T Helper Type 2 Cell Differentiation Occurs in the Presence of Interleukin 12 Receptor β2 Chain Expression and Signaling (original) (raw)
Abstract
The differentiation of CD4+ T cells into T helper type 1 (Th1) cells is driven by interleukin (IL)-12 through the IL-12 receptor β2 (IL-12Rβ2) chain, whereas differentiation into Th2 cells is driven by IL-4, which downregulates IL-12Rβ2 chain. We reexamined such differentiation using IL-12Rβ2 chain transgenic mice. We found that CD4+ T cells from such mice were able to differentiate into Th2 cells when primed with IL-4 or IL-4 plus IL-12. In the latter case, the presence of IL-4 suppressed interferon (IFN)-γ production 10–100-fold compared with cells cultured in IL-12 alone. Finally, in studies of the ability of IL-12 to convert Th2 cells bearing a competent IL-12R to the Th1 cells, we showed that: (a) T cells bearing the IL-12Rβ2 chain transgene and primed under Th2 conditions could not be converted to Th1 cells by repeated restimulation under Th1 conditions; and (b) established Th2 clones transfected with the IL-12Rβ2 chain construct continued to produce IL-4 when cultured with IL-12. These studies show that IL-4–driven Th2 differentiation can occur in the presence of persistent IL-12 signaling and that IL-4 inhibits IFN-γ production under these circumstances. They also show that established Th2 cells cannot be converted to Th1 cells via IL-12 signaling.
Keywords: cytokine, interleukin 4, reversibility, signal transducer and activator of transcription 4, T helper type 1
Introduction
An important goal of recent immunological study is the elucidation of the molecular events governing the differentiation of naive CD4+ T cells into Th1 and Th2 cells. For the most part, such study has focused on the effects of various cytokines on developing T cells 1. As a result, it is now clear that IL-4 induces naive CD4+ T cells to differentiate into Th2 cells 2 3 4 5 6, in large measure through its ability to activate signal transducer and activator of transcription (STAT) 6 7 8 9, a transcription factor that binds to the IL-4 gene 10. Other transcription factors such as c-Maf and GATA-3 are also induced during Th2 cell differentiation 11 12, but it is not yet known how these factors interact with STAT6, if they do so at all. It is also now clear that IL-12 induces naive CD4+ T cells to differentiate into Th1 cells 13 14 15 and that such differentiation is affected by the activation of STAT4 16 17 18 19, a transcription factor that binds to the IFN-γ gene 20.
Although in separate cultures of naive T cells, IL-4 induces Th2 cells and IL-12 induces Th1 cells, when both cytokines are present in the same culture the IL-4 effect is predominant and Th2 cells develop in the presence of IL-12 13 14. These observations have been explained by the effect of the various cytokines on IL-12R expression, or, more specifically, the expression of the IL-12Rβ2 chain, the receptor component that is rapidly upregulated by TCR stimulation and is required for STAT4 activation 21 22 23 24 25. In particular, it has been postulated that IL-4 downregulates the expression of the IL-12Rβ2 chain and in so doing prevents IL-12 from transducing its signal; this then allows unopposed IL-4–directed differentiation 1. Conversely, IFN-γ (in mice) maintains IL-12Rβ2 chain expression even in the presence of IL-4 and hence sustains IL-12 responsiveness 1 23 26. In this view, the regulation of the IL-12Rβ2 chain is a critical regulator of Th1/Th2 differentiation.
However, it should be noted that the data so far obtained do not exclude the alternative possibility that although optimal Th1 cell differentiation requires IL-12Rβ2 chain expression and STAT4 activation, Th2 cell development can occur in spite of such expression and activation. To examine this possibility, we studied Th1/Th2 cell differentiation in CD4+ T cells obtained from IL-12Rβ2 chain transgenic mice that constitutively express the IL-12Rβ2 chain as well as in Th2 cell clones transfected with an IL-12Rβ2 chain construct. The results showed that CD4+ T cells stimulated via the TCR can be induced to become Th2 cells by IL-4 despite continuing expression of IL-12Rβ2 chain and IL-12–induced STAT4 activation. Thus, while expression of the IL-12Rβ2 chain may be a critical regulator of Th1 differentiation, it cannot turn off an IL-4–initiated Th2 differentiation program.
Materials and Methods
Reagents.
Culture medium used for D10.G4.1 (D10) cells was Click's medium (Biosource International) with 10% FCS, 100 μg/ml streptomycin, 100 U/ml penicillin, 50 μM 2-ME, 10 U/ml human IL-2 (Life Technologies), and 6% rat Con A supernatants 27. Culture medium used for primary cell lines was RPMI 1640 (BioWhittaker) with 10% FCS, 100 μg/ml streptomycin, 100 U/ml penicillin, 15 mM Hepes, pH 7.0, 5% NCTC-109 (Biosource International). Murine IL-4, human IL-2, and murine IL-12 were purchased from PeproTech, Life Technologies, and R&D Systems, respectively. Anti–mouse IL-4 mAb (clone 11B11) was purchased from National Cancer Institute. Biotinylated mouse anti–mouse IL-12Rβ1 chain mAb was provided by Dr. C.Y. Wu (National Institute of Allergy and Infectious Diseases). Hamster anti–mouse IL-12Rβ2 chain mAb (PDL-HAM10B9) was made by immunizing hamster with Chinese hamster ovary (CHO) cells transfected with mouse IL-12Rβ2 chain cDNA. The specificity of PDL-HAM10B9 was confirmed by flow cytometry analysis of CHO cells transfected with mouse IL-12Rβ2 chain cDNA, Con A–stimulated splenocytes of wild-type and IL-12Rβ2 chain knockout mice (Hoffman-La Roche). OVA peptide (323–339) was purchased from American Peptide Company, Inc.
Cell Culture.
D10 cells were purchased from American Type Culture Collection 27, and were stimulated every 2–3 wk with 0.5 × 106/ml 35 Gy–irradiated AKR splenocytes and 100 μg/ml conalbumin (Sigma-Aldrich). Splenocytes from OVA-TCR and IL-12Rβ2 chain double transgenic mice or OVA-TCR single transgenic mice were isolated, and CD4+ cells were purified by mouse CD4 columns that are based on negative selection for CD4+ cells (R&D Systems). CD4+CD62Lhigh cells were further purified by FACS® sorting with CD4-FITC and CD62L-PE by the National Institute of Allergy and Infectious Diseases Flow Cytometry Unit. The isolated cells (>98% CD4+CD62Lhigh) were stimulated with 1.5 × 106/ml 30 Gy–irradiated T cell–depleted Balb/c splenocytes and 0.3 μM OVA peptide. The additional cytokines and anticytokine mAb are IL-4 (200 U/ml), IL-12 (2 ng/ml), and anti–IL-4 mAb 11B11 (10 μg/ml). T cell lines were expanded with 20 U/ml human IL-2 and additives 72 h after stimulation. T cell lines were stimulated weekly with OVA peptide and APCs as described above except that irradiated whole splenocytes were used as APCs at the concentration of 2.5 × 106 /ml. T cell–depleted Balb/c splenocytes were made by complement lysis with guinea pig complement (Life Technologies), anti-CD8α mAb (clone 2.43), anti-CD4 mAb (clone GK1.5), anti-Thy1.2 mAb (clone 30-H12), and anti–rat IgG mAb (clone MAR18.5). After T cell depletion treatment, CD3ε1 cells were <3% by flow cytometry analysis.
Plasmid Construction.
VAhCD2 minigene vector 28 and mouse IL-12Rβ2 chain cDNA 21 were provided by Dr. D. Kioussis (National Institute for Medical Research, London, UK) and Dr. U. Gubler (Hoffman-La Roche Inc., Nutley, NJ), respectively. FLAG epitope tag sequence (5′-GATTACAAGGACGACGATGACAAG-3′) was inserted before the stop codon of mouse IL-12Rβ2 chain cDNA by PCR, and confirmed by sequencing with an ABI PRISM 377 sequencer. The FLAG-tagged mouse IL-12Rβ2 cDNA was inserted into the VAhCD2 minigene vector using conventional subcloning technique (VAhCD2IL-12Rβ2FLAG). For making transgenic mice, we inserted 2.8-kb NheI–AflII fragment of mouse IL-12Rβ2 cDNA into the VAhCD2 minigene vector after performing EcoRI linker ligation (VAhCD2IL-12Rβ2). We then isolated a 14.2-kb fragment after digesting SalI–NotI for microinjection into C57BL/6 OVA. The total cDNA sequence of the final clone was confirmed by sequencing.
Mice.
Balb/c and AKR mice were purchased from National Cancer Institute. The Balb/c background OVA-TCR transgenic mouse DO11.10, which recognizes the 323–339 peptide of OVA in the context of I-Ad, was provided by Dr. D. Loh (Washington University School of Medicine, St. Louis, MO [29]). IL-12Rβ2 chain transgenic mice were created by microinjection of VAhCD2IL-12Rβ2 construct into C57BL/6 OVA in the National Institute of Allergy and Infectious Diseases Transgenic Facility, resulting in two founders: 1818 (about six copies) and 1826 (less than one copy). From the founder 1818, the transgenes were segregated, and we used three lines with about two to three copies for backcrossing to Balb/c. Transgenic mice were maintained in heterozygous condition for the IL-12Rβ2 chain transgene. After backcrossing two to three times to Balb/c and checking MHC class II type of peripheral blood by flow cytometry, IL-12Rβ2 chain transgenic mice (heterozygous for the transgene) homozygous for I-Ad were mated with Balb/c background OVA-TCR transgenic mice DO11.10 (homozygous for the OVA-TCR transgene). Screening for IL-12Rβ2 chain transgene was performed by PCR with tail DNA using primers 5′-GCATACGTTCACGTTCTTGG-3′ and 5′-CAGAAGCGCCTTTTGAGTTG-3′. In each experiment, spleens from two to three mice, 4–12 wk old, were pooled for isolation of CD4+ cells and littermates were used as control.
