Expression Levels of B Cell Surface Immunoglobulin Regulate Efficiency of Allelic Exclusion and Size of Autoreactive B-1 Cell Compartment (original) (raw)
Abstract
Surface-expressed immunoglobulin (Ig) has been shown to have a critical role in allelic exclusion of Ig heavy (H) and light (L) chains. Although various degrees of suppression of endogenous Ig expression are observed in Ig transgenic (Tg) mice, it was not clear whether this difference is due to different onsets of Tg expression or to different levels of Tg expression, which are obviously affected by integration sites of the transgene. In this study we generated antierythrocyte antibody Tg mice that carry tandem joined H and L chain transgenes (H+L) and confirmed that homozygosity of the transgene loci enhances the level of transgene expression as compared with heterozygosity. Suppression of endogenous H and L chain gene expression was stronger in homozygous than in heterozygous Tg mice. Similar results were obtained in control Tg mice carrying the H chain only. These results suggest that there is a threshold of the B cell receptor expression level that induces allelic exclusion. In addition, despite the same B cell receptor specificity, the size of Tg autoreactive B-1 cell compartment in the peritoneal cavity is larger in homozygous than in heterozygous mice, although the number of the Tg B-2 cell subset decreased in the spleen and bone marrow of homozygous Tg mice as compared with heterozygous Tg mice. By contrast, homozygosity of the H chain alone Tg line, which does not recognize self-antigens, did not increase the size of the peritoneal B-1 subset. These results suggest that the size of the B-1 cell subset in the Tg mice may depend on strength of signals through B cell receptors triggered by self-antigens.
Keywords: transgenic lines, homozygosity, flow cytometry, anti-RBC antibody
Productive VHDHJH recombination in the Ig H chain locus of one allele of the B cell results in suppression of VHDHJH recombination in the other allele. Thus, a B cell expresses generally only one H chain gene out of two alleles 1. This phenomenon is called H chain allelic exclusion. Surface expression of the μ chain has a critical role in allelic exclusion of the H chain locus because targeted disruption of the μ chain membrane exon results in loss of H chain allelic exclusion 2. The essential role of the cell surface μ chain expression in allelic exclusion is also supported by studies using Ig transgenic (Tg)1 mice 3. Mice expressing the membrane-form μ chain transgene inhibit expression of the endogenous μ chain, whereas Tg mice with the secreted form μ chain do not show such inhibition 4 5 6. In addition, expression of Ig L chain transgenes also exerts suppression on the rearrangement and expression of the endogenous L chain Ig locus 6 7. However, the suppression of endogenous H and L chain gene expression in Ig Tg mice are generally not complete, and efficiencies of the suppression are variable among different lines of Tg mice 8 9 10 11 12 13 14 15 16 17. Variable integration sites of transgenes may influence the onset and level of transgene expression, which may affect the efficiency of allelic exclusion. Since the V(D)J recombination event in each B cell is all or none, it has not been clear whether allelic exclusion can be induced by a small number of surface μ chain or if it requires a relatively higher number of surface μ chain (a threshold). To examine these possibilities, quantitative comparison between the levels of transgene expression and suppression of endogenous H and L chain gene expression should be carried out using Tg lines with the same integration site to avoid the difference due to the developmental onset of transgene expression.
We have generated Tg mice that produce an autoantibody (4C8) to RBCs, and have analyzed the mechanisms of B cell tolerance and B-1 cell activation 18 19 20 21 22 23 24. Since autoreactive B cells are eliminated at the immature stage in the bone marrow, the number of self-reactive B cells is markedly decreased in the bone marrow, peripheral blood, spleen, and lymph nodes in the Tg mice. In contrast, the peritoneal cavities of the Tg mice contain a normal number of autoreactive B-1 cells that can be eliminated by interaction with RBCs 18 19. B-1 cells show distinct surface antigen expression and anatomical localization from conventional B (B-2) cells 25 26 27 28. Furthermore, the VH gene usage, N region diversity, and antigen specificity of B-1 cells are also unique 29 30 31 32 33 34. B-1 cells are thought to be generated from fetal liver cells, and two independent studies have demonstrated that adult bone marrow cells do not give rise to CD5+ B-1 cells after transfer to irradiated hosts 35 36. In contrast, Wortis and colleagues 37 38 39 have shown that adult bone marrow contains precursors for CD5+ cells and that CD5 expression in splenic B cells is induced by surface IgM cross-linking, suggesting that B-1 cells are generated from common precursors to conventional B cells. Arnold, Clarke, and colleagues 40 41 have reported that B-1 cells differentiate from B-0 cells after expression of specific antigen receptors. Thus, it is not yet clear whether B-1 cells constitute a B cell lineage distinct from conventional B cells. In addition, even if some B-1 cells are derived from activation of B-2 cells, it is not clear whether a certain threshold level of B cell receptor (BCR) expression is required for B-1 cell differentiation or if the receptor specificity alone plays a major role.
To answer these questions we generated new lines of anti-RBC antibody Tg mice that carried tandem joined H and L chain (H+L) transgenes, and we compared the levels of allelic exclusion and the size of B-1 cell compartment between homozygotes and heterozygotes that differ only in the level of the surface Ig expression. Suppression of endogenous H and L chain gene expression was more prominent in homozygotes than in heterozygotes, suggesting that allelic exclusion depends on a certain level of surface Ig expression. In addition, the size of the Tg B-1 cell compartment in the peritoneal cavity is larger in homozygous than in heterozygous H+L mice, suggesting that the increase in the B-1 cell compartment in the Tg mice may be due to augmentation of signals through surface Ig by the self-antigen (RBC).
Materials and Methods
Tg Mice.
Tg mouse lines carrying either H or L chain gene for the anti-RBC mAb (4C8 mAb) have been established previously 18. Double Tg (H×L) mice with H and L chain transgenes were obtained by mating H and L chain Tg mice. In this study we generated new lines of 4C8 mAb Tg mice that carried tandem joined H and L chain transgenes. To construct tandem joined transgenes, a 5.6-kb BamHI fragment of pMO-κ4C8 was first subcloned in the BamHI site of pSP73 Vector (Promega Corp.). Next, a 15.5-kb XhoI fragment of pMO-μ4C8 was subcloned between the SalI and XhoI sites. The pSP73 plasmid containing both H and L chain genes was designated as pSP73-κμ4C8. Tandem joined H and L chain Tg (H+L) mice were generated by injecting a 22.0-kb PvuI–XhoI fragment of pSP73-κμ4C8 (see Fig. 1 A). Heterozygous mice were mated to get homozygous mice in two representative lines, H+L5, with 4–12 copies, and H+L6, with 1–3 copies of the transgene. The presence of the transgenes and homozygosity of the Tg loci were screened by PCR and Southern blot analysis. Tg mice were maintained under conventional conditions in our animal facility.
