High Level Expression of Cd43 Inhibits T Cell Receptor/CD3-Mediated Apoptosis (original) (raw)
Abstract
In a screen designed to identify genes that regulate T cell receptor (TCR)/CD3-mediated apoptosis, we found that high level expression of CD43 protected T cell hybridomas from activation-induced cell death. The protection appears to result from its capacity to block Fas-mediated death signals rather than from inhibition of the upregulation of Fas and/or Fas ligand after T cell stimulation. We found that peripheral CD4+ T cells can be divided into two subsets based on the level of CD43 surface expression. The CD4+CD43low subset exhibits a naive T cell phenotype, being CD62LhighCD45RBhighCD44low, whereas CD4+CD43high cells exhibit a memory phenotype, being CD62LlowCD45RBlowCD44high. Recent studies have demonstrated that engagement of TCR and Fas induces naive CD4+ T cells to undergo apoptosis, and the same treatment enhances the proliferation of memory CD4+ T cells. We confirm here that peripheral CD4+CD43high T cells are resistant to TCR/CD3-mediated cell death. These results suggest that the expression levels of CD43 on naive and memory CD4+ T cells determine their susceptibility to Fas-dependent cell death and that high level expression of CD43 may be used as a marker to define CD4+ memory T cells. Expression of CD43 provides a novel mechanism by which tumor cells expressing abnormally high levels of CD43 may escape Fas-mediated killing.
Keywords: CD43, activation-induced apoptosis, memory CD4+ T cells, Fas
After generation of a successful T cell response, a majority of the activated T cells die, and a minority survive to become resting memory cells 1. Memory T cells differ from naive T cells not only in the expression of surface markers but also in their functionality. Compared with naive T cells, memory T cells express lower levels of CD62L and CD45RB and higher levels of CD44. Memory T cells also respond faster, have a lower activation threshold, and secrete a wider range of cytokines than naive T cells. In addition, recent studies have demonstrated that, compared with naive CD4+ T cells, memory CD4+ T cells are resistant to TCR-mediated, Fas-dependent apoptosis 2 3 4. Engagement of TCR or TCR plus Fas on naive CD4+ T cells induces these cells to undergo apoptosis, whereas Fas engagement may enhance the proliferation of memory CD4+ T cells 2 3 4. Studies on activation-induced cell death (AICD) of T cell hybridomas have been fruitful for understanding the molecular mechanisms of T cell apoptosis. After TCR stimulation, T cell hybridomas upregulate Fas and FasL (ligand). Autocrine stimulation of Fas by FasL triggers the death pathway via the recruitment of adaptors to the cytoplasmic tail of Fas 5. CD43 (leukosialin, sialophorin) is a highly glycosylated transmembrane protein expressed on the surfaces of all hematopoietic cells except mature B cells and erythrocytes 6. Previous studies suggest that CD43 may regulate multiple cellular functions, such as cell adhesion, activation, and proliferation as well as cell survival and apoptosis. However, the precise function of CD43 remains unknown due to conflicting results. Cross-linking of CD43 may enhance T cell proliferation 7, and it can act as a costimulatory molecule independent of CD28 8. However, T cells from CD43-deficient mice are hyperresponsive after both in vivo and in vitro activation, indicating a negative regulatory role for CD43 in T cell activation 9. Although an anti-CD43 mAb has been shown to inhibit T cell binding to lymph node and Peyer's patch high endothelial venule and homing from the blood into second lymphoid tissues 10, T cells from CD43-deficient mice homed significantly more frequently to secondary lymphoid organs compared with wild-type T cells 11. Certain studies have shown that engagement of CD43 induces apoptosis of T cells and hematopoietic progenitor cells 12 13; CD43 was also shown to promote B cell survival when ectopically expressed 14.
In a search for genes that regulate TCR/CD3-mediated AICD, we found that CD43 can protect T cells when expressed at high levels. This protection appears to be due to a blockade of Fas function by CD43. Our findings shed new light on the significance of CD43 expression in lymphoid and nonlymphoid tissues.
Materials and Methods
Cell Lines.
A subclone of DO11.10 T hybridoma cells with a low survival rate in PMA plus ionomycin was used for expression cloning. φNX-Ampho is a retrovirus packaging cell line 15. DO11.10 cell line expressing a tailless version of human CD2 (DO11.10hCD2) as control was described previously 16. DO11.10 cells expressing high levels of CD43 were generated by transduction of mouse CD43 cDNA using the pMX retrovirus vector, followed by FACS® sorting for CD43 expression. Cells were cultured in DMEM containing 10% FCS, 2 mM glutamine, 25 mM Hepes, 50 μM β-ME, 100 U/ml penicillin, and 100 μg/ml streptomycin.
mAbs and Reagents.
