Freshly Isolated Peyer's Patch, but Not Spleen, Dendritic Cells Produce Interleukin 10 and Induce the Differentiation of T Helper Type 2 Cells (original) (raw)
Abstract
Orally administered antigens often generate immune responses that are distinct from those injected systemically. The role of antigen-presenting cells in determining the type of T helper cell response induced at mucosal versus systemic sites is unclear. Here we examine the phenotypic and functional differences between dendritic cells (DCs) freshly isolated from Peyer's patches (PP) and spleen (SP). Surface phenotypic analysis of CD11c+ DC populations revealed that PP DCs expressed higher levels of major histocompatibility complex class II molecules, but similar levels of costimulatory molecules and adhesion molecules compared with SP DCs. Freshly isolated, flow cytometrically sorted 98–100% pure CD11c+ DC populations from PP and SP were compared for their ability to stimulate naive T cells. First, PP DCs were found to be much more potent in stimulating allogeneic T cell proliferation compared with SP DCs. Second, by using naive T cells from ovalbumin peptide–specific T cell receptor transgenic mice, these ex vivo DCs derived from PP, but not from SP, were found to prime for the production of interleukin (IL)-4 and IL-10 (Th2 cytokines). In addition, PP DCs were found to prime T cells for the production of much lower levels of interferon (IFN)-γ (Th1) compared with SP DCs. The presence of neutralizing antibody against IL-10 in the priming culture dramatically enhanced IFN-γ production by T cells stimulated with PP DCs. Furthermore, stimulation of freshly isolated PP DCs via the CD40 molecule resulted in secretion of high levels of IL-10, whereas the same stimulus induced no IL-10 secretion from SP DCs. These results suggest that DCs residing in different tissues are capable of inducing distinct immune responses and that this may be related to the distinct cytokines produced by the DCs from these tissues.
Keywords: antigen presentation, cytokine, mucosal immunity
Peyer's patches (PP)1 represent the primary site for uptake and presentation of ingested antigens in the intestine. One of the unique features of mucosal lymphoid tissues such as PP is their capacity to induce Th cells producing type 2 (IL-4, IL-5, and IL-10; Th2) 1 and type 3 (TGF-β; Th3) 2 cytokines. Induction of these Th cell responses is important for the production of IgA and the generation of regulatory cell–mediated oral tolerance, two prominent unique features of immune responses to oral antigens. However, despite this capacity to generate Th2/Th3 responses in PP, distinct Th1 responses can be induced in the mucosa, particularly after intestinal infection with pathogenic microorganisms. For instance, IFN-γ secretion by PP T cells has been observed after gastrointestinal infection with Salmonella typhimurium 3 4 5 6 and Toxoplasma gondii 7 . In addition, high dose antigen feeding results in a transient Th1 response before T cell clonal deletion 8 9.
The mechanisms that determine the ability of PP to generate Th2/Th3 responses, yet allow for the differentiation of Th1 responses after infection with pathogenic organisms, are not known. One possibility is that the cytokine environment in the intestinal mucosa favors the differentiation of Th2/Th3 cells (e.g., high levels of IL-4 and TGF-β), but that this pattern is overridden by strong signals from pathogens, such as those that directly induce IL-12 from APCs. Another important factor may be the nature of the resident APCs. In this regard, it has recently been suggested that resident DCs may differ in their capacities to drive T cell differentiation 10.
An earlier study from our laboratory identified different populations of PP dendritic cells (DCs) by immunohistochemistry. We characterized two distinct populations of DCs in PP, namely those that reside just underneath the follicle-associated epithelium in the subepithelial dome (SED) region of the PP, and those that are present in the interfollicular T cell regions (IFR) 11. The SED DCs express the DC marker CD11c, but not other DC markers, such as DEC-205 and the intracellular antigen recognized by mAb M342. These cells are anatomically ideally situated for taking up luminal antigens transported by M cells. On the other hand, populations of DCs in the IFR express CD11c as well as DEC-205 and stain with M342. The expression of DEC-205, as well as the M342 antigen, correlates with DC differentiation in vitro, and these antigens are expressed by interdigitating DCs from other lymphoid organs, suggesting that the IFR DCs are more mature or differentiated than those in the SED. In addition, these cells are more likely responsible for priming T cells because they come in close contact with naive T cells at this site, much like interdigitating DCs in the spleen (SP) or peripheral lymph node. Whether SED DCs are immature DCs that migrate to the IFR of the PP to become IFR DCs or whether these two subsets of DCs have entirely separate lineages and functions in PP is unknown.
In this report, we address the issue of whether freshly isolated DCs from PP are different from DCs from SP in both their surface phenotype and their ability to induce T cell differentiation. To decipher the functions of unmanipulated DCs from tissues, we have opted to use an isolation method based on selection by magnetic beads that requires no in vitro tissue culture step. Cells isolated in this manner from SP and PP were further purified by flow cytometric sorting to obtain 98–100% pure CD11c+ DCs. We show that PP DCs express higher levels of MHC class II compared with SP DCs, whereas the levels of expression of costimulatory molecules CD80 and CD86 were low in both DC populations. A similar percentage of PP DCs and SP DCs expressed CD8α and DEC-205. In functional studies, we show that freshly isolated PP DCs are much more potent stimulators of both allogeneic and antigen-specific T cell proliferation compared with SP DCs. Moreover, using naive OVA-specific TCR transgenic T cells, PP DCs, but not SP DCs, primed T cells for the production of Th2 cytokines. In addition, SP DCs primed T cells for the production of much higher levels of IFN-γ compared with PP DCs. Finally, stimulation of freshly isolated DCs with CD40L trimer induced secretion of IL-10 exclusively from those isolated from PP but not from SP.
Materials and Methods
Animals.
6–8-wk-old female BALB/c mice and B10.A mice were obtained from the National Cancer Institute. Mice transgenic for TCR that recognize OVA323–339 peptide in the context of I-Ad (DO11.10TCR-α/β transgenic mice) on a BALB/c background were produced as previously described 12 and were provided by Dr. Dennis Loh (Washington University, St. Louis, MO). Mice transgenic for TCR specific for pigeon cytochrome c (PCC) 88–104 in the context of I-Ek on a B10.A background crossed to RAG2−/− mice were obtained from Taconic Farms, Inc.
Tissue Culture Media.
RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, 3 mM l-glutamine, and 50 μM β-ME was used for primary DC–T cell coculture and DC culture, as well as for secondary T cell stimulation culture.
Peptide.
Peptides corresponding to residues 323–339 of OVA (ISQAVHAAHAEINEAGR) and 88–104 of PCC (KAERADLIAYLKQATAK) were synthesized by the National Institute of Allergy and Infectious Diseases Laboratory of Molecular Structure.
Reverse Transcriptase PCR and Competitive Reverse Transcriptase PCR.
Total RNA was isolated from sorted DC populations by RNA isolation column (Qiagen), and subsequently digested with RNase-free DNase (GIBCO BRL) to remove contaminating genomic DNA. Single-stranded cDNA was synthesized using SuperScript preamplification system (GIBCO BRL), and PCR was carried out for 35 cycles using primer pairs for CD3∈ (forward, 5′ CACTTTCTGGGGCATCCTGT 3′; reverse, 5′ CAGTACTCACACACTCGAGC 3′), CD19 (forward, 5′ TCTCTATTGTCAAAGAGCCT 3′; reverse, 5′ CTTCCTCTGGACCCATGGGC 3′), TGF-β (forward, 5′ ACCGCAACAACGCCATC-TAT 3′; reverse, 5′ GTAACGCCAGGAATTGTTGC 3′), and β2-microglobulin (β2m; forward, 5′ TGACCGGCTTGTATGCTATC 3′; reverse, 5′ CAGTGTGAGCCAGGATATAG 3′). Oligonucleotides were synthesized by Operon Technologies. Competitive RT-PCR was carried out as previously described 13. In brief, equivalent amounts of cDNA from sorted DCs were added to each PCR reaction tube. Serial dilutions (total of 10 fourfold dilutions) of known amounts of competitive plasmid pMCQ (provided by Dr. David Shire, Sanofi Recherche, Montpellier, France) were added to the reaction tubes containing target cDNA. The competitive plasmid DNA contained the same primer templates as the target cDNA, and served as an internal standard. PCR was carried out for 35 cycles as described above and the products were analyzed on 1% agarose gels. The quantity of the target cDNA template for TGF-β and β2m was determined by the number of competitive plasmid molecules present in the lane in which the band intensities of the target cDNA and the competitor plasmid DNA are equivalent. The β2m ratio was calculated as (No. of molecules of TGF-β template in cDNA) / (No. of molecules of β2m template in cDNA) for each cDNA sample.
