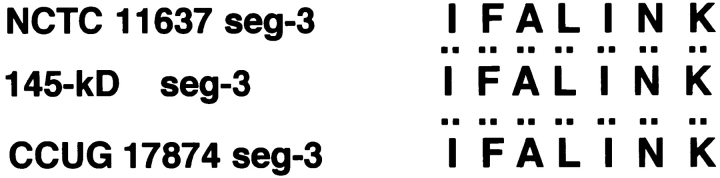Helicobacter pylori Caga Protein Can Be Tyrosine Phosphorylated in Gastric Epithelial Cells (original) (raw)
Abstract
Attachment of Helicobacter pylori to gastric epithelial cells induces various cellular responses, including the tyrosine phosphorylation of an unknown 145-kD protein and interleukin 8 production. Here we show that this 145-kD protein is the cagA product of H. pylori, an immunodominant, cytotoxin-associated antigen. Epithelial cells infected with various H. pylori clinical isolates resulted in generation of tyrosine-phosphorylated proteins ranging from 130 to 145 kD in size that were also induced in vitro by mixing host cell lysate with bacterial lysate. When epithelial cells were infected with [35S]methionine-labeled H. pylori, a radioactive 145-kD protein was detected in the immunoprecipitates with antiphosphotyrosine antibody or anti-CagA (cytotoxin-associated gene A) antibody. Consistently, the 145-kD protein recognized by the anti-CagA and antiphosphotyrosine antibodies was induced in epithelial cells after infection of wild-type H. pylori but not the cagA::Km mutant. Furthermore, the amino acid sequence of the phosphorylated 145-kD protein induced by H. pylori infection was identical to the H. pylori CagA sequence. These results reveal that the tyrosine-phosphorylated 145-kD protein is H. pylori CagA protein, which may be delivered from attached bacteria into the host cytoplasm. The identification of the tyrosine-phosphorylated protein will thus provide further insights into understanding the precise roles of CagA protein in H. pylori pathogenesis.
Keywords: bacterial infection, bacterial adhesion, bacterial protein, protein tyrosine kinase, signal transduction
Introduction
Helicobacter pylori, a spiral, Gram-negative, microaerophilic bacterium, is a human pathogen responsible for chronic active gastritis, and infection with this organism is an important risk factor for the development of peptic ulcer, gastric cancer, and mucosa-associated lymphoid tissue lymphoma 1 2 3 4 5. Several putative H. pylori virulence factors have been identified, including urease, the vacuolating cytotoxin VacA, and the cytotoxin-associated gene A antigen CagA 6 7. Most of the clinical isolates of H. pylori from patients suffering from peptic ulcer or malignant disease express these virulence-associated factors 8 9. H. pylori strains may be divided into two broad families, type I and type II, based on whether or not they possess the cag pathogenicity island (PAI). Type I strains are those that contain the cag PAI, whereas type II strains lack functional cag PAI 10. Serological studies have indicated that patients with duodenitis, duodenal ulcers, and gastric tumors are most often infected by type I H. pylori strains. In the mouse model, type I H. pylori strains can induce visible gastric damage, whereas type II strains do not induce dramatic changes 9. These studies thus suggest that Cag proteins, including CagA encoded by the cag PAI, play important roles in the pathogenicity of H. pylori 8 9 11 12.
H. pylori attaches specifically and tightly to gastric epithelial cells. The adherence of H. pylori to the gastric epithelial cells is an important determinant of pathogenesis. Bacterial attachment causes microvilli effacement, actin rearrangement, pedestal formation, and induction of IL-8 release 13, although the precise mechanisms of H. pylori adherence are still poorly understood. Previous studies have reported that attachment of H. pylori to cultured gastric epithelial cells such as AGS cells can induce tyrosine phosphorylation of a 145-kD host protein and accumulation of F-actin beneath the bacterium 14 15 and also the subsequent evoked activation of nuclear factor (NF)-κB and release of IL-8 16 17 18. The ability of bacteria to induce protein tyrosine phosphorylation, IL-8 production, and the rearrangement of actin cytoskeletons is closely correlated with the presence of the ∼40-kb cag PAI 7 14 15. It has been suggested that the PAI DNA was inherited by horizontal transfer from an unknown microorganism, and the 31 genes of cag are thought to be encoded by a putative type IV secretion system 19 20. For example, the cag homologues of VirB4 (CagE), VirB7 (CagT), VirB9 (_cag_ORF528), VirB10 (_cag_ORF527), VirB11 (_cag_ORF525), and VirD4 (_cag_ORF524) of Agrobacterium tumefaciens have been indicated to be assembled as a complex and form the type IV transport machinery 19 20. Although the notion must still be verified in H. pylori, it suggests the potential ability of H. pylori to deliver bacterial effector molecules through the putative type IV secretion system into the attached host cells, thus enabling the bacteria to alter host cell signaling such as that required for protein tyrosine phosphorylation, stimulation of IL-8 release, and induction of actin dynamics 13 14 15. Although the signaling pathways involved in induction of the various cellular responses remain unclear, persistent host cellular responses to bacterial infection are thought to be the basis of chronic active gastritis, peptic ulcer disease, and perhaps the oncogenic transformation that are the hallmarks of symptomatic H. pylori infection. In this sense, elucidation of the bacterial effector molecules as well as the host cellular responses including signaling pathways must be important. Although the role of IL-8 in promotion of inflammatory reaction to H. pylori infection has been well studied 16 18 21, the identity and function of the tyrosine-phosphorylated 145-kD protein remains unknown.
In this context, we have attempted to characterize the tyrosine-phosphorylated 145-kD protein induced in gastric epithelial cells by H. pylori infection. In our study, we provide evidence that the tyrosine-phosphorylated protein is not a host cellular protein but is rather the cagA gene product of H. pylori.
Materials and Methods
Bacterial Strains, Cell Lines, and Media.
H. pylori standard strains NCTC11637, NCTC11916, and ATCC43579, and its cagA::Km mutant and clinical isolates of H. pylori from patients with gastric ulcer (GU301, GU303, GU304, GU305, and GU306) or gastric cancer (GC401 and GC402) from Fukui Medical University in Japan were used. Before each experiment, H. pylori strains were passaged on 5% sheep blood agar plates (Nippon Becton Dickinson Co., Ltd.) by incubation in an atmosphere consisting of 5% O2, 15% CO2, and 80% N2 for 2–4 d at 37°C. Bacteria were cultured in brucella broth (Difco Labs., Inc.) supplemented with 5% FBS (GIBCO BRL) under the same conditions for 12–24 h at 37°C with agitation (80–100 rpm/min). Human gastric adenocarcinoma epithelial MKN45 and AGS cells (ATCC CRL1739) were from the Japanese Cancer Research Resources Bank and the American Type Culture Collection, respectively. MKN45 and AGS cells were cultured in RPMI 1640 (Sigma Chemical Co.) and DME (Nihonseiyaku) containing 10% FBS, respectively.
Antibodies.