Anti–human CD2 mAb 35.1 (American Type Culture Collection) was purified and labeled in our laboratory. The following mAbs were purchased from PharMingen: purified or PE–anti-CD3 (145-2C11), FITC– or PE–anti-CD4 (GK1.5), FITC–, PE–, or biotin–anti-CD8α (53-6.7), FITC– or PE–anti-CD43 (S7), PE–anti-CD69 (H1.2F3), biotin–anti-FasL (Kay-10), PE–anti-Fas (Jo2), biotin–anti-CD62L (MEL-14), biotin–anti-CD45RB (16A), and biotin–anti-CD44. Ceramide and staurosporine were purchased from Calbiochem Corp. Recombinant human IL-2 was purchased from Chiron Corp.
Expression Cloning.
Expression cloning was performed as described 16. In brief, a thymocyte cDNA library in the retroviral vector pMX 17 was transfected into the φNX-Ampho packaging cell line using CaPO4 precipitation, and retrovirus-containing supernatant was harvested 2 d later. DO11.10 cells were infected with the retrovirus supernatant. A total of 5 × 106 DO11.10 cells were infected, as assessed using control pMX-hCD2 retrovirus infection. 2 d after the infection, DO11.10 cells were placed into 96-well plates (5 × 104 cells/well) and selected for survival and growth in the presence of PMA (10 ng/ml) plus ionomycin (0.2 μg/ml). Resistant clones were identified after 2–4 wk in culture. cDNA inserts were amplified by reverse transcriptase–PCR and sequenced.
Flow Cytometric Analyses.
Before staining, cells were incubated with a mAb to FcR (2.4G2) and then sequentially stained with an excess of biotinylated mAb, PE–streptavidin, or FITC– and PE–conjugated mAbs on ice for 30 min and washed with PBS containing 0.1% BSA. Data were collected on 1–5 × 104 cells on a FACScan™ flow cytometer (Becton Dickinson) using CELLQuest™ software.
Apoptosis Assay.
24- or 96-well tissue culture plates were precoated with rabbit anti–hamster antiserum (50 μg/ml) overnight at 37°C, washed with HBSS, and coated with anti-CD3 mAb (145-2C11) at the amount indicated in figure legends. Hybridoma cells were added to the plates for the indicated time (see figure legends), harvested, and analyzed. Alternatively, PMA plus ionomycin was added to the culture instead of anti-CD3 mAb. For the thymidine incorporation assay, 2 × 104 hybridoma cells per well were added to 96-well plates, cultured for 20 h, and labeled with [3H]thymidine (1 μCi/well, 25 Ci/mmol; New England Nuclear) for a further 4 h. For TCR-induced apoptosis of CD4+ cells, splenocytes were activated with Con A (5 μg/ml) for 2 d, treated with methyl-α-d-mannopyranoside (10 mg/ml) at 37°C for 30 min, washed twice with PBS, and then cultured in 100 U/ml human IL-2 for an additional 2 d. The cells were then harvested and plated on an anti-CD3–coated plate. 16–24 h later, cells were quantitated by trypan blue exclusion and FACS® analysis.
Results
Identification of CD43 as an Inhibitor of AICD.
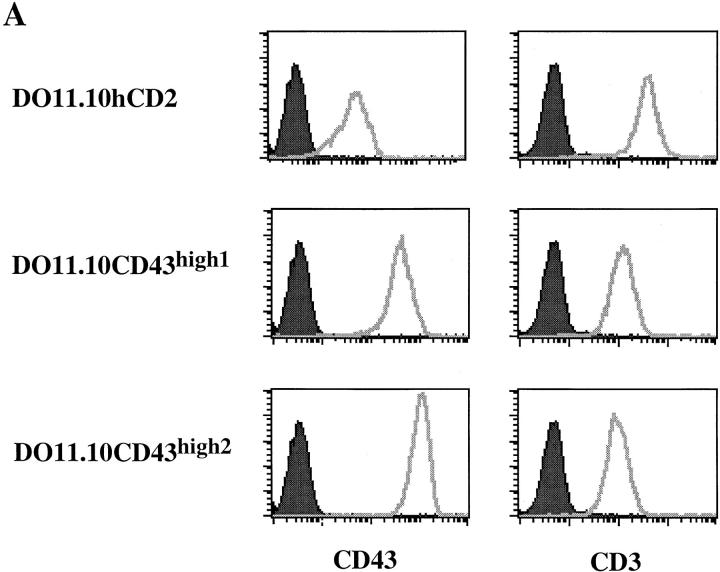
We employed an expression cloning strategy to identify genes that regulate AICD in a T cell hybridoma. We infected DO11.10 cells with a thymocyte cDNA library packaged in a retrovirus and selected for cells that could grow in the presence of PMA plus ionomycin. cDNA inserts from resistant clones were sequenced, revealing that one of the inserts encoded a full length CD43. To confirm the antiapoptotic effect of CD43, we transduced DO11.10 cells with either the full length CD43 or a tailless human CD2 as control. DO11.10 cell lines expressing high levels of CD43 were established by fluorescence-activated cell sorting. As shown in Fig. 1 A, DO11.10hCD2 control cells express a low level of endogenous CD43 on their surfaces. After transduction with pMXCD43, we isolated DO11.10 cell lines expressing ∼6–20-fold higher surface levels of CD43 compared with control cells (Fig. 1 A). Interestingly, DO11.10CD43high cells expressed two- to threefold lower levels of CD3 on their surfaces compared with DO11.10hCD2 control cells (Fig. 1 A). The lower CD3 surface staining was not due to nonspecific blockade of cell surface accessibility, as the expression levels of other surface molecules such as CD2 and CD5 were not changed (data not shown).