Transfection.
107 D10 cells in 0.4 ml were transfected by electroporation with 20 μg VAhCD2IL-12Rβ2FLAG and 1 μg pSV2Neo (a gift of Dr. J. Cohen, National Institute of Allergy and Infectious Diseases). The electroporation was performed with a BTX electroporator at 280 V, 1,050 μF, and R10 in 0.4-cm-gap cuvette. Immediately after electroporation, cells were transferred to culture medium, incubated overnight, and stimulated with irradiated AKR mice splenocytes and conalbumin. 48 h after stimulation, the transfected cells were plated in 96-well plates with 500 μg/ml G418 (Life Technologies). Positive clones were screened by FACS® analysis with anti–mouse IL-12Rβ2 chain mAb and Western blotting for FLAG epitope. The transfectants were maintained in culture medium with 200 μg/ml G418.
ELISA.
To measure cytokines, 5 × 105/ml T cells were stimulated with coated anti–mouse CD3ε (5 μg/ml; PharMingen) plus soluble anti–mouse CD28 (1 μg/ml; PharMingen), mouse IL-12 (5 ng/ml) plus mouse IL-18 (100 ng/ml; R&D Systems), and irradiated APCs plus antigen (IL-12 plus IL-18 stimulation was used to get maximal IFN-γ production through IL-12R instead of IL-12 stimulation). IL-4 and IFN-γ concentrations in the supernatants were measured by Endogen ELISA minikits after the supernatants were diluted to be measured in linear range. The samples are measured in duplicate, and averages were shown.
Flow Cytometry.
Flow cytometry was performed using standard procedures using rat anti–mouse CD4-FITC (PharMingen), rat anti–mouse CD62L-PE (PharMingen), biotinylated mouse anti–mouse IL-12Rβ1 chain mAb, hamster anti–mouse IL-12Rβ2 chain mAb (PDL-HAM10B9), hamster anti-TNP mAb (PharMingen), biotinylated goat anti–hamster IgG (H+L) (Jackson ImmunoResearch Laboratories), and streptavidin-PE (PharMingen). For staining for mouse IL-12Rβ2 chain, cells were stained sequentially with PDL-HAM10B9, with biotinylated goat anti–hamster IgG (H+L; Jackson ImmunoResearch Laboratories Inc.), and with streptavidin-PE (PharMingen) and rat anti–mouse CD4-FITC (PharMingen). The analysis was performed on a Becton Dickinson FACScan™ flow cytometer with CELLQuest™ II software.
Proliferation Assay.
T cells were plated at the concentration of 0.5 × 106/ml for primary cell lines and 0.2 × 106/ml for D10 cell transfectants, and cultured with varying concentrations of IL-12. The cultures were incubated for 24 h and pulsed with 1 μCi of [3H]thymidine for the last 6 h.
Intracellular Cytokine Staining.
T cells subjected to intracellular cytokine staining were cells from T cell lines from double and single transgenic mice as described above. 7 d after antigen stimulation, 98–99% of these cells expressed CD4 and OVA-TCR clonotype recognizing mAb KJ1-26 as determined by flow cytometry (data not shown). T cell lines were washed extensively and stimulated with 20 ng/ml PMA (Sigma-Aldrich), 1 μM ionomycin (Calbiochem Corp.), and 2 μM monensin (Calbiochem Corp.) for 6 h. The stimulated cells were treated with 20 μg/ml DNaseI (Roche Molecular Biochemicals) for 5 min, washed, incubated with FcBlock (PharMingen), and fixed with 4% formaldehyde (Polysciences Inc.) in PBS. The fixed cells were treated with PBS containing 5% nonfat dry milk, 0.1% saponin, 1 mM CaCl2, 1 mM MgSO4, 0.05% NaN3, 1% BSA, 10 mM Hepes, pH 7.0 for 30 min, incubated for 30 min with 2 μg/ml anti–mouse IFN-γ–FITC (XMG1.2; PharMingen) and 2 μg/ml anti–mouse IL-4–PE (BVD4-1D11; PharMingen), and then washed with the same buffer without nonfat dry milk 30. As negative controls, directly labeled isotype-matched mAbs were used. Flow cytometric analysis was performed as described above.
Immunoprecipitation and Western Blotting.
Cells were washed extensively, resuspended at 4 × 106/ml with 0.5% FCS Click's medium, and incubated for 1 h. 20 min after addition of mouse IL-12 (5 ng/ml), the cells were washed two times with cold PBS and lysed with 200 μl of lysis buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM EDTA, 1 mM EGTA, 10 mM NaF, 1 mM Na3VO4, and protein inhibitor cocktail; Roche Molecular Biochemical). After 30 min incubation at 4°C, the lysates were microcentrifuged for 30 min. The supernatants were collected, and mixed with 1 μg of normal rabbit IgG and 20 μl of protein A–agarose for 15 min. After centrifugation, the supernatants were incubated with 1 μg of anti-STAT4 (Santa Cruz Biotechnology) or anti-STAT3 antibody (Santa Cruz Biotechnology) for 1 h, added with protein A–agarose, and incubated overnight. The protein A–agarose was washed four times with lysis buffer without proteinase inhibitor, then eluted with SDS sample buffer at 95°C for 5 min. The samples were run in SDS-PAGE in reducing conditions, and transferred to Protran nitrocellulose membrane (Schleicher & Schuell). The blotted membranes were blocked with 2% BSA TBS-T, probed with 0.2 μg/ml horseradish peroxidase–labeled antiphosphotyrosine mAb (4G10; Upstate Biotechnology Inc.), and developed by Supersignal Chemiluminescence kit (Pierce Chemical Co.). After stripping, the membranes were reprobed with anti-STAT antibodies.
For the detection of the FLAG epitope tag of D10 cells transfected with VAhCD2IL-12Rβ2FLAG, 106 cells were lysed in 20 μl of lysis buffer (0.5% Triton X-100, 300 mM NaCl, 50 mM Tris-HCl, pH 7.5, proteinase inhibitor cocktail (Roche Molecular Biochemical), incubated for 45 min, centrifuged for 15 min, and 0.2% SDS/0.2% sodium deoxycholate (final concentration) was added to collected supernatants. The lysates were mixed with the SDS sample buffer, boiled for 5 min, gel electrophoresed in reducing conditions, and transferred to the nitrocellulose as described above. The blot was blocked with 5% nonfat dry milk TBS-T, probed with rabbit anti-FLAG antibody (Santa Cruz Biotechnology), and developed as described above.
Northern Blotting.
Total RNA was isolated with RNA STAT-60 RNA isolation kit (Tel-Test, Inc.). Mouse c-Maf cDNA (550 bp), which was PCR amplified with primers 5′-GAGCAGTTGGTGACCATGTC-3′ and 5′-CACCACAAGCTTGGTGTGTG-3′ and verified by sequencing, ClaI–NcoI fragment (1.1 kb) of mouse GATA-3 cDNA (provided by Dr. J. Engel, Northwestern University, Evanston, IL), and mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA (905 bp; Ambion Inc.) were used for probe synthesis. The probes were made with random primer kits (Amersham Pharmacia Biotech) with [α-32P]dCTP (3,000 Ci/mmol; Amersham Pharmacia Biotech). 10 μg of total RNA for D10 cells and 6 μg for primary T cell lines were separated on formaldehyde gel, and transferred to Nytran nylon membrane (Schleicher & Schuell) by capillary blotting. The blotted membranes were prehybridized and hybridized according to the manufacturer's protocol. After washing, the blot was exposed to a PhosphorImager® plate (Molecular Dynamics).
Results
Effect of Persistent IL-12R Expression on Naive T Cell Differentiation into Th2 Cells.
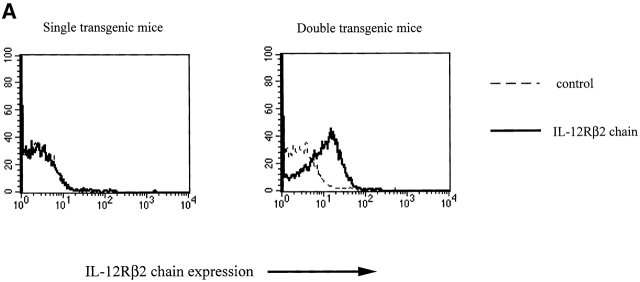
In initial studies, we determined the capacity of naive CD4+ T cells that constitutively express the IL-12Rβ2 chain to undergo Th2 cell differentiation (here defined by the ability of the CD4+ T cells to produce IL-4). For this purpose, we constructed IL-12Rβ2 chain transgenic mice expressing the IL-12Rβ2 chain on T cells under the control of the human CD2 promoter/enhancer (the VAhCD2 minigene vector) 21 28. As shown in Fig. 1 A, spleen CD4+ T cells from such transgenic mice do in fact manifest constitutive expression of the IL-12Rβ2 chain, as determined by flow cytometric analysis of cells stained with an IL-12Rβ2 chain–specific mAb (PDL-HAM10B9). To obtain CD4+ T cells expressing the IL-12Rβ2 chain transgene that respond to a particular antigen (OVA peptide), we made double transgenic mice by crossing the IL-12Rβ2 chain transgenic mice (backcrossed for two to three generations with Balb/c mice) to DO11.10 Balb/c mice expressing the TCR specific for OVA peptide in the context of I-Ad 29.
Figure 1.
