Normal Regulatory α/β T Cells Effectively Eliminate Abnormally Activated T Cells Lacking the Interleukin 2 Receptor β in Vivo (original) (raw)
Abstract
Although interleukin 2 (IL-2) has been thought to be the most important cytokine for T cell growth, animals lacking IL-2 or a component of its receptor molecules have more expanded T cells with activated memory phenotype, indicating an indispensable role for the IL-2/IL-2 receptor system in regulating the size and activity of the T cell population. In this study, we investigated the possible mechanism of abnormal expansion of activated T cells in IL-2 receptor β chain (IL-2Rβ)−/− mice using the systems of bone marrow transplantation and T cell transfer. Here, we show that IL-2Rβ2/− T cells in mice reconstituted with a mixture of IL-2Rβ2/− and IL-2Rβ1/+ bone marrow cells did not develop into an abnormally activated stage, and that already activated IL-2Rβ2/− T cells were effectively eliminated by IL-2Rβ1/+ T cells when both cells were cotransferred to T cell–deficient host mice. This regulation and/or elimination was dependent on T cells bearing α/β type T cell receptor, especially on CD8+ T cells and independent of the Fas–Fas ligand (FasL) system. IL-2Rβ1/+ T cells that eliminated activated IL-2Rβ2/− T cells expressed FasL, perforin, granzyme B, and tumor necrosis factor α/β. These results indicate a novel function of IL-2Rβ that is necessary for the induction of regulatory T cells acting to eliminate activated T cells.
Keywords: T lymphocytes; mice, knockout; CD8+ T lymphocytes; bone marrow transplantation; adoptive transfer
Interleukin (IL)-2 is one of the earliest discovered and most extensively studied cytokines. It was first characterized as a growth factor for T cells, and its main function was thought to be the induction of T cell proliferation and expansion 1 2. Since the discovery of IL-2, the receptor for IL-2 (IL-2R) has also been extensively studied. IL-2R is expressed not only in T cells, but also in a wide variety of hematopoietic cells, including NK cells, B cells, monocytes, and neutrophils. At least three subunit molecules forming the “IL-2 receptor complex” have been identified so far. They are designated α, β, and γ chain. γ chain is known as γc (common γ), and works as a subunit of many different cytokine receptors. β chain is also shared with the receptor for IL-2 and IL-15 3 4. The overlapping usage of the receptor subunits and the expression of the receptor in various cell types complicate the action of IL-2, and in consequence, the overall function of IL-2 has not yet been fully characterized 5 6.
In this decade, the targeted disruption of genes of interest by homologous recombination has been applied to mammalian biology and has revealed much information about the function of hundreds of gene products. Studies of cytokines have been accelerated by this technique. Mice disrupted with a gene encoding a cytokine molecule or its receptor have been created and extensively studied. IL-2 and its receptor are not the exception: IL-2–deficient mice were first created and described in 1991 7, followed by IL-2Rα– and β–deficient mice, both of which were demonstrated in 1995 8 9. A striking phenotype of these gene-targeted mice was the expansion of activated T cells and autoimmune-like disorders, which might be difficult to interpret with the previously identified function of the IL-2/IL-2R system. Some studies on IL-2–deficient mice and IL-2Rα–deficient mice have introduced a possible mechanism to explain the abnormal expansion of activated T cells in these mice. It was reported earlier that IL-2 is necessary to induce activation-induced cell death of T cells 10. Based on this theory, activated T cells produced by stimulation with either self-antigens or nonself-antigens may not die, but survive long after IL-2 fails to function. Actually, T cells in IL-2–deficient mice and IL-2Rα–deficient T cells were both shown to be resistant to Fas-mediated activation-induced cell death 11 12. In contrast to IL-2– or IL-2Rα–deficient mice, T cells in IL-2Rβ2/− mice were shown to be normal in superantigen-induced and Fas-mediated apoptosis 13. This discrepancy may simply be due to the involvement of other cytokines, including IL-15, which uses the IL-2Rβ for its receptor component. However, for a better explanation of abnormal expansion of activated T cells in these gene-targeted mice, one must consider another, more important underlying function of the IL-2/IL-2R systems.
In this study, we investigated the relationship between IL-2Rβ2/− T cells and normal T cells, and found a possible regulatory activity of normal T cells against IL-2Rβ2/− T cells. We propose here that the most indispensable function of IL-2Rβ is the induction of regulatory T cells, the loss of which may lead to the abnormalities seen in IL-2Rβ2/− mice.
Materials and Methods
Mice.
IL-2Rβ2/− mice described previously 9 were maintained in our animal facility. All IL-2Rβ2/− mice used in this study had been back-crossed at least 10 times to C57BL/6 (B6) mice, providing a pure genetic background. These mice were further crossed with B6/CD45.1/CD45.1 congenic strain to make IL-2Rβ2/− mice with the CD45.1 allotype marker. B6 recombination activating gene (RAG)-2−/− (B6.RAG-2−/−) mice were provided by the Central Institute for Experimental Animals (Kawasaki, Japan) with the permission of Dr. F.W. Alt (Harvard Medical School, Boston, MA). B6_lpr/lpr_ mice were purchased from Japan SLC, Inc. B6_gld/gld_ mice were provided by Dr. K. Okumura (Juntendo University, Tokyo, Japan). TCR-β2/− mice were provided by Dr. Y. Yoshikai (Nagoya University). Lymphocytic choriomeningitis virus (LCMV)-specific TCR transgenic mice were provided by Dr. R. Zinkernagel (Institute of Experimental Immunology, University Hospital, Zürich, Switzerland) and mated with RAG-2−/− mice to generate TCR transgenic mice with RAG-2−/− background.
Antibodies and Flow Cytometry.
FITC-conjugated anti–mouse CD69 mAb (clone H1.2F3), PE-conjugated anti-CD62L antibody (clone MEL14), FITC- or biotin-conjugated anti-CD45.1 antibody (clone A20), FITC- or biotin-conjugated anti-CD45.2 antibody (clone 104), FITC- or PE-conjugated anti–Thy-1.2 antibody (clone 30-H12), PE-conjugated anti-B220 antibody (clone RA3-6B2), and FITC-conjugated anti–Gr-1 antibody (clone RB6-8C5) were purchased from PharMingen. FITC- or PE-conjugated anti-CD4 antibody (clone H129.19) and FITC- or PE-conjugated anti-CD8α antibody (clone 53-6.7) were purchased from Sigma Chemical Co. Cells were stained with antibodies for 20 min on ice, and analyzed using a FACSCalibur™ (Becton Dickinson). Biotin-conjugated antibodies were visualized by secondary staining with streptavidin-conjugated RED670 (GIBCO BRL).
Bone Marrow Transplantation.
Bone marrow cells were obtained by flushing out the femoral bone marrow of 3-wk-old mice. In a combination of bone marrow cell preparation, T cells were depleted by treatment with anti–Thy-1.2 antibody and rabbit complement (ICN Pharmaceuticals, Inc.). A total of 2 × 106 bone marrow cells were intravenously injected into an irradiated (9 Gy) B6.RAG-2−/− mouse.
T Cell Transfer.
Lymph node cells from IL-2Rβ2/− mice or spleen cells and lymph node cells from other types of mice were collected and passed through nylon wool columns. Some of the nylon wool–passed cells were analyzed before transfer. The remaining cells were either mixed or unmixed, and a total of 1–4 × 107 cells were injected intravenously to sublethally irradiated (4 Gy) B6.RAG-2−/− mice.
