Potent Immune Response against HIV-1 and Protection from Virus Challenge in hu-PBL-SCID Mice Immunized with Inactivated Virus-pulsed Dendritic Cells Generated in the Presence of IFN-α (original) (raw)
Abstract
A major challenge of AIDS research is the development of therapeutic vaccine strategies capable of inducing the humoral and cellular arms of the immune responses against HIV-1. In this work, we evaluated the capability of DCs pulsed with aldrithiol-2–inactivated HIV-1 in inducing a protective antiviral human immune response in SCID mice reconstituted with human PBL (hu-PBL-SCID mice). Immunization of hu-PBL-SCID mice with DCs generated after exposure of monocytes to GM-CSF/IFN-α (IFN-DCs) and pulsed with inactivated HIV-1 resulted in a marked induction of human anti–HIV-1 antibodies, which was associated with the detection of anti-HIV neutralizing activity in the serum. This vaccination schedule also promoted the generation of a human CD8+ T cell response against HIV-1, as measured by IFN-γ Elispot analysis. Notably, when the hu-PBL-SCID mice immunized with antigen-pulsed IFN-DCs were infected with HIV-1, inhibition of virus infection was observed as compared with control animals. These results suggest that IFN-DCs pulsed with inactivated HIV-1 can represent a valuable approach of immune intervention in HIV-1–infected patients.
Keywords: antigen presenting cell, vaccine, neutralizing antibodies, CD8+ T lymphocytes, AIDS
Introduction
The immunopathogenesis of HIV-1 infection involves multiple interactions between the virus and cells of the immune system, which progressively lead to immune dysfunctions and subsequently to AIDS (1). Even though the recent advances in the development of antiretroviral treatment have dramatically reduced mortality and morbidity of HIV-1–infected patients, the achievement of a long-term immune control of viral replication still remains a major challenge of AIDS research. In fact, viral rebound generally occurs upon discontinuation of highly active antiviral therapy (HAART; references 2–5), which is unlikely to eradicate HIV replication within a reasonable period of time. In fact, it has been estimated that the clearance of the T cell viral reservoir may take up to 60 yr of infection containment with continuous HAART (2). Therefore, an increasing interest is now focused on the efforts to develop therapeutic vaccination strategies to be combined with HAART to achieve a durable immune control of HIV replication.
The development of an effective therapeutic anti-HIV vaccine requires not only the characterization of the relevant virus antigens potentially important for achieving immune protection but also the identification of potent adjuvants, which are necessary for inducing suitable levels of neutralizing antibodies as well as for ensuring the generation of a vigorous antiviral CD8+ T cell response. In recent years, a special attention has been given to the use of DCs as potentially ideal cellular adjuvants for the development of therapeutic vaccines (6, 7).
DCs are professional antigen-presenting cells capable of stimulating naive T cells for the initiation of a primary immune response and of processing extracellular antigens for presentation by MHC class I molecules (8). Although the use of DCs as cellular adjuvants for the preparation of therapeutic vaccines against some human malignancies has become a frequent experimental approach on the basis of promising results generated in animal tumor models (7), DC-based vaccination strategies in patients with chronic infectious diseases, such as hepatitis B and C or HIV-1 infection, are still at a very early stage of development. In a previous paper, we showed that aldrithiol-2 (AT-2)–inactivated HIV-pulsed DCs generated after a 3-d treatment of monocytes with GM-CSF and IFN-α were highly effective in inducing a primary immune response against HIV-1 in vitro as well as in SCID mice reconstituted with human PBL (9). However, no information was available on the capability of this DC-based immunization to induce important immune correlates of protection against HIV-1, such as neutralizing antibodies and virus-specific CD8+ T cells. Likewise, it was important to establish whether DC-based vaccination of hu-PBL-SCID mice could result in the in vivo control of HIV-1 replication after virus challenge.
In the present report, we demonstrate that immunization of hu-PBL-SCID mice with HIV-1–pulsed DCs generated after exposure of monocytes to GM-CSF/IFN-α results in a remarkable induction of both human anti–HIV-1 antibodies and CD8+ T cells reactive against HIV-1. Moreover, we report that this DC-based vaccination protocol induces inhibition of virus replication after HIV-1 challenge of hu-PBL-SCID mice.
Materials and Methods
Cell Separation and Culture.
Peripheral blood mononuclear cells were obtained from heparinized blood of healthy donors by Ficoll density gradient centrifugation (Seromed). Monocytes were isolated by column magnetic immunoselection (MACS Cell Isolation Kits; Miltenyi Biotec). Positively selected CD14+ monocytes (>98% as assessed by flow cytometry) were plated at the concentration of 2 × 106 cells/ml in RPMI 1640 (GIBCO BRL), supplemented with 10% LPS-free FCS, 500 U/ml GM-CSF, and either 250 U/ml IL-4 (R&D Systems) or natural IFN-α (Alfaferone; AlfaWasserman) at the concentration of 10,000 U/ml, and cultured for 3 d as described previously (9). Negatively selected PBLs were used to reconstitute SCID mice.
hu-PBL-SCID Mouse Model.