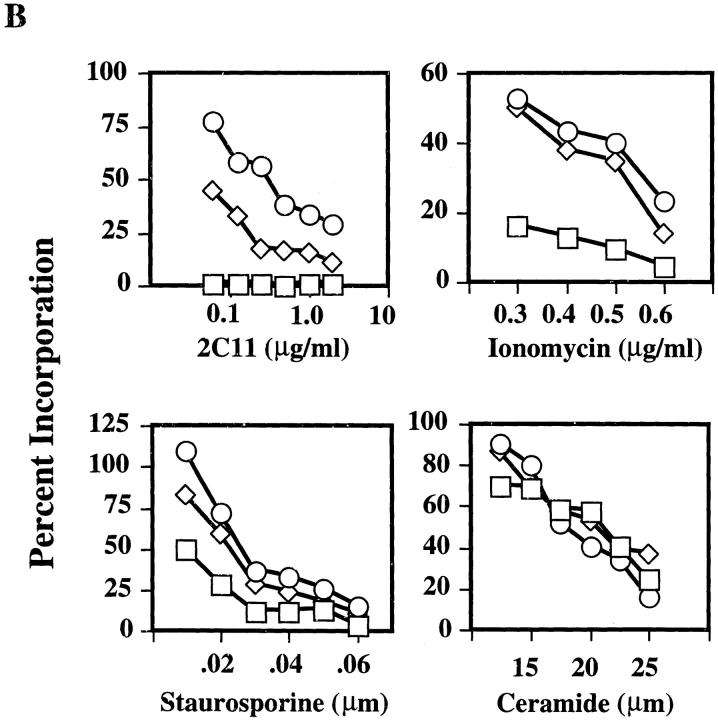
Figure 1.
High level expression of CD43 protects T hybridoma cells from AICD. (A) FACS® analysis of surface expression of CD43 and CD3 on DO11.10hCD2 control and DO11.10CD43high cell lines. Shown are the histogram profiles of DO11.10hCD2 control and two individual DO11.10CD43high cell lines. Histograms on the left represent isotype-matched antibody staining for background control. (B) DO11.10hCD2 control cells (□) and two DO11.10CD43high (⋄, number 1; ○, number 2) cell lines as shown in A were cultured in plates coated with 2C11, PMA (10 ng/ml) plus various amounts of ionomycin, or different concentrations of staurosporine and ceramide and assayed for their [3H]thymidine incorporation. Incorporation of individual cell lines cultured in medium alone is calculated as 100%.
To examine the effect of high level expression of CD43 on activation-induced apoptosis, we measured [3H]thymidine uptake of DO11.10hCD2 control and DO11.10CD43high cells in the absence or presence of anti-CD3 mAb or PMA plus ionomycin. [3H]thymidine incorporation by these cells closely correlated with their viability, measured by either propidium iodide uptake or trypan blue exclusion 16. As shown in Fig. 1 B, DO11.10 cells expressing high levels of CD43 were protected from anti-CD3–induced death. In contrast, control cells were readily induced to undergo apoptosis. We further determined whether the antiapoptotic effect in DO11.10CD43high cells could be due to a lowered TCR/CD3 surface expression by using PMA plus ionomycin as a stimulus for AICD, which bypasses the TCR. DO11.10CD43high cells were also refractory to apoptosis induced by PMA plus ionomycin (Fig. 1 B). The protection from AICD by CD43 correlated with its expression level, and the dosage effect was more pronounced when these cells were stimulated with anti-CD3, possibly due to some steric hindrance of CD43 imposed on the interaction of TCR/CD3 with the plate-bound anti-CD3 mAb. Next, we tested the effect of high level expression of CD43 on apoptosis induced by other stimuli. DO11.10 cells expressing high levels of CD43 were protected from apoptosis only in the presence of low amounts of staurosporine and were not protected from ceramide-induced killing (Fig. 1 B).
