the CFU-EMkMc–derived cells adhered with high frequency (>54% after overnight incubation) to murine fibroblasts (Fig. 6) but expressed an immature phenotype characterized by lack of reactivity with alcian blue (unpublished data) and low levels of FcɛRI expression (Fig. 6 G). These results suggest that mast cell precursors within the CFU-EMkMc–derived colonies are unable to complete differentiation.
Differentiation of BMMC Derived from Hemopoietic Tissues of GATA-1low Mice Is Defective In Vitro.
Mast cell differentiation has seldom been reported in cultures of normal murine hemopoietic tissues at day 7 (12) but many mast cell–containing colonies were detected at this time in semisolid cultures of tissues from the GATA-1low mice (Figs. 5 and 6). Because CFU-EMkMc represent a unique class of progenitor cells found only in the tissues of the GATA-1low mice, it is possible that the defective mast cells they contain are the result of “abortive differentiation” under nonpermissive culture conditions. To directly compare the maturation of mast cells differentiated in vitro from normal and GATA-1low mice, marrow (and spleen) cells were cultured under conditions specific for the generation of BMMC (10, 12). The results obtained are presented in Fig. 7 . Under these conditions, a homogeneous population of BMMC (95% of which were c-kithigh/FcɛRIhigh) was observed after 21 d in normal cell cultures. In contrast, marrow cells from the GATA-1low mice yielded a BMMC population, the majority (87%) of which expressed high levels of c-kit, that was composed both by FcɛRI− (16.3% ± 3.7%) and FcɛRI+ cells (83.7% ± 3.7%, P < 0.001 with respect to the frequencies observed in cultures obtained from normal mice; Fig. 7 A). In addition, the GATA-1low BMMC expressed less FcɛRI on their surface than normal cells (median fluorescence intensity 120 vs. 150 arbitrary units, respectively; Fig. 7 A).
Figure 7.
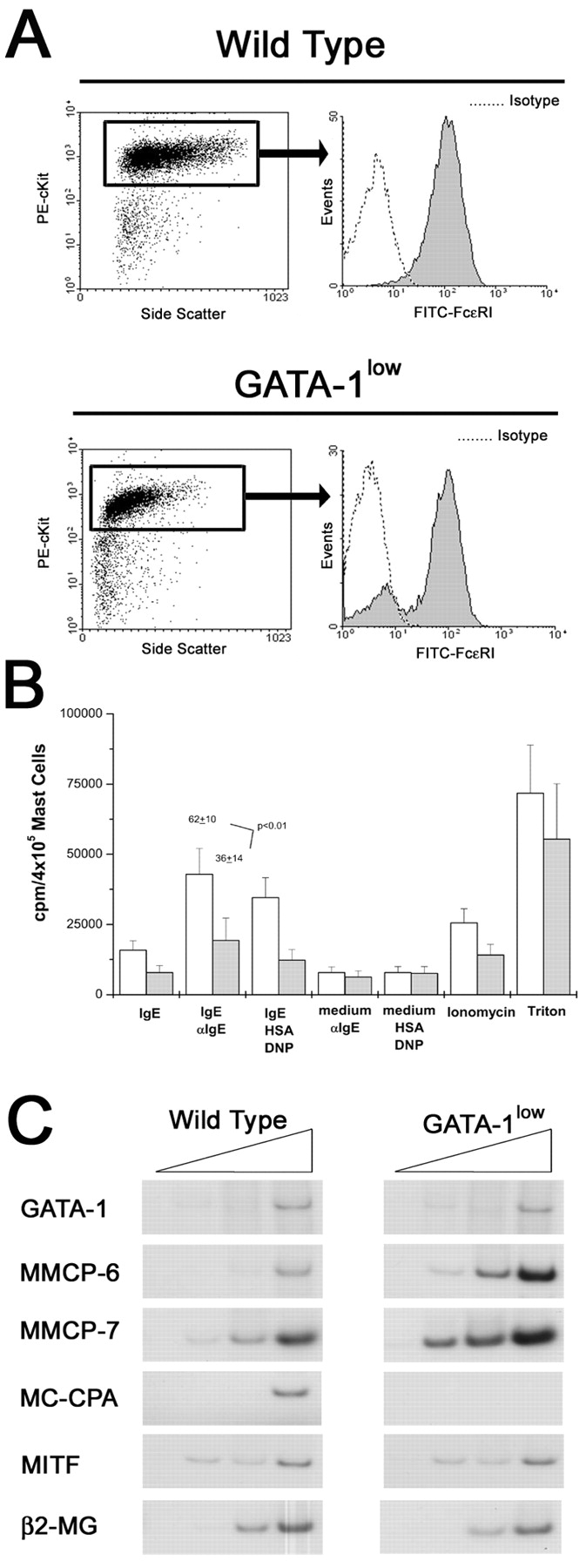
BMMCs obtained from marrow of GATA-1low mice express lower levels of FcɛRI and MC-CPA and release less [3H]serotonin after IgE-αIgE stimulation than the normal cells. (A) FACS® analysis for the expression of c-kit and FcɛRI of BMMC obtained after 21 d of culture under BMMC-specific conditions from normal mice and their GATA-1low littermates are presented on the top and bottom panels, as indicated. Dot plots for side scatter and c-kit expression and histograms for FcɛRI expression of the c-kithigh gated cells are presented on the left and right, respectively. Negative controls were represented by cells labeled with an irrelevant isotype-matched antibody and for convenience are only presented for the FcɛRI analysis. The results are representative of those obtained in five separate experiments (each one started with cells harvested from a different animal). (B) Level of serotonin (as cpm/105 cells) released upon IgE-αIgE stimulation by BMMC obtained after 21 d of culture under specific culture conditions from GATA-1low mice (gray bars) and their normal littermates (open bars). Positive and negative controls were represented by cells stimulated with IgE alone, medium plus αIgE, and medium, human serum albumin, DNP, and Ionomycin, as indicated. The levels of total serotonin that