T Helper Cell Type 2 Cytokine–Mediated Comitogenic Responses and Ccr3 Expression during Differentiation of Human Mast Cells in Vitro (original) (raw)
Abstract
Mast cells (MCs) arise in situ from circulating stem cell factor (SCF)-dependent committed progenitors (PrMCs) and accumulate at sites of allergic mucosal inflammation. We hypothesized that human (h)PrMCs and their mature counterparts might share overlapping patterns of chemokine and cytokine receptor utilization with eosinophils, basophils, and T helper type 2 (Th2) lymphocytes for their homing and allergy-associated hyperplasia. We have characterized committed hPrMCs and fully mature hMCs derived in vitro from cord blood for their functional responses to chemokine and cytokine agonists germane to allergic inflammation and for their maturation-related expression of the corresponding receptors. After 4 wk of culture in the presence of recombinant stem cell factor (SCF), interleukin (IL)-6, and IL-10, the cells were characterized as hPrMCs based upon their uniform surface expression of c-kit and CD13, low-level expression of Fc∈RIα, absence of CD14 and CD16 expression, and immunoreactivity for MC chymase in >80%, and about half were immunoreactive for tryptase and metachromatic with toluidine blue. By week 9, the cells had matured into hMCs, identified by higher levels of c-kit, continued expression of CD13 and low-level Fc∈RIα, uniform toluidine blue metachromasia, and uniform immunoreactivity for both tryptase and chymase. The 4-wk-old hPrMCs expressed four chemokine receptors (CXCR2, CCR3, CXCR4, and CCR5). Each receptor mediated transient rapid calcium fluxes in response to its respective ligand. Both recombinant human eotaxin and stromal cell–derived factor 1α elicited chemotaxis of hPrMCs. Only CCR3 was retained on the mature 9-wk-old hMCs from among these chemokine receptors, and hMCs responded to eotaxin with a sustained calcium flux but without chemotaxis. The Th2 cytokines IL-3, IL-5, IL-6, IL-9, and granulocyte/macrophage colony-stimulating factor each augmented the SCF-dependent proliferation of hPrMCs and hMCs. In contrast, the prototypical Th1 cytokine, interferon γ, suppressed SCF-driven proliferation of both hPrMCs and hMCs. Thus, throughout their development in vitro, hMCs obey SCF-dependent, cytokine-driven mitogenic responses that reflect a Th2-type polarization characteristic of allergy and asthma. Furthermore, committed hPrMCs have a unique profile of chemokine receptor expression from among reported hematopoietic cells, including CCR3, which is shared with the other cells central to allergic inflammation (eosinophils, basophils, and Th2 lymphocytes).
Keywords: chemokines, asthma, HIV, calcium flux, stem cell factor
Mast cells (MCs)1 are ubiquitously distributed to mucosal and cutaneous surfaces, as well as to microvascular sites in connective tissues. This distribution facilitates their role in adaptive immune responses to intestinal helminthic parasites and in innate host resistance to gram-negative bacterial infections in experimental models 1 2. In humans, MCs activated by IgE-dependent or idiosyncratic mechanisms release potent bioactive inflammatory mediators with direct pathobiologic roles in asthma, rhinitis, urticaria, and anaphylaxis 3 4 5 6 7. Tissue MCs differentiate and mature in situ from committed progenitor cells (PrMCs), which arise in the marrow compartment from pluripotent hematopoietic progenitors 8 9 and circulate as mononuclear leukocytes lacking characteristic secretory granules 10 11. Circulating PrMCs differ from monocytes by their surface expression of the receptor for stem cell factor (SCF), c-kit 10, by their SCF-dependent proliferation in vitro that is strongly augmented by IL-3 in both humans 12 and mice 11, and by their lack of CD14 expression 13. The normal development of all tissue MC populations in vivo requires SCF, which is constitutively produced by stromal cells. Additional factors that are derived from T lymphocytes, including IL-3 14, are required for the development of the MC subpopulation that normally resides in the intestinal epithelium in both mice and humans but not for MC development in the skin or connective tissues. Intraepithelial MCs are depleted in the absence of T lymphocytes 15 16. Conversely, their numbers increase in intestinal helminthic infections in mice 14 and in bronchial wall inflammation in humans with asthma 17 18 19. The regulation of tissue localization, proliferation, and viability of hPrMCs, as well as their terminal differentiation into hMCs, must include constitutive mechanisms that ensure normal numbers of tissue hMCs within connective tissues and inducible mechanisms that maintain and/or increase the supply of hPrMCs within intraepithelial compartments for T cell–dependent reactive hMC responses.
The hyperplasia of mucosal MCs, both in human asthma and in helminthic infections in mice, occurs concomitantly with local recruitment and activation of T lymphocytes bearing the T helper type 2 (Th2) phenotype, with attendant local production of IL-3, IL-4, IL-5, IL-6, IL-9, IL-13, GM-CSF, and other cytokines, and with a tissue eosinophilia 20. Cytokines, derived from Th2 cells orchestrate several aspects of allergic inflammation and emanate from a genetic locus on the long arm of chromosome 5, containing several genes with candidate roles in asthma and allergic disease 21 22. IL-3, GM-CSF, and IL-5 are implicated in tissue eosinophilia, as each supports eosinophil development 23, sustains eosinophil viability 24, and converts mature eosinophils from a resting phenotype to a functionally primed state 25. Composite data from animal models of allergic inflammation indicate that these cytoprotective cytokines, particularly IL-5, act cooperatively with chemoattractive cytokines (chemokines) to orchestrate tissue eosinophilia 26 27. Several CC chemokines induce the directed migration of eosinophils from the peripheral blood and produce their activated adhesion, both of which are necessary for net cell accumulation 28. Of the eosinophil-active chemokines, eotaxin (ETX) is particularly implicated in the mucosal inflammation of allergic diseases, being expressed by epithelial and endothelial cells at sites of allergic inflammation 29. Increases in lung ETX levels accompany bronchial eosinophilia in humans with asthma 30 and are elicited by allergen challenge by a T cell–dependent mechanism in experimental models 31. The recent additional demonstration of the ETX receptor (CCR3) 32 on human peripheral blood basophils 33 and human Th2 lymphocytes 34 suggests that ETX may particularly recruit several blood-borne cells that participate in allergic inflammation. Completion of the cellular profile of allergic tissue inflammation would likely include both chemokine-induced attraction and Th2 cytokine–mediated hyperplasia of SCF-dependent PrMCs.
Although hMCs were previously developed from cultures of bone marrow cells 9, fetal liver cells 35, and cord blood mononuclear cells 36 using SCF with or without IL-6, there has been no definition of the membrane phenotype of the hPrMCs or hMCs derived in vitro with regard to cytokine and chemokine receptor expression. Furthermore, although IL-3, IL-4, IL-5, and IL-6 were each cytoprotective for hMCs 37, the potential role for these and other Th2 cytokines as mitogens or comitogens with SCF has not been characterized during developmental progression of hMCs. Given that reactive MC hyperplasia is a frequent concomitant of Th2-polarized immune responses, we hypothesized that MC might obey overlapping patterns of chemokine and cytokine receptor utilization with eosinophils, basophils, and Th2 lymphocytes. We recently reported the development of homogeneously pure PrMCs (metamastocytes) from uncommitted mouse bone marrow cells using a triad of SCF, IL-6, and IL-10 38. Using the analogous combination of recombinant human growth factors, we now report the characterization of chemokine and cytokine receptor utilization by hPrMCs derived in vitro from cord blood and maturation-related alterations in these parameters during their differentiation into fully mature hMCs. hPrMCs express CCR3, CXCR4 (the receptor for the constitutively produced lymphocyte chemoattractant stromal cell–derived factor [SDF]-1α) 39, CCR5 (the receptor for the Th1-active chemokine macrophage inflammatory protein (MIP)-1α) 40, and an IL-8 receptor, CXCR2 28. These receptors each mediate intracellular calcium flux in hPrMCs in response to their respective ligands, and both ETX and SDF-1α promote chemotaxis in a dose-dependent fashion. The presence of these receptors, along with CD4, on hPrMCs could provide a previously unrecognized cellular reservoir for HIV. Additionally, the SCF-driven mitogenesis of both hPrMCs and their mature counterparts is augmented by several members of the Th2 cytokine gene cluster that are implicated as potential candidate genes in human asthma (IL-3, IL-5, IL-6, IL-9, and GM-CSF), whereas a Th1 cytokine, IFN-γ, inhibits mitogenesis. The expression of CXCR4 by hPrMCs during their lineage development suggests that SDF-1α may contribute to the stromal distribution of hPrMC under basal conditions, whereas the presence of both functional CCR3 and Th2 cytokine responses provides a potentially critical link between T cell activation, allergic inflammation, and the hyperplasia of intraepithelial MCs in human allergic disease.
Materials and Methods
Cytokines and Chemokines.
The recombinant human cytokines SCF and IL-6 were provided by Amgen. Recombinant human IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, IL-12, IL-13, IFN-γ, GM-CSF, granulocyte (G)-CSF, and macrophage (M)-CSF were purchased from Endogen; and ETX, IL-8, MIP-1α, and SDF-1α were from PeproTech, Inc.
Culture of hPrMCs and hMCs.
hPrMCs were derived from cord blood mononuclear cells cultured in the presence of SCF, IL-6, and IL-10, as previously described for the development of mouse PrMCs 38. Heparin-treated umbilical cord blood was obtained from placentas after routine cesarean section deliveries. After dextran sedimentation of the blood to remove erythrocytes, the interfaces containing mononuclear cells were obtained by centrifugation of the buffy coats through a cushion of Ficoll-Hypaque® (1.77 g/ml; Pharmacia). Residual erythrocytes were removed by hypotonic lysis, and the mononuclear cells were suspended in RPMI 1640 (GIBCO BRL) supplemented with 10% fetal bovine serum (Sigma Chemical Co.), 2 mM l-glutamine, 0.1 mM nonessential amino acids, 0.2 μM 2-ME, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 μg/ml gentamycin. The cell suspensions were seeded at a density of 106 cells/ml and cultured in the presence of 100 ng/ml SCF, 50 ng/ml IL-6 (added because of its synergistic effects for SCF-driven hMC development from cord blood mononuclear cells; reference 36), and 10 ng/ml IL-10, which was included because of its synergistic effect for mouse MC development with SCF and IL-3 41 42 and because of its suppressive effect on endogenous production of GM-CSF and resultant proliferation of granulocytes and monocytes 43. Cultures were carried for up to 9 wk. The entire volume of cytokine-supplemented medium was replaced on a weekly basis, and the adherent fraction of cells was discarded weekly by the transfer of the nonadherent cells to fresh culture flasks. Every week, aliquots of 2 × 104 cultured cells were spun onto glass slides in a cytocentrifuge (Cytospin® 2; Shandon) and stained with toluidine blue (which elicits a metachromatic reaction only in the granules of MCs and basophils) as previously described 38.
