B Cell Antigen Receptor Specificity and Surface Density Together Determine B-1 versus B-2 Cell Development (original) (raw)
Abstract
Mice expressing the immunoglobulin (Ig) heavy (H) chain variable (V) region from a rearranged VH12 gene inserted into the IgH locus generate predominantly B-1 cells, whereas expression of two other VH region transgenes (VHB1-8 and VHglD42) leads to the almost exclusive generation of conventional, or B-2, cells. To determine the developmental potential of B cells bearing two distinct B cell antigen receptors (BCRs), one favoring B-1 and the other favoring B-2 cell development, we crossed VH12 insertion mice with mice bearing either VHB1-8 or VHglD42. B cells coexpressing VH12 and one of the other VH genes are readily detected in the double IgH insertion mice, and are of the B-2 phenotype. In mice coexpressing VH12, VHB1-8 and a transgenic κ chain able to pair with both H chains, double H chain–expressing B-2 cells, and B-1 cells that have lost VHB1-8 are generated, whereas VHB1-8 single producers are undetectable. These data suggest that B-1 but not B-2 cells are selected by antigenic stimuli in whose delivery BCR specificity and surface density are of critical importance.
Keywords: B-1 cells, B-2 cells, immunoglobulin heavy chain, allelic inclusion, gene targeting
Two major B cell subsets, designated B-1 and B-2, exist in humans and mice. B-1 and B-2 cells can be distinguished by their cell surface phenotypes and anatomical localization. B-1 cells, found mainly in the pleural and peritoneal cavities, express high levels of surface IgM, low levels of B220 and IgD, and moderate levels of CD5. In addition, they do not express CD23. In contrast, conventional, or B-2, cells are the predominant B cells found in the spleen and lymph nodes; they express high levels of B220, IgD, and CD23 and moderate levels of IgM, and lack surface CD5 expression (for review see references 1, 2).
Different VH genes are preferentially expressed in B-1 and B-2 cells 3 4. The distinct VH repertoire that is found in B-1 cells has led to the hypothesis that the specificity of the B cell antigen receptor (BCR)1 may in fact determine the differentiation of B cells into this subset 5 6. It is known that antibodies that react with phosphatidyl choline (PtC) are produced largely by B-1 cells 7 and are mostly encoded by either of two H and L chain combinations, namely VH12 and Vκ4 or VH11 and Vκ9. Consistent with the view that BCR specificity plays a role in the development of B-1 cells, transgenic mice expressing VH12 alone, or in combination with Vκ4, generate mostly B-1 cells in all of the peripheral lymphoid organs, including spleens and lymph nodes 8.
We have generated by gene targeting various strains of Ig H chain insertion mice carrying different H chain variable (V) region genes targeted into their physiological position in the IgH locus. The inserted VHDHJH elements include the segments designated VH12 8, VHB1-8 9, and VHglD42 10. Similar to conventional VH12 transgenic mice, VH12 insertion mice develop mainly B-1 cells. In contrast, mice whose IgH alleles were engineered to express VHB1-8 or VHglD42 develop mainly B-2 cells. This again suggests that BCR specificity may play a determining role in the differentiation of B-1 cells. If indeed signals transmitted through a BCR of a certain specificity lead to the generation of B-1 cells, then interference with the cell surface expression of that specific BCR may alter the differentiation process of these cells. Here, we generated IgH double (VH12 and VHB1-8 or VH12 and VHglD42) insertion mice to test whether the expression of a second H chain in VH12-expressing B cells may act in a dominant negative manner to perturb the generation of B-1 cells.
Materials and Methods
Mice.
The B1-8f 11 and glD42i 10 IgH insertion mice were generated in the laboratory in Cologne and in collaboration with D. Eilat's group at Hebrew University (Jerusalem, Israel), and have been described previously (references 10 and 11, as indicated behind each mouse strain). The generation of the VH12f mice will be described elsewhere (Lam, K.-P., and K. Rajewsky, manuscript in preparation). The conventional Vκ4 transgenic mice 8 were obtained from Stephen Clarke (University of North Carolina, Chapel Hill, NC). Mice used were 2–4 mo old and maintained in a conventional animal facility.
Antibodies.
The following mAbs used in this study were produced and conjugated to fluorochromes in our laboratory: anti-B220 (RA3-6B2); anti-IgM (R33-24.12); anti-IgD (1.3-5); anti-CD43 (S7); anti-μa(RS3.1); anti-μb (MB86); anti–VHB1-8 (Ac146); and anti-VH12 (5C5). The anti-CD5 and anti-CD23 mAbs were purchased from PharMingen.
FACS® Analyses and Cell Sorting.
