Mouse Plasmacytoid Cells: Long-lived Cells, Heterogeneous in Surface Phenotype and Function, that Differentiate Into CD8+ Dendritic Cells Only after Microbial Stimulus (original) (raw)
Abstract
The CD45RAhiCD11cint plasmacytoid predendritic cells (p-preDCs) of mouse lymphoid organs, when stimulated in culture with CpG or influenza virus, produce large amounts of type I interferons and transform without division into CD8+CD205− DCs. P-preDCs express CIRE, the murine equivalent of DC-specific intercellular adhesion molecule 3 grabbing nonintegrin (DC-SIGN). P-preDCs are divisible by CD4 expression into two subgroups differing in turnover rate and in response to Staphylococcus aureus. The kinetics of bromodeoxyuridine labeling and the results of transfer to normal recipient mice indicate that CD4− p-preDCs are the immediate precursors of CD4+ p-preDCs. Similar experiments indicate that p-preDCs are normally long lived and are not the precursors of the short-lived steady-state conventional DCs. However, in line with the culture studies on transfer to influenza virus-stimulated mice the p-preDCs transform into CD8+CD205− DCs, distinct from conventional CD8+CD205+ DCs. Hence as well as activating preexistant DCs, microbial infection induces a wave of production of a new DC subtype. The functional implications of this shift in the DC network remain to be determined.
Keywords: plasmacytoid cells, dendritic cells, CD8, CpG, microbial infection
Introduction
The ‘plasmacytoid T cell’ or ‘plasmacytoid monocyte’ of human blood and lymphoid tissues puzzled pathologists for decades. Eckert and Schmid (1) first suggested that these cells were novel antigen presenting cells and indeed Grouard (2) later identified these CD4+CD11c− plasmacytoid (p) cells as predendritic cells (preDCs)* that, on culture with IL-3, yielded functional DCs. Similar differentiation to DCs was then shown to be induced by CD40 ligand (L) and by viral and bacterial stimuli. These same stimuli were subsequently found to induce production of type I IFNs (3–5). It has long been known that the p-preDCs migrate to inflamed lymph nodes and cluster around high endothelial venules (for a review, see reference 6), and it is now known that once there, they produce large amounts of type I IFN (4, 5). Thus, as well as being preDCs, the plasmacytoid cells proved to be the ‘natural IFN-producing cells’ which had evaded virologists for many years (6). The response of these p-preDCs to microbial stimuli appears to depend on a discrete set of Toll-like receptors (TLRs; references 7–9) and possibly other pattern recognition receptors. These findings, of a dual antigen-presenting and IFN-producing role in response to microbial invasion, have initiated investigations of the role of p-preDCs in various infectious diseases (10–14).
The identification of the murine equivalent of the human p-preDCs paves the way to a detailed study of the functions of this unusual cell type in models of microbial infections, tumors, and autoimmune disease. Several groups simultaneously identified mouse CD11cloB220+ cells of plasmacytoid morphology as type I IFN-producing preDCs (15–17). In a search for the precursors of CD8+ DCs we recently reported the isolation of p-preDCs from mouse blood and showed they are closely related to the human blood p-preDCs (17a). We now report the characteristics of these cells in mouse spleen, thymus, and lymph nodes, and find that they express a unique pattern of surface markers, including the murine equivalent of DC-specific intercellular adhesion molecule 3 grabbing nonintegrin (DC-SIGN). We find they are separable by CD4 expression into two functionally distinct subtypes that represent different stages of differentiation. We demonstrate that in uninfected mice these p-preDCs are long-lived and not the precursors of the short-lived steady-state conventional CD8+ DCs. However, upon bacterial or viral stimulation in vivo, as well as in vitro, they then produce a separate wave of a different subtype of CD8+ DCs. This points to a significant change in the nature of the DCs present in uninfected versus infected individuals.
Materials and Methods
Mice.
Mice were bred under specific pathogen free conditions in the Walter and Eliza Hall Institute (WEHI) animal breeding facility. Male and female C57BL/6J WEHI mice (Ly 5.2) were used at 6–9 wk of age. T cells used in allostimulatory mixed leukocyte reactions (allo-MLR) were from CBA CaH WEHI mice. The recipients used for cell transfer experiments were age and sex-matched mice of the C57BL/6 Ly-5.1 Pep3b strain.
Immunofluorescent Labeling of DCs.
Most of the mAb, the fluorescent conjugates, and the multicolor labeling procedures have been specified previously (18). For sorting CD11c+CD45RA− conventional DCs and CD11cintCD45RAhi p-preDCs from lymphoid organs, anti-CD11c (N418)-FITC conjugate, and anti-CD45RA (clone 14.8)-PE conjugate (BD Biosciences) were used. For sorting p-preDC sub-populations the following mAb were used: anti-CD45RA (clone 14.8)-PE, anti-CD4 (GK1.5)-FITC, and anti-CD8α (YTS169.4)-Cy-5. For surface phenotype analyses the following mAb conjugates were used: anti-41BBLigand (TKS-1)-biotin, anti–BP-1 (6C3)-biotin, anti-CD1d (1B1)-FITC, anti-CD4 (GK1.5)-Alexa 594 or -FITC, anti-CD5 (53–7.3)-biotin, anti-CD8α (YTS169.4)-Cy5, anti-CD8β (53–5.8)-biotin, anti-CD11b (M1/70)-FITC, anti-CD11c (N418)-Cy5, -FITC, or -Alexa-594, anti-CD14 (RmC5–3)-FITC, anti-CD16/CD32 (2.4G2)-FITC, anti-CD18 (M18/2.a.12.17)-biotin, anti-CD19 (ID3)-FITC or -biotin, anti-CD24 (M1/69)-FITC, anti-CD25 (PC61)-FITC, anti-CD36 (clone 63)-FITC, anti-CD38 (NIM-R5)-biotin, anti-CD40 (FGK45.5)-FITC or biotin, anti-CD43 (S7)-FITC, anti-CD45R (RA3–6B2)-FITC, anti-CD45.2 (anti-Ly5.2, clone ALI-4A2)-biotin, anti-CD45RA (14.8)-FITC, -PE, or -Cy-5, anti-CD45RB (MB23G2)-biotin, anti-CD49d (PS/2)-FITC, anti-CD54 (YN1/1.7.4)-biotin, anti-CD62L (MEL-14)-FITC, anti-CD80 (16–10A1)-FITC, anti-CD86 (GL1)-FITC, anti-CD115 (AFS98)-FITC, anti-CD127 (A7R34–2.2)-biotin, anti-CD205 (DEC205, clone NLDC-145)-FITC, anti-CIRE (murine DC-SIGN, clone 5H10)-biotin, anti-Ep-CAM (G8.8)-biotin, anti-F4/80 (F4/80)-FITC, anti-Ly6C.2 (5075–3.6)-FITC or -biotin, anti-Ly6G (RB68C5)-FITC or -biotin, anti-MHC class II (N22 or M5/114)-FITC or -Alexa 594 (the conjugation levels were deliberately less than maximal to ensure the strong staining for class II MHC on DCs at saturation did not cause color compensation problems in other channels), anti-NK cell (DX-5)-biotin, anti-Sca-1 (E13161–7)-FITC, and anti–Sca-2 (E381–2.4)-biotin. Propidium iodide (PI) was included at 1 μg/ml in the final wash after immunofluorescent staining to label dead cells.
Flow Cytometric Sorting and Analyses.
For sorting conventional DC and p-preDC populations a MoFlo instrument (Cytomation Inc.) was used. Reanalysis was routinely performed and populations were only used for further functional analyses when the purity was >95%. For cell transfer experiments care was taken to use only p-preDCs that were >98% pure. In most cases an autofluorescent presort of DC preparations was performed on the Mo-Flo instrument as described previously (18). This involved sorting the cells before staining with any antibodies, but after PI staining. Only those cells that were PI negative and which did not fluoresce in the FITC or PE channels were collected, yielding a preparation free of autofluorescent cells and of dead cells. Most analyses were performed on a FACStarPlus™ instrument (Becton Dickinson) as described previously (18), using up to four fluorescent channels for the immunofluorescent staining (FL1 for FITC, FL2 for PE, FL3 for Cy5, and FL4 for Alexa 594), with the FL5 channel set to exclude any residual PI-positive dead cells or autofluorescent cells.
Purification of p-preDCs and DCs from Mouse Lymphoid Organs.