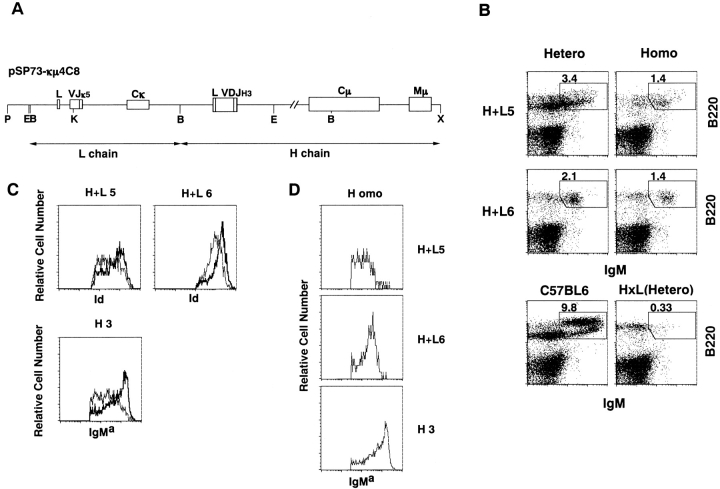
Figure 1.
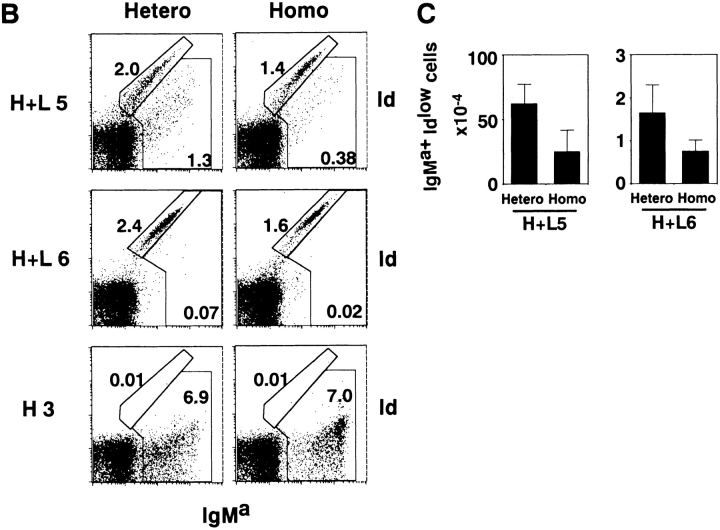
Structure and expression of anti-RBC transgene. (A) Tg construct with tandem joined H and L chain genes. DNA fragments of the L and H chain genes are shown by arrows. Exons are represented by open boxes. Relevant restriction sites are shown: B, BamHI; E, EcoRI; X, XhoI; K, KpnI; P, PvuI. (B) Flow cytometric analysis of bone marrow cells in Tg mice. Bone marrow cells were isolated from heterozygous (hetero) H+L5, homozygous (homo) H+L5, hetero H+L6, homo H+L6, C57BL/6, and H×L mice at 10–14 wk of age and stained with biotin-conjugated goat anti–mouse IgM antibody, followed with FITC-conjugated anti-B220 antibody and Cy-Chrome®–conjugated SA. The percentages of B220+IgM+ cells of the gated region in the total viable cells are indicated in each panel. (C) Flow cytometric analysis for surface expression of the Tg Ig in heterozygotes and homozygotes. Bone marrow cells were isolated from heterozygous (thin line) or homozygous Tg mice (thick line) of H+L5, H+L6, and H3 mice at 10–14 wk of age. Bone marrow cells were stained with either biotin-conjugated anti-Id antibody (S54) followed by PE-conjugated SA or FITC-conjugated antiallotypic antibody for IgMa (Tg). (D) Flow cytometric analysis of relative surface Tg expression in homozygous Tg lines. Staining was done as described in panel C using FITC-conjugated anti-IgMa antibody.
PCR Analysis.
Mice were typed by PCR analysis of tail DNA with set of primers to the H and L chain genes. PCR was carried out for 30 cycles consisting of denaturation (for 1 min at 94°C), annealing (for 1 min at 55°C), and extension (for 2 min at 72°C). The primer pairs for PCR were as follows: 5′Hc, 5′-CTACGCATTTAGTAGTGACTGG-3′; JH3, 5′-TGCAGAGACAGTGACCAGAG-3′; IgL5′-2, 5′-CTGCAAGTCCAGTCAGAGCC-3′; IgL3′-2, 5′-CAGCACCGAACGTGAGAAAG-3′.
Immunological Reagents.
FITC- or Cy-Chrome®–conjugated anti–mouse CD45R (B220), FITC- or PE-conjugated anti–mouse IgMa (Igh-6a), FITC- or PE-conjugated anti–mouse IgMb (Igh-6b), PE-conjugated anti–mouse CD11b (Mac-1 α chain), biotin-conjugated anti–mouse λ1 and λ2 L chain mAbs, and FITC- or Cy-Chrome®–conjugated streptavidin (SA) were purchased from PharMingen. FITC- or biotin-conjugated F(ab′)2 fragments of goat anti–mouse IgM were from ICN Pharmaceuticals (Cappel). PE-conjugated SA was from Dako. Anti-idiotype (Id) mAb (S54) against the transgene-encoded anti-RBC antibody (4C8) 18 was conjugated with _N_-hydroxysuccinimidobiotin (Pierce Chemical Co.) according to the directions provided by the manufacturer.
Flow Cytometric Analysis.
Single cell suspension from bone marrow was prepared by flashing femur bones with a staining buffer (PBS containing 2% FCS and 0.05% sodium azide), gently pipetted, and washed with the staining buffer. Single cell suspensions from bone marrow, spleen, and peritoneal cavity were prepared with the staining buffer and pretreated with 10% heat-inactivated normal rat serum. After 15 min of incubation, FITC- and/or biotin-conjugated mAbs at appropriate dilutions in the staining buffer were added directly. After 30 min of incubation, PE-conjugated mAbs and/or FITC- or PE-conjugated SA was added. Before and after each step of incubation, cells were washed with the staining buffer. Stained cells were then applied to FACSCalibur® (Becton Dickinson). After excluding dead cells by propidium iodide staining, cells present in the lymphocyte gate defined by forward and side light scatters were analyzed.
