Compromised Ox40 Function in Cd28-Deficient Mice Is Linked with Failure to Develop Cxc Chemokine Receptor 5–Positive Cd4 Cells and Germinal Centers (original) (raw)
Abstract
Mice rendered deficient in CD28 signaling by the soluble competitor, cytotoxic T lymphocyte–associated molecule 4–immunoglobulin G1 fusion protein (CTLA4-Ig), fail to upregulate OX40 expression in vivo or form germinal centers after immunization. This is associated with impaired interleukin 4 production and a lack of CXC chemokine receptor (CXCR)5 on CD4 T cells, a chemokine receptor linked with migration into B follicles. Germinal center formation is restored in CTLA4-Ig transgenic mice by coinjection of an agonistic monoclonal antibody to CD28, but this is substantially inhibited if OX40 interactions are interrupted by simultaneous injection of an OX40-Ig fusion protein. These data suggest that CD28-dependent OX40 ligation of CD4 T cells at the time of priming is linked with upregulation of CXCR5 expression, and migration of T cells into B cell areas to support germinal center formation.
Keywords: CD28, OX40/OX40 ligand, germinal center, chemokine, T cell migration
Studies of the timing and location of T and B cell activation show that the primary site of CD4 cognate help for B cells is in the outer T zone 1 2. After this engagement, two distinct processes evolve. In the first process, activated B cells migrate to extrafollicular foci where they proliferate extensively and differentiate into plasma cells 3. Although the Ab produced is of relatively low affinity, production is rapid. In the second process, antigen-specific B and T cells migrate into follicles and form germinal centers (GCs)1 where somatic mutation and affinity maturation occur. Unlike differentiation of plasmablasts in the extrafollicular foci, the efficient selection of rare B cell mutants in GCs is highly dependent on the provision of T cell help. Approximately 10% of lymphocytes in GCs are antigen-specific CD4 T cells 4, and their deletion leads to the rapid dissolution of GCs 5. As a consequence of this process, high-affinity Ab is produced, albeit relatively slowly.
We have investigated the molecular events that control T cell help for GCs. A starting point for our studies was the observation that T cell signaling through CD28 is clearly important for GC development. CD28-deficient mice lack GCs and fail to develop class-switched high-affinity Ab 6 7, and this is linked with selectively poor Th2 development in vivo 8 and in vitro 9. Despite this, CD28-deficient mice make some low-affinity T cell–dependent Ab in the extrafollicular response, and T cell–independent responses are normal 7. This implies that CD28 signaling of CD4 T cells plays a crucial nonredundant role in GC formation.
In vitro in both humans 10 and mice 11, CD28 costimulation by itself seems to be a poor signal for the production of IL-4 in naive CD4 T cells. However, we and others have recently reported that in mice 11 and humans 10, costimulation through OX40 and CD28 promotes IL-4 expression in naive CD4 T cells in vitro. This combination also upregulates expression of CXC chemokine receptor (CXCR)5 mRNA 11, linking CD4 T cell IL-4 production with the capacity to migrate into B follicles and help GC formation. This conclusion is supported by transgenic mice that constitutively express OX40 ligand on dendritic cells (DCs) (CD11c-OX40L tg mice). CD4 T cells receive a default OX40 signal when they are primed in these mice, and this is associated with greatly increased numbers of CD4 T cells in splenic B cell areas 12. Taken together, these experiments strongly implicate OX40 signaling in the migration of CD4 T cells into GCs, and the development of a cytokine profile conducive for B cell help.
In this paper, we have examined the interrelationship between CD28 and OX40 signaling. We report deficient expression of OX40 and IL-4 in mice that express the soluble CD28 competitor, CTL-associated molecule 4–IgG1 fusion protein (CTLA4-Ig tg mice [7]), and show that these mice lack an activated subset of CD4 T cells that express CXCR5. In contrast_,_ CD11c-OX40L tg mice have increased numbers of CXCR5+ T cells, but this phenotype is dependent on CD28 signaling. GC formation is restored in CTLA4-Ig tg mice by coinjection of agonistic CD28 mAb, but this is substantially dependent on OX40 signaling, as injection of a fusion protein between murine OX40 and human IgG1 (OX40-Ig) inhibits GC development but not the generation of extrafollicular plasma cell foci. These experiments reveal that there is a hierarchy of costimulatory signals that regulate T cell help for B cell GCs. CD28 signaling is permissive for OX40 signaling that, in addition to costimulatory effects, is specifically linked with T cell migration to B cell areas and the formation of GCs.
Materials and Methods
Preparation and Stimulation of CD4_+_CD62Lhigh Cells.
CD4 CD62Lhigh cells were prepared from BALB/c mice aged 6–12 wk by MACS® (Miltenyi Biotec), as described elsewhere 11. CD4 T cells which were consistently >95% pure for CD4 and CD62Lhigh expression were activated in 24-well plates (Nunc) previously coated with 10 μg/ml anti–murine CD3 mAb (2C11; PharMingen). The cultures were incubated with or without a 10% final concentration of a supernatant of anti-CD28 mAb (clone 37.51; a gift of Dr. Jim Allison, University of California at Berkeley, Berkeley, CA).
Mice.
BALB/c, CD11c-OX40L, and CTLA4-Ig tg experimental animals were bred and maintained in accordance with animal house guidelines.
Semiquantitative Reverse Transcription PCR on CTLA4-Ig Tg Mice and Control Littermates.