The antiphosphotyrosine antibodies (mAb PY-20 and RC-20; conjugated with horseradish peroxidase) were purchased from Transduction Labs., Inc. Normal rabbit and normal mouse IgG was purchased from Santa Cruz Biotechnology, Inc. Rabbit polyclonal anti-CagA antibody has been described 22.
Preparation of Cell Lysates and Immunoprecipitation.
AGS and MKN45 cells (5 × 106 per plate) cultured to 80% confluence were washed once with 0.1 M PBS, pH 7.5, and then 4 ml of fresh, antibiotic-free DME was added to each 100-mm dish. Cells were infected with H. pylori at a multiplicity of infection of 20–50. After incubation in a 5% CO2 atmosphere for the time indicated in each figure, 5 × 106 infected cells were lysed in ice cold 1% Triton X-100 buffer (50 mM Tris/HCl, pH 7.4, 1% Triton X-100, 5 mM EDTA, 1 mM Na3VO4, 10 μg/ml leupeptin, 10 μg/ml aprotinine, 100 μM _p_-tosyl-l-phenylalanine chloromethyl ketone [TPCK], 100 μM _p_-tosyl-l-lysine chloromethyl ketone [TLCK], and 1 mM PMSF). The cell lysates were centrifuged at 10,000 g for 10 min at 4°C, and the supernatant (1% Triton X-100–soluble fraction) was subsequently immunoprecipitated with the antibodies indicated in each figure or control normal IgG for 2 h at 4°C, after which protein A–Sepharose (Pharmacia Biotech) was added for 30 min at 4°C. The precipitates were washed three times with lysis buffer and once with 10 mM Hepes/NaOH, pH 8.0, and then boiled with electrophoresis SDS sample buffer (2% SDS, 10% glycerol, 5% 2-ME, 0.003% bromophenol blue, and 62.5 mM Tris/HCl, pH 6.8) for 3 min.
Immunoblot Analysis.
Equal amounts of samples from whole cell lysates or immunoprecipitates were separated by SDS-PAGE (6.5% or 7.5% polyacrylamide) and blotted onto Immobilon P (Millipore Corp.). The membranes were blocked with 5% skim milk in T-PBS (PBS containing 0.05% Tween 20) and incubated with a primary antibody in T-PBS for 1–2 h at room temperature. After washing with T-TBS (10 mM Tris/HCl, pH 7.4, 100 mM NaCl, 0.05% Tween 20), the membranes were incubated with horseradish peroxidase–conjugated goat anti–rabbit IgG polyclonal antibodies in T-TBS for 30 min and visualized with an enhanced chemiluminescence (ECL) detection system as directed by the manufacturer (Amersham Corp.). In the case of using antiphosphotyrosine RC-20, after decantation of the primary antibody solution, the membrane was washed with T-TBS and visualized with the ECL detection systems described above. The antiphosphotyrosine immunoblots of anti-CagA immunoprecipitates were stripped by incubating the membranes in a removed buffer (2% SDS, 62.5 mM Tris/HCl, pH 6.8, and 100 mM 2-ME) for 30 min at 50°C and reprobed with the anti-CagA polyclonal antibody.
In Vitro Phosphorylation Assay.
AGS or MKN45 cells (3 × 107) were lysed in 1 ml of ice cold 1% NP-40 buffer (20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 50 mM NaF, 10 mM ammonium molybdate, 1 mM Na3VO4, 10 μg/ml leupeptin, 10 μg/ml aprotinine, 100 μM TLCK, 100 μM TPCK, and 1 mM PMSF) and homogenized in an Ultrasonic Disrupter UD-201 (Tomy) three times for 45 s each time at 4°C. Similarly, H. pylori standard or clinical isolated strains (3 × 109) were lysed in 1 ml of cold 1% NP-40 buffer and homogenized by the disrupter. The host cell lysates were incubated together with the bacterial lysates for 10 min at 30°C in a phosphorylation reaction buffer (50 mM Hepes, 40 μM ATP, 5 mM MnCl2, 100 mM MgCl2, 100 μM Na3VO4). The lysates were boiled with the sample buffer and separated by SDS-PAGE, followed by immunoblotting with antiphosphotyrosine antibody (RC-20) as described above.
Phosphorylation by c-Src Protein Tyrosine Kinase or Epidermal Growth Factor Receptor Protein Tyrosine Kinase In Vitro.
H. pylori (3 × 109 bacteria per milliliter) suspended in 1% NP-40 (40 μl) was added into 5 μl of 10× phosphorylation reaction buffer (250 mM Tris/HCl, pH 7.2, 400 μM ATP, 62.5 mM MnCl2, 312.5 mM MgCl2, 5 mM EGTA, 625 μM Na3VO4) together with 5 μl of human c-Src protein tyrosine kinase (15 U; Upstate Biotechnology Inc.) and incubated for 10 min at 30°C. H. pylori (3 × 109 bacteria per milliliter) suspended in 1% NP-40 (30 μl) was added to 20 μl of 2.5× phosphorylation reaction buffer (50 mM Hepes, pH 7.4, 100 μM ATP, 5 mM MnCl2, 12.5 mM MgCl, 5 mg/ml BSA, 125 μM Na3VO4) together with 10 μl of epidermal growth factor (EGF) receptor protein tyrosine kinase (1.39 U; Biomol Research Labs., Inc.) and incubated for 10 min at 30°C.
Labeling of H. pylori and Host Cells with [35S]Methionine.
H. pylori (109) were incubated in 100 μl of methionine- and FBS-free RPMI medium containing 200 μCi [35S]methionine (NEN Life Science Products, Inc.) in an atmosphere consisting of 5% O2, 15% CO2, and 80% N2 for 5 h at 37°C. Then, [35S]methionine-labeled H. pylori were washed three times with PBS and resuspended in fresh RPMI containing 10% FBS. After the addition of [35S]methionine-labeled H. pylori (2.5 × 108), nonlabeled host cells (5 × 106 per plate) were incubated for 5 h at 37°C and washed three times with PBS. The 1% Triton X-100–soluble fraction was immunoprecipitated with antiphosphotyrosine antibody (mAb PY-20) and separated by SDS-PAGE (6.5% polyacrylamide). The dried gels were scanned by an radioanalytic imaging system (Fuji BAS1500; Fuji Photo Film Co.). For labeling of host epithelial cells with [35S]methionine, epithelial cells (5 × 106 per plate) were incubated in 2 ml of methionine- and FBS-free RPMI medium containing 200 μCi [35S]methionine for 5 h at 37°C. After washing with PBS at room temperature, the labeled cells were resuspended in fresh RPMI containing 10% FBS and added to nonlabeled H. pylori (2.5 × 108).
Construction of cagA::Km Mutant of H. pylori.
The cagA::Km mutant of H. pylori ATCC43579 was constructed by the following method: A 2.0-kb green fluorescent protein gene and kanamycin-resistant cassette were ligated into the HincII site (2,352 nucleotides) of the cagA gene (pCR_cagA_-GK; reference 22), and the resulting plasmid, designated pCR_cagA_-GK, was introduced into ATCC43579 by electroporation. Kanamycin-resistant transformants were screened for allelic exchange of _cagA_-GK with the wild-type cagA gene by PCR. The disruption of the cagA gene was further confirmed by Southern hybridization using a _cagA_-specific DNA probe and by immunoblot with an anti-CagA polyclonal antibody 22.