DC preparations, containing conventional CD11chi DCs and p-preDCs, were isolated from spleen, thymus, and subcutaneous and mesenteric lymph nodes using methods similar to those described previously (18, 19). This involved mild collagenase digestion at room temperature, isolation of light density cells, then immunomagnetic bead depletion of non-DC lineages. To include B220+p-preDCs in the enriched DC preparation, the critical difference was to replace anti-B220 (RA3–6B2) in the mAb depletion cocktail with anti-CD19 (ID3). Increasing the density of Nycodenz or leaving out anti-Gr-1 (RB6-8C5) from the depletion cocktail did not increase the yield or change the phenotype of the p-preDCs recovered. For purification and segregation of the populations of conventional and p-preDCs, the presorted DC preparation, containing both cell types, was labeled with anti-CD11c-FITC and anti-CD45RA-PE, and the two distinct CD11cintCD45RAhi (p-preDC) and CD11c+CD45RA− (conventional DC) populations were sorted using the MoFlo instrument. Alternatively, to purify discrete sub-populations of p-preDCs, the DC preparation was labeled with anti-CD45RA-PE, anti-CD4-FITC, and anti-CD8-Cy-5 and the four CD45RAhi subpopulations were sorted: CD4−CD8−, CD4+CD8−, CD4+CD8+, and CD4−CD8+.
Cytokines and Stimulants of preDCs.
Murine recombinant (r)GM- CSF (used at 200 U/ml), murine (m)-rIL-3 (used at 100 U/ml), m-rTNF-α (used at 100 U/ml), and m-rIL-4 (used at 100 U/ml) were gifts from Immunex Corporation, Seattle, WA. Recombinant rat IFN-γ (used at 20 ng/ml and bioactive with mouse cells) was purchased from PeproTech. Murine rIL-12 p70 was purchased from R&D Systems. Oligonucleotides containing a fully phosphorothioated CpG motif were synthesized by GeneWorks Pty Ltd. according to a published sequence (CpG1668 [20]) and used at 25–1,000 nM. All CpG sequences and their modifications display differing effects on immune cells (for a review, see reference 21). The phosphorothiate CpG was initially chosen for its extended half life in vitro and in vivo and then routinely used due to the stimulatory effects it had on p-preDCs, which were less intense but otherwise mimicked the response to flu virus. Pansorbin (fixed and heat killed Staphylococcus aureus [SAC]) was purchased from Calbiochem-Novabiochem. LPS and polyinosinic-polycytidylic acid (poly I:C) were purchased from Sigma-Aldrich. Beta propiolactone-inactivated (BPL) influenza A viruses (PR/8/34 and A/Guangdong/25/93) were provided by Michael Hocart, Influenza Process Development, CSL Ltd., Melbourne, Australia. Viable A/Guangdong/25/93 virus was from the Department of Microbiology and Immunology, The University of Melbourne.
Differentiation and Activation of DCs in Culture.
Conventional DCs and p-preDCs were incubated at 0.5 × 106 cells/ml in U–bottom wells of 96-well tissue culture plates, or in flat-bottom wells of 96- or 24-well tissue culture plates, in a humidified 10% CO2-in-air incubator at 37°C for between 8 h and 6 wk. Modified, mouse osmolarity, RPMI-1640 medium (22) was used, together with the appropriate cytokines and stimulants as specified in the text.
Cell Cycle Analysis.
The cell cycle distribution of sorted p-preDCs, freshly isolated or after overnight culture with 0.5 μM CpG, was performed as described previously (23). Briefly, cells were fixed with 70% ethanol, treated with 0.5 μg/ml DNase-free RNase A (Boehringer) for 20 min at room temperature, and stained with 69 μM PI in 0.1 M sodium citrate (pH 7.4) for 30 min at 4°C. Flow cytometric analysis was performed using a FACScan™ II and cell cycle distribution was determined with the Cellfit program (Becton Dickinson).
Phase Contrast Microscopy.
Cells were cultured for 8 h to 6 wk in wells of 96- or 24-well flat-bottom culture plates. The cells were visualized using the ×20 or ×40 objective of a Nikon Phase Contrast inverted microscope and photographed with a connected Nikon SLR camera using Eastman Kodak Co. 64T film.
MLR Cultures for T Cell Stimulation Capacity.
CD4+ T cells were purified from pooled mesenteric, axillary, brachial, and inguinal lymph nodes of CBA/CaH mice as described previously (24). Freshly isolated CD11cintCD45RAhi preDC (1–4 × 103) or CD11chiCD45RA− conventional DC (1–4 × 103) were added to U-bottom wells containing 2 × 104 T cells. Total culture volume was 100 μl in the modified RPMI 1640 medium described above or in the same medium containing 200 U/ml GM-CSF and 0.1 μM CpG. Replicate culture trays were incubated at 37°C in 10% CO2-in-air for 3–6 d. At days 3, 4, 5, 6, and 7 a culture tray was pulsed with [3H]thymidine, 1 μCi/well, for 6 h, then frozen. The trays were thawed, the cells harvested onto glass fiber filters, and the thymidine incorporated was counted by liquid scintillation. All cultures at all times were at least in triplicate and background controls, with T cells or preDCs only and with and without added stimulants, were included at each time point.
Quantitation of Cytokine Production.
The analysis of IL-12 production in culture supernatants was performed by two-site ELISA as described previously (25, 26). The detection of IL-6 also employed a two-site ELISA, using as capture antibody clone MP5-20F3 and as detection antibody biotinylated clone MP5–32C11. The IL-6 standard used was an IL-6 transfectant supernatant (27) and the readout of the ELISA was exactly as that described for IL-12. A bioassay for type I IFN was performed as described previously (26). In addition, IFN-α was assayed by a two-site ELISA, using as a capture antibody anti-mouse IFN-α (clone RMMA-1; PBL Biomedical Laboratories) and as detection antibody a rabbit anti-mouse IFN-α polyclonal (PBL Biomedical Laboratories), followed by a F(ab′)2 fragment donkey-anti–rabbit-HRPO conjugate (Jackson Immunoresearch Laboratories). The IFN-α standard used was recombinant mouse IFN-αA (PBL Biomedical Laboratories) and the readout of the ELISA was exactly as that described for IL-12.
BrdU Treatment of Mice and Analysis of BrdU-labeled Cells.
Mice were given an initial intraperitoneal injection of BrdU (100 μg in 100 μl PBS) at zero time and BrdU was administered continuously in drinking water for up to 14 d as described previously (28). DCs were purified from treated mice as described above and presorted to remove autofluorescent cells using the Mo-Flo instrument. The non-autofluorescent cells were stained with surface markers and then fixed in 70% ethanol, followed by intracellular staining for BrdU exactly as described previously (28), using the anti-BrdU antibody B44 (BD Biosciences). The cells were then analyzed using a FACStarPlus™ instrument.
Transfer of p-preDCs to Ly 5.1+ Recipients.
Sorted p-preDCs from C57BL/6 Ly5.2+ mice were washed in serum-free PBS and injected either intravenously (1.5–2.5 × 106 cells/mouse, 200 μl volume) or into the footpad (0.4 × 106 cells/footpad, 15 μl volume) or intrathymically (0.25 × 106 cells/lobe, 10 μl volume) into nonirradiated Ly5.1+ recipients (three mice per group).
Treatment of Mice with Influenza Viruses.
Groups of at least three mice were injected intravenously with BPL-PR/8/34 or BPL-Guandong/93, at doses of 40 to 4,000 hemagglutinating units (HAU)/mouse, either once or on two consecutive days. Mice that also received p-preDCs (as above), received virus 2 h after transfer of p-preDCs.
Results
Isolation of p-preDC from Mouse Lymphoid Organs.
We recently isolated CD45RA+ p-preDCs from mouse blood (17a) and showed that on stimulation in culture with CpG (oligonucleotides that mimic bacterial CpG motifs), these cells rapidly produced large amounts of type I IFN, and also became DCs and acquired surface CD8α. This raised the question of whether the p-preDCs were precursors of the CD8+ DCs present in the lymphoid organs of normal laboratory mice (18, 19). We therefore examined in detail the p-preDCs present in mouse spleen, thymus, and lymph nodes and designed experiments to determine if they were the immediate precursors of the CD8+ DCs of these organs.
To prepare the p-preDCs we first employed isolation and enrichment procedures similar to those used to purify mouse DCs, including selection of light-density cells and immunomagnetic bead depletion of irrelevant cells. Importantly, to remove B cells, we replaced anti-CD45R (B220) mAb with anti-CD19, as we and others (15–17) found that mouse p-preDCs express B220. Others have reported that mouse p-preDCs stain with anti-Ly6G (GR-1), perhaps due to cross-reactivity with other Ly6 molecules (17). However, we obtained variable and always low staining of mouse p-preDCs with anti-GR-1. We found no drop in p-preDC yield when anti–GR-1 was included in the depletion cocktail nor any appearance of high anti–GR-1 staining on p-preDCs when it was omitted. Accordingly, anti–GR-1 was usually included in the depletion mAb cocktail.