Results
Surface Expression of the Transgene Is Higher in Homozygous than in Heterozygous Mice.
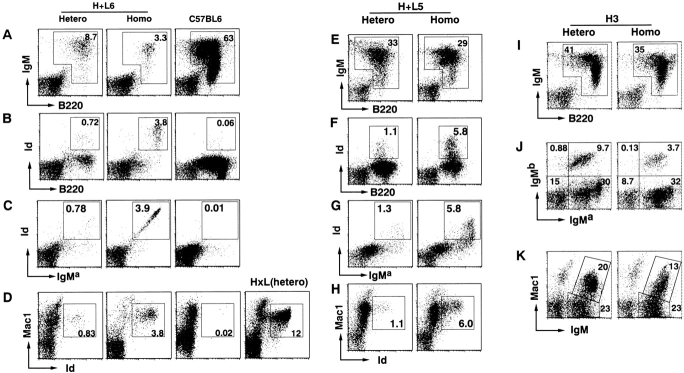
To assess whether the levels of transgene expression are higher in homozygous than in heterozygous mice, we analyzed the bone marrow cells of H+L5 and H+L6 mice that carried tandem joined H and L chain transgenes cording for the 4C8 anti-RBC antibody (Fig. 1 A). In H×L mice, B220+IgM+ bone marrow cells almost completely disappeared 18. In contrast, both lines of H+L mice had significant numbers of B220+IgM+ cells in bone marrow (Fig. 1 B). The majority of these cells were stained both with antiallotypic mAb for IgMa (Tg) and with antiidiotypic mAb (S54) for the anti-RBC antibody. We compared the fluorescence intensity of Id+ bone marrow cells in homozygous mice to that in heterozygous mice (Fig. 1 C). The mean fluorescence intensities (MFI) of Id+ cells in heterozygous and homozygous H+L5 mice are 481.70 and 717.42, respectively. Similarly, the MFI of Id+ cells in heterozygous and homozygous H+L6 mice are 997.76 and 1670.11, respectively. These results indicate that in both H+L5 and H+L6 lines the levels of transgene expression are clearly higher in homozygous than in heterozygous mice. We also examined surface Ig expression on the bone marrow cells of H3 Tg mice that had only H chain transgene. Fluorescence intensity of antiallotypic mAb for IgMa (Tg) of bone marrow cells was also stronger in homozygous (MFI = 908.77) than in heterozygous (MFI = 319.25) mice. In a parallel experiment, anti-IgMa mAb MFI of homozygous H+L5 and H+L6 were 173.07 and 250.72, respectively, indicating that homozygous H+L B cells had not saturated the capacity of surface Ig expression (Fig. 1 D). Taken together, homozygosity of Tg Ig loci enhances the level of transgene expression on B cell surface.
Inhibition of Endogenous H Chain Expression Is Stronger in Homozygous than in Heterozygous Mice.
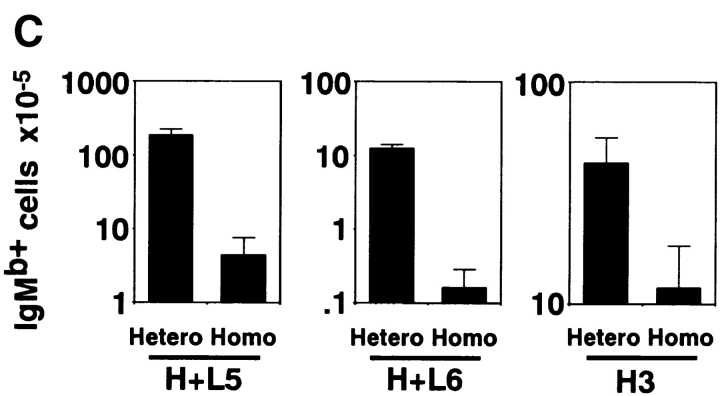
To test whether inhibition of endogenous H chain expression is stronger in homozygous than in heterozygous mice, we analyzed lymphocytes in bone marrow and spleen of H+L5, H+L6, and H3 mice by flow cytometry. We stained bone marrow and spleen cells with antiallotypic mAbs (which can clearly distinguish IgM of the control mice; Fig. 2A and Fig. B) for IgMa (Tg) and IgMb (endogenous). In all heterozygous Tg mice, the numbers of IgMa+IgMb+ cells (allelic inclusion) were small but significant, whereas these cells almost completely disappeared in all homozygous Tg mice. This conclusion is further confirmed by the finding that both bone marrow and spleen B cells with only endogenous H chains (IgMa−IgMb+) decreased in homozygous Tg mice as compared with heterozygous Tg mice (Fig. 2A and Fig. B). In all lines of Tg mice, total numbers of endogenous H chain–expressing (IgMb+) spleen cells were lower in homozygous than in heterozygous mice (Fig. 2 C). These results indicate that inhibition of endogenous H chain expression (allelic exclusion) is stronger in homozygous than in heterozygous mice. Taken together, higher levels of Ig transgene expression appear to cause stronger inhibition of endogenous H chain expression, suggesting that a certain level of surface Ig expression is required for allelic exclusion of the H chain locus.
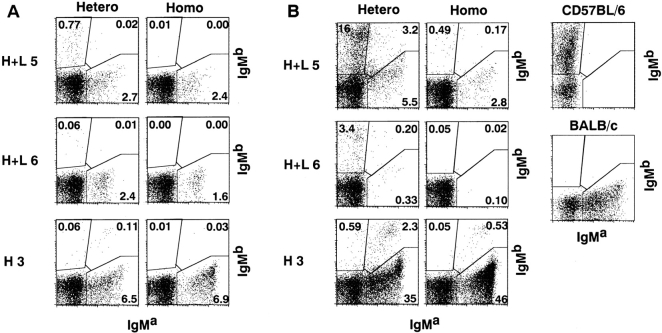
Figure 2.
Tg H chain expression in B cells of heterozygous or homozygous H+L and H3 Tg mice. Cells were isolated from bone marrow (A) and spleen (B) of indicated mice at 10–14 wk of age and stained with FITC-conjugated antiallotypic antibody for IgMa (Tg) and PE-conjugated antiallotypic antibody for IgMb (endogenous). Control stainings of IgMa (BALB/c) and IgMb (C57BL/6) spleen cells are shown. The percentages of cells of each gated region in total viable cells are indicated. Total numbers of the cells with endogenous H chains in heterozygous or homozygous H+L5, H+L6, and H3 Tg mice are shown (C). Spleen cells were stained and analyzed from at least three mice of each Tg line. Numbers of indicated cells were calculated by (percentage of indicated cells in viable cells) × (No. of viable cells). Results are expressed as mean ± SD.