CB17 scid/scid female mice (Charles River Laboratories) were used at 4 wk of age and kept under specific pathogen-free conditions. Hu-PBLs were obtained from the peripheral blood of healthy donors. All donors were screened for HIV-1 and hepatitis before donation. The hu-PBLs were obtained as described previously (9). Three or four mice for each group were injected i.p. with 30–40 × 106 cells and resuspended in 0.5 ml RPMI 1640 medium.
Immunization and Challenge of hu-PBL-SCID Mice.
HIV-1 (SF-162 strain) was propagated in PBMCs from HIV-1–negative donors and virus titers were determined on PHA-stimulated PBMC blasts. To prepare the inactivated HIV-1 for immunization experiments, different SF-162 HIV-1 stocks were inactivated by treatment for 1 h at 37°C with 2,2′-dithiodipyridine (aldrithiol-2 [AT-2]) as described previously (9). The virus was ultracentrifugated to remove treatment agents and contaminants, resuspended in RPMI 1640 medium, and frozen at −80°C until use. 3 d after reconstitution with autologous human PBLs, hu-PBL-SCID mice were injected i.p. with 2.5 × 106 autologous DCs, pulsed for 2 h at 37°C with AT-2–inactivated HIV-1 (100 ng p24). Mice were further immunized with the inactivated virus-pulsed DCs on day 7 and subjected to a final vaccination boost consisting of AT-2–inactivated HIV alone (100 ng p24) on day 14. In some experiments, we also immunized the hu-PBL-SCID mice by three weekly injections of free AT-2–inactivated virions (100 ng p24). 1 wk later, mice were challenged i.p. with 103 TCID50 of HIV-1 SF162 strain.
ELISA for Human Immunoglobulins.
Sera from control and vaccinated hu-PBL-SCID mice were tested for antibodies to HIV-1 using a commercial ELISA kit detecting IgG, IgM, and IgA specific to a series of envelope and core HIV-1 peptides (Abbott Murex HIV-1.2.O). An ELISA system was used to quantify human immunoglobulins to the ELDKWAS HIV-1 gp41 epitope in the sera of the chimeras by using anti–human total Igs (Cappel-Cooper Biomedical). All ELISAs were performed in duplicate and laboratory standards were included on each plate. Sera from nonimmunized hu-PBL-SCID mice were used as negative controls of all the ELISA determinations. Values represent mean adsorbance of each individual serum tested in duplicate. The cutoff value was calculated as mean adsorbance value of all the control sera plus 0.100 O.D. Sera showing A490 values higher than this threshold were considered positive for anti-HIV antibodies.
Western Blot.
Sera from hu-PBL-SCID mice immunized with AT-2/HIV-1–pulsed DCs were assayed by a commercial Western blot kit (HIV kit; Cambridge Biotech). In brief, individual nitrocellulose strips were incubated overnight with different mouse serum samples (diluted 1:20) or with human positive or negative control sera (diluted 1:1,000; Cambridge Biotech). Sera from nonimmunized hu-PBL-SCID mice were included as experimental negative controls. Visualization of the human Igs specifically bound to HIV-1 proteins was obtained by incubation with substrate chromogen after the addition of biotin-conjugated goat anti–human IgG and streptavidin-conjugated horseradish peroxidase.
Neutralization Assay.
Sera were serially diluted in RPMI 1640 medium containing 15% FCS in 96-well plates (Costar) in duplicate, and 100 TCID50 of HIV-1 SF162 strain in a volume of 50 μl was added to each dilution. After a 2-h incubation at 37°C, 2 × 105 PHA-activated PBMCs were added to each well in the presence of 50 U/ml IL-2. After 3 d, cells were washed three times and 50 U/ml IL-2 (Roche Diagnostic) were added. Culture supernatants were tested for p24 production after 7 d by a HIV-1 p24 ELISA commercial kit (NEN Life Science Products). Sera from nonimmunized hu-PBL-SCID mice were used as negative controls for neutralizing activity.
Cell Recovery from Peritoneal Cavity and Organs of the SCID Mice.
hu-PBL-SCID mice were killed 7–10 d after viral challenge. Cells were collected from the peritoneal cavity and spleen. Spleens were disrupted, connective tissue and debris were allowed to settle, and the single cell suspensions were washed twice in RPMI 1640 medium. Human cells from mouse spleens were enriched by Ficoll density gradient centrifugation and used for subsequent analyses.
Elispot Assay.