Peptide Mapping, Amino Acid Sequence, and Nucleotide Sequence.
For peptide mapping of the tyrosine-phosphorylated 145-kD protein, the 145-kD protein sample was prepared by immunoprecipitation with antiphosphotyrosine mAb PY-20 from lysates of epithelial cells infected by H. pylori (NCTC11637; in vivo sample) or from the reaction mixtures containing bacterial and epithelial cell lysates incubated in the phosphorylation reaction buffer in vitro (in vitro sample). The samples were separated on a 7.5% polyacrylamide gel and stained with Coomassie Brilliant Blue R-250. The 145-kD protein bands were then subjected to direct peptide sequencing analysis as follows: The 145-kD protein bands excised from the gel were treated with 50 mM Tris/HCl buffer, pH 8.5, containing lysyl endopeptidase for 20 h at 35°C using the in-gel digestion method described by Rosenfeld et al. 23. The digestion solution was subjected to reverse-phase HPLC (column: TSKgel ODS-80Ts QA, 2.0 × 250 mm; TOSOH), and the peptide fragments were resolved. For amino acid sequencing of internal portions of the 145-kD protein, three major digested peptide fragments from in vitro or in vivo samples designated for sequences 1, 2, and 3 were subjected to an amino acid sequence analyzer (HP G1005A Protein Sequencing Systems; Hewlett-Packard Co.). For nucleotide sequencing of the DNA segments encoding sequences 1, 2, and 3, each of the DNA sequences were amplified by PCR from the chromosomal DNA of H. pylori NCTC11637 using oligonucleotide primers (sequence 1: sense, 5′-AAGGAGAACAATGACTAACGAA-3′, and antisense, 5′-CTGCAAAAGATTGTTTGGCAGA-3′; sequence 2: sense, 5′-GGCAATGGTGGTCCTGGAGCTAGGC-3′, and antisense, 5′-GGAAATCTTTAATCTCAGTTCGG-3′; sequence 3: sense, 5′-ATTTCAAATACACCAACGCCTCCA-3′, and antisense, 5′-TTGCTTGCGTTACCTTGCTG-3′).
PCR was performed under the following conditions: 95°C for 5 min; 25 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min; and 72°C for 7 min. PCR products were purified using concentrator columns (Centricon-100; Amicon, Inc.) according to the manufacturer's instructions. Direct sequencing was performed on both strands using the fluorescent dideoxy terminator method (ABI Prism Dye Terminator Cycle Sequencing Ready Reaction Kit with AmpliTaq DNA polymerase, FS; Perkin-Elmer Corp.). Sequences were analyzed using a DNA sequencer (model 310; Applied Biosystems, Inc.) according to the manufacturer's protocol.
Results
H. pylori Attachment to Gastric Epithelial Cells Induces Tyrosine Phosphorylation of a 145-kD Protein.
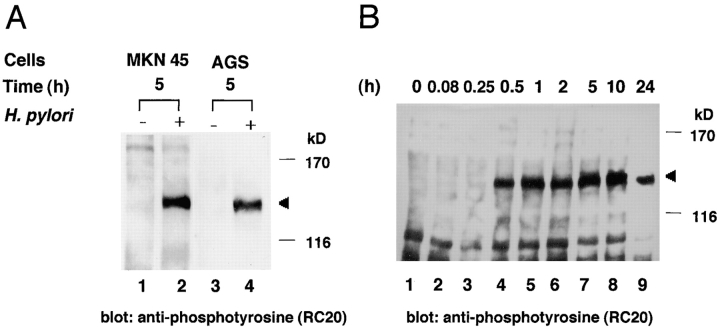
To characterize the tyrosine phosphorylation of a 145-kD protein induced in epithelial cells infected with H. pylori, MKN45 or AGS cells were infected with H. pylori NCTC11637, and 5 h after infection, the epithelial cells lysates were examined for protein tyrosine phosphorylation by immunoblotting with an antiphosphotyrosine antibody (RC-20). Upon attachment of H. pylori to the epithelial cells, a tyrosine-phosphorylated 145-kD protein was detected, whereas no phosphorylated protein ∼145 kD in size was detected in either of the cell lines without H. pylori infection (Fig. 1 A). To further characterize the appearance of the phosphorylated 145-kD protein in the epithelial cells, the MKN45 cells were sampled 15 min–24 h after infection and examined for protein phosphorylation by immunoblotting with antiphosphotyrosine antibody RC-20. As shown in Fig. 1 B, the tyrosine phosphorylation of a 145-kD protein could be seen at 15 min, increased to a maximum level at 5–10 h, and declined to a low level 24 h after infection.
Figure 1.
Immunoblot analysis of gastric epithelial cells infected with H. pylori by antiphosphotyrosine antibody RC-20. (A) Tyrosine phosphorylation of a 145-kD protein in MKN45 or AGS cells induced by H. pylori NCTC11637 infection. Lanes 1 and 2, lysates of uninfected MKN45 or MKN45 infected with H. pylori for 5 h; lanes 3 and 4, lysates of uninfected AGS or AGS infected with H. pylori for 5 h. Arrowhead indicates the 145-kD protein. (B) Time course for the expression of protein tyrosine phosphorylation in MKN45 cells infected with H. pylori NCTC11637. Uninfected (lane 1) or infected (lanes 2–9) MKN45 cells were examined at the indicated times (h) after bacterial infection. Effect of genistein (C) or vanadate (D) on the 145-kD protein tyrosine phosphorylation in MKN45 cells infected with H. pylori NCTC11637. Cells were treated with various concentrations of genistein or vanadate for 30 min at 37°C before the infection. The epithelial cells infected with H. pylori for 5 h were sampled at the indicated concentration, and the protein tyrosine phosphorylation in the cell lysates was examined by the same methods as in A.
To confirm that the 145-kD protein was tyrosine phosphorylated, MKN 45 cells were treated with various concentrations of genistein, a tyrosine kinase inhibitor, and the cells were infected by H. pylori for 5 h. As shown in Fig. 1 C, phosphorylation of the 145-kD protein was abrogated at 100 μg/ml (370 μM). In contrast, treatment by various concentrations of orthovanadate, a tyrosine phosphatase inhibitor, increased the level of tyrosine phosphorylation of the 145-kD protein (Fig. 1 D), implying that protein tyrosine kinase and protein tyrosine phosphatase activities are involved in the phosphorylation of the 145-kD protein.
Infection of Gastric Epithelial Cells by H. pylori Clinical Isolates Induces Diverse Tyrosine-Phosphorylated Proteins.