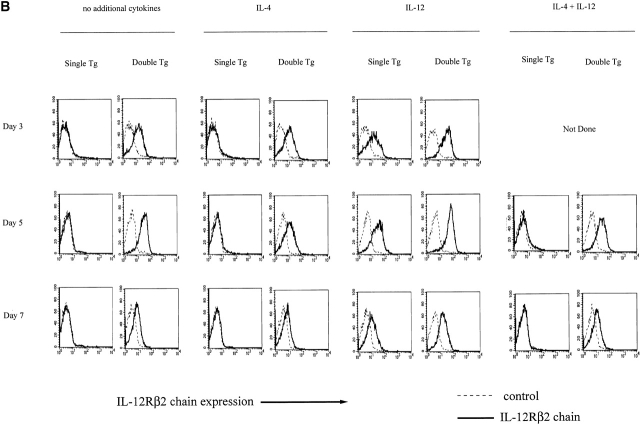
(A) IL-12Rβ2 chain expression on CD4+ cells from double transgenic mice (bearing an IL-12Rβ2 chain transgene and an OVA-TCR transgene) and single transgenic mice (bearing an OVA-TCR transgene only). Histograms of gated CD4+ cells are shown. (B) IL-12Rβ2 chain expression on T cell lines primed in vitro. CD4+CD62Lhigh splenocytes from double transgenic mice bearing an IL-12Rβ2 chain transgene and an OVA-TCR transgene (double Tg) and single transgenic mice bearing an OVA-TCR transgene only (single Tg) were stimulated with antigen (OVA peptide) and APCs without any cytokine addition, with IL-4 (200 U/ml), with IL-12 (2 ng/ml), or with IL-4 (200 U/ml) plus IL-12 (2 ng/ml), and stained for IL-12Rβ2 chain on CD4+ cells on day 3, 5, and 7 after priming. Each group of cells was stained with anti–mouse IL-12Rβ2 chain mAb (solid line) and control mAb (dashed line). Histograms of gated CD4+ cells are shown.
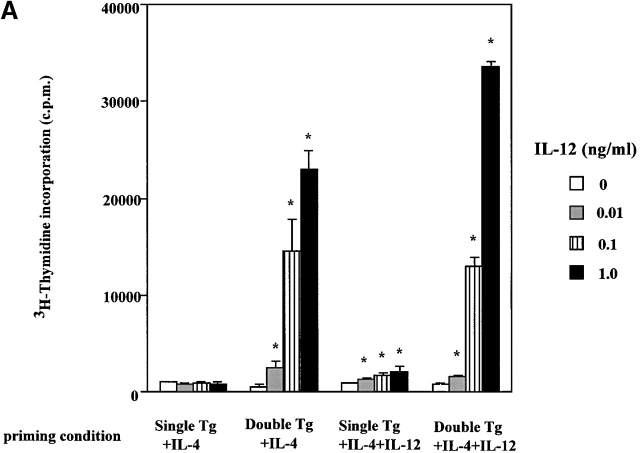
With the above double transgenic mice (as well as single transgenic littermates bearing the OVA-TCR transgene in the absence of the IL-12Rβ2 chain transgene) in hand, we stimulated flow-sorted naive CD4+ splenocytes from the transgenic mice with OVA peptide presented by irradiated syngeneic Balb/c T cell–depleted APCs in the presence of IL-4 (200 U/ml). As shown in Table , after culture for 1 wk under these conditions, cells obtained from both the double transgenic and single transgenic mice produce large amounts of IL-4 and only small amounts of IFN-γ and had thus differentiated into Th2 cells. Nevertheless, as shown in Fig. 1 B, CD4+ T cells from the double transgenic mice (double transgenic T cells) continued to express the IL-12Rβ2 chain on days 3, 5, and 7 after OVA stimulation in the presence of IL-4, whereas control CD4+ T cells from single transgenic mice (single transgenic T cells) expressed little or no IL-12Rβ2 chain. In additional studies, we showed that the CD4+ T cells that develop into Th2 cells from double transgenic mice express a functional IL-12R. Thus, as shown in Fig. 2a and Fig. b, these cells proliferate in response to exogenous IL-12 added on day 7 after initiation of culture and display STAT4 tyrosine phosphorylation, whereas cells from the single transgenic mice exhibit little or no such function. Overall, these studies provide initial evidence that early Th2 cell differentiation and IL-4 production can occur in the presence of persistent expression of a competent IL-12R. In addition, they confirm that unlike IFN-γ, IL-12 does not suppress Th2 cell proliferation 31 32 33.
Table 1.
Cytokine Production in Cultures of CD4+ T Cells from IL-12Rβ2 Chain and OVA-TCR Double Transgenic Mice 7 d after Antigen Stimulation
| IFN-γ | IL-4 | ||||
|---|---|---|---|---|---|
| Antigen | CD3 +CD28 | IL-12 +IL-18 | Antigen | CD3 +CD28 | |
| Single Tg + IL-4 | 0 | 2 | 3 | 36 | 349 |
| Double Tg + IL-4 | 2 | 8 | 2 | 40 | 352 |
| Single Tg + IL-12 | 2,480 | 2,499 | 388 | 6 | 0 |
| Double Tg + IL-12 | 2,713 | 3754 | 689 | 1 | 0 |
Figure 2.
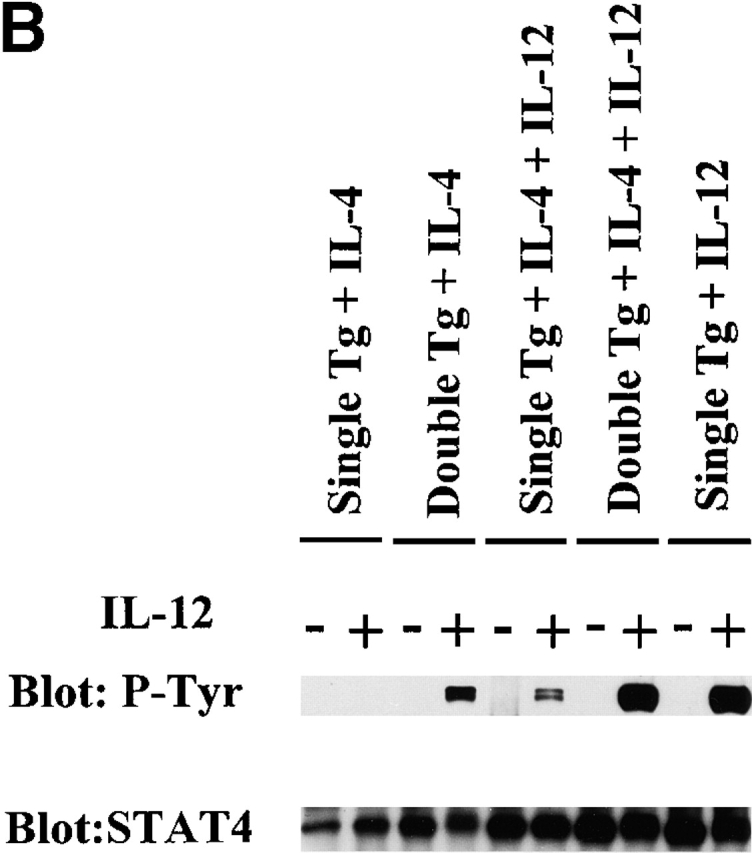
(A) Proliferation of primary T cell lines in response to IL-12. T cell lines from single and double transgenic mice primed with IL-4 (200 U/ml) or with IL-4 (200 U/ml) plus IL-12 (2 ng/ml) were harvested 7 d after priming, washed extensively, and recultured with varying concentrations of IL-12 (0–1.0 ng/ml) for 24 h; cultures were pulsed with 1 μCi of [3H]thymidine during the last 6 h. Error bars indicate SEM of triplicate cultures. Values obtained with varying IL-12 concentrations are compared with values obtained without IL-12 by Student's t test (*P < 0.05). (B) Immunoprecipitation Western blots for detection of tyrosine phosphorylation of STAT4 in primary T cell lines. Single transgenic T cells and double transgenic T cells were primed with IL-4, with IL-4 and IL-12, or with IL-12, harvested 7 d after priming, washed extensively, and stimulated with (+) or without (−) IL-12 (5 ng/ml) for 20 min. The same blot was stripped and reprobed with anti-STAT4 antibody.
One potential objection to the above results arises from the possibility that Balb/c APCs are poor IL-12 producers and thus that there was not enough endogenous IL-12 generated in the cultures to prevent Th2 cell development even though competent IL-12R was being expressed; we therefore determined Th2 cell differentiation in double transgenic T cells in the presence of exogenous IL-12 13 14. In this experiment, we added IL-12 (2 ng/ml; i.e., enough IL-12 to induce a robust Th1 cell response in the absence of IL-4) to cultures of naive CD4+ T cells from double and single transgenic mice primed with antigen in the presence of IL-4 (200 U/ml). As also shown in Fig. 2a and Fig. b, CD4+ T cells from double transgenic mice continued to express functional IL-12Rβ2 chain, as evidenced by their ability to manifest STAT4 tyrosine phosphorylation and T cell proliferation in the presence of IL-12. In contrast, under the same conditions CD4+ T cells from single transgenic mice expressed greatly reduced amounts of functional IL-12Rβ2 chain. Furthermore, as shown in Table , IL-4 production in cultures of double transgenic T cells in the presence of exogenous IL-12 was similar to (albeit slightly lower than) IL-4 production by such T cells in the absence of IL-12. Finally, although addition of IL-12 did induce low-level IFN-γ production in double transgenic T cells cultured with IL-4 plus IL-12, such production was ∼10–100-fold lower than that exhibited by such T cells stimulated by antigen in the presence of IL-12 alone.
Table 2.