Purification of Cells Using Magnetic Beads.
Spleen and lymph node cells were stained with biotin-conjugated anti-CD4 or anti-CD8 antibody, and secondarily incubated with streptavidin microbeads (MACS; Miltenyi Biotec). The following column work was performed according to the manufacturer's protocol (Miltenyi Biotec).
Reverse-transcribed PCR.
RNA was extracted from collected cells using RNAzol (Tel-Test), and cDNA was created using the RNA LA PCR kit (Takara). 30 cycles of PCR reaction were performed under the following conditions: 94°C, 30 s; 60°C, 30 s; 72°C, 90 s. PCR primers used in this study were as follows: 5′ β-actin, TGG AAT CCT GTG GCA TCC ATG AAA C; 3′ β-actin, TAA AAC GCA GCT CAG TAA CAG TCC G; 5′ IL-2, TGA TGG ACC TAC AGG AGC TCC TGA G; 3′ IL-2, GAG TCA AAT CCA GAA CAT GCC GCA G; 5′ IL-4, CGA AGA ACA CCA CAG AGA GTC AGC T; 3′ IL-4, GAC TCA TTC ATG GTG CAG CTT ATC G; 5′ IFN-γ, AGC GGC TGA CTG AAC TCA GAT TGT AG; 3′ IFN-γ, GTC ACA GTT TTC AGC TGT ATA GGG; 5′ TNF-α, GGC AGG TCT ACT TTG GAG TCA TTG C; 3′ TNF-α, ACA TTC GAG GCT CCA GTG AAT TCG G; 5′ TNF-β, TGG CTG GGA ACA GGG GAA GGT TGA C; 3′ TNF-β, CGT GCT TTC TTC TAG AAC CCC TTG G; 5′ Fas ligand (FasL), GGT CAG CAC TGG TAA GAT TG; 3′ FasL, GAG TTC ACC AAC CAA AGC CT; 5′ granzyme B, GCC CAC AAC ATC AAA GAA CAG; 3′ granzyme B, AAC CAG CCA CAT AGC ACA CAT; 5′ perforin, GTC ACG TCG AAG TAC TTG GTG; and 3′ perforin, AAC CAG CCA CAT AGC ACA CAT.
Statistical Analysis.
The statistical analysis was performed using StatView J-4.5 software.
Results
IL-2Rβ–deficient T Cells in Bone Marrow Chimera Reconstituted with a Mixture of IL-2Rβ1/+ and IL-2Rβ2/− Cells Are Not Abnormally Activated.
To investigate the mechanism of abnormal development of multiple hematopoietic cells in IL-2Rβ2/− mice, we first examined bone marrow chimeric mice reconstituted with IL-2Rβ2/− bone marrow cells. When lymphocyte-deficient RAG-2−/− mice were reconstituted with IL-2Rβ2/− bone marrow cells, T cells arising from the transferred bone marrow cells later than 6 wk showed a markedly activated memory phenotype of CD69+CD62Llo (Fig. 1, IL-2Rβ2/− BM chimera), CD44hi, and CD45RBlo (data not shown), all of which were characteristic features of T cells in IL-2Rβ2/− mice (Fig. 1, IL-2Rβ2/− [9]). These mice also showed phenotypes of decreased B220+ cells in lymphatic organs, increased Gr-1+ cells in bone marrow, and anemia (data not shown), all of which were observed in IL-2Rβ2/− mice 9, indicating that most phenotypes caused by IL-2Rβ deficiency were restored by bone marrow reconstitution. However, when RAG-2−/− mice were reconstituted with a mixture of IL-2Rβ1/+ and IL-2Rβ2/− bone marrow cells, T cells arising from IL-2Rβ2/− bone marrow cells, which were distinguished from IL-2Rβ1/+–derived cells by CD45 allotype-specific antibody and were shown to exist with a number similar to that in simple IL-2Rβ2/− bone marrow chimera, showed no sign of activation (Fig. 1, IL-2Rβ1/+ + IL-2Rβ2/− BM chimera). B cells, granulocytes, and erythrocytes were also normal in these mice (data not shown), and inflammatory bowel disease was never observed. This result indicates that IL-2Rβ2/− T cells do not develop into an abnormally activated phenotype when they exist together with normal IL-2Rβ1/+ cells, which may have the activity to regulate the abnormal activation of IL-2Rβ2/− T cells.
Figure 1.
No abnormal T cell activation in chimeric mice reconstituted with a mixture of IL-2Rβ2/− and IL-2Rβ1/+ bone marrow (BM) cells. Bone marrow of B6.RAG-2−/− mice was reconstituted with IL-2Rβ1/+ alone (top row), a mixture of IL-2Rβ2/− and IL-2Rβ1/+ (second row), or IL-2Rβ2/− alone (third row), and their lymph node cells were analyzed for the expression of CD69 and CD62L. Lymph node cells obtained from the indicated mice 6 wk after bone marrow transplantation were stained with a mixture of FITC-conjugated anti-CD69, PE-conjugated anti–Thy-1.2, and biotin-conjugated anti-CD45.1 antibodies, or with a mixture of FITC-conjugated anti–Thy-1.2, PE-conjugated anti-CD62L, and biotin-conjugated anti-CD45.1 antibodies, then secondarily stained with streptavidin-conjugated RED670. Bold lines represent the expression of CD69 or CD62L in cells gated to Thy-1.2+CD45.1+ population (IL-2Rβ2/−–derived T cells). Thin lines represent the expression in cells gated to Thy-1.2+CD45.1− population (IL-2Rβ1/+–derived T cells). The bottom row shows the expression of CD69 and CD62L in T cells from a 6-wk-old IL-2Rβ2/− mouse.
Skewing of IL-2Rβ1/+–derived Cells towards CD8+ T Cells in Mixed Bone Marrow Chimeric Mice.
In mixed bone marrow chimeric mice, the percentages of cells derived from IL-2Rβ1/+ or IL-2Rβ2/− bone marrow were examined for some different lineage of hematopoietic cells, and the result was summarized in Table . Although the bone marrow cells of two different mice were mixed at a 1:1 ratio and were injected into host mice, the ratio of the origin in differentiated hematopoietic cells in the individual bone marrow chimeric mice varied. The first striking finding was the difference in the contribution of IL-2Rβ1/+ (or IL-2Rβ2/−) cells between B cells or granulocytes and T cells. All the mixed bone marrow chimeric mice showed a higher contribution of IL-2Rβ1/+ cells in T cells than in B cells or granulocytes (i.e., there were more IL-2Rβ2/− cells in B cells and granulocytes than in T cells). This skewing of T cells to IL-2Rβ1/+ cells was more striking in CD8+ T cells than in CD4+ T cells. This difference in CD4+ cells and CD8+ cells also reflected the difference in the CD4+/CD8+ ratio between IL-2Rβ1/+–derived and IL-2Rβ2/−–derived cells. The average ratio was 2.68 for IL-2Rβ2/−–derived cells and 1.25 for IL-2Rβ1/+–derived cells. When bone marrow cells had been depleted with T cells (Table , A), or when bone marrow cells from a neonatally thymectomized IL-2Rβ2/− mouse were used (Table , B), the skewing was consistently observed, excluding the possibility of skewing due to the difference in hematopoietic potential influenced by mature T cells infiltrating the bone marrow.