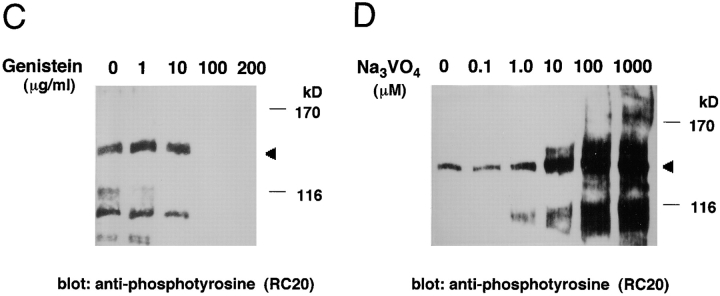
To further investigate the tyrosine-phosphorylated protein, H. pylori clinical isolates from Japanese patients with gastric ulcer or gastric cancer were examined for their ability to induce tyrosine phosphorylation in MKN45 or AGS cells. All of the clinical isolates elicited tyrosine phosphorylation of 130–135-kD proteins in both epithelial cells (Fig. 2), although the level of tyrosine phosphorylation was lower than that displayed by NCTC11637 or NCTC11916, indicating that the size variation in the tyrosine-phosphorylated proteins was dependent on the infecting H. pylori strains but not on the epithelial cell lines.
Figure 2.
Diversity of tyrosine-phosphorylated large proteins induced in epithelial cells infected with various H. pylori strains. Protein tyrosine phosphorylation in MKN45 (A) and AGS cells (B) infected with various clinical isolates of H. pylori for 5 h. Lane 1, uninfected control; lanes 2–7 represent protein tyrosine phosphorylation induced in epithelial cells infected with NCTC11916, NCTC11637, GU301, GU303, GC401, and GC402, respectively. Arrowhead indicates the tyrosine-phosphorylated 145-kD protein; arrow indicates the phosphorylated large proteins in epithelial cells infected with various clinical isolates.
In Vitro Phosphorylation Assay of Tyrosine-Phosphorylated Proteins.
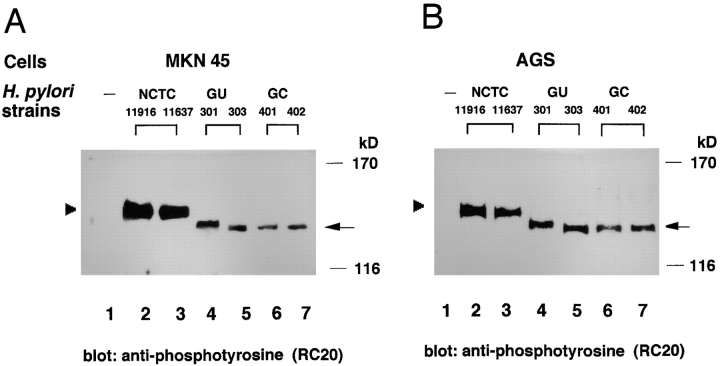
To determine whether the 145-kD phosphorylated protein evoked in the epithelial cells infected with H. pylori is a host cellular protein 13, reconstitution experiments were carried out using H. pylori and epithelial cell lysates in vitro. MKN45 or AGS cell lysates were or were not combined with H. pylori lysates (from NCTC11637), incubated in a phosphorylation reaction buffer containing ATP and cations for 10 min at 37°C (see Materials and Methods), analyzed by SDS-PAGE, and immunoblotted with antiphosphotyrosine RC-20. As shown in Fig. 3 A, a 145-kD protein was only tyrosine phosphorylated in a combination of H. pylori and epithelial cell lysates. Similarly, when bacterial lysates from each of seven H. pylori clinical isolates combined with MKN45 cell lysates were incubated in the phosphorylation reaction buffer, diverse phosphorylated proteins ∼130–135 kD in size were detected (Fig. 3 B). Importantly, the size variations with GU301, GU303, GC401, or GC402 were comparable to those seen in the epithelial cells infected with the corresponding H. pylori strains (Fig. 2). Under the same conditions, neither H. pylori lysate alone nor the epithelial cell lysate alone in the phosphorylation reaction buffer induced protein tyrosine phosphorylation (data not shown), strongly suggesting that the size variation of the phosphorylated protein was dependent on the bacterial strain.
Figure 3.
In vitro tyrosine phosphorylation of the 145-kD protein. (A) Tyrosine phosphorylation of a 145-kD protein induced by a combination of H. pylori NCTC11637 and MKN45 (or AGS) cell lysates. Epithelial cell lysates with or without bacterial lysates were added to a phosphorylation reaction buffer and incubated for 10 min at 30°C. The 145-kD protein was detected by immunoblotting with antiphosphotyrosine antibody RC-20. (B) Tyrosine phosphorylation of a large protein induced by the combination of various H. pylori strains and MKN45 cell lysates. Lanes 1–9: control (without H. pylori lysate), NCTC11637, GU301, GU303, GU304, GU305, GU306, GC401, and GC402, respectively. Arrowhead and arrow indicate the tyrosine-phosphorylated 145-kD and other proteins, respectively.
To verify that the 145-kD proteins phosphorylated in vitro and in vivo were identical, the 145-kD protein was collected by immunoprecipitation with antiphosphotyrosine mAb PY-20 from in vitro or in vivo samples and digested with lysyl endopeptidase using an in-gel digestion method (see Materials and Methods). The HPLC profiles of each digested segment of the 145-kD proteins were similar (data not shown). To further ascertain whether the tyrosine-phosphorylated protein was of H. pylori origin, NCTC11637 bacterial lysate was phosphorylated in phosphorylation reaction buffer in vitro using nonreceptor protein tyrosine kinase, c-Src kinase, or receptor protein tyrosine kinase EGF receptor kinase. Indeed, a 145-kD protein was tyrosine phosphorylated, as determined by immunoblotting with antiphosphotyrosine RC-20 (data not shown), raising the possibility that the 145-kD tyrosine-phosphorylated protein induced in epithelial cells infected with H. pylori is from the infecting bacteria.
Demonstration of the 145-kD Protein from H. pylori.