Cytokine Production of CD4+ T Cell Lines Primed with IL-4 plus IL-12
| IFN-γ | IL-4 | ||||
|---|---|---|---|---|---|
| Antigen | CD3 +CD28 | IL-12 +IL-18 | Antigen | CD3 +CD28 | |
| Single Tg + IL-4 | 0 | 1 | 0 | 55 | 272 |
| Double Tg + IL-4 | 0 | 35 | 8 | 73 | 640 |
| Single Tg + IL-4 + IL-12 (2) | 0 | 0 | 0 | 78 | 777 |
| Double Tg + IL-4 + IL-12 (2) | 17 | 294 | 171 | 60 | 328 |
| Single Tg + IL-4 + IL-12 (25) | 0 | 63 | 0 | 60 | 378 |
| Double Tg + IL-4 + IL-12 (25) | 49 | 451 | 311 | 42 | 217 |
| Single Tg + IL-12 | 5,612 | 1,556 | 1,303 | 0 | 0 |
| Double Tg + IL-12 | 7,240 | 4,490 | 1,438 | 0 | 0 |
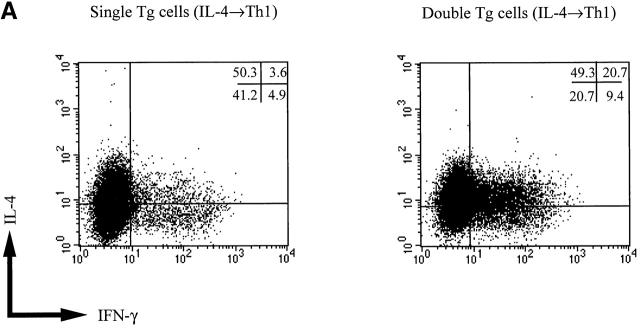
In further studies, we analyzed Th2 cell differentiation in cultures of double and single transgenic T cells at the single cell level by performing flow cytometry on cultured cells stained for detection of intracellular IL-4 and IFN-γ. As shown in Fig. 3, double transgenic T cells cultured with IL-4 alone gave rise to at least as many and perhaps more IL-4+/IFN-γ− cells (45.5%) as single transgenic T cells (27.4%). Perhaps more importantly, while double transgenic T cells cultured with IL-4 and IL-12 (2 ng/ml) gave rise to a greater number of IFN-γ+ (IL-4+ or IL-4−) T cells (12.3%) than did single transgenic T cells (2.2%), both cell populations gave rise to the same number of IL-4+/IFN-γ− cells (∼36%). Finally, we determined IL-12Rβ2 chain expression in double transgenic cells stimulated with IL-4 and IL-12 using triple staining for IFN-γ, IL-4, and IL-12Rβ2 chain and confirmed that cells in each quadrant of the relevant panels of Fig. 3 expressed similar level of IL-12Rβ2 chain (data not shown). This provides direct proof that both IL-4+/IFN-γ− and IL-4+/IFN-γ+ cells cultured in the presence of IL-12 retain IL-12Rβ2 chain expression.
Figure 3.
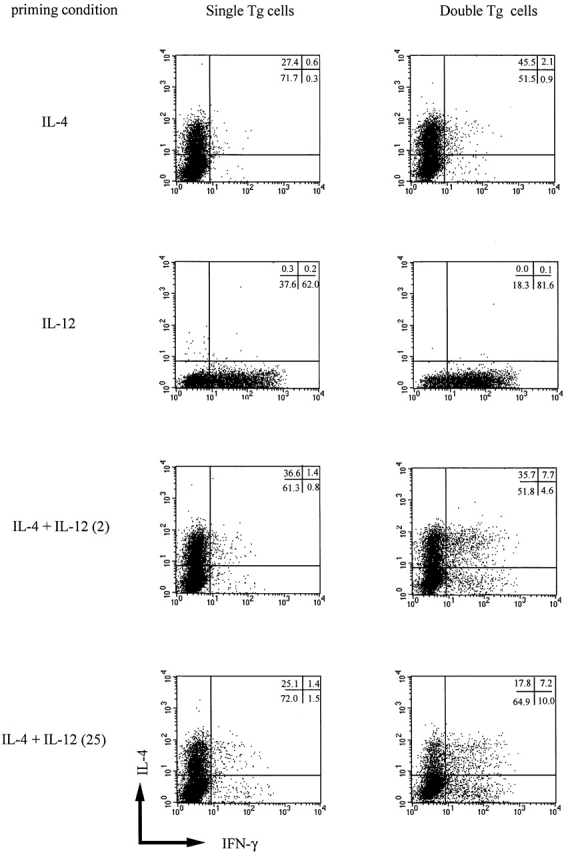
Intracellular cytokine staining for IL-4 and IFN-γ in T cell lines obtained from single and double transgenic mice. T cell lines were harvested 7 d after priming with IL-4 (200 U/ml), with IL-12 (2 ng/ml), with IL-4 (200 U/ml) plus IL-12 (2 ng/ml), or with IL-4 (200 U/ml) plus IL-12 (25 ng/ml), washed extensively, and stimulated with PMA and ionomycin as described in Materials and Methods. Cell populations were assigned quadrants based on isotype-matched labeled control mAbs. The percentage of cells in each quadrant is also shown. Representative data of three independent experiments are shown.
In a final series of studies along these lines, we also determined if high doses of IL-12 added to cultures of double transgenic T cells could reverse IL-4–induced Th2 differentiation (Table , and Fig. 3). In these studies, the effect on T cell differentiation was again measured by cytokine secretion in culture supernatant measured by ELISA and by intracellular cytokine expression measured by flow cytometry. As shown in Table , although addition of high doses of IL-12 (25 ng/ml) to cultures of double transgenic T cells containing IL-4 led to a decrease in IL-4 production, the latter was still the dominant cytokine being secreted. In addition, as shown in Fig. 3, while double transgenic T cells cultured with IL-4 and high doses of IL-12 (25 ng/ml) gave rise to about half the number of cells producing IL-4 alone (17.8%) as cells cultured with low doses of IL-12 (2 ng/ml), this population still remained the main population in this condition.
Taken together, these studies show that the presence of a competent IL-12R containing the IL-12Rβ2 chain does not prevent Th2 cell differentiation induced by IL-4 even when IL-12 is present and stimulates the T cell via its receptor.
Effect of Persistent IL-12R Expression on Established Th2 Cells.
Having established that neither expression of the IL-12Rβ2 chain nor signaling through the IL-12R can shut down naive CD4+ T cell differentiation into IL-4–producing cells driven by IL-4, we turned our attention to whether such expression and signaling could induce the conversion of Th2 cells into Th1 cells. In initial studies along these lines, we transfected an established mouse Th2 cell clone, D10.G4.1 (D10), with a mouse IL-12Rβ2 chain cDNA (containing a FLAG tag sequence just before the terminal codon) under a CD2 promoter/enhancer (see Materials and Methods). As shown in Fig. 4a and Fig. b, the stable transfectants thus obtained did express IL-12Rβ2 chain as determined by Western blotting with an anti-FLAG antibody and by flow cytometry using an anti–mouse IL-12Rβ2 chain–specific antibody. In addition, as shown in Fig. 4 B, using flow cytometry we showed that D10 cells express endogenous IL-12Rβ1 chain. Thus, the transfected cells could express a competent IL-12R. To confirm the latter point, we stimulated transfected D10 cells with IL-12 and showed that such stimulation results in tyrosine phosphorylation of both STAT3 and STAT4 in transfected cells but not in mock-transfected cells; in addition, such stimulation leads to proliferation of transfected D10 cells (data not shown). Therefore, we concluded that the transfected D10 cells did in fact express functional IL-12Rs and that reconstituted IL-12 signaling in differentiated Th2 cell induces the T cells to proliferate.
Figure 4.