Human cells recovered from either the spleen or peritoneal lavages of hu-PBL-SCID mice of each group were pooled (three or four mice per group). The presence of HIV-1–reactive CD8+ T lymphocytes was tested using as stimulators/targets autologous EBV-transformed B-lymphoblastoid cell lines (BLCLs) or DCs, infected with either HIV-1 SF162 strain or recombinant vaccinia virus vectors encoding gag and pol antigens from the IIIB strain of HIV-1. Stimulator/target cells were infected with the HIV-I SF162 strain (MOI = 0.1) or recombinant HIV-1 vaccinia virus vectors (MOI = 3) for 48 and 12 h, respectively, washed, irradiated, and used as antigen-presenting cells. Control uninfected BLCLs, vaccinia vector infected BLCLs, or uninfected DCs were used as stimulators/targets for the calculation of background spots to be subtracted for the evaluation of the specific number of IFN-γ–spot-forming cells. PBMCs cultures treated with 1 μg/ml PHA served as positive controls. The cells were added at 106 per well and incubated at 37°C overnight in a final volume of 2 ml of medium (RPMI 1640 supplemented with 2 mM l-glutamine and 10% heat-inactivated FCS).
After incubation with autologous BLCLs at a responder/stimulator ratio of 4:1, CD8+ T cells were positively selected by MACS Micro Beads (Miltenyi Biotec) and tested in an ELISPOT assay for the production of IFN-γ (Euroclone Ltd.). In brief, 105 CD8+ T cells (100 μl /well) were dispensed in a 96-well anti–IFN-γ antibody-coated plate; after overnight incubation and cell lysis, trapped cytokine molecules were revealed by a secondary biotinylated detection antibody and developed by incubating with streptavidin–alkaline phosphatase, followed by incubation with BCIP substrate in a gel overlay. Colored spots were enumerated on an inverted microscope at a magnification of 40. Analysis of the CD8+ T cell response to the HLA-A*0201–restricted CTL epitope SL9 (10, 11) was performed by pulsing BLCL cells with 5 × 10−6 M of the synthetic peptide SL9 (SLYNTVATL).
Detection of HIV-1 Infection.
PCR detection of HIV-1 proviral sequences. DNA was extracted from spleens and lymph nodes of the hu-PBL-SCID mice. The presence of human sequences was determined by DNA-PCR using specific primers for the HLA-DQα gene, whereas HIV-1 proviral DNA was detected by specific amplification of HIV-1 gag sequences as described previously (12). In each experiment, the sensitivity of the assay was tested by amplifying serial dilutions of DNA prepared from 8E5 cells (which harbor one proviral copy per cell). 8E5 DNA was serially diluted into SCID mouse cell DNA.
Co-cultivation Assay.
105 cells from the peritoneal lavages were cocultured with 105 human allogeneic T cells, preventively activated with 5 μg/ml PHA, and cultivated in the presence of 50 U/ml IL-2. Virus replication was determined after 10 d of culture by detection of p24 gag antigen in culture supernatant using a commercial ELISA kit (Dupont).
Results and Discussion
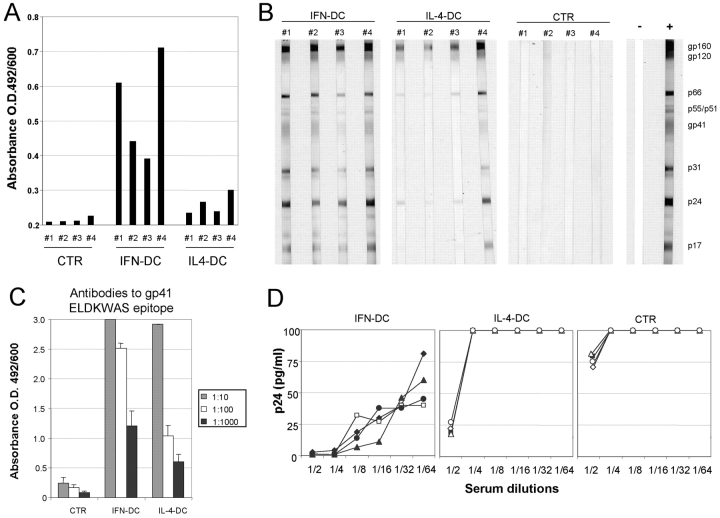
All the mice immunized with AT-2/HIV-1–pulsed IFN-DCs exhibited a clear-cut antibody response to HIV-1, as revealed by a commercial ELISA kit detecting IgG, IgM, and IgA specific to a series of envelope and core peptides (Fig. 1A) as well as by Western blot analysis (Fig. 1 B), which revealed the presence of antibodies to virtually all HIV proteins. A detectable, though even less intensive, antibody response was also observed in sera from mice vaccinated with AT-2/HIV-1–pulsed IL-4–DCs (Fig. 1, A and B). Notably, sera from immunized animals did not show any reactivity against irrelevant antigens, such as recombinant HCV proteins, whereas very low and comparable levels of human antibodies against tetanus toxoid were detected in both control and immunized xenochimeras (unpublished data), ruling out a nonspecific adjuvant or mitogenic effect by the HIV vaccine preparation. In contrast, hu-PBL-SCID mice immunized with virus-pulsed IFN-DCs exhibited high levels of antibodies reacting against the gp41 ectodomain epitope ELDKWAS (Fig. 1 C), also recognized by the 2F5 mAb, which is known to exert cross-neutralization of some divergent HIV-1 isolates (13). Of interest, all the sera from mice immunized with virus-pulsed IFN-DCs showed in vitro anti–HIV-1 neutralizing activity at dilutions up to 1:64 (Fig. 1 D). A considerably lower neutralizing activity was detected in the sera from mice immunized with virus-pulsed IL-4–DCs, whereas sera from control hu-PBL-SCID mice did not show any activity, even at the 1:2 dilution (Fig. 1 D).