Immunocytochemistry.
Cytocentrifugation slides were prepared as described above, air dried, and fixed in Carnoy's fluid (60% ethanol, 30% chloroform, and 10% glacial acetic acid) for 10 min at room temperature. After being washed with PBS three to four times, the slides were blocked with 2% chicken egg albumin (Sigma Chemical Co.) for 30 min at room temperature and incubated with a 1:200 dilution of a mouse anti–human IgG1 mAb that detects both human α and β tryptases (Chemicon International, Inc.) 44 with an affinity-purified rabbit anti–human chymase antibody (provided by Dr. N. Schechter, University of Pennsylvania, Philadelphia, PA) 16 or irrelevant isotype-matched mouse monoclonal or rabbit IgG antibodies. These antibody probes were chosen because of their specificity as MC markers. Immunocytochemical procedures were carried out as previously described with alkaline phosphatase as the chromogenic reporter 45. The cells exhibiting strong immunoreactivity as indicated by a red staining reaction were expressed as a percentage of 300 cells counted on each slide.
Flow Cytometry.
Flow cytometric analysis was carried out as previously described 46. All analyses were carried out in the presence of cold HBSS containing 2% fetal bovine serum, 0.1% human serum, and 0.01% sodium azide (FACS buffer). 105 cells were incubated with mouse anti–human mAbs specific for the following epitopes known to be expressed by mature MCs: c-kit (the receptor for SCF, expressed by PrMCs, abundantly by MCs, and to a lesser degree by basophils 47 and eosinophils 46, recognized by SR-1, an IgG2a antibody provided by Dr. V. Broudy, University of Washington, Seattle, WA) 48; Fc∈RIα (the α subunit of the high-affinity IgE receptor expressed by MCs and basophils, recognized by 22E7, an IgG1 mAb provided by Dr. R. Chizzonite, Hoffmann-LaRoche, Nutley, NJ) 49; CD13 (an epitope homologous to the K1 membrane aminopeptidase that distinguishes mouse MCs from basophils 50 51, recognized by an IgG1 from PharMingen); and β3 integrin (a component of the vitronectin receptor expressed by mature dispersed hMCs from skin, lung, and uterus [52], as well as by macrophages and some peripheral blood monocytes [53, 54], recognized by an IgG1 from PharMingen); the α subunit of the IL-3 receptor (IL-3Rα, lacked by lung hMCs [55] but likely expressed by hPrMCs from peripheral blood based on their synergistic response to IL-3 with SCF [12], recognized by an IgG1 from PharMingen); CD4 (interacts with CCR3, CCR5, and CXCR4 to facilitate HIV entry [56, 57]; not known to be expressed by hPrMCs or hMCs, recognized by an IgG1 from PharMingen); the α subunit of the IL-5 receptor (IL-5Rα, recognized by an IgG1 from PharMingen); CD14 (a monocyte marker, recognized by an IgG2a from PharMingen); and CD16 (a neutrophil marker, recognized by an IgG1 from PharMingen). The cells were also stained with the following mAbs against chemokine receptors having the indicated known cell specificities 58: CCR1 (a receptor for MIP-1α, monocyte chemotactic proteins [MCP]-3 and -4 and RANTES [regulated on activation, normal T cell expressed and secreted], expressed by eosinophils, monocytes, dendritic cells and activated T lymphocytes; IgG1; R & D Systems, Inc.); CCR2 (a receptor for MCP-1, -2, -3, -4, and -5, expressed by basophils, monocytes, activated T cells, dendritic cells, and NK cells; IgG1; R & D Systems, Inc.); CCR3 (the receptor for ETX and MCP 2-4, expressed by eosinophils, basophils, and Th2, recognized by 7B11, an IgG2a provided by the National Institutes of Health (NIH) AIDS Repository, Bethesda, MD); CCR4 (a receptor for thymus- and activation-regulated chemokine, expressed by activated T cells and dendritic cells; IgG1 hybridoma supernatant [1G1] provided by Lijun Wu, LeukoSite, Inc.); CCR5 (a receptor for MIP-1α, MIP-1β, and RANTES, expressed by monocytes, activated T cells, dendritic cells, and NK cells recognized by 2D7, an IgG2a from the NIH AIDS Repository); CCR6 (a receptor for MIP-3α, expressed by dendritic cells, recognized by an IgG1 hybridoma supernatant [11A9]; provided by P. Ponath, LeukoSite, Inc.); CXCR1 (a receptor for IL-8 and GCP-2, expressed by neutrophils, monocytes, basophils, a subset of T lymphocytes and recently reported on the surface of the transformed MC leukemia line [HMC-1] and dispersed skin hMCs 59, clone 5A12, IgG2b; PharMingen); CXCR2 (a receptor for IL-8, granulocyte chemotactic protein 2, growth-related oncogene, epithelial neutrophil-activating peptide, neutrophil-activating peptide 2 and LPS-induced CXC chemokine, with a distribution similar to CXCR1; clone 6C6, IgG1; PharMingen); CXCR3 (receptor for IP-10, monokine induced by IFN-γ, and IFN-inducible T cell α attractant, expressed by NK cells and Th1, recognized by an IgG1 hybridoma supernatant [11A9]; provided by P. Ponath, LeukoSite, Inc.); and CXCR4 (receptor for SDF-1α, expressed by naive T cells, monocytes, and dendritic cells, recognized by 12G5, an IgG2a from the NIH AIDS Repository). Negative controls included an IgG1 hybridoma culture supernatant (P3; provided by Dr. M. Hemler, Harvard Medical School, Boston, MA) and irrelevant mouse IgG2a or IgG2b (PharMingen). After exposure to the mAbs, the cells were stained with FITC-conjugated sheep anti–mouse IgG (Calbiochem Corp.) and then analyzed using FACSort™ (Becton Dickinson). The results are presented as overlaid histograms.
Functional Assays.
[3H]thymidine was incorporated by 5 × 104 cells in triplicate experiments. The cytokines used in the assays and their plateau concentrations were SCF (100 ng/ml), IL-6 (50 ng/ml), IL-10 (10 ng/ml), IL-3 (5 ng/ml), IL-2 (5 ng/ml), IL-4 (10 ng/ml), IL-5 (5 ng/ml), G-CSF (5 ng/ml), M-CSF (5 ng/ml), GM-CSF (5 ng/ml), IL-9 (50 ng/ml), IFN-γ (10 ng/ml), IL-12 (10 ng/ml), or IL-13 (10 ng/ml). Cells were cultured at 37°C and 5% CO2 for 6 d in the presence of the indicated cytokines. The cultures were pulsed for the final 16 h with 1 μCi/well of [3H]thymidine (NEN Life Science Products; specific activity, 20 Ci/mmol), harvested with Harvester 96® MACH II (Tomtec, Inc.), and analyzed in triplicate with a 1205 Betaplate™ Liquid Scintillation Counter (Pharmacia). Because the trends for [3H]thymidine incorporation were consistent but the absolute values varied considerably among experiments, the results for each cytokine were normalized relative to the incorporation in response to SCF alone in each experiment, thereby allowing mean ± SEM to be expressed for the cumulative data obtained in different experiments.
Changes in the cytosolic free Ca2+ concentration was measured using Fura-2–loaded hPrMCs and hMCs. The cells were resuspended in HBSS containing 1 mM CaCl2, 1 mM MgCl2, and 0.1% BSA and loaded with Fura-2 AM (Molecular Probes, Inc.) for 30 min at 37°C. After labeling, the cells (5 × 106) were washed and resuspended in the above buffer. [Ca2+]i was measured using excitation at 340 and 380 nm in a fluorescence spectrophotometer (Hitachi F-4500) after stimulation with recombinant chemokines (1–100 nM each), and the relative ratio of fluorescence emitted at 510 nm was recorded.
The chemotaxis of hPrMC was measured using Transwell® tissue culture inserts with an 8-μm pore size (Corning Costar Corp.). The cell suspensions (105) and chemokine dilutions were made in RPMI 1640 supplemented with 20 mM Hepes, pH 7.5, and 1% human plasma albumin (Sigma Chemical Co.). Migration was allowed to proceed for 1 h at 37°C in a 5% CO2–humidified atmosphere. The membrane was then removed, washed on the upper side with PBS, fixed, and stained with Diff-Quik® (Baxter Corp.). The migrated cells were counted in five randomly selected fields at a magnification of 400. Spontaneous migration was also determined in the absence of chemokine and subtracted from chemokine-induced migrated cells. The chemotactic responses were consistently three- to fourfold greater than the background cell migration.
Reverse Transcriptase PCR.