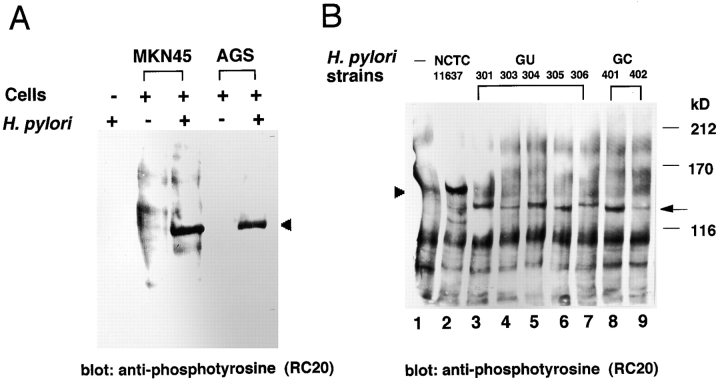
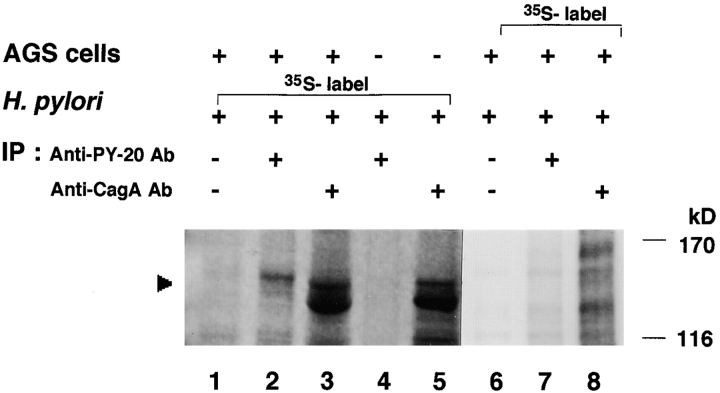
To directly demonstrate that the tyrosine-phosphorylated 145-kD protein was of H. pylori origin, H. pylori strain NCTC11637 and AGS cells were labeled with [35S]methionine separately, and then the nonlabeled epithelial cells were infected with the [35S]methionine-labeled bacteria for 5 h (the [35S]methionine-labeled epithelial cells were infected with nonlabeled bacteria for 5 h). The infected AGS cells were lysed with 1% Triton X-100, and the soluble fraction was immunoprecipitated with antiphosphotyrosine mAb PY-20. Analysis of the precipitates revealed a 145-kD protein labeled by [35S]methionine (Fig. 4, lane 2). Although the radioactivity of the tyrosine-phosphorylated protein was low, the labeled 145-kD protein was reproducibly detected in three independent experiments. Under the same conditions, the 145-kD protein was not detected in antiphosphotyrosine mAb PY-20 immunoprecipitates from labeled bacteria alone (Fig. 4, lane 4) or from labeled AGS cells infected with nonlabeled H. pylori (lane 7). As the CagA protein of H. pylori ranges in size from 120 to 140 kD in different strains 11 22 and our data suggested that the 145-kD protein was derived from H. pylori, we reasoned that the tyrosine-phosphorylated protein was CagA. The lysates of epithelial cells infected with the [35S]methionine-labeled H. pylori were immunoprecipitated by an anti-CagA polyclonal antibody. As shown in Fig. 4 (lane 3), a protein corresponding to 145 kD that was labeled by [35S]methionine was immunoprecipitated with anti-CagA antibody.
Figure 4.
Demonstration of the tyrosine-phosphorylated 145-kD protein derived from H. pylori. AGS cells were infected with [35S]methionine-labeled or nonlabeled H. pylori for 5 h at 37°C, and the cell lysates prepared with 1% Triton X-100 were immunoprecipitated by an antiphosphotyrosine mAb PY-20 or an anti-CagA polyclonal antibody. The precipitated proteins were separated by SDS-PAGE and analyzed using a radioanalytic imaging system. Arrowhead indicates the 145-kD protein. Lanes 1–3, AGS infected with [35S]methionine-labeled H. pylori; lanes 4 and 5, [35S]methionine-labeled H. pylori alone; lanes 6–8, [35S]methionine-labeled AGS infected with nonlabeled H. pylori; lanes 1 and 6, the 1% Triton X-100–soluble fraction precipitated by control normal IgG; lanes 2, 4, and 7, the 1% Triton X-100–soluble fraction precipitated by antiphosphotyrosine mAb PY-20; and lanes 3, 5, and 8, the 1% Triton X-100–soluble fraction precipitated by anti-CagA polyclonal antibody. Note that the [35S]methionine-labeled band being lower than that of 145-kD protein in lanes 3 and 5 may be bacterial protein(s) cross-reacted with the polyclonal anti-CagA antibody.
Amino Acid Sequence of the 145-kD Protein.
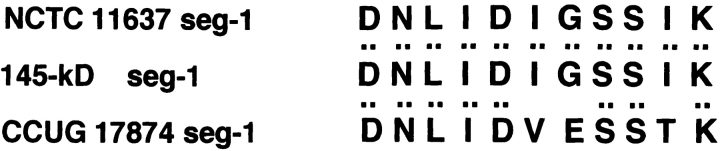
The amino acid sequence of three internal segments of the 145-kD protein prepared from in vitro– or in vivo–phosphorylated sample was determined. The 145-kD phosphorylated protein was digested with lysyl endopeptidase as described above, and the resulting three internal segments, named segment 1, 2, and 3, were subjected to amino acid sequence analysis by Edman chemistry (see Materials and Methods). The amino acid sequences in segment 1, 2, and 3 yielded the sequences DNLIDIGSSIK, NQQGDNVATLINVHMK, and IFALINK, respectively, which were identical to the deduced sequences of the three internal cagA sequences of H. pylori NCTC11637 that were amplified by PCR using three sets of oligonucleotide primers, as described in Materials and Methods (Fig. 5). These sequences were further confirmed to be present in the CagA sequence deduced from the whole cagA gene sequence of NCTC11637 (available from EMBL/GenBank/DDBJ under accession nos. NCTC11637, AF202973 and GC401, AF202972). The amino acids of segments 1, 2, and 3 were almost identical to the respective internal amino acid sequences of the CagA protein deduced from the nucleotide sequences of the cagA gene of H. pylori CCUG17874 11; sequence 1 (segment 1), sequence 2 (segment 2), and sequence 3 (segment 3) of the CagA protein from NCTC11637 corresponded to the internal CagA sequences of Asp97–Lys107, Asn339–Lys 354, and Ile655–Lys661, respectively. Based on the results, we concluded that the 145-kD tyrosine-phosphorylated protein induced in the epithelial cells infected with H. pylori is the CagA protein.
Figure 5.
Amino acid sequences of the tyrosine-phosphorylated 145-kD protein. Amino acid sequences of the three internal segments of the tyrosine-phosphorylated 145-kD protein (145-kD seg-1, -2, and -3) from in vitro sample (the reactant of NCTC11637 and MKN45 cell lysates incubated in the phosphorylation reaction buffer in vitro), the amino acid sequences deduced from nucleotide sequences of the DNA segments encoding sequences 1, 2, and 3 from NCTC11637 (NCTC 11637 seg-1, -2, and -3), or the amino acid sequences deduced from the nucleotide sequences of the corresponding cagA sequence from CCUG17874 (CCUG 17874 seg-1, -2, and -3; reference 11).
Intracellular CagA Protein Can Only Be Tyrosine Phosphorylated.