For in vivo studies of mRNA expression, footpad immunizations were carried out using 50 μg alum-precipitated chicken γ-globulin resuspended in 200 μl normal saline, or heat-killed Bordetella pertussis (5 × 108) resuspended in 200 μl normal saline as indicated. Immunized mice were killed 3 and 7 d after immunization by CO2 asphyxiation. RNA and cDNA samples were prepared from draining popliteal LNs as described 13.
cDNA prepared from the tissue sections was diluted to a final volume of 100 μl. The PCR β-actin signal was used to correct for differences in the amount of starting cDNA from each sample. The specific primers were: β-actin (Stratagene), 5′-agcgggaaatcgtgcgtg and 5′-CAGGGTACATGGTGGTGCC; IL-4 14, 5′-GAATGTACCAGGAGCCATATC and 5′-CTCAGTACTACGAGTAATCCA; IFN-γ 14, 5′-AACGCTACACACTGCATCTTGG and 5′-GACTTCAAAGAGTCTGAGG; murine OX40, 5′-TGTATGTGTGGGTTCAGCAGCC and 5′-ccctcaggagtcaccaaggtggg; and murine OX40L, 5′-atggaaggggaaggggttcaacc and 5′-TCACAGTGGTACTTGGTTCACAG.
For semiquantitative reverse transcription PCR of OX40, OX40L, IL-4, and IFN-γ mRNA, ∼10 fewer cycles than were required to detect PCR product using ethidium bromide gels were performed, so that quantitation could be performed by PCR Southern blot or Southern dot blot analysis. For each set of primers, three different numbers of PCR cycles were performed, to ensure that amplification was logarithmic and that the conditions were not saturating. The PCR product was separated on a 1.5% agarose gel and transferred onto prewetted Hybond-N+ membrane (Nycomed Amersham plc) by capillary transfer under alkaline conditions. The membrane was hybridized with a 32P-labeled purified PCR product used as a probe and imaged using a PhosphorImager® (Molecular Dynamics).
Using ImageQuant® software (Molecular Dynamics), a grid was laid over PCR bands with individual fields covering the central 50% of a band. The signal in each field was calculated and these figures transferred to spreadsheet software to sort the randomized files to the correct order. The average of the three PCRs with different cycle number for each gene was taken and divided by the average of the three corresponding β-actin PCRs. These values represent the relative amount of mRNA for each gene per cell. This value was multiplied by the size of the section area (determined by microscopy on adjacent sections to those taken for cDNA using the point counting technique [15]) to give mRNA amount per section.
Abs.
A polyclonal rabbit antiserum prepared by immunizing with CXCR5 peptides was a gift of Dr. Jason Cyster, University of California San Francisco, San Francisco, CA 16. The specificity of this Ab was confirmed in cross-blocking studies with a rat mAb against mouse CXCR5.
Preparation of Fusion Proteins and mAbs.
OX40-Ig was prepared by using protein A purification of supernatant cultures from a hybridoma expressing this fusion protein 11. Control Ig was prepared in a similar way. Anti-CD28 mAb was prepared by protein G purification of supernatants of clone 37.51. These proteins were sterilized by filtration through 0.2-μm filters, and frozen in aliquots before use.
Experimental Immunizations Where CD28 Signaling Was Restored by CD28 mAb.
Mice expressing soluble CTLA4-Ig or littermate controls were immunized in the intraperitoneal cavity with 50 μg alum-precipitated 4-hydroxy-3-nitrophenyl acetyl (NP)-KLH resuspended in 200 μl of saline. KLH (Sigma Chemical Co.) was haptenated as described elsewhere 17. Assuming a molecular weight of 3 million to KLH, there are ∼400 molecules of NP conjugated to each KLH molecule. Anti-CD28 mAb (100 μg) was injected intraperitoneally where indicated 24 h after antigen administration. OX40-Ig or control Ig (100 μg) was injected intraperitoneally on days 1, 2, and 3. Animals were killed after 9 d, then bled, and their spleens were taken for frozen section analysis and flow cytometry.
Flow Cytometry.
mAbs used for flow cytometry analysis were FITC-conjugated rat anti–mouse CD4 (Southern Biotechnology), PE-conjugated rat anti–mouse OX40 (Serotec), biotin-conjugated rat anti–mouse CD62L (Southern Biotechnology), and rabbit anti–mouse Burkitt's lymphoma receptor 1 (BLR1 [CXCR5]). Secondary step reagents were streptavidin-cychrome (PharMingen) and anti–rabbit PE (Jackson ImmunoResearch Labs).
Immunohistology and Quantitative Analysis.
Animals were killed by CO2 asphyxiation, and their spleens were removed, snap-frozen in liquid N2, and stored at −70°C until use. 5-μm cryostat sections from these tissues were mounted onto 4-spot glass slides that were air dried for 1 h, fixed in acetone at 4°C for 20 min, dried again, and stored in sealed polythene bags at −20°C.
Tissue sections were double-stained for IgD (sheep anti–mouse IgD; Binding Site) with CD3 (KT3), CD4 (GK1.5), or CD8 (2.43) (American Type Culture Collection). Slides were stained as described elsewhere 4. In brief, second step reagents were donkey anti–sheep peroxidase (Binding Site) and biotinylated rabbit anti–rat (DAKO) followed by StreptABComplex alkaline phosphatase (DAKO). NP-binding cells were identified using NP conjugated to rabbit IgG. Secondary Abs were biotinylated swine anti–rabbit and biotinylated rabbit anti–rat. Sheep anti–mouse IgD was detected using peroxidase-conjugated donkey anti–sheep Ig (Binding Site).
Substrate for peroxidase was 3-3′ diaminobenzidine (Sigma Chemical Co.), and for alkaline phosphatase the substrate Naphthol AS-MX phosphate and chromogen Fast Blue BB salt/levamisole (Sigma Chemical Co.) were used to block endogenous alkaline phosphatase activity.
For quantitative analysis, GC size was assessed by counting the number of graticule intercepts (units) at 400× magnification of IgD− B cell areas within B follicles 15. The numbers of CD3+ T cells were counted in each GC.
Results
Costimulatory Signals through CD28 Upregulate OX40 on CD3-activated Naive CD4 T Cells.