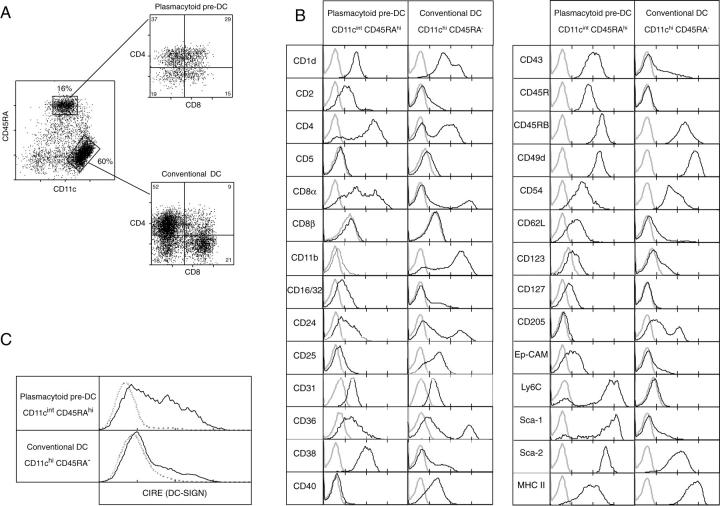
To finally purify the p-preDCs, autofluorescent cells were first eliminated in a presort and the remaining cells were stained and sorted as CD45RAhiCD11cint cells. We found CD45RA to stain more brightly than CD45R (B220). We also found the p-preDCs from mouse lymphoid tissues stained at intermediate levels for CD11c, higher than the levels found in mouse blood p-preDCs, and differing from the reported negative staining of CD11c on human p-preDCs (2). In all mouse lymphoid tissues this produced a discrete population of p-preDCs, well segregated from the CD45RA−CD11chi conventional DCs (Fig. 1 A). The ratio of CD45RAhiCD11cint p-preDCs to CD45RA−CD11chi DCs was 1:3 to 1:4 for spleen (Fig. 1 A), 1:3 for thymus and 1:1 for lymph nodes (unpublished data).
Figure 1.
Surface phenotype of mouse spleen p-preDCs compared with conventional DCs. Mouse spleen p-preDCs and conventional DCs were purified as described in Materials and Methods. The enriched preparation, after removal of autofluorescent cells, was stained for surface expression of CD11c and CD45RA, together with staining for one or two other surface molecules. The p-preDCs (CD45RAhiCD11cint) and DCs (CD45RA−CD11chi) populations were gated as shown in A. The distribution of CD4 and CD8 on these (gated) populations is shown as a dot plot in A and as histograms in B. The fluorescence distributions of a range of surface markers on the p-preDCs and conventional DC populations are shown in B; the broken lines give the background fluorescence with only the relevant stain omitted. Not shown are the stains for the following surface molecules that were negative (like CD40, as shown) on the preDC populations: 41BBLigand, CD14, CD18, CD19, CD80, CD86, CD115, NK cell marker (DX-5). Surface staining for CIRE, the putative mouse DC-SIGN homologue is shown in C. The data of A are representative of more than 10 analyses, the data of B and C are representative of 2–5 analyses.
Morphology of p-preDCs in Mouse Lymphoid Organs.
Microscopy and light-scatter characteristics indicated the mouse p-preDCs were round cells intermediate in size between lymphocytes and conventional DCs. Electron microscopy revealed cells with plasmacytoid morphology, without dendritic processes, rather than conventional DC morphology (data not shown). Endoplasmic reticulum was not prominent in the majority of splenic p-preDCs, although some cells showed the extensive endoplasmic reticulum reported for human p-preDCs and that we have found for mouse blood p-preDCs.
CD4 and CD8 Expression by Mouse p-preDCs.
By using 3 or 4-color staining, the surface phenotype of the p-preDCs was examined in detail. The first notable feature was that the freshly isolated CD45RAhiCD11cint population, although homogeneous by many markers, included cells expressing CD4 and CD8α (but not CD8β; Fig. 1). All combinations of CD4 and CD8 expression were obtained, allowing segregation into four populations (Fig. 1 A). This was common to all mouse lymphoid tissue sources, which all displayed similar proportions of the four populations, but contrasted with mouse blood where we found all p-preDCs were CD4−CD8− (17a).
Surface Markers on Mouse p-preDCs.
The staining for a range of other markers on mouse spleen p-preDCs is directly compared with that of pooled conventional spleen DCs in Fig. 1 B. This data confirms that we are studying the same mouse p-preDC population as other groups who have reported such a distinct pattern of markers, and also introduces some new aspects of the p-preDC surface. The p-preDCs expressed low surface levels of MHC II rather than the moderate levels on quiescent conventional DCs; however, p-preDCs showed intracellular MHC II by confocal microscopy (unpublished data). The p-preDCs also lacked detectable expression of the costimulator molecules CD40 (Fig. 1 B), CD80, and CD86 (data not shown), in contrast to quiescent conventional DCs which expressed low levels. This makes it unlikely that p-preDCs in an unstimulated form could activate naive T cells.
The lack of expression of various myeloid markers (CD11b [Fig. 1 B], CD14, F4/80 [data not shown]) and the expression of some markers found on mature or early lymphoid cells (CD2, CD45R, Sca-1, Sca-2; Fig. 1 B) hints at a possible lymphoid origin. Of note, although these cells expressed CD45RA and CD45R (B220; Fig. 1 B), they did not express other B lineage markers such as BP-1, CD21, CD19, or surface Ig (data not shown). Nor did they express on the surface the NK-cell marker DX5, nor the T cell markers TCR αβ, TCR γδ, nor CD3 chains (data not shown).
One difference to human p-preDCs is the much lower expression of CD123 (IL-3 receptor) on mouse p-preDCs, although it is positive (Fig. 1 B). CD127 (IL-7 receptor) is low positive on the mouse p-preDCs.
A notable feature of the mouse p-preDCs is the high expression of Sca-1 (Ly 6A/E) and of Sca-2 (Ly-6E, TSA-1). These are markers on early developmental stages of lymphoid lineage cells. Despite the high staining of these Ly-6 family members, including Ly 6C, our staining with GR-1 (Ly-6G) gave erratic and usually low to negative results, much lower than the high staining of granulocytes, even when GR-1 depletion was omitted from the isolation protocol. Similar results were obtained using a separate batch of anti–GR-1 mAb provided by Drs. Asselin-Paturel and G. Trinchieri (Schering-Plough, Dardilly, France).
One important difference from conventional DCs is that as both CD11b and CD205 (DEC-205) are not expressed by p-preDCs (Fig. 1 B), the expression of these markers did not correlate with the CD8− and CD8+ subdivisions as is seen with conventional splenic DCs (18).
CIRE (DC-SIGN) on Mouse Spleen p-preDCs.
A novel DC-specific C-type lectin originally termed CIRE was just identified on CD8− DCs and cloned in this laboratory by Caminschi et al. (29). CIRE appears to be the closest murine equivalent of human DC-SIGN, a ligand of intercellular adhesion molecule (ICAM)-2 and ICAM-3 and also a receptor for HIV-1 that facilitates transinfection of T cells (29–31). The availability of a mAb against CIRE (unpublished data) enables its expression on DC lineage cells to be monitored. CIRE (DC-SIGN) is expressed by the majority of spleen p-preDCs (Fig. 1 C). Cross-correlation studies showed that only the fraction of splenic p-preDCs lacking expression of both CD4 and CD8 did not express CIRE (data not shown). The level of CIRE expression on p-preDC was higher than on conventional quiescent DCs, where expression is limited to a subset of the CD8− DCs.
Culture of p-preDCs with Cytokines.
To determine if certain exogenous cytokines alone could induce p-preDCs to secrete cytokines, or to differentiate into DCs, the CD45RAhiCD11cint fraction from spleen was incubated with various combinations of cytokines (Table I). These cells died rapidly in culture in medium alone. GM-CSF and IL-3, separately or together, enhanced survival, but did not induce growth, differentiation to DCs, or cytokine production, even on prolonged culture. However a modest increase in surface MHC II expression was noted. IL-4 and TNF-α, factors used to produce and mature DCs from monocytes, merely increased the rate of cell death. IL-6, a factor found to induce DC production from bone marrow cells (32), was without effect. No combination of the cytokines used activated these p-preDCs to cytokine production or produced cells with the shape or surface markers of DCs, in contrast to results with human p-preDCs where IL-3 alone induced some differentiation to DCs (3).
Table I.