Antibodies.
Purified monoclonal rat anti–mouse IL-10 (JES5-16E3), hamster anti–mouse CD3∈ (145-2C11), and hamster anti–mouse CD28 (37.51) were purchased from PharMingen. Monoclonal anti–TGF-β antibody was purchased from Genzyme Corp. For staining of epithelial cells, FITC-conjugated anti–pan cytokeratin antibody (PCK-26) was purchased from Sigma Chemical Co. Surface phenotype of DCs was analyzed with anti-CD11c (HL3), anti-CD80 (16-10A1), anti-CD86 (GL1), anti-CD11b (M1/70), anti-CD8α (53-6.7), anti-CD45R (RA3-6B2), anti–I-Ad (AMS-32.1), anti-DEC205 (NLDC-145), anti–ICAM-1 (3E2), anti–ICAM-2 (3C4), and anti-CD40 (HM-43). Naive T cells from OVA TCR transgenic mice were stained with anti-CD4–FITC (GK1.5) and anti-LECAM-1–PE (MEL-14). Before staining, Fc receptors (FcγRIII/II) were blocked using anti–mouse CD16/CD32 (2.4G2). The antibodies used for DC and T cell surface markers were purchased from PharMingen, except for NLDC-145, which was purified from hybridoma obtained from the American Type Culture Collection (HB290). Isotype-matched controls used for staining DCs or in cytokine neutralization studies include mouse IgG1, κ (107.3), rat IgG2a, κ (R35-95), rat IgG2b, κ (R35-38 or A95-1), rat IgG1, κ (R3-34), hamster IgG (G235-2356), and hamster IgM (G235-1), all of which were purchased from PharMingen.
Preparation of DCs.
DCs were prepared from SP and PP of naive 6–10-wk-old mice in parallel. PP were treated with media containing dithiothreitol 145 μg/ml (Sigma Chemical Co.), 25 mM Hepes (Biofluids Inc.), 10% FCS (Biofluids Inc.), and 5 mM EDTA (Biofluids Inc.) in HBSS for 90 min at 37°C to remove epithelial cells, and were washed extensively with HBSS. Both SP and PP were digested with collagenase D (400 U/ml; Boehringer Mannheim) and DNase (15 μg/ml; Boehringer Mannheim), and incubated in the presence of 5 mM EDTA at 37°C for 5 min. Single cell suspension was prepared and cells were incubated with anti–mouse CD11c-coated magnetic beads (Miltenyi Biotech) and selected on MACS separation columns. Cells selected on the basis of CD11c expression were then stained with PE-labeled anti-CD11c antibody and FITC-labeled anti-B220 antibody. CD11c+/B220− cells were isolated by flow cytometric sorting performed on a FACStar™ sorter (Becton Dickinson). Sorted DCs were routinely 98–100% positive for CD11c. The sorted population was rigorously screened for contamination by B and T lymphocytes by performing RT-PCR on RNA derived from sorted DCs using specific primer pairs for CD19 and CD3∈, respectively, using 35 cycles. Neither PP nor SP DC–sorted populations contained macrophage contamination because CD11c−/lo macrophages are excluded by sorting cells that expressed only high levels of CD11c 14.
Mixed Lymphocyte Reaction.
Allogeneic T cells were prepared from SP of B10.A mice by negative selection using T cell enrichment columns (R&D Systems). T cells (H-2k) at 105 cells per well were mixed with flow cytometrically sorted pure DCs from BALB/c (H-2d) mice at various concentrations in 96-well microtiter plates for 48 h. Proliferation was measured by [3H]thymidine incorporation during the last 8 h of incubation.
Preparation of TCR Transgenic T Cells.
SP T cells from DO11.10 OVA TCR transgenic mice or PCC TCR transgenic mice were prepared by negative selection on T cell enrichment columns (R&D Systems) according to the manufacturer's instruction. Since the PCC TCR transgenic mice contained no other lymphocytes (RAG2−/−), T cell–enriched column fraction (90% CD3+) was used directly for DC–T cell cultures. From OVA TCR transgenic mice, T cells were first enriched by negative selection as above, followed by isolation of CD4+/MEL14+ T cells by flow cytometric sorting using FITC-labeled anti-CD4 and PE-labeled anti–LECAM-1 antibodies. Sorted T cell populations were typically 99% positive for the two markers.
Stimulation of TCR Transgenic T Cells by DCs.
In vitro T cell differentiation assays were performed according to previously established methods 15. In brief, primary stimulation cultures were established by coincubation of purified T cells (5 × 104 cells per well) and sorted CD11c+/B220− DCs from SP or PP (5 × 103 cells per well) pulsed with the corresponding peptide (3 μM), and 1 ng/ml recombinant human IL-2 (Genzyme Corp.) in a 96-well plate at 200 μl/well. In some cultures 20 μg/ml of a neutralizing anticytokine antibody or isotype control antibody was added. After 48 h, cells were transferred to 24-well plates and allowed to expand for 3–4 d in fresh medium without additional cytokines or antibodies. T cells were then washed and 2 × 105 cells were plated on 96-well microtiter plates coated with anti-CD3∈ (10 μg/ml) in the presence of soluble anti-CD28 antibody (1 μg/well). Supernatants from restimulated T cells were collected for detection of IL-4 at 24 h and IL-10 and IFN-γ were collected at 48 h. Proliferation of T cells was assayed by incorporation of [3H]thymidine during the final 8 h of a 48-h incubation.
Stimulation of DCs by CD40L Trimer.
FACS®-purified CD11c+/B220− DCs (105 per well) were incubated overnight in the presence of recombinant murine CD40L trimer (10 μg/ml; Immunex Corp.) in a total volume of 200 μl per well of 96-well microtiter plate. Supernatants were collected and IL-10 and IL-12 p40 levels were measured by ELISA.
Measurement of Cytokine Production.
IL-10 and IFN-γ secretion was assayed by a specific sandwich ELISA using antibody pairs according to the manufacturer's instructions (PharMingen). The lower limit of detection was 10 pg/ml for IL-10 and 50 pg/ml for IFN-γ. IL-4 was measured using a sandwich ELISA kit from Endogen. The lower limit of sensitivity for IL-4 ELISA was 10 pg/ml. IL-12 p40 was measured using the OptEIA™ set (PharMingen), which had a lower limit of detection at 30 pg/ml.
Statistical Analysis.
Normally distributed continuous variable comparisons were done using Student's t test.
Results
Phenotypic Analysis of Freshly Isolated DCs from PP and SP.
In our previous study, we identified distinct subsets of DCs in murine PP by immunohistochemical analysis 11. When DCs were isolated from murine PP by transient plastic adherence, a technique that requires overnight culture at 37°C, we found that their capacity to process and present soluble antigens was equivalent to that of DCs similarly derived from the SP. In addition, we found that the surface expression of DC markers as well as costimulatory molecules were largely similar between transiently adherent SP and PP DCs. The one exception to this rule was the expression of MHC class II, which was found to be 5–10-fold higher on PP DCs. However, since it is known that overnight culture of DCs can result in their differentiation, cells isolated by this technique do not necessarily reflect the state of DC differentiation in vivo. To overcome this problem, we developed techniques to study freshly isolated DCs. This allowed us to more accurately compare the phenotype and function of DCs from PP and SP.