Figure 1.
Characterization of the antibody response against HIV-1 in hu-PBL-SCID mice immunized with DCs pulsed with AT-2–inactivated HIV-1. (A) Immune reactivity toward HIV envelope and core proteins in individual xenochimeric mice. Undiluted sera from control and vaccinated hu-PBL-SCID mice were tested for antibodies to HIV-1 using a commercial ELISA kit detecting IgG, IgM, and IgA specific to a series of envelope and core peptides (ABBOTT Murex HIV-1.2.O). Each bar represents the anti-HIV antibody reactivity in individual mouse sera collected 7 d after completion of the immunization schedule. Cutoff value has been calculated as recommended by the manufacturer and has been subtracted. (B) Western blot characterization of the human humoral response to viral antigens in individual sera from immunized xenochimeric mice. The assay was performed on mouse sera collected 7 d after completion of the immunization schedule as described in Materials and Methods. Sera from nonimmunized hu-PBL-SCID mice were included as experimental negative control (CTR) together with both human positive (+) and negative (−) control sera. (C) ELISA detection of antibodies to the HIV-1 gp41 ectodomain epitope ELDKWAS in the sera from immunized hu-PBL-SCID. 10-fold dilutions of the sera from four mice in each experimental group were tested. Each bar represents the mean ± SE of values of four serum samples from individual mice. (D) HIV-neutralizing activity of sera from immunized hu-PBL-SCID mice. Twofold serial dilutions of mouse sera were tested for inhibition of HIV infection of PHA-activated T cells in vitro as described in Materials and Methods. Sera from nonimmunized hu-PBL-SCID were used as controls. The figure shows the results from one representative experiment (four mice per group) out of five.
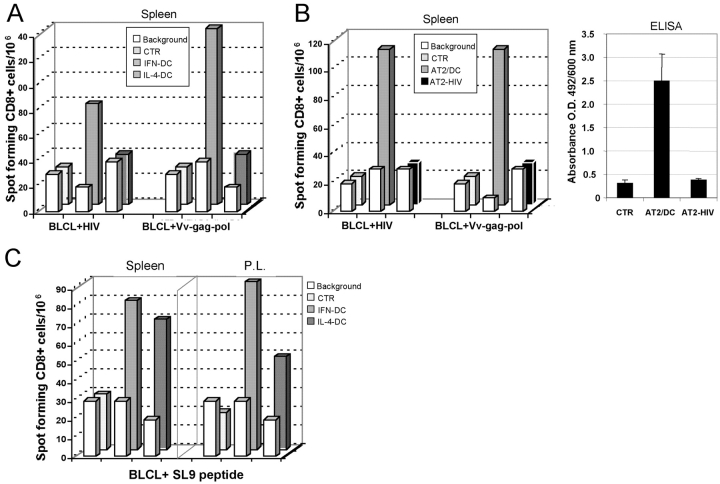
We evaluated whether the immunization of hu-PBL-SCID mice with AT-2/HIV-pulsed DCs could result in the generation of human anti-HIV CD8+ T cells, which are considered as an important immune correlate of protection (14–16). As shown in Fig. 2A, vaccination of hu-PBL-SCID mice with virus-pulsed IFN-DCs resulted in a clear-cut generation of HIV-1–specific CD8+ T cells. A significant CD8+ T cell response against the gag and pol proteins of HIV-1 was also observed in animals immunized with AT-2/HIV-1–pulsed IL-4–DCs, even though the extent of the response was lower than that detected in mice immunized with virus-pulsed IFN-DCs (Fig. 2 A). Of note, no CD8 T response to HIV-1 was observed when hu-PBL-SCID mice were immunized with free AT-2–inactivated virus, which was also unable to induce detectable levels of specific antibodies against HIV-1 (Fig. 2 B). In a subsequent experiment, we also evaluated the HLA-A*0201–restricted response to the CTL epitope SL9 (SLYNTVATL) of the p17 HIV-1 gag protein using human cells recovered from either the spleen or the peritoneal cavity of hu-PBL-SCID mice vaccinated with virus-pulsed DCs. As illustrated in Fig. 2 C, vaccination of SCID mice reconstituted with PBLs from an HLA-A*0201 individual with virus-pulsed IFN-DCs elicited a strong CD8+ T cell response toward the conserved SL9 epitope. A similar, even though less pronounced, CD8+ T cell response directed against the CTL epitope SL9 was also observed in hu-PBL-SCID mice immunized with virus-pulsed IL-4–DCs. Notably, in HIV-1–infected patients, this immunodominant HIV-gag p17-derived epitope elicits strong CTL response, which is maintained, even in the presence of strong selective pressure to viral escape (10, 11).