Tissues and cell preparations for flow cytometric analyses and cell sorting were prepared as previously described 12. In brief, spleen cells were prepared by dissociation between frosted glass slides. Peritoneal cavity and bone marrow cells were obtained by injecting staining medium (PBS containing 3% FCS and 0.1% NaN3) into the peritoneal cavity and femurs and tibia, respectively, using a 1-ml syringe with a 26-gauge needle. All cells were treated with RBC lysing solution (0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM Na2EDTA) to eliminate erythrocytes. For FACS® analyses, cells were stained with optimal amounts of FITC-, PE-, and biotin-conjugated mAbs for 10 min on ice and washed three times with staining medium. Biotin-conjugated mAbs were revealed with streptavidin-Cychrome. Flow cytometry analyses were performed on a FACScan™ (Becton Dickinson) and cell sorting was done on a FACStarPLUS™.
Southern Blot Analysis.
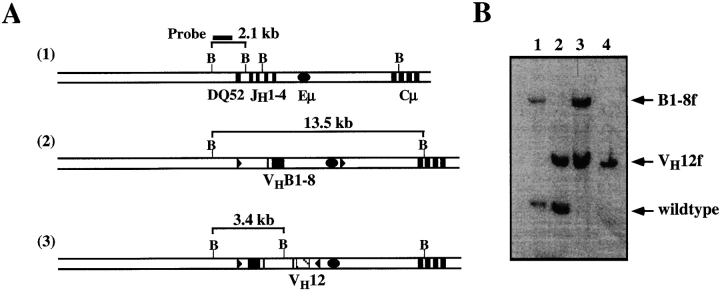
Genomic DNA was prepared from mouse livers and sorted splenic B cells 13, digested with BamHI and fractionated on a 1% agarose gel. After capillary transfer, the membrane was hybridized with a random-primed α-P32–labeled specific probe, as shown in Fig. 5.
Figure 5.
Single VH12, Vκ4–expressing B cells in VH12f/B1-8f, Vκ4tg mice have lost the targeted B1-8f H chain allele. (A) Structures of the wild-type IgH locus 1, the targeted locus bearing the B1-8 VDJ 2, and the targeted locus bearing the VH12 VDJ 3 are shown together with the size of the respective restriction fragments. Genomic DNA was digested with BamHI and hybridized with the indicated probe. Maps are not drawn to scale. (B) Southern blot analysis of DNA from livers of B1-8f/+, Vκ4tg (lane 1); VH12f/+, Vκ4tg (lane 2); and VH12f/B1-8f, Vκ4tg (lane 3) mice; and FACS® sorted 5C5+Ac146− splenic B cells of VH12f/B1-8f, Vκ4tg mice (lane 4).
Results
Different B Cell Populations Are Generated in glD42i, B1-8f, and VH12f Mice.
We used gene targeting to generate a series of IgH insertion mice in which the JH locus was replaced by distinct VHDHJH segments 14. These segments were taken from the 4-hydroxy-3-nitrophenyl acetyl–binding antibody, B1-8 9; the antibody glD42, which is a reduced affinity mutant of the DNA-binding antibody, D42 10; and the anti-PtC antibody, VH12 8. The corresponding mice were designated B1-8f, glD42i, and VH12f respectively. The B1-8f 11 and glD42i 10 mice have been described previously, whereas the generation of the VH12f mice will be described elsewhere (Lam, K.-P., and K. Rajewsky, manuscript in preparation).
Flow cytometric characterizations of the B cell populations in the spleens of wild-type, glD42i, B1-8f, and VH12f mice are depicted in Fig. 1. The majority of the B cells present in glD42i (Fig. 1 A) and B1-8f (Fig. 1 B) mice are B-2 cells in that they express high levels of CD23 (shown for glD42i mice), IgD (shown for B1-8f mice), and B220, the pan-B cell marker. In addition, they do not express CD5, a marker found on T and B-1 cells. In contrast, VH12f mice generate cells predominantly of the B-1 phenotype in that they express intermediate levels of CD5, low levels of B220 and IgD, and no detectable CD23. These data are consistent with a previous report that showed the preferential generation of B-1 cells in the lymphoid organs of conventional VH12-transgenic mice 8. Thus, different VH gene specificity seems to bias the generation and/or selection of different B cell subsets in the mouse.
Figure 1.