had been incorporated by the cells was measured by lysing the BMMC in Triton X-100. The results are presented as the mean (±SD) of at least five separate experiments performed in duplicate. (C) Semiquantitative RT-PCR analysis for the expression of β2 microglobulin (β2-MG), GATA-1, GATA-2, MITF, MMCP6, MMCP7, and MC-CPA genes in cells obtained after 21 d under BMMC-specific culture conditions. Each product was amplified for an increasing number of cycles (20, 25, 30, and 35), as indicated by the triangle on the top of the panel. Similar results were obtained in two additional experiments.
In vitro maturation of GATA-1low mast cells was analyzed further by comparing the levels of [3H]serotonin incorporated and released upon appropriate stimulation with values obtained with normal BMMC (Fig. 7 B). Cells obtained in 21 d of culture from tissues of GATA-1low mice and normal littermates incorporated comparable high levels of [3H]serotonin. However, GATA-1low BMMC released significantly less [3H]serotonin after IgE-αIgE stimulation than cells obtained from the normals (36 ± 14 vs. 62 ± 10, respectively; P < 0.01).
In addition, the protease expression profile of GATA-1low and normal BMMC was compared by semiquantitative RT-PCR analysis (Fig. 7 C). Although more MMCP-6 and MMCP-7 fragments were amplified with cDNA from GATA-1low than from wild-type BMMC, MC-CPA fragments were amplified only with cDNA from wild-type cells (Fig. 7 C).
These results indicate that the GATA-1low mast cells mature poorly in vitro even under specific culture conditions.
Forced GATA-1 Expression Restores the Mast Cell Differentiation Potential of GATA-1low BMMC In Vitro.
To clarify whether the defective mast cell differentiation observed in cultures from the GATA-1low mice was a direct consequence of the mutation affecting GATA-1 expression, we examined whether reexpression of GATA-1 would restore the in vitro maturation potential of GATA-1low BMMC. GATA-1low cells, obtained at day 7 under BMMC-specific conditions, were cocultured either with NIH 3T3 or with the NIH 3T3–derived cells producing the PGK-GATA-1 virus (42). 48 h later, nonadherent cells were harvested and transferred into fresh medium. At days 10 and 17 after infection, cells were analyzed for their surface antigenic profile and GATA-1 expression. The expression of mast cell–specific differentiation markers (metachromasia after toluidine blue, reactivity to alcian blue and expression of MITF, MMCP6, and MMCP7) was also analyzed.
Before infection, the cells used in these experiments contained both E (TER-119+) and mast cell (c-kithigh/SCA-1+) precursors (Fig. 8 E and unpublished data). After coculture with the murine fibroblasts, TER-119+ cells were not detectable (unpublished data) and almost all (>95%) of the cells grown under these conditions expressed high levels of c-kit at 10–17 d. All BMMC cocultured with NIH 3T3 (mock-infected) became c-kit+ SCA-1+ after 17 d whereas some (35%) of the PGK-GATA-1–infected BMMC were SCA-1− (Fig. 8 E). Because expression of SCA-1 is lost during differentiation of hemopoietic cells, these findings suggested that transduction with a GATA-1–containing retrovirus might have induced maturation of the GATA-1low BMMC.
Figure 8.