Figure 2.
Detection of HIV-reactive human CD8+ T cells in immunized hu-PBL-SCID mice. (A) Elispot analysis of anti–HIV-1 CD8+ T cell response. Human cells were recovered from three spleens of hu-PBL-SCID mice from each group and pooled. The assay was performed using as stimulators autologous BLCL targets, infected with either HIV-1 SF162 strain (BLCL + HIV) or recombinant vaccinia virus vectors encoding gag and pol antigens from the IIIB HIV-1 strain (BLCL + Vv-gag-pol). Bars represent the CD8+ T cell response from hu-PBL-SCID mice immunized with virus-pulsed IFN-DCs or IL-4–DCs, as compared with the basal CD8+ T cell response in control nonimmunized xenochimeric mice (CTR). The white bars in the first row represent the background values after stimulation with uninfected BLCL targets for each experimental condition. The panel shows the results of one representative experiment out of four. (B) Comparative analysis of the anti–HIV-1 immune response after vaccination with either virus-pulsed IFN-DCs (AT2/DC) or free inactivated HIV virions (AT2-HIV) as immunogen. The xenochimeric mice were vaccinated and received two boost immunizations at 7-d intervals, according to the immunization schedule described in Materials and Methods. Mice were killed 7 d after the completion of the vaccination schedule. Elispot analysis of the anti–HIV-1 CD8+ T cell response. Human cells were recovered from three spleens hu-PBL-SCID mice from each group and pooled. The assay was performed using as stimulators autologous BLCL targets, infected with either HIV-1 SF162 strain (BLCL + HIV) or recombinant vaccinia virus vectors encoding gag and pol antigens from the IIIB HIV-1 strain (BLCL + Vv-gag-pol). Control uninfected BLCL were used as stimulators/targets for the calculation of background spots for each experimental condition (white bars). Serum ELISA reactivity toward HIV envelope and core proteins. Undiluted sera from control and vaccinated hu-PBL-SCID mice were tested for antibodies to HIV-1 using a commercial ELISA kit detecting IgG, IgM, and IgA specific to a series of envelope and core peptides (ABBOTT Murex HIV-1.2.O). Mouse sera were collected 7 d after completion of the immunization schedule. Each bar represents the mean of the values of sera from three mice ± SE for each experimental group. (C) Elispot analysis of the HLA-A*0201–restricted CD8+ T cell response to the CTL epitope “SL9” (SLYNTVATL) using human cells recovered from spleens and peritoneal lavages (P.L.) of vaccinated xenochimeras. Elispot assay was performed after coculture of the human cells with peptide-pulsed target cells as described in Materials and Methods. Bars represent the CD8+ T cell response from hu-PBL-SCID mice immunized with virus-pulsed IFN-DCs or IL-4–DCs, as compared with the basal CD8+ T cell response in control nonimmunized xenochimeric mice (CTR). The white bars in the first row represent the background values after stimulation with unpulsed BLCL targets for each experimental condition. The panel shows the results of one representative experiment out of three.
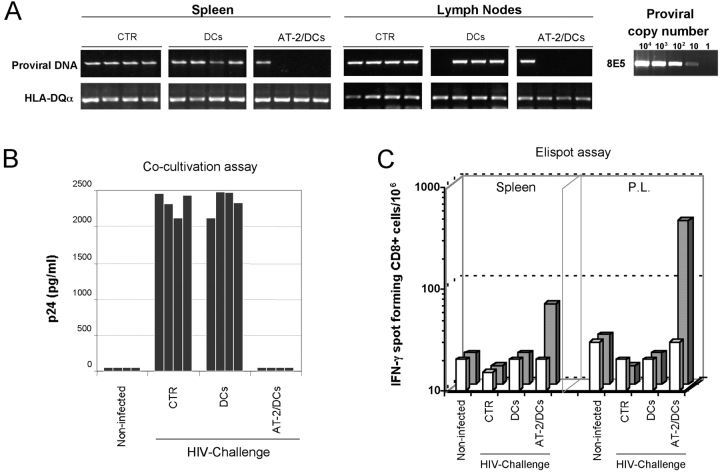
In a second set of experiments, hu-PBL-SCID mice were challenged i.p. with 103 TCID50 of SF162 HIV-1 7 d after a complete immunization schedule. 1 wk later, the animals were assayed for the extent of viral infection/replication by measuring the proviral load in spleen and lymph nodes as well as by testing the capability to rescue infectious virus after coculture of cells recovered from the infected mice with HIV-susceptible target cells. As shown in Fig. 3, all the mice injected with unpulsed IFN-DCs proved to be infected by HIV-1, as revealed by both PCR analysis of DNA extracted from mouse tissues and cocultivation assay, similarly to the nontreated hu-PBL-SCID mice. In contrast, only one out of the four mice immunized with virus-pulsed IFN-DCs exhibited evidence of infection by HIV-1 PCR analysis (Fig. 3 A) and no infectious HIV could be rescued from the cells recovered from any of these animals (Fig. 3 B). In a total set of four experiments, in which hu-PBL-SCID mice were vaccinated with virus-pulsed IFN-DCs, only 4 out of 16 mice proved to be infected by HIV, whereas all the reconstituted and infected control mice (16/16) showed signs of infection by HIV-1 PCR analysis of mouse tissues. Of interest, only the hu-PBL-SCID mice vaccinated with AT-2/HIV–pulsed DCs showed the presence of an anti-HIV CD8+ T cell response (Fig. 3 C).