Table 1.
Granulocytes, B Cells, and T Cell Subsets in Bone Marrow Chimeric Mice Reconstituted with a Mixture of IL-2Rβ1/+ and IL-2Rβ2/− Cells
| Mouse no. | Source of BM | Weeks after BMT | Percentage of CD45.1− (IL-2Rβ1/+) cells in | CD4+/CD8+ ratio in | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gr-1+ | B220+ | Thy-1+ | CD4+ | CD8+ | CD45.1+ (IL-2Rβ2/−) | CD45.1− (IL-2Rβ1/+) | |||
| 1 | A | 4 | 33.1 | 27.4 | 75.7 | 72.9 | 80.7 | 2.35 | 1.52 |
| 2 | A | 8 | 27.6 | 32.8 | 59.7 | 48.5 | 77.5 | 2.70 | 0.73 |
| 3 | B | 4 | 28.3 | 29.0 | 51.6 | 43.5 | 63.4 | 3.10 | 1.39 |
| 4 | B | 8 | 30.9 | 24.9 | 58.7 | 52.9 | 66.0 | 2.68 | 1.55 |
| 5 | C | 6 | 22.6 | 19.2 | 44.2 | 34.2 | 58.0 | 2.42 | 0.89 |
| 6 | C | 12 | 25.9 | 19.4 | 44.9 | 35.4 | 58.1 | 2.46 | 0.97 |
| 7 | D | 6 | 86.5 | 80.5 | 91.9 | 91.3 | 93.8 | 2.00 | 1.37 |
| 8 | E | 6 | 19.9 | 27.6 | 59.4 | 50.5 | 71.1 | 3.60 | 1.50 |
| 9 | E | 20 | 19.4 | 24.9 | 47.6 | 38.6 | 57.7 | 2.44 | 1.13 |
| 10 | F | 32 | 52.2 | 42.1 | 71.6 | 68.3 | 78.3 | 3.06 | 1.42 |
| Ave. 1–10 | 10.6 | 34.6 | 32.8 | 60.5 | 53.6 | 70.5 | 2.68 | 1.25 | |
| 11 | D | 12 | 81.4 | 84.6 | 94.3 | ND | ND | ND | ND |
| 12 | E | 20 | 18.2 | 16.2 | 51.8 | ND | ND | ND | ND |
| 13 | G | 4 | 53.1 | 49.2 | 80.4 | ND | ND | ND | ND |
| 14 | G | 6 | 48.5 | 51.7 | 76.5 | ND | ND | ND | ND |
| 15 | G | 8 | 48.1 | 49.2 | 73.2 | ND | ND | ND | ND |
| Ave. 1–15 | 10.4 | 39.7 | 38.6 | 65.4 | – | – | – | – |
These profiles of cell origin in mixed bone marrow chimeric mice (Table ) are striking, but should be interpreted carefully. Although we mixed the same numbers of nucleated cells from IL-2Rβ2/− and IL-2Rβ1/+ bone marrow, the frequency of hematopoietic stem cells might have varied in individual donor mice, resulting in the diverse contribution rate of two different donors for individual chimeric mice. Skewing of B cells and granulocytes towards IL-2Rβ2/− cells as a phenotype intrinsic to IL-2Rβ2/− should be excluded for the following reason. In IL-2Rβ2/− mice, the number of granulocytes (Gr-1+ cells) is greatly increased, whereas the number of B220+ cells is markedly decreased, both of which are secondary phenomena affected by abnormal T cells 9. Therefore, the numbers of B cells and granulocytes should not have moved in the same direction when these cells were affected by an IL-2Rβ deficiency. The contribution of host-derived Gr-1+CD45.1− cells could be neglected, because no change was observed in the CD45.1+/CD45.1− ratio in Gr-1+ cells compared with that in B220+ cells. With these considerations in mind, we concluded that the IL-2Rβ2/−/IL-2Rβ1/+ ratio in B cells and granulocytes reflected the ratio in hematopoietec stem cells. Accordingly, the skewing of T cells towards IL-2Rβ1/+ cells was a real event, and reflected either a decrease in IL-2Rβ2/−–derived T cells or an increase in IL-2Rβ1/+–derived T cells.
The CD4+/CD8+ ratio was also significantly different between IL-2Rβ1/+ and IL-2Rβ2/− cells (Table ). This difference was mainly caused by a decrease in the CD4+/CD8+ ratio in IL-2Rβ1/+ cells, because that ratio in mixed bone marrow chimeric mice was significantly lower than that in mice simply reconstituted with IL-2Rβ1/+ bone marrow (1.25 ± 0.29, n = 10 vs. 1.84 ± 0.22, n = 5; P < 0.01, unpaired two group _t_ test), whereas the CD4+/CD8+ ratio of IL-2Rβ2/− cells in mixed bone marrow chimeric mice was similar to that in simple IL-2Rβ2/− bone marrow chimeric mice (2.68 ± 0.46, _n_ = 10 vs. 2.74 ± 0.28, _n_ = 5; _P_ > 0.5). Therefore, an especially significant skewing of CD8+ T cells to IL-2Rβ1/+–derived cells was more likely to be due to an increase in IL-2Rβ1/+ CD8+ cells, rather than to a decrease in IL-2Rβ2/− CD8+ cells.
Functional T Cells Are Needed, but Fas/FasL Is Unnecessary for Regulation of IL-2Rβ2/− T Cells.
We performed the examination of mixed bone marrow chimera using some different types of mutant mice as partners for IL-2Rβ2/−. As shown in Fig. 2, when bone marrow cells of B6_lpr/lpr_ mice which lacked the functional Fas molecule were mixed with IL-2Rβ2/−, the resulting IL-2Rβ2/− T cells were normal in their activation and memory phenotype. Functional FasL-deficient B6_gld/gld_ cells also had the same effect on the regulation of IL-2Rβ2/− T cells. In contrast with these Fas/FasL mutant strains, when TCR-β2/− bone marrow cells were mixed with IL-2Rβ2/− and reconstituted the RAG-2−/− host, the resulting CD4+ or CD8+ T cells were all IL-2Rβ2/−–derived, and showed a striking activated phenotype similar to those in IL-2Rβ2/− mice or simple IL-2Rβ2/− bone marrow chimeras (see Fig. 1). T cells in LCMV-specific TCR transgenic mice with RAG-2−/− background, which consequently express TCR molecules with a single specificity, showed no activity to regulate the activation of IL-2Rβ2/− T cells (Fig. 2, TCR tg (RAG-2−/−)). All of these chimeric mice were effectively reconstituted with each partner of bone marrow cells, because the percentages of mutant partner–derived T cells per total T cells were not significantly different from those of mice reconstituted with wild-type B6 and IL-2Rβ2/− bone marrow (P > 0.5 for every combination). Percentages of CD45.1− cells (partners for IL-2Rβ2/−) per total T cells recovered from mixed bone marrow chimera of each combination were 60.5 ± 15.3 (n = 5), 60.6 ± 10.5 (n = 4), 58.2 ± 10.0 (n = 4), 53.7 ± 5.1 (n = 3), for B6, B6_lpr/lpr_, B6_gld/gld_, and TCR transgenic, respectively, and that of CD45.1− cells per total B cells was 58.0 ± 11.5 (n = 3) for TCR-β2/−. These results indicated that functional T cells with an adequate TCR repertoire were required, but that Fas or FasL was unnecessary to regulate the abnormal activation of IL-2Rβ2/− T cells.