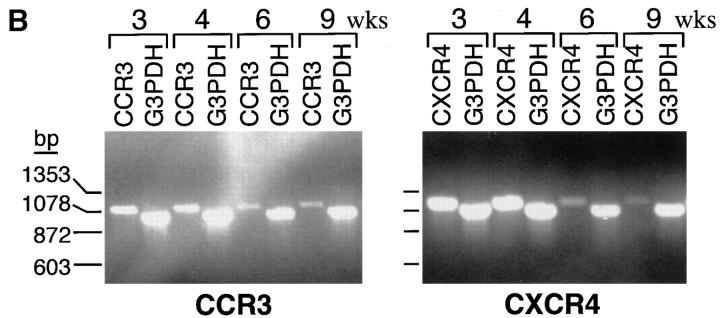
Total RNA was extracted from cultured cells at 3, 4, 6, and 9 wk using TRI Reagent (Molecular Research) as previously described 60. Each sample of total RNA was reverse transcribed after oligo dT priming according to the manufacturer's protocol of a commercial reverse transcriptase (RT) kit (Invitrogen Corp.). The following deoxyoligonucleotide primers were designed for PCR amplification of human CCR3: sense strand primer “CCR3A”: 5′-ATG-ACA-ACC-TCA-CTA-GAT-ACA-GTT-G-3′; and antisense strand primer “CCR3B”: 5′-CTA-AAA-CAC-AAT-AGA-GAG-TTC-CGG-C-3′. For the amplification of CXCR4, the primers were: sense strand primer “CXCR4A”: 5′-ATG-GAG-GGG-ATC-AGT-ATA-TAC-ACT-TC-3′; and antisense strand primer “CXCR4B”: 5′-GCT-GGA-GTG-AAA-ACT-TGA-AGC-TC-3′. The primer locations were chosen to encompass two exons, thus ensuring that any amplified genomic contaminant could be distinguished from the transcript of interest based on size. 10 μl of the reverse transcription reaction mixture was amplified by 35 cycles in an automated thermal cycler (Perkin-Elmer Cetus Instruments) under the following conditions: 1× PCR buffer (Boehringer Mannheim) containing 1.5 mM MgCl2, 200 μM dCTP, dTTP, dGTP, and dATP, 10 pmols of each primer, and 2.5 U of Taq polymerase (Perkin-Elmer Corp.). The parameters were 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min. Chain elongation was continued after the last cycle for 10 min. Duplicate samples of each RT mixture were also amplified using commercially prepared oligonucleotides for human glyceraldehyde 3-phosphate dehydrogenase (G3PDH; Clontech) as an internal standard with the same parameters. The PCR reaction products were resolved on a 1% agarose gel containing ethidium bromide. The specificity of the resultant appropriately sized PCR products was confirmed using an automated sequencing reaction (Dana-Farber Cancer Institute Molecular Biology Core Facility, Boston, MA).
Results
Immunocytochemical and Cytofluorographic Characteristics of Cultured hPrMCs and hMCs.
As defined by metachromatic staining with toluidine blue dye, no hMCs were identified among the starting cultures of cord blood mononuclear cells. During culture in the presence of SCF/IL-6/IL-10, both the total number of cells and the total number of toluidine blue–positive cells arising from 3 × 107 cord blood mononuclear cells increased in all experiments, with total cells reaching a maximum at week 3 (7.0 ± 1.6 × 107 cells) and total toluidine blue–positive cells reaching a peak at week 5 (3.4 ± 0.3 × 107 cells), both declining thereafter (Fig. 1 A). The decline in total cell number was more rapid than the decline in toluidine blue–positive cell number. As a result, the percentage of cells exhibiting toluidine blue metachromasia progressively increased, reaching >75% of the cultured cells by week 6 and 100% by week 9 (Fig. 1 B). The percentages of cells that were positive for tryptase (Fig. 1 C) paralleled the toluidine blue staining and comprised 43 ± 2% at week 4, 92 ± 2% at week 6, and 100 ± 0% at week 9 (mean ± SEM; n = 3). Chymase immunoreactivity was evident in 85 ± 3% of the cells at week 4, 100 ± 0% at week 6, and 100 ± 0% at week 9 (mean ± SEM; n = 3; Fig. 1 C).
Figure 1.
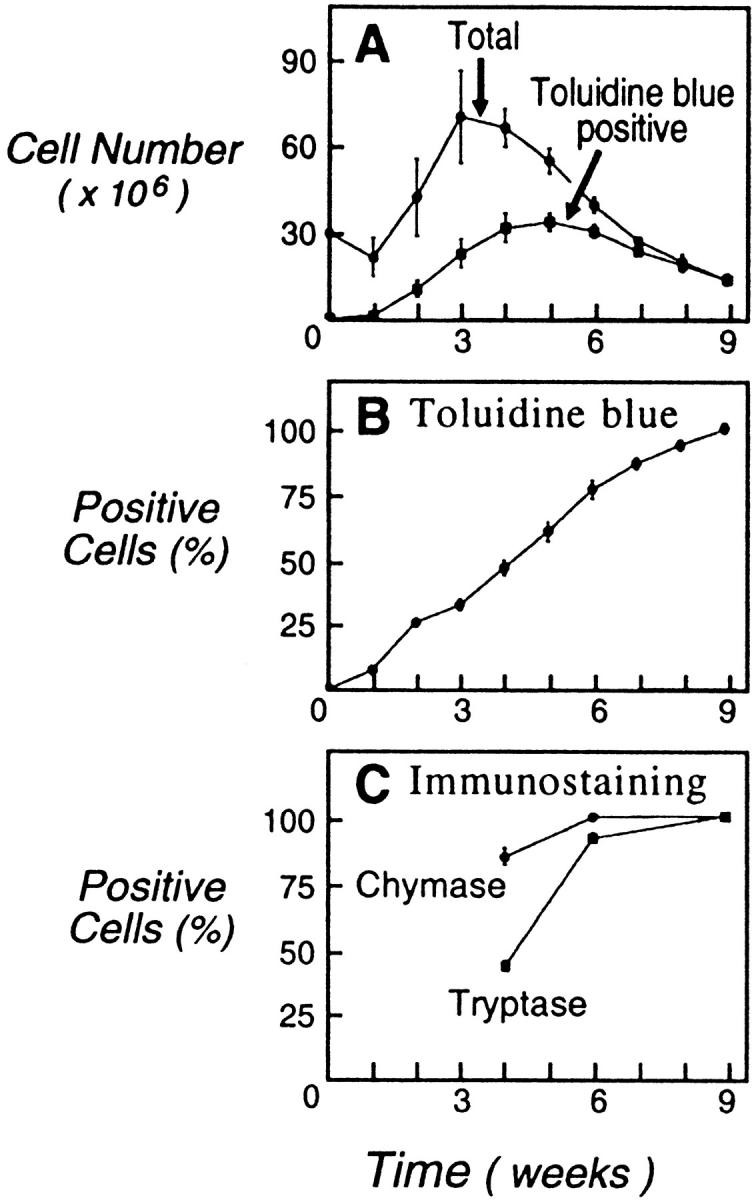
(A) Total cell numbers (•) and numbers of cells staining metachromatically with toluidine blue (▪) arising over 9 wk from cultures of 3 × 107 cord blood mononuclear cells in the presence of SCF/IL-6/IL-10. (B) Progressive increase in the percentage of cells with toluidine blue metachromasia. (C) Percentages of cells with tryptase (▪) and chymase immunoreactivity (•) at weeks 4, 6, and 9 of culture. Results are the means ± SEM of three experiments.
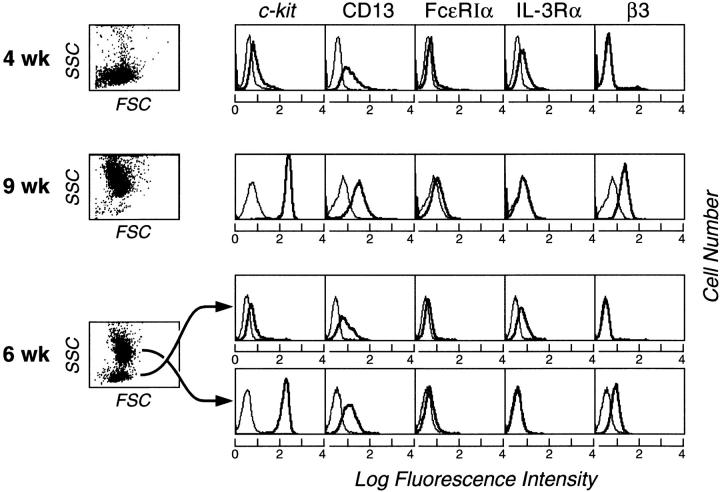
In all experiments, the cells had reached homogeneity for light scatter properties by week 4 (Fig. 2, top row, left), with monophasic expression of c-kit and CD13 and low-level monophasic expression of Fc∈RIα (as shown for a representative experiment, Fig. 2). These 4-wk-old ungated cells also expressed IL-3Rα monophasically but lacked the β3 integrin. Neither CD14 nor CD16 was detectable at week 4 (n = 3; data not shown). At week 9, the cells also showed uniform light scatter but of substantially higher intensity (Fig. 2, second row, left). 100% of the ungated 9-wk-old cells strongly expressed c-kit and CD13, were again weakly Fc∈RIα-positive, and were IL-3Rα–negative but had become strongly β3 integrin–positive (Fig. 2). At week 6, the cells segregated into two populations based on differences in side angle light scatter (SSC) (Fig. 2, third row, left); a population of low SSC was indicative of low granularity and a population of high SSC indicated high granularity. Separate cytofluorographic gating revealed that the low granularity 6-wk-old population (c-kit/CD13/Fc∈RIα/IL-3Rα–positive, β3 integrin negative) resembled the 4-wk-old population, whereas the high granularity 6-wk-old population (c-kit/CD13/Fc∈RIα/β3 integrin–positive, IL-3Rα–negative) resembled the 9-wk-old population in its distribution of surface epitopes.
Figure 2.
Cytofluorographic characteristics of 4- (top row) and 9-wk-old (second row) cells, each representing uniform populations by light scatter (left). Representative histograms are shown for c-kit, CD13, Fc∈RIα, IL-3Rα, and β3 integrin. At week 6, two cell subpopulations are indicated by distinct groups that differ in terms of the height of SSC. Separate gating and analysis revealed that the population of lesser SSC (low granularity; third row) resembled the 4-wk-old cells in its surface phenotype, whereas the higher SSC population (high granularity; fourth row) resembled the 9-wk-old cells. Expression of each surface epitope is represented by the bold tracings, and the isotype-matched negative controls are represented by the lighter tracings. Results are representative of independent experiments performed with cells from three different donors.
Sorting, Characterization, and Culture of Low and High Granularity Cell Populations at Week 6.
To determine whether the c-kit/CD13/Fc∈RIα/IL-3Rα–positive, β3 integrin–negative cells of low granularity were progenitors of the c-kit/CD13/Fc∈RIα/β3 integrin–positive, IL-3Rα–negative cells of high granularity, the two cell populations were separated by FACSorting at 6 weeks (Fig. 3 A). Approximately 20% of the original cells were recovered after the sorting procedure. 15 ± 6% of the low granularity cells were toluidine blue positive (a representative field shown in Fig. 3 B), and 11 ± 1% were tryptase positive (Fig. 3 C), whereas 96 ± 2% and 94 ± 3% stained for chymase and chloroacetate esterase, respectively (Fig. 3D and Fig. E; n = 2; mean ± half range for all parameters). In contrast, all of the cells separated into the high granularity group were strongly positive for toluidine blue (Fig. 3 F), immunoreactive tryptase (Fig. 3 G), and chymase (Fig. 3 H) and chloroacetate esterase activity (Fig. 3 I) (n = 2).