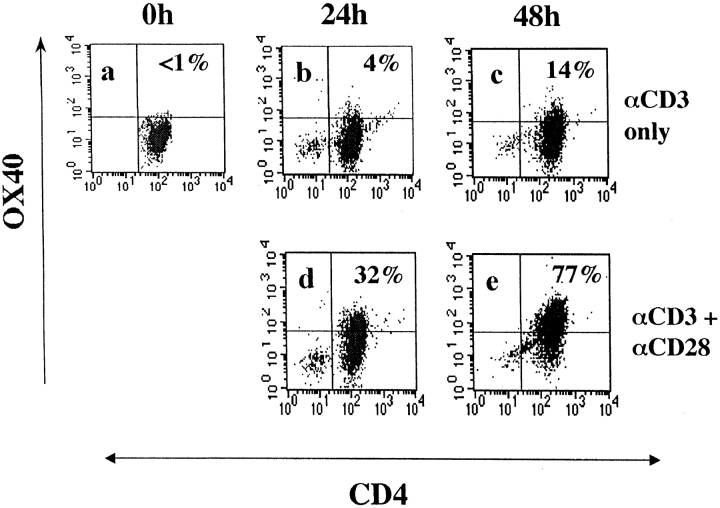
To test for the CD28 dependence of OX40 expression, naive CD62Lhigh CD4 T cells were purified from mouse spleens 11 and activated with anti-CD3 mAb, with and without agonistic CD28 mAb. Unstimulated CD4 cells did not express detectable levels of OX40 compared with a control isotype-matched mAb (Fig. 1 a). In addition, the specificity of staining was checked by cold competition with unlabeled anti-OX40 mAb. This could not inhibit fluorescence on unstimulated CD4 T cells, indicating undetectable levels of OX40 protein as assessed by flow cytometry. By 24 h after activation through CD3, naive CD4 T cells with (Fig. 1 d) and without (Fig. 1 b) agonistic CD28 mAb were equivalently enlarged in size (data not shown) but it was already clear that CD28 costimulation augmented OX40 expression (32 vs. 4% positive CD4 T cells). The differences were even more pronounced after 48 h of culture (compare Fig. 1 e with Fig. 1 c). Specificity of staining was shown by the capacity of unlabeled OX40 mAb to inhibit binding of OX40-PE–specific mAb (data not shown). These results appear to contradict reports that OX40 expression in vitro is relatively independent of signaling through CD28 10 18. Because differences in strength of signaling through CD3 with mAb could account for this discrepancy, we went on to investigate the induction of OX40 expression in vivo.
Figure 1.
Costimulation dependence of OX40 expression on CD3-activated naive CD4 T cells. CD62Lhigh CD4 T cells were stained with OX40 mAb at different times after activation with CD3 mAb in the absence (a, b, and c) or presence of agonistic mAb to CD28 (d and e). Flow gates were set on an isotype-matched control PE-conjugated mAb (PharMingen).
In Vivo OX40 Expression Is Dependent on CD28 Signaling.
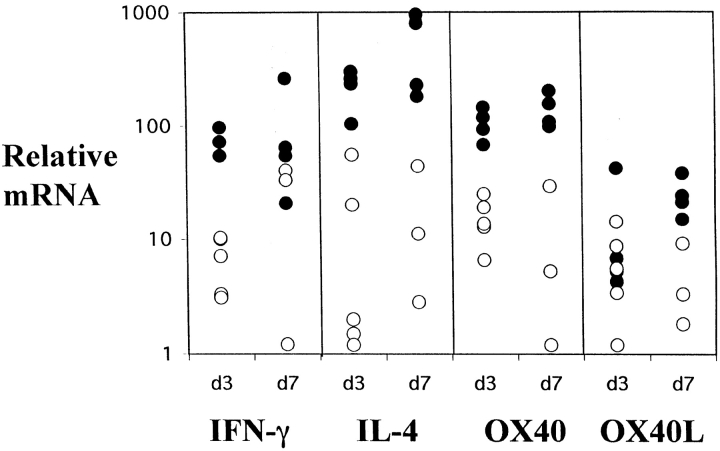
To test for the CD28 dependence of OX40 expression in vivo, we compared OX40 induction in CTLA4-Ig tg mice and nontransgenic littermates. Mice were immunized in the left footpad with the Th2-inducing antigen, alum-precipitated, chicken γ-globulin 13, and the draining popliteal nodes were examined 3 or 7 d later. Levels of mRNA for OX40 and its ligand, together with the cytokines IL-4 and IFN-γ, were quantified in the draining LN of transgenic and normal littermate mice (Fig. 2). Previous studies have shown that upregulation of OX40L occurs on day 2, and IL-4, IFN-γ, and OX40 are induced by day 3 11 13. There was less mRNA for IL-4, IFN-γ, and OX40 in CTLA4-Ig tg mice compared with control littermates on day 3, although OX40L expression was comparable. In particular, in CTLA4-Ig tg mice, median levels of day 3 OX40 and IL-4 mRNA were similar to those in day 0 unimmunized controls (data not shown). After 7 d, CTLA4-Ig tg mice made comparable amounts of IFN-γ as has been reported elsewhere 8, but there was still much less mRNA for IL-4 and OX40, and less OX40L in transgenic animals. CTLA4-Ig mice immunized with B. pertussis, which evokes IL-4 and IFN-γ, and a combination of IgG1 and IgG2a Abs in normal mice 13 had comparable levels of IFN-γ at day 3 (data not shown). These data show there is a relative defect in IL-4 compared with IFN-γ, and this is correlated with deficient OX40 expression in CTLA4-Ig tg mice. This suggests that in vivo OX40 expression is CD28 dependent, and that poor OX40 induction correlates with reduced IL-4 levels.
Figure 2.
Relative mRNA levels for IFN-γ, IL-4, OX40, and OX40L in the draining LNs of normal (•) and CTLA4-Ig (○) mice at days 3 and 7 after immunization in the footpad with alum-precipitated chicken γ-globulin. Levels are corrected for differences in expression of β-actin. Average day 3 levels of OX40 and IL-4 in CTLA4-Ig tg mice were similar to levels in day 0 control unimmunized mice (data not shown).