High Level Expression of CD43 Does Not Inhibit T Hybridoma Activation and Upregulation of Fas and FasL.
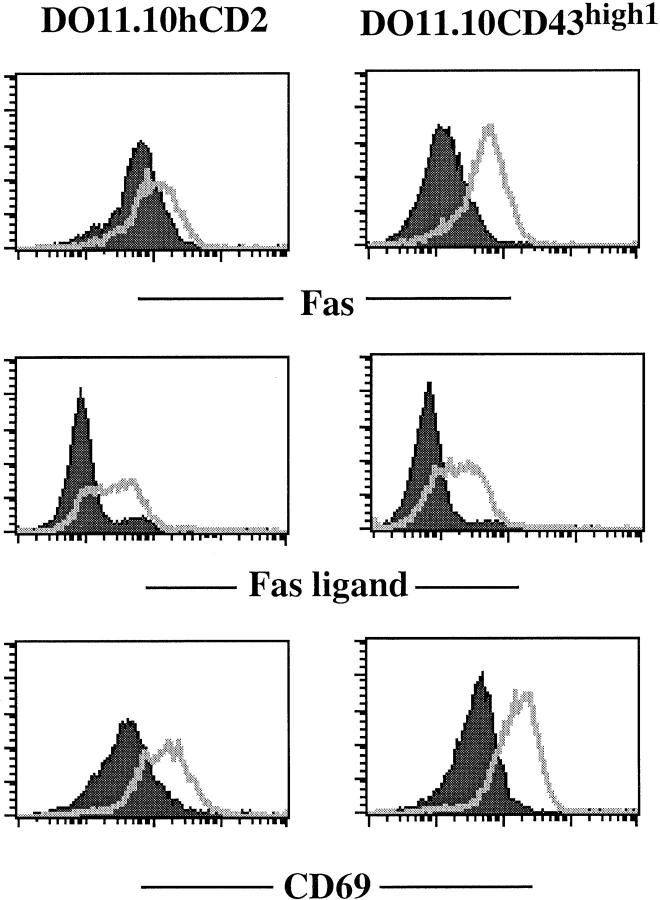
Previous studies have demonstrated that CD43 acts as a negative regulator of T cell activation, presumably due to its highly charged nature and its large size 9. To examine the effect of high level expression of CD43 on T cell hybridoma activation, we stimulated control DO11.10hCD2 and DO11.10CD43high cells with either plate-bound anti-CD3 mAb or PMA plus ionomycin for 6 h and monitored the upregulation of Fas, FasL, and CD69 by FACS® analysis. Before TCR stimulation, the expression of Fas on DO11.10CD43high cells was lower compared with control cells (Fig. 2). However, both Fas and FasL were upregulated by anti-CD3 stimulation of DO11.10CD43high cells (Fig. 2), even though these cells express lower levels of CD3 (Fig. 1 A). The T cell activation marker CD69 was also upregulated to a similar level in both cell types (Fig. 2). Similarly, PMA plus ionomycin upregulated expression of these three markers in control and CD43high cells (data not shown). These data indicate that high levels of CD43 expression did not obviously affect the activation of these T hybridoma cells and the upregulation of Fas and FasL and suggest that high level expression of CD43 may interfere with the death signal initiated through the Fas molecule.
Figure 2.
Effect of high level expression of CD43 on the upregulation of Fas, FasL, and CD69. FACS® analysis of cell surface expression of Fas, FasL, and CD69 in DO11.10hCD2 control or DO11.10CD43high cell line after TCR-mediated activation. Cells were activated on 2C11-coated plates for 5 h, and the cell surface phenotype was examined (filled curve, before activation; unfilled curve, after activation).
CD4_+_CD43high Peripheral T Cells Exhibit a Memory Phenotype.