Inhibition of Endogenous L Chain Expression Is Stronger in Homozygous Mice than in Heterozygous Mice.
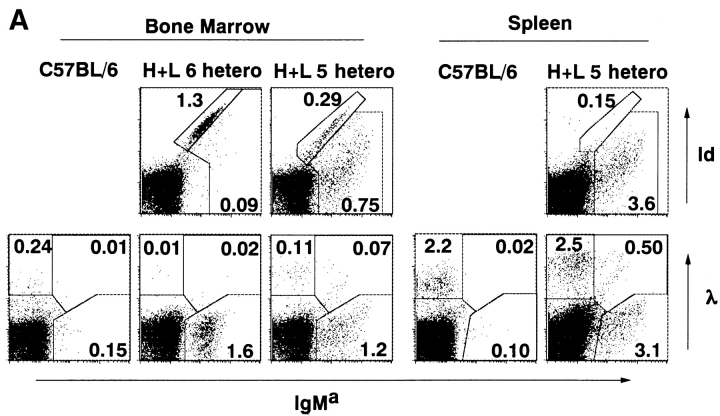
Next, we asked whether homozygosity of Tg loci also reduces endogenous L chain expression. Since the allotypic specificity for the Tg Lκ chain is not known, we estimated the degree of endogenous L chain expression by difference in expression of Tg H chain (IgMa) and Id (the combined epitope of the H and L chains of the 4C8 anti-RBC mAb) that is recognized by the S54 mAb. In the H+L5 line, two populations of IgMa+ cells were observed: one stained with both anti-IgMa and anti-Id mAbs diagonally, and the other stained more weakly with anti-Id mAb than with anti-IgMa mAb (Fig. 3a, Fig. B, and Fig. D, tops). As a control, IgMa+ cells from H3 mice did not contain the population stained with the S54 mAb (Fig. 3B and Fig. D, bottoms). We presume that the cells that stained diagonally with both mAbs express the Tg H and L chains equally, and that the IgMa+Idlow cells express the Tg H chain with both endogenous and Tg L chains. In support of this assumption, IgMa+ bone marrow cells in heterozygous H+L6 mice, which consisted of almost exclusively IgMa+Id+ cells and only few IgMa+Idlow cells, contained <1.2% λ+ cells, which represent a fraction of B cells that express endogenous L chains (Fig. 3 A). In contrast, IgMa+ bone marrow cells in heterozygous H+L5 mice, which consisted of ∼28% IgMa+Id+ and ∼72% IgMa+Idlow cells, contained >5.5% λ+ cells (Fig. 3 A). Furthermore, IgMa+ spleen cells in heterozygous H+L5 mice, which consisted of ∼4% IgMa+Id+ and ∼96% IgMa+Idlow cells, contained >14% λ+ cells (Fig. 3 A). The total number of cells expressing endogenous L chains (IgMa+Idlow) in bone marrow (Fig. 3B and Fig. C) and spleen (Fig. 3D and Fig. E) was less in homozygous than in heterozygous Tg lines, indicating that homozygosity of the Tg loci enhances the inhibition of endogenous L chain expression as compared with heterozygosity.
Figure 3.
Tg Id expression in B cells of heterozygous or homozygous H+L5, H+L6 and H3 Tg mice. Cells were isolated from bone marrow (A and B) and spleen (A and D) of mice at 10–14 wk of age and stained with FITC-conjugated antiallotypic antibody for IgMa (Tg) and biotin-conjugated anti-Id (A, B, and D) or biotin-conjugated anti-λ antibody (A) coupled with PE-conjugated SA. The percentages of the cells of each gated region in total viable cells are indicated. Note that results from different mice are shown for A than for B–E. Total numbers of the bone marrow (C) and spleen (E) cells expressing endogenous L chain and Tg H chain in heterozygous or homozygous H+L5 and H+L6 Tg mice. Cells were analyzed from at least three mice in each line. Numbers of indicated cells were calculated by (percentage of indicated cells in viable cells) × (No. of viable cells). Results are expressed as mean ± SD.
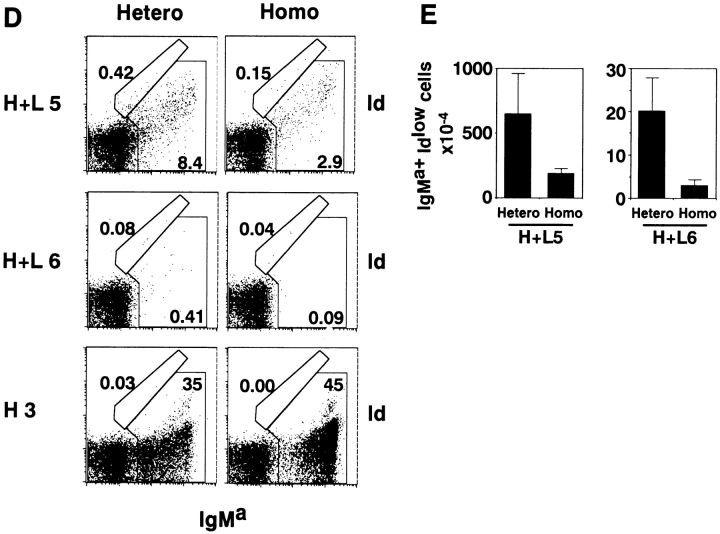
The Size of an Autoreactive B1 Cell Compartment Is Larger in Homozygous Mice than in Heterozygous H+L Mice.