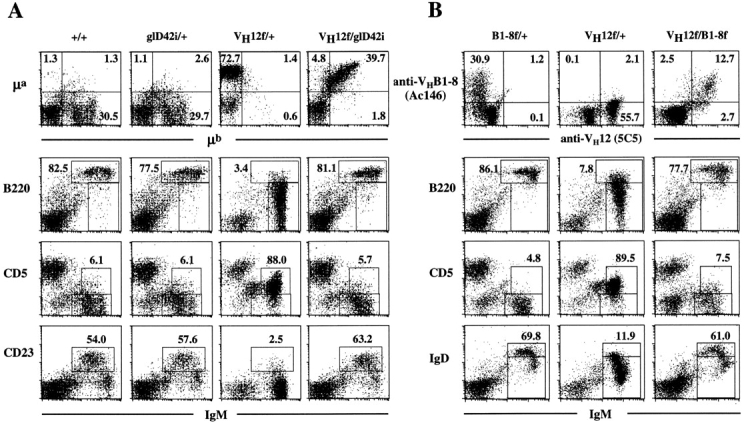
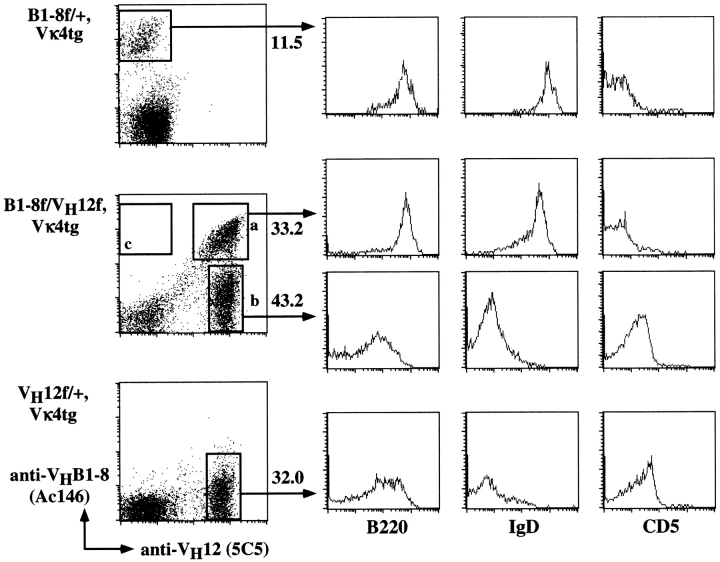
Phenotype of splenic B cells in (A) wild-type, glD42i/+, VH12f/+, and VH12f/glD42i; and (B) B1-8f/+, VH12f/+, and VH12f/B1-8f IgH insertion mice. Spleen cells obtained from wild-type and various Ig H chain insertion mice were stained with fluorochrome-conjugated allotypic (A, top) and idiotypic (B, top) antibodies as well as antibodies that recognize various cell surface markers used to define B-1 and B-2 cells. Numbers indicate percentage of total lymphocytes in the top panels and percentage of total B220+ cells in the others.
VH12-expressing B Cells Bearing a Second In-frame V Region Gene Develop into Conventional B Cells.
B cell development under the condition of H chain allelic inclusion had previously been analyzed in mice bearing VHB1-8 and VHglD42 15. In these double IgH insertion mice, B cells expressing two functional VH alleles are readily generated in the bone marrow and are not counter-selected in the peripheral lymphoid organs. Here, we cross VH12f mice with glD42i and B1-8f mice to examine the developmental potential of B cells bearing two distinct BCRs, one that is preferentially expressed in B-1 and the other in B-2 cells.
Expression of the VH12 and glD42 alleles in B cells can be identified by the expression of their constant regions as the former is of the a and the latter, of the b allotype. Flow cytometric analyses of the B cells in double VH12f/glD42i mice revealed that the majority of the cells in the spleen (Fig. 1 A), bone marrow, and lymph nodes (data not shown) of these mice coexpress both VH genes. Similar results were also obtained when VH12f mice were crossed with B1-8f mice. Expression of VH12 and VHB1-8 can be distinguished by staining with the anti-idiotype (Id) mAb 5C5 8 and Ac146 9 respectively. The 5C5 mAb recognizes VH12 independent of the L chains 8 whereas the Ac146 mAb recognizes VHB1-8 in association with λ and the majority (∼80%) of the κ L chains 9. As shown in Fig. 1 B, the majority of the splenic B cells in VH12f/B1-8f mice coexpress both Ids, indicating that they are double-IgH expressors. This is also true for the B cells in the bone marrow and lymph nodes of these mice (data not shown). Taken together, these data indicate that VH12-expressing B cells can coexpress another VH gene.
Surprisingly, phenotypic characterization of the IgH “double-producers” in VH12f/glD42i and VH12f/B1-8f mice revealed that these B cells express high levels of B220 and IgD (shown for VH12f/B1-8f mice; Fig. 1 B); and the majority of them are also CD23-positive (shown for VH12f/glD42i mice; Fig. 1 A). In addition, these double-producers do not express CD5 on their cell surfaces. Thus, in contrast to B cells that express VH12 only, B cells that coexpress VH12/VHglD42, or VH12/VHB1-8 assumed a phenotype that is characteristic of B-2 cells.