CTLA4-Ig Tg Mice Lack Activated CD4 T Cells that Express the Chemokine Receptor, CXCR5, and This Subset Is Increased in CD11c-OX40L Tg Mice.
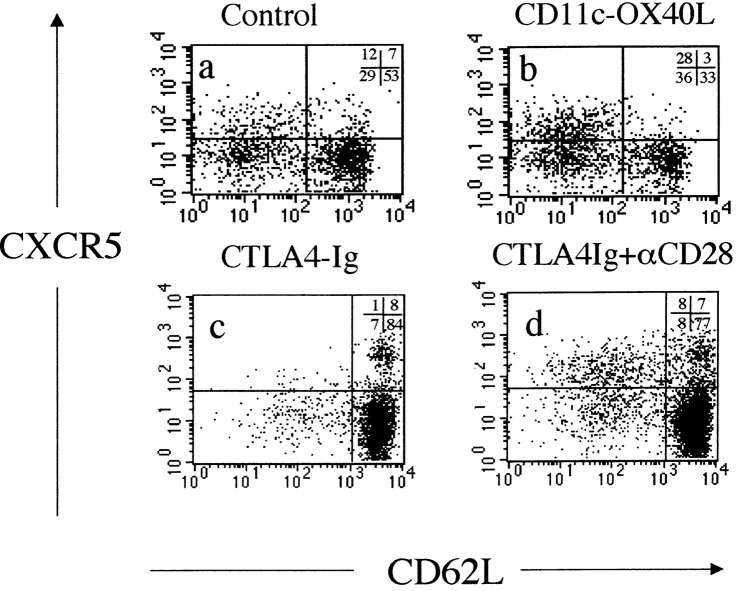
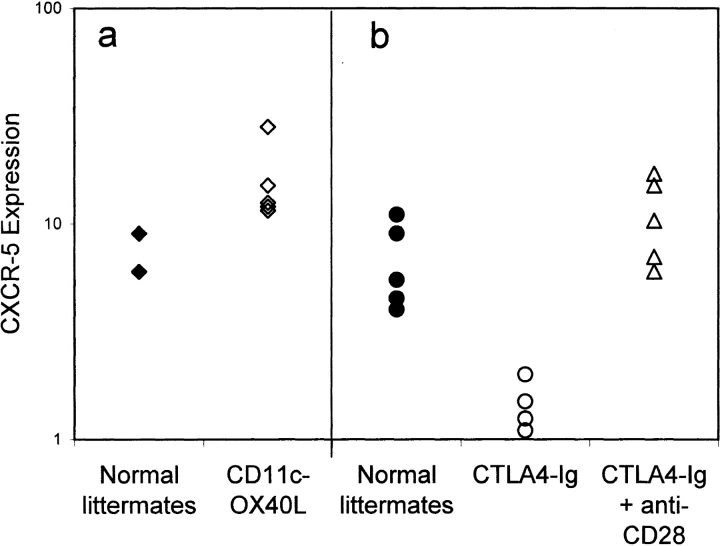
Previously, we reported that ligation of OX40 on CD4 T cells upregulates mRNA for CXCR5 11, a chemokine receptor which appears to direct lymphocytes to B cell follicles 19 20. Using mAb and polyclonal rabbit antiserum to CXCR5, we confirm previous reports 19 that a subset of memory CD4 T cells, which are low in expression of L-selectin (CD62L), expresses this chemokine receptor (Fig. 3 a). We also found a proportion of CD62Lhigh cells expressing CXCR5 as has been reported in humans 19. This population may represent an intermediately activated CD4 T cell population. Alternatively, some CD62Llow cells may revert to a CD62Lhigh phenotype. Mice which constitutively express OX40L on their DCs (CD11c-OX40L tg mice) are characterized by increased numbers of CD4 T cells in B cell follicles 12. Here we show that this phenotype is linked to an expansion of CXCR5+CD62Llow CD4 cells (Fig. 3 b, and Fig. 4 a). In contrast, immunized CTLA4-Ig tg mice, which lack CD28 costimulation, have some CD62Llow CD4 cells, but these are strikingly deficient in expression of CXCR5 (Fig. 3 c, and Fig. 4 b). However, if the CD80/CD86 blockade is bypassed by injection of an agonistic CD28 mAb, then this subset is rapidly restored (Fig. 3 d, and Fig. 4 b). These data link CD28 and OX40 signaling with CXCR5 expression on CD4 T cells.
Figure 3.
Expression of CD62L and CXCR5 on gated splenic CD4 T cells from normal and transgenic mice immunized 10 d previously with alum-precipitated protein antigens: (a) normal mice; (b) CD11c-OX40L tg mice; (c) CTLA4-Ig tg mice; and (d) CTLA4-Ig tg mice coinjected with agonistic mAb to CD28 (100 μg) 1 d after immunization. Staining is shown from two separate experiments: b should be compared with a, and d compared with c, as staining for CD62L is slightly different for the two experiments.
Figure 4.
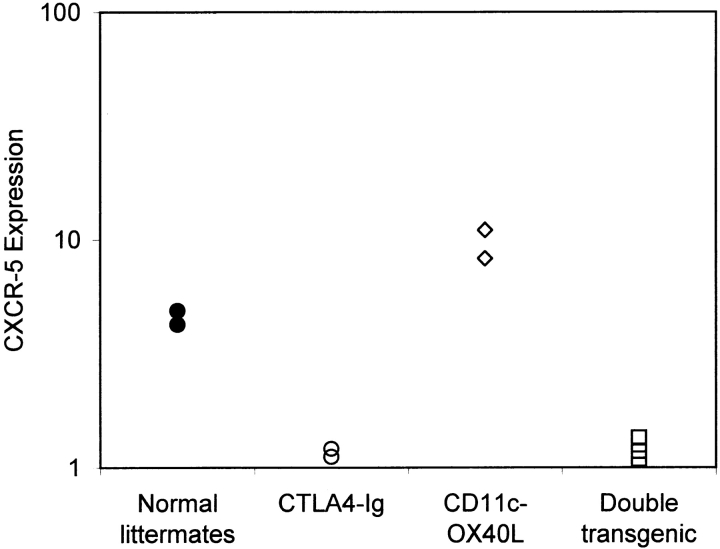
Percentage of gated splenic CD4 T cells that were CD62Llow, but expressed CXCR5 by flow cytometry 10 d after intraperitoneal immunization with alum-precipitated protein antigens: normal littermates for CD11c-OX40L tg mice (♦); CD11c-OX40L tg mice (⋄); normal littermates for CTLA4-Ig tg mice (•); CTLA4-Ig tg mice (○); CTLA4-Ig tg mice after coinjection with CD28 mAb (▵). Results are representative of (a) three and (b) eight separate experiments.