Figure 3.
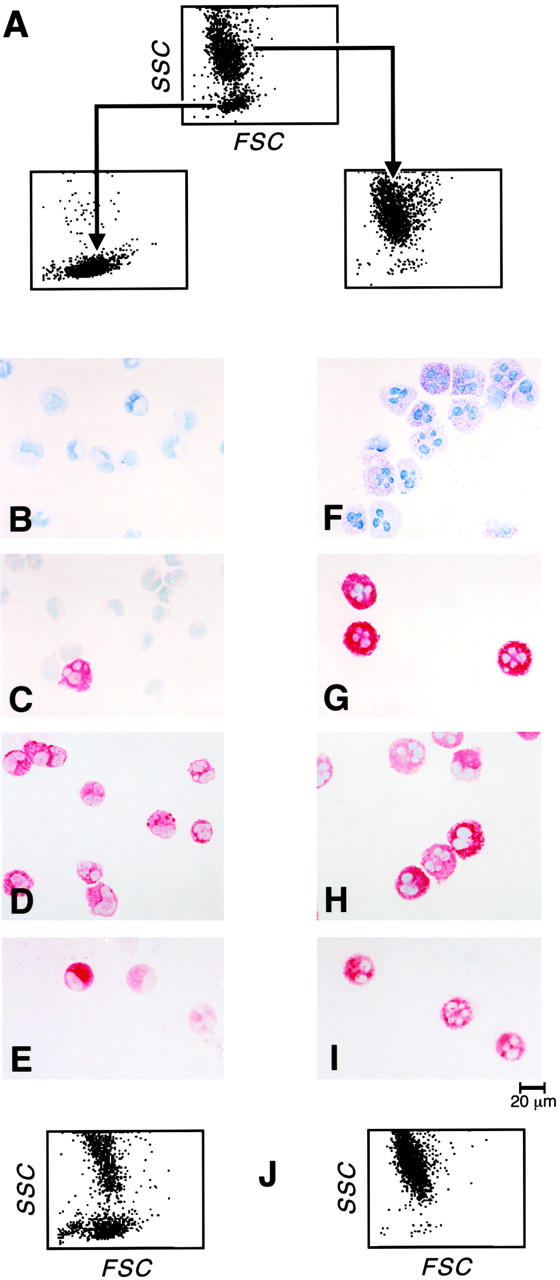
FACSorting of 6-wk-old cells into low and high granularity populations by their light scatter profiles (A). Toluidine blue metachromasia (B, F), tryptase immunoreactivity (C, G), chymase immunoreactivity (D, H), and chloroacetate esterase activity (E, I) are depicted for the low granularity (left) and high granularity (right) populations. Dot plot density light scatter profile of cells derived after 2 wk of culture from the original sorted 6-wk-old populations (J) of low granularity (left) and high granularity (right). The results are representative of two experiments, and the fields depict the profile presented quantitatively in the text.
The respective 6-wk-old separated populations were maintained for 2 wk of further culture in the presence of SCF/IL-6/IL-10 and then reanalyzed for their light scatter characteristics, surface expression of c-kit, CD13, Fc∈RIα, IL-3Rα, and β3 integrin, and metachromatic staining properties. The FACSorted 6-wk-old high granularity population retained its original level of granularity after the two additional weeks of culture (Fig. 3 J, right), retained its original surface distribution of c-kit, CD13, Fc∈RIα, and β3 integrin, and remained uniformly toluidine blue positive (not shown). The FACSorted 6-wk-old low granularity population gave rise to two distinct populations on subsequent culture that segregated by differences in granularity (Fig. 3 J, left). Cytofluorographic analysis revealed that the population of lower SSC was identical in surface phenotype to the original 6-wk-old purified low granularity population (c-kit/CD13/Fc∈RIα/IL-3Rα–positive, β3 integrin–negative), whereas the newly derived population of higher SSC was cytofluorographically identical to the original sorted high granularity population (c-kit/CD13/Fc∈RIα/ β3–integrin positive, IL-3Rα–negative; not shown). The evolution of the 6-wk-old sorted low granularity cells into two populations was accompanied by an increase in the proportion of cells with toluidine blue positivity (from 15 ± 6% to 59 ± 3%; n = 2). Thus, the low granularity population, observed uniformly at week 4 and fractionally at week 6, were designated hPrMCs and viewed as providing a population of higher granularity, designated hMCs, observed initially at week 6 and uniformly at week 9.
Proliferative Responses of hPrMCs and hMCs to Th2 Cytokines and Reinduction of IL-3Rα.
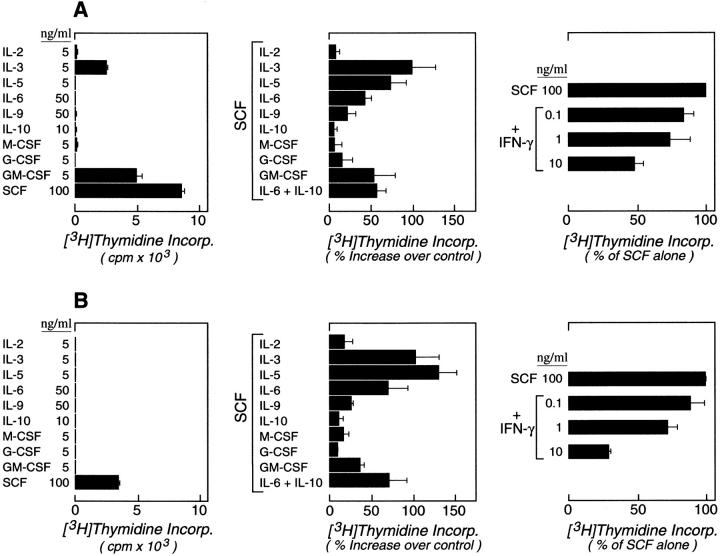
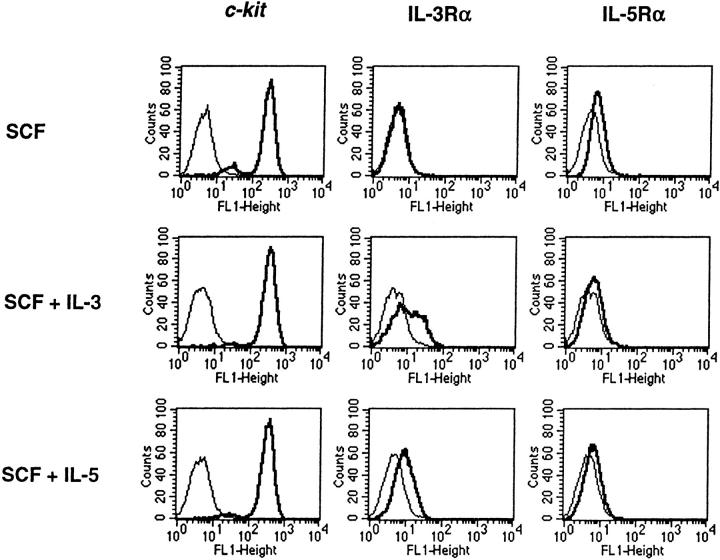
Thymidine incorporation in response to various hematopoietic cytokines was studied at weeks 4 and 9. At week 4, the cultured cells responded maximally to SCF among the cytokines tested alone and also responded to GM-CSF (51 ± 11% of the SCF response) and IL-3 (27 ± 3%) alone (n = 3, as shown for a representative experiment; Fig. 4 A, left). The 4-wk-old cells did not respond to M-CSF, G-CSF, IL-2, IL-5, IL-6, IL-9, or IL-10 alone. No proliferative response was seen to IL-4, IL-13, IFN-γ, or IL-12 (data not shown). Combinations of SCF with IL-3 (100 ± 28%), IL-5 (74 ± 18%), IL-6 (43 ± 8%), GM-CSF (54 ± 26%), and the developmental combination of IL-6 and IL-10 (58 ± 10%) significantly augmented proliferation relative to SCF alone (P < 0.05, means ± SEM; n = 3 for all conditions; Fig. 4 A, center). Small comitogenic effects were seen for IL-4 (31 ± 15%; n = 2; not shown), and IL-9 (22 ± 10%), whereas IL-2, IL-10, M-CSF, IL-12, G-CSF, and IL-13 (not shown) had no costimulatory activity. In contrast, IFN-γ suppressed the SCF-driven proliferation of hPrMCs in a dose-dependent manner (Fig. 4 A, right), with 10 ng/ml IFN-γ suppressing SCF-driven proliferation by 52 ± 6% (n = 2). At week 9, the cells incorporated thymidine only in response to SCF among the cytokines tested alone (Fig. 4 B, left). The costimulatory effects of IL-3 (104 ± 28%), IL-5 (131 ± 22%), IL-6 (71 ± 23%), and GM-CSF (37 ± 4%) (Fig. 4 B, center; P < 0.05 for each; n = 3) were retained by these mature hMCs. IL-9 again had a small comitogenic effect (21 ± 5%; mean ± SEM range; n = 3). No comitogenic effect was observed for IL-4 (n = 3; data not shown). The suppressive effect of IFN-γ (71 ± 1% at 10 ng/ml) was more pronounced for hMCs (Fig. 4 B, right) than for hPrMC (Fig. 4 A, right). None of the comitogenic or suppressive cytokines elicited a change in the 9-wk-old cell populations in light scatter or surface epitope distribution, except for IL-3 and IL-5, both of which caused an induction of IL-3Rα (Fig. 5), and IL-4, which diminished the signal for c-kit (not shown). The absolute quantity of SCF-driven thymidine incorporated was consistently 50–75% lower in the 9-wk-old cells than in the 4-wk-old cells.
Figure 4.
Incorporation of [3H]thymidine by 4-wk-old hPrMCs (A) and 9-wk-old hMCs (B) that were cultured for six subsequent days in the presence of the indicated concentrations of cytokines (ng/ml). Results for individual cytokines (left) are expressed as the means ± SEM of triplicates and are representative of experiments performed with the cells of three different donors. The results for comitogenic responses (center) are expressed as the percentage increases above the responses to SCF alone and are the means ± SEM for three independent experiments. IFN-γ–mediated suppression of SCF-driven proliferation of hPrMCs (A, right; means ± half range, n = 2) and hMCs (B, right; means ± SEM, n = 3) is expressed as the percentage of thymidine incorporation occurring in response to 100 ng/ml of SCF alone.