Development of B Lymphocytes that Coexpress VH12 and VHB1-8 into Conventional B-2 Cells Is Not Due to Restricted Light Chain Usage.
The specificity of the BCR is determined by the variable regions of the H and L chains. Thus, the loss of the B-1 phenotype in cells coexpressing VH12 and VHB1-8 or VH12 and VHglD42 may be due to altered Ig L chain usage. It is conceivable that the L chains that associate with both VH12 and VHB1-8 or VH12 and VHglD42 under the condition of H chain allelic inclusion are different from those that normally associate with VH12 alone. This altered L chain usage could affect the specificity of the VH12 receptor and thus could influence the generation and/or selection of B-1 cells. To examine this possibility, we crossed Vκ4 L chain transgenic (tg) mice 8 with VH12f/+, B1-8f/+, and VH12f/B1-8f mice. The Vκ4 gene used in the generation of the transgenic mice was initially isolated from a CD5+ B lymphoma cell line that together with VH12 recognizes PtC 8. In addition, this Vκ4 L chain can also associate with the B1-8 H chain to form a BCR of innocuous specificity. Association of the Vκ4 L chain with either or both VH12 and VHB1-8 is demonstrated in Fig. 2. We had previously shown that the bone marrow pre-B cell compartment is absent in Ig transgenic mice whose H and L chains pair to form a BCR of an innocent specificity 16. This probably reflects the fact that precursor cells carrying functional Ig H and L chain transgenes rapidly differentiate into IgM+ B cells. We have used this phenomenon to examine the association of Vκ4 with both VH12 and VHB1-8. As shown in Fig. 2, B220+ CD43− pre-B cells are present in wild-type and in the various single and double IgH tg mice and represent ∼8% of the cells present. However, this population is fivefold reduced in the B1-8f/+, VH12f/+, and B1-8f/VH12f H chain tg mice that also carry the Vκ4 L chain transgene. This suggests that the Vκ4 L chain can associate efficiently with both VHB1-8 and VH12. Association of Vκ4 with VHB1-8 is also evident in the splenic B cell population of B1-8f/+, Vk4tg mice, as the cells expressing this H and L chain combination are all Ac146 Id+ (Fig. 3, top).
Figure 2.
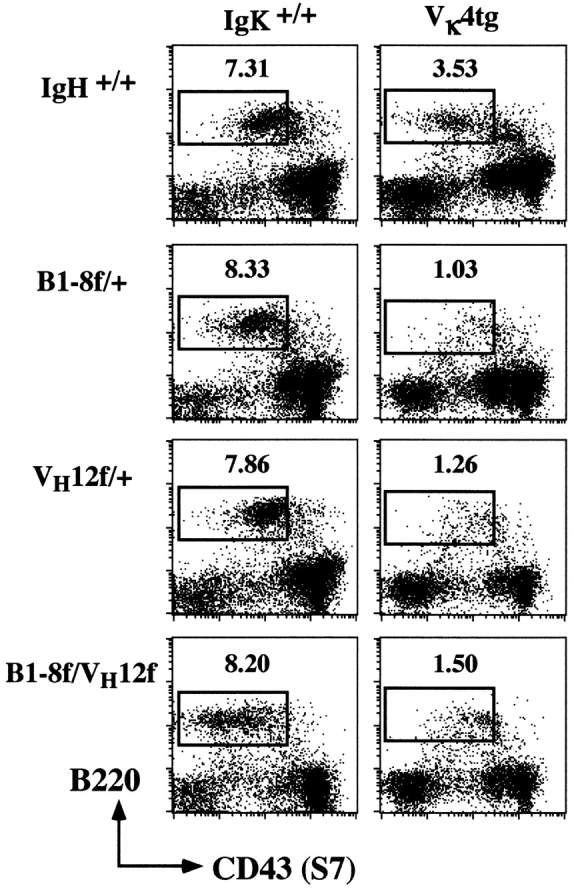
Association of the Vκ4 L chain with the VH12 and VHB1-8 H chains. Bone marrow cells from wild-type, VH12f/+, B1-8f/+, or VH12f/B1-8f mice with or without the Vκ4 L chain transgene were stained with anti-B220, anti-CD43, and anti-IgM mAbs. The figure depicts the surface IgM− cells and the boxed area indicates the pre-B cell compartment in the bone marrow. Numbers indicate percentage of total lymphocytes.
Figure 3.