Figure 2.
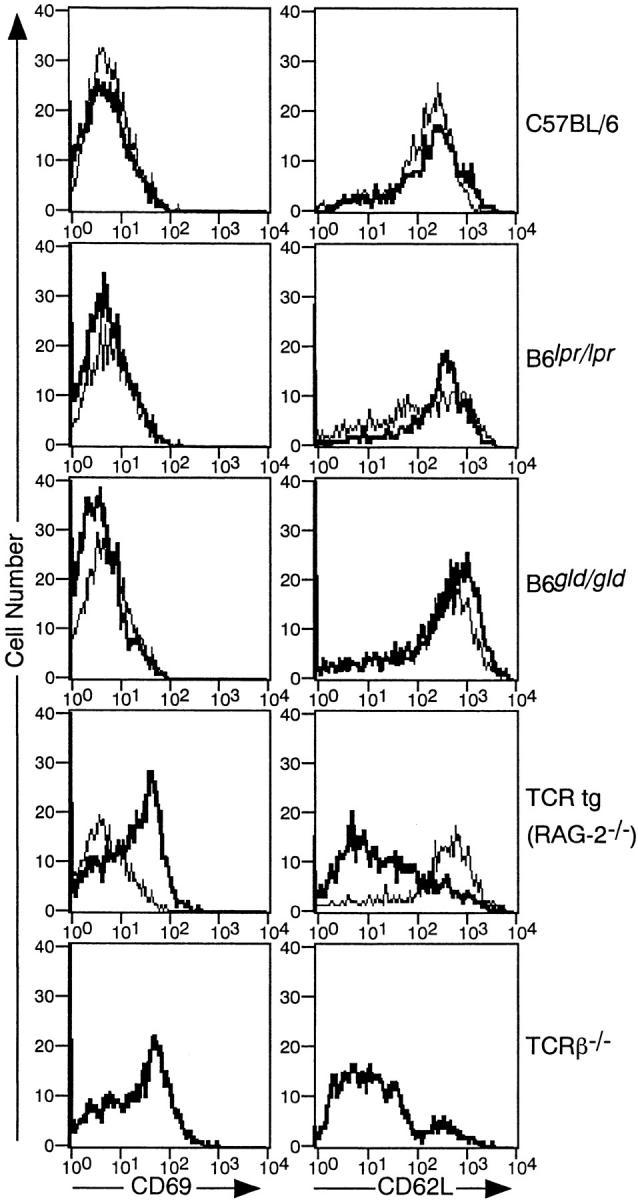
Requirement of functional α/β TCR but not Fas–FasL for T cells to regulate the activation of IL-2Rβ2/− T cells. B6.RAG-2−/− mice were reconstituted with a mixture of bone marrow cells from IL-2Rβ2/− mice and B6, B6_lpr/lpr_, B6_gld/gld_, and TCR transgenic (tg) mice with RAG-2−/− background or TCR-β2/− mice. Lymph node cells obtained from chimeric mice 6 wk after bone marrow transplantation were analyzed. The staining procedure was the same as that in the legend to Fig. 1, except that FITC- or PE-conjugated anti-CD4 and anti-CD8 antibodies were used instead of anti–FITC- or PE-conjugated anti–Thy-1.2 antibody. Bold lines represent the expression of CD69 or CD62L in cells gated to CD4 or CD8+CD45.1+ population (IL-2Rβ2/−–derived T cells); thin lines represent that in cells gated to the CD4 or CD8+CD45.1− population (IL-2Rβ1/+–derived T cells).
Activated IL-2Rβ2/− T Cells Are Eliminated by IL-2Rβ1/+ T Cells.
To investigate the mechanism of regulation of IL-2Rβ2/− T cells in more detail, we performed a transfer of purified T cells to RAG-2–deficient host mice. Nylon wool column–passed lymph node cells (Thy-1+ cells > 90%) from IL-2Rβ1/+ and IL-2Rβ2/− mice were mixed with 1:1 ratio (Fig. 3, top middle panel) and transferred to RAG-2−/− mice. Here, we used IL-2Rβ2/− mice >6 wk, and almost all T cells from these mice were expressing activated memory phenotypes with CD69+ and CD62Llo (see Fig. 1). 7 d later, cells were recovered from the spleen and lymph nodes, and their origin was examined. As shown in the middle panels of Fig. 3, recovered IL-2Rβ2/− T cells were much fewer than IL-2Rβ1/+ T cells. This phenomenon was consistently observed because the percentage of CD45.1+ (IL-2Rβ2/−) cells per total T cells was 2.6 ± 1.1% (n = 10, from five independent experiments). When IL-2Rβ1/−CD45.1+ T cells and IL-2Rβ1/+CD45.1− T cells were mixed and transferred to the RAG-2−/− host, no increase or decrease in either population was observed 7 d after transfer (data not shown), excluding the possibility that IL-2Rβ1/+ T cells reacted to CD45.1 antigen, Neor gene product, or some minor antigenic differences which had been carried from embryonic stem cells and had not been eliminated by back-crossing to B6 mice. In the recovered IL-2Rβ1/+ T cells that had been transferred with IL-2Rβ2/− T cells, the CD4/CD8 ratio was skewed towards a CD8+ dominant phenotype (Fig. 3, bottom panels), whereas no such skewing was observed in IL-2Rβ1/+ T cells simply transferred to RAG-2−/− hosts without IL-2Rβ2/− T cells. The CD4+/CD8+ ratio of IL-2Rβ1/+ cells recovered from mice transferred with a mixture of IL-2Rβ1/+ and IL-2Rβ2/− T cells was significantly lower (0.96 ± 0.27, n = 10, from five independent experiments; P < 0.001, unpaired two group _t_ test) than that of donor IL-2Rβ1/+ mice (1.85 ± 0.14, _n_ = 5), whereas that from mice transferred with single IL-2Rβ1/+ T cells was not (1.69 ± 0.36, _n_ = 4, from four independent experiments; _P_ > 0.3).
Figure 3.

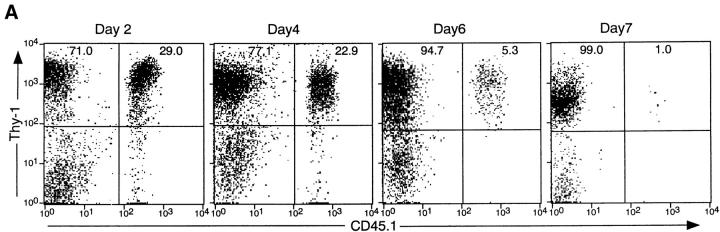
Elimination of activated IL-2Rβ2/− T cells by IL-2Rβ1/+ T cells. T cells isolated from IL-2Rβ2/− (CD45.1+) or IL-2Rβ1/+ (CD45.2+) mice by passing through a nylon wool column were stained with FITC-conjugated anti-CD4 plus PE-conjugated anti-CD8 antibodies, and analyzed (top left and top right). T cells from two different types of mice were mixed and checked for their contents by staining with FITC-conjugated anti-CD45.2 plus biotin-conjugated anti-CD45.1, which was visualized by the secondary staining with streptavidin-conjugated RED670 (top middle). IL-2Rβ1/+ T cells alone (lower left), IL-2Rβ2/− T cells alone (lower right), or their mixture (lower middle) were injected into B6.RAG-2−/− mice. 7 d later, lymph node cells were analyzed for their CD45 allotype markers (middle row) and for their CD4/CD8 ratio (bottom row). Cells recovered from mice transferred with a mixture of IL-2Rβ1/+ and IL-2Rβ2/− were triple stained with anti-CD4, anti-CD8, and anti-CD45.2 antibodies, and CD4/CD8 expression in cells gated to CD45.2+ (IL-2Rβ1/+) was shown (bottom middle). Numbers in the panels represent percentages of cells in the indicated quadrants.