Previous studies have shown that TCR/CD3-mediated apoptosis of CD4+ T cells in vitro is primarily induced through Fas, whereas CD8+ T cell death is primarily induced through TNFR 18. Given the antiapoptotic effect exhibited by high level expression of CD43 on T hybridoma cells, we examined its expression on peripheral T lymphocytes. As shown in Fig. 3 A, splenic CD4+ T cells of unimmunized, young adult C57BL/6 mice can be divided into two subsets based on CD43 expression. CD4+CD43high cells consist of 10–15% of the total CD4+ T cells, and the surface expression of CD43 on these cells is six- to eightfold higher than that on CD4+CD43low cells (Fig. 3 A). Interestingly, splenic CD8+ T cells exhibit uniformly high levels of CD43 on their surfaces (Fig. 3 A). To further determine the phenotype of CD4+CD43high and CD4+CD43low T cells, we analyzed these cells with three-color staining using mAbs directed against CD62L, CD45RB, and CD44. Surprisingly, the two CD4+ subsets expressing high or low levels of CD43 exhibited a characteristic phenotype of memory or naive T cells, respectively (Fig. 3 B). CD4+CD43high T cells express high levels of CD44 and low levels of CD62L and CD45RB, whereas CD4+CD43low T cells express low levels of CD44 and high levels of CD62L and CD45RB. Peripheral CD4+ and CD8+ T cells from lymph nodes exhibited a similar phenotype to splenic T cells in the above analysis (data not shown). These results suggest that CD43 may be used as a marker to define memory CD4+ T cells.
Figure 3.
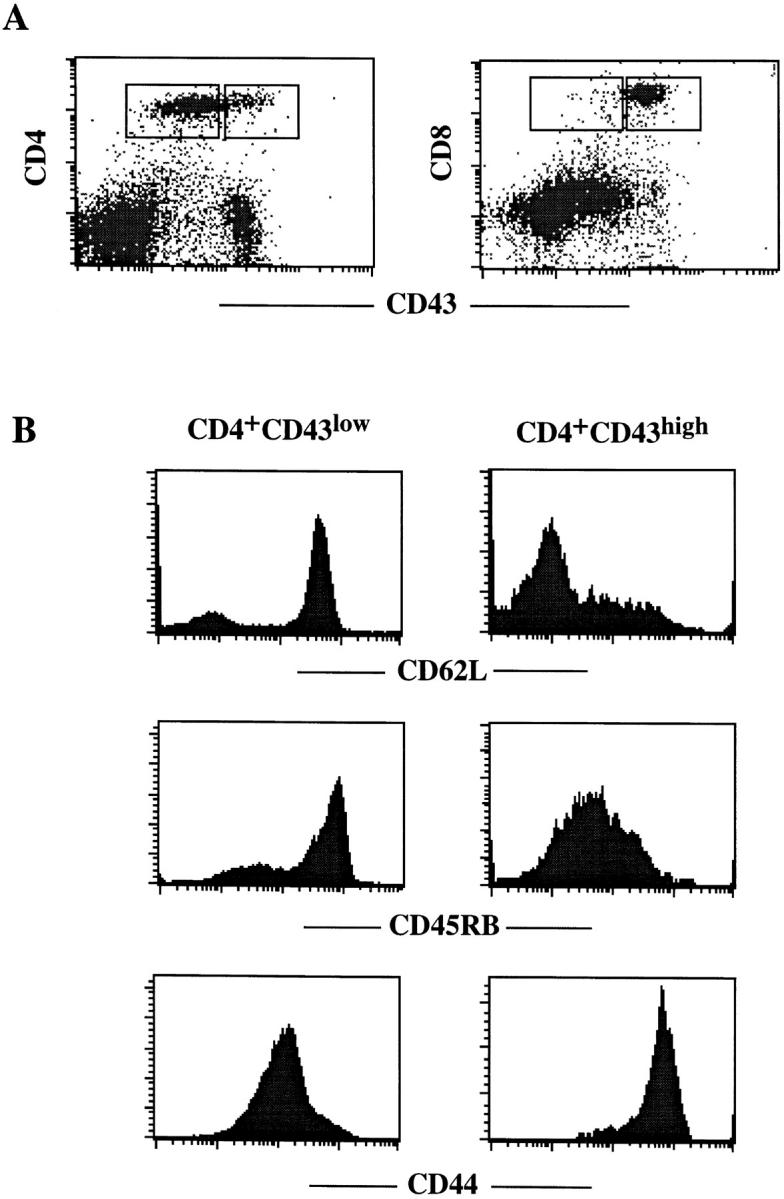
Phenotypic analysis of CD4+ T cells in the spleens of C57BL/6 mice. (A) Expression of CD43 on CD4 and CD8 T cells. Shown are the dot plot profiles of splenocytes double stained with anti-CD43 plus anti-CD4 or anti–CD8. Gated regions represent CD43low and CD43high cells. (B) Expression of CD62L, CD45RB, CD44, and CD43 on CD4+ T cells. Shown are histogram profiles of CD62L, CD45RB, and CD44 staining on CD4+CD43low and CD4+CD43high cells as gated in A.