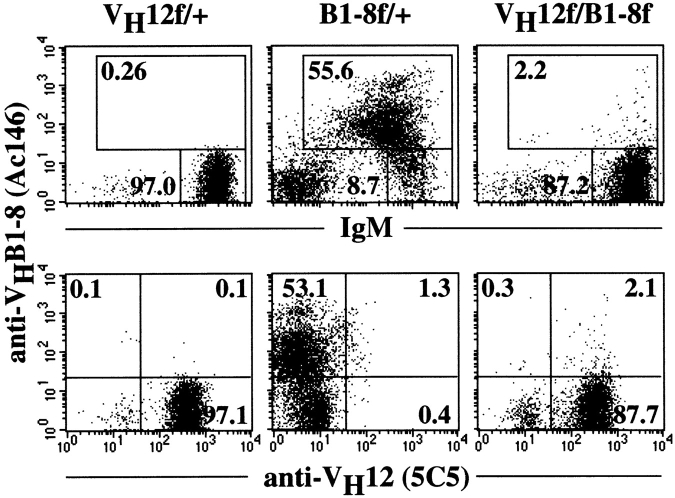
B cells coexpressing VH12, Vκ4, and VHB1-8, Vκ4 assume a conventional B cell phenotype. Phenotype of the various splenic B cell populations found in B1-8f/+, Vκ4tg; VH12f/+, Vκ4tg; and VH12f/B1-8f, Vκ4tg mice. Cells were stained with antiidiotypic Ac146 and 5C5 mAbs, and with anti-B220, anti-IgD, and anti-CD5 mAbs. Numbers indicate percentage of total splenic lymphocytes.
Phenotypic analyses of splenic B lymphocytes in B1-8f/+, Vκ4tg mice indicate that these cells are predominantly B-2 cells (Fig. 3, top) as they are B220high(hi), IgDhi, and CD5+. In comparison, splenic B cells present in VH12f/+, Vκ4tg mice are of the B-1 phenotype as indicated by their lower levels of B220 and IgD expression. Furthermore, these cells are CD5+ (Fig. 3, bottom), in agreement with previous published data 8.
Interestingly, two populations of B cells are present in the spleens of VH12f/B1-8f, Vκ4tg mice (Fig. 3, middle). The Ac146+5C5+ population (fraction a) represents double producers that coexpress VH12, Vκ4, and VHB1-8, Vκ4 receptors. The Ac146−5C5+ population (fraction b) seems to have lost the surface expression of the B1-8 H chain and appears to express only VH12, Vκ4. In contrast, Ac146+ 5C5− cells (fraction c) that express only VHB1-8, Vκ4 are not seen in these mice.
FACS® analyses of the Ac146+5C5+ double producers indicate that these cells are conventional B cells in phenotype, as they express high levels of B220 and IgD and lack CD5 expression. They are indistinguishable from the B cells found in B1-8f/+; B1-8f/+, Vκ4tg; or VH12f/B1-8f and VH12f/glD42i mice. Thus, the development of allelically included VH12-expressing B lymphocytes into conventional B cells is not likely to be due to altered L chain usage, as it occurs also in the presence of the Vκ4 transgene.
B Cells Expressing VH12 only in VH12f/B1-8f Mice Have Lost the B1-8f Allele.
The Ac146−5C5+ B cells present in the spleens of VH12f/B1-8f, Vκ4tg mice appear to have lost the surface expression of the B1-8 H chain. Thus, they are de facto single VH12, Vκ4 expressors and, not surprisingly, have a B-1 phenotype (Fig. 3, middle).
Further analyses revealed that B cells expressing VH12 only can also be found in the peritoneal cavities of VH12f/B1-8f (Fig. 4) and VH12f/glD42i (data not shown) mice that do not carry the Vκ4 L chain transgene. As shown in Fig. 4, the Ac146−5C5+ cells comprise a large fraction (>90%) of the B cells present in the peritoneal cavity of these mice and possess, as expected, a B-1 cell phenotype (data not shown). Although we cannot exclude the possibility that cells bearing VHB1-8 but not expressing the Ac146 Id (due to pairing with certain L chains) are also included in this population, such cells should represent a minor fraction. This is supported by FACS® analysis of control B1-8f/+ mice (Fig. 4), which suggests that the majority of the B cells (>80%) in the peritoneal cavity express the Ac146 Id.
Figure 4.
B cell populations found in the peritoneal cavity of B1-8f/+, VH12f/+, and VH12f/B1-8f mice. Peritoneal cavity cells of the various single and double IgH insertion mice were stained with antiidiotypic Ac146 and anti-IgM (top) and Ac146 and 5C5 (bottom) mAbs. Numbers indicate percentage of total lymphocytes.