CD4 T Cell CXCR5 Expression and Enhanced Migration into B Cell Areas in CD11c-OX40L Tg Mice Is Dependent on CD28 Signaling.
CD11c-OX40L tg mice, which constitutively express OX40L on DCs, have increased numbers of CXCR5+CD62Llow CD4 cells and increased numbers of follicular T cells. To test the CD28 dependence of this phenotype, we crossed CTLA4-Ig tg mice (heterozygous for the transgene) with CD11c-OX40L tg mice (heterozygous for the transgene). On average, 25% of the offspring of this pairing are normal (nontransgenic), 25% are transgenic for CTLA4-Ig, 25% transgenic for CD11c-OX40L, and 25% express both CTLA4-Ig and CD11c-OX40L. CXCR5 expression was compared in all four groups of animals immunized with alum-precipitated protein antigens. Normal mice form GCs by day 10 (data not shown) and have CXCR5+CD62Llow CD4 T cells (Fig. 5). CTLA4-Ig tg mice lack CXCR5+ CD62Llow CD4 cells (Fig. 5), and do not form GCs 7. CD11c-OX40L tg mice have increased CXCR5+ CD4 cells (Fig. 5) and form GCs 12. However, double transgenic mice have the phenotype of their CTLA4-Ig tg littermates: they lack CXCR5-expressing CD62Llow CD4 T cells (Fig. 5), and do not develop GCs (data not shown). This clearly indicates that the phenotype in CD11c-OX40L tg mice requires CD28 signaling, and is consistent with the expression of OX40 being CD28 dependent in vivo. However, this does not exclude the possibility that other CD28-dependent signals induce CXCR5 expression on CD4 T cells.
Figure 5.
CD28 dependence of CXCR5 expression in CD11c-OX40L tg mice. Percentage of CD4 T cells that were CD62Llow, but expressed CXCR5 10 d after intraperitoneal immunization with alum-precipitated protein antigens: normal littermates (•); CTLA4-Ig tg mice (○); CD11c-OX40L tg mice (⋄); double transgenic mice coexpressing CD11c-OX40L and CTLA4-Ig tg (□). Data are shown from one litter resulting from crossing CTLA4-Ig tg and CD11c-OX40L tg mice, and are representative of two separate experiments.
Rescue of GC Formation by mAb to CD28 in CTLA4-Ig Tg Mice Is Partially Dependent on OX40 Interactions.
The lack of GC formation in CTLA4-Ig tg mice can be reversed by a single injection of agonistic anti-CD28 Ab (Fig. 6 a). These mice develop large GCs, unlike CTLA4-Ig tg mice given control hamster Ig (data not shown). Production of specific IgG Ab is also restored, and there is evidence of affinity maturation (data not shown). Blocking OX40 interactions with a fusion protein between murine OX40 and human IgG1 (OX40-Ig) allowed us to test our hypothesis that the restoration of GC formation by anti-CD28 treatment is dependent on OX40 signaling. CTLA4-Ig tg mice were immunized with alum-precipitated protein intraperitoneally, and 1 d later were given anti-CD28 mAb (100 μg) with either OX40-Ig (100 μg in three doses on days 1, 2, and 3) or a control Ig fusion protein. Because CTLA4-Ig tg mice have no GCs, injection of mAb to CD28 allows accurate timing of their onset. Also, because CTLA4-Ig tg mice are tolerant to fusion proteins between human IgG1 and self-proteins, blockade is more likely to persist and be effective. The presence of CTLA4-Ig, which binds to CD80 and CD86 on activated B cells, controls for potential artifacts due to OX40-Ig binding to B cells, independent of its effect on OX40/OX40L blockade.
Figure 6.