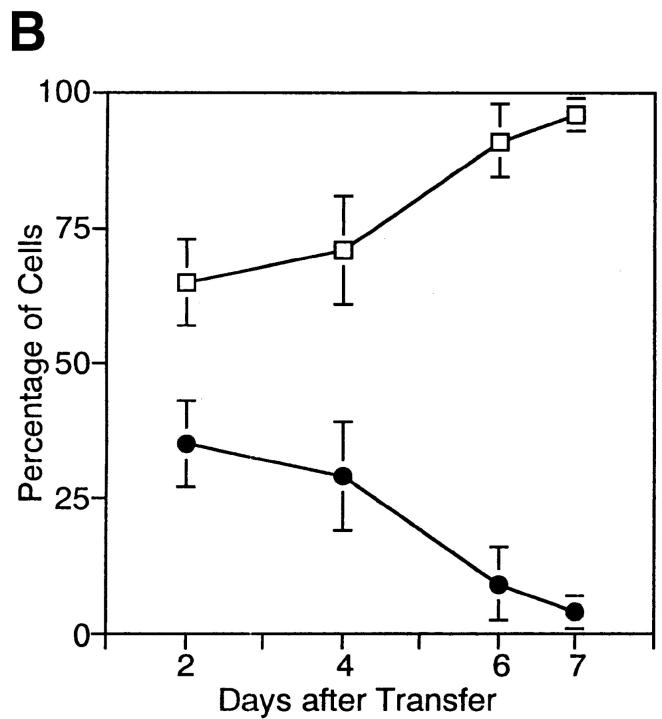
Analyses of mice at several different time points after T cell transfer revealed that the change in the IL-2Rβ2/−/IL-2Rβ1/+ cell ratio was gradually taking place in vivo in the initial 6–7 d after T cell transfer to RAG-2−/− host mice (Fig. 4). At time points later than 7 d, the IL-2Rβ2/−/IL-2Rβ1/+ cell ratio was consistently low and not significantly changed from that of day 7 (data not shown). These results suggested that normal IL-2Rβ1/+ T cells had the activity to eliminate abnormally activated IL-2Rβ2/− T cells.
Figure 4.
Time course of elimination of IL-2Rβ2/− T cells cotransferred with IL-2Rβ1/+ T cells. B6.RAG-2−/− mice were transferred with mixtures of IL-2Rβ1/+ (CD45.1−) and IL-2Rβ2/− (CD45.1+) T cells, and analyzed at the indicated time after T cell transfer. (A) Representative flow cytometric profiles. Total cells recovered from spleen and lymph nodes were stained with anti-CD45.1 and anti–Thy-1.2 antibodies. Numbers in the panels represent percentages of cells in the indicated quadrant per total Thy-1.2+ cells. (B) Data obtained from a total of 12 mice, including 3 independent mice analyzed at the same time point, are demonstrated. Open squares represent percentages of IL-2Rβ1/+ cells per total Thy-1.2+ cells recovered, and filled circles represent percentages of IL-2Rβ2/− cells per total Thy-1.2+ cells. Data are represented by the mean value of three independent mice analyzed, and their SD by error bars.
IL-2Rβ2/− T Cell Elimination Activity Is Fas–FasL Independent, and Stronger in CD8+ T Cells.
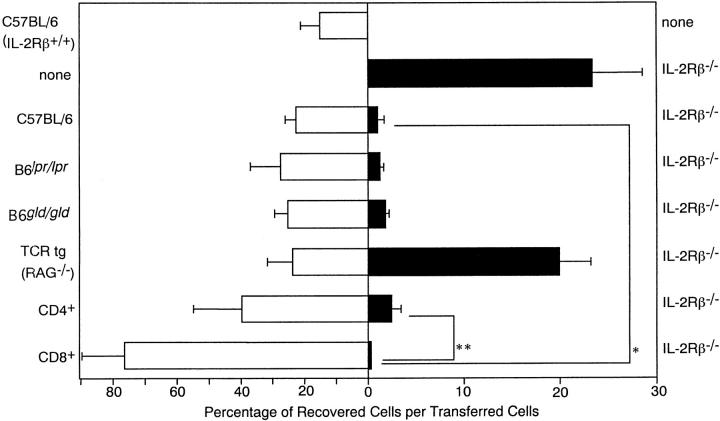
T cells from some different types of mutant mice were used for the partner of IL-2Rβ2/− T cells, and were transferred to RAG-2−/− mice. 7 d later, recovered cells were analyzed for their origin, and percentages of recovered cell numbers per transferred cell numbers were calculated. As shown in Fig. 5T cells from B6_lpr/lpr_ mice and B6_gld/gld_ mice showed an almost identical action to B6 T cells in eliminating IL-2Rβ2/− T cells. On the other hand, T cells from LCMV-specific TCR transgenic mice with RAG-2−/− background showed no such elimination activity. When normal T cells were separated into CD4+ cells and CD8+ cells, and their activity was measured independently, CD8+ cells showed a significantly stronger effect than total T cells or CD4+ T cells alone. Interestingly, CD8+ T cells showed a markedly increased recovery rate when CD8+ T cells were purified and transferred with IL-2Rβ2/− T cells.
Figure 5.
Fas–FasL-independent and more effective elimination of IL-2Rβ2/− T cells by CD8+ cells. B6.RAG-2−/− mice were transferred with 1:1 mixtures of IL-2Rβ2/− T cells and T cells from B6, B6_lpr/lpr_, B6_gld/gld_, or TCR transgenic (tg) mice with RAG-2−/− background. CD4+ cells and CD8+ cells were obtained from normal B6 mice using biotin-conjugated anti-CD4 or anti-CD8 antibody and streptavidin microbeads (MACS), then mixed with IL-2Rβ2/− T cells (1:1) and transferred to B6.RAG-2−/− mice. 7 d later, spleen and lymph node cells were recovered and analyzed by antibody staining and flow cytometry. The total number of T cells of indicated origin was calculated as follows: (total number of cells recovered from spleen and lymph nodes) × (percentage of T cells bearing the CD45 allotype marker). Three independent experiments including two recipient mice for each experiment were performed for each combination of transfer, and the data are represented by mean percentages of recovered cell numbers per transferred cell numbers for indicated T cell types, and their SD with error bars (n = 6). *Significantly different (t test, P < 0.05); **significantly different (P < 0.01).
T Cells Eliminating IL-2Rβ2/− T Cells Express TNFs, FasL, Granzyme B, and Perforin.