To determine the nature of the lack of B1-8 gene expression in these VH12-only cells, we first sorted 5C5+Ac146− cells from the spleen of VH12f/B1-8f, Vκ4tg mice and analyzed the targeted IgH loci by Southern blotting using a probe located 5′ of the DQ52 region. The wild-type, targeted VH12f and B1-8f alleles should yield fragment sizes of 2.3, 3.4, and 13.5 kb (Fig. 5A and Fig. B, lanes 1–3), respectively. In the 5C5+Ac146− B cells sorted from the spleen of VH12f/B1-8f, Vκ4tg mice, the band corresponding to the targeted B1-8f allele is missing (Fig. 5 B, lane 4), suggesting that the gene has been replaced. Similar results were also obtained from 5C5+Ac146− B cells isolated from the peritoneal cavities of VH12f/B1-8f mice (data not shown). Thus, 5C5+Ac146− B cells in the spleens and peritoneal cavity of VH12f/B1-8f, Vκ4tg or in the peritoneal cavity of VH12f/B1-8f have lost VHB1-8 and consequently express only VH12. Loss of the B1-8f allele could occur by rearrangement of upstream V or D gene segments into the B1-8 VDJ and resulting in a nonfunctional allele 17 18 19. It is not known presently at which developmental stage the replacement of the B1-8f allele occurs. FACS® analyses of the bone marrow of VH12f/B1-8f or VH12f/B1-8f, Vκ4tg mice suggest that the immature and mature B lymphocytes in this compartment are predominantly double producers (data not shown).
Reduced Expansion of B Lymphocytes in Mice Coexpressing VH12 and Another H Chain.
Assessment of the number of B cells in wild-type and various IgH insertion mice revealed that VH12-expressing B lymphocytes undergo pronounced cellular expansion. As shown in Table , 5H12f/+ mice kept in a conventional animal facility generally have 2- and 20-fold more B cells in the spleen and peritoneal cavity respectively, compared with wild-type, B1-8f/+, or glD42i/+ mice. Interestingly, the number of splenic B cells in mice coexpressing VH12 and either VHB1-8 or VHglD42 is similar to that in wild-type, B1-8f/+, or glD42i/+ mice, suggesting that the expansion of VH12-expressing B cells is curtailed in these mice. However, the number of peritoneal B cells in VH12f/B1-8f or VH12f/glD42i mice is similar to that in VH12f/+ mice. This probably reflects the fact that the B cells that accumulate in the peritoneal cavities of the double IgH insertion mice are mainly VH12 expressors (see Fig. 4) that have lost expression of the other H chain (see Fig. 5).
Table 1.
Number of B Cells in the Spleen and Peritoneal Cavity of Wild-type and Various Ig tg Mice
| Genotype | Spleen (× 107) | Peritoneal cavity (× 106) | |
|---|---|---|---|
| +/+ | 2.45 ± 0.83 | 0.85 ± 0.28 | |
| VH12f/+ | 5.76 ± 0.26 | 15.40 ± 6.72 | |
| B1-8f/+ | 2.47 ± 0.42 | 0.24 ± 0.11 | |
| glD42i/+ | 1.08 ± 0.47 | 0.76 ± 0.26 | |
| VH12f/B1-8f | 2.00 ± 0.13 | 11.63 ± 0.38 | |
| VH12f/glD42i | 1.60 ± 0.21 | 10.58 ± 0.32 | |
| +/+ | Vκ4tg | 2.20 ± 0.69 | 0.95 ± 0.33 |
| B1-8f/+ | Vκ4tg | 1.60 ± 0.40 | 0.31 ± 0.10 |
| VH12f/+ | Vκ4tg | 15.19 ± 1.71 | 13.20 ± 3.70 |
| VH12f/B1-8f | Vκ4tg | 6.96 ± 1.90 | 11.20 ± 2.53 |
The presence of a Vκ4 L chain transgene leads to an even greater increase in the number of splenic B cells as VH12f/+, Vκ4tg mice have threefold more cells than do VH12f/+ mice and eightfold more cells than do either Vκ4tg or B1-8f/+, Vκ4tg mice. This is probably due to ligand-mediated clonal expansion, as VH12 together with Vκ4 recognizes PtC 8. Again, this expansion is modulated in VH12/B1-8f, Vκ4tg mice (Table ). The three- to fivefold increase in the number of splenic B cells in this mouse strain compared with Vκ4tg or B1-8f/+, Vκ4tg mice is probably due to the fact that >50% of these cells in VH12f/B1-8f, Vκ4tg mice are single VH12 expressors (Fig. 3).
Discussion
VH12 insertion mice, like conventional VH12 transgenic mice 8, generate mostly B-1 cells, whereas VHB1-8 and VHglD42 insertion mice produce predominantly conventional, or B-2, cells. This is in line with the concept that BCR specificity is a major determinant in B-1 versus B-2 cell development. The novel findings in this study are that the coexpression of VH12 with either of the two other VH region genes in double IgH insertion mice (which express wild-type κ chains or a Vκ4 transgene) results in the generation of a population of double-producing B-2 cells. In addition, in such animals a population of single-producing B cells appears, namely, B-1 cells expressing only VH12.