CD4+CD43 high T Cells Are Resistant to TCR/CD3-mediated Cell Death.
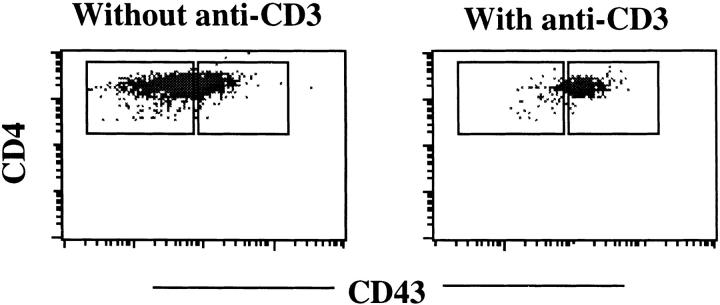
Recent studies have demonstrated that naive CD4+ T cells are more susceptible to TCR-mediated, Fas-dependent cell death compared with memory phenotype CD4+ T cells 2 3 4. To test whether the CD4+CD43high primary cells are resistant to TCR/CD3-mediated cell death, we used an in vitro assay for AICD of CD4+ T cells. Splenocytes from normal C57BL/6 mice were activated with Con A for 2 d, followed by culture in a high concentration of IL-2 for another 2 d. The activated cells were then restimulated with plate-bound anti-CD3, and cells that survived the restimulation were analyzed by FACS®. After restimulation with plate-bound anti-CD3, a majority of the CD4+ T cells were readily induced to undergo apoptosis, whereas CD8+ T cells did not suffer obvious cell loss in 16–24-h assay. When live cells from the restimulation plates were stained with CD4 and CD43, it was observed that only those CD4+ cells expressing high levels of CD43 survived anti-CD3 stimulation (Fig. 4). These results demonstrate that CD4+CD43high T cells are resistant to TCR/CD3-mediated cell death and imply that CD43 may determine this property of memory CD4+ T cells.
Figure 4.
CD4+CD43high T cells are resistant to TCR/CD3-mediated cell death. Splenocytes were activated with Con A, cultured in IL-2, and restimulated with plate-bound 2C11 for 18 h in the presence of IL-2. Live cells were electronically gated and analyzed for their expression of CD43 and CD4. Control cells were cultured in IL-2 alone.
Discussion
Several recent studies have demonstrated that CD4+ memory T cells are resistant to TCR-mediated, Fas-dependent cell death, whereas CD4+ naive T cells succumb 2 3 4. In this report, we show that high level expression of CD43 protects T cell hybridomas from AICD. Peripheral CD4+CD43low T cells exhibit a naive phenotype, whereas CD4+CD43high exhibit a memory phenotype, suggesting that the surface expression levels of CD43 on CD4+ naive and memory T cells determine their susceptibility to TCR-mediated, Fas-dependent cell death.
How is the inhibition of TCR/CD3-mediated cell death by high level expression of CD43 achieved? CD43 has been shown to have multiple, sometimes contradictory functions. This may be due to its structural features and the systems that have been used to study its function. CD43 is arguably the most abundant protein on the T cell surface 19. It extends 45 nm from the cell surface, at least six times the height of the TCR. It is also highly _O_-glycosylated and bears numerous sialic acid residues. The antiadhesive and antiproliferative effects mediated by CD43 are thought to result from a physical barrier formed by this highly negatively charged and rigid rod–like structure. However, this functional model of CD43 has been challenged by recent experiments showing that the cytoplasmic domain of CD43 plays an important role in its function 20 21. Moreover, the extracellular portion of CD43 may also be highly dynamic and actively interact with other surface structures. In support of this notion, a recent study showed that CD43 moves away from the contact sites of T cells and APCs, whereas CD45, similarly large in size and strongly negatively charged, does not 22. In addition, CD43 was reported to be physically associated with the TCR/CD3 complex 23. Several mechanisms can be envisioned for the anti-apoptotic effect caused by high level expression of CD43. First, highly expressed CD43 might block Fas signaling by physically preventing the interaction between FasL and Fas. Alternatively, it may prevent Fas from forming trimers. The fact that high level expression of CD43 on DO11.10 specifically decreases the expression of TCR/CD3 and Fas indicates that they may physically interact or form surface complexes with each other. Furthermore, high level expression of CD43 may interfere with the recruiting of death signaling molecule by Fas or inhibit the activation of caspases. Overexpression of Toso, a surface protein that contains an Ig domain, was shown to inhibit Fas signaling by upregulating the caspase-8 inhibitor cFLIP (cellular FLICE [FADD-like IL-1β–converting enzyme]-inhibitory protein; reference 15). We have not observed differences in the expression of cFLIP in DO11.10 control cells and CD43high–expressing cells (our unpublished observation). It is interesting to note that although CD43highCD4+ T cells from spleen and lymph nodes express six- to eightfold higher levels of CD43 on their surfaces than CD43lowCD4+ cells, these cells are much easier to activate by either anti-CD3 or Con A than CD43low CD4+ cells (our unpublished data), in agreement with previous studies showing that memory T cells have a lower threshold for activation 1. These data further argue against the model that CD43 negatively regulates T cell activation by increasing the stimulation threshold or by physically hindering the interaction between T cells and APCs. Our attempt to investigate whether the cytoplasmic tail of CD43 is required for the inhibition of AICD was inconclusive. A tailless CD43 retaining two amino acids of the cytoplasmic tail can be expressed at only two- to threefold higher levels than the endogenous CD43 on DO11.10 cells, and this tailless version of CD43 had no obvious antiapoptotic effect (our unpublished data).