H×L mice show almost complete deletion of autoreactive B cells from bone marrow and spleen, whereas a normal level of B-1 cells is found in the peritoneal cavity 18. Bone marrow of both lines of H+L mice contained some autoreactive B cells (IgMa+Id+), which have B-2 cell phenotypes (CD5−B220highIgMlow), whereas autoreactive B cells in spleen almost completely disappeared in H+L mice (Fig. 3B and Fig. D). We examined whether homozygosity of the Ig transgene loci influences the size of the autoreactive B-1 cell compartment in the peritoneal cavities of H+L5 and H+L6 mice. Peritoneal cells of H+L mice had a limited number of B220+Id+ cells, almost all of which were IgMa+ (Fig. 4A–C and Fig. E–G). Almost all Id+ cells in the peritoneal cavity were Mac1+ and therefore belong to the B-1 subset (Fig. 4D and Fig. H). Thus, IgMa+Id+ cells are most likely to be autoreactive B-1 cells (Fig. 4C and Fig. G). These B220+Id+ cells in the peritoneal cavity were much more abundant in homozygous H+L mice than in heterozygous H+L mice, indicating that the size of the autoreactive B-1 cell subset is larger in homozygous than in heterozygous H+L mice. The relative size of the B-1 cell compartment is directly proportional to the B-1 cell number because total cell numbers in the peritoneal cavity did not vary between homozygous and heterozygous Tg mice. These results suggest that a higher expression level of the autoantibody facilitates the increase in autoreactive B-1 cells in the peritoneal cavity.
Figure 4.
Flow cytometric analysis of peritoneal cells of heterozygous or homozygous H+L5, H+L6, H×L, and H3 Tg mice. Cells were isolated from peritoneal cavity of mice at 10–14 wk of age and stained with FITC-anti mouse IgM and Cy-Chrome®–conjugated anti–mouse B220 (A, E and I); biotin-conjugated anti-Id antibody followed by FITC-conjugated SA and Cy-Chrome®–conjugated anti-B220 antibody (B and F); biotin-conjugated anti-Id antibody followed by FITC-conjugated SA and PE-conjugated antiallotypic antibody for IgMa (C and G); biotin-conjugated anti-Id antibody followed by FITC-conjugated SA and PE-conjugated anti–mouse Mac-1 (D and H); FITC-conjugated antiallotypic antibody for IgMb (endogenous) and PE-conjugated antiallotypic antibody for IgMa (Tg) (J); and FITC-conjugated goat anti–mouse IgM antibody and PE-conjugated anti–mouse Mac-1 (K). The percentages of the cells of each gated region in total viable cells are indicated. Total peritoneal cell numbers were constant between heterozygous and homozygous Tg mice.
To test whether autoreactivity of Ig expressed on B cells is crucial to enlargement of the B-1 cell subset, we carried out similar studies on the peritoneal B cells in H3 Tg mice, the vast majority of which are not autoreactive due to the absence of the Tg L chain. The majority of peritoneal B cells expressed the Tg H chain (IgMa+), in agreement with analysis of bone marrow and spleen cells (Fig. 4I and Fig. J). Indeed, B cells expressing endogenous H chains were less in homozygous than in heterozygous H3 mice (Fig. 4 J), probably because of more efficient allelic exclusion due to stronger expression of the transgene product on homozygous B cells (Fig. 1 C). Nonetheless, the percentage of IgM+Mac1+ cells in the peritoneal cavity did not increase in homozygous H3 mice as compared with those in heterozygous H3 mice (Fig. 4 K). These results indicate that increased surface Ig expression of non-auto-reactive B cells did not facilitate enlargement of the B-1 cell subset in the peritoneal cavity. Taken together, enhancement of autoreactive surface Ig expression appears to be crucial to increase the B-1 cell subset in the peritoneal cavity.
Discussion
In this study, we generated new lines of the anti-RBC antibody Tg mice that carry tandem joined H and L chain transgenes, and compared B cell phenotypes between heterozygous and homozygous Tg mice to evaluate quantitatively the effect of surface Ig level on allelic exclusion and B-1 cell development. Higher levels of surface Tg Ig expression resulted in (a) stronger clonal deletion of B-2 cells from bone marrow and spleen; (b) reduction of B cells expressing endogenous H or L chains; and (c) enlargement of the autoreactive B-1 cell subset in the peritoneal cavity.
Several lines of evidence suggest that surface expression of the μ chain is critical for H chain allelic exclusion 2 4 5 6. In addition, pre-BCR, which is the H chain paired with the surrogate L chain, is suggested to mediate H chain allelic exclusion through downregulation of recombination-activating genes 42 43 44 45 46. Igα and Igβ, which associate with surface-expressed H chains, have also been shown to trigger the signals inducing allelic exclusion 47 48 49. Taken together, expression of the H chain as a pre-BCR on the cell surface may induce signals mediated by Igα and Igβ, resulting in H chain allelic exclusion. In this study we have demonstrated that homozygosity of the transgene Ig loci increases surface Ig expression on bone marrow B cells and causes stronger allelic exclusion, as compared with heterozygosity. Since allelic exclusion is all or none in each B cell, the present results suggest that there is a threshold of the pre-BCR signal intensity that induces allelic exclusion.
There are several other possibilities to explain our results. First, B cells with allelic inclusion may be negatively selected. However, Sonoda and Rajewsky 50 have shown that B cells with allelic inclusion can normally expand in the periphery using double Ig knock-in mice, indicating the absence of negative selection against allelic inclusion B cells. In addition, we have shown that endogenous only B cells (IgMa−IgMb+) are also reduced in homozygotes (Fig. 2). Second, reduction of allelic inclusion B cells could be due to selective expansion of higher surface Ig-expressing cells by a self-antigen. However, the self-antigen (RBC) can kill self-reactive B cells 19. In fact, Id+ B cells of H+L mice decreased in homozygotes as compared with heterozygotes in spleen as well as in bone marrow (Fig. 3B and Fig. D). In addition, we have shown that non–self-reactive (H chain alone expressing) Tg (H3) B cells also show suppression of endogenous Ig expression (Fig. 2). These results cannot be explained by positive selection of self-reactive Ig expressing B cells by the self-antigen. Third, excess Tg Ig expression on surface may inhibit detection of endogenous Ig expression. It is unlikely that endogenous and Tg Ig molecules compete for surface expression in H+L5 and H+L6 spleen cells. This is because the surface Ig levels of homozygous H3, H+L6, and H+L5 cells are 909, 251, and 173 (MFI using the anti-IgMa Ab), respectively (Fig. 1 D), indicating that H+L5 and H+L6 B cells have not hit the ceiling of the surface Ig expression level. Sonoda and Rajewsky 50 have shown that there is no inhibitory mechanism for the H chain on the surface as long as it can associate with the L chain. It is inconceivable that the surface expression efficiency differs between H chains derived from the transgene and endogenous gene. The possibility that a lower detection efficiency by anti-IgMb Ab staining in the presence of large amounts of IgMa is also unlikely because the FACS® profiles of H3 spleen cells expressing large amounts of IgMa clearly show the presence of IgMa IgMb double-positive cells even in homozygotes (Fig. 2 B). Finally, the suppression efficiency of the endogenous locus may be increased in homozygotes simply because the frequency of silencing the transgene locus is reduced by doubling the number of the transgene locus in homozygotes. Assuming that this is the case, the efficiency of silencing of the transgene locus can be calculated from the endogenous H chain only cells shown in Fig. 2 A: H3, 0.06 / (0.06 + 0.11 + 6.50) = 0.009 in heterozygotes and 0.00144 = 0.01 / (0.01 + 0.03 + 6.9) in homozygotes. The value in homozygotes is ∼18 times of the expected value (0.009 × 0.009 = 0.000081) based on the simple statistics. Similar discrepancy was seen in the H+L5, H+L6, and H3 spleen cells (Fig. 2 B). In addition, homozygosity alters the number of Tg Id+ B cells in spleen and peritoneal cavity to the opposite direction. These considerations make the final possibility unlikely.