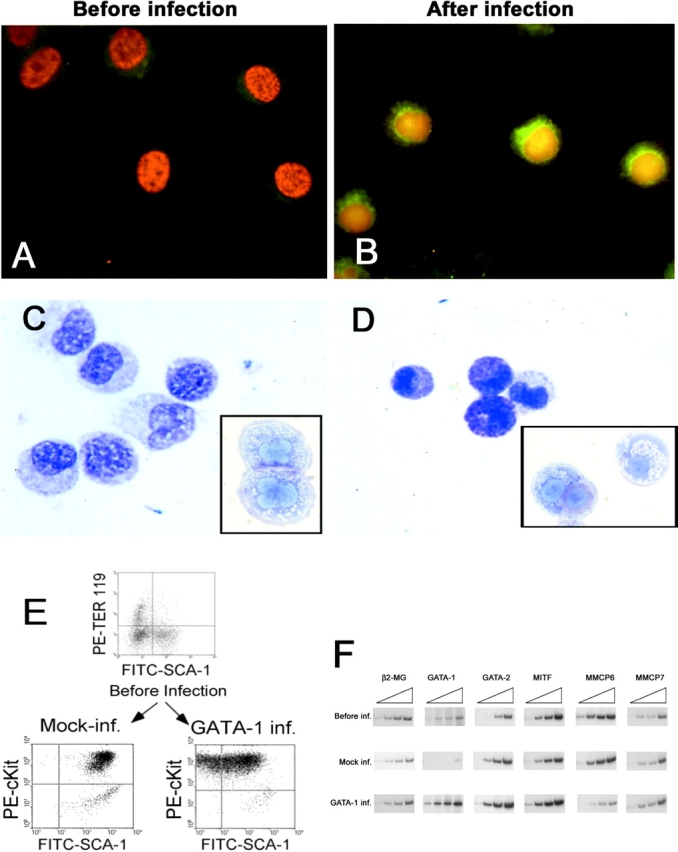
Infection of GATA-1low BMMC with the PGK-GATA-1 retrovirus increases the level of GATA-1 expression and restores in vitro maturation potential. (A and B) GATA-1–specific immunofluorescent staining of BMMC either before or 17 d after their coculture with the PGK-GATA-1–producing cell line, as indicated. Cell nuclei are counterstained with DAPI. (C and D) Alcian blue staining of the same cells shown above. Toluidine blue staining of representative cells are presented in the inserts. ×100 in all of the cases. (E) Immunophenotype of day 7 BMMC before infection and 17 d after the 48-h coculture with either NIH 3T3 (Mock-inf.) or the PGK-GATA-1–producing cell line (GATA-1 inf.). Day 7 BMMC were immunophenotyped with TER-119/SCA-1 whereas cocultured BMMC were immunophenotyped with c-Kit/Sca-1. (F) Semiquantitative RT-PCR analysis for the expression of β2 microglobulin (β2-MG, positive control), GATA-1, GATA-2, MITF, MMCP6, and MMCP7 genes in cells obtained after 7 d under BMMC-specific culture conditions (before infection) or 17 d after the BMMC had been cocultured with either NIH 3T3 (Mock inf.) or the PGK-GATA-1–producing cell line (GATA-1 inf.; the same cells as presented above). Each product was amplified for an increasing number of cycles (20, 25, 30, and 35), as indicated by the triangle on the top of the panel. Similar results were obtained in two additional experiments.
The morphological analysis of transduced BMMC is presented in Fig. 8. Very low levels of GATA-1 protein were detectable by immunofluorescence in the GATA-1low BMMC before infection (Fig. 8 A) or after coculture with NIH 3T3 cells (not depicted). In contrast, high levels of GATA-1 expression were expressed by >65% of the cells that had been cocultured with the PGK-GATA-1–producing cell line (Fig. 8 B). This result indicates that the majority of the GATA-1low cells being analyzed were infected by the retrovirus. Forced GATA-1 expression resulted in marked changes in the maturation profile of the GATA-1low BMMC. These changes included a reduction in cell size and the appearance of cytoplasmic alcian blue+ metachromatic+ granules (Fig. 8 D). These results indicate that forced GATA-1 expression restored the in vitro maturation potential of GATA-1low BMMC.
Increased GATA-1 expression in transduced GATA-1low BMMC was confirmed by semiquantitative RT-PCR analysis (Fig. 7 F). Before infection, day 7 BMMC expressed GATA-1 and GATA-2, as well as the mast cell–specific transcription factor MITF and tryptases MMCP6 and MMCP7, which represent markers for cells at earlier and later times in BMMC-specific cultures, respectively. (53). Mock-infected cells expressed high levels of GATA-2 and very low levels of GATA-1 whereas the levels of expression of the mast cell–specific genes remained similar to that observed at baseline. On the other hand, cells cocultured with the PGK-GATA-1 cell line expressed high levels of GATA-1 whereas the levels of expression of GATA-2 increased only modestly over baseline. Of note, these cells expressed lower levels of MMCP6 and higher levels of MMCP7 than mock-infected cells.