Figure 5.
Reinduction of IL-3Rα on fully mature hMCs at week 9 of culture. hMCs were transferred to fresh medium containing SCF (100 ng/ml) alone (top row), SCF plus IL-3 (5 ng/ml; center row), or SCF plus IL-5 (5 ng/ml; bottom row). Cytofluorographic analysis was performed 1 wk later for the expression of c-kit (left column), IL-3Rα (center column), and IL-5Rα (right column). The results are representative of the two experiments performed.
Expression of Chemokine Receptors by hPrMCs and hMCs.
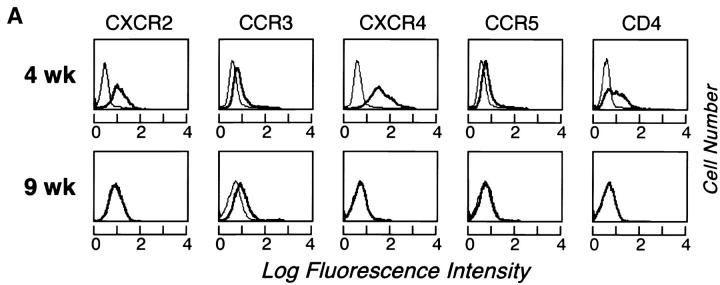
At week 4, CXCR2, CCR3, CXCR4, and CCR5 (n = 3 each) were expressed in monophasic distributions, with highest relative expression of CXCR4 (as shown for a representative experiment; Fig. 6 A, top). CD4, which acts in concert with CCR3, CXCR4, and CCR5 to facilitate HIV entry into T cells and monocytes, was also expressed at week 4 (Fig. 6 A, top). At week 9, CCR3 was present in a monophasic distribution (Fig. 6 A, bottom), but CD4, CXCR2, CXCR4, and CCR5 had reverted to negative (Fig. 6 A, bottom). CXCR1, CXCR3, CCR1, CCR2, CCR4, CCR5, and CCR6 were not expressed by hPrMCs or hMCs in any experiment (n = 2 each; data not shown). RT-PCR revealed that both CCR3 and CXCR4 mRNA were present at weeks 3 and 4 and progressively diminished at weeks 6 and 9 (Fig. 6 B). Signals for the internal standard G3PDH were comparable from lane to lane.
Figure 6.
(A) Cytofluorographic expression of CXCR2, CCR3, CXCR4, CCR5, and CD4 by hPrMCs at week 4 (top row) and by hMCs at week 9 (bottom row). Expression of each surface epitope is represented by the bold tracings, and isotype-matched negative controls are represented by the lighter tracings. Results are representative of three experiments with different donor cells (n = 2 for CXCR2). (B) CCR3 (left) and CXCR4 (right) mRNA expression determined by RT-PCR analysis at 3, 4, 6, and 9 wk of culture compared with the internal standard, G3PDH.
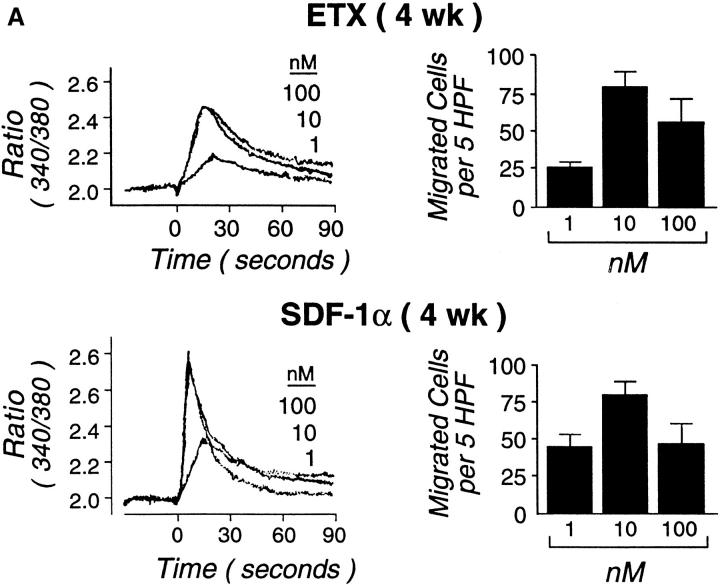
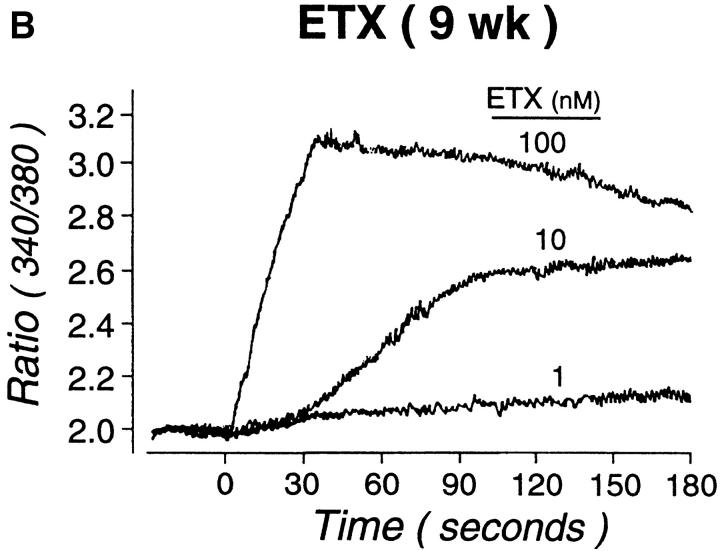
Neither SDF-1α nor ETX (1–100 nM each) were mitogenic, either alone or in combination with SCF at weeks 4 or 9 (not shown). Both ETX and SDF-1α (1–100 nM) elicited the rapid intracellular flux of calcium in Fura-2–loaded hPrMCs studied at week 4. MIP-1α and IL-8 also elicited dose-dependent calcium flux at a 1–100 nM concentration range (data not shown). SDF-1α consistently elicited a higher and sharper peak of calcium flux than did ETX (Fig. 7 A), MIP-1α, or IL-8 (data not shown). When stimulation was performed sequentially with maximally effective doses of ETX and SDF-1α (100 nM of each), there was neither potentiation nor inhibition of calcium flux elicited by the second agonist (not shown). In preliminary experiments, checkerboard analyses revealed that both ETX and SDF-1α elicited directional migration and not chemokinesis. In subsequent transwell migration assays, ETX and SDF-1α elicited similar dose responses from hPrMCs and inhibition at high doses, with the net migration of 26 ± 4, 79 ± 10, and 55 ± 16 hPrMCs per high-power field in response to 1, 10, and 100 nM ETX, respectively, and 45 ± 8, 80 ± 9, and 47 ± 14 hPrMCs per high-power field in response to 1, 10, and 100 nM SDF-1α, respectively (mean ± SEM; n = 3 for each agonist; Fig. 7 A). Neither ETX nor SDF-1α elicited chemotaxis of 9-wk-old hMCs at concentrations as high as 100 nM. However, ETX provoked a marked and sustained calcium flux by 9-wk-old hMC (Fig. 7 B). The ETX-induced calcium flux was blocked by prior application of the mAb 7B11 (not shown), indicating that it was mediated entirely through CCR3. ETX did not elicit histamine release from either hPrMCs or hMCs (not shown).
Figure 7.
Functionality of CXCR4 and CCR3. Both ETX (A, top) and SDF-1α (A, bottom) caused transient dose-dependent calcium flux of Fura-loaded hPrMCs (left panels) and promoted their directed migration (right panels). At week 9, ETX elicited marked, sustained calcium flux (B) without chemotaxis (not shown). The calcium flux assays depicted are representative of three independent analyses at both weeks 4 and 9. The chemotaxis assays were analyzed by counting the numbers of migrating cells per five high power fields (HPF) and are the means ± SEM for three experiments.
Discussion
Among the major effector cells of allergic inflammation, MCs are unique for their homing to tissues as committed progenitors and development into mature cells in situ. Because the circulating levels of PrMCs are small and their direct detection is difficult, little is understood regarding their homing mechanisms and the regulation of their subsequent T cell–dependent reactive hyperplasia at sites of allergic mucosal inflammation. We reasoned that these monocyte-like hPrMCs could be developed in vitro using an SCF-dependent culture system, with IL-6 as a comitogenic cytokine as previously reported for hMCs 36 and with IL-10 added to suppress monocyte development 43. This approach, analogous to our prior observations in the mouse 38, permitted the first cytofluorographic characterization of membrane phenotype changes during the SCF-dependent maturation of hPrMCs into hMCs, including their expression of chemokine receptors, and the study of their comitogenic responses to Th2 cytokines.
As indicated by flow cytometry, the cultured cord blood–derived cells were homogeneous in cell size and granularity by week 4, with monophasic low expression of c-kit and Fc∈RIα and monophasic high expression of CD13 and IL-3Rα but no expression of the β3 integrin associated with monocytes and macrophages 53 54 and dispersed mature tissue hMCs 52 (Fig. 2). Neither the monocyte marker CD14 nor the CD16 epitope expressed by neutrophils and NK cells was detected on the 4-wk-old cells, possibly reflecting IL-10–mediated suppression of granulocyte/monocyte growth 43. The inclusion of IL-10 may also account for the immunoreactivity for chymase observed in 85% of cells at week 4 (Fig. 1 C), when <50% were tryptase-positive or metachromatic (Fig. 1B and Fig. C), as mouse IL-10 induces the expression of two MC chymases in bone marrow–derived mouse MCs 61 62. Progressive increases in the proportion of mature hMCs occurred with continued culture, as indicated by increases in metachromasia, tryptase positivity, and chymase positivity at week 6 (77, 92, and 100%, respectively) and week 9 (virtually 100% for all three markers), concomitantly with a decline in cell numbers (Fig. 1 A). The uniformly metachromatic, tryptase- and chymase-positive hMCs comprised a single cell population of high granularity, as indicated by cytofluorographic SSC at week 9 (Fig. 2), and expressed high levels of c-kit, CD13, and β3 integrin and low levels of Fc∈RIα but no detectable IL-3Rα. The presence of the β3 integrin and the lack of IL-3Rα are each consistent with the surface phenotype reported for hMCs dispersed from skin, uterus, and lung 52 55.