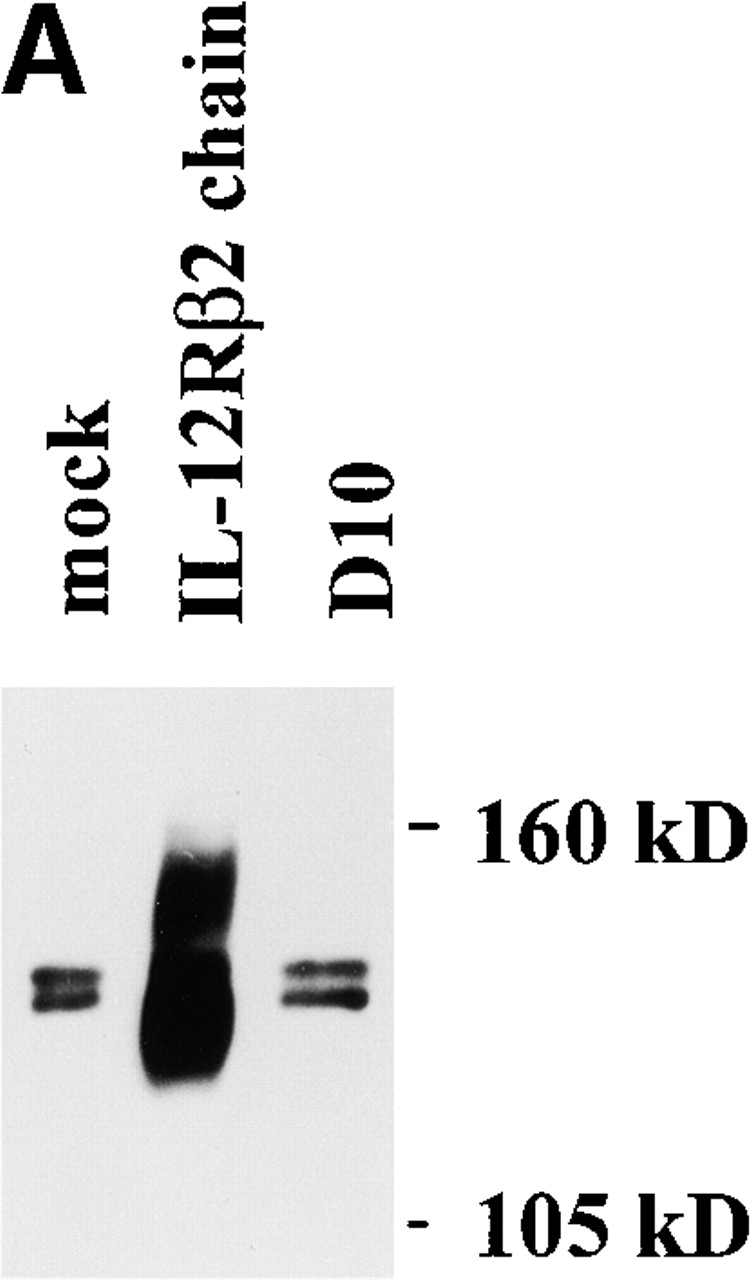
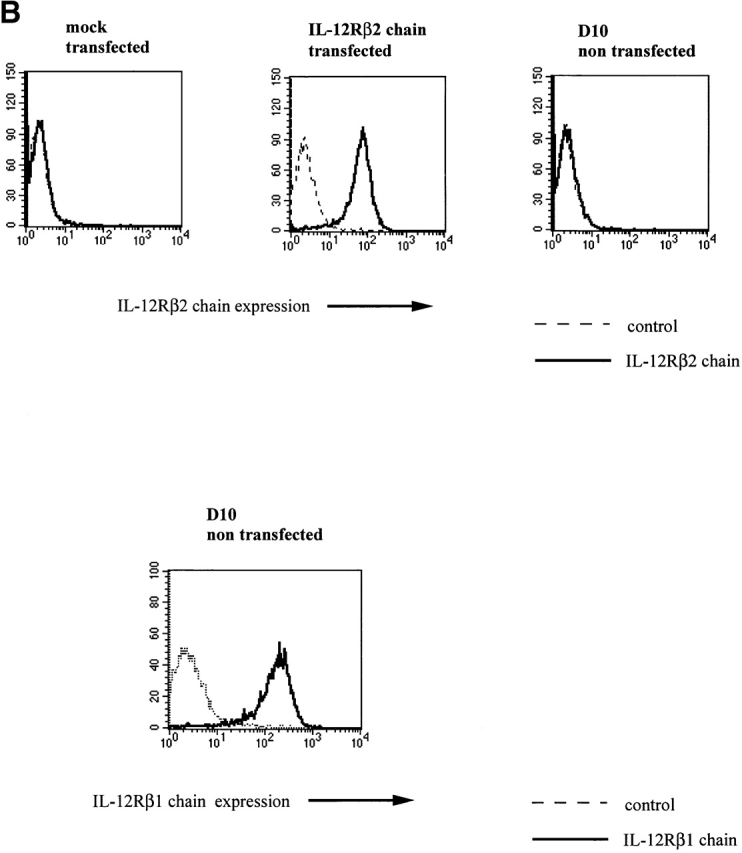
(A) Western blot for detection of IL-12Rβ2 chain FLAG epitopes in D10 cells transfected with VAhCD2IL-12Rβ2FLAG. Two broad specific bands (130 and 150 kD) were detected in the transfected cells with VAhCD2IL-12Rβ2FLAG (IL-12Rβ2 chain) compared with mock-transfected cells (mock) or parental cells (D10). After PNGase F (New England Biolabs) treatment of lysates and repeat Western blot, they gave rise to one sharp specific band of 96 kD, the expected size from amino acid residues of mouse IL-12Rβ2 chain cDNA (data not shown). (B) IL-12Rβ2 chain expression of D10 cell transfectants and endogenous IL-12Rβ1 chain expression of D10 cells by flow cytometry. D10 cell transfectants were stained with anti–mouse IL-12Rβ2 chain mAb 7 d after antigen (conalbumin) stimulation. For endogenous IL-12Rβ1 chain expression, nontransfected D10 cells were stained with biotinylated mouse anti–mouse IL-12Rβ1 chain mAb and streptavidin-PE, or with biotinylated mouse anti-TNP mAb as a negative control. Histogram of gated CD3ε1 cells is shown.
In further studies, we determined if signaling of D10 cells via the transfected IL-12Rβ2 chain could convert these cells from Th2 cells making large amounts of IL-4 but little or no IFN-γ into Th1 cells that make large amounts of IFN-γ 34. Accordingly, we stimulated transfected and control nontransfected or vector-transfected D10 cells with either anti-CD3ε plus anti-CD28 with or without IL-12, IL-12 plus IL-18, or irradiated APCs plus antigen (conalbumin) with or without IL-12. As shown in Table , we detected IFN-γ production at a modest level in only one out of three transfectants (clone NE7), mainly after anti-CD3ε plus anti-CD28 stimulation. Interestingly, all of the transfectants (including the IFN-γ–producing clone) still produced large amounts of IL-4 at a level similar to that of the parental cells or mock-transfected cells. Thus, these data show that IL-4 production cannot be extinguished and a high level of IFN-γ production cannot be induced in an established Th2 clone simply by reacquisition of the IL-12Rβ2 chain and IL-12 signaling.
Table 3.
Cytokine Production of Mouse Th2 Clone D10 Cells Expressing a Transfected IL-12Rβ2 Chain Gene
| − | CD3+CD28 | CD3+CD28+IL-12 | IL-12+IL-18 | Antigen | Antigen+IL-12 | |
|---|---|---|---|---|---|---|
| IFN-γ | ||||||
| D10 (parental clone) | 0 | 0.03 | 0 | 0 | 0.56 | 0.91 |
| VAG1 (mock transfected) | 0 | 0 | 0 | 0 | 0.65 | 1.05 |
| 19C1 (IL-12Rβ2 chain+) | 0 | 0 | 0 | 0 | 0.04 | 0.65 |
| ND2 (IL-12Rβ2 chain+) | 0 | 0 | 0 | 0 | 0 | 0.33 |
| NE7 (IL-12Rβ2 chain+) | 0 | 10.57 | 47.32 | 0.46 | 3.02 | 1.69 |
| IL-4 | ||||||
| D10 (parental clone) | 0 | 427 | 419 | ND | 35 | 32 |
| VAG1 (mock transfected) | 0 | 237 | 243 | ND | 32 | 31 |
| 19C1 (IL-12Rβ2 chain+) | 0 | 278 | 504 | ND | 22 | 13 |
| ND2 (IL-12Rβ2 chain+) | 0 | 179 | 191 | ND | 4 | 4 |
| NE7 (IL-12Rβ2 chain+) | 0 | 126 | 251 | ND | 41 | 38 |
Effect of Persistent IL-12R Expression on Primary Th2 Cell Lines.
To investigate the convertibility of a newly established Th2 cell line, we turned again to cells obtained from IL-12Rβ2 chain transgenic mice. In these studies, we first established Th2 cell lines from double and single transgenic mice expressing both the IL-12Rβ2 chain transgene and an OVA-specific TCR transgene or only an OVA-specific TCR transgene, respectively 35. This was accomplished by stimulation of naive CD4+ T cells in the presence of IL-4 and then culturing the Th2 cell lines obtained under Th1 cell conditions (IL-12 [2 ng/ml] and anti–IL-4 mAb [10 μg/ml]) for three successive antigen stimulation cycles spaced 6 d apart. As shown in Table , although IFN-γ production elicited in T cell lines derived from double transgenic mice was greater than that elicited in single transgenic mice, the level of IFN-γ was still ∼7–30-fold less than obtained in double transgenic T cells primed in the presence of IL-12. Perhaps more importantly, IL-12 signaling of the Th2 cells did not reduce the high level of IL-4 production in double transgenic T cells, and the latter continued to produce as much IL-4 as observed in single transgenic T cells. In addition, as shown in Fig. 5 A, by using intracellular cytokine staining, we showed that while IFN-γ+ cells (IL-4−/IFN-γ+ and IL-4+/IFN-γ+ cells) arose in cultures of both single and double transgenic cells (8.5 and 30.1%, respectively), IL-4+/IFN-γ− cells were still the predominant subpopulation in both single and double transgenic cell populations (50.3 and 49.3%, respectively). Interestingly, two thirds of IFN-γ–producing cells in double transgenic cells were IL-4+/IFN-γ+ cells. As shown in Fig. 5 B, the Th2 cells from the double transgenic mice continued to exhibit IL-12 signaling indicated by STAT4 tyrosine phosphorylation. Thus, these studies show that even in newly differentiated Th2 cells, IL-4 production was not terminated by IL-12Rβ2 chain expression and IL-12 signaling.
Table 4.
Newly Differentiated Th2 Cells from Double Transgenic Mice (Expressing an IL-12Rβ2 Chain Transgene) Maintain Th2 Phenotype after Repeated Restimulation under Th1 Conditions
| IFN-γ | IL-4 | ||||
|---|---|---|---|---|---|
| Antigen | CD3 +CD28 | IL-12 +IL-18 | Antigen | CD3 +CD28 | |
| Single Tg + IL-4 | 1 | 27 | 0 | 56 | 195 |
| Double Tg + IL-4 | 8 | 194 | 3 | 125 | 533 |
| Single Tg (IL-4 →Th1) | 213 | 290 | 78 | 98 | 164 |
| Double Tg (IL-4 →Th1) | 634 | 1,524 | 1,101 | 222 | 214 |
| Single Tg + IL-12 | 16,411 | 21,250 | 5,306 | 0 | 0 |
| Double Tg + IL-12 | 20,766 | 24,395 | 7,710 | 0 | 0 |
Figure 5.