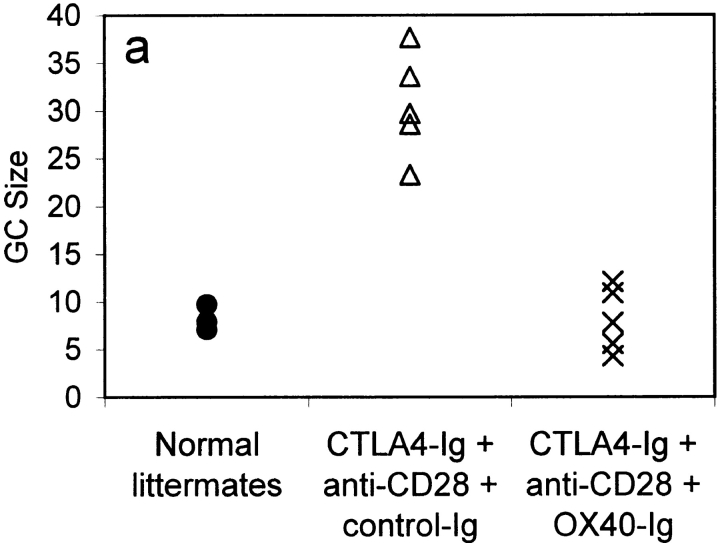
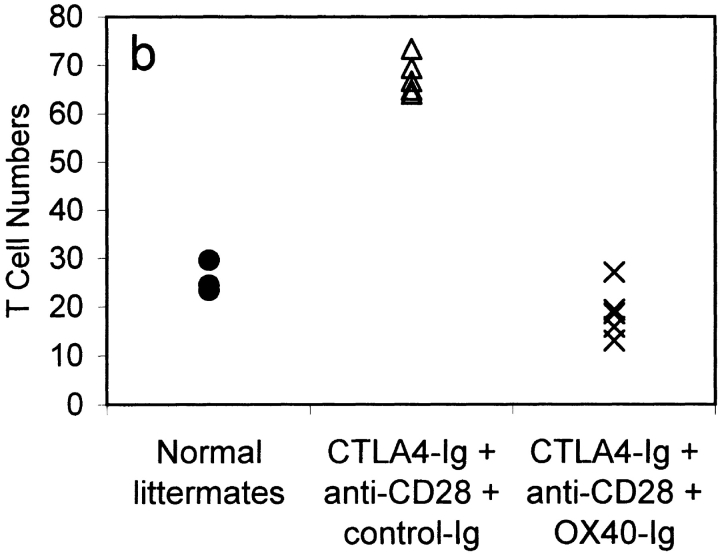
GC size and average number of T cells per GC in mice immunized 9 d previously with 100 μg alum-precipitated protein antigen. Results are shown for normal littermate controls (•); CTLA4-Ig tg mice treated with anti-CD28 mAb and either a control Ig fusion protein (control-Ig, 100 μg; ▵) or OX40-Ig (100 μg; X). Results show the mean GC size (a) and T cells/GC (b). In each mouse, 20 consecutive GC areas were measured, and the numbers of individual CD3 cells within GCs were counted. Results are representative of two separate experiments.
CTLA4-Ig tg mice treated with anti-CD28 mAb and control Ig had GCs with a mean size of 30.6 U (see Materials and Methods; Fig. 6 a) with 67.3 T cells per GC (Fig. 6 b). In marked contrast, CTLA4-Ig tg mice treated with OX40-Ig had much smaller but not absent GCs with a mean size of 8.1 U (Fig. 6 a), and a mean number of T cells per GC of 18.7 (Fig. 6 b). Although GC size was diminished in OX40-Ig–injected mice, the extrafollicular anti-NP plasma cell responses were consistently increased (data not shown). These data link CD4 T cell OX40 signaling with GC development, but not with the extrafollicular plasma cell response.
Discussion
We have investigated the sequence of molecular instructions that control CD4 T cell migration and differentiation to provide GC T cell help. We recently published evidence that OX40 signaling was linked with induction of IL-4 expression 11, and studies in humans have yielded a similar conclusion 10. We also found that OX40 ligation was linked with upregulation of CXCR5 on CD4 T cells, a chemokine receptor linked with lymphocyte migration to B cell areas. This idea was supported by the phenotype of CD11c-OX40L tg mice, which have increased CD4 T cell numbers within GCs 12. CD28-deficient mice are also deficient in their capacity to provide B cell help: they have a relatively selective deficit in Th2 development 8, and lack GCs 7 21. In keeping with the idea that CXCR5+ CD4 cells are implicated in GC development, we report here that CTLA4-Ig tg mice have a selective deficit in these cells. In contrast, CD11c-OX40L tg mice, which have greatly increased CD4 T cells in B cell areas, have the opposite phenotype, with an increased proportion of CXCR5 CD4+CD62Llow cells.
To understand the relationship between CD28 and OX40 signaling in development of CXCR5+ CD4 T cells and GCs, we investigated the CD28 dependence of OX40 expression. Although it has been reported that OX40 expression in vitro is independent of CD28 costimulation 10 18, in our hands we found that levels of OX40 in vitro and in vivo were greatly augmented by CD28 signaling. We hypothesized that poor expression of OX40 in CD28-deficient mice was linked with the lack of GC development 7 8. To test this, we acutely reversed the CD28 signaling defect by injection of agonistic CD28 mAb. GCs reappear in Ab-treated mice, and this is correlated with the appearance of CXCR5+CD62Llow CD4 T cells. If, however, OX40 interactions were blocked by OX40-Ig, GC formation was greatly reduced, although significantly the extrafollicular plasma cell response was augmented (data not shown). These data strongly support the idea that CD28-costimulated CD4 T cells primed in the outer T zone by DCs upregulate OX40, which allows them to respond to OX40L expressed by either CD40-activated DCs 12 22 or activated B cells 23. This sequential signal then activates a program of events that includes upregulation of IL-4, CXCR5 expression, and migration into follicles to help B cells form GCs. This model predicts that the enhanced accumulation of CD4 T cells in B cell follicles in CD11c-OX40L tg mice would be dependent on CD28 signaling, which indeed proved to be the case in our study.
Although our data implicate ligation of OX40 on T cells in CD4 migration into B follicles, injection of OX40-Ig reduced rather than abolished GC formation. Preliminary experiments show OX40-deficient mice can develop GCs albeit at a slower tempo (our unpublished observations), indicating that other molecules can substitute for OX40. CD70 (CD27L), whose receptor is expressed on resting CD4 T cells 24, is a good candidate, as in vitro it has similar effects on CD4 T cell differentiation and, like OX40L, is expressed on both CD40-activated B cells and DCs (our unpublished observations). This situation is analogous to CD80 and CD86, which can both costimulate T cells through CD28, but perhaps because of temporal differences in expression, exert subtle differences on T cell immune responses.
Our model of the role of OX40 in CD4 T cell differentiation is different from that proposed elsewhere 23 25, which suggests that ligation of B cell OX40L by CD4 T cell OX40 promotes the extrafollicular plasma cell response, but does not influence GC development. In the in vivo experiments described 25, a polyclonal rabbit antiserum to OX40 was used to block OX40/OX40L interactions. We speculate that this antiserum might signal OX40-expressing CD4 T cells to migrate into B follicles, as in the CD11c-OX40L tg mice 12. In our experiments, injection of OX40-Ig would allow signaling through B cell OX40L and the development of the extrafollicular plasma cell response, but by blocking OX40 ligation of CD4 T cells, would inhibit T cell migration and reduce GC formation.