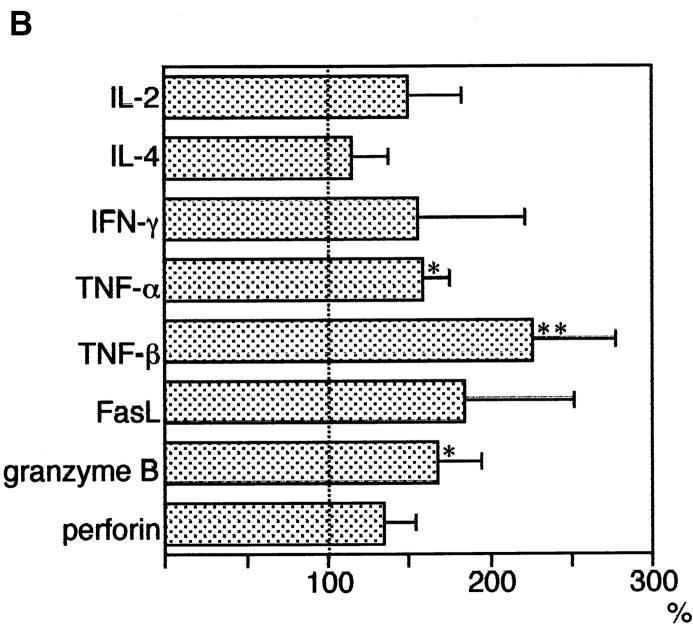
Our study of mixed T cell transfer showed an elimination of IL-2Rβ2/− T cells by normal T cells, and an especially strong elimination activity in CD8+ T cells suggested a cytotoxic mechanism in this process. Accordingly, we next examined the expression of genes involved in T cell cytotoxic activity. T cells, which were prepared from normal B6 (IL-2Rβ1/+) mice and IL-2Rβ2/− mice, were mixed in equal numbers and transferred to B6.RAG-2−/− host mice. 7 d later, B6-derived T cells were recovered and purified by sorting cells stained with anti-CD45.2 and anti-CD4/CD8 antibodies, and the expressions of IL-2, IL-4, IFN-γ, TNF-α, TNF-β, FasL, granzyme B, and perforin were analyzed by the reverse transcription PCR method. The expression in T cells recovered from mice transferred with IL-2Rβ2/− T cells was compared with that in T cells simply transferred without IL-2Rβ2/− T cells and recovered. As shown in Fig. 6 A, recovered T cells expressed all the genes tested, including TNF-α, TNF-β, FasL, granzyme B, and perforin. Among these, the expression levels of TNFs, especially TNF-β, were higher in cells transferred with IL-2Rβ2/− T cells (Fig. 6 B). These results indicated the possibility that normal T cells eliminated IL-2Rβ2/− T cells by using these molecules associated with T cell cytotoxic activity.
Figure 6.

Expression of genes involved in T cell cytotoxic activity. (A) T cells from normal B6 (IL-2Rβ1/+) mice were either unmixed (single) or mixed (mixed) with T cells from IL-2Rβ2/− mice, and were transferred to B6.RAG-2−/− host mice. 7 d later, B6 mice–derived T cells (CD45.2+CD4 or CD8+) were recovered using a cell sorter (EPICS elite; Coulter), and RNA was extracted. After cDNA was synthesized from each RNA sample, PCR was performed using primers described in Materials and Methods to amplify the specific gene products. PCR products were run in 1.5% agarose gel containing 0.5 μg/ml ethidium bromide. The intensity of bands was analyzed using the Molecular Analyst program (Bio-Rad). Numbers (right) indicate relative intensity of bands of mixed (T cells transferred with IL-2Rβ2/− T cells) compared with single (T cells transferred alone, without IL-2Rβ2/− T cells). (B) Increase of each gene expression (shown as a percentage) adjusted with that of β-actin was calculated as follows: (relative band intensity of indicated gene of mixed compared with single) / (relative band intensity of β-actin of mixed compared with single) × 100. Three independent experiments (one recipient mouse per experiment) were performed, and the data are represented by mean values and their SD with error bars. *Significantly different compared with β-actin (paired two group t test, P < 0.01); **significantly different (P < 0.001).
Discussion
Demonstration That Regulatory T Cells Are Strictly IL-2Rβ Dependent in Eliminating Activated T Cells.
It was surprising to find that a disruption of one of the components of the IL-2/IL-2 receptor system caused a significant activation and increase of T cells 8 9 14 15 16. This finding proved that the IL-2/IL-2R system is important for regulating and maintaining the homeostasis of immune systems, although the precise mechanism of such regulation was not clarified. Studies on IL-2–deficient and IL-2Rα–deficient mice have demonstrated some insights into the possible mechanism. T cells in IL-2–deficient mice were shown to be more resistant to apoptosis induced by reactivation or Fas triggering 11. IL-2Rα–deficient T cells were also shown to be apoptosis resistant 12 17. This failure of spontaneous death may be a possible mechanism explaining the expansion of abnormally activated T cells under the condition in which IL-2 or IL-2R never functions. However, studies on IL-2Rβ–deficient mice revealed that T cells in these mice were normally sensitive to Fas-mediated or superantigen-induced cell death 13. This finding indicated the existence of another essential mechanism causing the accumulation of abnormally activated T cells in IL-2Rβ–deficient mice.
In this study, we have demonstrated by two different experimental systems that normal T cells regulate the abnormal activation of IL-2Rβ2/− T cells. Our experimental result of bone marrow transplantation appears to be a “prevention of abnormal activation,” while that of T cell transfer seems more like an “elimination of already activated cells,” possibly demonstrating that two different mechanisms are involved in regulating abnormal T cells. However, these two experiments showed some coincidental results: (a) lack of Fas or FasL molecules does not affect regulatory activity; (b) cells with transgenic TCR lack regulatory activity; and (c) CD8+ cells from IL-2Rβ1/+ mice are increased when they regulate IL-2Rβ2/− T cells. These coincidences may indicate that the two experimental systems reflected the same event, and it would only be logical to consider that a common mechanism worked to express two different-looking phenomena. Based on this assumption, the suppression of activation observed in bone marrow–transplanted mice could be maintained by continuous elimination of activated T cells. Depletion of T cells lacking IL-2Rβ, observed in the T cell transfer experiment, is not due to mere “general weakness” of those cells compared with normal IL-2Rβ1 T cells, because IL-2Rβ2/− T cells, although their number is slightly reduced, do coexist with IL-2Rβ1 T cells in the mixed bone marrow chimeric mice, and do not decrease when transferred with TCR transgenic T cells. Therefore, lack of IL-2Rβ itself cannot trigger the elimination; however, some sign of the activated state could do so.
Krämer et al. also performed a study of mixed bone marrow transplantation using IL-2–deficient mice, and found that IL-2−/−–derived T cells did not develop into an abnormal state in mixed chimera containing 30% IL-2+ lymphocytes 18. Although their finding is similar to our observation in mixed bone marrow chimera of IL-2Rβ2/− and IL-2Rβ1 cells, it is unclear whether the same mechanism works for the normalization of mutant T cells in the mixed bone marrow transplantation system of IL-2−/−and that of IL-2Rβ2/−, because no information, such as the detailed distribution of the lymphocyte population in IL-2−/− mixed bone marrow chimera and the result of the mixed T cell transfer experiment for IL-2−/−, is provided. Furthermore, in the case of the IL-2−/− system, because IL-2 could be secreted from normal T cells and act on IL-2−/− T cells, it is impossible to identify whether the normalizing mechanism is a paracrine action of IL-2, or the regulatory activity of wild-type T cells. In our case, because IL-2 could never act on receptor-deficient T cells, a regulatory mechanism other than the action of IL-2 on the activated T cells must be working between normal T cells and IL-2Rβ–deficient T cells. By adding the system of the T cell transfer experiment, we have postulated the mechanism to be the elimination of activated T cells.