Discussion
We have demonstrated that mast cell differentiation is defective in mice harboring a targeted deletion that removes upstream enhancer and promoter elements of the GATA-1 gene. The mutation affects all stages of mast cell differentiation. More specifically, it alters the commitment process by inducing the formation of high numbers of a unique class of progenitor cells (the CFU-EMkMc), induces an increase in proliferation that is coupled by increased cell death (but on balance generating more live cells than dead) of the precursor cells, and finally, impairs the expression of the FcɛRI receptor and of the MC-CPA in mature mast cells. Our findings, therefore, implicate GATA-1 as a critical transcription factor for proper mast cell development.
At the level of the progenitor cell compartments, the most striking alteration was reflected by the presence of a unique class of cells, the CFU-EMkMc, which are apparently committed toward the E, Mk, and mast cell lineage (Fig. 5). The CFU-EMkMc gave rise to colonies in only 7 d of culture and their frequency was as high as 800 ± 150 and 315 ± 65 progenitor cell per 105 mononuclear bone marrow and spleen cells, respectively (60–70% of all the progenitor cells present in these organs). Because CFU-EMkMc were not detected from normal mice, their relationship to the pathway of normal hemopoietic differentiation is unclear. In the hierarchy of normal hemopoietic cells, mast cell differentiation potential is thought to be lost before the restriction toward E/Mk differentiation occurs. In fact, mast cells are detected in single cell deposition cultures of purified common myeloid progenitors and not in those of the purified common E/Mk ones (54). It is possible, however, that reduced GATA-1 expression at the progenitor cell level might alter the commitment process itself, thereby allowing E/MK progenitor cells to retain mast cell differentiation potential (55).
At the precursor level, approximately three times more mast cells were found in the connective region of the skin (Figs. 1 and 2) and in peritoneal lavage (Table I) of the mutant mice. Conversely, granule-containing cells were observed in the connective region but not in the mucosa of the stomach (unpublished data). An increased number of mast cell precursors might result from either increased proliferation capacity and/or a block in terminal maturation. The first possibility is supported by the presence of many (0.3%) dividing mast cells in peritoneal lavage and the high proliferative capacity exhibited by GATA-1low BMMC in vitro (>98% of which were capable to proliferate in single cell cloning experiments). However, cultured mast cells typically have high proliferative capacity (12) and even normal mature cells purified from the tissues of the mice may regain proliferative potential once induced to degranulate (i.e., they are de-differentiated; reference 56). The fact that c-kithigh FCɛRI− cells were still detectable after 21 d in BMMC-specific cultures seeded with marrow (and spleen) cells of mutant origin (Figs. 6 and 7) favors the possibility that the increased proliferation is a direct result of a block in the cellular maturation induced by the mutation.
In spite of the increased frequency of mast cell precursors in tissues of the GATA-1low mice, the number of mature alcian blue+ safranin+ cells in the same tissues was not significantly higher or only modestly (≅20–50%) higher than normal (Fig. 1). On the other hand, high numbers of apoptotic (TUNEL+; Fig. 4) precursor cells were detected in tissues. It is possible that mutant mast cell precursors, as mutant BMMC in culture, are unable to progress in their maturation process and therefore activate an apoptotic program. Adult GATA-1low mice maintain normal hemoglobin and hematocrit as increased apoptosis observed at the pro-erythroblasts level is counter balanced by high output from the progenitor cell compartment (38, 39). Because in the case of the erythroblasts, apoptosis is a direct consequence of reduced GATA-1 expression (57), we suggest here that GATA-1 is also involved in preventing death of mast cell precursors.
The mature mast cells identified in connective tissue and peritoneal lavage exhibit altered morphology that includes large size, abnormal chromatin organization, and the presence of heterogeneously sized cytoplasmic granules (Fig. 2). In addition, they expressed lower levels of FcɛRI on their surface and did not expressed MC-CPA (Fig. 3). These findings are superficially reminiscent of abnormalities in megakaryocyte maturation in the absence of GATA-1. Therefore, we considered whether mast cell maturation is blocked in the GATA-1low mice. In both semisolid cultures and under BMMC-specific culture conditions, cells with the profile of mast cell precursors (c-kithigh, CD34+) were detected as early as day 7 of culture of mutant cells compared with 21 d in cultures of normal cells. Furthermore, maturation of mutant cells was incomplete. Cells with alcian blue+ metachromatic granules were not seen (Figs. 6, 8, and unpublished data). Additionally, GATA-1low BMMC expressed lower levels of FCɛRI (Figs. 6, F and G, and 7 A), released less [3H]serotonin after IgE-αIgE stimulation (Fig. 6 B) than normal BMMCs and did not express MC-CPA (Fig. 7), which is the only mast cell–specific protease whose expression has been demonstrated to be under GATA-1 control (32).