Much controversy exists in the literature concerning the role of CD28 signaling in immunosuppression strategies. In general, mice rendered deficient from birth in CD28 signaling, by either gene deletion of CD28 6 or constitutive expression of CTLA4-Ig 7, make surprisingly normal immune responses. They can reject grafts (26; and our unpublished observations), resist intracellular infections such as Leishmania 27, and mount many normal viral immune responses 6 28. The one consistent immune deficit is the capacity to make B cell GCs 7 21, and this is linked to selective deficiency in Th2 responses 8 29. However, if CD28 is blocked acutely in normal mice by injecting CTLA4-Ig, very different results can be obtained 30. CD4 T cell responsiveness is regulated by a balance between CD28 (positive) and CTLA4 (negative) signaling 26, and the differences observed with acute and chronic blockade of CD28 by CTLA4-Ig could be due to individual T cells tuning their responsiveness to the costimulatory environment in which they mature. In this paper, we found that acute reversal of CD28 signaling in CTLA4-Ig tg mice led to massive GC formation, which is in keeping with the idea that CD4 T cells in these mice are hyperresponsive when CD28 blockade is reversed 31 32. However, our data also provide a potential explanation for why the timing of CD28 blockade may have very different consequences for the subsequent immunological response. We have shown that CD28 signaling is permissive for other costimulatory signals such as OX40. We speculate that delaying CD28 blockade allows OX40 signaling to occur at the onset of immune responses, which allows Th2 CD4 T cells to develop 30 33, resulting in less damaging autoimmune and transplant responses. It could also explain why blocking CD86 aggravates experimental allergic encephalitis, a model in which pathology is largely Th1 mediated 34. CD86 is constitutively expressed on DCs and could provide crucial early CD28 signals required for OX40 expression.
Evidence that OX40 blockade modifies the course of inflammatory disorders 35 36 indicates roles for OX40 other than in development of GCs. In addition to its expression on activated DCs and B cells, OX40L is expressed on vascular endothelium 37. An intriguing possibility is that ligation of OX40 here is involved in recruitment of activated T cells to inflammatory sites. Both CTLA4-Ig 38 and OX40-Ig 35 abrogate susceptibility to experimental allergic encephalitis. However, CTLA4-Ig does not block priming of encephalogenic T cells 38. This might be explained if CTLA4-Ig blocked OX40 expression, hence inhibiting migration into the central nervous system. Therefore, the effects of OX40 ligation may depend on the context in which CD4 T cells find themselves.
Acknowledgments
We are very grateful to Jason Cyster for providing the polyclonal CXCR5_-_specific antiserum and for his many helpful comments on the manuscript. We would also like to thank Ian MacLennan, David Sansom, Matt Cook, Kai Toellner, and Graham Anderson for reading the manuscript and making many helpful suggestions. These experiments could not have been done without the expert help of the staff of the Birmingham University Animal Facility (BMSU).
This work was supported by grants from the Wellcome Trust to P. Lane. S. Flynn is supported by a Medical Research Council Ph.D. studentship. T. Brocker is supported by the Deutsche Forschungsgemeinschaft Leibniz-Program (to M. Reth).
Footnotes
1used in this paper: CD11c-OX40L tg mice, transgenic mice expressing OX40L driven by the CD11c promoter; CTLA, CTL-associated molecule; CTLA4-Ig tg mice, transgenic mice expressing the soluble CD28 competitor, CTLA4-Ig; CXCR, CXC chemokine receptor; DC, dendritic cell; GC, germinal center; NP, 4-hydroxy-3-nitrophenyl acetyl; OX40-Ig, murine OX40–human IgG1 fusion protein; OX40L, OX40 ligand
References
- Jacob J., Kassir R., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 1991;173:1165–1176. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Zhang J., Lane P.J., Chan E.Y., MacLennan I.C. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- MacLennan I.C.M., Gulbranson-Judge A., Toellner K.M., Casamayor-Palleja M., Chan E., Sze D.M.Y., Luther S.A., Orbea H.A. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol. Rev. 1997;156:53–66. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- Gulbranson-Judge A., MacLennan I. Sequential antigen-specific growth of T cells in the T zones and follicles in response to pigeon cytochrome C. Eur. J. Immunol. 1996;26:1830–1837. doi: 10.1002/eji.1830260825. [DOI] [PubMed] [Google Scholar]
- Han S., Hathcock K., Zheng B., Kepler T.B., Hodes R., Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- Shahinian A., Pfeffer K., Lee K.P., Kundig T.M., Kishihara K., Wakeham A., Kawai K., Ohashi P.S., Thompson C.B., Mak T.W. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- Lane P., Burdet C., Hubele S., Scheidegger D., Müller U., McConnell F., Kosco-Villbois M. B cell function in mice transgenic for mCTLA4-Hγ1lack of germinal centers correlated with poor affinity maturation and class switching despite normal priming of CD4+ T cells. J. Exp. Med. 1994;179:819–830. doi: 10.1084/jem.179.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D.J., Herold K.C., Rhee L., Patel B., Koons A., Qin H.-Y., Fuchs E., Singh B., Thompson C.B., Bluestone J.A. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- Manickasingham S.P., Anderton S.M., Burkhart C., Wraith D.C. Qualitative and quantitative effects of CD28/B7-mediated costimulation on naive T cells in vitro. J. Immunol. 1998;161:3827–3835. [PubMed] [Google Scholar]