The fact that the 6-wk-old cell population clearly segregated into distinct subpopulations (Fig. 2, third and fourth rows) of low granularity (resembling the 4-wk-old cells in surface phenotype) and high granularity (resembling the 9-wk-old cells), respectively, provided the opportunity to demonstrate that the low granularity group contained progenitors of the high granularity group by sorting and subsequent culture. Although the sorted low granularity group at week 6 had lower mean SSC (Fig. 3 A), fewer metachromatic cells (Fig. 3 B), and lower proportions of tryptase-immunoreactive cells (Fig. 3 C) than the 4-wk-old cells (Fig. 1) of the same membrane phenotype, they gave rise to high granularity cells after 2 wk of continued culture with the same staining and cytofluorographic characteristics as the 9-wk-old hMCs (Fig. 3 J). As it is possible that the low granularity population, comprising the majority of cells at week 4 and a subfraction at week 6, contained both hPrMCs and another lineage, we used a mitogenic analysis to establish concordance of the cytokine-mediated responses of hPrMCs at week 4 and hMCs at week 9.
As determined by incorporation of [3H]thymidine, the 9-wk-old hMCs exhibited mitogenic responses only to SCF among the cytokines tested alone (Fig. 4 B, left) that was approximately half of the response observed for the 4-wk-old hPrMCs. Although the response of the week 4 population to IL-3 or GM-CSF alone (27 and 51% of the SCF response, respectively) could reflect the presence of some early granulocyte/monocyte or pluripotent progenitor cells possibly representing the small chymase-negative fraction (Fig. 1 C), the absence of a mitogenic response to IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, M-CSF, G-CSF, or IFN-γ alone argues against substantial contamination with hematopoietic lineages other than MCs. The synergy observed between SCF and IL-3 is also recognized for the proliferation and development of MCs from human peripheral blood CD34-positive cells 12 and for proliferation of mouse fetal blood promastocytes 11 and mouse metamastocytes developed in vitro 38 and is therefore consistent with a response from committed hPrMC. The costimulatory effect of IL-3 was still observed on the homogeneously mature 9-wk-old hMCs, even though IL-3Rα was not detected by cytofluorographic analysis (Fig. 2). However, IL-3Rα was readily detected after culture of the hMC for 7 d with SCF plus IL-3 or SCF plus IL-5 (Fig. 5). This is the first cytofluorographic demonstration of IL-3Rα on hMCs and its upregulation in response to two important Th2 cytokines. The comitogenic effect of IL-6 36 38 for SCF-driven MC development has been previously recognized in both humans and mice, and the comitogenic action of IL-9 was appreciated in a transgenic mouse model 63 but has not previously been noted for hMCs. Comitogenic responses to IL-5 were not previously reported for hMCs but are apparently mediated through low-level surface expression of a functional IL-5Rα (Fig. 5). This observation is consistent with the recognition by other investigators that mature hMCs derived in the presence of SCF and IL-6 express mRNA for IL-5Rα that encodes a functional protein, as indicated by IL-5–mediated cytoprotection of hMCs 37.
A critical point in this study is that the same cytokines elicit comitogenic responses with SCF for hPrMCs at week 4 (Fig. 4 A) and for hMCs at week 9 (Fig. 4 B), compatible with the retention or reinduction of the required receptors during MC development and consistent with a role for each of these cytokines in the amplification of hMC responses in allergic inflammation. At the same time, the inhibition of SCF-driven proliferation of hPrMCs (Fig. 4 A, right) and hMCs (Fig. 4 B, right) by IFN-γ indicates that this cytokine directly counteracts MC proliferation, simultaneously with its polarizing effects on T cells toward a Th1 profile of cytokine production. Finally, the lack of responses to IL-13 and IL-12 reflects selectivity among the cytokines within the polarized T cell armamentarium for their actions on MCs.
Unlike IL-3, IL-5, IL-6, and GM-CSF, IL-4 had relatively small comitogenic effects that were observed only for hPrMCs. Previous studies have yielded conflicting data on the role of IL-4 as an MC mitogen. IL-4 suppressed SCF-driven hMC development in vitro from fetal liver cells 64 and PBMCs 65 but was comitogenic with SCF for MC colony formation from mouse committed progenitors 66 and synergistically induced [3H]thymidine incorporation of cord blood–derived hMCs when combined with both SCF and IL-6 67. The fact that IL-4 potently primes mature hMCs for Fc∈RI expression 67, IgE-dependent histamine release 67, and IL-13 production 68 suggests that this cytokine is a key factor in regulating MC maturation and function in allergic disease, irrespective of its mitogenic actions, which vary depending on progenitor cell source and maturational stage.
Both connective tissue and T cell–dependent mucosal MC subpopulations arise from a single SCF-dependent lineage as revealed in mice 11 69. The cytofluorographic identification of chemokine receptors during development has not been addressed for MCs in the mouse due to the lack of reagents. Importantly, those chemokine receptors noted here could have implications for hPrMC development and distribution. CXCR4, CCR3, and CD4 were expressed in monophasic distributions at week 4 (Fig. 6 A, top), as were CXCR2 and CCR5, whereas CXCR1, CXCR3, CCR1, CCR2, CCR4, CCR5, and CCR6 were absent. At week 9, only CCR3 was still expressed from among the chemokine receptors, and CD4 was absent (Fig. 6 A, bottom). Although steady-state expression of chemokine receptor mRNA was below the limits of detection by Northern analysis, RT-PCR confirmed the maturation-related reductions in CCR3 and CXCR4 mRNA (Fig. 6 B), indicating the lack of a quantitative relationship between steady-state mRNA levels and the levels of the corresponding surface proteins. The transition from hPrMC to mature hMC is therefore accompanied by a change in chemokine receptor profile, with initial expression of CXCR2, CCR3, CXCR4, and CCR5, as well as CD4, but retention of CCR3 only from among these immunodetectable proteins. The CXCR2/CCR3/CXCR4/CCR5-positive profile of chemokine receptor expression for hPrMCs is unique among hematopoietic cells 58 and may explain the distribution of MCs under basal conditions as well as their recruitment to diverse sites of inflammation. The retention of only CCR3 by hMCs may reflect the importance of CCR3 and its ligands (ETX, MCP 2-4, RANTES) in allergic inflammation. Furthermore, the expression of all three HIV coreceptors 56 57 and CD4 by hPrMCs suggests that these cells could carry HIV into tissues.
Both ETX and SDF-1α induced rapid, transient concentration–dependent calcium fluxes and chemotactic responses of 4-wk-old hPrMCs (Fig. 7), as did MIP-1α and IL-8 (not shown). SDF-1α consistently induced a sharper and higher increase in intracellular calcium than did ETX in 4-wk-old hPrMCs (Fig. 7 A, bottom and top, respectively), but the two chemokines elicited nearly equal migration, with superimposable dose–response curves at 1–100 nM (Fig. 7 A). Although ETX did not elicit chemotaxis of 9-wk-old hMCs, it did cause a marked, sustained dose-dependent calcium flux (Fig. 7 B). Whereas chemokines typically elicit transient calcium fluxes that are required for chemotactic responses, sustained calcium fluxes are associated with cell differentiation and translocation of NF-AT (nuclear factor of activated T lymphocytes) transcription factors 70. The observations imply that ETX may have functions for stationary hMCs within tissues that are distinct from its actions on blood-borne hPrMCs.
The findings of this study, analogous to eosinophil trafficking in allergic inflammation, favor a model of cognate functions for chemokines and Th2 cytokines in regulating the levels of tissue MCs in allergic diseases. The transit of hPrMCs from the circulation to various sites within the tissues may be regulated by their expression of CXCR2, CCR3, CXCR4, and CCR5 and the local availability of the corresponding respective ligands. SDF-1α, for example, is constitutively expressed by stromal cells in various tissues (which are also a source of the required MC growth factor SCF), and participates via CXCR4 in basal trafficking of naive T cells 40 71. Lung expression of the CCR5 ligand MIP-1α is rapidly upregulated in response to inhaled allergen challenge and is linked to the mobilization of leukocytes to the bronchial tissue in the first few hours after challenge 72. Furthermore, MIP-1α and MIP-1β are implicated in the recruitment of MCs to regional lymph nodes in response to experimentally elicited cutaneous contact hypersensitivity in mice 73. The expression of CXCR2, which was previously localized to the granules of skin hMCs and to the surface of the primitive MC line, HMC-1 59, may permit IL-8–induced recruitment of hPrMCs; indeed, elevated levels of IL-8 are reported in both the sera and lung tissue of asthmatics and correlate with disease severity 74. The expression of CCR3 throughout hMC development, accompanied by the losses of CXCR2, CXCR4, and CCR5, is compatible with the proposed critical role for ETX in allergic inflammation. ETX, expressed by epithelial cells 29, may provide an important stimulus for hPrMC movement toward mucosal surfaces. Furthermore, the loss of chemokine receptor expression or ligand-initiated migration may be a mechanism for tissue retention of MCs. IL-3, IL-5, and GM-CSF are locally available within and near the epithelial surface in asthma patients 75, and each may augment local SCF-dependent proliferation of hPrMCs and mature hMCs in addition to their cytoprotective functions. The IL-9 gene is implicated as a candidate gene for asthma in humans 22 and for bronchial hyperresponsiveness in mice 76 based on linkage studies and causes a marked hyperplasia of intraepithelial MCs accompanied by methacholine hyperresponsiveness when overexpressed in the bronchial epithelia of mice 77. Thus, Th2 cytokine–driven cytoprotection, proliferation, and migration of hPrMCs and hMCs may account for mucosal MC hyperplasia; and the shared responses of MCs, eosinophils, and basophils to Th2-derived cytokines are part of the integrated profile of allergic/asthmatic inflammation in which this mucosal MC hyperplasia is observed. Conversely, the lack of intraepithelial intestinal MCs in humans with T cell immunodeficiencies 16 and the lack of mucosal MC hyperplastic responses in T cell–deficient mice 15 likely reflect deficiencies in several local cytokines and perhaps an additional lack of T cell–dependent ETX production by local epithelial cells 31.
Acknowledgments
This work was supported by National Institutes of Health grants AI-01304-02, AI-31599-06, AI-22531, and HL-36110 and by grants from the Hyde and Watson Foundation and the Immunology Research Institute of New England. Dr. Boyce is the recipient of a Basic Investigator Award from Glaxo-Wellcome.