The similarities between the defects at the level of the mast cell lineage and those observed in E and Mk cells suggest but do not prove that the impaired mast cell differentiation observed in the GATA-1low mice was the direct consequence of a decreased GATA-1 expression in the cells of this lineage. The GATA-1low animals compensate for the defective hemopoiesis induced by the mutation by establishing a complex homeostatic mechanism that includes increased growth factor gene expression (such as TGF-β and platelet-derived growth factor) in the marrow microenvironment (40) and extensive extramedullary hemopoiesis in the spleen (39). It remained possible that the alterations in the mast cell differentiation pathway observed in these animals might be secondary to the mutation itself and the consequence of the alterations observed in the microenvironment. Although homogeneous, and therefore deprived of accessory cells BMMC populations obtained from the mutant mice did not progress properly along the differentiation pathway even when cultured for an extensive period of time (>45 d) under highly specific culture conditions, it could be argued that such a lack of differentiation was the result of an in vivo priming by “nonpermissive” growth factor stimulation. To determine if the abnormal differentiation we observed in vitro was due to insufficient expression of GATA-1, we transduced GATA-1low mast cells with a retrovirus harboring GATA-1 cDNA. After infection, full mast cell differentiation was restored. Thus, the defects we describe in GATA-1low mast cells in vitro result from lower than normal GATA-1 expression. Whether cells entirely lacking GATA-1 would differ in phenotype is under study.
In conclusion, mice with a mutation in the upstream enhancer and promoter of the GATA-1 gene exhibit a complex phenotype that includes impaired mast cell differentiation. From our findings we infer that GATA-1 has a critical role in the proper differentiation of mast cells. In some respects this requirement mimics the role of GATA-1 in both E and Mk. The recent report that targeted deletion of the high affinity binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo (26) and our current data suggest that GATA-1 function is important in the control of inflammation and allergic reactions.
Acknowledgments
The authors gratefully acknowledge Dr. Peter Besmer for guidance in the characterization of the mast cells, Dr. David Bodine for providing the PGK-GATA-1–producing cell line, and Dr. Thalia Papayannopoulou for helpful discussion. Luca Marsilli, Antonio Di Virgilio, and Giovanna Grifone are acknowledged for technical help and Monia Cieri for editorial assistance. SCF and TPO were generously provided by Amgen (Transfer of material agreement no. 19982634).
This study was supported by Progetto Strategico Oncologia CNR Ministero Istruzione Universitâ Ricerca legge 449/99, Progetti di Ricerca di Interesse Nazionale 2000, 2001, and 2002 from the Ministry of Health; grant number E1172 from the Telethon Foundation; and a grant from Associazione Italiana per le Leucemie “30 ore,” Florence. L. Bianchi is a fellow of Fondazione Italiana Ricerca sul Cancro, Milan, Italy. S.H. Orkin is an Investigator of the Howard Hughes Medical Institute.
Footnotes
*
Abbreviations used in this paper: BFU, burst-forming unit; BMMC, bone marrow–derived mast cells; E, erythroid; EPO, erythropoietin; GM, granulocytic-monocytic; MC-CPA, mast cell carboxypeptidase A; Mk, megakaryocytic; PGK, phosphoglicerate kinase gene; SCF, stem cell factor; TPO, thrombopoietin; TUNEL, terminal deoxy transferase uridine triphosphate nick-end labeling.