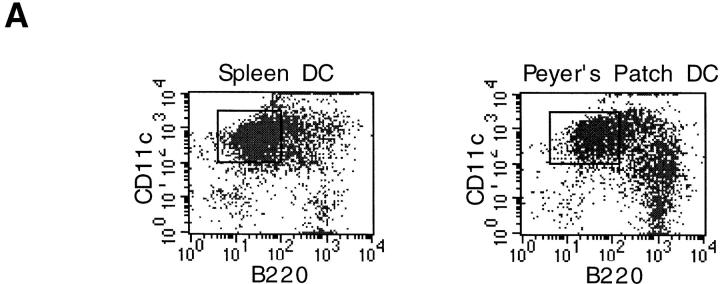
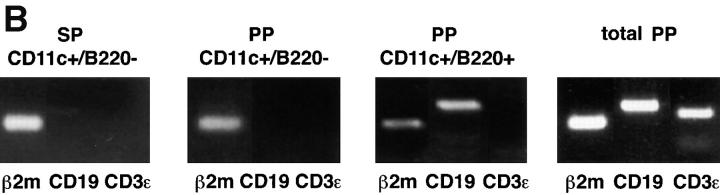
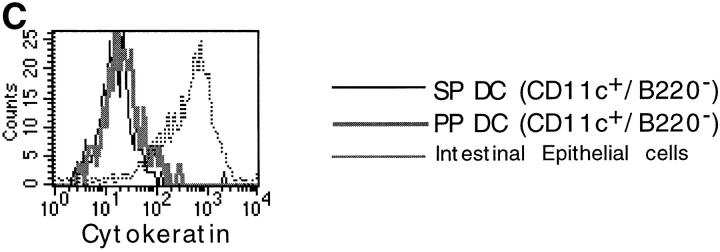
In initial experiments, we analyzed the expression of T and B cell markers on DC-enriched populations positively selected from PP and SP on the basis of their expression of CD11c, a well-established marker for DCs in mice. As shown in Fig. 1 A, we found that a significant proportion of anti-CD11c magnetic bead–selected cells from both PP (30%) and SP (9%) expressed B220, whereas cells expressing CD3 were undetectable (data not shown). We next determined that at least some of these B220+ cells were indeed B cells, since RT-PCR analysis of flow cytometrically sorted CD11c+/B220+ cells demonstrated the presence of mRNA for CD19 (Fig. 1 B). In contrast, we found that the sorted CD11c+/B220− cell population contained no detectable mRNA for CD19, nor did it contain detectable mRNA for CD3 (Fig. 1 B). Thus, to exclude B cell contamination from DC preparations, magnetically sorted CD11c+ cells were purified by flow cytometric sorting on the basis of CD11c+/B220− expression (Fig. 1 A) and were routinely screened by RT-PCR for expression of B (CD19) and T cell (CD3) markers. Moreover, neither PP DC or SP DC sorted populations contained macrophage contamination since CD11c−/lo macrophages are excluded by sorting cells that expressed only high levels of CD11c 14. Finally, since PP cell preparations often contain large numbers of intestinal epithelial cells, it was necessary to exclude contamination of the sorted DCs by epithelial cells. Although PP were pretreated with dithiothreitol- and EDTA-containing media to remove epithelial cells, it was theoretically possible that any remaining epithelial cells could be selected by nonspecific binding to the anti-CD11c antibody-coupled magnetic beads. To exclude this possibility, sorted CD11c+/B220− cells were stained with an anti–pan cytokeratin antibody, which recognizes a broad spectrum of cytokeratin proteins expressed by epithelial cells. As shown in Fig. 1 C, cytokeratin-specific staining was not observed in DCs isolated from either PP or SP, whereas freshly isolated intestinal epithelial cells were strongly stained by the same antibody. Taken together, these initial experiments confirmed that the CD11c+/B220− populations of cells isolated by the technique used here were highly purified DCs.
Figure 1.
Phenotypic analysis of sorted DC populations from SP and PP. (A) Cells from PP or SP were isolated using magnetic beads as described in Materials and Methods, and were dual stained with the FITC-conjugated anti-B220 and PE-conjugated anti-CD11c antibodies. Cells that subsequently were sorted by FACS® and used for T cell stimulation are indicated by the enclosed square (CD11c+/B220−). (B) RT-PCR analysis of B and T cell contamination in sorted DC populations. Magnetically selected DCs from SP and PP were further purified by flow cytometric sorting into either CD11c+/B220− or CD11c+/B220+ cells. Total RNA was extracted from sorted DCs and analyzed by RT-PCR for expression of B (CD19) and T (CD3) cell markers. RT-PCR of β2m was included as a positive control for mRNA in each sample. An RT-PCR profile of total PP tissue was included to indicate that B and T cells were present in the starting population (right). (C) Exclusion of epithelial cells from sorted DC populations. CD11c+/B220− cells from SP and PP were stained for the pan-epithelial cell marker, cytokeratin. In parallel, freshly isolated intestinal epithelial cells were stained with the same antibody (dotted line) as a positive control.
We next analyzed the expression of DC surface antigens on CD11c+/B220− cells from PP and SP. As shown in Fig. 2, we found that freshly isolated CD11c+/B220− PP DCs expressed 5–10-fold higher levels of MHC class II antigen compared with SP DCs, as we had previously observed with overnight cultured transiently adherent DCs 11. Next, we analyzed the expression of lymphoid DC markers. Lymphoid DCs, which are thought to be involved in the inhibition of peripheral T cell responses, have been shown to express the α chain dimer of CD8 and high levels of Fas ligand 16. Moreover, in some reports CD8α chain expression by DCs correlates strongly with expression of DEC-205, suggesting that DEC-205 may be an additional or independent marker of lymphoid-derived DCs 17. As it has been shown that lymphoid DCs are highly adherent cells that are only released from tissues and adherent T cells by treatment with EDTA 17, we routinely incorporated treatment with EDTA before magnetic bead isolation, as previously described by Vremec et al. 18, to ensure release of all DC subsets. As shown in Fig. 2, when CD11c+/B220− DCs from PP and SP were analyzed, comparable percentages of cells were found to express CD8α and DEC-205 (Fig. 2), although Fas ligand expression was undetectable in either population (data not shown). In addition, we stained for CD11b, a β2 integrin that has been associated with DCs and which may be derived from a myeloid, as opposed to lymphoid, precursor. Although we could discern a clear population of CD11bhi cells from both PP and SP DCs, the majority of cells from both groups expressed moderate levels of CD11b. Finally, we determined the expression of costimulatory molecules and adhesion molecules, as these may influence T cell differentiation. We found that CD80 and CD86, as well as CD40, were expressed at low to moderate, but equivalent, levels on freshly isolated SP and PP DCs. Moreover, high levels of adhesion molecule ICAM-1 were expressed by both SP and PP DCs, whereas ICAM-2 expression was negligible in both DC populations. Therefore, with the exception of MHC class II antigen, the DCs freshly isolated from PP and SP appeared to have a similar surface phenotype.
Figure 2.
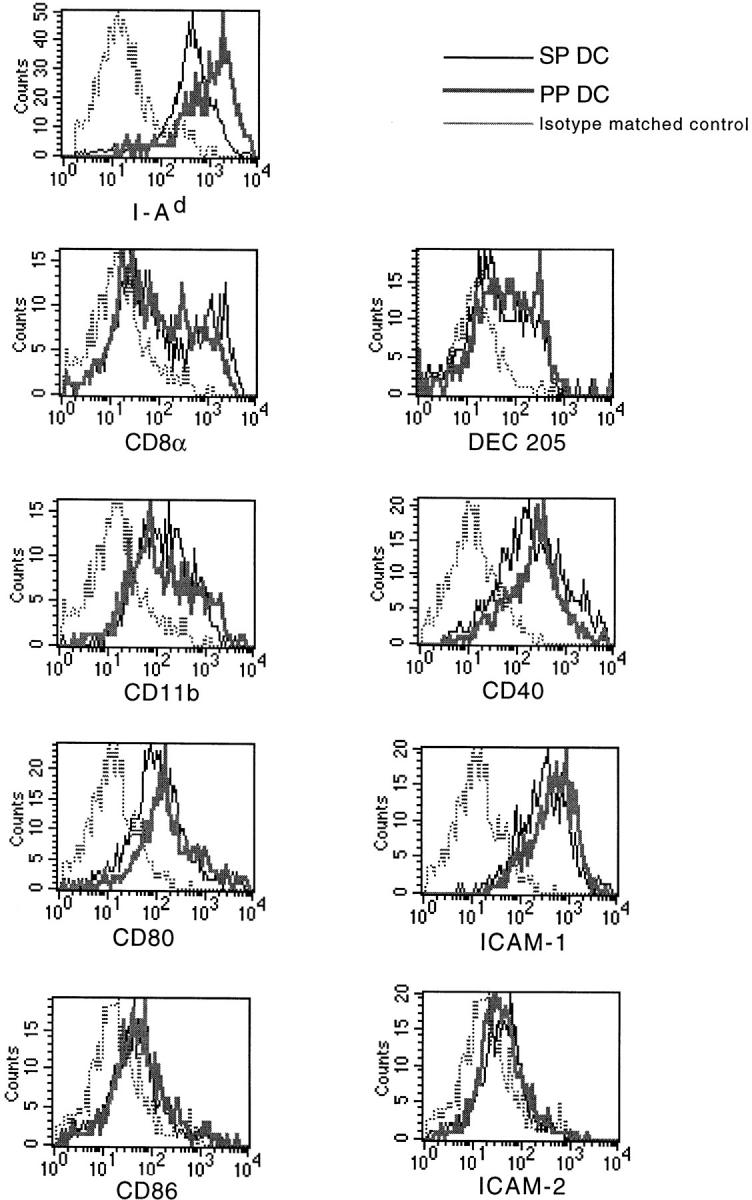
Surface phenotype analysis of sorted DC populations from SP and PP. Sorted CD11c+/B220− DCs from SP or PP were analyzed for expression of various surface molecules. The results are shown as histograms with fluorescence intensity on the x-axis and cell number on the y-axis. The thin lines represent staining of SP DCs, and the thick lines staining of PP DCs. Isotype-matched control is indicated for each antibody set with a dotted line. The data depicted here represents four independent experiments producing similar results.