Culture of Mouse Spleen Plasmacytoid Cells
| Viable cellrecovery (% input)a | Cytokineproduction day 1 | |||||
|---|---|---|---|---|---|---|
| Stimulusand cytokines | Day 1 | Day 3 | Type 1 IFN | IL-6 | IL-12p70 | Transformationto DC |
| Medium alone | 30 | <10 | − | − | − | − |
| IL-4 | 17 | <10 | − | − | − | − |
| TNFα | 22 | <10 | − | − | − | − |
| IL-4 + TNFα | 37 | <10 | − | − | − | − |
| IL-6 | 32 | <10 | − | ND | − | − |
| GM-CSF | 51 | 25 | − | − | − | − |
| IL-3 | 45 | 28 | − | − | − | − |
| GM-CSF + IL-3 | 64 | 30 | − | − | − | − |
| CpG | 69 | 50 | +++ | ++ | + | +++ |
| CpG + GM-CSF + IL-3 | 75 | 60 | +++ | ++ | + | +++ |
| CpG + GM-CSF + anti–IL-6 | 77 | 61 | +++ | ND | + | +++ |
| CpG + GM-CSF + IL-3 + IFN-γ + IL-4 | 71 | 58 | +++ | ++ | ++ | +++ |
| LPS + GM-CSF + IL-3 | 54 | 30 | − | − | − | − |
| Poly I:C + GM-CSF + IL-3 | 61 | 25 | − | − | − | − |
| SAC + GM-CSF + IL-3 | 62 | 32 | + | +/− | +/− | + |
Induction of Cytokine Production by CpG.
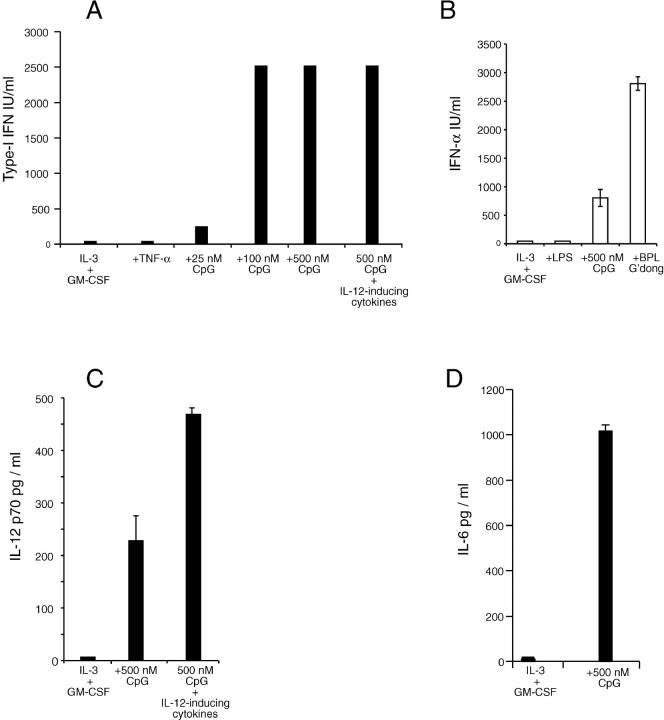
Stimulation of the CD45RAhiCD11cint fraction with CpG alone activated the cells to production of type I IFN at high levels, as well as to production of IL-6 and under appropriate conditions to production of moderate levels of IL-12p70. In addition the survival of the cells in culture was markedly enhanced, including the reversal of the death-inducing effects of IL-4 (Table I). These results were obtained with p-preDCs from all murine sources tested, including blood. This result indicated a close functional similarity to human p-preDCs (3–5).
The conditions for optimal cytokine production induced by CpG were then examined in detail (Table I). The presence of GM-CSF and/or IL-3 usually gave some improvement in cell survival but did not alter type I IFN production. As well as the bioassay, a specific ELISA confirmed that IFN-α was present in the culture supernatants. IL-6, assayed by a specific ELISA, was produced under all conditions that induced IFN-α production. The level of production of IL-12p70 was low with CpG alone, but was enhanced under the conditions established as optimal for IL-12p70 production by conventional CD8+ DCs, namely CpG plus GM-CSF, IFN-γ, and IL-4 (25). The level of IL-12p70 produced was still less than that obtained with conventional splenic CD8+ DCs and in contrast to CD8+ DCs a very high level of IL-12p40 (more than 0.1 μg/ml) was still produced even in the presence of IL-4 (data not shown). Production of IL-10 was not detected under any of the culture conditions. These results were also obtained for each individual fraction when the p-preDCs (CD45RAhiCD11cint) were first segregated into four groups on the basis of CD4 and CD8 expression (as in Fig. 1 A) before culture.
Stimulation of DC Development by CpG.
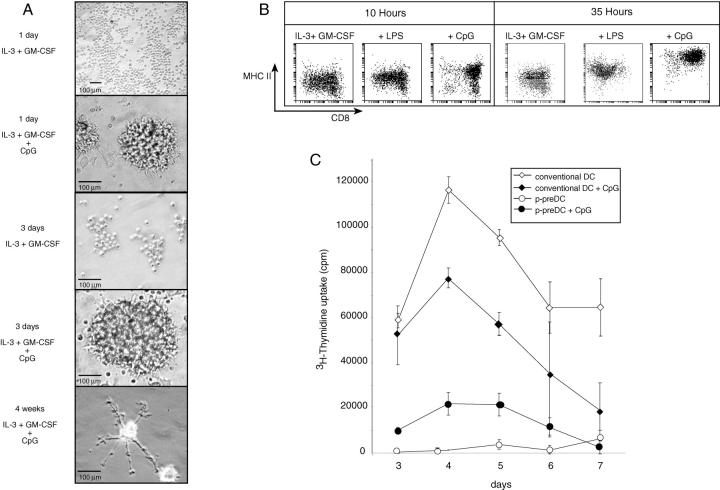
In contrast to the results with cytokines alone, culture of the CD45RAhiCD11cint fraction with CpG rapidly changed the appearance of the cells; they clustered and within 1 d differentiated into cells with DC morphology (Fig. 2 A). Similar results have been obtained with the equivalent fraction from blood. This demonstrates that these cells are indeed preDCs. The morphological change was even more dramatic on prolonged culture with CpG (Fig. 2 A). After 3 d some DC clusters produced long spindle-like DCs, many of which were still viable with this extreme morphology after 4 wk (Fig. 2 A), although the majority of DCs in clusters had died by this stage. This long survival of some DCs derived from preDCs is in marked contrast to the rapid death in culture of conventional splenic CD8+ DCs (28).
Figure 2.
Phenotype of p-preDCs after culture. (A) Images of p-preDCs after 1 d, 3 d, or 4 wk culture in the presence of IL-3 and GM-CSF, with or without CpG. Many cells died in the long term stimulated cultures, the results at 4 wk representing surviving cells. The original magnification was ×20 (top panel) and ×40 (all other panels). The images are representative of cultures from more than 20 preparations of sorted p-preDCs. (B) The MHC II and CD8α surface phenotype of cells after 10 h culture of sorted spleen p-preDCs in IL-3 and GM-CSF alone, or together with LPS or CPG, is shown, along with the phenotype of sorted p-preDCs after 35 h culture with IL-3 and GM-CSF alone, or together with LPS or CPG. The analyses are representative of more than 10 similar experiments. The recoveries of cells are indicated in Table I. (C) The ability of 2 × 103 p-preDCs to stimulate 2 × 104 CBA CD4+ T cells in an MLR in the presence or absence of CpG. The stimulatory capacity of p-preDCs was compared with that of 2 × 103 conventional DCs (purified at the same time), in the presence or absence of CpG. The average cpm of triplicate values is shown for each time point, the error bars representing the range of triplicate values. The data shown are from a single experiment; similar results were obtained in a second experiment.
There was no increase in cell numbers after culture with CpG (Table I) which, together with the speed of the transition, suggested no cell expansion was involved. Indeed PI staining of fixed samples of both freshly isolated CD45RAhiCD11cint cells, and the cells after 12 h culture with CpG, revealed only a sharp single 2n peak of DNA, with no evidence of any cells in division cycle (data not shown).
The Phenotype of DCs Generated in Culture from p-preDCs.
The surface phenotype of the DCs produced at various times of culture was analyzed (Fig. 2 B). In contrast to even prolonged culture with cytokines such as IL-3 and GM-CSF, which resulted in only small increases in surface MHC II, culture with CpG overnight resulted in a marked increase in surface MHC II, together with increased levels of CD11c and decreased levels of CD45RA (not shown); these effects were amplified with extended culture times, to produce a surface phenotype resembling by these markers that of conventional splenic DCs, except for the persistence of CD45RA (Fig. 2 B, and data not shown). This induction of DC morphology and phenotype was not caused by the IL-6 production, as it was also obtained in the presence of excess anti-IL-6 in the culture (data not shown). With respect to expression of CD8, this increased on culture with CpG; around 90% of the cells expressed moderate to high levels of CD8α after overnight culture and this increased on extended culture (Fig. 2 B). At the same time the level of CD4 decreased, dropping to very low levels on prolonged culture (data not shown), as is the case for CD4 expression by conventional DCs. Thus, all p-preDCs served as precursors of CD4−/loCD8+ DCs in culture. However no CD205 was expressed, so the DCs differed in this respect from conventional splenic CD8α+ DCs. Similar results were obtained with p-preDCs from all murine sources tested, including mouse blood (data not shown).