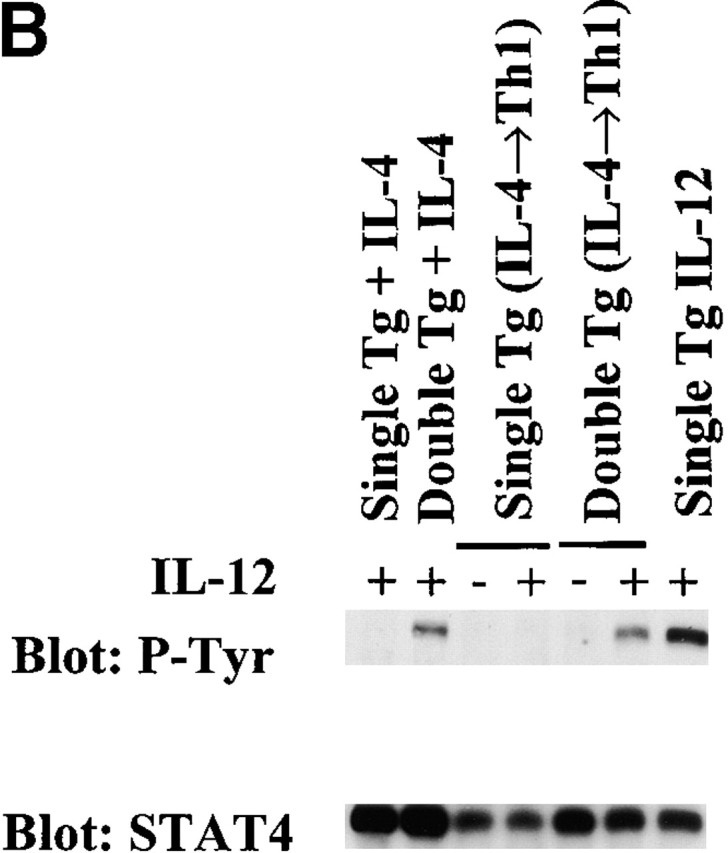
(A) Intracellular cytokine staining for IL-4 and IFN-γ in IL-4–primed cell lines from single and double transgenic mice restimulated three times with OVA peptide, APCs, and IL-12 (2 ng/ml) and anti–IL-4 mAb (10 μg/ml). T cell lines were harvested 7 d after final antigen stimulation and treated as described in the legend to Fig. 3. Representative data of three independent experiments are shown. (B) Immunoprecipitation Western blot for detection of STAT4 tyrosine phosphorylation in Th2 cells cultured for three cycles under Th1 conditions. IL-4–primed single and double transgenic cells were restimulated with OVA peptide, APCs, IL-12 (2 ng/ml), and anti–IL-4 mAb (10 μg/ml) over three cycles (IL-4→Th1); 7 d after final stimulation, T cells were harvested and restimulated with (+) or without (−) IL-12 (5 ng/ml). As controls, cells cultured with IL-4 or IL-12 for four cycles are also shown. The same blots were stripped and reprobed with anti-STAT4 antibody.
GATA-3 and c-Maf Expression in IL-12Rβ2 Chain Transgenic Th2 Cells.
GATA-3 and c-Maf are transcription factors associated with Th2 cells and have been implicated in the termination of IL-12 signaling 11 12 36. Therefore, we determined the expression of GATA-3 and c-Maf in D10 cells and Th2 cells expressing the IL-12Rβ2 chain and then stimulated in the presence of IL-12. As shown in Fig. 6 A, transfected D10 cells express a similar level of GATA-3 and c-Maf in mRNA in relation to parental cells or mock-transfected cells. In addition, as shown in Fig. 6 B, although IL-4–primed T cells from single and double transgenic mice express both GATA-3 and c-Maf mRNA, the addition of IL-12 in the culture did not shut down the expression of such mRNA previously induced by IL-4. Thus, IL-4–induced GATA-3 and c-Maf expression is not significantly decreased by IL-12 signaling via the IL-12Rβ2 chain.
Figure 6.
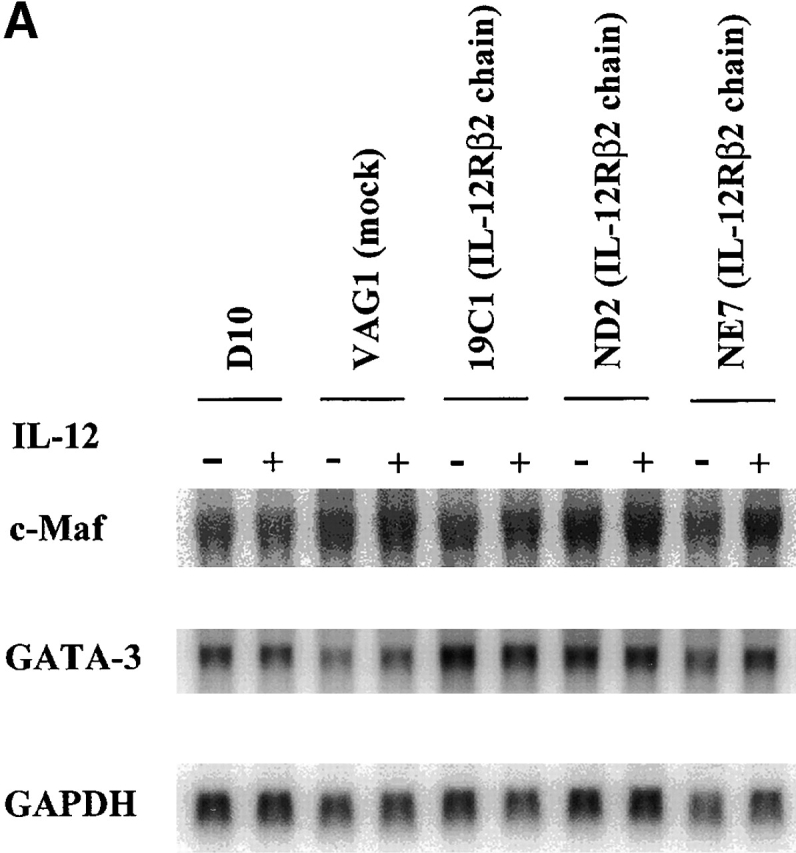
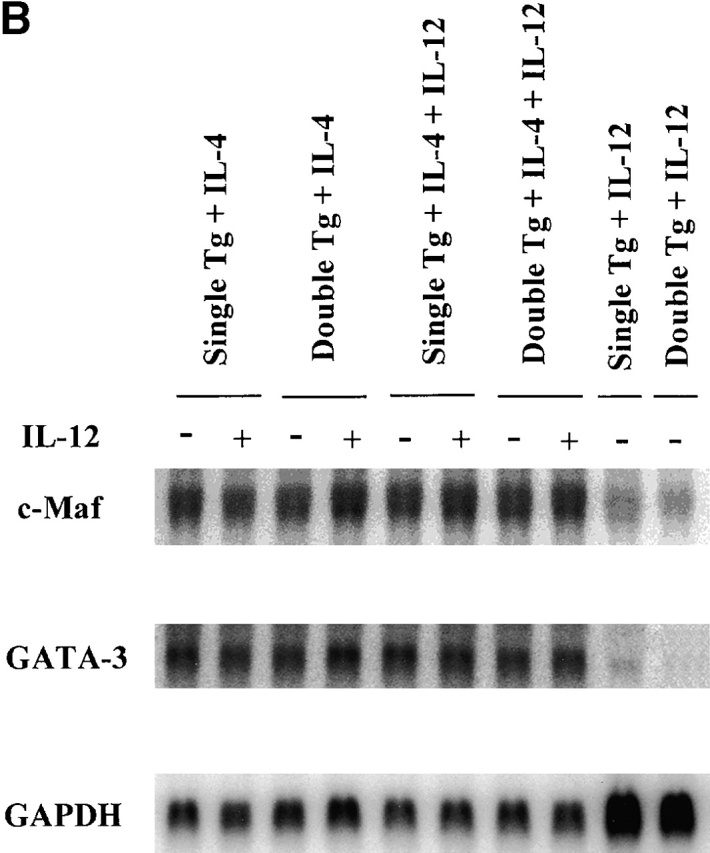
(A) Northern blot studies for detection of c-Maf and GATA-3 mRNA in D10 cells. D10 cells and transfectants (VAG1 [mock]; 19C1, ND2, and NE7 [IL-12Rβ2 chain]) were harvested 10 d after antigen (conalbumin) stimulation, washed extensively, and then restimulated with surface-bound anti-CD3ε (5 μg/ml) plus soluble anti-CD28 (1 μg/ml) with (+) or without (−) IL-12 (5 ng/ml). 6 h after restimulation, total RNA was isolated and subjected to Northern blotting. 10 μg of total RNA was loaded in each lane. The same blot was probed with GATA-3, c-Maf, and GAPDH sequentially. (B) Northern blot studies for detection of c-Maf and GATA3 mRNA in T cell lines from single and double transgenic mice. T cell lines primed with IL-4 (200 U/ml), with IL-4 (200 U/ml) plus IL-12 (2 ng/ml), or with IL-12 (2 ng/ml) from single and double transgenic mice were harvested 7 d after priming and restimulated with surface-bound anti-CD3ε (5 μg/ml) plus soluble anti-CD28 (1 μg/ml) with (+) or without (−) IL-12 (5 ng/ml). 6 h after restimulation, total RNA was isolated and subjected to Northern blotting. 6 μg of total RNA was loaded in each lane. The same blot was probed with c-Maf, GATA-3, and GAPDH sequentially.