We propose that high level expression of CD43 be used as a surface marker to define CD4+ memory T cells in C57BL/6 mice. Our results further suggest that CD43 may protect activated cells from AICD. In addition, we note that, correlating with their resistance to Fas-mediated killing, CD8+ T cells uniformly express high levels of CD43.
The protection against Fas-mediated killing by high level expression of CD43 may provide a novel mechanism for its role in tumorigenesis. CD43 has been shown to be overexpressed in Friend erythroleukemia cells 24 and abnormally expressed in nonhematopoietic tumor lines such as colon carcinoma and adenoma cells 25 26 27. Surface expression of CD43 diminishes susceptibility of target cells to T cell–mediated cytolysis 28, and the obvious implication is that CD43 expression on tumor cells protects them against lymphoid effectors bearing FasL.
Acknowledgments
This study was supported by the National Institutes of Health and the Howard Hughes Medical Institute.
References
- Dutton R.W., Bradley L.M., Swain S.L. T cell memory. Annu. Rev. Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- Desbarats J., Wade T., Wade W.F., Newell M.K. Dichotomy between naive and memory CD4+ T cell responses to Fas engagement. Proc. Natl. Acad. Sci. USA. 1999;96:8104–8109. doi: 10.1073/pnas.96.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M., Kurasawa K., Mamura M., Kumano K., Saito Y., Iwamoto I. Primed T cells are more resistant to Fas-mediated activation-induced cell death than naive T cells. J. Immunol. 1999;163:1315–1320. [PubMed] [Google Scholar]
- Kishimoto H., Sprent J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4+ T cells. J. Immunol. 1999;163:1817–1826. [PubMed] [Google Scholar]
- Lenardo M.J. The molecular regulation of lymphocyte apoptosis. Semin. Immunol. 1997;9:1–5. doi: 10.1006/smim.1996.0050. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Rosen F.S. Sialophorin (CD43) and the Wiskott-Aldrich syndrome. Immunodefic. Rev. 1990;2:151–174. [PubMed] [Google Scholar]
- Park J.K., Rosenstein Y.J., Remold-O'Donnell E., Bierer B.E., Rosen F.S., Burakoff S.J. Enhancement of T-cell activation by the CD43 molecule whose expression is defective in Wiskott-Aldrich syndrome. Nature. 1991;350:706–709. doi: 10.1038/350706a0. [DOI] [PubMed] [Google Scholar]
- Sperling A.I., Green J.M., Mosley R.L., Smith P.L., DiPaolo R.J., Klein J.R., Bluestone J.A., Thompson C.B. CD43 is a murine T cell costimulatory receptor that functions independently of CD28. J. Exp. Med. 1995;182:139–146. doi: 10.1084/jem.182.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath N., Correa M., Ardman M., Ardman B. Negative regulation of T-cell adhesion and activation by CD43. Nature. 1995;377:535–538. doi: 10.1038/377535a0. [DOI] [PubMed] [Google Scholar]
- McEvoy L.M., Sun H., Frelinger J.G., Butcher E.C. Anti-CD43 inhibition of T cell homing. J. Exp. Med. 1997;185:1493–1498. doi: 10.1084/jem.185.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton B.M., Cheng G., Manjunath N., Ardman B., von Andrian U.H. Negative regulation of T cell homing by CD43. Immunity. 1998;8:373–381. doi: 10.1016/s1074-7613(00)80542-7. [DOI] [PubMed] [Google Scholar]
- Bazil V., Brandt J., Tsukamoto A., Hoffman R. Apoptosis of human hematopoietic progenitor cells induced by crosslinking of surface CD43, the major sialoglycoprotein of leukocytes. Blood. 1995;86:502–511. [PubMed] [Google Scholar]