A hallmark of a mature DCs is the ability to stimulate proliferation in allogeneic naive T cells in culture. Similar to our results with mouse blood p-preDCs (17a), the freshly isolated CD45RAhiCD11cint fraction from spleen did not stimulate T cells in MLR culture (Fig. 2 C). However, when CpG (which produced detectable morphological changes in p-preDCs within 2 h) was added directly to the MLR cultures, the resultant activated p-preDCs did stimulate T cell proliferation, albeit to a lesser extent than that obtained with conventional splenic CD11c+ DCs, either freshly isolated or on culture with CpG (Fig. 2 C). A similar enhancement of T cell stimulation capacity was seen by preincubation of blood p-preDCs for 8 h with CpG, before washing and testing the cells in the MLR cultures.
Differential Responses of p-preDCs to Different Microbial Stimuli.
A range of microbial and other stimuli were tested to determine if they, like CpG, would activate mouse p-preDCs (Table I and Fig. 3) . In all cases stimuli found to induce type I IFN production in culture also induced full differentiation to DCs, and the pattern of response was the same for spleen, thymus, and lymph node p-preDCs. Neither LPS nor poly I:C activated the CD45RAhiCD11cint fraction to produce IFN-α, nor did soluble or surface bound anti-CD40 antibody FGK45.5 (Fig. 3, and data not shown). However influenza virus Guangdong strain, either live or inactivated, was a potent activator and induced 2–3 times more IFN-α than did CpG. The effectiveness of inactivated virus and the lack of response to poly I:C indicated that viral replication was not required for this activation. The surface phenotype of the DC induced after virus activation was identical to that induced by CpG.
Figure 3.
Activated spleen p-preDC produce IFN-α, IL-12, and IL-6. (A) Bioassay for the production of type I IFN from p-preDCs cultured for 14 h with IL-3 and GM-CSF alone, or with additional cytokines and stimulants. The “IL-12 inducing cytokines” were IL-3, GM-CSF, rat IFN-γ, and IL-4. The values shown represent the means of duplicate samples; similar results were obtained in three separate experiments. Culturing of p-preDCs for up to 80 h yielded similar supernatant levels of type I IFN. The production of type I IFN from p-preDCs stimulated with BPL-Guangdong and live Guangdong virus was also shown by bioassay in a single experiment; the levels being higher than with CpG. (B) ELISA for the production of IFN-α from p-preDCs cultured for 14 h in the presence of IL-3 and GM-CSF together with the stimulants shown. The results shown were similar to those obtained in three separate experiments for LPS and BPL-Guangdong and in five separate experiments for IL-3 and GM-CSF with or without CpG. The values shown are the means of duplicate samples and the error bars represent the range. (C) ELISA for the production of IL-12 p70 from spleen p-preDCs cultured for 14 h with IL-3 and GM-CSF alone, or together with additional cytokines and stimulants. IL-12 inducing cytokines were IL-3, GM-CSF, rat IFN-γ, and IL-4. The values shown are the means of duplicate samples and the error bars represent the range; similar results were obtained in three separate experiments. (D) ELISA for the production of IL-6 from spleen p-preDCs cultured for 14 h with IL-3 and GM-CSF alone, or together with CpG. The values shown are the means of duplicate samples and the error bars represent the range; similar results were obtained in three separate experiments.
The Developmental Kinetics of Mouse p-preDCs and DCs.
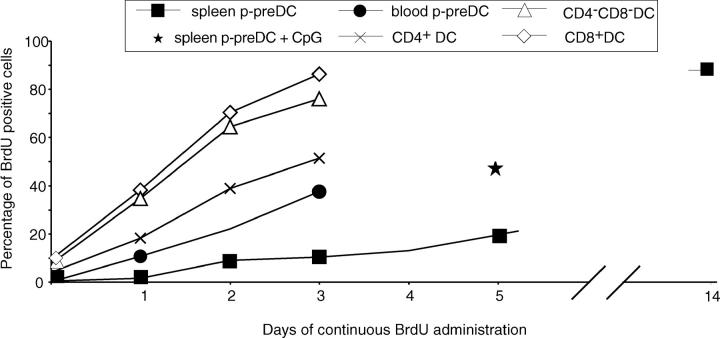
The culture studies indicated that the CD45RAhiCD11cint cells, whether CD4− or CD4+, could transform into CD4−CD8+ DCs, albeit with some differences in surface phenotype from conventional spleen CD4−CD8+ DCs. This raised the question of whether the p-preDCs served as the immediate precursors of the conventional CD4−CD8+ DCs in the lymphoid organs of normal laboratory mice. As the CD45RAhiCD11cint p-preDCs and the CD4−CD8+ conventional DCs were present in similar numbers in spleen, and as neither population was dividing, it was possible to check precursor-product relationship by following the rate of acquisition of labeled cells during continuous administration of BrdU. As previously documented (28) and now confirmed (Fig. 4) , all the mature DC populations of spleen had a fast turnover. The CD4−CD8+ splenic DC population had a very rapid turnover, 90% being positive for BrdU uptake by 3 d. In marked contrast, the turnover of the CD45RAhiCD11cint p-preDC population was slow, only 10% being labeled by 3 d; it took 14 d of continuous labeling before 90% of the cells were BrdU positive. Thus, it was not numerically possible that the CD45RAhiCD11cint cells were transforming directly into the CD4−CD8+ conventional splenic DCs in vivo. Nor could they be precursors of the other splenic DC subsets. To check if both conventional splenic DCs and splenic p-preDCs could be independent products of the CD45RAhiCD11clo p-preDCs of blood, the BrdU labeling kinetics of these latter cells was examined. These rare blood p-preDCs (0.025% of mouse peripheral blood mononuclear cells) did label faster than spleen p-preDCs, with nearly 40% labeled after 3 d. However, although they could conceivably be the direct precursors of the p-preDCs in spleen, they still did not turn over rapidly enough to be the direct precursors of splenic CD8+ DCs.
Figure 4.
Spleen p-preDCs are long-lived cells compared with the short-lived spleen DCs. BrdU was continuously administered to normal, unstimulated, uninfected mice and at times from 2 h (the first time point) to 14 d of labeling, spleen p-preDCs and conventional DCs were isolated, stained for surface markers to delineate the subsets and stained for intra-cellular BrdU to determine their BrdU-labeling kinetics. The labeling of the three splenic conventional DC subsets is compared with that of the total splenic p-preDCs and total blood p-preDCs; a single 5 d point compares the labeling of spleen p-preDCs from mice injected once with CpG (0.3 μmol) at time zero. Results are the mean values from two separate experiments, giving similar results, except for the CpG and 14 d points which represent a single experiment.
Overall, mouse p-preDCs appeared to be long-lived and were developmentally distinct from the conventional DCs normally present in mouse spleen. As the culture studies indicated that a microbial stimulus was required to induce DC production from p-preDCs, we checked if injection of CpG would increase the turnover of p-preDCs. Injection of CpG (0.3 μmol per mouse) did increase the turnover of p-preDCs, with around twice as many cells labeled at day 5 (Fig. 4). In addition there was a threefold increase in total p-preDCs and of conventional DCs in the spleens (data not shown). However, this enhanced turnover was still not sufficient to generate the normal CD4−CD8+ subset.
Influenza Virus as a Potent Stimulus of p-preDCs In Vivo.
It seemed impossible that in steady-state noninfected mice the p-preDCs were generating the CD4−CD8+ DC population. This agreed with the culture data showing that microbial stimuli, rather than endogenous cytokines, were required to activate the p-preDCs. Although CpG was a potent stimulus in culture, injection of CpG produced only a modest activation of p-preDCs, as judged by size increase and increase in CD11c and CD86 expression. However, we found inactivated influenza virus, a more potent stimulus in culture, produced marked activation and when injected intravenously at low levels (4 × 102 HAU) appeared to induce DC formation. At high levels (4 × 103 HAU), rapid activation was followed by a rapid loss of p-preDCs and conventional DCs from spleen, to 25% control levels (data not shown). The fate of these cells lost from the spleen was not explored and the lower dose was used in subsequent experiments.
Adoptive Transfer to Test for Virus-activated or Steady-State PreDCs to DC Transformation.
The complex shifts in the DC population balance made it impossible to follow DC generation from p-preDCs in response to influenza virus stimulation within a single mouse. We therefore used an adoptive transfer approach, injecting purified p-preDCs from a Ly5.2 donor into a Ly5.1 nonirradiated recipient, either unstimulated or virus-injected, and tracking the fate of the transferred cells from 12 h to 4 d after transfer.
When 106 p-preDCs from spleen were transferred intravenously, it was possible to recover in the recipient spleen a distinct population of donor (Ly5.2) type cells in the p-preDCs enriched fraction at all time points that corresponded to at least 3% of the original transferred cells. When the p-preDCs were transferred into nonstimulated normal recipients, >98% of the recovered cells were unchanged even after 4 d, with maintenance of the surface phenotype CD45RAhiCD11cint and no indication of maturation to a DC phenotype, such as up-regulation of CD11c or MHC II (Fig. 5 , and data not shown). This agrees with the BrdU results indicating a very slow turnover of the p-preDC population in normal mice. Similar transfers were made by injecting thymic p-preDCs directly into a recipient thymus; again the phenotype of the donor-derived cells recovered from the recipient thymus was unchanged and they had not become the typical thymic CD8+ DCs (data not shown).