We have shown that H+L Tg mice have B cells expressing endogenous L chains together with the Tg H chain. These cells, expressing endogenous L chains in H+L mice, may be generated either by incomplete allelic exclusion or by receptor editing because the B cells which express the transgenes are self-reactive 51 52. Nemazee and colleagues 53 54 55 have suggested that interaction with autoantigens leads IgMlowIgD− bone marrow cells to undergo receptor editing but IgMhighIgD+ cells to undergo rapid apoptosis. On the other hand, Rusconi et al. 16 generated B cell hybridomas from anti-trinitrophenol antibody (anti-non-self) Tg mice that carried tandem joined H and L chain transgenes and demonstrated that the B cell hybridomas secreting the Tg antibody expressed the Tg L chain at about one-tenth of the level of coexpressed endogenous L chains. Endogenous L chain expression in their Tg mice is probably due to incomplete allelic exclusion because these B cells are unlikely to receive BCR stimulation by self-antigens to trigger receptor editing. Although we cannot exclude the possibility that receptor editing is involved in the appearance of B cells with endogenous L chains in our Tg mice, we think it less likely because of the following reason. If the expression of endogenous L chain is due to receptor editing induced by stimulation with the self-antigen, the mechanism to reduce the number of B cells with endogenous L chains by enhanced expression of self-reactive Ig should be clonal deletion. However, when B cells expressed more self-reactive Ig on surface, B cells with both Tg and endogenous L chains (IgMa+Idlow) are more efficiently reduced than Tg only B cells (IgMaId+), which are most likely eliminated by clonal deletion (Fig. 3). This observation is somewhat opposed to the expected efficiency of clonal deletion by the self-antigen because stronger BCR signaling will be induced in IgMaId+ cells than IgMaIdlow cells.
Although it is still controversial whether B-1 cells belong to an ontogenetically different B cell lineage from conventional B cells 34 35 36 37 38 39 40, B-1 and B-2 cells clearly constitute different subsets of B cells. In this study we have shown that the size of the Tg B-1 cell compartment is larger in homozygous than in heterozygous H+L mice (Fig. 4). Since Tg B-1 cells in heterozygous and homozygous mice show the same antigen specificity, our results suggest that the level of surface Ig expression directly influences the size of the Tg B-1 cell compartment. Our findings are consistent with the previous observations that defects of BCR signaling cause reduction of the B1 cell 56 57, and loss of a BCR inhibitory molecule, SHP-1, increases the B1 cell number 58 59 60 61 62. It is important to note that increased levels of autoreactive BCR induced augmented clonal deletion of B-2 cells in bone marrow and spleen but expansion of B-1 cells in the peritoneal cavity (Fig. 4). Increased expression of nonautoreactive Ig (H3) enhanced neither clonal deletion of B-2 cells nor expansion of B-1 cells. These results suggest that there are at least three levels of BCR signaling that regulate self-reactive B1 and B2 cell differentiation. At a lower level, self-reactive B-2 cells can be stimulated to induce receptor editing or to become anergic 53 54 55 63. At an intermediate signaling level, B-2 cells are clonally deleted and B-0 40 41 and/or B-2 cells are induced to differentiate into B-1 cells, which migrate into the peritoneal cavity. At a strong signaling level, B-1 cells are also clonally deleted 19. It is tempting to speculate that at least a sizable fraction of peritoneal B-1 cells originate and expand from autoreactive B cells that are stimulated by self-antigens to a level strong enough to be activated but weak enough to avoid apoptosis.
Acknowledgments
We thank Ms. Y. Kobayashi and Ms. T. Taniuchi for their technical assistance, and Ms. Y. Takahashi for manuscript preparation.
This work was supported by grants from the COE program from the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
1used in this paper: BCR, B cell receptor; H+L mice, tandem joined H and L chain transgenic mice; H×L mice, double transgenic mice with H and L chain transgenes; Id, idiotype; MFI, mean fluorescence intensity; SA, streptavidin; Tg, transgenic
S. Nisitani's present address is Howard Hughes Medical Institute, University of California, Los Angeles, 5-720 MRL, 675 Circle Dr. South, Box 951662, Los Angeles, CA 90095-1662.