Discussion
In the view of T cell differentiation, the important concept has been put forward that IL-4 counterregulates Th1 cell development by controlling expression of the β2 chain of the heterodimeric IL-12R, i.e., the receptor component necessary for STAT4 activation and IFN-γ production 21 22 23 24. Thus, in the model of Leishmania infection occurring in Balb/c mice, it has been speculated that a burst of IL-4 production at an early stage of infection induces downregulation of the IL-12Rβ2 chain on reacting T cells, which then leads to a dominant Th2 response and the nonresolving infection characteristic of this mouse strain 37 38. However, it should be noted that any concept that implies that the IL-12Rβ2 chain is a molecular switch controlling Th1/Th2 cell development must contend with the fact that the IL-12Rβ2 chain is upregulated and sustained by IL-12 or IFN-γ (in the mouse) 23, so that its expression could reflect the fact that it is merely one of several Th1 phenotypic markers rather than a controlling differentiation factor. In fact, in this study we provide evidence in support of the latter possibility in that we show that although IL-12Rβ2 chain downregulation may occur during Th2 cell differentiation (defined by the ability of the CD4+ T cells to produce IL-4), it is not necessary for such differentiation. More particularly, we demonstrated that naive CD4+ T cells expressing a transgene for the IL-12Rβ2 chain under a human CD2 promoter/enhancer and stimulated in the presence of IL-12 and IL-4 develop into IL-4–producing cells despite continued IL-12 signaling via the IL-12R and STAT4 tyrosine phosphorylation. In addition, we showed with Northern blotting that the Th2-specific transcription factors GATA-3 and c-Maf induced by IL-4 are not shut down by sustained IL-12 signaling. Finally, we demonstrated that although IFN-γ production was dependent on IL-12Rβ2 chain expression and IL-12, IFN-γ production by CD4+ T cells bearing the IL-12Rβ2 transgene was ∼10–100-fold less when primed with IL-4 and IL-12 than when primed with IL-12 alone, even though the level of IL-12Rβ2 expression and STAT4 tyrosine phosphorylation was the same under these two conditions. Taken together, these findings strongly suggest that IL-4 activates a mechanism to suppress IFN-γ production that is independent of IL-12 signaling and/or suppression of the IL-12Rβ2 chain. One candidate mechanism for such IL-4–mediated suppression is the induction of GATA-3 and/or c-Maf, IL-4–induced transcriptional factors that have been shown to be suppressive of IFN-γ production 36 39. However, GATA-3 has suppressive effects on IL-12Rβ2 chain expression and suppressive effects by c-Maf are overcome by the addition of IL-12; therefore, how much these factors contribute to the direct suppressive effects of IL-4 on IFN-γ production needs further analysis.
The conclusion drawn above, that Th2 differentiation can proceed in the presence of IL-12 signaling, should not be taken to imply that IL-12 signaling has no influence on such differentiation. Thus, in cultures of naive single and double transgenic T cells in the presence of IL-4 and the absence of exogenous IL-12, the double transgenic T cells that emerge produce more IL-4 than their single transgenic counterparts (Table , and Fig. 3). This can be explained by assuming that APCs in these cultures produce some endogenous IL-12, and the latter through its capacity to reduce the expression of the cdk inhibitor p27Kip1, via STAT4 activation 40, results in increased T cell proliferation; in turn, this leads to greater IL-4 production because IL-4 gene activation is highly cell cycle dependent 41. On the other hand, double transgenic T cells cultured with IL-4 plus exogenous IL-12 produce less IL-4 than single transgenic T cells (Table , and Fig. 3). This suggests that IL-12 signaling can work against IL-4 gene activation when cells are exposed to higher levels of IL-12. Whether the inhibitory effect is a direct effect of IL-12 on Th2 cell differentiation or an indirect effect of IL-12 mediated by IFN-γ 31 32 33 or by induction of nitric oxide synthetase 2 42 requires further study. Finally, it should be noted that cultures of double transgenic cells with IL-4 plus exogenous IL-12 leads to the appearance of modest numbers of IL-4+/IFN-γ+ cells. This may reflect the more frequent occurrence of IL-4+/IFN-γ+ cells in humans than in mice 43 44, since in humans IFN-α can directly transduce a STAT4 signal independently of IL-12 and the IL-12Rβ2 chain and thus induce naive human CD4+ T cells to become IFN-γ–producing cells even in the presence of IL-4 24 25.
In the studies reported here, we used a newly developed mAb specific for the mouse IL-12Rβ2 chain to assess expression of the latter in transgenic T cells and transfected T cells. The data collected with the use of this antibody require some comment. First, we noted that although transgenic CD4+ T cells cultured under a variety of conditions express high levels of surface IL-12Rβ2 chain 3 d after stimulation, such expression declines with continued culture and is considerably reduced by 7 d (Fig. 1 B). However, it should be noted that despite this decline, IL-12 induces significant proliferation (Fig. 2 A) and high levels of STAT4 tyrosine phosphorylation (Fig. 2 B). Thus, it is apparent that either STAT4 tyrosine phosphorylation is a more sensitive index of the presence of a competent IL-12R function than is flow cytometry with this antibody or that even a low level of IL-12Rβ2 chain expression is sufficient for maximal transduction of the IL-12 signal. These considerations make it unlikely that IL-4–induced downregulation of the IL-12Rβ2 chain transgene and the emergence of a subpopulation of cells during culture that lack IL-12Rβ2 chain expression explain the results obtained in these studies. Further evidence in support of the latter conclusion is that IL-12Rβ2 chain expression on cells was manifest as a single peak on flow cytometry throughout the period of study and that analysis of IL-12Rβ2 chain expression on IL-4+/IFN-γ− and IL-4+/IFN-γ+ cells using intracellular cytokine staining showed that these cells retained similar levels of IL-12Rβ2 chain expression as IL-4−/IFN-γ+ cells.
A second area addressed by these studies is the role of IL-12Rβ2 chain and IL-12 signaling on the maintenance of Th1/Th2 phenotypes 45. Previous work in this area has shown that repetitive stimulation of CD4+ T cells via the TCR under Th1 or Th2 cytokine conditions causes fixation of the Th1 or Th2 phenotype, respectively 26 35 46, and suggested that the basis of such fixation with regard to Th2 cells was again the loss of IL-12Rβ2 chain expression. We obtained two types of data that address the latter hypothesis. First, we showed that an established Th2 clone (D10) stably transfected with the IL-12Rβ2 chain and capable of transducing STAT4/STAT3 tyrosine phosphorylation does not produce large amounts of IFN-γ and (more importantly) does not manifest decreased IL-4 production upon stimulation in the presence of IL-12; thus, this T cell clone does not convert to a Th1 cell. Such lack of conversion may be due to any of several factors. One is that IL-4 signaling negatively affects IFN-γ production independently of IL-12Rβ2 chain expression, as mentioned above. Another is that differentiation of Th2 cells is accompanied by epigenetic changes such as methylation of the IFN-γ promoter and establishment of DNaseI hypersensitivity sites in cytokine gene regions that, in effect, make back-differentiation a nonregulatable and irreversible process 34 47 48 49. In any case, the effects of Th2 differentiation are not absolute in that one clone of D10 cells expressing the IL-12R did make small amounts of IFN-γ. Finally, it is clear that not all IL-12 signaling pathways in D10 cells are inoperative, as D10 cells proliferate in response to IL-12 stimulation.
Another and perhaps more cogent way in which we examined the convertibility of the Th2 cell was to examine early Th2 cells produced by a single round of stimulation of naive CD4+ T cells from IL-12Rβ2 transgenic mice with IL-4, although IL-4–primed CD4+ T cells lines contain few IFN-γ–producing cells and IL-4−/IFN-γ− cells, which means that the starting population was not a pure Th2 cell line 26 35. In these studies, we showed that repeated restimulation of such cells in the presence of IL-12 and anti–IL-4 mAb (i.e., in the presence of Th1 condition) did not reverse their basic Th2 phenotype or IL-4 production capability even when IL-12 signaling is maintained. Therefore, these studies show that the irreversible commitment to IL-4 production occurs early and occurs irrespective of signaling via the IL-12R. As in the case of established D10 Th2 clones, IFN-γ production of double transgenic Th2 cell lines cultured sequentially with exogenous IL-12 and anti–IL-4 mAb was only about a tenth of that of T cell lines cultured with IL-12 (Table ). However, it should be noted that the IL-12Rβ2 chain transgene driven by the human CD2 promoter/enhancer used in these studies appeared to be somewhat downregulated by IL-4 (Fig. 1 B) and possibly by other factors as well. Thus, the IFN-γ production difference might be due either to suppression of IFN-γ gene expression by IL-4–related factors or to lower expression of IL-12Rβ2 chain in the double transgenic Th2 lines cultured sequentially with exogenous IL-12 and anti–IL-4 mAb.
In summary, using IL-12Rβ2 chain transgenic mice and anti–mouse IL-12Rβ2 mAb, we demonstrated that Th2 cell differentiation can occur in the presence of IL-12Rβ2 chain and IL-12 signaling, and that once the IL-4 gene is activated IL-4 production cannot be shut down by IL-12 signaling.
Acknowledgments
We would like to thank Dr. U. Gubler for providing mouse IL-12Rβ2 chain cDNA, Dr. D. Kioussis for providing VAhCD2 minigene vector, and Dr. C.Y. Wu for providing biotinylated mouse anti–mouse IL-12Rβ1 chain mAb. We also appreciated technical support of Ms. L. Penoyer for DNA sequencing, and the technical support of Dr. J. Hewitt and the National Institute of Allergy and Infectious Diseases Transgenic Facility Staff for making IL-12Rβ2 chain transgenic mice, maintaining the mouse colony, and making tail DNAs. Finally, we acknowledge the technical support from Ms. S. Barbieri for FACS® sorting and Ms. K. Nishikomori for ELISA assays, helpful discussions with Dr. C. Prussin regarding intracellular cytokine staining, and critical comments on the manuscript by Dr. R. Seder and Dr. J. O'Shea.
Footnotes
Abbreviations used in this paper: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; STAT, signal transducer and activator of transcription.