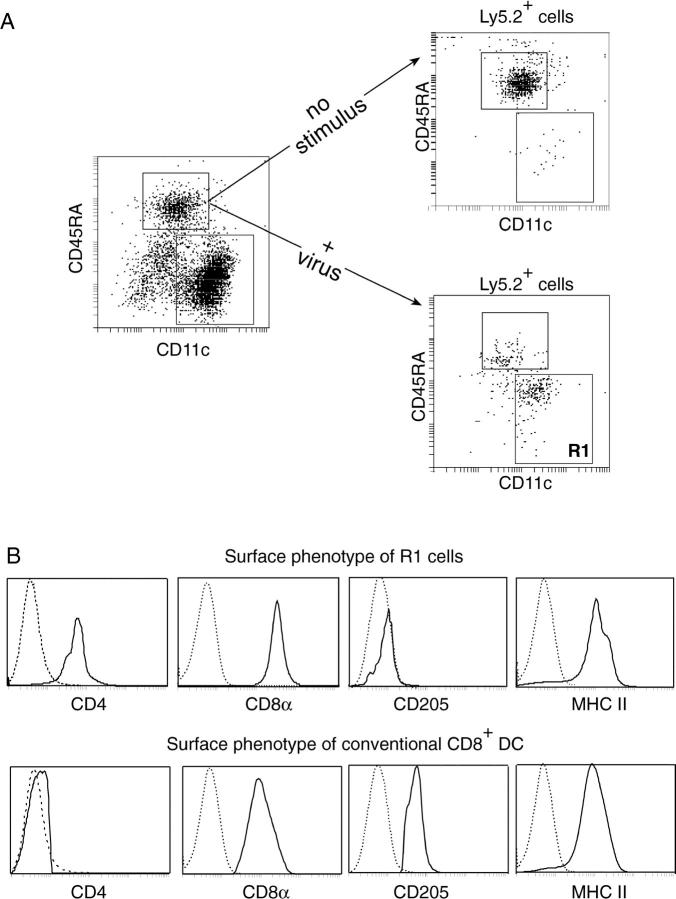
Figure 5.
Mouse p-preDCs do not generate DCs on transfer to an unstimulated recipient, but generate CD8+ DCs on transfer to an influenza virus-injected recipient. Ly5.2+CD11cintCD45RA+ p-preDCs (106) were intravenously transferred to Ly5.1+ recipient mice that received either no stimulus or a single intravenous dose of 400 HAU of BPL-Guangdong virus 2 h after cell transfer. 12 h after cell transfer the p-preDCs and conventional DCs were purified from the recipient mice and autofluorescent cells were removed by presorting. (A) The p-preDCs and conventional DCs were stained with antibodies to Ly5.2, CD11c, and CD45RA. The surface phenotypes of the gated Ly5.2+ cells from mice that received either no stimulus or inactivated virus are compared. (B) After virus treatment many of the Ly5.2+ cells displayed a changed phenotype (Region 1(R1) cells). The surface phenotypes of the donor-derived R1 cells and host conventional CD8+ DCs, both from mice that received inactivated virus, were compared. The cells were stained with antibodies against the following markers: Ly5.2, CD45RA, and CD11c, together with CD8 or CD205 or MHC II or CD4. R1 cells were gated as in panel A. Host conventional CD8+ DCs were gated as CD11c+CD45RA− cells expressing either CD8 or CD205, although for MHC II staining these cells were gated as CD11c+CD45RA− only since the level of MHC II was the same on all of the conventional DCs within this gate. The broken lines give the background fluorescence with only the relevant stain omitted. Similar results were obtained in five experiments for the transfer of p-preDCs into hosts that received no stimulus. Similar results were obtained in two experiments for the transfer of p-preDCs into hosts that received 400 HAU of BPL-Guangdong virus and in a third experiment where hosts received 400 HAU of BPL-PR/8/34 virus.
In contrast, a single intravenous injection of inactivated influenza virus (4 × 102 HAU) 2 h after intravenous transfer of p-preDCs induced a change in surface phenotype of the donor cells recovered in the recipient spleen 10 h later (Fig. 5). Over 50% of the recovered donor-type cells showed a reduction in CD45RA to intermediate levels and an increase in CD11c expression, to a phenotype intermediate between p-preDCs and conventional splenic DCs. In addition, these activated p-preDCs all expressed levels of MHC II as high as on conventional DCs, and all expressed CD8α but only low levels of CD4. These cells did not express CD205, in contrast to the CD8+ DCs normally present in the spleen. The in vivo progeny of the influenza virus stimulated CD45RA+CD11cint fraction therefore closely resembled the DCs formed in culture in response to microbial stimuli.
Overall it appeared that only when there was a microbial stimulus did the p-preDCs develop into DCs, and they then produced a new wave of CD8+ DCs, distinct from the CD8+ DCs normally present in an uninfected, unstimulated laboratory mouse.
Two Developmental Stages of Mouse p-preDCs.
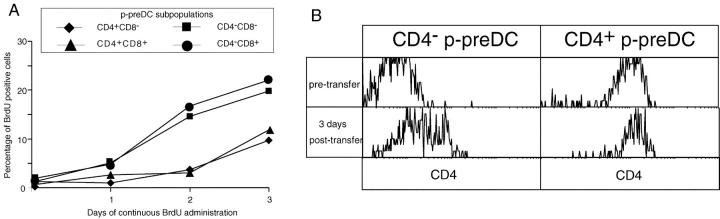
To check if the subsets of mouse spleen p-preDC segregated on the basis of CD4 and CD8 expression represented sequential steps in development, their relative rates of generation from dividing precursors was examined. BrdU was continuously administered to mice and the p-preDC fraction isolated as in Fig. 4, but the individual subsets of p-preDC separated as in Fig. 1 before analysis for BrdU incorporation. The results are shown in Fig. 6 A. While segregation by CD8 expression showed no differences in the rate of BrdU labeling, the CD4− and CD4+ p-preDCs showed a marked difference in kinetics. The CD4− cells (whether CD8+ or CD8−) showed a more rapid labeling while the CD4+ cells (whether CD8+ or CD8−) showed a pronounced labeling lag. This suggested a precursor-product relationship between CD4− and CD4+ splenic p-preDCs with expression of CD8 being irrelevant. As there was a lag in labeling of the CD4− splenic p-preDCs and blood p-preDCs (CD4−CD8−) labeled even faster, this suggests a sequence: blood p-preDCs → spleen CD4− p-preDCs → spleen CD4+ p-preDCs. Note that none of these subtypes of p-preDCs showed a sufficiently fast turnover to generate and maintain the conventional splenic CD8+ DC population.
Figure 6.
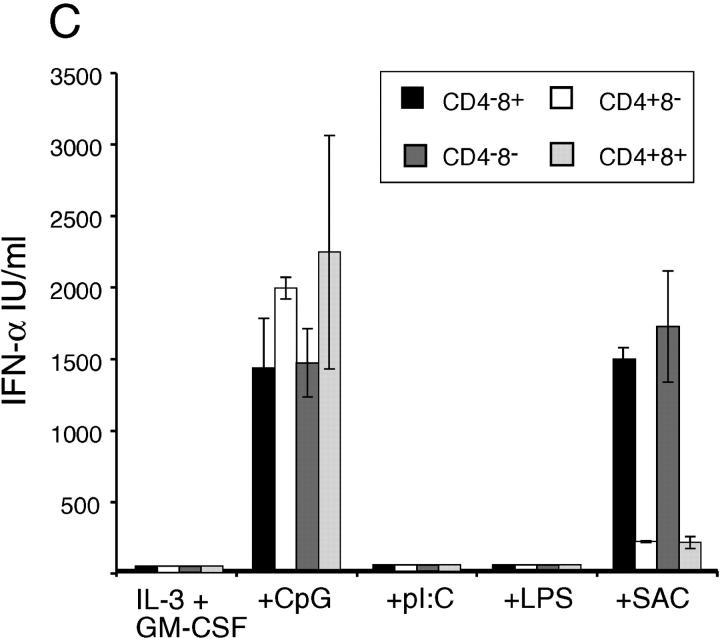
Spleen p-preDCs subsets differ in function and developmental kinetics. (A) BrdU-labeling was performed as in Fig. 4 and p-preDCs were divided into subsets based on CD4 and CD8 expression. Results are the mean values from two separate experiments, giving similar results. (B) Sorted CD4+ or CD4− p-preDCs were transferred to Ly5.1+ mice (without stimulus) as in Fig. 5. 3 d later conventional and p-preDC subsets were harvested and cells were stained with antibodies against the following markers: Ly5.2, CD4, CD45RA, and CD11c. Cells were gated based on high Ly5.2 expression and the CD4 expression level on these cells is shown. All Ly5.2+ cells from both sets of mice had maintained the original phenotype CD11cintCD45RAhi. (C) Spleen p-preDCs sorted on the basis of CD4 and CD8 expression into four subsets, then cultured for 36 h in the presence of IL-3 and GM-CSF alone or together with 500 nM CpG, 100 μg/ml poly I:C, 10 μg/ml LPS, or 10 μg/ml SAC. The cell supernatants were tested by ELISA for the production of IFN-α. The values shown are the means of duplicate samples and the error bars represent the range of these samples; similar results were obtained in three separate experiments.