References
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Rajewsky K. Targeted disruption of μ chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992;356:154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- Storb U. Ig gene expression and regulation in Ig transgenic mice. In: Honjo T., Alt W.F., editors. Immunoglobulin Genes. Academic Press; London: 1995. pp. 345–363. [Google Scholar]
- Nussenzweig M.C., Shaw A.C., Sinn E., Danner D.B., Holmes K.L., Morse H.C., III, Leder P. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin μ. Science. 1987;236:816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M.C., Shaw A.C., Sinn E., Campos-Torres J., Leder P. Allelic exclusion in transgenic mice carrying mutant human IgM genes. J. Exp. Med. 1988;167:1969–1974. doi: 10.1084/jem.167.6.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz J., Denis K., Witte O., Brinster R., Storb U. Feedback inhibition of immunoglobulin gene rearrangement by membrane μ, but not by secreted μ heavy chains. J. Exp. Med. 1988;168:1363–1381. doi: 10.1084/jem.168.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K.A., Brinster R.L., Storb U. Allelic exclusion and control of endogenous immunoglobulin gene rearrangement in κ transgenic mice. Nature. 1984;312:517–520. doi: 10.1038/312517a0. [DOI] [PubMed] [Google Scholar]
- Stall A.M., Kroese F.G.M., Gadus F.T., Sieckmann D.G., Herzenberg L.A., Herzenberg L.A. Rearrangement and expression of endogenous immunoglobulin genes occur in many murine B cells expressing transgenic membrane IgM. Proc. Natl. Acad. Sci. USA. 1988;85:3546–3550. doi: 10.1073/pnas.85.10.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Rüther U., Vieira P., Hombach J., Reth M., Rajewsky K. Membrane-bound IgM obstructs B cell development in transgenic mice. Eur. J. Immunol. 1989;19:923–928. doi: 10.1002/eji.1830190520. [DOI] [PubMed] [Google Scholar]
- Kenny J.J., Finkelman F., Macchiarini F., Kopp W.C., Storb U., Longo D.L. Alteration of the B cell surface phenotype, immune response to phosphocholine and the B cell repertoire in M167 μ plus κ transgenic mice. J. Immunol. 1989;142:4466–4474. [PubMed] [Google Scholar]
- Rath S., Durdik J., Gerstein R.M., Selsing E., Nisonoff A. Quantitative analysis of idiotypic mimicry and allelic exclusion in mice with a μ Ig transgene. J. Immunol. 1989;143:2074–2080. [PubMed] [Google Scholar]
- Forni L. Extensive splenic B cell activation in IgM-transgenic mice. Eur. J. Immunol. 1990;20:983–989. doi: 10.1002/eji.1830200506. [DOI] [PubMed] [Google Scholar]
- Grandien A., Coutinho A., Andersson J. Selective peripheral expansion and activation of B cells expressing endogenous immunoglobulin in μ-transgenic mice. Eur. J. Immunol. 1990;20:991–998. doi: 10.1002/eji.1830200507. [DOI] [PubMed] [Google Scholar]
- Iacomini J., Yannoutsos N., Bandyopadhay S., Imanishi-Kari T. Endogenous immunoglobulin expression in μ transgenic mice. Int. Immunol. 1991;3:185–196. doi: 10.1093/intimm/3.2.185. [DOI] [PubMed] [Google Scholar]
- Lam K.P., Herzenberg L.A., Stall A.M. A high frequency of hybridomas from M54 μ heavy chain transgenic mice initially co-express transgenic and rearranged endogenous μ genes. Int. Immunol. 1993;5:1011–1022. doi: 10.1093/intimm/5.9.1011. [DOI] [PubMed] [Google Scholar]
- Rusconi S., Köhler G. Transmission and expression of a specific pair of rearranged immunoglobulin μ and κ genes in a transgenic mouse line. Nature. 1985;314:330–334. doi: 10.1038/314330a0. [DOI] [PubMed] [Google Scholar]
- Carmack C.E., Camper S.A., Mackle J.J., Gerhard W.U., Weigert M.G. Influence of a Vκ8 L chain transgene on endogenous rearrangements and the immune response to the HA(Sb) determinant on influenza virus. J. Immunol. 1991;147:2024–2033. [PubMed] [Google Scholar]
- Okamoto M., Murakami M., Shimizu A., Ozaki S., Tsubata T., Kumagai S., Honjo T. A transgenic model of autoimmune hemolytic anemia. J. Exp. Med. 1992;175:71–79. doi: 10.1084/jem.175.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Tsubata T., Okamoto M., Shimizu A., Kumagai S., Imura H., Honjo T. Antigen-induced apoptotic death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature. 1992;357:77–80. doi: 10.1038/357077a0. [DOI] [PubMed] [Google Scholar]
- Nisitani S., Tsubata T., Murakami M., Okamoto M., Honjo T. The bcl-2 gene product inhibits clonal deletion of self-reactive B lymphocytes in the periphery but not in the bone marrow. J. Exp. Med. 1993;178:1247–1254. doi: 10.1084/jem.178.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Tsubata T., Shinkura R., Nisitani S., Okamoto M., Yoshioka H., Usui T., Miyawaki S., Honjo T. Oral administration of lipopolysaccharides activates B-1 cells in the peritoneal cavity and lamina propria of the gut and induces autoimmune symptoms in an autoantibody transgenic mouse. J. Exp. Med. 1994;180:111–121. doi: 10.1084/jem.180.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisitani S., Tsubata T., Murakami M., Honjo T. Administration of interleukin-5 or -10 activates peritoneal B-1 cells and induces autoimmune hemolytic anemia in anti-erythrocyte autoantibody-transgenic mice. Eur. J. Immunol. 1995;25:3047–3052. doi: 10.1002/eji.1830251110. [DOI] [PubMed] [Google Scholar]
- Murakami M., Nakajima K., Yamazaki K., Muraguchi T., Serikawa T., Honjo T. Effects of breeding environments on generation and activation of autoreactive B-1 cells in anti-red blood cell autoantibody transgenic mice. J. Exp. Med. 1997;185:791–794. doi: 10.1084/jem.185.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisitani S., Sakiyama T., Honjo T. Involvement of IL-10 in induction of autoimmune hemolytic anemia in anti-erythrocyte Ig transgenic mice. Int. Immunol. 1998;10:1039–1047. doi: 10.1093/intimm/10.8.1039. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Parks D.R., Herzenberg L.A. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J. Exp. Med. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldschmidt T.J., Kroese F.G., Tygrett L.T., Conrad D.H., Lynch R.G. The expression of B cell surface receptors. III. The murine low-affinity IgE Fc receptor is not expressed on Ly 1 or “Ly 1-like” B cells. Int. Immunol. 1991;3:305–315. doi: 10.1093/intimm/3.4.305. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Stall A.M., Herzenberg L.A., Herzenberg L.A. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur. J. Immunol. 1986;16:1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- Marcos M.A., Huetz F., Pereira P., Andreu J.L., Martinez A.C., Coutinho A. Further evidence for coelomic-associated B lymphocytes. Eur. J. Immunol. 1989;19:2031–2035. doi: 10.1002/eji.1830191110. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Honda M., Herzenberg L.A., Steinberg A.D., Herzenberg L.A. Ly-1 B cellsfunctionally distinct lymphocytes that secrete IgM autoantibodies. Proc. Natl. Acad. Sci. USA. 1984;81:2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalor P.A., Morahan G. The peritoneal Ly-1 (CD5) B cell repertoire is unique among murine B cell repertoires. Eur. J. Immunol. 1990;20:485–492. doi: 10.1002/eji.1830200305. [DOI] [PubMed] [Google Scholar]