References
- 1.Costa, J.J., P.F. Weller, and S.J. Galli. 1997. The cells of the allergic response: mast cells, basophils, and eosinophils. JAMA. 278:1815–1822. [PubMed] [Google Scholar]
- 2.Gurish, M.F., and K.F. Austen. 2001. The diverse roles of mast cells. J. Exp. Med. 194:F1–F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitamura, Y., M. Yokoyama, H. Matsuda, T. Ohno, and K.J. Mori. 1981. Spleen colony-forming cell as common precursor for tissue mast cells and granulocytes. Nature. 291:159–160. [DOI] [PubMed] [Google Scholar]
- 4.Kirshenbaum, A.S., J.P. Goff, T. Semere, B. Foster, L.M. Scott, and D.D. Metcalfe. 1999. Demonstration that human mast cells arise from a progenitor cell population that is CD34+, c-kit+, and expresses aminopeptidase N (CD13). Blood. 94:2333–2342. [PubMed] [Google Scholar]
- 5.Rodewald, H.R., M. Dessing, A.M. Dvorak, and S.J. Galli. 1996. Identification of a committed precursor for the mast cell lineage. Science. 271:818–822. [DOI] [PubMed] [Google Scholar]
- 6.Stevens, R.L., D.S. Friend, H.P. McNeil, V. Schiller, N. Ghildyal, and K.F. Austen. 1994. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc. Natl. Acad. Sci. USA. 91:128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakahata, T., and M. Ogawa. 1982. Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc. Natl. Acad. Sci. USA. 79:3843–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi, T., T. Nakano, T. Nakahata, H. Asai, Y. Yagi, K. Tsuji, A. Komiyama, T. Akabane, S. Kojima, and Y. Kitamura. 1986. Formation of mast cell colonies in methylcellulose by mouse peritoneal cells and differentiation of these cloned cells in both the skin and the gastric mucosa of W/Wυ mice: evidence that a common precursor can give rise to both “connective tissue-type” and “mucosal” mast cells. J. Immunol. 136:1378–1384. [PubMed] [Google Scholar]
- 9.Dayton, E.T., P. Pharr, M. Ogawa, W.E. Serafin, K.F. Austen, F. Levi-Schaffer, and R.L. Stevens. 1988. 3T3 fibroblasts induce cloned interleukin 3-dependent mouse mast cells to resemble connective tissue mast cells in granular constituency. Proc. Natl. Acad. Sci. USA. 85:569–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nocka, K., J. Buck, E. Levi, and P. Besmer. 1990. Candidate ligand for the c-kit transmembrane kinase receptor: KL, a fibroblast derived growth factor stimulates mast cells and erythroid progenitors. EMBO J. 9:3287–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durand, B., G. Migliaccio, N.S. Yee, K. Eddleman, T. Huima-Byron, A.R. Migliaccio, and J.W. Adamson. 1994. Long-term generation of human mast cells in serum-free cultures of CD34+ cord blood cells stimulated with stem cell factor and interleukin-3. Blood. 84:3667–3674. [PubMed] [Google Scholar]
- 12.Rottem, M., S. Barbieri, J.P. Kinet, and D.D. Metcalfe. 1992. Kinetics of the appearance of FcɛRI-bearing cells in interleukin-3-dependent mouse bone marrow cultures: correlation with histamine content and mast cell maturation. Blood. 79:972–980. [PubMed] [Google Scholar]
- 13.Nakano, T., T. Sonoda, C. Hayashi, A. Yamatodani, Y. Kanayama, T. Yamamura, H. Asai, T. Yonezawa, Y. Kitamura, and S.J. Galli. 1985. Fate of bone marrow–derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell–deficient W/Wυ mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J. Exp. Med. 162:1025–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nocka, K., J.C. Tan, E. Chiu, T.Y. Chu, P. Ray, P. Traktman, and P. Besmer. 1990. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 9:1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki, K., H. Nakajima, N. Watanabe, S. Kagami, A. Suto, Y. Saito, T. Saito, and I. Iwamoto. 2000. Role of common cytokine receptor gamma chain (gamma(c))- and Jak3-dependent signaling in the proliferation and survival of murine mast cells. Blood. 96:2172–2180. [PubMed] [Google Scholar]
- 16.Fukao, T., T. Yamada, M. Tanabe, Y. Terauchi, T. Ota, T. Takayama, T. Asano, T. Takeuchi, T. Kadowaki, J.J. Hata, et al. 2002. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat. Immunol. 3:295–304. [DOI] [PubMed] [Google Scholar]
- 17.Morii, E., H. Ogihara, D.K. Kim, A. Ito, K. Oboki, Y.M. Lee, T. Jippo, S. Nomura, K. Maeyama, M.L Lamoreux, et al. 2001. Importance of leucine zipper domain of mi transcription factor (MITF) for differentiation of mast cells demonstrated using mice/mice mutant mice of which MITF lacks the zipper domain. Blood. 97:2038–2044. [DOI] [PubMed] [Google Scholar]
- 18.Hodgkinson, C.A., K.J. Moore, A. Nakayama, E. Steingrimsson, N.G. Copeland, N.A. Jenkins, and H. Arnheiter. 1993. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 74:395–404. [DOI] [PubMed] [Google Scholar]
- 19.Daheshia, M., D.S. Friend, M.J. Grusby, K.F. Austen, and H.R. Katz. 2001. Increased severity of local and systemic anaphylactic reactions in gp49B1-deficient mice. J. Exp. Med. 194:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orkin, S.H. 2001. Transcription factors that regulate lineage decisions. The Molecular Basis of Blood Diseases. G. Stamatoyannopoulos, P.W. Majerus, R. Perlmutter, and H. Varmus, editors. WB Saunders, Philadelphia, PA. pp. 80–102.