- Brown T.J., Shuford W.W., Wang W.C., Nadler S.G., Bailey T.S., Marquardt H., Mittler R.S. Characterization of a CD43/leukosialin-mediated pathway for inducing apoptosis in human T-lymphoblastoid cells. J. Biol. Chem. 1996;271:27686–27695. doi: 10.1074/jbc.271.44.27686. [DOI] [PubMed] [Google Scholar]
- Misawa Y., Nagaoka H., Kimoto H., Ishii Y., Kitamura K., Tsunetsugu-Yokota Y., Shibuya M., Takemori T. CD43 expression in a B cell lymphoma, WEHI 231, reduces susceptibility to G1 arrest and extends survival in culture upon serum depletion. Eur. J. Immunol. 1996;26:2573–2581. doi: 10.1002/eji.1830261106. [DOI] [PubMed] [Google Scholar]
- Hitoshi Y., Lorens J., Kitada S.-I., Fisher J., LaBarge M., Ring H.Z., Francke U., Reed J.C., Kinoshita S., Nolan G.P. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. 1998;8:461–471. doi: 10.1016/s1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- He Y.-W., Deftos M.L., Ojala E.W., Bevan M.J. RORγt, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity. 1998;9:797–806. doi: 10.1016/s1074-7613(00)80645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deftos M.L., He Y.-W., Ojala E.W., Bevan M.J. Correlating notch signaling with thymocyte maturation. Immunity. 1998;9:777–786. doi: 10.1016/s1074-7613(00)80643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Fisher G., Miller R.E., Peschon J., Lynch D.H., Lenardo M.J. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- Shaw A.S., Dustin M.L. Making the T cell receptor go the distancea topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- Thurman E.C., Walker J., Jayaraman S., Manjunath N., Ardman B., Green J.M. Regulation of in vitro and in vivo T cell activation by CD43. Int. Immunol. 1998;10:691–701. doi: 10.1093/intimm/10.5.691. [DOI] [PubMed] [Google Scholar]
- Walker J., Green J.M. Structural requirements for CD43 function. J. Immunol. 1999;162:4109–4114. [PubMed] [Google Scholar]
- Sperling A.I., Sedy J.R., Manjunath N., Kupfer A., Ardman B., Burkhardt J.K. Cutting edgeTCR signaling induces selective exclusion of CD43 from the T cell-antigen-presenting cell contact site. J. Immunol. 1998;161:6459–6462. [PubMed] [Google Scholar]
- Alvarado M., Klassen C., Cerny J., Horejsi V., Schmidt R.E. MEM-59 monoclonal antibody detects a CD43 epitope involved in lymphocyte activation. Eur. J. Immunol. 1995;25:1051–1055. doi: 10.1002/eji.1830250429. [DOI] [PubMed] [Google Scholar]
- Misawa Y., Shibuya M. Amplification and rearrangement of melF/mouse CD43 (leukosialin) gene encoding a highly glycosylated membrane protein gp120 in Friend erythroleukemia cells. Oncogene. 1992;7:919–926. [PubMed] [Google Scholar]
- Baeckstrom D., Zhang K., Asker N., Ruetschi U., Ek M., Hansson G.C. Expression of the leukocyte-associated sialoglycoprotein CD43 by a colon carcinoma cell line. J. Biol. Chem. 1995;270:13688–13692. doi: 10.1074/jbc.270.23.13688. [DOI] [PubMed] [Google Scholar]
- Santamaria M., Lopez-Beltran A., Toro M., Pena J., Molina I.J. Specific monoclonal antibodies against leukocyte-restricted cell surface molecule CD43 react with nonhematopoietic tumor cells. Cancer Res. 1996;56:3526–3529. [PubMed] [Google Scholar]
- Sikut R., Nilsson O., Baeckstrom D., Hansson G.C. Colon adenoma and cancer cells aberrantly express the leukocyte-associated sialoglycoprotein CD43. Biochem. Biophys. Res. Commun. 1997;238:612–616. doi: 10.1006/bbrc.1997.7334. [DOI] [PubMed] [Google Scholar]
- McFarland T.A., Ardman B., Manjunath N., Fabry J.A., Lieberman J. CD43 diminishes susceptibility to T lymphocyte-mediated cytolysis. J. Immunol. 1995;154:1097–1104. [PubMed] [Google Scholar]