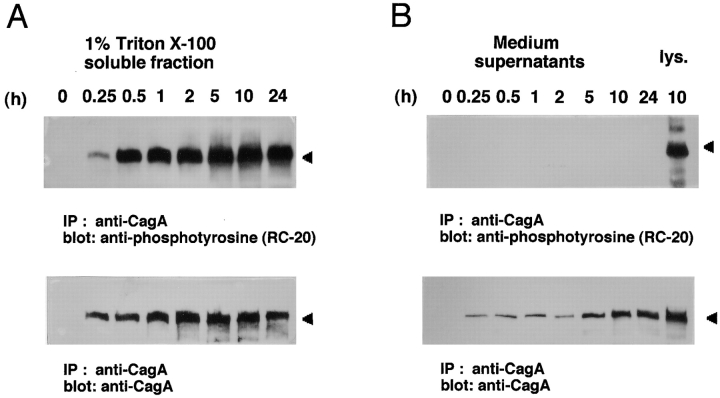
To confirm the above results, the 145-kD tyrosine-phosphorylated protein induced in AGS cells infected with H. pylori NCTC11637 was reinvestigated by immunoprecipitation with anti-CagA antibody. As shown in Fig. 6 A, the 145-kD protein precipitated with anti-CagA antibody was detected by immunoblotting with antiphosphotyrosine RC-20. As the CagA protein of H. pylori is secreted 24 25 26 27 28, we examined whether the 145-kD protein was also secreted into the culture supernatant during infection. Immunoblot analysis with the anti-CagA antibody revealed that the 145-kD protein was present in the culture medium, the levels of which increased by 5–10 h after infection (Fig. 6 B). Importantly, protein tyrosine phosphorylation could not be detected in the culture supernatant at all (Fig. 6 B), indicating that the intracellular CagA protein can only undergo tyrosine phosphorylation.
Figure 6.
Tyrosine phosphorylation of CagA protein induced in H. _pylori_–infected epithelial cells. (A) Detection of the tyrosine phosphorylation of CagA protein in AGS cells induced by H. pylori infection. AGS cells infected with H. pylori NCTC11637 at indicated times after infection were lysed in a 1% Triton X-100 lysis buffer, and the soluble fraction was immunoprecipitated with an anti-CagA polyclonal antibody. The precipitates were separated by SDS-PAGE and then immunoblotted with antiphosphotyrosine antibody RC-20 or anti-CagA polyclonal antibody. (B) Absence of tyrosine-phosphorylated CagA protein in the culture supernatants. The culture supernatants used for the AGS cells infected with H. pylori NCTC11637 at the indicated times were subjected to the same immunoblot analysis as in A. The rightmost lane represents the tyrosine phosphorylation of CagA protein in AGS cells induced by H. pylori 10 h after infection. (C) Tyrosine phosphorylation of CagA protein in AGS cells induced by infection with H. pylori strains NCTC11637, GU303, and GC401. The leftmost lane shows noninfected control, and the second lane on the left shows NCTC11637 control. Top panel: tyrosine-phosphorylated CagA proteins detected in AGS cells infected with H. pylori; bottom panel: the same blot reprobed with the anti-CagA polyclonal antibody. (D) Tyrosine phosphorylation of CagA protein in AGS cells induced by infection with ATCC43579 and the cagA::Km mutant. Arrowhead indicates the 145-kD protein.
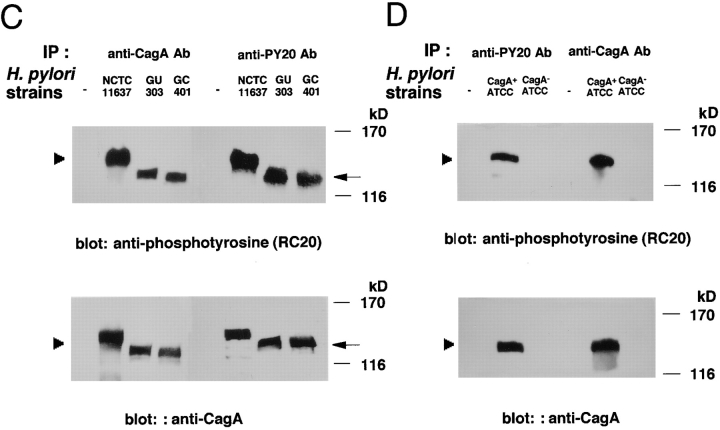
We reexamined the tyrosine-phosphorylated proteins induced in epithelial cells infected with H. pylori GU303 or GC401 by immunoblotting with the anti-CagA antibody. The 130-kD phosphorylated proteins were also detected by the anti-CagA antibody (Fig. 6 C). H. pylori ATCC43579 and its isogenic cagA::Km mutant were similarly tested. Whereas the wild-type H. pylori gave rise to the 145-kD tyrosine-phosphorylated protein, the isogenic cagA::Km mutant failed to generate the phosphorylated protein in the infected epithelial cells (Fig. 6 D).
Discussion
In this study, we investigated the tyrosine-phosphorylated 145-kD protein induced in gastric epithelial cells infected with H. pylori and identified the phosphorylated protein as the cagA gene product of H. pylori. Our conclusion was based on the following results: (a) attachment of different clinical isolates, including several standard H. pylori strains, to epithelial cells evoked diverse sizes of tyrosine-phosphorylated proteins ranging from 130 to 145 kD; (b) when bacterial lysates from the clinical isolates were combined with host cell lysates in vitro, diverse sizes of tyrosine-phosphorylated proteins were also evoked; (c) when epithelial cells were infected with [35S]methionine-labeled H. pylori NCTC11637, a radiolabeled 145-kD protein was immunoprecipitated by antiphosphotyrosine mAb PY-20 and anti-CagA polyclonal antibody; (d) infection of epithelial cells by a cagA::Km mutant of H. pylori failed to induce the tyrosine phosphorylation of the 145-kD protein; and (e) the amino acid sequence of the tyrosine-phosphorylated 145-kD protein was identical to the CagA sequence of H. pylori.
Adherence of H. pylori to gastric epithelial cells can induce host cellular responses, including the reorganization of actin cytoskeletons 29, the tyrosine phosphorylation of a 145-kD protein 13, and release of IL-8 15 18 21. The 145-kD phosphorylated protein induced in gastric epithelial cells infected with H. pylori strain 87A300 was originally reported by Segal and colleagues, who proposed that the 145-kD protein was a host cellular component 13 14. In a subsequent study, they examined type I H. pylori strains together with various cag insertion mutants (cagE, cagF, cagG, cagH, cagI, cagL, cagN, and cagM) for their ability to induce tyrosine phosphorylation of the 145-kD protein or IL-8 production and showed that whereas type I strains were able to induce protein phosphorylation and IL-8 production, all of the cag insertion mutants except for cagN failed to induce the protein phosphorylation and IL-8, indicating that for all of the cag PAI mutants, there was a correlation between the ability to phosphorylate the 145-kD protein and induction of IL-8 15. Based on the effects of various kinase inhibitors on phosphorylation of the 145-kD protein and IL-8 induction as well as on the different inhibitory effect, Segal and colleagues proposed that two distinct signal pathways participate independently in stimulation of IL-8 release and protein tyrosine phosphorylation. Censini et al. 7 reported that KATO-III cells infected with the cagA or cagN mutant were still able to stimulate IL-8 release, whereas the other cag mutants (cagE, cagG, cagH, cagI, cagL, and cagM) failed to induce IL-8 production and NF-κB activation 15 30. Therefore, these studies have suggested that some of the cag genes in the cag PAI, including the cagA gene, could be involved in the induction of phosphorylation of the 145-kD protein 7.