Recognition and Effector Molecules of the Regulatory T Cells.
The experiment using TCR transgenic mice showed a coincidental result in bone marrow transplantation and T cell transfer. T cells with transgenic TCR showed no activity to regulate activated IL-2Rβ2/− T cells. This result may indicate that α/β-type functional TCR is directly involved in the process of regulation and/or elimination as the recognition molecule on the regulatory T cells. However, it is also possible that T cells in TCR transgenic mice are functionally monotonous, and may not include the regulatory T cell population. A significant increase in Vβ12 TCR–using cells in CD8+ T cells that had eliminated IL-2Rβ2/− T cells in mixed T cell transferred mice (our unpublished observations) may indicate that CD8+ T cells bearing Vβ12 TCR are the key population responding to IL-2Rβ2/− T cells. However, the increase in Vβ12+ T cells is still small (control = 1.92 ± 0.10% vs. posttransfer = 3.20 ± 0.40%, n = 3; P < 0.01), suggesting that the responsible T cells are more likely to be multiclonal.
What molecules are involved in the process for the regulatory cells to recognize abnormally activated T cells remains an important question. They could be T cell activation–linked molecules potentially presented to the regulatory cells by the activated cells. IL-2Rβ2/− T cells express increased levels of many cytokines, including IL-2, IL-4, IL-10, IFN-γ, TNF-α, and TGF-β (13; our unpublished observations), and cell surface molecules such as CD69 and CD44. These molecules could be working for recognition by the regulatory T cells.
The additional question arises as to what molecules are working in the effector phase. We performed an analysis of the expression of several genes which may possibly be involved in the effector phase of cytotoxic T cells 19 20. A Fas–FasL deficiency causes expansion of T cells and autoimmune-like abnormalities, suggesting a possible involvement of this system in the regulation of activated T cells 21. Although FasL expression was clearly identified in the T cells eliminating IL-2Rβ2/− T cells (Fig. 6), our study proved that Fas and FasL interaction was not indispensable in the regulation and/or elimination of IL-2Rβ–deficient T cells (Fig. 5). Similarly, expression of TNFs, perforin, and granzyme B was observed, and the level of TNF-β expression was elevated in T cells eliminating IL-2Rβ2/− T cells. Mice lacking the activity of TNFs, perforin, or granzyme B molecules show no sign of an increase in activated T cells 22 23 24, suggesting that the defect of a single molecule may be indecisive in the regulatory activity. Taken together, these molecules involved in the cytotoxic T cell function may compensate for one another, and IL-2Rβ may be the key molecule controlling the central function or development of cells using these molecules.
Cell Surface Phenotypes of Regulatory T Cells.
Our study indicated that CD8+ cells are more effective regulators against the activated IL-2Rβ2/− T cells. The regulatory cells found in this study may be related to the suppressor T cell subsets that have been described in previous reports. Both CD8+ and CD4+ subsets were reported to include suppressor T cells 25 26 27, and the suppressor activity is sometimes related to Fas–FasL dependency 28. In our study, however, the regulatory activity is stronger in CD8+ cells than in CD4+ cells, and Fas–FasL independent. CD4+ cells alone are also sufficient to achieve the regulatory activity (Fig. 5), indicating the heterogeneity of the responsible cells. This elimination by CD4+ cells is not due to any minor contamination of CD8+ cells, <5% of which remain after purification with anti-CD8 antibody and MACS, because a small number of CD8+ T cells (one tenth of IL-2Rβ2/− cells) is not sufficient to eliminate IL-2Rβ2/− T cells (data not shown).
The “regulatory T cells” described in more recent studies seem to expand to both the CD4+ and CD8+ populations 29 30 31 32 33 34 35, indicating that a variety of cells perform the immunoregulatory activity. CD4+ regulatory cells described in several studies constitutively express CD25 (IL-2Rα 36 37 38 39 40). These CD25+CD4+ regulatory cells suppress the IL-2 production from CD4+CD25− cells, and only respond to a high dose of IL-2. CD38+CD4+ regulatory cells proliferate when subject to TCR stimulation in the presence of IL-2 41. Although the cell surface phenotypes are varied, these findings of IL-2R expression and responsiveness to IL-2 may agree with our data, further indicating the importance of the IL-2/IL-2R system in the regulation of T cell activity. The importance of the regulatory T cells found in our analysis is that they have the activity to eliminate abnormally activated T cells. Such elimination activity has not been reported in the majority of previously described regulatory T cells, except CD8+ regulatory T cells, which were shown to eliminate activated Vβ8+CD4+ cells in a Qa-1–restricted manner 42. The elimination of activated IL-2Rβ2/− T cells may be related to cytotoxic activity, because such elimination is related to CD8+ population, and the eliminating cells express molecules related to the cytotoxic effector function. Further characterization and purification of the T cells regulating IL-2Rβ2/− cells by the in vitro culture system may establish the mechanism of this elimination process.
In this study, we investigated the relationship between IL-2Rβ2/− T cells and normal T cells, and found a mechanism by which normal T cells could regulate activated IL-2Rβ2/− T cells. We propose that IL-2Rβ is essential for the development of the regulatory TCR-α/β T cells that effectively eliminate activated T cells. Lack of such regulatory activity may result in an accumulation of activated T cells such as those observed in IL-2Rβ–deficient mice.
Acknowledgments
We thank Dr. H. Yagita (Juntendo University) for providing us with mice, and Ms. S. Aritake and Ms. Y. Umeda for their experimental assistance.
This work was supported by grants from the Ministry of Science, Education, Sports and Culture of Japan; Nitto Industry Co.; and the Takeda Science Foundation.
Footnotes
Abbreviations used in this paper: B6, C57BL/6; LCMV, lymphocytic choriomeningitis virus; RAG, recombination activating gene.