- Ohshima Y., Yang L.P., Uchiyama T., Tanaka Y., Baum P., Sergerie M., Hermann P., Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4+ T cells into high IL-4-producing effectors. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- Flynn S., Toellner K.-M., Raykundalia C., Goodall M., Lane P. CD4 T cell cytokine differentiationthe B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J. Exp. Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker T., Gulbranson-Judge A., Flynn S., Riedinger M., Raykundalia C., Lane P. CD4 T cell traffic controlin vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells into B follicles. Eur. J. Immunol. 1999;29:1610–1616. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Toellner K., Luther S., Sze D.M.-Y., Choy R.K.-W., Taylor D.R., MacLennan I.C.M., Acha-Orbea H. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J. Exp. Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetic A., Finkelman F.D., Jian Y.C., Dieffenbach C.W., Scott D.E., McCarthy K.F., Steinberg A.D., Gause W.C. Cytokine gene-expression after in vivo primary immunization with goat antibody to mouse IgD antibody. J. Immunol. 1991;147:2391–2397. [PubMed] [Google Scholar]
- Weibel E.R. Principles and methods for the morphometric study of the lung and other organs. Lab. Invest. 1963;12:131–155. [PubMed] [Google Scholar]
- Schmidt K.N., Hsu C.W., Griffin C.T., Goodnow C.C., Cyster J.G. Spontaneous follicular exclusion of SHP1-deficient B cells is conditional on the presence of competitor wild-type B cells. J. Exp. Med. 1998;187:929–937. doi: 10.1084/jem.187.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G.J., Karvelas M. Soluble antigen abrogates the appearance of anti-protein IgG1-forming cell precursors during primary immunization. Proc. Natl. Acad. Sci. USA. 1990;87:1615–1619. doi: 10.1073/pnas.87.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaglia I., Weinberg A.D., Lemon M., Croft M. Ox-40 liganda potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- Förster R., Emrich T., Kremmer E., Lipp M. Expression of the G-protein–coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- Förster R., Mattis A.E., Kremmer E., Wolf E., Brem G., Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Ferguson S.E., Han S., Kelsoe G., Thompson C.B. CD28 is required for germinal center formation. J. Immunol. 1996;156:4576–4581. [PubMed] [Google Scholar]
- Ohshima Y., Tanaka Y., Tozawa H., Takahashi Y., Maliszewski C., Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J. Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- Stüber E., Neurath M., Calderhead D., Fell H.P., Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–521. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- Gravestein L.A., Blom B., Nolten L.A., Devries E., Vanderhorst G., Ossendorp F., Borst J., Loenen W.A.M. Cloning and expression of murine CD27comparison with 4-1BB, another lymphocyte-specific member of the nerve growth factor receptor family. Eur. J . Immunol. 1993;23:943–950. doi: 10.1002/eji.1830230427. [DOI] [PubMed] [Google Scholar]
- Stüber E., Strober W. The T cell–B cell interaction via OX40–OX40L is necessary for the T cell–dependent humoral immune response. J. Exp. Med. 1996;183:979–989. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D.J., Walunas T.L., Bluestone J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Brown D.R., Green J.M., Moskowitz N.H., Davis M., Thompson C.B., Reiner S.L. Limited role of CD28-mediated signals in T helper subset differentiation. J. Exp. Med. 1996;184:803–810. doi: 10.1084/jem.184.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann C., Seiler P., Lane P., Zinkernagel R.M. Antiviral immune responses in CTLA4 transgenic mice. J. Virol. 1997;71:1802–1807. doi: 10.1128/jvi.71.3.1802-1807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.L., Jia X.L., June C.H., Abe R., Lee K.P. CD28-deficient mice generate an impaired Th2 response to Schistosoma mansoni infection. Eur. J. Immunol. 1996;26:2448–2455. doi: 10.1002/eji.1830261027. [DOI] [PubMed] [Google Scholar]
- Sayegh M.H., Akalin E., Hancock W.W., Russell M.E., Carpenter C.B., Linsley P.S., Turka L.A. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J. Exp. Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchese F., Hausmann B., Hubele S., Lane P. Mice transgenic for a soluble form of murine CTLA-4 show enhanced expansion of antigen-specific CD4+ T cells and defective antibody production in vivo. J. Exp. Med. 1994;179:809–817. doi: 10.1084/jem.179.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P., Haller C., McConnell F. Evidence that induction of tolerance in vivo involves active signaling via a B7 ligand-dependent mechanismCTLA4-Ig protects Vβ8+ T cells from tolerance induction by the superantigen staphylococcal enterotoxin B. Eur. J. Immunol. 1996;26:858–862. doi: 10.1002/eji.1830260420. [DOI] [PubMed] [Google Scholar]
- Lenschow D.J., Ho S.C., Sattar H., Rhee L., Gray G., Nabavi N., Herold K.C., Bluestone J.A. Differential effects of anti–B7-1 and anti–B7-2 monoclonal antibody on the development of diabetes in the nonobese diabetic mouse. J. Exp. Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchroo V.K., Das M.P., Brown J.A., Ranger A.M., Zamvil S.S., Sobel R.A., Weiner H.L., Nabavi N., Glimcher L.H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathwaysapplication to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- Weinberg A.D., Vella A.T., Croft M. OX-40life beyond the effector T cell stage. Semin. Immunol. 1998;10:471–480. doi: 10.1006/smim.1998.0146. [DOI] [PubMed] [Google Scholar]
- Higgins L.M., McDonald S.A., Whittle N., Crockett N., Shields J.G., MacDonald T.T. Regulation of T cell activation in vitro and in vivo by targeting the OX40-OX40 ligand interactionamelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J. Immunol. 1999;162:486–493. [PubMed] [Google Scholar]
- Imura A., Hori T., Imada K., Ishikawa T., Tanaka Y., Maeda M., Imamura S., Uchiyama T. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J. Exp. Med. 1996;183:2185–2191. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A.H., Girard T.J., Giacoletto K.S., Evans R.J., Keeling R.M., Lin R.F., Trotter J.L., Karr R.W. Long-term inhibition of murine experimental autoimmune encephalomyelitis using CTLA4-Fc supports a key role for CD28 costimulation. J. Clin. Invest. 1995;95:2783–2789. doi: 10.1172/JCI117982. [DOI] [PMC free article] [PubMed] [Google Scholar]