- Mercolino T.J., Arnold L.W., Haughton G. Phosphatidyl choline is recognized by a series of Ly-1+ murine B cell lymphomas specific for erythrocyte membranes. J. Exp. Med. 1986;163:155–165. doi: 10.1084/jem.163.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S.D., Ward M.M., Apicella M.A., Ward R.E. The primary B cell response to the O/core region of bacterial lipopolysaccharide is restricted to the Ly-1 lineage. J. Immunol. 1991;146:327–331. [PubMed] [Google Scholar]
- Forster I., Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur. J. Immunol. 1987;17:521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- Tornberg U.C., Holnberg D. B-1a, B-1b and B-2 B cells display unique VHDJH repertoires formed at different stages of ontogeny and under different selection pressures. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:1680–1689. doi: 10.1002/j.1460-2075.1995.tb07157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Herzenberg L.A., Herzenberg L.A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor A.B., Stall A.M., Adams S., Herzenberg L.A. Differential development of progenitor activity for three B-cell lineages. Proc. Natl. Acad. Sci. USA. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y.Z., Rabin E., Wortis H.H. Treatment of murine CD5- B cells with anti-Ig, but not LPS, induces surface CD5two B-cell activation pathways. Int. Immunol. 1991;3:467–476. doi: 10.1093/intimm/3.5.467. [DOI] [PubMed] [Google Scholar]
- Huang C.A., Henry C., Iacomini J., Imanishi-Kari T., Wortis H.H. Adult bone marrow contains precursors for CD5+ B cells. Eur. J. Immunol. 1996;26:2537–2540. doi: 10.1002/eji.1830261039. [DOI] [PubMed] [Google Scholar]
- Berland R., Wortis H.H. An NFAT-dependent enhancer is necessary for anti-IgM-mediated induction of murine CD5 expression in primary splenic B cells. J. Immunol. 1998;161:277–285. [PubMed] [Google Scholar]
- Arnold L.W., Pennell C.A., McCray S.K., Clarke S.H. Development of B-1 cellssegregation of phosphatidyl choline–specific B cells to B-1 population occurs after immunoglobulin gene expression. J. Exp. Med. 1994;179:1585–1595. doi: 10.1084/jem.179.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S.H., Arnold L.W. B-1 cell developmentevidence for an uncommitted immunoglobulin (Ig)M+ B cell precursor in B-1 cell differentiation. J. Exp. Med. 1998;187:1325–1334. doi: 10.1084/jem.187.8.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of λ 5 protein in B cell development. Cell. 1992;69:823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Grawunder U., Leu T.M., Schatz D.G., Werner A., Rolink A.G., Melchers F., Winkler T.H. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–608. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- Löffert D., Ehlich A., Müller W., Rajewsky K. Surrogate light chain expression is required to establish immunoglobulin heavy chain allelic exclusion during early B cell development. Immunity. 1996;4:133–144. doi: 10.1016/s1074-7613(00)80678-0. [DOI] [PubMed] [Google Scholar]
- Shaffer A.L., Schlissel M.S. A truncated heavy chain protein relieves the requirement for surrogate light chains in early B cell development. J. Immunol. 1997;159:1265–1275. [PubMed] [Google Scholar]
- ten Boekel E., Melchers F., Rolink A.G. Precursor B cells showing H chain allelic inclusion display allelic exclusion at the level of pre-B cell receptor surface expression. Immunity. 1998;8:199–207. doi: 10.1016/s1074-7613(00)80472-0. [DOI] [PubMed] [Google Scholar]
- Papavasiliou F., Misulovin Z., Suh H., Nussenzweig M.C. The role of Ig β in precursor B cell transition and allelic exclusion. Science. 1995;268:408–411. doi: 10.1126/science.7716544. [DOI] [PubMed] [Google Scholar]
- Papavasiliou F., Jankovic M., Suh H., Nussenzweig M.C. The cytoplasmic domains of immunoglobulin (Ig) α and Ig β can independently induce the precursor B cell transition and allelic exclusion. J. Exp. Med. 1995;182:1389–1394. doi: 10.1084/jem.182.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh Y.M., Neuberger M.S. The immunoglobulin (Ig)α and Igβ cytoplasmic domains are independently sufficient to signal B cell maturation and activation in transgenic mice. J. Exp. Med. 1997;185:1753–1758. doi: 10.1084/jem.185.10.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E., Pewzner-Jung Y., Schwers S., Taki S., Jung S., Eilat D., Rajewsky K. B cell development under the condition of allelic inclusion. Immunity. 1997;6:225–233. doi: 10.1016/s1074-7613(00)80325-8. [DOI] [PubMed] [Google Scholar]
- Tiegs S.L., Russell D.M., Nemazee D. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D., Saunders T., Camper S., Weigert M. Receptor editingan approach by autoreactive B cells to escape tolerance. J. Exp. Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed D., Nemazee D. Self-antigen does not accelerate immature B cell apoptosis, but stimulates receptor editing as a consequence of developmental arrest. Proc. Natl. Acad. Sci. USA. 1997;94:9267–9272. doi: 10.1073/pnas.94.17.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz M., Nemazee D. BCR ligation induces receptor editing in IgM+IgD− bone marrow B cells in vitro. Immunity. 1997;6:429–436. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- Melamed D., Benschop R., Cambier J.C., Nemazee D. Developmental regulation of B lymphocyte immune tolerance compartmentalizes clonal selection from receptor selection. Cell. 1998;92:173–182. doi: 10.1016/s0092-8674(00)80912-5. [DOI] [PubMed] [Google Scholar]
- Zhang R., Alt F.W., Davidson L., Orkin S.H., Swat W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 1995;374:470–473. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]
- Leitges M., Schmedt C., Guinamard R., Davoust J., Schaal S., Stabel S., Tarakhovsky A. Immunodeficency in protein kinase Cβ-deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- Sidman C.L., Shultz L.D., Hardy R.R., Hayakawa K., Herzenberg L.A. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science. 1986;232:1423–1425. doi: 10.1126/science.3487115. [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Schweitzer P.A., Rajan T.V., Yi T., Ihle J.N., Matthews R.J., Thomas M.L., Beier D.R. Mutation at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- Tsui H.W., Siminovitch K.A., de Souza L., Tsui F.W.L. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat. Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- Cyster J.G., Goodnow C.C. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Pani G., Kozlowski M., Cambier J.C., Mills G.B., Siminovitch K.A. Identification of the tyrosine phosphatase PTP1C as a B cell antigen receptor-associated protein involved in the regulation of B cell signaling. J. Exp. Med. 1995;181:2077–2084. doi: 10.1084/jem.181.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C.C., Crosbie J., Adelstein S., Lavoie T.B., Smith-Gill S.J., Brink R.A., Pritchard-Briscoe H., Wotherspoon J.S., Loblay R.H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]