To test the hypothesis that the splenic CD4− p-preDCs could be precursors of the splenic CD4+ p-preDCs, we transferred purified, sorted samples of each population from Ly5.2+ mice into normal, nonirradiated, and nonstimulated Ly5.1 recipient mice. As indicated in Fig. 6 B, CD4− p-preDCs did increase surface expression of CD4 so that 3 d after transfer 20% expressed medium to high levels, whereas the CD4+ p-preDCs remained CD4+ and also increased CD4 expression levels. This timing agrees with the BrdU labeling kinetic differences between the populations. Thus, CD4− p-preDCs do become CD4+ p-preDCs in normal, unstimulated mice, but neither become a mature DC without microbial stimulation.
Functional Differences Between Developmental Stages of p-preDCs.
We examined the four subsets of spleen p-preDCs to determine if any of the subtypes or developmental stages differed in response or function. Each of the microbial stimuli of Fig. 3 had an identical activation effect, or lack of effect, on the four subsets segregated on the basis of CD4 and CD8 expression. Indeed, CpG induced similar levels of IFN-α production (Fig. 6 C) by all four p-preDC subsets and in all such cultures the cells became CD11c+MHCIIhiCD8+ DCs (data not shown). CpG induced down-regulation of CD4 in both CD4+ subsets and did not induce surface expression of CD4 by the CD4− subsets (data not shown). This response to CpG did not reveal any functional heterogeneity. In contrast, the response to SAC revealed a functional heterogeneity according to CD4 expression (Fig. 6 C). Both CD4− p-preDC subsets produced 10-fold more IFN-α, with levels of cytokine similar to those induced by CpG. In these SAC-activated cultures the CD4− p-preDCs but not the CD4+ p-preDCs produced cells of DC morphology, although they did not form such large DC clusters nor express as high levels of MHC II as those obtained by CpG stimulation. Indeed the levels of MHC II were similar on CD4− and CD4+ p-preDCs, despite a lack of IFN-α production by this latter subset. The production of IFN-α in response to SAC was independent of the expression of CD8. Thus, the biological response to one stimulus correlated with the developmental sequence above, the earlier CD4− p-preDCs showing greater response to SAC than their later CD4+ p-preDC progeny.
Discussion
In common with three other groups (15–17), and following our own studies on mouse blood (17a) we have found the murine equivalent of the human plasmacytoid cell, which we have termed p-preDCs. The key features of this cell are that it is a major producer of type I IFN and simultaneously a precursor of DCs, both functions being activated by microbial stimuli. Although there are some differences from other reports, such as the extent of staining with anti-GR1, most of the features align well with those of other studies. We also present some novel features such as the high levels of the early lymphoid and stem cell markers Sca-1 and Sca-2, members of the Ly6 family. Also of particular interest is the expression of the putative human DC-SIGN homologue CIRE, at levels higher than on conventional DCs, on all p-preDCs except those lacking both CD4 and CD8 expression.
Our study is the first to indicate that, despite many common features, the p-preDC population is heterogeneous, not just in surface phenotype but in aspects of the biology of these cells. Whether CD4− or CD4+, all of the p-preDCs acquired CD8 expression after stimulus with CpG or virus. As high levels of IL-6 and IFN-α were also produced under these conditions, we tested whether these cytokines per se influenced CD8 expression. High concentrations of antibodies to IL-6 added to p-preDC cultures before addition of CpG did not affect the acquisition of CD8 expression. Likewise, mice which lacked expression of the type I IFN receptor, and thus unable to respond to type I IFN, harbored p-preDCs expressing all combinations of CD4 and CD8 and indeed CpG or viral stimulus of these cells resulted in the acquisition of CD8 expression on the CD8− populations (data not shown). Only the CD4− splenic p-preDC subgroup shows a vigorous IFN-α response to SAC although the response of CD4− and CD4+ p-preDCs to CpG is equivalent. The CD4− subgroup also shows a faster DNA-precursor labeling and faster turnover than the CD4+ subgroup. It appears that these differences represent developmental stages of one lineage, rather than totally separate cell types. The transfer studies indicate that CD4− p-preDCs with time and without microbial stimulation acquire CD4, whereas the CD4+ p-preDCs remain CD4+ unless subjected to microbial stimuli. The DNA-precursor labeling kinetics suggests a developmental sequence from blood CD4− p-preDCs to spleen CD4− p-preDCs to spleen CD4+ p-preDCs. Tracking such development further back to earlier dividing precursors and to hematopoietic precursor cells is underway.
The difference in response to SAC during p-preDC development is most likely due to differences in the expression of Toll-like or other pattern-recognition receptors. Despite this difference, there must be much that remains common in the distribution of pattern-recognition receptors on the p-preDCs during their development, as they all respond to influenza virus and CpG, but in contrast to conventional DCs do not respond to LPS or poly I:C. The response to virus is presumably due to recognition of an envelope protein rather than viral RNA, as inactivated virus is effective and poly I:C is not.
Our main interest in these plasmacytoid cells was as precursors of DCs. As others had found, once effectively activated in vitro the p-preDCs not only secreted type I IFN, but also rapidly transform into a cell with DC form and function, with no evidence of cell expansion or entry of the p-preDCs into the cell division cycle. The DCs so produced are all CD8+ DCs, but they differ from the CD4−CD8+ DCs found in normal mouse spleen in lacking expression of CD205, and in retaining some surface CD45RA and CD4.
Our in vitro data raised the question as to whether these plasmacytoid cells were the precursors of any of the DCs, and particularly of the CD8+ DCs, present in the lymphoid organs of normal, noninfected laboratory mice. If they did not act as precursors of normal DCs, was the production of DCs in culture merely a laboratory artifact? Similar questions have been asked of the human plasmacytoid cells, questions that could not readily be answered without the BrdU labeling and adoptive transfer approaches we applied to the murine p-preDCs.
Both the BrdU labeling studies on the intact mice and the transfer studies using nonirradiated uninfected normal recipient mice indicated that both splenic and thymic murine p-preDC are relatively long-lived cells which in a normal noninfected laboratory mouse maintain their plasmacytoid state and do not transform into DCs. They could not be the precursors of the rapidly turning-over conventional CD8+ DCs found in normal mice. In kinetic studies we would not be able to detect a small proportion of p-preDCs producing the normal CD8+ DCs via extensive cell division, but we found no evidence of such division in either the p-preDC or the conventional CD8+ DC populations, and the transfer studies negated this model. The immediate precursors of the conventional mouse CD8+ DCs are not p-preDCs. However, a recent publication by Martinez del Hoyo et al. (33) describes a CD11c+MHC-II− cell population of mouse blood that likely contains the immediate precursors of both CD8+ and CD8− DCs. Whether this newly identified population is homogeneous, meaning that identical precursor cells give rise to CD8+ and CD8− DCs, or is heterogeneous, containing separate precursors for these two DC populations, is not yet clear.
In response to stimulation by influenza virus the p-preDCs did transform into CD4loCD8+ DCs within recipient mice, indicating that they can generate DCs in vivo if provided with an appropriate microbial stimulus. This is in complete agreement with the culture studies. These microbial stimulus induced DCs represent a new wave of CD8+ DCs differing in origin from those present in the steady-state, and indeed their surface phenotype differs from steady-state CD8+ DCs in several aspects, including CD205 expression. Although it served to demonstrate the principal of microbial induced p-preDCs to DC transformation in vivo, our system involving the injection of low levels of inactivated influenza virus intravenously is not an appropriate model of infection by virus, as influenza infection would normally be initiated in the lung. It is clear that the localization, activation, and life span of the p-preDCs and their DC progeny will have to be considered in detail for different infectious disease situations.