Footnotes
1used in this paper: ETX, eotaxin; MCs, mast cells; MCP, monocyte chemotactic proteins; MIP, macrophage inflammatory protein; Pr, progenitor; RANTES, regulated on activation, normal T cell expressed and secreted; RT, reverse transcriptase; SCF, stem cell factor; SDF, stromal cell–derived factor; SSC, side angle light scatter
References
- Donaldson L.E., Schmitt E., Huntley J.F., Newlands G.F., Grencis R.K. A critical role for stem cell factor and c-kit in host protective immunity to an intestinal hel–minth. Int. Immunol. 1996;8:559–567. doi: 10.1093/intimm/8.4.559. [DOI] [PubMed] [Google Scholar]
- Echtenacher B., Mannel D.N., Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–79. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- O'Sullivan S., Dahlen B., Dahlen S.E., Kumlin M. Increased urinary excretion of the prostaglandin D2 metabolite 9α, 11 β-prostaglandin F2 after aspirin challenge supports mast cell activation in aspirin-induced airway obstruction. J. Allergy Clin. Immunol. 1996;98:421–432. doi: 10.1016/s0091-6749(96)70167-7. [DOI] [PubMed] [Google Scholar]
- Casale T.B., Wood D., Richerson H.B., Zehr B., Zavala D., Hunninghake G.W. Direct evidence of a role for mast cells in the pathogenesis of antigen-induced bronchoconstriction. J. Clin. Invest. 1987;80:1507–1511. doi: 10.1172/JCI113234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells M., Schwartz L.B. Tryptase levels in nasal lavage fluid as an indicator of the immediate allergic response. J. Allergy Clin. Immunol. 1988;82:348–355. doi: 10.1016/0091-6749(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Robuschi M., Gambaro G., Sestini P., Pieroni M.G., Refini R.M., Vaghi A., Bianco S. Attenuation of aspirin-induced bronchoconstriction by sodium cromoglycate and nedocromyl sodium. Am. J. Respir. Crit. Care Med. 1997;155:1461–1464. doi: 10.1164/ajrccm.155.4.9105094. [DOI] [PubMed] [Google Scholar]
- Cockroft D.W., Murdock K.Y. Comparative effects of inhaled salbutamol, sodium cromoglycate, and beclomethasone dipropionate on allergen-induced early asthmatic responses, late asthmatic responses, and increased bronchial responsiveness to histamine. J. Allergy Clin. Immunol. 1987;79:734–740. doi: 10.1016/0091-6749(87)90204-1. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- Kirshenbaum A.S., Kessler S.W., Goff J.P., Metcalfe D.D. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J. Immunol. 1991;146:1410–1415. [PubMed] [Google Scholar]
- Castells M.C., Friend D.S., Bunnell C.A., Hu X., Kraus M., Osteen R.T., Austen K.F. The presence of membrane-bound stem cell factor on highly immature nonmetachromatic mast cells in the peripheral blood of a patient with aggressive systemic mastocytosis. J. Allergy Clin. Immunol. 1996;98:831–840. doi: 10.1016/s0091-6749(96)70133-1. [DOI] [PubMed] [Google Scholar]
- Rodewald H.R., Dessing M., Dvorak A.M., Galli S.J. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- Rottem M., Okada T., Goff J.P., Metcalfe D.D. Mast cells cultured from the peripheral blood of normal donors and patients with mastocytosis originate from a CD34+/Fc∈R1− cell population. Blood. 1994;84:2489–2496. [PubMed] [Google Scholar]
- Agis H., Willheim M., Sperr W.R., Wilfing A., Kromer E., Kabrna E., Spanblochl E., Strobl H., Geissler K., Spittler A. Monocytes do not make mast cells when cultured in the presence of SCF. Characterization of the circulating mast cell progenitor as a c-kit +, CD34+, Ly−, CD14−, CD17−, colony-forming cell. J. Immunol. 1993;151:4221–4227. [PubMed] [Google Scholar]
- Lantz C.S., Boesiger J., Song C.H., Mach N., Kobayashi T., Mulligan R.C., Nawa Y., Dranoff G., Galli S.J. Role for interleukin-3 in mast cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- Ruitenberg E.J., Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976;264:258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- Irani A.A., Craig S.S., DeBlois G., Elson C.O., Schechter N.M., Schwartz L.B. Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J. Immunol. 1987;138:4381–4386. [PubMed] [Google Scholar]
- Gibson P.G., Allen C.J., Yang J.P., Wong J.O., Dolovich J., Denburg J., Hargreave F.E. Intraepithelial mast cells in allergic and nonallergic asthma. Assessment using bronchial brushings. Am. Rev. Respir. Dis. 1993;148:80–86. doi: 10.1164/ajrccm/148.1.80. [DOI] [PubMed] [Google Scholar]
- Pesci A., Foresi A., Bertorelli G., Chetta A., Oliveri D. Histochemical characteristics and degranulation of mast cells in epithelium and lamina propria in bronchial biopsies from asthmatic and normal subjects. Am. Rev. Respir. Dis. 1993;147:684–689. doi: 10.1164/ajrccm/147.3.684. [DOI] [PubMed] [Google Scholar]
- Laitinen L.A., Laitinen A., Haahtela T. Airway mucosal inflammation even in patients with newly diagnosed asthma. Am. Rev. Respir. Dis. 1993;147:697–704. doi: 10.1164/ajrccm/147.3.697. [DOI] [PubMed] [Google Scholar]
- Finkelman F.D., Shea-Donohue T., Goldhill J., Sullivan C.A., Morris S.C., Madden K.B., Gause W.C., Urban J.F., Jr. Cytokine regulation of host defense against parasitic gastrointestinal nematodes. Annu. Rev. Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- Marsh D.G., Neely J.D., Breazeale D.R., Gosh B., Friedhoff L.R., Erlich-Kautzky E., Shou C., Krishnaswamy G., Beaty T.H. Linkage analysis of IL-4 and other chromosome 5q31.1 markers and total serum immunoglobulin E concentrations. Science. 1994;264:1152–1156. doi: 10.1126/science.8178175. [DOI] [PubMed] [Google Scholar]
- Noguchi E., Shibasaki M., Arinami T., Takeda K., Maki T., Miyamoto T., Kawashima T., Kobayashi K., Hamaguchi H. Evidence for linkage between asthma/atopy in childhood and chromosome 5q31-q33 in a Japanese population. Am. J. Respir. Crit. Care Med. 1997;156:1390–1393. doi: 10.1164/ajrccm.156.5.9702084. [DOI] [PubMed] [Google Scholar]
- Clutterbuck E.J., Sanderson C.J. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow culturescomparison and interaction with IL-1, IL-3, IL-6, and GM-CSF. Blood. 1989;73:1504–1512. [PubMed] [Google Scholar]
- Simon H.U., Yousefi S., Schranz C., Schapowal A., Bachert C., Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J. Immunol. 1997;139:3902–3908. [PubMed] [Google Scholar]
- Owen W.F., Jr., Rothenberg M.E., Silberstein D.S., Gasson J.C., Stevens R.L., Austen K.F., Soberman R.J. Regulation of human eosinophil viability, density, and function by granulocyte/macrophage colony-stimulating factor in the presence of 3T3 fibroblasts. J. Exp. Med. 1987;166:129–141. doi: 10.1084/jem.166.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P.D., Maleau S., Griffiths-Johnson D.A., Jose P.J., Williams T.J. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J. Exp. Med. 1995;182:1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould A.W., Matthaei K.I., Young I.G., Foster P.S. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J. Clin. Invest. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Ponath P.D., Qin S., Ringler D.J., Clark-Lewis I., Wang J., Kassam N., Smith H., Shi X., Gonzalo J.A., Newman W. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding and functional properties suggest a mechanism for the selective recruitment of eosinophils. J. Clin. Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkhioued B., Renzi P.M., Abi-Younes S., Garcia-Zepada E.A., Allakhverdi Z., Ghaffar O., Rothenberg M.E., Luster A.D., Hamid Q. Increased expression of eotaxin in bronchoalveolar lavage and airways of asthmatics contributes to the chemotaxis of eosinophils to the site of inflammation. J. Immunol. 1997;159:4593–4601. [PubMed] [Google Scholar]
- MacLean J.A., Ownbey R., Luster A.D. T cell–dependent regulation of eotaxin in antigen-induced pulmonary eosinophilia. J. Exp. Med. 1996;184:1461–1469. doi: 10.1084/jem.184.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponath P.D., Qin S., Post T.W., Wang J., Wu L., Gerard N.P., Newman W., Gerard C., Mackay C.R. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J. Exp. Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uguccioni M., Mackay C.R., Ochensberger B., Loetscher P., Rhis S., LaRosa G.J., Rao P., Ponath P.D., Baggiolini M., Dahinden C.A. High expression of the chemokine receptor CCR3 in human blood basophils Role in activation by eotaxin, MCP- 4,and other chemokines. J. Clin. Invest. 1001997. 1137 1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Mackay C.R., Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- Irani A.A., Nillson G., Miettinen U., Craig S.S., Ashman L.K., Ishizaka T., Zsebo K.M., Schwartz L.M. Recombinant human stem cell factor stimulates differentiation of mast cells from dispersed human fetal liver cells. Blood. 1992;80:3009–3021. [PubMed] [Google Scholar]
- Saito H., Ebisawa M., Tachimoto H., Shichijo M., Fukagawa K., Matsumoto K., Iikura Y., Awaji T., Tsujimoto G., Yanagida M. Selective growth of human mast cells induced by steel factor, interleukin 6 and prostaglandin E2 from cord blood mononuclear cells. J. Immunol. 1996;157:343–350. [PubMed] [Google Scholar]
- Yanagida M., Fukamachi H., Ohgami K., Kuwaki T., Ishii H., Uzumaki H., Amano K., Tokiwa T., Mitsui H., Saito H. Effects of T-helper 2-type cytokines, interleukin 3 (IL-3), IL-4, IL-5, and IL-6 on the survival of cultured human mast cells. Blood. 1995;86:3705–3714. [PubMed] [Google Scholar]
- Yuan Q., Gurish M.F., Friend D.S., Austen K.F., Boyce J.A. Identification of a novel stem cell factor-dependent mast cell progenitor. J. Immunol. 1998;161:5143–5146. [PubMed] [Google Scholar]