- 21.Tsai, S.F., D.I. Martin, L.I. Zon, A.D. D'Andrea, G.G. Wong, and S.H. Orkin. 1989. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 339:446–451. [DOI] [PubMed] [Google Scholar]
- 22.Romeo, P.H., M.H. Prandini, V. Joulin, V. Mignotte, M. Prenant, W. Vainchenker, G. Marguerie, and G. Uzan. 1990. Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature. 344:447–449. [DOI] [PubMed] [Google Scholar]
- 23.Martin, D.I., L.I. Zon, G. Mutter, and S.H. Orkin. 1990. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 344:444–447. [DOI] [PubMed] [Google Scholar]
- 24.Crotta, S., S. Nicolis, A. Ronchi, S. Ottolenghi, L. Ruzzi, Y. Shimada, A.R. Migliaccio, and G. Migliaccio. 1990. Progressive inactivation of the expression of an erythroid transcriptional factor in GM- and G-CSF-dependent myeloid cell lines. Nucleic Acids Res. 18:6863–6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vannucchi, A.M., S. Linari, C.S. Lin, M.J. Koury, M.C. Bondurant, and A.R. Migliaccio. 1999. Increased expression of the distal, but not of the proximal, Gata1 transcripts during differentiation of primary erythroid cells. J. Cell. Physiol. 180:390–401. [DOI] [PubMed] [Google Scholar]
- 26.Yu, C., A.B. Cantor, H. Yang, C. Browne, R.A. Wells, Y. Fujiwara, and S.H. Orkin. 2002. Targeted deletion of a high-affinity binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 195:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pevny, L., M.C. Simon, E. Robertson, W.H. Klein, S.F. Tsai, V. D'Agati, S.H. Orkin, and F. Costantini. 1991. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 349:257–260. [DOI] [PubMed] [Google Scholar]
- 28.Pevny, L., C.S. Lin, V. D'Agati, M.C Simon, S.H. Orkin, and F. Costantini. 1995. Development of hematopoietic cells lacking transcription factor GATA-1. Development. 121:163–172. [DOI] [PubMed] [Google Scholar]
- 29.Harigae, H., S. Takahashi, N. Suwabe, H. Ohtsu, L. Gu, Z. Yang, F.Y. Tsai, Y. Kitamura, J.D. Engel, and M. Yamamoto. 1998. Differential roles of GATA-1 and GATA-2 in growth and differentiation of mast cells. Genes Cells. 3:39–50. [DOI] [PubMed] [Google Scholar]
- 30.Tsai, F.Y., and S.H. Orkin. 1997. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 89:3636–3643. [PubMed] [Google Scholar]
- 31.Jippo, T., H. Mizuno, Z. Xu, S. Nomura, M. Yamamoto, and Y. Kitamura. 1996. Abundant expression of transcription factor GATA-2 in proliferating but not in differentiated mast cells in tissues of mice: demonstration by in situ hybridization. Blood. 87:993–998. [PubMed] [Google Scholar]
- 32.Zon, L.I., M.F. Gurish, R.L. Stevens, C. Mather, D.S. Reynolds, K.F. Austen, and S.H. Orkin. 1991. GATA-binding transcription factors in mast cells regulate the promoter of the mast cell carboxypeptidase A gene. J. Biol. Chem. 266:22948–22953. [PubMed] [Google Scholar]
- 33.Nishiyama, C., T. Yokota, K. Okumura, and C. Ra. 1999. The transcription factors Elf-1 and GATA-1 bind to cell-specific enhancer elements of human high-affinity IgE receptor α-chain gene. J. Immunol. 163:623–630. [PubMed] [Google Scholar]
- 34.Gachter, T., D.R. Moritz, J. Gheyselinck, and R. Klemenz. 1998. GATA-dependent expression of the interleukin-1 receptor-related T1 gene in mast cells. Mol. Cell. Biol. 18:5320–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDevitt, M.A., Y. Fujiwara, R.A. Shivdasani, and S.H. Orkin. 1997. An upstream, DNase I hypersensitive region of the hematopoietic-expressed transcription factor GATA-1 gene confers developmental specificity in transgenic mice. Proc. Natl. Acad. Sci. USA. 94:7976–7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shivdasani, R.A., Y. Fujiwara, M.A. McDevitt, and S.H. Orkin. 1997. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 16:3965–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vyas, P., K. Ault, C.W. Jackson, S.H. Orkin, and R.A. Shivdasani. 1999. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood. 93:2867–2875. [PubMed] [Google Scholar]
- 38.McDevitt, M.A., R.A. Shivdasani, Y. Fujiwara, H. Yang, and S.H. Orkin. 1997. A “knockdown” mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc. Natl. Acad. Sci. USA. 94:6781–6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vannucchi, A.M., L. Bianchi, C. Cellai, F. Paoletti, V. Carrai, A. Calzolari, L. Centurione, R. Lorenzini, C. Carta, E. Alfani, et al. 2001. Accentuated response to phenylhydrazine and erythropoietin in mice genetically impaired for their GATA-1 expression (GATA-1low mice). Blood. 97:3040–3050. [DOI] [PubMed] [Google Scholar]
- 40.Vannucchi, A.M., L. Bianchi, C. Cellai, F. Paoletti, R.A. Rana, R. Lorenzini, G. Migliaccio, and A.R. Migliaccio. 2002. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1low mice). Blood. 100:1123–1132. [DOI] [PubMed] [Google Scholar]
- 41.Pearse, A.G.E. 1978. Histochemistry. Theoretical and Applied. Little, Brown & Company, Boston. 754 pp.