Figure 3.
Protection from HIV-1 challenge of hu-PBL-SCID mice vaccinated with IFN-DCs pulsed with AT-2–inactivated HIV-1. hu-PBL-SCID mice were challenged i.p. with 103 TCID50 of SF162 HIV-1 7 d after a complete immunization schedule with IFN-DCs pulsed with AT-2–inactivated HIV-1 as described in Materials and Methods. A control group of hu-PBL-SCID mice was neither immunized nor challenged (noninfected), whereas virus-infected control groups were represented by nonimmunized hu-PBL-SCID mice (CTR) and mice injected with unpulsed DCs. 1 wk after HIV-1 infection, proviral load was analyzed in spleen and lymph nodes from infected hu-PBL-SCID mice by PCR for viral gag sequences (A). The sensitivity of the assay was tested by amplifying serial dilutions of DNA prepared from 8E5 cells that harbor one proviral copy/cell (A). The presence of infectious HIV-1 was assessed by cocultivation of peritoneal cells rescued from individual mice with PHA-activated T lymphocytes and determination of the p24 protein in culture supernatants (B) as described in Materials and Methods. The human CD8+ T cell response to HIV antigens (C) was evaluated in cells pooled from the spleen or peritoneal lavages (P.L.) of four hu-PBL-SCID mice per group by IFN-γ Elispot analysis as described in Materials and Methods. Target/stimulator cells used in these experiments were autologous DCs. Bars represent the CD8+ T cell response from hu-PBL-SCID mice immunized with virus-pulsed IFN-DCs, as compared with the CD8+ T cell response in control nonimmunized xenochimeric mice (CTR) and to hu-PBL-SCID mice injected with unpulsed DCs. The white bars in the first row represent the background values after stimulation with uninfected DC targets for each experimental condition. The figure shows the data from one representative experiment out of four. Four SCID mice were included in each experimental group.
In the present work, we have provided the first in vivo evidence on the efficacy of antigen-pulsed human DCs in inducing an anti–HIV-1 protection, which is associated with two main immune correlates, such as anti-HIV antibodies and CD8+ T cells. Little information is available so far on DC-mediated anti–HIV-1 immunity and only three papers in virus-infected monkeys have provided some evidence suggesting the advantage of using DCs for the development of anti–HIV-1 vaccines (17–19). In our work, the induction of an antibody response to HIV was invariantly associated with the detection of HIV-neutralizing activity of mouse sera. Even though neutralizing antibodies have been shown to exert poor effects on the control of established HIV-1 infection in vivo (20), they may contribute in limiting virus cell-to-cell spread during the chronic infection phase. Notably, passive transfer of antibodies has been shown to confer protection against HIV challenge in animal models (21, 22). However, such protection was obtained with antibody concentrations higher than those commonly achievable by vaccination.
In the present paper, we have also demonstrated the development of an efficient HIV-1–specific CD8+ T cell response in vaccinated hu-PBL-SCID mice. Several papers have supported an important role of CD8+ T cells in the control of HIV infection (14–16). CTL induction in HIV-1–infected patients generally precedes the production of any neutralizing antibody and has been considered instrumental in reducing viral load during primary infection (14). Of note, an increase in CTL frequencies has been shown to correlate with decline of viremia (16) and the importance of CD8+ T cells has been well-documented by depletion experiments in monkey models (15). Notably, the IFN-DCs used in the present paper proved to be powerful cellular adjuvants for the generation of neutralizing antibodies and CD8+ T cells against HIV-1, whereas the immature monocyte-derived DCs generated in the presence of IL-4 showed a lower activity. The higher activity of IFN-DCs could be explained by the strong Th-1 type of immune response elicited by these cells (9) and is consistent with data on the type I IFN-induced enhancement of antibody production in mouse models (23, 24) as well as with recent findings showing that human IFN-DCs induce a potent CTL response against HLA-class I–restricted EBV antigens (25). Thus, the use of highly active DCs, such as the IFN-DCs, could explain, at least in part, the powerful immune responses observed in our vaccination experiments in hu-PBL-SCID mice. Second, the use of AT-2–inactivated HIV-1 as immunogen for pulsing DCs may have represented an advantage for inducing a broad spectrum of immune response with respect to the use of purified virus antigens, as suggested by recent papers (26, 27).