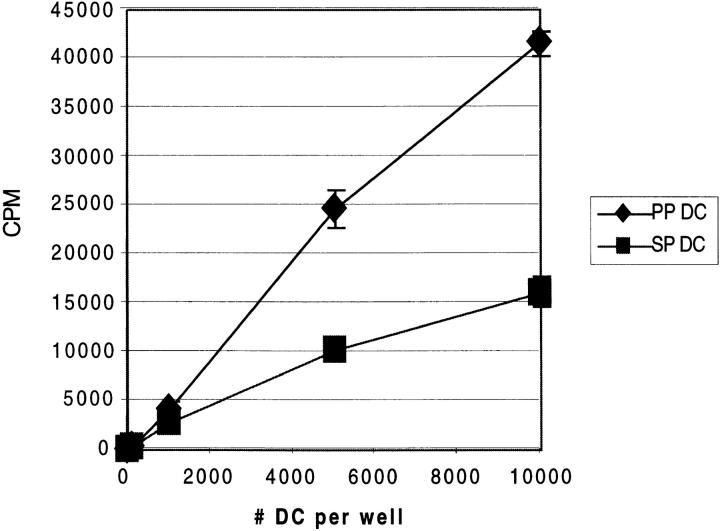
Highly Purified Fresh CD11c+ DCs from PP Are More Potent Stimulators of Allogeneic T Cells.
We next determined whether PP and SP DCs also had a similar capacity to induce primary T cell responses by measuring the ability of freshly isolated CD11c+/B220− cells to stimulate allogeneic T cells in vitro. Varying numbers of BALB/c (H-2d) DCs were cultured with T cells purified from B10.A (H-2k) mice. Proliferation of allogeneic T cells was then determined by [3H]thymidine incorporation. As shown in Fig. 3, we found that PP DCs were much more potent for stimulating allogeneic T cells than were SP DCs. We next determined whether this enhanced ability to induce primary T cell proliferation could be restricted to a particular MHC background of the stimulating or responding populations. We found that PP DCs were consistently more potent stimulators of allogeneic T cell proliferation compared with SP DCs in all the mouse strain combinations that have been tested to date [B10.A (H-2k) DCs + C57B/6 (H-2b) T cells, B10.A DCs + BALB/c T cells, BALB/c DCs + C57B/6 (H-2b) T cells] (data not shown).
Figure 3.
DCs from PP are more potent stimulators of allogeneic T cell proliferation than are those from SP. Mixed lymphocyte reaction was carried out with varying numbers of purified BALB/c DCs (H-2d) and 105 T cells from B10.A (H-2k) mice per well in 96-well microtiter plates. Proliferation was measured by [3H]thymidine uptake during the last 8 h of a 48-h culture. Results are represented as the mean cpm of triplicate cultures on the y-axis, with the number of DCs per well on the x-axis. Similar results were obtained in three separate experiments conducted in the same manner.
PP DCs Prime OVA TCR Transgenic T Cells (BALB/c) to Produce Th2 Cytokines, Whereas SP DCs Prime only for a Th1 Response.
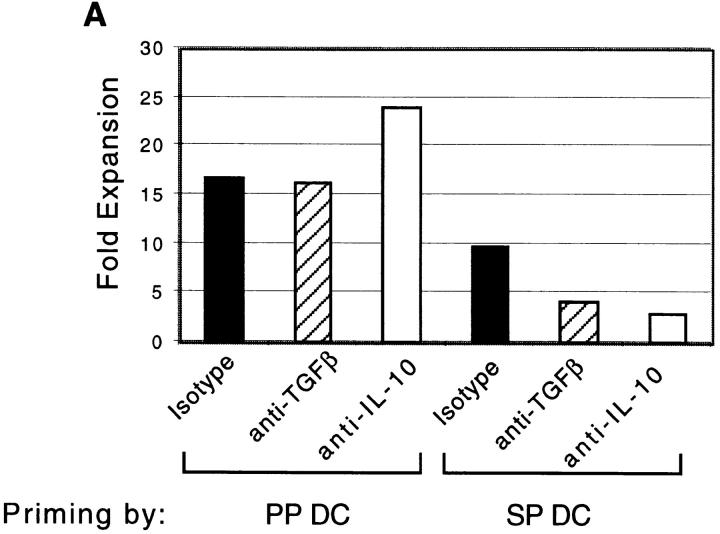
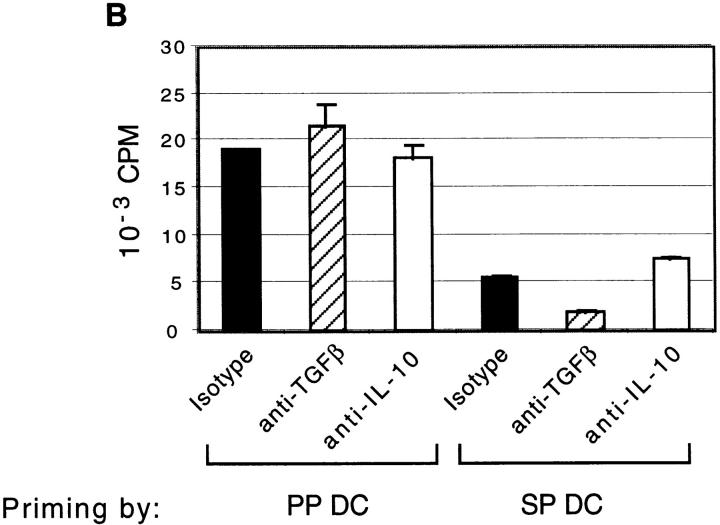
We next determined whether PP and SP DCs had different capacities for inducing Th cell differentiation. This was of particular interest because two unique functions of the mucosal immune system, production of secretory IgA and suppression of responses to ubiquitous environmental antigens, are both Th cell–dependent processes. Specifically, optimal IgA-B cell differentiation 19 20 21, as well as the effector functions of regulatory T cells induced after low dose antigen feeding 22 23, have been shown to depend on Th2 and Th3 (TGF-β) cytokines. Moreover, B cell switching to IgA requires a CD40L signal from activated T cells 21. Thus, to further characterize the antigen-presenting capacity of DCs isolated from these tissues, FACS®-sorted CD11c+/B220− cells from BALB/c SP and PP were used to stimulate naive CD4+/LECAM-1hi FACS®-sorted T cells from OVA TCR transgenic mice in the presence of OVA 323–339 peptide. As indicated in Fig. 4 A, PP DCs induced two- to threefold higher expansion of naive T cells than did SP DC during primary culture, consistent with the enhanced capacity to drive allogeneic T cell proliferation that was noted above. Interestingly, as shown in Fig. 4 B, we also found that secondary stimulation of PP DC–primed T cells with plate-bound anti-CD3∈ and soluble anti-CD28 in the absence of APCs consistently resulted in 10-fold higher proliferation when compared with SP DC–primed T cells. Although the reasons for this enhanced proliferation during secondary culture are not yet clear, this finding suggests that T cells initially stimulated with PP DCs are fundamentally different than those stimulated with SP DCs.
Figure 4.
PP DCs induced higher expansion of OVA TCR transgenic T cells during primary and secondary culture than did SP DCs. (A) Naive CD4+/MEL14+ FACS®-sorted OVA TCR transgenic T cells (5 × 104 per well) were coincubated with CD11c+ DCs sorted from PP or SP (5 × 103 per well) for 5–6 d in the presence of blocking antibodies against TGF-β, IL-10, or isotype control antibody. Live cells were counted using hemacytometer by exclusion of dead cells with Trypan blue staining. The figure depicts the factor by which the number of T cells multiplied during priming by either PP or SP DCs on the y-axis. (B) OVA TCR transgenic T cells primed with DCs as described in A were restimulated with plate-bound anti-CD3∈ and soluble anti-CD28 antibodies for 48 h. Proliferation of T cells was determined by incorporation of [3H]thymidine, which was added to the culture wells during the last 8 h of incubation. Data is representative of four separate experiments producing similar results.