In this study, we confirmed that infection of gastric epithelial cells by H. pylori strains such as NCTC11637, NCTC11916, and ATCC43579 induced tyrosine phosphorylation of a 145-kD protein. Interestingly, when AGS cells were infected with various H. pylori clinical isolates, tyrosine-phosphorylated proteins ranging from 130 to 135 kD in size were evoked that were also reproducibly induced in the infected MKN45 cells, raising the possibility that the tyrosine-phosphorylated protein was not a host cellular protein but rather derived from H. pylori. This notion agreed with the results of in vitro protein phosphorylation assays using bacterial lysates and AGS cell lysates, where the sizes of the phosphorylated proteins were comparable to those induced in epithelial cells infected with H. pylori clinical isolates (Fig. 3). As the CagA protein displayed considerable size variation ranging from 120 to 140 kD in different H. pylori clinical isolates, a consequence of the intragenic repeat sequences in the 3′ portion of the cagA gene 11, we reasoned that the tyrosine-phosphorylated 145-kD protein, including the diverse phosphorylated proteins induced in epithelial cells infected with NCTC11637 or various clinical isolates, H. pylori strains would be the cagA product. Examination of the tyrosine-phosphorylated 145-kD protein using anti-CagA polyclonal antibody and the direct amino acid sequencing supported our premise. Interestingly, the levels of phosphorylation of the 130–135-kD protein (identified as CagA) also varied among H. pylori clinical isolates, with somewhat lower levels of phosphorylation than those induced by H. pylori NCTC11637 or NCTC11916. The CagA sequences deduced from the nucleotide sequencing of the cagA gene in H. pylori CCUG1784 (128-kD CagA) and ACTT43526 (138-kD CagA) had two and five copies of repeat Glu-Pro-Ile-Tyr-Ala (EPIYA) sequences at the 3′ region, respectively 11 22. Similarly, the CagA sequences deduced from the nucleotide sequencing of the cagA gene in NCTC11637 and GC401 revealed that CagA of NCTC11637 (139-kD CagA) and GC401 (131-kD CagA) contained five and three copies of EPIYA sequences at the 3′ region, respectively. Interestingly, one of the putative tyrosine phosphorylation sites in the EPIYA sequence, which is part of the Pro(Leu)-Glu-Glu-Pro-Ile-Tyr-Ala (PEEPIYA or LEEPIYA) sequence, is similar to the PEEHIYD sequence in the Tir protein of enteropathogenic Escherichia coli (EPEC; reference 31). Notably, the tyrosine phosphorylation at the PEEHIYD sequence of the EPEC Tir protein is essential for EPEC induction of the actin nucleation activity beneath the bacterium and tight bacterial adherence to epithelial cells 32. Although the preferential tyrosine phosphorylation sites in the CagA protein remain to be determined, it is likely that the different levels of phosphorylation of the CagA proteins in epithelial cells induced by H. pylori clinical isolates could partly depend on the numbers of repeated EPIYA sequences present in each CagA protein.
As the CagA protein can be secreted onto the bacterial surface and also released into the culture supernatant even in the absence of a typical leader peptide sequence 11 24 25 26 27 28, CagA secretion is thought to be exported by a _sec_-independent secretion system 11 19. In this study, we confirmed that the 145-kD protein (and the 130–135-kD protein) was also secreted into the culture supernatant from various H. pylori strains when attached to the epithelial cells, and we identified the 145-kD protein (and the 130–135-kD proteins) as CagA. Whereas the extracellular 145-kD protein was not tyrosine phosphorylated at all, the intracellular protein was tyrosine phosphorylated. Although the precise mechanisms of CagA secretion from H. pylori into the bacterial environment or host cells remains unclear, it is tempting to speculate that H. pylori could directly deliver the CagA protein into the host cell cytoplasm through attachment to the cells, and the internalized CagA protein could then undergo tyrosine phosphorylation by host cellular tyrosine kinases. Importantly, some of the _cag_-encoded proteins such as CagE, CagT, _cag_ORF524, _cag_ORF525, _cag_ORF527, _cag_ORF528, and _cag_ORF996 have sequence similarities to well known proteins composing the type IV export machineries of E. coli, Bordetella pertussis, A. tumefaciens, Legionella pneumophila, Rickettsia prowazekii, and Brucella suis 19 20 33 34 35. The type IV export systems specialize in transfer of a variety of multimolecular complexes across the bacterial membrane to the extracellular space or into target host cells 19 20. Furthermore, Segal et al. showed that mutations in some of the cag genes such as cagE, cagF, cagG, cagH, cagI, cagL, and cagM failed to induce tyrosine phosphorylation of the 145-kD protein 15. It is thus intriguing to speculate that the delivery of CagA from H. pylori into the culture supernatant and the attached epithelial cells could be via the putative type IV export system.
Although the precise role of tyrosine phosphorylation of intracellular CagA protein in the pathogenesis of H. pylori remains to be elucidated, the behavior of CagA protein observed in this study is reminiscent of the EPEC Tir protein; the Tir protein can be directly delivered from EPEC into attached epithelial cells though the type III secretion machinery, and the translocated protein undergoes tyrosine phosphorylation in the host cell cytoplasm 31. In addition, both H. pylori and EPEC can attach tightly to the epithelial cells, an attachment through which pathogens can induce a local rearrangement of actin cytoskeletons, including pedestal formation beneath the bacterium and release of IL-8 13 15 36 37. However, CagA and Tir have no amino acid sequence similarity. Indeed, Tir possesses two transmembrane domains, whereas CagA has no such domains, and CagA is a very hydrophilic protein 11 31. Although the tyrosine-phosphorylated Tir protein has been implicated in induction of actin condensation beneath the bacterium and serves as the receptor for binding to the intimin protein, the tyrosine-phosphorylated CagA protein seems to have no such specific role 15 31 38. Instead, CagA has been implicated in determination of the intensity of inflammation in the gastric epithelium, a key event in the development of gastric diseases associated with H. pylori infection 9 12. Thus, based on our results, the phosphorylated CagA protein may also play a crucial role in promoting the inflammatory responses of gastric mucosa to H. pylori infection. The identification of the 145-kD tyrosine-phosphorylated protein as the cagA gene product of H. pylori should provide further insight into the precise role of the CagA protein in the pathogenesis of H. pylori as well as in the development of duodenitis, duodenal ulcers, and gastric cancer.
Acknowledgments
We thank Dr. Eiji Majima for help with amino acid analysis.
This work was supported by a Grant-in-Aid for General Scientific Research (10670148) from the Ministry of Education, Science, Sports and Culture of Japan and a grant from the Science Promotion Foundation for Fukui Prefectural University (both to M. Asahi), as well as a grant from the ‘Research for the Future’ Program of the Japan Society for the Promotion of Science to C. Sasakawa.
Footnotes
Chihiro Sasakawa, Dept. of Bacteriology, Institute of Medical Science, University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan. Phone: 81-3-5449-5252; Fax: 81-3-5449-5405; E-mail: sasakawa@ims.u-tokyo.ac.jp
Abbreviations used in this paper: EGF, epidermal growth factor; PAI, pathogenicity island.