- 42.Farina, S.F., L.J. Girard, E.F. Vanin, A.W. Nienhuis, and D.M. Bodine. 1995. Dysregulated expression of GATA-1 following retrovirus-mediated gene transfer into murine hematopoietic stem cells increases erythropoiesis. Blood. 86:4124–4133. [PubMed] [Google Scholar]
- 43.Markowitz, D., C. Hesdorffer, M. Ward, S. Goff and A. Bank. 1990. Retroviral gene transfer using safe and efficient packaging cell lines. Ann. New York Acad. Sci. 612:407–414. [DOI] [PubMed] [Google Scholar]
- 44.Jippo, T., E. Morii, K. Tsujino, T. Tsujimura, Y.M. Lee, D.K. Kim, H. Matsuda, H.M. Kim, and Y. Kitamura. 1997. Involvement of transcription factor encoded by the mouse mi locus (MITF) in expression of p75 receptor of nerve growth factor in cultured mast cells of mice. Blood. 90:2601–2608. [PubMed] [Google Scholar]
- 45.Morii, E., T. Jippo, T. Tsujimura, K. Hashimoto, D.K. Kim, Y.M. Lee, H. Ogihara, K. Tsujino, H.M. Kim, and Y. Kitamura. 1997. Abnormal expression of mouse mast cell protease 5 gene in cultured mast cells derived from mutant mi/mi mice. Blood. 90:3057–3066. [PubMed] [Google Scholar]
- 46.Ogihara, H., E. Morii, D.K. Kim, K. Oboki, and Y. Kitamura. 2001. Inhibitory effect of the transcription factor encoded by the mutant mi microphthalmia allele on transactivation of mouse mast cell protease 7 gene. Blood. 97:645–651. [DOI] [PubMed] [Google Scholar]
- 47.Shivdasani, E.A., M.F. Rosenblatt, D. Zucker-Franklin, C.W. Jackson, P. Hunt, C.J. Saris, and S.H. Orkin. 1995. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MDGF in megakaryocyte development. Cell. 81:695–704. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 1772 pp.
- 49.Churukian, C.J., and E.A. Schenk. 1981. A Toluidine blue method for demonstrating mast cells. J. Histotechnol. 4:85–86. [Google Scholar]
- 50.Dvorak, A.M. 2002. Ultrastructure of human mast cells. Int. Arch. Allergy Immunol. 127:100–105. [DOI] [PubMed] [Google Scholar]
- 51.Kitamura, Y., H. Nakayama, and J. Fujita. 1989. Mechanism of mast cell deficiency in mutant mice of W/Wv and Sl/Sld genotype. Mast Cell and Basophil Differentiation and Function in Health and Disease. S.J. Galli and K.F. Austen, editors. Raven Press, New York. p. 15.
- 52.Akashi, K., D. Traver, T. Miyamoto, and I.L. Weissman. 2000. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 404:193–195. [DOI] [PubMed] [Google Scholar]
- 53.McNeil, H.P., D.S. Reynolds, V. Schiller, N. Ghildyal, D.S. Gurley, K.F. Austen, and R.L. Stevens. 1992. Isolation, characterization, and transcription of the gene encoding mouse mast cell protease 7. Proc. Natl. Acad. Sci. USA. 89:11174–11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iwasaki, H., and K. Akashi. 2001. Mast cells originate from granulocyte/macrophage-committed progenitors. Blood. 98:275a. [Google Scholar]
- 55.McNgny, K., and T. Graf. 2002. Making eosinophils through subtle shifts in transcription factor expression. J. Exp. Med. 195:F43–F47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuriu, A., S. Sonoda, Y. Kanakura, Y. Jozaki, A. Yamatodani, and Y. Kitamura. 1989. Proliferative potential of degranulated murine peritoneal mast cells. Blood. 74:925–929. [PubMed] [Google Scholar]
- 57.Orkin, S.H., and M.J. Weiss. 1999. Apoptosis. Cutting red-cell production. Nature. 401:433–436. [DOI] [PubMed] [Google Scholar]