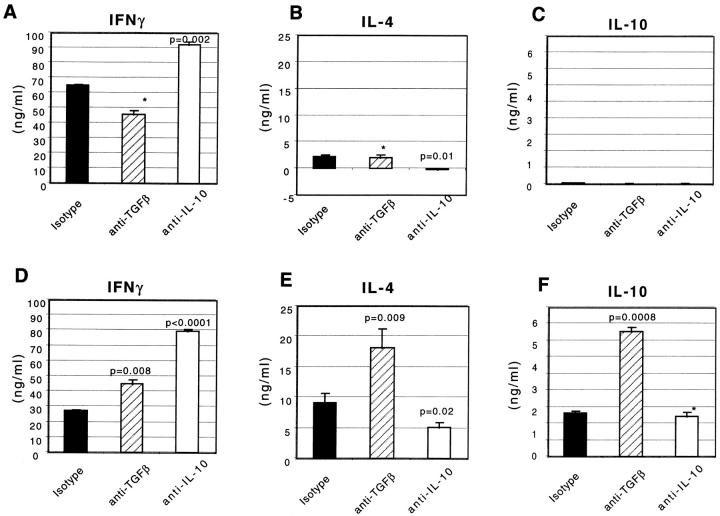
We next determined the cytokine secretion pattern of OVA TCR transgenic T cells primed with PP or SP DCs and restimulated with anti-CD3 and anti-CD28. As shown in Fig. 5T cells primed with PP DCs secreted fivefold higher levels of IL-4 than SP DC–primed T cells. Most strikingly, PP DCs also primed T cells to secrete IL-10, whereas IL-10 secretion was minimal from T cells stimulated with SP DCs. In contrast, IFN-γ secretion was two- to threefold higher in T cells primed with SP DCs than with PP DCs, although both T cell populations produced substantial amounts of IFN-γ. This reduction in IFN-γ production by PP DC–stimulated T cells was partially or fully reversed by addition of a neutralizing anti–TGF-β antibody and anti-IL-10 antibody, respectively (Fig. 5 D). The addition of anti–TGF-β antibody to PP DC–T cell culture also enhanced secretion of IL-4 and IL-10, but not in SP DC–T cell culture, suggesting that either PP DCs themselves secrete TGF-β, or that they induce T cells to secrete TGF-β that suppresses the production of IL-4, IFN-γ, and IL-10. Furthermore, in an effort to evaluate the overall capacity of DCs to differentiate and proliferate T cells, we normalized the levels of cytokines produced by T cells during the secondary stimulation to the expansion of T cells during the priming culture (Table ). By analyzing the data in this fashion, we determined that the overall difference in secretion of IL-4 and IL-10 between PP DC– or SP DC–primed T cells was even greater than we had previously determined (due to higher expansion of T cells by PP DCs). Moreover, the observed increase in IFN-γ secretion by neutralizing IL-10 was considerable only from T cells stimulated with PP DCs (fourfold higher than isotype control).
Figure 5.
Cytokine production by OVA TCR transgenic T cells during secondary stimulation. Naive CD4+/MEL14+ FACS®-sorted OVA TCR transgenic T cells were stimulated with purified CD11c+ DCs isolated from SP (A, B, and C) or PP (D, E, and F) in the presence of blocking antibodies against TGF-β, IL-10, or isotype control antibody for 5–6 d. T cells were restimulated with plate-bound anti-CD3∈ and soluble anti-CD28 antibodies for up to 48 h. Supernatants were harvested and IFN-γ (A and D), IL-4 (B and E), and IL-10 (C and F) levels were measured by ELISA at 24 (IL-4) or 48 h (IFN-γ and IL-10). The P values for cytokines secreted in the presence or absence of neutralizing antibody in the priming cultures are indicated with value labels or with an asterisk if P > 0.05. This experiment was repeated four times producing similar cytokine secretion patterns.
Table 1.
Cytokine Secretion from OVA TCR Transgenic T Cells Primed with Either PP or SP DCs Normalized to T Cell Expansion
| Priming by: | Antibody | IFN-γ | IL-4 | IL-10 |
|---|---|---|---|---|
| ng/ml | ng/ml | ng/ml | ||
| PP DCs | Isotype | 229.09 ± 10.10 | 76.61 ± 13.58 | 16.54 ± 0.78 |
| Anti–TGF-β | 367.01 ± 22.99 | 147.59 ± 24.33 | 45.12 ± 1.80 | |
| Anti–IL-10 | 956.42 ± 19.16 | 62.10 ± 10.74 | 21.43 ± 2.79 | |
| SP DCs | Isotype | 313.30 ± 14.85 | 10.59 ± 1.81 | 0.23 ± 0.02 |
| Anti–TGF-β | 95.66 ± 3.86 | 4.47 ± 1.24 | 0.05 ± 0.01 | |
| Anti–IL-10 | 138.67 ± 2.58 | −0.15 ± 0.10 | 0.03 ± 0.02 |
Splenic DCs Prime PCC TCR Transgenic T Cells (B10.A) to Produce Higher Levels of IFN-γ.
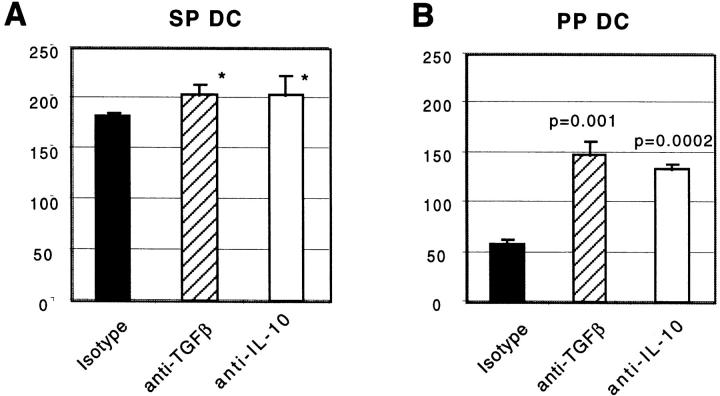
It is known that the default cytokine secretion pattern of T cells under a neutral priming condition is determined by the genetic background of mice from which the T cells are isolated 24. The OVA TCR transgenic mice we used are of the BALB/c background, in which the T cells are predisposed towards the Th2 cytokine production. To determine whether the Th cell phenotype differences induced with PP and SP DCs that were observed in our OVA TCR transgenic system priming also applied to a Th1-predisposed strain, we carried out the same set of experiments in the B10.A (Th1) background. T cells from PCC TCR transgenic mice (B10.A) were stimulated with purified, sorted B10.A CD11c+/B220− DCs from PP or SP. Cytokine production from T cells during secondary stimulation with plate-bound anti-CD3∈ and soluble anti-CD28 was assessed. It is evident from Fig. 6 that three to fourfold higher IFN-γ secretion was induced in SP DC–primed T cells compared with PP DC–primed T cells, as in the case of BALB/c mice (Fig. 5). In contrast, no IL-10 or IL-4 was detected in secondary cultures of T cells initially primed with PP or SP DCs. Thus, although PP DCs induce Th2 cytokines in BALB/c (Th2) mice, they are not capable of overcoming the Th1 bias of B10.A mice, despite the fact that PP DCs were less capable of inducing IFN-γ–secreting cells. Next, the role of TGF-β and IL-10 in T cell priming was assessed. As shown in Fig. 5 (BALB/c), IFN-γ secretion was enhanced from PP DC– but not from SP DC–primed T cells derived from B10.A mice in the presence of neutralizing antibody against either TGF-β or IL-10 in priming culture (Fig. 6). Taken together, in two separate strains of mice, (a) PP DCs were found to prime T cells to produce lower levels of IFN-γ compared with SP DC–primed T cells, and (b) both TGF-β and IL-10 suppressed IFN-γ production in PP DC–T cell, but not SP DC–T cell, coculture.
Figure 6.
SP DCs induce higher IFN-γ secretion by PCC TCR transgenic T cells than do PP DCs. Naive PCC TCR transgenic T cells were stimulated with purified CD11c+ DCs isolated from SP (A) or PP (B) in the presence of blocking antibodies against TGF-β, IL-10, or isotype control antibody for 5 d. T cells were restimulated with plate-bound anti-CD3∈ and soluble anti-CD28 antibodies for up to 48 h. Supernatants were harvested and IFN-γ level was measured by ELISA at 48 h. The P values for cytokines secreted in the presence or absence of neutralizing antibody in the priming cultures are indicated either as a number or an asterisk if P > 0.05. The same experiment was repeated three times producing similar results.
Detection of TGF-β Secreted by PP and SP DCs.