Why do VH12-expressing B cells that coexpress a second H chain not differentiate into B-1 cells? This can perhaps, best be explained by postulating that signaling via a BCR of a certain specificity, expressed at the cell surface at high density, is required to drive the differentiation of B cells into the B-1 subset. In our experiments, the provision of a second H chain of a different specificity presumably acts in a “dominant-negative” manner to dilute out the VH12-containing BCR complexes on the cell surface. Assuming equivalent production of H chains from the various inserted VHDHJH segments and equivalent pairing of the H and L chains involved, only 25% of the Ig molecules on the cell surface of double-producing cells would carry VH12 regions on both H chains. This reduced density of B-1–specific BCRs may not provide sufficient signal for the development of B-1 phenotype.
The hypothesis that BCR signaling is responsible for the development of the B-1 cell phenotype has been supported by experiments that show that under certain conditions the cross-linking of sIg on splenic B cells may lead to development of a B-1 cell phenotype on B-2 cells 20. The skewed development of B-1 and B-2 cell subsets in many gene-targeted mice with mutations in specific signaling molecules is also consistent with this hypothesis. For example, CD19-deficient mice 21 22 and xid 23 mice that have a mutation in the btk gene have reduced numbers and a lack of B-1 cells, respectively. In contrast, lyn-deficient mice 24 25 and motheaten mice that have a mutation in SHP-1 26 have increased numbers of B-1 cells. More significantly, the introduction of the xid defect into VH12 conventional transgenic mice leads to the predominance of VH12-expressing B cells with a B-2 cell phenotype 27, compared with wild-type VH12 transgenic mice that generate mostly B-1 cells.
The appearance of large numbers of VH12-expressing B-1 cells in the double mutants that have lost expression of the second H chain is of particular interest. Such loss variants are rare in VHB1-8/VHglD42 mice 15, emphasizing the stability of these targeted IgH loci in B cell development. This suggests that the VH12-only cells in the present system are strongly selected, in accord with the concept that B-1 cell development is driven by BCR signaling. It will be of interest to determine at which stage of development the loss of the second IgH allele occurs in these cells; and whether its loss in mature (B-2) double producers will change their phenotype to that of B-1 cells.
It is apparent that B-2 cell development depends to a lesser extent than that of B-1 cells on density of BCRs of certain specificities at the cell surface (reference 15 and the data presented here). This might reflect a lesser dependence of B-2 cells on positive selection by (self)-antigens. The requirement of BCR expression for B-2 cell survival 11 would then largely be a cell-autonomous phenomenon.
Acknowledgments
We thank Drs. S. Clarke and G. Haughton for the Vκ4 transgenic mice and the anti-VH12 Id mAb (5C5), respectively; and C. Koenigs, A.-T. Tan, C. Kanellopoulou, and C. Gottlinger for expert technical help.
The project was initiated in the Rajewsky laboratory where K.-P. Lam was an EMBO and HFSP fellow and completed in the latter's laboratory at IMCB where he is now an independent investigator. This work was supported by the Volkswagen Foundation, the Deutsche Forschungsgemeinschaft through SFB 243, the Human Frontier Science Program, the Körber Foundation, the Land Nordrhein-Westafalen, and by the National Science and Technology Board of Singapore.