In this report, the induction of the anti–HIV-1 immune response was associated with a protection from HIV-1 infection/replication when the animals were challenged with the homologous SF-162 viral strain. Several other studies, including the evaluation of the possible protection from the infection with divergent HIV strains, need to be performed in this practical animal model. In fact, the hu-PBL-SCID mouse exhibits the unique characteristic of allowing the use of human DCs for vaccination studies. However, one important limitation of the model is represented by the rapid in vivo infection of human cells, which does not allow to perform therapeutic immune interventions in chronic infected animals under conditions closely mimicking the natural HIV-1 infection occurring in patients. Nevertheless, the ensemble of the results presented here represent the first “proof of concept” on the potential in vivo efficacy of human DC–based anti-HIV vaccines. Although our experiments have been designed to evaluate the efficacy of DC-based vaccines in a prophylactic context because of the characteristics of the hu-PBL-SCID mouse model, the ensemble of results may be more relevant to the design of therapeutic vaccination strategies, as this potentially highly effective vaccination strategy is not practicable for large scale preventive immunization. We suggest that the combined use of inactivated HIV-1 as immunogen and IFN-DCs as cellular adjuvant can offer new perspectives for the design of therapeutic vaccination strategies in HIV-1–infected patients. IFN-DC–based therapeutic immunization could be associated to HAART in the treatment of acute HIV infection, to preserve both DCs and specific activated CD4+ T lymphocytes taking advantage of a still-intact CD4 repertoire. In the course of an established chronic infection, IFN-DC–based immunization could be beneficial for the persistence of CD8+ T cell response during HAART, thus preventing specific CD8+ lymphocyte reduction, which generally parallels viral load drop after antiviral therapy.
Acknowledgments
We are grateful to C. Gasparrini and A. Ferrigno for excellent secretarial assistance.
This work was supported in part by grants from the Italian Project on AIDS (Istituto Superiore di Sanità-Ministry of Health) and from the special project on Cytokines as Vaccine Adjuvants (Italian Ministry of Health).
References
- 1.Pantaleo, G., and A.S. Fauci. 1996. Immunopathogenesis of HIV infection. Annu. Rev. Microbiol. 50:825–854. [DOI] [PubMed] [Google Scholar]
- 2.Finzi, D., J. Blankson, J.D. Siliciano, J.B. Margolick, K. Chadwick, T. Pierson, K. Smith, J. Lisziewicz, F. Lori, C. Flexner, et al. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5:512–517. [DOI] [PubMed] [Google Scholar]
- 3.Jubault, V., M. Burgard, E. Le Corfec, D. Costagliola, C. Rouzioux, and J.P. Viard. 1998. High rebound of plasma and cellular HIV load after discontinuation of triple combination therapy. AIDS. 12:2358–2359. [PubMed] [Google Scholar]
- 4.de Jong, M.D., R.J. de Boer, F. de Wolf, N.A. Foudraine, C.A. Boucher, J. Goudsmit, and J.M. Lange. 1997. Overshoot of HIV-1 viraemia after early discontinuation of antiretroviral treatment. AIDS. 11:F79–F84. [DOI] [PubMed] [Google Scholar]
- 5.Chun, T.-W., R.T. Davey Jr., D. Engel, H.C. Lane, and A.S. Fauci. 1999. Re-emergence of HIV after stopping therapy. Nature. 401:874–875. [DOI] [PubMed] [Google Scholar]
- 6.Hart, D.N. 2001. Dendritic cells and their emerging clinical applications. Pathology. 33:479–492. [DOI] [PubMed] [Google Scholar]
- 7.Steinman, R.M., and M. Dhodapkar. 2001. Active immunization against cancer with dendritic cells: the near future. Int. J. Cancer. 94:459–473. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811. [DOI] [PubMed] [Google Scholar]
- 9.Santini, S.M., C. Lapenta, M. Logozzi, S. Parlato, M. Spada, T. Di Pucchio, and F. Belardelli. 2000. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191:1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brander, C., K.E. Hartman, A.K. Trocha, N.G. Jones, R.P. Johnson, B. Korber, P. Wentworth, S.P. Buchbinder, S. Wolinsky, B.D. Walker, and S.A. Kalams. 1998. Lack of strong immune selection pressure by the immunodominant, HLA-A*0201-restricted cytotoxic T lymphocyte response in chronic human immunodeficiency virus-1 infection. J. Clin. Invest. 