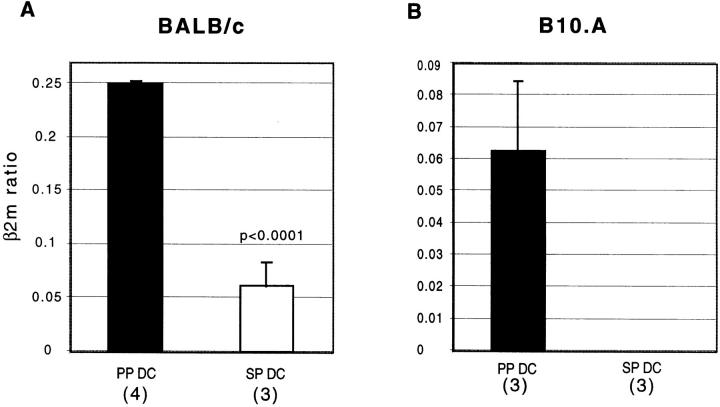
Because the presence of neutralizing antibody against TGF-β during the priming culture dramatically increased the secretion of cytokines by T cells stimulated with PP DCs, we reasoned that either TGF-β is being secreted by PP DCs and/or that PP DCs induce T cells to secrete TGF-β. To address this issue, we attempted to measure TGF-β secretion by ELISA from T cells alone (in the secondary T cell stimulation culture) or DC alone (in the DC stimulation culture). To mimic DC–T cell interaction, DCs were stimulated with a soluble, trimerized, recombinant form of murine CD40L, a molecule normally expressed on activated T cells. We could not detect secretion of TGF-β above background levels (>200 pg/ml) after stimulation of either T cells or DCs in serum-containing media. We also failed to detect TGF-β production by T cells or DCs in serum-free media, which was probably due to the poor viability of cells in these conditions. In the absence of serum, secretion of other cytokines such as IL-10 was also minimal (data not shown). However, when the expression of mRNA for TGF-β was assessed from freshly isolated DCs, much higher levels of TGF-β message was found in PP DCs compared with SP DCs in both strains of mice (Fig. 7). Moreover, there was no detectable mRNA for TGF-β in SP DCs in the B10.A mice even in the absence of competitive plasmid DNA in the PCR reaction (Fig. 7 B).
Figure 7.
Expression of TGF-β by freshly isolated PP DCs. cDNA samples prepared from freshly isolated PP and SP DCs from BALB/c (A) or B10.A (B) mice were analyzed by competitive RT-PCR for the expression of TGF-β and a control marker, β2m, as described in Materials and Methods. The ratios of TGF-β mRNA level to β2m mRNA level derived from the same cDNA sample are depicted on the y-axis as the mean values of at least three separate experiments ± SEM. The number of separate experiments done for each DC population are indicated in parentheses.
CD40-mediated Stimulation of PP DCs but not SP DCs Induces Secretion of IL-10.
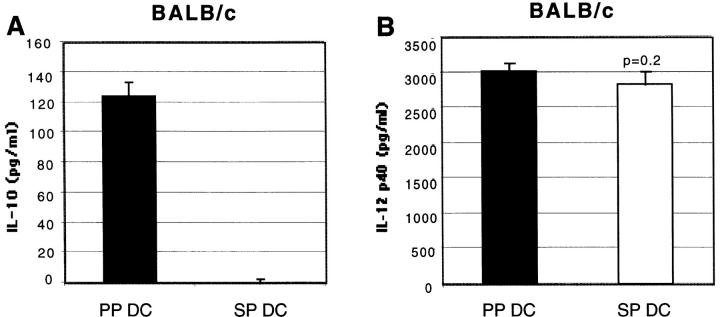
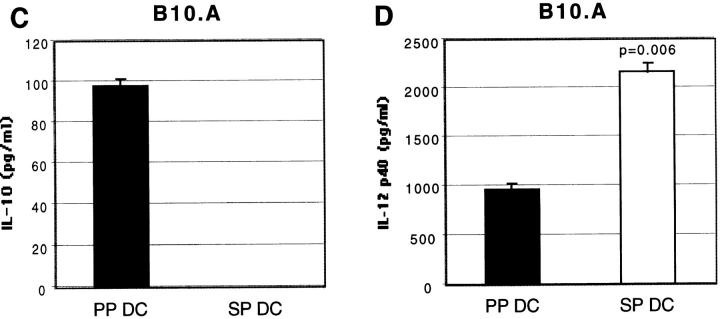
One possible mechanism underlying the distinct cytokine production patterns induced by stimulation of T cells with PP DCs (Th2) and SP DCs (Th1) may be that these DCs secrete discrete sets of cytokines upon activation by T cells. To address this possibility, cytokine production by purified DCs from BALB/c and B10.A mice in the absence of T cells was assessed. After overnight incubation of sorted CD11c+/B220− DCs with CD40L trimer, supernatants were assessed for the presence of IL-12 and IL-10. As shown in Fig. 8, we found that PP DCs secreted high levels of IL-10, whereas no detectable IL-10 secretion was observed from SP DCs under the same condition in both strains of mice. In contrast, similar levels of IL-12 p40 were detected from both SP and PP DC culture supernatants in BALB/c mice, whereas higher levels of IL-12 p40 was detected from SP than PP DCs in B10.A background. Levels of IL-12 p70 were below the level of detection by ELISA (∼30 pg/ml) in supernatants from both PP and SP DCs (data not shown).
Figure 8.
IL-10 is produced only by PP DCs after CD40L trimer stimulation, whereas similar levels of IL-12 p40 is detected from both DC types. FACS®-purified CD11c+ DCs (105 per well) from BALB/c (A and B) or B10.A (C and D) mice were incubated overnight in the presence of CD40L trimer (10 μg/ml). Supernatants were collected and IL-10 (A and C) and IL-12 p40 (B and D) levels were measured by ELISA. Each column is representative of four (A and B) or two (C and D) separate experiments.
Discussion
To study physiologically relevant DC populations from two distinct lymphoid tissues, namely PP and SP, we used freshly isolated DCs purified on the basis of their surface expression of CD11c. These highly purified DC populations devoid of B cells, T cells, and macrophages from PP and SP were strikingly different in their ability to stimulate T cells in vitro. First, PP DCs were much more potent in stimulating proliferation of both allogeneic (Fig. 3) and antigen-specific (Fig. 4) T cells compared with SP DCs. A possible explanation for these findings is that PP DCs were found to express 5–10-fold higher levels of MHC class II antigens than SP DCs (Fig. 2) and thus on a per cell basis are likely to provide a more potent signal to the responding T cell by engaging more TCRs. The enhanced ability to induce proliferation by PP DCs also suggested that these cells may be intrinsically more “mature” in their phenotype, since maturation has been shown to correlate with enhanced expression of MHC class II, as well as costimulatory molecules that could affect T cell proliferation. However, we found that CD80, CD86, and CD40 expression was low to modest, and similar for PP and SP DC populations. In addition, we found that levels of the adhesion molecules ICAM-1 and ICAM-2 were also similarly expressed by PP and SP DCs (Fig. 2). Thus, it appeared that neither population of cells was fully mature or differentiated, and that higher expression of these costimulatory and adhesion molecules could not explain the enhanced ability of PP DCs to induce primary T cell proliferation. The findings presented here are consistent with prior studies by Ruedl et al. that demonstrated that freshly isolated CD11c+ cells from PP are functionally immature 25. Thus, it was shown that upon overnight culture in the presence of either GM-CSF and TNF-α or anti-CD40 antibody, freshly isolated PP DCs matured as determined by their higher levels of expression of MHC class II, CD80, and CD86, and their loss of the ability to process intact antigens 25. Whether PP DCs express as yet unidentified costimulatory molecules or soluble factors responsible for the induction of increased T cell proliferation remains to be determined.
We next determined whether the tissue specificity of DCs influences the type of Th cell responses induced during antigen-specific stimulation. For these studies, we primed naive CD4+ T cells from OVA TCR transgenic mice in vitro with freshly isolated CD11c+/B220− DCs from PP or SP, and determined the phenotype of the primed cells by measuring cytokine production after secondary stimulation of T cells with anti-CD3 and anti-CD28 antibodies. Interestingly, we found that PP but not SP DCs primed T cells for the production of IL-4 and particularly IL-10. In addition, the level of IFN-γ produced by T cells primed with SP DCs was significantly higher than that produced by T cells primed with PP DCs. Furthermore, when cytokine production was normalized to T cell expansion, we found even greater differences in the amounts of cytokines produced by T cells primed with either PP or SP DCs.