Footnotes
1used in this paper: BCR, B cell receptor; Id, idiotype; PtC, phosphatidyl choline; tg, transgenic
References
- Herzenberg L.A., Stall A.M., Lalor P.A., Sidman C., Moore W.A., Parks D., Herzenberg L.A. The Ly-1 B cell lineage. Immunol. Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Kantor A.B., Herzenberg L.A. Origin of murine B cell lineages. Annu. Rev. Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- Lalor P.A., Morahan G. The peritoneal Ly-1 (CD5) B cell repertoire is unique among murine B cell repertoires. Eur. J. Immunol. 1990;20:485–492. doi: 10.1002/eji.1830200305. [DOI] [PubMed] [Google Scholar]
- Pennell C.A., Arnold L.W., Haughton G., Clarke S.H. Restricted Ig variable region gene expression among Ly-1+ B cell lymphomas. J. Immunol. 1988;141:2788–2796. [PubMed] [Google Scholar]
- Haughton G., Arnold L.W., Whitmore A.C., Clarke S.H. B-1 cells are made, not born. Immunol. Today. 1993;14:84–87. doi: 10.1016/0167-5699(93)90064-R. [DOI] [PubMed] [Google Scholar]
- Wortis H.H. Surface markers, heavy chain sequences and B cell lineages. Int. Rev. Immunol. 1992;8:235–246. doi: 10.3109/08830189209055576. [DOI] [PubMed] [Google Scholar]
- Mercolino T.J., Locke A.L., Afshari A., Sasser D., Travis W.W., Arnold L.W., Haughton G. Restricted immunoglobulin variable region gene usage by normal Ly-1 (CD5+) B cells that recognize phosphatidyl choline. J. Exp. Med. 1989;169:1869–1877. doi: 10.1084/jem.169.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L.W., Pennell C.A., McCray S.K., Clarke S.H. Development of B-1 cellssegregation of phosphatidyl choline–specific B cells to the B-1 population occurs after immunoglobulin gene expression. J. Exp. Med. 1994;179:1585–1595. doi: 10.1084/jem.179.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Hammerling G.J., Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57Bl/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur. J. Immunol. 1978;8:393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- Pewzner-Jung Y., Friedmann D., Sonoda E., Jung S., Rajewsky K., Eilat D. B cell deletion, anergy, and receptor-editing in “knock-in” mice targeted with a germline-encoded or somatically mutated anti-DNA heavy chain. J. Immunol. 1998;161:4634–4645. [PubMed] [Google Scholar]
- Lam K.-P., Kuhn R., Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Lam K.-P., Stall A.M. Major histocompatibility complex class II expression distinguishes two distinct B cell developmental pathways during ontogeny. J. Exp. Med. 1994;180:507–516. doi: 10.1084/jem.180.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P.W., Zijderveld A., Linders K., Rudnicki M.A., Jaenisch R., Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki S., Meiering M., Rajewsky K. Targeted insertion of a variable region gene into the immunoglobulin heavy chain locus. Science. 1993;262:1268–1271. doi: 10.1126/science.8235657. [DOI] [PubMed] [Google Scholar]
- Sonoda E., Pewzner-Jung Y., Schwers S., Taki S., Jung S., Eilat D., Rajewsky K. B cell development under the condition of allelic inclusion. Immunity. 1997;6:225–233. doi: 10.1016/s1074-7613(00)80325-8. [DOI] [PubMed] [Google Scholar]
- Pelanda R., Schwers S., Sonoda E., Torres R.M., Nemazee D., Rajewsky K. Receptor-editing in a transgenic mouse modelsite, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–775. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- Taki S., Schwenk F., Rajewsky K. Rearrangement of upstream DH and VH genes to a rearranged immunoglobulin variable region gene inserted into the DQ52-JH region of the immunoglobulin heavy chain locus. Eur. J. Immunol. 1995;25:1888–1896. doi: 10.1002/eji.1830250715. [DOI] [PubMed] [Google Scholar]
- Chen C., Nagy Z., Prak E.L., Weigert M. Immunoglobulin heavy chain gene replacementa mechanism of receptor editing. Immunity. 1995;3:747–755. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Cascalho M., Ma A., Lee S., Masat L., Wabl M. A quasi-monoclonal mouse. Science. 1996;272:1649–1652. doi: 10.1126/science.272.5268.1649. [DOI] [PubMed] [Google Scholar]
- Cong Y.Z., Rabin E., Wortis H.H. Treatment of murine CD5− B cells with anti-Ig, but not LPS, induces surface CD5two B-cell activation pathways. Int. Immunol. 1991;3:467–476. doi: 10.1093/intimm/3.5.467. [DOI] [PubMed] [Google Scholar]
- Rickert R., Rajewsky K., Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- Engel P., Zhou L.J., Ord D.C., Sato R., Koller B., Tedder T.F. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39–50. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- Satterthwaite A.B., Li Z., Witte O.N. Btk function in B cell development and response. Semin. Immunol. 1998;10:309–316. doi: 10.1006/smim.1998.0123. [DOI] [PubMed] [Google Scholar]
- Hibbs M.L., Tarlinton D.M., Armes J., Grail D., Hodgson G., Maglitto R., Stacker S.A., Dunn A.R. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Nishizumi H., Taniuchi I., Yamanashi Y., Kitamura D., Ilic D., Mori S., Watanabe T., Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Sidman C.L., Shultz L.D., Hardy R.R., Hayakawa K., Herzenberg L.A. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science. 1986;232:1423–1425. doi: 10.1126/science.3487115. [DOI] [PubMed] [Google Scholar]
- Clarke S.H., Arnold L.W. B-1 cell developmentevidence for an uncommitted immunoglobulin (Ig)M+ B cell precursor in B-1 cell differentiation. J. Exp. Med. 1998;187:1325–1334. doi: 10.1084/jem.187.8.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]