References
- Marshall B.J., Warren J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Goodwin C.S., Armstrong J.A., Marshall B.J. Campylobacter pyloridis, gastritis, and peptic ulceration. J. Clin. Pathol. 1986;39:353–365. doi: 10.1136/jcp.39.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M.J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J. Infect. Dis. 1990;161:626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G.D., Vandersteen D.P., Chang Y., Vogelman J.H., Orentreich N., Sibley R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Hansen S., Rodriguez L., Gelb A.B., Warnke R.A., Jellum E., Orentreich N., Vogelman J.H., Friedman G.D. Helicobacter pylori infection and gastric lymphoma. N. Engl. J. Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- Cover T.L., Tummuru M.K., Cao P., Thompson S.A., Blaser M.J. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J. Biol. Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- Censini S., Lange C., Xiang Z., Crabtree J.E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton J.C., Cao P., Peek R.M., Tummuru M.K., Blaser M.J., Cover T.L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- Marchetti M., Arico B., Burroni D., Figura N., Rappuoli R., Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- Pagliaccia C., De Bernard M., Lupetti P., Ji X., Burroni D., Cover T.L., Papini E., Rappuoli R., Telford J.L., Reyrat J.-M. The m2 form of the Helicobacter pylori cytotoxin has cell type-specific vacuolating activity. Proc. Natl. Acad. Sci. USA. 1998;95:10212–10217. doi: 10.1073/pnas.95.17.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M.J., Perez-Perez G.I., Kleanthous H., Cover T.L., Peek R.M., Chyou P.H., Stemmermann G.N., Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- Segal E.D., Falkow S., Thompkins L.S. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc. Natl. Acad. Sci. USA. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E.D. Consequences of attachment of Helicobacter pylori to gastric cells. Biomed. Pharmacother. 1997;51:5–12. doi: 10.1016/s0753-3322(97)87073-4. [DOI] [PubMed] [Google Scholar]
- Segal E.D., Lange C., Covacci A., Tompkins L.S., Falkow S. Induction of host signal transduction pathways by Helicobacter pylori . Proc. Natl. Acad. Sci. USA. 1997;94:7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J.E., Covacci A., Farmery S.M., Xiang Z., Tompkins S., Perry S., Lindley I.J., Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J. Clin. Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.A., Tummuru M.K., Blaser M.J., Kerr L.D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J. Immunol. 1998;160:2401–2407. [PubMed] [Google Scholar]
- Aihara M., Tsuchimoto D., Takizawa H., Azuma A., Wakebe H., Ohmoto Y., Imagawa K., Kikuchi M., Mukaida N., Matsushima K. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN45. Infect. Immun. 1997;65:3218–3224. doi: 10.1128/iai.65.8.3218-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacci A., Telford J.L., Giudice G.D., Parsonnet J., Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- Covacci A., Rappuoli R. Helicobacter pylorimolecular evolution of a bacterial quasi-species. Curr. Opin. Microbiol. 1998;1:96–102. doi: 10.1016/s1369-5274(98)80148-3. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., Kita M., Kodama T., Sawai N., Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]
- Maeda S., Kanai F., Ogura K., Yoshida H., Ikenoue T., Takahashi M., Kawabe T., Shiratori Y., Omata M. High seropositivity of anti-CagA antibody I Helicobacter pylori infected patients irrelevant to peptic ulcers and normal mucosa in Japan. Dig. Dis. Sci. 1997;42:1841–1847. doi: 10.1023/a:1018846723379. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J., Capdevielle J., Guillemot J.C., Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal. Biochem. 1992;203:173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- Kodama K., Fujioka T., Ito A., Kodama R., Nasu M. Toxigenicity of Helicobacter pylori isolates possessing cagA gene and vacuolating cytotoxin. J. Gastroenterol. 1998;33:14–17. [PubMed] [Google Scholar]
- Apel I., Jacobs E., Kist M., Bredt W. Antibody response of patients against a 120 kDa surface protein of _Campylobacter pylori_Zentralbl. Bakteriol. Mikrobiol. Hyg.. 268 1988. 271 276[A] [DOI] [PubMed] [Google Scholar]
- Crabtree J.E., Figura N., Taylor J.D., Bugnoli M., Armellini D., Tompkins D.S. Expression of 120 kilodalton protein and cytotoxicity in Helicobacter pylori . J. Clin. Pathol. 1992;45:733–734. doi: 10.1136/jcp.45.8.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T.L., Dooley C.P., Blaser M.J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect. Immun. 1990;58:603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Bugnoli M.G., Pucci A.M., Lusini P., Quaranta S., Barbieri A., Rossolini A., Di Tommasa A.L., De Magistris T., Rappuoli R. Pathogenic mechanisms of Helicobacter pyloriproduction of cytotoxin. In: Malfer-Theiner P., Ditschuneit H., editors. Helicobacter pylori, Gastritis and Peptic Ulcer. Springer-Verlag; Berlin: 1990. pp. 86–95. [Google Scholar]
- Smoot D.T., Resau J.H., Naab T., Desbordes B.C., Gilliam T., Bull-Henry K., Curry S.B., Nidiry J., Sewchand J., Milis-Robertson K. Adherence of Helicobacter pylori to cultured human gastric epithelial cells. Infect. Immun. 1993;61:350–355. doi: 10.1128/iai.61.1.350-355.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clocker E., Lange C., Covacci A., Bereswill S., Kist M., Pahl H.L. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-κB activation. Infect. Immunity. 1998;66:2346–2348. doi: 10.1128/iai.66.5.2346-2348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny B., Devinney R., Stein M., Reinscheid D.J., Frey E.A., Finlay B.B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- Kenny B. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol. Microbiol. 1999;31:1229–1241. doi: 10.1046/j.1365-2958.1999.01265.x. [DOI] [PubMed] [Google Scholar]
- Segal G., Shuman H.A. How is the intracellular fate of the Legionella pneumophila phagosome determined? Trends Microbiol. 1998;6:253–255. doi: 10.1016/s0966-842x(98)01308-0. [DOI] [PubMed] [Google Scholar]
- Andersson S.G., Zomorodipour A., Andersson J.O., Sicheritz-Ponten T., Alsmark U.C., Podowski R.M., Naslund A.K., Eriksson A.S., Winkler H.H., Kurland C.G. The genome sequence of Rickettsia prowazekki and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- Winans S.C., Burns D.L., Christie P.J. Adaptation of a conjugal transfer system for the export of pathogenic macromolecules. Trends Microbiol. 1996;4:64–68. doi: 10.1016/0966-842X(96)81513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Donnenberg M.S., Kaper J., Finlay B.B. Signal transduction between enteropathogenic Escherichia coli and epithelial cellsEPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Ruschkowski S., Stein M., Reinscheid D.J., Mills S.D., Finlay B.B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO (Eur. Mol. Biol. Organ.) J. 1996;11:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- Dytoc M., Gold B., Louie M., Huesca M., Fedorko L., Crowe S., Lingwood C., Brunton J., Sherman P. Comparison of Helicobacter pylori and attaching-effacing Escherichia coli adhesion to eukaryotic cells. Infect. Immun. 1993;61:448–456. doi: 10.1128/iai.61.2.448-456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]