In an effort to investigate the mechanisms underlying the particular ability of PP DCs to stimulate Th2 cytokine responses, we next determined whether TGF-β was preferentially expressed by PP DCs or generated in PP DC–T cell cultures, and whether this cytokine was affecting the differentiation of T cells into a Th2 pathway. This possibility was based on prior studies suggesting a role for TGF-β in directing Th2 responses in murine infection with Leishmania 26 as well as in driving Th2 immune deviation seen after antigen administration to the anterior chamber of the eye 27. We initially determined the ability of neutralizing anti–TGF-β antibodies added to the primary culture to alter the T cell phenotype induced by PP and SP DCs. Interestingly, neutralization of TGF-β resulted in increased levels of IFN-γ, IL-4, and IL-10 production by PP DC–, but not by SP DC–, primed T cells (Fig. 5). This increase in cytokine production did not appear to be due to enhanced expansion of cytokine-producing T cells, as [3H]thymidine incorporation during secondary stimulation was identical in cells treated and untreated with blocking antibodies (Fig. 4). We extended our finding of the inhibitory effects of TGF-β on IFN-γ production by PP DC–primed T cells to another TCR transgenic system of different genetic background (B10.A), confirming that the effect is not a BALB/c strain–dependent phenomenon (Fig. 6). Although the role of TGF-β in suppressing IFN-γ secretion has been well documented 26 28, its effect in modulating Th2 cytokine secretion from T cells is at best controversial. Our observations argue that TGF-β in the priming culture was suppressive to the induction of IFN-γ– as well as IL-4– and IL-10–producing T cells. Although there is evidence that the presence of TGF-β can suppress IL-4 and IL-5 secretion from purified T cells activated with anti-CD3 29, further investigation is required to clarify the direct effect of TGF-β in the differentiation of Th2 cells.
The source of TGF-β responsible for the observed suppression was either PP DCs and/or T cells primed with PP DCs. To address this possibility, we attempted to directly measure TGF-β production from freshly isolated PP and SP DCs. Although we were unable to detect TGF-β by ELISA from overnight cultures of freshly isolated DCs stimulated with CD40L, we did find that the level of expression of TGF-β mRNA was found to be much higher in PP DCs compared with SP DCs in both strains of mice (Fig. 7). Moreover, there was no detectable mRNA for TGF-β in SP DCs in the B10.A mice. Although TGF-β mRNA levels do not always correlate with the level of protein secretion 30 31, our data suggested that only PP DCs had constitutive TGF-β mRNA expression, and that the level of TGF-β produced is able to suppress both Th1 and Th2 cytokine secretion by T cells in the PP DC–T cell, but not SP DC–T cell, coculture. In PP, the presence of high levels of TGF-β, in combination with the Th2-inducing phenotype of PP DCs, may play an important role in regulating the local Th cell responses. In other words, the cytokine environment in PP may modify DCs as well as T cells to ensure that unwanted Th1 responses towards noninfectious materials such as food antigens are prevented.
We next determined the role of IL-10 in T cell differentiation by PP and SP DCs. We found that the addition of a neutralizing anti–IL-10 during priming cultures resulted in significantly enhanced IFN-γ secretion from the T cells primed with PP DCs, almost to the levels secreted by T cells stimulated with SP DCs (Fig. 5). This result is consistent with IL-10 being a potent inhibitor of Th1 development 32. It was also found that T cells primed by SP DCs in the presence of anti–IL-10 antibody secreted higher levels of IFN-γ. However, when cytokine secretion was normalized to the expansion of T cells during the priming culture, we found considerable increase in IFN-γ only from PP DC–primed T cells in the presence of anti–IL-10 antibody (Table ). Thus, significant suppression of IFN-γ was mediated by IL-10 present in the PP DC–T coculture. We next explored whether IL-10 produced directly from PP DCs could be responsible for the differentiation of T cells that secrete low levels of IFN-γ and high levels of IL-4 and IL-10. When IL-10 production by purified DCs was assessed upon stimulation with CD40L trimer, we found that PP but not SP DCs secrete high levels of IL-10. Therefore, the exclusive ability of PP DCs to generate IL-10–secreting T cells may at least be partially due to the production of IL-10 by PP DCs themselves. However, the fact that blocking IL-10 during T cell priming by PP DCs only partially converts T cell phenotype towards Th1 suggests that other factors might be involved in the Th2 development by PP DCs.
One final possible explanation for why PP and not SP DCs induce Th2 differentiation is related to the enhanced ability of PP DCs to stimulate T cell proliferation. Recently, it has been shown that naive murine CD4+ T cells have an intrinsic program for the transcription of the IL-4 gene that is directly related to the number of times the T cell divides. Thus, it was shown that the expression of IL-4 begins to occur after the third cell division, and that this expression is independent of exogenous IL-4 signaling, as similar results were found with T cells from STAT-6–deficient mice 33. This suggests that the enhanced proliferation of T cells stimulated by PP DCs may result in an intrinsic bias toward Th2 development, which, when occurring in an environment that has low levels of IL-12 due to the production of IL-10 by PP DCs, results in the selection or emergence of T cells with a Th2 phenotype.
Taken together, the data presented here provide the first demonstration of the ability of DCs from the murine PP to preferentially induce the differentiation of CD4+ T cells into a Th2 pathway. Previous studies have suggested that different APC types could direct Th responses to either Th1 or Th2 34 35 36 37. In addition, a recent study by Stumbles et al. suggested a similar Th2-inducing phenotype of DCs derived from the rat airway mucosa 28. In that study, repeated injections of OVA-pulsed rat respiratory tract DCs lead to increasing levels of IgG1 but not IgG2b in the rats known to be Th2- and Th1-dependent IgG subclasses, respectively 38 39. The data presented here supports the claim that resting tissue-resident DCs at mucosal surfaces have a unique ability to drive T cell responses towards the Th2 pathway. Finally, it has been reported recently that DCs that express the CD8α molecule (lymphoid-derived) drive predominant Th1 responses and that DCs lacking the CD8α molecule preferentially drive Th2 responses in vivo 40 41. Since we found that equivalent numbers of DCs from SP and PP express CD8α, a difference in DCs populations, per se, cannot offer an explanation for the differences in T cell differentiation shown here. We are currently investigating the potential differences in the functions of these DC subsets between PP and SP.
The mechanism by which tissue-specific DCs influence Th cell development after an oral antigenic challenge can be speculated as follows. Intestinal antigens transported via M cells are taken up by SED DCs, which migrate to the T cell region and become IFR DCs. During migration, SED DCs can undergo two distinct developmental pathways. If the antigen encountered is a noninfectious food antigen, the default pathway for IFR DCs is to generate Th2/Th3 responses. However, upon encounter with infectious agents, maturation of DCs is triggered by interaction with some components of the invading microorganism such as LPS 42 43. This maturation of DCs leads to secretion of high levels of IL-12, which induces T cells to secrete IFN-γ resulting in Th1 responses. In support of this hypothesis, IFN-γ secretion by PP T cells has been observed after gastrointestinal infection with microorganisms known to stimulate production of IL-12 by macrophages and DCs, such as Salmonella typhimurium 3 4 5 6 and Toxoplasma gondii 7. Efforts to test this hypothesis are currently underway by analyzing the phenotype of PP DCs in vivo after oral delivery of microbial stimuli. Such a default Th2/Th3 environment in the intestinal tract may be indispensable because aberrant Th1 induction in the intestine is strongly associated with pathogenesis of inflammatory bowel diseases such as Crohn's disease 44.
Acknowledgments
We thank Drs. R. Seder, S. Gurunathan, and W. Strober for their editorial assistance.