References
- Murphy K.M., Ouyang W., Szabo S.J., Jacobson N.G., Guler M.L., Gorham J.D., Gubler U., Murphy T.L. T helper differentiation proceeds through Stat1-dependent, Stat4-dependent and Stat4-independent phases. Curr. Top. Microbiol. Immunol. 1999;238:13–26. doi: 10.1007/978-3-662-09709-0_2. [DOI] [PubMed] [Google Scholar]
- Hsieh C.S., Heimberger A.B., Gold J.S., O'Garra A., Murphy K.M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T cell-receptor transgenic system. Proc. Natl. Acad. Sci. USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gros G., Ben-Sasson S.Z., Seder R., Finkelman F.D., Paul W.E. Generation of interleukin 4 (IL-4)–producing cells in vivo and in vitroIL-2 and IL-4 are required for in vitro generation of IL-4–producing cells. J. Exp. Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S.L., Weinberg A.D., English M., Huston G. IL-4 directs the development of Th2-like helper effectors. J. Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- Seder R.A., Paul W.E., Davis M.M., Fazekas de St. Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J. Exp. Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M., Le Gros G., Bachmann M., Lamers M.C., Bluethmann H., Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- Kaplan M.H., Schindler U., Smiley S.T., Grusby M.J. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- Shimoda K., van Deursen J., Sangster M.Y., Sarawar S.R., Carson R.T., Tripp R.A., Chu C., Quelle F.W., Nosaka T., Vignali D.A. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- Takeda K., Tanaka T., Shi W., Matsumoto M., Minami M., Kashiwamura S., Nakanishi K., Yoshida N., Kishimoto T., Akira S. Essential role of Stat6 in IL-4 signaling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- Kubo M., Ransom J., Webb D., Hashimoto Y., Tada T., Nakayama T. T cell subset-specific expression of the IL-4 gene is regulated by a silencer element and STAT6. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:4007–4020. doi: 10.1093/emboj/16.13.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Flavell R.A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Ho I.C., Hodge M.R., Rooney J.W., Glimcher L.H. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–983. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- Seder R.A., Gazzinelli R., Sher A., Paul W.E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc. Natl. Acad. Sci. USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.S., Macatonia S.E., Tripp C.S., Wolf S.F., O'Garra A., Murphy K.M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Magram J., Connaughton S.E., Warrier R.R., Carvajal D.M., Wu C.Y., Ferrante J., Stewart C., Sarmiento U., Faherty D.A., Gately M.K. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- Jacobson N.G., Szabo S.J., Weber-Nordt R.M., Zhong Z., Schreiber R.D., Darnell J.E., Jr., Murphy K.M. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J. Exp. Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M.H., Sun Y.L., Hoey T., Grusby M.J. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- Thierfelder W.E., van Deursen J.M., Yamamoto K., Tripp R.A., Sarawar S.R., Carson R.T., Sangster M.Y., Vignali D.A., Doherty P.C., Grosveld G.C., Ihle J.N. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- Carter L.L., Murphy K.M. Lineage-specific requirement for signal transducer and activator of transcription (Stat)4 in interferon gamma production from CD4+ versus CD8+ T cells. J. Exp. Med. 1999;189:1355–1360. doi: 10.1084/jem.189.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Sun Y.L., Hoey T. Cooperative DNA binding and sequence-selective recognition conferred by the STAT amino-terminal domain. Science. 1996;273:794–797. doi: 10.1126/science.273.5276.794. [DOI] [PubMed] [Google Scholar]
- Presky D.H., Yang H., Minetti L.J., Chua A.O., Nabavi N., Wu C.Y., Gately M.K., Gubler U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc. Natl. Acad. Sci. USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S.J., Jacobson N.G., Dighe A.S., Gubler U., Murphy K.M. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2:665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Szabo S.J., Dighe A.S., Gubler U., Murphy K.M. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogge L., Barberis-Maino L., Biffi M., Passini N., Presky D.H., Gubler U., Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J. Exp. Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogge L., D'Ambrosio D., Biffi M., Penna G., Minetti L.J., Presky D.H., Adorini L., Sinigaglia F. The role of Stat4 in species-specific regulation of Th cell development by type I IFNs. J. Immunol. 1998;161:6567–6574. [PubMed] [Google Scholar]
- Hu-Li J., Huang H., Ryan J., Paul W.E. In differentiated CD4+ T cells, interleukin 4 production is cytokine-autonomous, whereas interferon gamma production is cytokine-dependent. Proc. Natl. Acad. Sci. USA. 1997;94:3189–3194. doi: 10.1073/pnas.94.7.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C.A., Jr. Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J. Exp. Med. 1983;158:836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhumabekov T., Corbella P., Tolaini M., Kioussis D. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J. Immunol. Methods. 1995;185:133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
- Murphy K.M., Heimberger A.B., Loh D.Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Prussin C., Openshaw P.J. Detection of intracellular cytokines by flow cytometry Coligan J.E., Kruisbeek A.M., Margulies D.H., Shevach E.M., Strober W. Current Protocols in Immunology 1998. John Wiley & Sons, Inc; New York: 6.24.1–6.24.11. [Google Scholar]
- Gajewski T.F., Fitch F.W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J. Immunol. 1988;140:4245–4252. [PubMed] [Google Scholar]
- Pernis A., Gupta S., Gollob K.J., Garfein E., Coffman R.L., Schindler C., Rothman P. Lack of interferon gamma receptor beta chain and the prevention of interferon gamma signaling in TH1 cells. Science. 1995;269:245–247. doi: 10.1126/science.7618088. [DOI] [PubMed] [Google Scholar]
- Bach E.A., Szabo S.J., Dighe A.S., Ashkenazi A., Aguet M., Murphy K.M., Schreiber R.D. Ligand-induced autoregulation of IFN-gamma receptor beta chain expression in T helper cell subsets. Science. 1995;270:1215–1218. doi: 10.1126/science.270.5239.1215. [DOI] [PubMed] [Google Scholar]
- Young H.A., Ghosh P., Ye J., Lederer J., Lichtman A., Gerard J.R., Penix L., Wilson C.B., Melvin A.J., McGurn M.E. Differentiation of the T helper phenotypes by analysis of the methylation state of the IFN-gamma gene. J. Immunol. 1994;153:3603–3610. [PubMed] [Google Scholar]
- Murphy E., Shibuya K., Hosken N., Openshaw P., Maino V., Davis K., Murphy K., O'Garra A. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J. Exp. Med. 1996;183:901–913. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W., Ranganath S.H., Weindel K., Bhattacharya D., Murphy T.L., Sha W.C., Murphy K.M. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- Launois P., Maillard I., Pingel S., Swihart K.G., Xenarios I., Acha-Orbea H., Diggelmann H., Locksley R.M., MacDonald H.R., Louis J.A. IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6:541–549. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- Himmelrich H., Parra-Lopez C., Tacchini-Cottier F., Louis J.A., Launois P. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor beta 2-chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12. J. Immunol. 1998;161:6156–6163. [PubMed] [Google Scholar]
- Ho I.C., Lo D., Glimcher L.H. c-maf promotes T helper cell type 2 (Th2) and attenuates Th1 differentiation by both interleukin 4–dependent and –independent mechanisms. J. Exp. Med. 1998;188:1859–1866. doi: 10.1084/jem.188.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M.H., Daniel C., Schindler U., Grusby M.J. Stat proteins control lymphocyte proliferation by regulating p27Kip1 expression. Mol. Cell. Biol. 1998;18:1996–2003. doi: 10.1128/mcb.18.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird J.J., Brown D.R., Mullen A.C., Moskowitz N.H., Mahowald M.A., Sider J.R., Gajewski T.F., Wang C.R., Reiner S.L. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- Koblish H.K., Hunter C.A., Wysocka M., Trinchieri G., Lee W.M. Immune suppression by recombinant interleukin (rIL)-12 involves interferon γ induction of nitric oxide synthase 2 (iNOS) activityinhibitors of NO generation reveal the extent of rIL-12 vaccine adjuvant effect. J. Exp. Med. 1998;188:1603–1610. doi: 10.1084/jem.188.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chretien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J. Immunol. 1988;141:849–855. [PubMed] [Google Scholar]
- Maggi E., Del Prete G., Macchia D., Parronchi P., Tiri A., Chretien I., Ricci M., Romagnani S. Profiles of lymphokine activities and helper function for IgE in human T cell clones. Eur. J. Immunol. 1988;18:1045–1050. doi: 10.1002/eji.1830180712. [DOI] [PubMed] [Google Scholar]
- Coffman R.L., Mocci S., O'Garra A. The stability and reversibility of Th1 and Th2 populations. Curr. Top. Microbiol. Immunol. 1999;238:1–12. doi: 10.1007/978-3-662-09709-0_1. [DOI] [PubMed] [Google Scholar]
- Perez V.L., Lederer J.A., Lichtman A.H., Abbas A.K. Stability of Th1 and Th2 populations. Int. Immunol. 1995;7:869–875. doi: 10.1093/intimm/7.5.869. [DOI] [PubMed] [Google Scholar]
- Bix M., Locksley R.M. Independent and epigenetic regulation of the interleukin-4 alleles in CD4+ T cells. Science. 1998;281:1352–1354. doi: 10.1126/science.281.5381.1352. [DOI] [PubMed] [Google Scholar]
- Hollander G.A., Zuklys S., Morel C., Mizoguchi E., Mobisson K., Simpson S., Terhorst C., Wishart W., Golan D.E., Bhan A.K., Burakoff S.J. Monoallelic expression of the interleukin-2 locus. Science. 1998;279:2118–2121. doi: 10.1126/science.279.5359.2118. [DOI] [PubMed] [Google Scholar]
- Agarwal S., Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]