These findings add a new concept to the existing evidence that ‘danger’ signals and microbial infections shift the DC population from a quiescent, perhaps tolerogenic, state to an activated state initiating immunity (34–36). They indicate that, at least for the CD8+ DCs, microbial infection initiates a new wave of production of a new type of DCs from these p-preDCs. This could indicate a shift in balance from a population of tolerogenic DCs to a new set of immunogenic DCs, or a shift in the nature of the immune response that can be generated. If the DCs generated from the p-preDCs on stimulation in vivo survive as long as those that develop in culture, they could have the function of inducing and maintaining T cell memory. A careful study of the functional differences between the steady-state and the induced CD8+ DCs forms should now provide some answers to these crucial questions. The present study sets the scene and demonstrates clearly that the rapidly turning-over CD8+ DCs of normal, uninfected mice are not, as might have been thought, the progeny of the IFN-producing plasmacytoid cells.
Acknowledgments
We thank V. Lapatis, D. Kaminaris, C. Tarlinton, A. Holloway, C. Clark, and F. Battye for expert FACS® assistance.
Footnotes
*
Abbreviations used in this paper: DC, dendritic cell; p-preDC, plasmacytoid preDC; PI, propidium iodide; SAC, Staphylococcus aureus.
References
- 1.Eckert, F., and U. Schmid. 1989. Identification of plasmacytoid T cells in lymphoid hyperplasia of the skin. Arch. Dermatol. 125:1518–1524. [PubMed] [Google Scholar]
- 2.Grouard, G., M.C. Rissoan, L. Filgueira, I. Durand, J. Banchereau, and Y.J. Liu. 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegal, F.P., N. Kadowaki, M. Shodell, P.A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y.J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science. 284:1835–1837. [DOI] [PubMed] [Google Scholar]
- 4.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919–923. [DOI] [PubMed] [Google Scholar]
- 5.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1:305–310. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald-Bocarsly, P. 1993. Human natural interferon-alpha producing cells. Pharmacol. Ther. 60:39–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadowaki, N., S. Ho, S. Antonenko, R.W. Malefyt, R.A. Kastelein, F. Bazan, and Y.J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer, M., V. Redecke, J.W. Ellwart, B. Scherer, J.P. Kremer, H. Wagner, and G.B. Lipford. 2001. Bacterial CpG-DNA triggers activation and maturation of human CD11c−, CD123+ dendritic cells. J. Immunol. 166:5000–5007. [DOI] [PubMed] [Google Scholar]
- 9.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388–3393. [DOI] [PubMed] [Google Scholar]
- 10.Pashenkov, M., N. Teleshova, M. Kouwenhoven, T. Smirnova, Y.P. Jin, V. Kostulas, Y.M. Huang, B. Pinegin, A. Boiko, and H. Link. 2002. Recruitment of dendritic cells to the cerebrospinal fluid in bacterial neuroinfections. J. Neuroimmunol. 122:106–116. [DOI] [PubMed] [Google Scholar]
- 11.Ronnblom, L., and G.V. Alm. 2001. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J. Exp. Med. 194:F59–F63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman, S., D. Stein, S. Amrute, T. Denny, Z. Garcia, P. Kloser, Y. Sun, N. Megjugorac, and P. Fitzgerald-Bocarsly. 2001. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 101:201–210. [DOI] [PubMed] [Google Scholar]
- 13.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 98:2574–2576. [DOI] [PubMed] [Google Scholar]
- 14.Patterson, S., A. Rae, N. Hockey, J. Gilmour, and F. Gotch. 2001. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 75:6710–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakano, H., M. Yanagita, and M.D. Gunn. 2001. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorck, P. 2001. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor- treated mice. Blood. 98:3520–3526. [DOI] [PubMed] [Google Scholar]
- 17.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144–1150. [DOI] [PubMed] [Google Scholar]
- 17a.O'Keeffe, M., H. Hochrein, D. Vremec, B. Scott, P. Hertzog, L. Tatarczuch, and K. Shortman. 2002. Dendritic cell precursor populations of mouse blood: identification of the murine homologues of human blood plasmacytoid pre-DC2 and CD11c+ DC1 precursors. Blood. In press. [DOI] [PubMed] [Google Scholar]
- 18.Vremec, D., J. Pooley, H. Hochrein, L. Wu, and K. Shortman. 2000. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 164:2978–2986. [DOI] [PubMed] [Google Scholar]
- 19.Henri, S., D. Vremec, A. Kamath, J. Waithman, S. Williams, C. Benoist, K. Burnham, S. Saeland, E. Handman, and K. Shortman. 2001. The dendritic populations of mouse lymph nodes. J. Immunol. 167:741–748. [DOI] [PubMed] [Google Scholar]
- 20.Sparwasser, T., T. Miethke, G. Lipford, K. Borschert, H. Hacker, K. Heeg, and H. Wagner. 1997. Bacterial DNA causes septic shock. Nature. 386:336–337. [DOI] [PubMed] [Google Scholar]
- 21.Krieg, A.M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709–760. [DOI] [PubMed] [Google Scholar]
- 22.Kronin, V., H. Hochrein, K. Shortman, and A. Kelso. 2000. The regulation of T cell cytokine production by dendritic cells. Immunol. Cell Biol. 78:214–223. [DOI] [PubMed] [Google Scholar]
- 23.Grumont, R.J., I.J. Rourke, L.A. O'Reilly, A. Strasser, K. Miyake, W. Sha, and S. Gerondakis. 1998. B lymphocytes differentially use the Rel and nuclear factor kappaB1 (NF- kappaB1) transcription factors to regulate cell cycle progression and apoptosis in quiescent and mitogen-activated cells. J. Exp. Med. 187:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Keeffe, M., H. Hochrein, D. Vremec, J. Pooley, R. Evans, S. Woulfe, and K. Shortman. 2002. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood. 99:2122–2130. [DOI] [PubMed] [Google Scholar]
- 25.Hochrein, H., M. O'Keeffe, T. Luft, S. Vandenabeele, R.J. Grumont, E. Maraskovsky, and K. Shortman. 2000. Interleukin-4 is a major regulatory cytokine governing bioactive Interleukin-12 production by mouse and human dendritic cells. J. Exp. Med. 192:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochrein, H., K. Shortman, D. Vremec, B. Scott, P. Hertzog, and M. O'Keeffe. 2001. Differential production of IL-12, IFN-α, and IFN-γ by mouse dendritic cell subsets. J. Immunol. 166:5448–5455. [DOI] [PubMed] [Google Scholar]
- 27.Lee, F., C.P. Chiu, J. Wideman, P. Hodgkin, S. Hudak, L. Troutt, T. Ng, C. Moulds, R. Coffman, A. Zlotnik, et al. 1989. Interleukin-6. A multifunctional regulator of growth and differentiation. Ann. NY Acad. Sci. 557:215–228. [PubMed] [Google Scholar]
- 28.Kamath, A., J. Pooley, M. O'Keeffe, D. Vremec, Y. Zhan, A. Lew, A. D'Amico, L. Wu, D. Tough, and K. Shortman. 2000. The development, maturation and turnover rate of mouse spleen dendritic cell populations. J. Immunol. 165:6762–6770. [DOI] [PubMed] [Google Scholar]
- 29.Caminschi, I., K.M. Lucas, M.A. O'Keeffe, H. Hochrein, Y. Laabi, T.C. Brodnicki, A.M. Lew, K. Shortman, and M.D. Wright. 2001. Molecular cloning of a C-type lectin superfamily protein differentially expressed by CD8α− splenic dendritic cells. Mol. Immunol. 38:365–373. [DOI] [PubMed] [Google Scholar]
- 30.Park, C.G., K. Takahara, E. Umemoto, Y. Yashima, K. Matsubara, Y. Matsuda, B.E. Clausen, K. Inaba, and R.M. Steinman. 2001. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int. Immunol. 13:1283–1290. [DOI] [PubMed] [Google Scholar]
- 31.Geijtenbeek, T.B., R. Torensma, S.J. van Vliet, G.C. van Duijnhoven, G.J. Adema, Y. van Kooyk, and C.G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 100:575–585. [DOI] [PubMed] [Google Scholar]
- 32.Brasel, K., T. De Smedt, J.L. Smith, and C.R. Maliszewski. 2000. Generation of murine dendritic cells from flt-3-ligand-supplemented bone marrow cultures. Blood. 96:3029–3039. [PubMed] [Google Scholar]
- 33.Martinez del Hoyo, G.M., P. Martin, H.H. Vargas, S. Ruiz, C.F. Arias, and C. Ardavin. 2002. Characterization of a common precursor population for dendritic cells. Nature. 415:1043–1047. [DOI] [PubMed] [Google Scholar]
- 34.Gallucci, S., M. Lolkema, and P. Matzinger. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5:1249–1255. [DOI] [PubMed] [Google Scholar]
- 35.Shortman, K., and W.R. Heath. 2001. Immunity or tolerance? That is the question for dendritic cells. Nat. Immunol. 2:988–989. [DOI] [PubMed] [Google Scholar]
- 36.Steinman, R.M., and M.C. Nussenzweig. 2002. Inaugural Article: Avoiding horror autotoxicus: The importance of dendritic cells in peripheral T cell tolerance. Proc. Natl. Acad. Sci. USA. 99:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]