References
- Smith K.A. Interleukin-2inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Waldmann T.A. The IL-2/IL-2 receptor systema target for rational immune intervention. Immunol. Today. 1993;14:264–270. doi: 10.1016/0167-5699(93)90043-K. [DOI] [PubMed] [Google Scholar]
- Minami Y., Kono T., Miyazaki T., Taniguchi T. The IL-2 receptor complexits structure, function, and target genes. Annu. Rev. Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- Sugamura K., Asao H., Kondo M., Tanaka N., Ishii N., Nakamura M., Takeshita T. The common γ-chain for multiple cytokine receptors. Adv. Immunol. 1995;59:225–277. doi: 10.1016/s0065-2776(08)60632-x. [DOI] [PubMed] [Google Scholar]
- Theze J., Alzari P.M., Bertoglio J. Interleukin 2 and its receptorsrecent advances and new immunological functions. Immunol. Today. 1996;17:481–486. doi: 10.1016/0167-5699(96)10057-c. [DOI] [PubMed] [Google Scholar]
- Nelson B.H., Willerford D.M. Biology of the interleukin-2 receptor. Adv. Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- Schorle H., Holtschke T., Hünig T., Schimpl A., Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- Willerford D.M., Chen J., Ferry J.A., Davidson L., Ma A., Alt F.W. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kündig T.M., Furlonger C., Wakeham A., Timms E., Matsuyama T., Schmits R., Simard J.J., Ohashi P.S., Griesser H. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- Lenardo M.J. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- Kneitz B., Herrmann T., Yonehara S., Schimpl A. Normal clonal expansion but impaired Fas-mediated cell death and anergy induction in interleukin-2-deficient mice. Eur. J. Immunol. 1995;25:2572–2577. doi: 10.1002/eji.1830250925. [DOI] [PubMed] [Google Scholar]
- Van Parijs L., Biuckians A., Ibragimov A., Alt F.W., Abbas A.K. Functional responses and apoptosis of CD25 (IL-2R α)-deficient T cells expressing a transgenic antigen receptor. J. Immunol. 1997;158:3738–3745. [PubMed] [Google Scholar]
- Suzuki H., Hayakawa A., Bouchard D., Nakashima I., Mak T.W. Normal thymic selection, superantigen-induced deletion and Fas-mediated apoptosis of T cells in IL-2 receptor β chain-deficient mice. Int. Immunol. 1997;9:1367–1374. doi: 10.1093/intimm/9.9.1367. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A.C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Kühn R., Schorle H., Rajewsky K., Müller W., Horak I. Development and proliferation of lymphocytes in mice deficient for both interleukins-2 and -4. Eur. J. Immunol. 1994;24:281–284. doi: 10.1002/eji.1830240144. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Löhler J., Schorle H., Klebb G., Haber H., Sickel E., Noelle R.J., Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur. J. Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- Zheng L., Trageser C.L., Willerford D.M., Lenardo M.J. T cell growth cytokines cause the superinduction of molecules mediating antigen-induced T lymphocyte death. J. Immunol. 1998;160:763–769. [PubMed] [Google Scholar]
- Krämer S., Schimpl A., Hünig T. Immunopathology of interleukin (IL) 2–deficient micethymus dependence and suppression by thymus-dependent cells with an intact IL-2 gene. J. Exp. Med. 1995;182:1769–1776. doi: 10.1084/jem.182.6.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Okazaki T., Naito Y., Yokota T., Arai N., Ozaki S., Nakao K., Nagata S. Expression of the Fas ligand in cells of T cell lineage. J. Immunol. 1995;154:3806–3813. [PubMed] [Google Scholar]
- Zheng L., Fisher G., Miller R.E., Peschon J., Lynch D.H., Lenardo M.J. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- Cohen P.L., Eisenberg R.A. Lpr and gldsingle gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Pfeffer K., Matsuyama T., Kündig T.M., Wakeham A., Kishihara K., Shahinian A., Wiegmann K., Ohashi P.S., Kronke M., Mak T.W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Kagi D., Ledermann B., Burki K., Seiler P., Odermatt B., Olsen K.J., Podack E.R., Zinkernagel R.M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Heusel J.W., Wesselschmidt R.L., Shresta S., Russell J.H., Ley T.J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Rich R.R., elMasry M.N., Fox E.J. Human suppressor T cellsinduction, differentiation, and regulatory functions. Hum. Immunol. 1986;17:369–387. doi: 10.1016/0198-8859(86)90298-3. [DOI] [PubMed] [Google Scholar]
- Tada T., Hu F.Y., Kishimoto H., Furutani-Seki M., Asano Y. Molecular events in the T cell-mediated suppression of the immune response. Ann. NY Acad. Sci. 1991;636:20–27. doi: 10.1111/j.1749-6632.1991.tb33434.x. [DOI] [PubMed] [Google Scholar]
- Tada T., Inoue T., Asano Y. Suppression of immune responses by cloned T cells and their products. Behring Inst. Mitt. 1992;91:78–86. [PubMed] [Google Scholar]
- Noble A., Pestano G.A., Cantor H. Suppression of immune responses by CD8 cells. I. Superantigen-activated CD8 cells induce unidirectional Fas-mediated apoptosis of antigen-activated CD4 cells. J. Immunol. 1998;160:559–565. [PubMed] [Google Scholar]
- Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Kumar V., Sercarz E. Induction or protection from experimental autoimmune encephalomyelitis depends on the cytokine secretion profile of TCR peptide-specific regulatory CD4 T cells. J. Immunol. 1998;161:6585–6591. [PubMed] [Google Scholar]
- Van de Keere F., Tonegawa S. CD4(+) T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti–myelin basic protein T cell receptor transgenic mice. J. Exp. Med. 1998;188:1875–1882. doi: 10.1084/jem.188.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon B., Mason D. Regulatory T cells in the control of autoimmunitythe essential role of transforming growth factor β and interleukin-4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4(+)CD45RC− cells and CD4(+)CD8(−) thymocytes. J. Exp. Med. 1999;189:279–288. doi: 10.1084/jem.189.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauels H.G., Specht C., Becker C., Kölsch E. Suppression of tumour-specific cytotoxic T-cell responses against the syngeneic BALB/c plasmacytoma ADJ-PC-5 by tumour-induced CD8+ regulatory T cells via IFN-γ. Scand. J. Immunol. 1996;43:421–430. doi: 10.1046/j.1365-3083.1996.d01-56.x. [DOI] [PubMed] [Google Scholar]
- Ciubotariu R., Colovai A.I., Pennesi G., Liu Z., Smith D., Berlocco P., Cortesini R., Suciu-Foca N. Specific suppression of human CD4+ Th cell responses to pig MHC antigens by CD8+CD28− regulatory T cells. J. Immunol. 1998;161:5193–5202. [PubMed] [Google Scholar]
- Jiang H., Kashleva H., Xu L.X., Forman J., Flaherty L., Pernis B., Braunstein N.S., Chess L. T cell vaccination induces T cell receptor Vβ-specific Qa-1-restricted regulatory CD8(+) T cells. Proc. Natl. Acad. Sci. USA. 1998;95:4533–4537. doi: 10.1073/pnas.95.8.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M., Toda M., Sakaguchi N., Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri-Payer E., Amar A.Z., Thornton A.M., Shevach E.M. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- Thornton A., Shevach E. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin-2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Kuniyasu Y., Toda M., Sakaguchi N., Itoh M., Iwata M., Shimizu J., Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cellsinduction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Itoh M., Takahashi T., Sakaguchi N., Kuniyasu Y., Shimizu J., Otsuka F., Sakaguchi S. Thymus and autoimmunityproduction of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Read S., Mauze S., Asseman C., Bean A., Coffman R., Powrie F. CD38+ CD45RBlow CD4+ T cellsa population of T cells with immune regulatory activities in vitro. Eur. J. Immunol. 1998;28:3435–3447. doi: 10.1002/(SICI)1521-4141(199811)28:11<3435::AID-IMMU3435>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Jiang H., Ware R., Stall A., Flaherty L., Chess L., Pernis B. Murine CD8+ T cells that specifically delete autologous CD4+ T cells expressing Vβ8 TCRa role of the Qa-1 molecule. Immunity. 1995;2:185–194. doi: 10.1016/s1074-7613(95)80079-4. [DOI] [PubMed] [Google Scholar]