101:2559–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brander, C., O.O. Yang, N.G. Jones, Y. Lee, P. Goulder, R.P. Johnson, A. Trocha, D. Colbert, C. Hay, S. Buchbinder, et al. 1999. Efficient processing of the immunodominant, HLA-A*0201-restricted human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitope despite multiple variations in the epitope flanking sequences. J. Virol. 73:10191–10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizza, P., S.M. Santini, M. Logozzi, C. Lapenta, P. Sestili, G. Gherardi, R. Lande, M. Spada, S. Parlato, F. Belardelli, and S. Fais. 1996. T-cell dysfunctions in hu-PBL-SCID mice infected with human immunodeficiency virus (HIV) shortly after reconstitution: in vivo effects of HIV on highly activated human immune cells. J. Virol. 70:7958–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muster, T., R. Guinea, A. Trkola, M. Purtscher, A. Klima, F. Steindl, P. Palese, and H. Katinger. 1994. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J. Virol. 68:4031–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borrow, P., H. Lewicki, B.H. Hahn, G.M. Shaw, and M.B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin, X., D.E. Bauer, S.E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C.E. Irwin, J.T. Safrit, J. Mittler, L. Weinberger, et al. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus–infected macaques. J. Exp. Med. 189:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogg, G.S., X. Jin, S. Bonhoeffer, P.R. Dunbar, M.A. Nowak, S. Monard, J.P. Segal, Y. Cao, S.L. Rowland-Jones, V. Cerundolo, et al. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 279:2103–2106. [DOI] [PubMed] [Google Scholar]
- 17.Nehete, P.N., S. Chitta, M.M. Hossain, L. Hill, B.J. Bernacky, W. Baze, R.B. Arlinghaus, and K.J. Sastry. 2001. Protection against chronic infection and AIDS by an HIV envelope peptide-cocktail vaccine in a pathogenic SHIV-rhesus model. Vaccine. 20:813–825. [DOI] [PubMed] [Google Scholar]
- 18.Wagner, G.S., C.J. Miller, and M.B. McChesney. 2002. CD4+ T cells and monocytes elicited by immunization of rhesus monkeys with antigen-pulsed dendritic cells control SIV replication. AIDS Res. Hum. Retroviruses. 18:143–148. [DOI] [PubMed] [Google Scholar]
- 19.Lu, W., X. Wu, Y. Lu, W. Guo, and J.M. Andrieu. 2003. Therapeutic dendritic-cell vaccine for simian AIDS. Nat. Med. 9:27–32. [DOI] [PubMed] [Google Scholar]
- 20.Poignard, P., R. Sabbe, G.R. Picchio, M. Wang, R.J. Gulizia, H. Katinger, P.W. Parren, D.E. Mosier, and D.R. Burton. 1999. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity. 10:431–438. [DOI] [PubMed] [Google Scholar]
- 21.Gauduin, M.C., P.W. Parren, R. Weir, C.F. Barbas, D.R. Burton, and R.A. Koup. 1997. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 3:1389–1393. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto, Y., Y. Eda, A. Ogura, S. Shibata, T. Amagai, Y. Katsura, T. Asano, K. Kimachi, K. Makizumi, and M. Honda. 1998. In SCID-hu mice, passive transfer of a humanized antibody prevents infection and atrophic change of medulla in human thymic implant due to intravenous inoculation of primary HIV-1 isolate. J. Immunol. 160:69–76. [PubMed] [Google Scholar]
- 23.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D.F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 14:461–470. [DOI] [PubMed] [Google Scholar]
- 24.Proietti, E., L. Bracci, S. Puzelli, T. Di Pucchio, P. Sestili, E. De Vincenzi, M. Venditti, I. Capone, I. Seif, E. De Maeyer, et al. 2002. Type I interferon as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J. Immunol. 169:375–383. [DOI] [PubMed] [Google Scholar]
- 25.Santodonato, L., G. D'Agostino, R. Nisini, S. Mariotti, D.M. Monque, M. Spada, L. Lattanzi, M.P. Perrone, M. Andreotti, F. Belardelli, and M. Ferrantini. 2003. Monocyte-derived dendritic cells generated after a short-term culture with IFN-α and granulocyte-macrophage colony-stimulating factor stimulate a potent Epstein-Barr virus-specific CD8+ T cell response. J. Immunol. 170: 5195–5202. [DOI] [PubMed] [Google Scholar]
- 26.Lu, W., and J.M. Andrieu. 2001. In vitro human immunodeficiency virus eradication by autologous CD8(+) T cells expanded with inactivated-virus-pulsed dendritic cells. J. Virol. 75:8949–8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buseyne, F., S. Le Gall, C. Boccaccio, J.P. Abastado, J.D. Lifson, L.O. Arthur, Y. Riviere, J.M. Heard, and O. Schwartz. 2001. MHC-I-restricted presentation of HIV-1 virion antigens without viral replication. Nat. Med. 7:344–349. [DOI] [PubMed] [Google Scholar]