Footnotes
1used in this paper: β2m, β2 microglobulin; DC, dendritic cell; ICAM, intracellular adhesion molecule; IFR, interfollicular T cell regions; PCC, pigeon cytochrome c; PP, Peyer's patch(es); RT-PCR, reverse transcriptase PCR; SED, subepithelial dome; SP, spleen(s)
A. Iwasaki is a recipient of the Medical Research Council of Canada post doctoral fellowship.
References
- Daynes R.A., Araneo B.A., Dowell T.A., Huang K., Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J. Exp. Med. 1990;171:979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Inobe J., Marks R., Gonnella P., Kuchroo V.K., Weiner H.L. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- Hess J., Ladel C., Miko D., Kaufmann S.H. Salmonella typhimurium aroA-infection in gene-targeted immunodeficient micemajor role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J. Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- Everest P., Allen J., Papakonstantinopoulou A., Mastroeni P., Roberts M., Dougan G. Salmonella typhimurium infections in mice deficient in interleukin-4 productionrole of IL-4 in infection-associated pathology. J. Immunol. 1997;159:1820–1827. [PubMed] [Google Scholar]
- George A. Generation of gamma interferon responses in murine Peyer's patches following oral immunization. Infect. Immun. 1996;64:4606–4611. doi: 10.1128/iai.64.11.4606-4611.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karem K.L., Kanangat S., Rouse B.T. Cytokine expression in the gut associated lymphoid tissue after oral administration of attenuated Salmonella vaccine strains. Vaccine. 1996;14:1495–1502. doi: 10.1016/s0264-410x(96)00118-1. [DOI] [PubMed] [Google Scholar]
- Liesenfeld O., Kosek J.C., Suzuki Y. Gamma interferon induces Fas-dependent apoptosis of Peyer's patch T cells in mice following peroral infection with Toxoplasma gondii . Infect. Immun. 1997;65:4682–4689. doi: 10.1128/iai.65.11.4682-4689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth T., Strober W., Kelsall B.L. High dose oral tolerance in ovalbumin TCR-transgenic micesystemic neutralization of IL-12 augments TGF-beta secretion and T cell apoptosis. J. Immunol. 1996;157:2348–2357. [PubMed] [Google Scholar]
- Chen Y., Inobe J., Weiner H.L. Inductive events in oral tolerance in the TCR transgenic adoptive transfer model. Cell. Immunol. 1997;178:62–68. doi: 10.1006/cimm.1997.1119. [DOI] [PubMed] [Google Scholar]
- Stumbles P.A., Thomas J.A., Pimm C.L., Lee P.T., Venaille T.J., Proksch S., Holt P.G. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med. 1998;188:2019–2031. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsall B.L., Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer's patch. J. Exp. Med. 1996;183:237–247. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K.M., Heimberger A.B., Loh D.Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Bouaboula M., Legoux P., Pessegue B., Delpech B., Dumont X., Piechaczyk M., Casellas P., Shire D. Standardization of mRNA titration using a polymerase chain reaction method involving co-amplification with a multispecific internal control. J. Biol. Chem. 1992;267:21830–21838. [PubMed] [Google Scholar]
- Metlay J.P., Witmer-Pack M.D., Agger R., Crowley M.T., Lawless D., Steinman R.M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J. Exp. Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R.A., Paul W.E., Davis M.M., Fazekas de St. Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J. Exp. Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suss G., Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas ligand–induced apoptosis. J. Exp. Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D., Shortman K. Dendritic cell subtypes in mouse lymphoid organscross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J. Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- Vremec D., Zorbas M., Scollay R., Saunders D.J., Ardavin C.F., Wu L., Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleeninvestigation of the CD8 expression by a subpopulation of dendritic cells. J. Exp. Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P.H., Kagnoff M.F. Transforming growth factor-beta 1 is a costimulator for IgA production. J. Immunol. 1990;144:3411–3416. [PubMed] [Google Scholar]
- Lebman D.A., Lee F.D., Coffman R.L. Mechanism for transforming growth factor beta and IL-2 enhancement of IgA expression in lipopolysaccharide-stimulated B cell cultures. J. Immunol. 1990;144:952–959. [PubMed] [Google Scholar]
- McIntyre T.M., Kehry M.R., Snapper C.M. Novel in vitro model for high-rate IgA class switching. J. Immunol. 1995;154:3156–3161. [PubMed] [Google Scholar]
- Friedman A., Weiner H.L. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc. Natl. Acad. Sci. USA. 1994;91:6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Kuchroo V.K., Inobe J., Hafler D.A., Weiner H.L. Regulatory T cell clones induced by oral tolerancesuppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Hsieh C.S., Macatonia S.E., O'Garra A., Murphy K.M. T cell genetic background determines default T helper phenotype development in vitro. J. Exp. Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedl C., Hubele S. Maturation of Peyer's patch dendritic cells in vitro upon stimulation via cytokines or CD40 triggering. Eur. J. Immunol. 1997;27:1325–1330. doi: 10.1002/eji.1830270605. [DOI] [PubMed] [Google Scholar]
- Barral-Netto M., Barral A., Brownell C.E., Skeiky Y.A., Ellingsworth L.R., Twardzik D.R., Reed S.G. Transforming growth factor-beta in leishmanial infectiona parasite escape mechanism. Science. 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- Takeuchi M., Alard P., Streilein J.W. TGF-beta promotes immune deviation by altering accessory signals of antigen-presenting cells. J. Immunol. 1998;160:1589–1597. [PubMed] [Google Scholar]
- Takeuchi M., Kosiewicz M.M., Alard P., Streilein J.W. On the mechanisms by which transforming growth factor-beta 2 alters antigen-presenting abilities of macrophages on T cell activation. Eur. J. Immunol. 1997;27:1648–1656. doi: 10.1002/eji.1830270709. [DOI] [PubMed] [Google Scholar]
- Fargeas C., Wu C.Y., Nakajima T., Cox D., Nutman T., Delespesse G. Differential effect of transforming growth factor beta on the synthesis of Th1- and Th2-like lymphokines by human T lymphocytes. Eur. J. Immunol. 1992;22:2173–2176. doi: 10.1002/eji.1830220833. [DOI] [PubMed] [Google Scholar]
- Assoian R.K., Fleurdelys B.E., Stevenson H.C., Miller P.J., Madtes D.K., Raines E.W., Ross R., Sporn M.B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc. Natl. Acad. Sci. USA. 1987;84:6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst G.R., Smale G., Pencev D. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J. Cell. Physiol. 1989;140:396–402. doi: 10.1002/jcp.1041400226. [DOI] [PubMed] [Google Scholar]
- Fiorentino D.F., Zlotnik A., Mosmann T.R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- Bird J.J., Brown D.R., Mullen A.C., Moskowitz N.H., Mahowald M.A., Sider J.R., Gajewski T.F., Wang C.R., Reiner S.L. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- De Becker G., Moulin V., Tielemans F., De Mattia F., Urbain J., Leo O., Moser M. Regulation of T helper cell differentiation in vivo by soluble and membrane proteins provided by antigen-presenting cells. Eur. J. Immunol. 1998;28:3161–3171. doi: 10.1002/(SICI)1521-4141(199810)28:10<3161::AID-IMMU3161>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Everson M.P., Lemak D.G., McDuffie D.S., Koopman W.J., McGhee J.R., Beagley K.W. Dendritic cells from Peyer's patch and spleen induce different T helper cell responses. J. Interferon Cytokine Res. 1998;18:103–115. doi: 10.1089/jir.1998.18.103. [DOI] [PubMed] [Google Scholar]
- Duncan D.D., Swain S.L. Role of antigen-presenting cells in the polarized development of helper T cell subsetsevidence for differential cytokine production by Th0 cells in response to antigen presentation by B cells and macrophages. Eur. J. Immunol. 1994;24:2506–2514. doi: 10.1002/eji.1830241037. [DOI] [PubMed] [Google Scholar]
- Schmitz J., Assenmacher M., Radbruch A. Regulation of T helper cell cytokine expressionfunctional dichotomy of antigen-presenting cells. Eur. J. Immunol. 1993;23:191–199. doi: 10.1002/eji.1830230130. [DOI] [PubMed] [Google Scholar]
- Gracie J.A., Bradley J.A. Interleukin-12 induces interferon-gamma-dependent switching of IgG alloantibody subclass. Eur. J. Immunol. 1996;26:1217–1221. doi: 10.1002/eji.1830260605. [DOI] [PubMed] [Google Scholar]
- Saoudi A., Kuhn J., Huygen K., de Kozak Y., Velu T., Goldman M., Druet P., Bellon B. TH2 activated cells prevent experimental autoimmune uveoretinitis, a TH1-dependent autoimmune disease. Eur. J. Immunol. 1993;23:3096–3103. doi: 10.1002/eji.1830231208. [DOI] [PubMed] [Google Scholar]
- Pulendran B., Smith J.L., Caspary G., Brasel K., Pettit D., Maraskovsky E., Maliszewski C.R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Lopez R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. CD8α1 and CD8α2 subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzler C., Rovere P., Rescigno M., Granucci F., Penna G., Adorini L., Zimmermann V.S., Davoust J., Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C., Hieny S., Scharton-Kersten T., Jankovic D., Charest H., Germain R.N., Sher A. In vivo microbial stimulation induces rapid CD40 ligand–independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parronchi P., Romagnani P., Annunziato F., Sampognaro S., Becchio A., Giannarini L., Maggi E., Pupilli C., Tonelli F., Romagnani S. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am. J. Pathol. 1997;150:823–832. [PMC free article] [PubMed] [Google Scholar]