- Loetscher M., Geiser T., O'Reilly T., Zwahlen R., Baggiolini M., Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J. Biol. Chem. 1994;269:232–237. [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A., Mackay C.R. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol. Today. 1998;19:568–577. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- Thompson-Snipes L., Dhar V., Bond M.W., Mosmann T.R., Moore K.W., Rennick D.M. Interleukin 10a novel stimulatory factor for mast cells and their progenitors. J. Exp. Med. 1991;173:507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Austen K.F., Bingham C.O., III, Friend D.S., Penrose J.F., Arm J.P. Interleukin-3 regulates the development of the 5-lipoxygenase/leukotriene C4 synthase pathway in mouse mast cells. J. Biol. Chem. 1995;270:22653–22656. doi: 10.1074/jbc.270.39.22653. [DOI] [PubMed] [Google Scholar]
- Oehler L., Foedinger M., Koeller M., Kollars M., Reiter E., Bohle B., Skoupy S., Fritsch G., Lechner K., Geissler K. Interleukin-10 inhibits spontaneous colony-forming unit-granulocyte-macrophage growth from human peripheral blood mononuclear cells by suppression of endogenous granulocyte-macrophage colony-stimulating factor release. Blood. 1997;89:1147–1155. [PubMed] [Google Scholar]
- Schwartz L.B. Monoclonal antibodies against human mast cell tryptase demonstrate shared antigenic sites on subunits of tryptase and selective localization of the enzyme to mast cells. J. Immunol. 1985;134:526–531. [PubMed] [Google Scholar]
- Friend D.S., Ghildyal N., Austen K.F., Gurish M.F., Matsumoto R., Stevens R.L. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J. Cell Biol. 1996;135:279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q., Austen K.F., Friend D.S., Heidtman M., Boyce J.A. Human peripheral blood eosinophils express a functional c-kit receptor for stem cell factor that stimulates very late antigen 4 (VLA-4)-mediated cell adhesion to fibronectin and vascular cell adhesion molecule 1 (VCAM-1) J. Exp. Med. 1997;186:313–323. doi: 10.1084/jem.186.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbo M., Horowitz E.M., Botana L.M., MacGlashan D.W., Jr., Bochner B.S., Gillis S., Zsebo K.M., Galli S.J., Lichtenstein L.M. The human recombinant c-kit receptor ligand, SCF, induces mediator release from human cutaneous mast cells and enhances IgE-dependent mediator release from both skin mast cells and peripheral blood basophils. J. Immunol. 1992;149:599–608. [PubMed] [Google Scholar]
- Briddell R.A., Broudy V.C., Bruno E., Brandt J.E., Srour E.F., Hoffman R. Further phenotypic characterization and isolation of human hematopoietic progenitor cells using a monoclonal antibody to the c-kit receptor. Blood. 1992;79:3159–3167. [PubMed] [Google Scholar]
- Riske F., Hakimi J., Mallamaci M., Griffin M., Pilson B., Tobkes N., Lin P., Danho W., Kochan J., Chizzonite R. High affinity human IgE receptor (Fc∈R1). Analysis of functional domains of the α-subunit with monoclonal antibodies. J. Biol. Chem. 1991;266:11245–11251. [PubMed] [Google Scholar]
- Kinzer C.A., Keegan A.D., Paul W.E. Identification of Fc∈R1-negative mast cells in mouse bone marrow cell cultures Use of a monoclonal anti-p 161antibody. J. Exp. Med. 1821995. 575 579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Kinzer C.A., Paul W.E. p161, a murine membrane protein expressed on mast cells and some macrophages, is mouse CD13/aminopeptidase N. J. Immunol. 1996;157:2593–2600. [PubMed] [Google Scholar]
- Sperr W.R., Agis H., Czerwenka K., Klepetko W., Kubista E., Boltz-Nitulescu G., Lechner K., Valent P. Differential expression of cell surface integrins on human mast cells and human basophils. Ann. Hematol. 1992;65:10–16. doi: 10.1007/BF01715119. [DOI] [PubMed] [Google Scholar]
- Weerasinghe D., McHugh K.P., Ross F.P., Brown E.J., Gisler R.H., Imhof B.A. A role for the αvβ3 integrin in the transmigration of monocytes. J. Cell Biol. 1998;142:595–607. doi: 10.1083/jcb.142.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M., Savill J., Haslett C. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis Mediation by αvβ 3CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am. J. Pathol. 1491996. 911 921 [PMC free article] [PubMed] [Google Scholar]
- Valent P., Besemer J., Sillaber C., Butterfield J.H., Eher R., Majdic O., Kishi K., Klepetko W., Eckersberger F., Lechner K. Failure to detect IL-3-binding sites on human mast cells. J. Immunol. 1990;145:3432–3437. [PubMed] [Google Scholar]
- Sanchez X., Cousins-Hodges B., Aguilar T., Gosselink P., Lu Z., Navarro J. Activation of HIV-1 coreceptor (CXCR4) mediates myelosuppression. J. Biol. Chem. 1997;272:27529–27531. doi: 10.1074/jbc.272.44.27529. [DOI] [PubMed] [Google Scholar]
- Choe H., Farzan M., Sun Y., Sullivan N., Rollins B., Ponath P.D., Wu L., Mackay C.R., LaRosa G., Newman W. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- Luster A.D. Chemokines-chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Lippert U., Artuc M., Grutzkau A., Moller A., Kenderessy-Szabo A., Schadendorf D., Norgauer J., Hartmann K., Schweitzer-Stenner R., Zuberbier T. Expression and functional activity of the IL-8 receptor type CXCR1 and CXCR2 on human mast cells. J. Immunol. 1998;161:2600–2608. [PubMed] [Google Scholar]
- Boyce J.A., Lam B.K., Penrose J.F., Friend D.S., Parsons S., Owen W.F., Austen K.F. Expression of LTC4 synthase during the development of eosinophils from cord blood progenitors. Blood. 1996;88:4338–4346. [PubMed] [Google Scholar]
- Ghildyal N., Friend D.S., Nicodemus C.F., Austen K.F., Stevens R.L. Reversible expression of mouse mast cell protease 2 mRNA and protein in cultured mast cells exposed to IL-10. J. Immunol. 1993;151:3206–3214. [PubMed] [Google Scholar]
- Ghildyal N., McNeil H.P., Stechschulte S., Austen K.F., Silberstein D., Gurish M.F., Somerville L.L., Stevens R.L. IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J. Immunol. 1992;149:2123–2129. [PubMed] [Google Scholar]
- Godfraind C., Louahed J., Faulkner H., Vink A., Warnier G., Grencis R., Renauld J.C. Intraepithelial infiltration by mast cells with both connective tissue-type and mucosal-type characteristics in gut, trachea, and kidneys of IL-9 transgenic mice. J. Immunol. 1998;160:3989–3996. [PubMed] [Google Scholar]
- Xia H.Z., Du Z., Craig S., Klisch G., Noben-Trauth N., Kochan J.P., Huff T.H., Irani A.M., Schwartz L.B. Effect of recombinant human IL-4 on tryptase, chymase, and Fc epsilon receptor type I expression in recombinant human stem cell factor-dependent fetal liver-derived human mast cells. J. Immunol. 1997;159:2911–2921. [PubMed] [Google Scholar]
- Sillaber C., Sperr W.R., Agis H., Spanblochl E., Lechner K., Valent P. Inhibition of stem cell factor-dependent formation of human mast cells by interleukin 3 and interleukin 4. Int. Arch. Allergy Immunol. 1994;105:264–268. doi: 10.1159/000236767. [DOI] [PubMed] [Google Scholar]
- Rennick D., Hunte B., Holland G., Thompson-Snipes L. Cofactors are essential for stem cell factor-dependent growth and maturation of mast cell progenitorscomparative effects of interleukin-3 (IL-3), IL-4, IL-10, and fibroblasts. Blood. 1995;85:57–65. [PubMed] [Google Scholar]
- Toru H., Ra C., Nonoyama S., Suzuki K., Yata J., Nakahata T. Induction of the high-affinity IgE receptor (Fc epsilon RI) on human mast cells by IL-4. Int. Immunol. 1996;8:1367–1373. doi: 10.1093/intimm/8.9.1367. [DOI] [PubMed] [Google Scholar]
- Toru H., Pawankar R., Ra C., Yata J., Nakahata T. Human mast cells produce IL-13 by high-affinity IgE receptor cross-linkingenhanced IL-13 production by IL-4-primed human mast cells. J. Allergy Clin. Immunol. 1998;102:491–502. doi: 10.1016/s0091-6749(98)70140-x. [DOI] [PubMed] [Google Scholar]
- Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S.J. Fate of bone marrow–derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell–deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J. Exp. Med. 1985;162:1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman L.A., Clipstone N.A., Ho S.N., Northrop J.P., Crabtree G.R. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–840. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- Bleul C.C., Fuhlbrigge R.C., Casasnovas J.M., Aiuti A., Springer T.A. A highly efficacious lymphocyte chemoattractant, stromal cell–derived factor 1 (SDF-1) J. Exp. Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E.M., Kunkel S.L., Strieter R.M., Lukacs L.W. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J. Immunol. 1998;161:7047–7053. [PubMed] [Google Scholar]
- Wang H.-W., Tedia N., Lloyd A.R., Wakefield D., McNeil H.P. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J. Clin. Invest. 1998;102:1617–1626. doi: 10.1172/JCI3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shute J.K., Vrugt B., Lindley I.J., Holgate S.T., Bron A., Aalbers R., Djukanovic R. Free and complexed interleukin-8 in blood and bronchial mucosa in asthma. Am. J. Respir. Crit. Care Med. 1997;155:1877–1883. doi: 10.1164/ajrccm.155.6.9196089. [DOI] [PubMed] [Google Scholar]
- Wooley K.L., Alderoth E., Wooley M.J., Ramis I., Abrams J.S., Jordana M., O'Byrne P.M. Interleukin-3 in bronchial biopsies from nonasthmatics and patients with mild and allergen-induced asthma. Am. J. Respir. Crit. Care Med. 1996;153:350–355. doi: 10.1164/ajrccm.153.1.8542142. [DOI] [PubMed] [Google Scholar]
- Nicolaides N.C., Holroyd K.J., Ewart S.L., Eleff S.M., Kiser M.B., Dragwa C.R., Sullivan C.D., Grasso L., Zhang L.Y., Messler C.J. Interleukin 9a candidate gene for asthma. Proc. Natl. Acad. Sci. USA. 1997;94:13175–13180. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temann U.A., Geba G.P., Rankin J.A., Flavell R.A. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J. Exp. Med. 1998;188:1307–1320. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]